Abstract
In the Atlantic salmon (Salmo salar) aquaculture industry, gaping (the separation of muscle bundles from the connective tissue) is a major quality problem. This study characterized chondroitin sulfate (CS) and heparan sulfate (HS) in the connective tissue of intact and gaping salmon fillets from 30 salmon by mass spectrometry. Statistical difference was detected between gaping and intact tissues only when comparing pairwise samples from the same individual (n = 10). The gaping tissue had a lower content of monosulfated CS disaccharides (p = 0.027), and the relative distribution of CS disaccharides was significantly different (p < 0.05). The HS chains were short (average = 14.09, SD = 4.91), and the intact tissue seemed to have a more uniform HS chain structure compared to the gaping tissue. Time-series samples from the same individuals are recommended for future research to improve the understanding of reasons and implications of these differences.
Introduction
The fish muscle myotomes are arranged as folded sheets that are joined together in layers along the longitudinal axis of the fish by connective tissue, the myocommata. Gaping is defined as the phenomenon in which the connective tissue of fish fillets is weakened and fails to hold the muscle myotomes together, resulting in holes and slits in the fillet. Gaping poses a major problem for the aquaculture and fisheries industries due to the unappealing appearance and limitations in specialized food production of the fillets, which causes down-rating of the quality classification and hence affects pricing.1
The location of tearing has been found to be in the sarcolemma (the cell membrane of the muscle cells), which connects the muscle fibers to the myocommatal sheets of the extracellular matrix (ECM) and, in the innermost layer of the myocommata, the basement membrane.2,3 The myocommata–muscle fiber interface has a lower degree of connection as gaping increases.4,5 Both in the ECM and in the sarcolemma, the components collagen, proteoglycans (PGs), and glycosaminoglycans (GAGs) interact to form a structural network, which is known to influence strongly the textural properties of various tissue types including connective tissue.6,7 The main structural component of the ECM is collagen, and the solubility of major collagen types (I and V) has long been suspected to be higher in gaping tissue.8−10 However, collagen is not the only factor affecting the strength of the ECM. Research in many different fields have revealed that GAG chains of PGs contribute significantly to the formation of a functional collagen network and thus to the structural integrity of connective tissue.11−14
There are four main groups of GAGs: chondroitin sulfate (CS)/dermatan sulfate (DS), heparin/heparan sulfate (HS), keratan sulfate (KS), and hyaluronan. They are linear polysaccharides whose disaccharide building blocks consist of an amino sugar (N-acetylglucosamine or N-acetylgalactosamine) and an uronic acid (glucuronic acid or iduronic acid) or galactose. PGs are glycoproteins covalently modified with heparin, HS, CS/DS, and/or KS chains (see study of Iozzo and Schaefer7 for the nomenclature). The GAG chains are much larger than other types of glycans typically found on PGs, and they therefore tend to dominate the chemical properties of the PGs.6,15 Many PGs also exhibit differences in the number of GAG chains, their lengths, and the arrangement of sulfated residues along the chains depending on the PG’s tissue location and function.16,17 A study examining the effect of GAGs on matrix production, distribution, and functionality by inhibiting GAG incorporation18 showed that an incomplete proteoglycan network resulted in a decrease in collagen deposition and less cross-linked collagen. This work suggested that the GAG incorporation in PGs contributes to the formation of a functional collagen network. In addition, a major function of PGs is binding collagen fibers together and anchoring cells to collagen or basement membranes.7,13,19−22 Some researchers claim that the degradation of the myocommata is caused mainly by the degradation of the PGs.23 The content of sulfated GAGs has been shown to differ between fish species, which are differently prone to gaping.24 Also, content of various PGs known to interact with collagen were shown to vary among these species,21 and the degradation of the intramuscular connective tissue in Pacific rockfish (Sebastes sp.) has also been related to both increased solubility of collagen and degradation of the GAGs.11 However, most published studies that analyzed the PG and GAG content in connection with fillet quality in other fish species and salmon fillets (e.g., refs (25) and (26)) have not focused on gaping.
