Abstract
A novel flexible ion-selective sensor for potassium and sodium detection was proposed. Flexible ion-selective electrodes with pseudo-liquid internal solution on contrary to the system with a solid contact provided a more stable analytical signal. Such advantages were achieved because of polyelectrolyte (PEI/PSS) layers adsorption on the conduct substrate with a layer-by-layer technique. Such an approach demonstrated that ion-selective electrodes save sensitivity with Nernstian dependence: 56.2 ± 1.4 mV/dec aNa+ and 56.3 ± 1.9 mV/dec aK+, as well as a fast time of response for potassium (5 s) and sodium (8 s) was shown. The sensing platform proposed demonstrates a better time of response and is close to the Nernstian value of sensitivity with a sensor low cost. The results proposed confirm a pseudo-liquid junction for the ion-selective electrode. Biocompatibility of an ion-selective sensing platform was demonstrated at potassium potentiometric measurements in Escherichia coli biofilms. Potassium levels in a biofilm were measured with potentiometry and showed agreement with the previous results.
Introduction
Real-time, wearable, minimally invasive monitoring is used in medical diagnosis and professional sport.1−5 The application on sensors becomes hard because of dilution of the biomarkers of interest, as well as variability in salinity, pH, and other physicochemical variables which directly impact the readout of real-time biosensors.6−13 Such parameters as sodium (Na+),14 potassium (K+),15 calcium (Ca2+), and others16−18 are analyzed in professional sports. Electrolyte ions in aqueous solutions are usually measured using a potentiometric method with ion-sensitive membranes.18 Potentiometric ion-selective electrodes (ISEs) have already been in use for half-a-century, mainly in physiological studies.19 Typical ISEs are liquid contact electrodes. Until recently, ISE devices were noncompatible with the principles of miniaturization and portability. Both the internal solution and the internal electrode are strived to be eliminated to be replaced by the solid contact.20,21 However, elimination of the internal reference system and its replacement with the solid contact resulted in insufficient long-term stability of ISE potentials and in poor piece-to-piece reproducibility.19,22 We suppose that polyelectrolyte multilayers formed by layer-by-layer assembly23,24 have hydration activity25−28 and can serve as the inner electrode solution in potentiometric sensors.29−31 This paper describes the design and fabrication of a flexible ion-sensing electrochemical adhesive tape (AT)-based analytical device for potentiometric measurements of potassium and sodium ions. This sensing platform has a carbon AT that contains ISEs with conventional ion-selective membranes (ISMs) immobilized within a polyelectrolyte multilayer as the pseudo-inner solution and ion-to-electron transducer. Within the polyelectrolyte layers, potassium chloride is used as an ion source. Carbon ATs are cheap flexible electronic conductors. The required selectivity for the target analyte is achieved by using a suitable ionophore.
Because of unique properties such as light weight, low cost, high flexibility, excellent elasticity, and figure moulding, the flexible carbon conductive AT could serve as an ideal platform for personalized wearable devices.
Results and Discussion
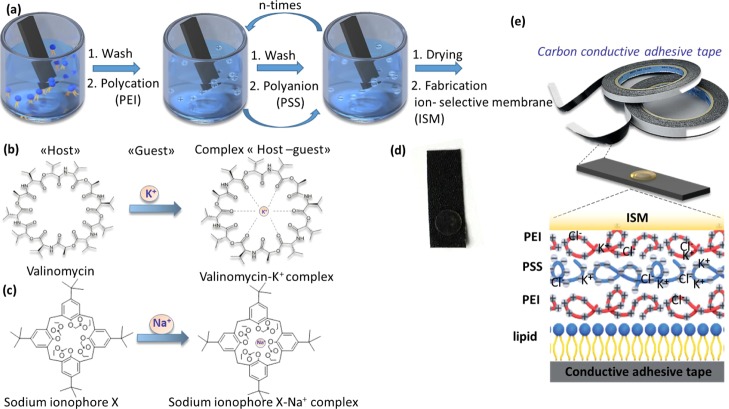
Flexible ISEs consisted of ISMs and ATs modified through lipid and polyelectrolyte layers (Figure 1a–e). The adhesive carbon tape was used as a substrate because of its mechanical reliability, adhesive effect, and conductivity. Carbon conductivity allows this material to be used as an inner electrode.32 The scheme of the ion-selective AT used as a flexible conducting platform (0.8 × 3.5 cm) is presented in Figure 1d.
Figure 1.
Experimental design, device architecture, and fabrication procedure of the self-powered wearable noninvasive sensor. (a) The polyelectrolyte LBL assembly on the adhesive type. (b) Structure of the antibiotic valinomycin, which was used as the ionophore for the potassium ISM with a complex “host–guest”. (c) Sodium ionophore X and its complexation with the sodium ion. (d) Carbon conductive AT as the initial substrate. (e) Common scheme of the ISM, composition with polyelectrolytes multilayers, lipid layer, carbon conductive AT.
Deposition of the polyelectrolyte layers was carried out by a layer-by-layer method,26,33 which is more preferable for polyelectrolyte adsorption, as it does not require specific expensive equipment31 and is more accessible. This method allows to create fairly uniform and thin films on the surface of the substrate. The number of layers was taken from the literature data.
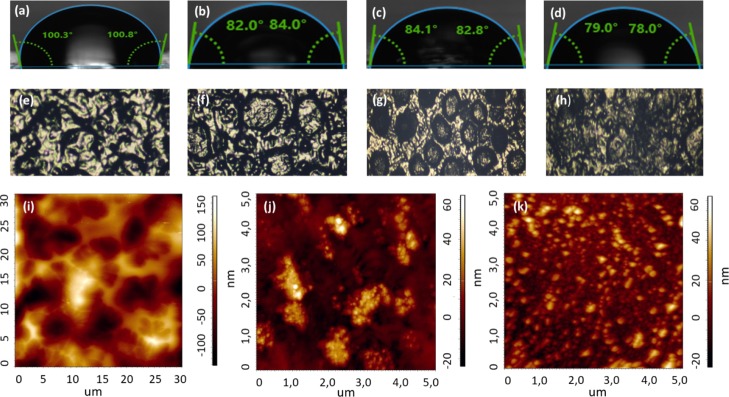
The polyelectrolyte layers were deposited on the substrates with a partially negative or positive charge.34 The surface of the AT did not have a partial charge; therefore, to obtain polyelectrolyte composites, lipid vesicles were deposited as the first modifying layer.35 The stage of the lipid layer setting was monitored by measuring the water contact angle. As seen from Figure 2, a hydrophobic substrate with a water contact angle of 100–110° (Figure 2a) became more hydrophilic with a contact angle of 82–84° after the assembly of the lipid layer (Figure 2b). Overlaying the lipid layer gave better wettability of the AT. Hydrophobic hydrocarbon tails were attached to the surface of the AT, and hydrophilic negatively charged heads were faced outside and interacted with the positively charged polyelectrolytes. When the positively charged (PEI) polyelectrolytes assembled on the lipid layer, the contact angle slightly increased (Figure 2c), but after adsorption of the PSS (negatively charged polyelectrolyte), the contact angle decreased to 79–78° (Figure 2d).
Figure 2.
Characterization of morphology surface of electrodes. Water contact angles of wetting for (a) bare adhesive tape (AT), (b) lipid bilayer deposited on top of AT (AT/lip), (c) composite with polyelectrolyte layer of PEI, deposited on top of lipid layer (AT/lip/PEI) (d) composite with polyelectrolyte layer of PSS, deposited on top of PEI layer; optic images of (e) AT, (f) AT/lip, (g) AT/lip/PEI (h) AT/lip/PEI/PSS/PEI; AFM images of (i) AT/lip/PEI, (j) ITO/lip/PEI, (k) ITO/lip/PEI/PSS.
Each step of the multilayer architecture assembly was controlled by morphology by atomic force microscopy (AFM).36 This method allows the control of surface parameters such as roughness and smoothness. These characteristics influence further stages of surface modification. For the ISM immobilization need not only be wetted surface but uniform. According to the AFM data on the surface of the AT, it is not always possible to create a sufficiently even layer of polyelectrolytes. This could be explained by the original structure of the AT surface. As can be seen from Figure 2e, a layer of glue on the surface of the substrate applied to the carbon tape rather unevenly. The thickness of the adhesive in some places of the AT sometimes exceeds the thickness of the polyelectrolyte layer, therefore, it is not possible to see the real structure of the substrate surface with polyelectrolytes deposited. In order to compare the AFM images obtained for the AT, AFM photographs with polyelectrolyte layers, deposited on the indium tin oxide (ITO) flexible substrate, were removed. As seen from Figure 2i–k, the structure of the deposited polyelectrolytes on the AT and on the substrate is quite similar. Thus, according to the AFM data obtained for the two different substrates with the same quantity of polyelectrolytes, the PEI layer is deposited on the substrate by small conglomerates, and the PSS layer precipitates fairly evenly. Deposition of polyelectrolyte layers on top of the AT/lipid layer composite leads to an increase of roughness.
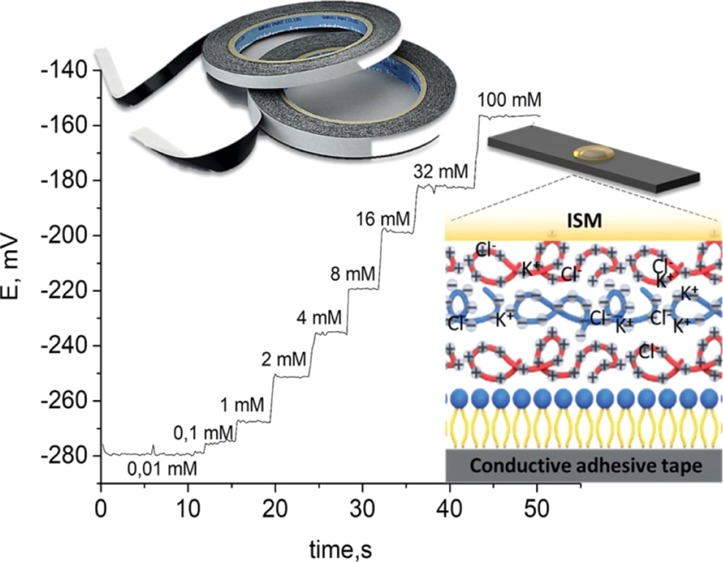
The adsorbed polyelectrolyte layers are hydrated25,37 and can be used as a liquid internal reference system. For this purpose, we provide polyelectrolyte layer adsorption from the potassium chloride decimolar solution. This technique allows adding more water in a layer as polyelectrolytes swell38 in saline solution. The number of layers was set to form a charge barrier between the inner solution and membrane.39 PEI,39 as the positively charged polyelectrolyte, prevents diffusion of potassium ions from the inner solution to outer media. This approach allows stable pseudo-inner electrode potential. Thus, we used consistent PEI/PSS/PEI layers. To avoid capacitor-like behavior, we did not prepare more layers. By adding potassium chloride into the polyelectrolyte layer, we achieved the pseudo-internal solution as a part of ISEs. This pseudo-internal electrode solution coupled with a carbon paste on the AT forms an ion-to-electron transfer system. This approach enables the production of low cost ISEs with high potential stability and signal reproducibility.