The aim of this study was to examine the content and structure of GAGs in gaping and intact salmon fillets in detail. Although all GAG types are of importance, CS and HS were of most interest in this instance as they are parts of PGs and they were estimated to be present in the tissue in adequate amounts for MS measurements. Liquid chromatography–mass spectrometry (LC–MS) methods are capable of providing detailed knowledge of the structural diversities of GAGs in tissue.27 These methods provide insight into variations in phenotypic distribution of GAGs in tissue of different origin, developmental stage, or disease stage.27−35 The research presented here reveals the content and distribution of structural phenotypes of the two GAGs, chondroitin sulfate (CS) and heparan sulfate (HS), as well as comparisons between gaping and intact connective tissues in farmed salmon (Salmo salar) fish fillets.
Results and Discussion
Chondroitin Sulfate
When digested with chondroitinase ABC, the average amount of CS disaccharides detected was 0.9147 μg/mg (SD = 0.4559) dried tissue. By comparison, extraction from fish heads, bones, or other organs containing a higher content of GAGs than muscle tissue generally resulted in higher amounts of extracted CS.36,37 The amounts of CS recovered from various diamond squid tissue types were between 0.021 and 3.482 μg/mg defatted tissue,38 while another study39 detected 0.11 μg/mg sulfated CS disaccharides in rat muscle. Therefore, the amounts recovered from salmon muscle tissue in this study were at levels comparable with those found in similar tissue of other animal species. In addition to disaccharides, the digestion of CS resulted in a variety of mono- and oligosaccharides. In-source dimer formation of nonsulfated and singly sulfated CS disaccharides during ionization was also registered and included in the analysis. The dimers were identified by having the same size exclusion chromatography (SEC) retention times as the respective disaccharides but with m/z values corresponding to dimers (Table 1). To our knowledge, there have not been any previous reports on in-source dimer formation of glycosaminoglycans. However, multimer formation in MS analyses of various compounds is not uncommon.40,41 All chondroitin sulfates detected and included in the comparative analysis are listed in Table 1, with m/z values, retention time, description of composition by shorthand (adapted from ref (42)), and ion species detected.
Table 1. Chondroitin Sulfate Saccharides Included in the Comparative Analysisa.
| composition |
||||||||
|---|---|---|---|---|---|---|---|---|
| saccharide units | m/z [M – H]−1 | retention time (min) | ΔHexA | HexA | GalN | SO3 | Ac | ion species |
| dp1 | 302.29 | 102.5 | 0 | 0 | 1 | 1 | 1 | [0,0,1,1,1] |
| dp2 | 378.1 | 106 | 1 | 0 | 1 | 0 | 1 | [1,0,1,0,1] |
| 468.01 | 106 | 1 | 0 | 1 | 0 | 1 | modificationb | |
| 757.216 | 106 | 1 | 0 | 1 | 0 | 1 | dimer | |
| dp2 | 458.06 | 97 | 1 | 0 | 1 | 1 | 1 | [1,0,1,1,1] |
| 917.13 | 97 | 1 | 0 | 1 | 1 | 1 | dimer | |
| dp2 | 537.02 | 90.5 | 1 | 0 | 1 | 2 | 1 | [1,0,1,2,1] |
| 268.51 | 90.5 | 1 | 0 | 1 | 2 | 1 | z = 2 | |
| 458.06 | 90.5 | 1 | 0 | 1 | 2 | 1 | [1,0,1,2,1]c | |
| dp3 | 634.09 | 93.5 | 1 | 1 | 1 | 1 | 1 | [1,1,1,1,1] |
| 839.07 | 86.5 | 0 | 1 | 2 | 3 | 2 | sat. [0,1,2,3,2] | |
| 419.03 | 86.5 | 0 | 1 | 2 | 3 | 2 | z = 2 | |
| dp4 | 757.22 | 96 | 1 | 1 | 2 | 0 | 2 | [1,1,2,0,2] |
| 837.17 | 86 | 1 | 1 | 2 | 1 | 2 | [1,1,2,1,2] | |
| 997.09 | 105.5 | 1 | 1 | 2 | 3 | 2 | [1,1,2,3,2] | |
| 498.04 | 105.5 | 1 | 1 | 2 | 3 | 2 | z = 2 | |
| dp5 | 568.65 | 91 | 0 | 2 | 3 | 2 | 3 | sat. [0,2,3,2,3], z = 2 |
| dp6 | 1136.33 | 106 | 1 | 2 | 3 | 0 | 3 | [1,2,3,0,3] |
| dp8 | 757.22 | 92 | 1 | 3 | 4 | 0 | 4 | [1,3,4,0,4], z = 2 |
Description of composition by shorthand adapted from ref (30). Abbreviations: ΔHexA, Δ-4,5-unsaturated hexuronic acid; HexA, saturated hexuronic acid; GalN, galactosamine; SO3, sulfate group; Ac, acetyl group; sat., saturated.