The potentiometric measurements of electrolytes such as K+ and Na+ require the addition of an ISM which is generally a plasticized polymer doped with an ionophore. It is a compound that can selectively bind to the ions of interest via coordinate bonds. In our investigation, an ISM was prepared by mixing a polymeric phase (PVC) with a plasticizer (o-NPOE). To make this material sensitive to Na+ and K+ ions, it was doped with a highly hydrophobic ionic site (KTpCIPB), sodium ionophore X, and potassium ionophore I, respectively. AT-based ISEs for two target analytes, namely, K+ and Na+, were prepared and tested individually.
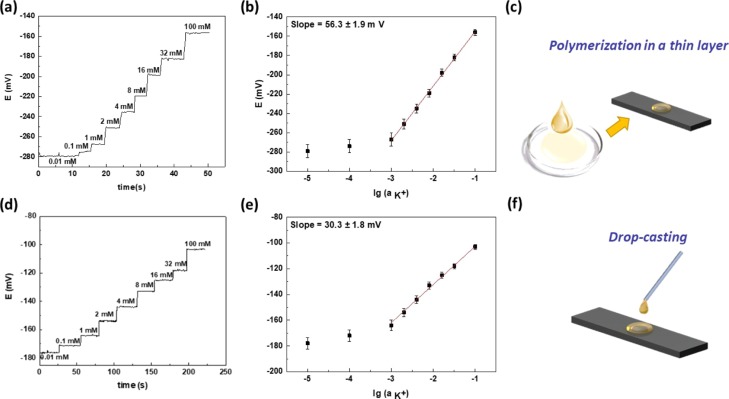
In each experiment, potassium and sodium ISEs were dipped into standard solutions of salt (NaCl and KCl respectively), while the concentration of the solution was changed in steps and the open-circuit potentials were monitored continuously using a potentiostat. Electromotive force (EMF) was measured between the ISE and a commercial Ag/AgCl reference electrode. Both Figures 3a and 4a show the time trace of potentiometric response for the prepared AT-based electrodes of K+ and Na+ ions, when the activity of the primary analyte increased. K+ ion concentration in human sweat lies in the range of 1 to 16 mM. The calibration plots for potassium ions (Figure 3b) show a Nernstian response between 10–3 and 10–1 M indicating that the membrane is working as expected.
Figure 3.
Analytical measurements of ISEs: (a) potentiometric response ISEs with ISMs which are prepared by polymerization a thin layer method, (b) calibration plot for the potassium ISE with an ISM which is prepared by polymerization in the thin layer method (y = 56.3x – 98.9, n = 3 P = 0.95), (c) representation of the preparation of the ISM by polymerisation in a thin film layer with a Petri dish, (d) potentiometric response ISE with an ISM which is prepared by a drop casting method (e) calibration plot for the potassium ISE with an ISM which is prepared by a drop casting method (y = 30.3x – 71.7, n = 3 P = 0.95), (f) representation of the drop casting method on the adhesive type.
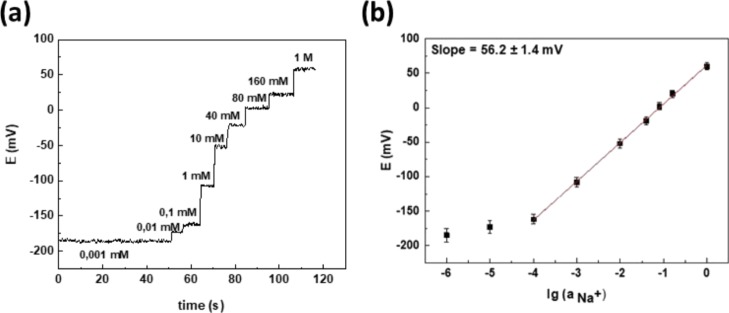
Figure 4.
(a) Potentiometric response for sodium ISE and (b) corresponding calibration plot after addition of the standard sodium chloride solution (y = 56.2x + 61.8).
The method of preparation and deposition of the ISM on AT/Lip/Poly was found to have an effect on sensor performance. The best sensitivity (56.3 ± 1.9 mV/decade) and stability of potential were obtained using a polymerization method in a thin film (Figure 3a–c). From the above, it was concluded that it is better to make a membrane in a Petri dish (Figure 3d–f).
Na+ ion concentration in human sweat is in the range of 10 to 160 mM (Figure 4b). Average sensitivity of ISEs in this range (10–4 to 1 M) was found to be 56.2 ± 1.4 mV/decade, which was close to the value expected for the detection of monovalent ions using ideal ISMs (59.2 mV/decade) (Figure 4b). Analytical characteristics of ISEs are shown in Table 1. The results obtained showed good agreement with the literature.20,40 The proposed sensing platform demonstrated a better time of response and was close to the Nernstian value of sensitivity with a low sensor cost. The results confirm the proposed pseudo-liquid junction for the ISE. The sodium ISE demonstrates lower response time in comparison with the carbon tattoo sensor of the solid contact type.41 Sensitivity of the proposed sodium sensor is close to other sensors based on the same carbon material17,41,42 and flexible textile.43 Linear range for sodium potentiometric sensor was higher, because was constructed pseudo-inner solution, which provide stable work of sensor. This pseudo-inner solution potassium ISE showed a much lower response time (5 s) in contrast with the carbon textile-based ion-selective sensor (60 s).44 Sensitivity, limit of detection, and linear range of the potassium-selective electrode with pseudo-inner solution were compared with ion-selective solid contact sensors based on carbon42−44 and flexible substrates such as textile43 and cotton.44
Table 1. Analytical Characteristics of the Wearable ISEs for Potassium and Sodium Detectiona.
| substrate material | type of transducing | sensitivity, mV/dec aNa+ | linear range, M | limit of detection | response time, s | reference |
|---|---|---|---|---|---|---|
| Sodium | ||||||
| adhesive carbon tape | inner solution | 56.2 ± 1.4 | 10–4 to 100 | 4.0 × 10–4 | 8 | present work |
| textile/polyurethane/CNTs | solid contact | 59.4 | 10–4 to 10–1 | 10–4.9 | (43) | |
| carbon tattoo | solid contact | 63.7 | 10–4 to 10–1 | 10 | (41) | |
| carbon/PEDOT/PET | solid contact | 54.8 | 10–4 to 10–1 | (42) | ||
| Potassium | ||||||
| adhesive carbon tape | inner solution | 56.3 ± 1.9 | 10–3 to 10–1 | 3.9 × 10–5 | 5 | present work |
| textile/polyurethane/CNTs | solid contact | 56.5 | 10–4 to 10–1 | 10–4.9 | (43) | |
| carbon/PEDOT/PET | solid contact | 53.9 | 10–4 to 10–1 | (42) | ||
| CNTs/cotton | solid contact | 54.9 | 10–4 to 10–1 | 10-5 | 60 | (44) |
CNTs—carbon nanotubes, PEDOT—poly(3,4-ethylenedioxythiophene); PET—polyethylene terephthalate.
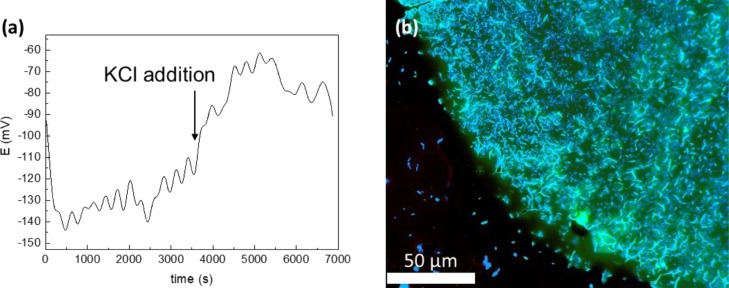
To demonstrate sensor application for monitoring in real samples, oscillations45 in the bacteria biofilm under expansion were observed.46 Such oscillations are usually observed by using a Nernstian fluorescent dye.47 Bacteria have many ion channels, such as sodium, chloride, calcium-gated, and potassium ones and ionotropic glutamate receptors, similar to those found in neurons. Escherichia coli was used to control potassium ions under biofilm living. For this purpose, the biofilm was directly grown on the ISE surface. EMF was refined because of resistance increase before measurements and after biofilm growth. Then, potentiometric measurements of such systems were provided (Figure 5). Standard potassium chloride (0.1 M, 100 μL) addition to bacteria was performed to create disturbance. According to the potassium ISE calibration curve, potassium concentration in the biofilm was calculated and established as 190 mM. This value corresponds with the potassium level in the biofilm, varying from 200 to 400 mM,48 found in the literature. After standard potassium addition, its concentration increased dramatically to 1.3 M. Such essential and disproportional potassium levels could be explained with potassium pumping by the biofilm from the solution to the gap between the ISE and biofilm. Nevertheless, the proposed sensor demonstrated good stability and biocompatibility during measurements, as the E. coli biofilm showed life-sustaining activity after potentiometric measurements (Figure 5b).
Figure 5.
(a) Potentiometric potassium chloride disruption response for the E. coli colony on the conductive sensor and (b) bacteria imaging with biomarkers thioflavin S and propidium.
Conclusions
We offer a wearable sensor by using PEI/PSS multilayers as inner ISE solution together with a low-cost adhesive carbon tape as a sensitive transducer. Its high sensitivity and durability are obtained using a novel approach to the preparation of polyelectrolyte multilayers as a unique platform for sensing application. The sodium and potassium ion-selective sensor shows combined superiority of high sensitivity, fast response, and high durability. In addition, the low-cost strain sensor based on the adhesive carbon tape shows sensitive response to bacteria potassium oscillations. This wearable sensing platform can monitor various human health indicators, including electrolytes. The sensitivity and suitability for making potentiometric sensors of the demonstrated sensing platform may enable a wide range of applications in intelligent devices.