Ion with unknown modification assigned as [1,0,1,0,1] based on chromatographic retention time.
Loss of SO3.
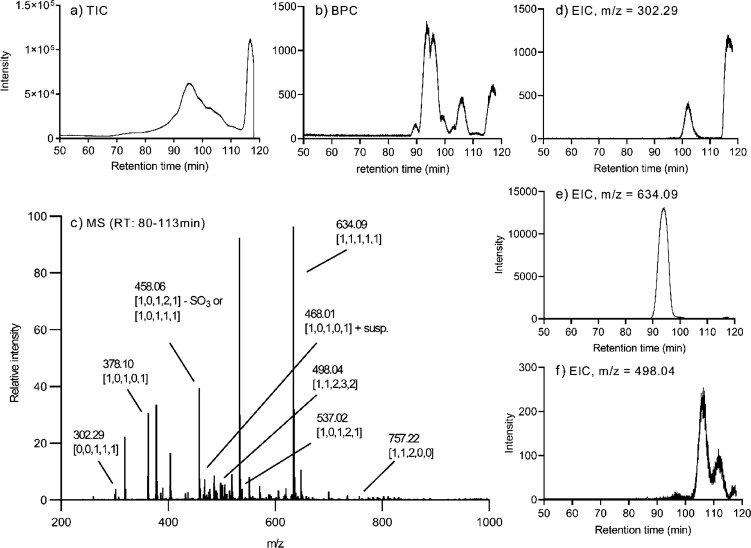
The total ion chromatograms (TICs) showed a considerable amount of noise when samples were examined by SEC-HPLC, as illustrated by a representative CS TIC in Figure 1a. An attempt to reduce the noise by precipitating CS in chilled ethanol prior to chondroitinase digestion proved unsuccessful. However, the base peak chromatograms (Figure 1b) show that relevant MS peaks could be analyzed without any problems. A representative MS spectrum with various peaks labeled is illustrated in Figure 1c. Representative extracted ion chromatograms (EICs) of the most abundant dp1, dp3, and dp4 ion species are illustrated in Figure 1d–f.
Figure 1.
Representative extracted MS spectrum and chromatograms for chondroitinase ABC-digested CS extracted from salmon muscle and connective tissues. (a) TIC, (b) BPC, and (c) extracted MS spectrum from SEC-LC/MS analysis. (c) Various peaks analyzed are labeled with m/z values and description of composition using the nomenclature described previously.30 EICs of relatively abundant CS oligosaccharides are illustrated in (d) dp1 [0,0,1,1,1], (e) dp3 [1,1,1,1,1], and (f) dp4 [1,1,2,3,2].
EICs of every ion species listed in Table 1 were measured for all samples, and comparisons were made between gaping and intact tissue samples. No significant differences were found in the abundances of the measured CS ion species, the CS chain length, or the relative dp2 distribution when comparing all gaping and intact samples. All measurements and statistical values for the comparison between intact and gaping tissues are listed in Table 2.