Methods
Chemicals
Valinomycin (potassium ionophore I), 4-tert-butylcalix[4]arene-tetraacetic acid tetraethyl ester (sodium ionophore X), potassium tetrakis(4-chlorophenyl)borate (KTpCIPB), 2-nitrophenyl octyl ether (o-NPOE), and poly(vinyl chloride) high molecular weight (PVC) were all purchased from Sigma-Aldrich. Tetrahydrofuran (THF) and hexane were purchased from Ekos-1, Russia. Potassium chloride (KCl), sodium chloride (NaCl), and phosphate-buffered saline were from Merk. Liquid soya lecithin Lecisoy 400 was purchased from Cargill, USA. Branched polyethyleneimine (PEI, Mw 70 kDa) 30% water solution was purchased from Alfa Aesar, and polysterenesulfonate (PSS, Mw 500 kDa)—from Polysciences, Inc.
Fabrication of the AT-Based Platform (ATP)
A conventional conductive double-sided carbon tape (8 mm width, 3.5 cm height, 150 μm thickness) purchased from Tescan (Czech Republic) was used as the initial substrate. To increase the hydrophilicity the AT was immersed in a dispersion of lipid vesicles.
To prepare small unilamellar vesicle (SUV) solution, the following procedure was performed: soy lecithin solution in hexane (10 mg mL–1) was kept under vacuum for solvent evaporation at least for 3 h. After removing any hexane traces, a thin lipid film on the bottom of the vessel was obtained. The resulting lipid film was rehydrated by using distilled water for final 10 mg/mL concentration with simultaneous sonication in an ultrasonic bath for 15 min.
To obtain the results, the lipid bilayer on top of the conductive double-sided adhesive carbon tape was immersed in a 10 mg/mL dispersion of SUV for 1 h. Vesicles attached to the oppositely charged surface of the AT then ruptured, fused, and spread on the surface forming a continuous bilayer. The procedure described was followed by rinsing with large quantity of distilled water and drying under an air steam.
The polyelectrolyte multilayer film was deposited on top of the lipid bilayer. Polyelectrolytes films were assembled using a layer-by-layer technique. Three layers of positively charged PEI and negatively charged PSS were assembled from solutions with a polyelectrolyte concentration of 2 mg mL–1 in 0.1 M KCl. A concentration of 2 mg/mL of polyelectrolytes was used, as it gives optimum thickness films. For each layer deposition, surfaces were incubated during 15 min at room temperature in the polyelectrolyte solution. Each step of polyelectrolyte deposition was followed by a washing step with distilled water.
Fabrication of the ISM
The potassium ISM contained 1.4 wt % of valinomycin, 0.3 wt % of KTpCIPB, 32.8 wt % of PVC, and 65.5 wt % NPOE. The sodium ISM contained 0.99 wt % of sodium ionophore X, 0.25 wt % of KTpClPB, 32.92 wt % of PVC, and 65.84 wt % of o-NPOE. The membranes were prepared by dissolving the mixture into 1.5–4 mL of THF. ISM coatings on the ATP were prepared by two different methods: drop casting and thin film polymerization. For thin film polymerization, we poured the THF solution into a Petri dish and allowed the THF to evaporate over 24 h. We then cut the membrane into small circular pieces (3 mm in diameter) and conditioned them by soaking overnight in chloride solutions of the corresponding ions (10–3 M K+, 10–1 M Na+). A volume of 40 μL of the membrane cocktail was applied at once by drop casting onto the electrode (into the orifice left by the plastic mask). The membrane was dried for 24 h. The volume of the membrane cocktail applied was optimized for the fabrication of a membrane approximately 3 mm in diameter. The electrodes were conditioned in proper saline solution: 0.001 M KCl or 0.1 NaCl prior to and between measurements, which were performed at room temperature.
E. coli ATCC Growing on the Potassium ISE
E. coli ATCC night culture was used for biofilm preparation. The biofilm was grown on the potassium ISE for 24 h at 38 °C in an LB broth (LENNOX). Prior to potentiometric measurements, the ISE with the biofilm was rinsed with deionized water.
Characterization of the AT-Based ISE
Characterization of the electrode surface morphology was observed by using an atomic force microscope Solver Next (NT-MDT, Russia) in semi-contact mode. Wettability of the obtained composites was characterized by contact angle measurements using a drop shape analyzer Kruss DSA25 (Germany).
Electrochemical measurements were performed using a CompactStat instrument (Ivium, Netherlands) in a standard two-electrode cell at room temperature (22 °C). The AT-based electrode was used as the working electrode and a 3 M Ag/AgCl/KCl (type 6.0733.100, Metrohm AG) as the reference electrode. The membrane was fully covered by the solution, but there was no direct contact between the exposed carbon adhesive-tape and the solution. The activity coefficients were calculated by the Debye–Hückel approximation. After the measurements, the electrodes were air-dried and stored with using storage corresponding salt solution. Several calibration curves with the primary analyte in highly concentrated background standard solutions (1–16 mM for potassium ions, 20–160 mM for sodium ions) were prepared. Knowing the concentration of the standard solutions and the limit of detection, the selectivity coefficient was obtained. The electrochemical cell volume was 20 mL. The background solution for bacteria measurements was phosphate-buffered solution (pH 7.2).
Acknowledgments
This work was supported by the Russian Foundation for Basic Research under the research project no. 18-38-20182. Dr. Koshel and her microbiology group members at ChemBio Cluster ITMO University are acknowledged for all the advices and help with biofilm growth and imaging which was done at the Chromas Core Facility.
The authors declare no competing financial interest.
References
- Gao W.; Ota H.; Kiriya D.; Takei K.; Javey A. Flexible Electronics toward Wearable Sensing. Acc. Chem. Res. 2019, 52, 523–533. 10.1021/acs.accounts.8b00500. [DOI] [PubMed] [Google Scholar]
- Jeong Y. R.; Lee G.; Park H.; Ha J. S. Stretchable, Skin-Attachable Electronics with Integrated Energy Storage Devices for Biosignal Monitoring. Acc. Chem. Res. 2019, 52, 91–99. 10.1021/acs.accounts.8b00508. [DOI] [PubMed] [Google Scholar]
- Yang Y.; Gao W. Wearable and flexible electronics for continuous molecular monitoring. Chem. Soc. Rev. 2019, 48, 1465–1491. 10.1039/c7cs00730b. [DOI] [PubMed] [Google Scholar]
- Koga H.; Nagashima K.; Huang Y.; Zhang G.; Wang C.; Takahashi T.; Inoue A.; Yang H.; Kanai M.; He Y.; Uetani K.; Nogi M.; Yanagida T. Paper-based disposable molecular sensor constructed from oxide nanowires, cellulose nanofibers, and pencil-drawn electrodes. ACS Appl. Mater. Interfaces 2019, 11, 15044. 10.1021/acsami.9b01287. [DOI] [PubMed] [Google Scholar]
- Hamedi M. M.; Ainla A.; Güder F.; Christodouleas D. C.; Fernández-Abedul M. T.; Whitesides G. M. Integrating Electronics and Microfluidics on Paper. Adv. Mater. 2016, 28, 5054–5063. 10.1002/adma.201505823. [DOI] [PubMed] [Google Scholar]
- Shiwaku R.; Matsui H.; Nagamine K.; Uematsu M.; Mano T.; Maruyama Y.; Nomura A.; Tsuchiya K.; Hayasaka K.; Takeda Y.; Fukuda T.; Kumaki D.; Tokito S. A Printed Organic Amplification System for Wearable Potentiometric Electrochemical Sensors. Sci. Rep. 2018, 8, 3922. 10.1038/s41598-018-22265-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brothers M. C.; DeBrosse M.; Grigsby C. C.; Naik R. R.; Hussain S. M.; Heikenfeld J.; Kim S. S. Achievements and Challenges for Real-Time Sensing of Analytes in Sweat within Wearable Platforms. Acc. Chem. Res. 2019, 52, 297–306. 10.1021/acs.accounts.8b00555. [DOI] [PubMed] [Google Scholar]
- Sekar M.; Pandiaraj M.; Bhansali S.; Ponpandian N.; Viswanathan C. Carbon fiber based electrochemical sensor for sweat cortisol measurement. Sci. Rep. 2019, 9, 403. 10.1038/s41598-018-37243-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y.; Wang X.; Lu W.; Yuan Q.; Zheng Y.; Yao B. A thin film polyethylene terephthalate (PET) electrochemical sensor for detection of glucose in sweat. Talanta 2019, 198, 86–92. 10.1016/j.talanta.2019.01.104. [DOI] [PubMed] [Google Scholar]
- Smith R. E.; Totti S.; Velliou E.; Campagnolo P.; Hingley-Wilson S. M.; Ward N. I.; Varcoe J. R.; Crean C. Development of a novel highly conductive and flexible cotton yarn for wearable pH sensor technology. Sens. Actuators, B 2019, 287, 338–345. 10.1016/j.snb.2019.01.088. [DOI] [Google Scholar]
- Li P.; Zhang D.; Wu Z. Flexible MoS2 sensor arrays for high performance label-free ion sensing. Sens. Actuators, A 2019, 286, 51–58. 10.1016/j.sna.2018.12.026. [DOI] [Google Scholar]
- Oh S. Y.; Hong S. Y.; Jeong Y. R.; Yun J.; Park H.; Jin S. W.; Lee G.; Oh J. H.; Lee H.; Lee S.-S.; Ha J. S. Skin-Attachable, Stretchable Electrochemical Sweat Sensor for Glucose and pH Detection. ACS Appl. Mater. Interfaces 2018, 10, 13729–13740. 10.1021/acsami.8b03342. [DOI] [PubMed] [Google Scholar]
- Bae C. W.; Toi P. T.; Kim B.-Y.; Lee W. I.; Lee H. B.; Hanif A.; Lee E. H.; Lee N.-E. Fully Stretchable Capillary Microfluidics-integrated Nanoporous Gold Electrochemical Sensor for Wearable Continuous Glucose Monitoring. ACS Appl. Mater. Interfaces 2019, 11, 14567. 10.1021/acsami.9b00848. [DOI] [PubMed] [Google Scholar]
- Buono M. J.; Ball K. D.; Kolkhorst F. W. Sodium ion concentration vs. sweat rate relationship in humans. J. Appl. Physiol. 2007, 103, 990–994. 10.1152/japplphysiol.00015.2007. [DOI] [PubMed] [Google Scholar]
- Kassal P.; Sigurnjak M.; Steinberg I. M. Paper-based ion-selective optodes for continuous sensing: Reversible potassium ion monitoring. Talanta 2019, 193, 51–55. 10.1016/j.talanta.2018.09.031. [DOI] [PubMed] [Google Scholar]
- Promphet N.; Rattanawaleedirojn P.; Siralertmukul K.; Soatthiyanon N.; Potiyaraj P.; Thanawattano C.; Hinestroza J. P.; Rodthongkum N. Non-invasive textile based colorimetric sensor for the simultaneous detection of sweat pH and lactate. Talanta 2019, 192, 424–430. 10.1016/j.talanta.2018.09.086. [DOI] [PubMed] [Google Scholar]
- Parrilla M.; Cuartero M.; Crespo G. A. Wearable potentiometric ion sensors. TrAC, Trends Anal. Chem. 2019, 110, 303–320. 10.1016/j.trac.2018.11.024. [DOI] [Google Scholar]
- Cuartero M.; Parrilla M.; Crespo G. A. Wearable potentiometric sensors for medical applications. Sensors 2019, 19, 363. 10.3390/s19020363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker E.; Pretsch E. Nanoscale potentiometry. TrAC, Trends Anal. Chem. 2008, 27, 612–618. 10.1016/j.trac.2008.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan W.-J.; Zou X. U.; Hamedi M. M.; Hu J.; Parolo C.; Maxwell E. J.; Bühlmann P.; Whitesides G. M. Paper-Based Potentiometric Ion Sensing. Anal. Chem. 2014, 86, 9548–9553. 10.1021/ac5018088. [DOI] [PubMed] [Google Scholar]
- Sempionatto J. R.; Martin A.; García-Carmona L.; Barfidokht A.; Kurniawan J. F.; Moreto J. R.; Tang G.; Shin A.; Liu X.; Escarpa A.; Wang J. Skin-worn Soft Microfluidic Potentiometric Detection System. Electroanalysis 2019, 31, 239–245. 10.1002/elan.201800414. [DOI] [Google Scholar]
- Jaworska E.; Lewandowski W.; Mieczkowski J.; Maksymiuk K.; Michalska A. Critical assessment of graphene as ion-to-electron transducer for all-solid-state potentiometric sensors. Talanta 2012, 97, 414–419. 10.1016/j.talanta.2012.04.054. [DOI] [PubMed] [Google Scholar]
- Tan Y.; Yildiz U. H.; Wei W.; Waite J. H.; Miserez A. Layer-by-Layer Polyelectrolyte Deposition: A Mechanism for Forming Biocomposite Materials. Biomacromolecules 2013, 14, 1715–1726. 10.1021/bm400448w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suran S.; Balasubramanian K.; Raghavan S.; Varma M. M. Spatially resolved observation of water transport across nanomembranes using bright-field nanoscopy. Appl. Phys. Lett. 2018, 113, 043701. 10.1063/1.5030082. [DOI] [Google Scholar]
- Schönhoff M.; Ball V.; Bausch A. R.; Dejugnat C.; Delorme N.; Glinel K.; Klitzing R. v.; Steitz R. Hydration and internal properties of polyelectrolyte multilayers. Colloids Surf., A 2007, 303, 14–29. 10.1016/j.colsurfa.2007.02.054. [DOI] [Google Scholar]
- Skorb E. V.; Volkova A. V.; Andreeva D. V. Layer-by-Layer Approach for Design of Chemical Sensors and Biosensors. Curr. Org. Chem. 2015, 19, 1097–1116. 10.2174/1385272819666150318221914. [DOI] [Google Scholar]
- Farhat T.; Yassin G.; Dubas S. T.; Schlenoff J. B. Water and Ion Pairing in Polyelectrolyte Multilayers. Langmuir 1999, 15, 6621–6623. 10.1021/la990631a. [DOI] [Google Scholar]
- Ryzhkov N. V.; Mamchik N. A.; Skorb E. V. Electrochemical triggering of lipid bilayer lift-off oscillation at the electrode interface. J. R. Soc., Interface 2019, 16, 20180626. 10.1098/rsif.2018.0626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva D. V.; Kollath A.; Brezhneva N.; Sviridov D. V.; Cafferty B. J.; Möhwald H.; Skorb E. V. Using a chitosan nanolayer as an efficient pH buffer to protect pH-sensitive supramolecular assemblies. PCCP 2017, 19, 23843–23848. 10.1039/c7cp02618h. [DOI] [PubMed] [Google Scholar]
- Prudnikova K.; Utz M.. Polyelectrolyte hydrogels as electromechanical transducers. In Frontiers in Sensing: From Biology to Engineering; Barth F. G., Humphrey J. A. C., Srinivasan M. V., Eds.; Springer Vienna: Vienna, 2012; pp 351–361. [Google Scholar]
- Rivero P. J.; Goicoechea J.; Arregui F. J. Layer-by-layer nano-assembly: A powerful tool for optical fiber sensing applications. Sensors 2019, 19, 683. 10.3390/s19030683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mikhelson K. N.Modern Trends in the ISEs Theory and Applications. In Ion-Selective Electrodes; Mikhelson K. N., Ed.; Springer Berlin Heidelberg: Berlin, Heidelberg, 2013; pp 125–134. [Google Scholar]
- Ermakov S. S.; Nikolaev K. G.; Tolstoy V. P. Novel electrochemical sensors with electrodes based on multilayers fabricated by layer-by-layer synthesis and their analytical potential. Russ. Chem. Rev. 2016, 85, 880–900. 10.1070/rcr4605. [DOI] [Google Scholar]
- Lu W.; Luo Y.; Chang G.; Liao F.; Sun X. Layer-by-layer self-assembly of multilayer films of polyelectrolyte/Ag nanoparticles for enzymeless hydrogen peroxide detection. Thin Solid Films 2011, 520, 554–557. 10.1016/j.tsf.2011.06.085. [DOI] [Google Scholar]
- Elizarova I. S.; Luckham P. F. Layer-by-layer adsorption: Factors affecting the choice of substrates and polymers. Adv. Colloid Interface Sci. 2018, 262, 1–20. 10.1016/j.cis.2018.11.003. [DOI] [PubMed] [Google Scholar]
- Kozhuharov S.; Maroni P.; Borkovec M. In situ Imaging of Single Polyelectrolyte Chains with the Atomic Force Microscope. Chimia 2019, 73, 17–20. 10.2533/chimia.2019.17. [DOI] [PubMed] [Google Scholar]
- Andreeva D. V.; Skorb E. V.; Shchukin D. G. Layer-by-Layer Polyelectrolyte/Inhibitor Nanostructures for Metal Corrosion Protection. ACS Appl. Mater. Interfaces 2010, 2, 1954–1962. 10.1021/am1002712. [DOI] [Google Scholar]
- Brezhneva N.; Nikitina A.; Ryzhkov N.; Klestova A.; Vinogradov A. V.; Skorb E. V. Importance of buffering nanolayer position in Layer-by-Layer assembly on titania based hybrid photoactivity. J. Sol-Gel Sci. Technol. 2019, 89, 92–100. 10.1007/s10971-018-4728-5. [DOI] [Google Scholar]
- Nestler P.; Paßvogel M.; Ahrens H.; Soltwedel O.; Köhler R.; Helm C. A. Branched Poly(ethylenimine) as Barrier Layer for Polyelectrolyte Diffusion in Multilayer Films. Macromolecules 2015, 48, 8546–8556. 10.1021/acs.macromol.5b01065. [DOI] [Google Scholar]
- Novell M.; Parrilla M.; Crespo G. A.; Rius F. X.; Andrade F. J. Paper-Based Ion-Selective Potentiometric Sensors. Anal. Chem. 2012, 84, 4695–4702. 10.1021/ac202979j. [DOI] [PubMed] [Google Scholar]
- Bandodkar A. J.; Molinnus D.; Mirza O.; Guinovart T.; Windmiller J. R.; Valdés-Ramírez G.; Andrade F. J.; Schöning M. J.; Wang J. Epidermal tattoo potentiometric sodium sensors with wireless signal transduction for continuous non-invasive sweat monitoring. Biosens. Bioelectron. 2014, 54, 603–609. 10.1016/j.bios.2013.11.039. [DOI] [PubMed] [Google Scholar]
- Alizadeh A.; Burns A.; Lenigk R.; Gettings R.; Ashe J.; Porter A.; McCaul M.; Barrett R.; Diamond D.; White P.; Skeath P.; Tomczak M. A wearable patch for continuous monitoring of sweat electrolytes during exertion. Lab Chip 2018, 18, 2632–2641. 10.1039/c8lc00510a. [DOI] [PubMed] [Google Scholar]
- Parrilla M.; Cánovas R.; Jeerapan I.; Andrade F. J.; Wang J. A Textile-Based Stretchable Multi-Ion Potentiometric Sensor. Adv. Healthcare Mater. 2016, 5, 996–1001. 10.1002/adhm.201600092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guinovart T.; Parrilla M.; Crespo G. A.; Rius F. X.; Andrade F. J. Potentiometric sensors using cotton yarns, carbon nanotubes and polymeric membranes. Analyst 2013, 138, 5208–5215. 10.1039/c3an00710c. [DOI] [PubMed] [Google Scholar]
- Liu J.; Prindle A.; Humphries J.; Gabalda-Sagarra M.; Asally M.; Lee D.-y. D.; Ly S.; Garcia-Ojalvo J.; Süel G. M. Metabolic co-dependence gives rise to collective oscillations within biofilms. Nature 2015, 523, 550. 10.1038/nature14660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prindle A.; Liu J.; Asally M.; Ly S.; Garcia-Ojalvo J.; Süel G. M. Ion channels enable electrical communication in bacterial communities. Nature 2015, 527, 59. 10.1038/nature15709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kralj J. M.; Hochbaum D. R.; Douglass A. D.; Cohen A. E. Electrical Spiking in Escherichia coli Probed with a Fluorescent Voltage-Indicating Protein. Science 2011, 333, 345. 10.1126/science.1204763. [DOI] [PubMed] [Google Scholar]
- Sharma R.; Shimada T.; Mishra V. K.; Upreti S.; Sardesai A. A. Growth Inhibition by External Potassium of Escherichia coli Lacking PtsN (EIIANtr) Is Caused by Potassium Limitation Mediated by YcgO. J. Bacteriol. 2016, 198, 1868. 10.1128/jb.01029-15. [DOI] [PMC free article] [PubMed] [Google Scholar]