Table 2. Chondroitin Sulfate Measurementsa.
| all samples (n = 32) (μg/mg dried tissue) |
intact samples (n = 14) (μg/mg dried tissue) |
gaping samples (n = 18) (μg/mg dried tissue) |
|||||
|---|---|---|---|---|---|---|---|
| chondroitin sulfate | average | SD | average | SD | average | SD | statistics (Welch’s t-test) |
| total dp2 | 0.9148 | 0.2683 | 0.9833 | 0.317 | 0.8615 | 0.1983 | p = 0.238 |
| dp2 [1,0,1,0,1] | 0.3573 | 0.0994 | 0.3541 | 0.953 | 0.3598 | 0.1023 | p = 0.876 |
| dp2 [1,0,1,1,1] | 0.4786 | 0.1706 | 0.5419 | 0.2192 | 0.4293 | 0.0939 | p = 0.081 |
| dp2 [1,0,1,2,1] | 0.0798 | 0.0452 | 0.0894 | 0.0546 | 0.0722 | 0.0343 | p = 0.331 |
| dp1 | 0.032 | 0.011 | 0.0321 | 0.0129 | 0.032 | 0.0093 | p = 0.985 |
| dp3 [0,1,2,3,2] | 0.00111 | 0.00137 | 0.00103 | 0.00101 | 0.00126 | 0.0016 | p = 0.786 |
| dp3 [1,1,1,1,1] | 1.505 | 0.2203 | 1.4917 | 0.1269 | 1.5154 | 0.2755 | p = 0.749 |
| dp4 | 0.0494 | 0.0309 | 0.0398 | 0.0281 | 0.057 | 0.031 | p = 0.123 |
| dp5 | 0.00201 | 0.00112 | 0.0079 | 0.00111 | 0.00206 | 0.00111 | p = 0.807 |
| dp6 | 0.00026 | 0.00032 | 0.0003 | 0.00043 | 0.00022 | 0.00019 | p = 0.515 |
| dp8 | 0.00066 | 0.00103 | 0.00041 | 0.00099 | 0.00086 | 0.00103 | p = 0.230 |
| chain length | 99.8 | 23.65 | 104.3 | 29.57 | 96.6 | 18.82 | p = 0.385 |
| dp2 distribution (%) | |||||||
|---|---|---|---|---|---|---|---|
| [1,0,1,0,1] | 39.6 | 7.0 | 37.2 | 7.1 | 41.5 | 6.5 | p = 0.088 |
| [1,0,1,1,1] | 52.0 | 5.7 | 54.1 | 5.5 | 50.4 | 5.5 | p = 0.071 |
| [1,0,1,2,1] | 8.4 | 2.3 | 8.8 | 2.5 | 8.1 | 2.2 | p = 0.446 |
Welch’s t-test was used to test for differences between intact (n = 14) and gaping (n = 18) samples. The chain length was estimated by dividing the total CS saccharide measurements adjusted to disaccharide units with the amount detected of saturated dp1, dp3, and dp5, which originate from the nonreducing end.
Chondroitin sulfate has a normal chain length ranging from 40 to 120 disaccharide units, depending on tissue context,43 although shorter chains also have been reported.30 Therefore, the average CS chain length of 99.8 disaccharide units estimated in this study is within the expected range. The length of the CS chain and molecular weight distribution have been reported to have a considerable effect on CS function and interaction with other molecules.44 The average CS chain length of intact tissue was higher than that of gaping tissue, but the difference was not significant (Table 2).
The dp3 composition [1,1,1,1,1] (see Table 1) accounted for between 93.3 and 99.8% of the various mono- and oligosaccharides, excluding the dp2s. An earlier study45 reported the presence of CS trisaccharides resistant to chondroitinase digestion and concluded that they originated from the reducing terminal end of CS chain and their release caused by either alkaline treatment of the PG or presence of tissue endo-β-d-glucuronidases. Another study46 also detected these trisaccharides in shark cartilage and suggested that they were generated by breakdown of the CS chain or during commercial processing. Because sampling for this study took place 7 days postmortem, some degradation might well have occurred, although it is difficult to estimate to what degree since no such analysis has been made of fresh salmon muscle and connective tissues for comparison. The cause of such a high concentration of these trisaccharides does not appear to be related to the connective tissue degradation causing gaping as no significant difference was detected between their content in intact and gaping tissues.
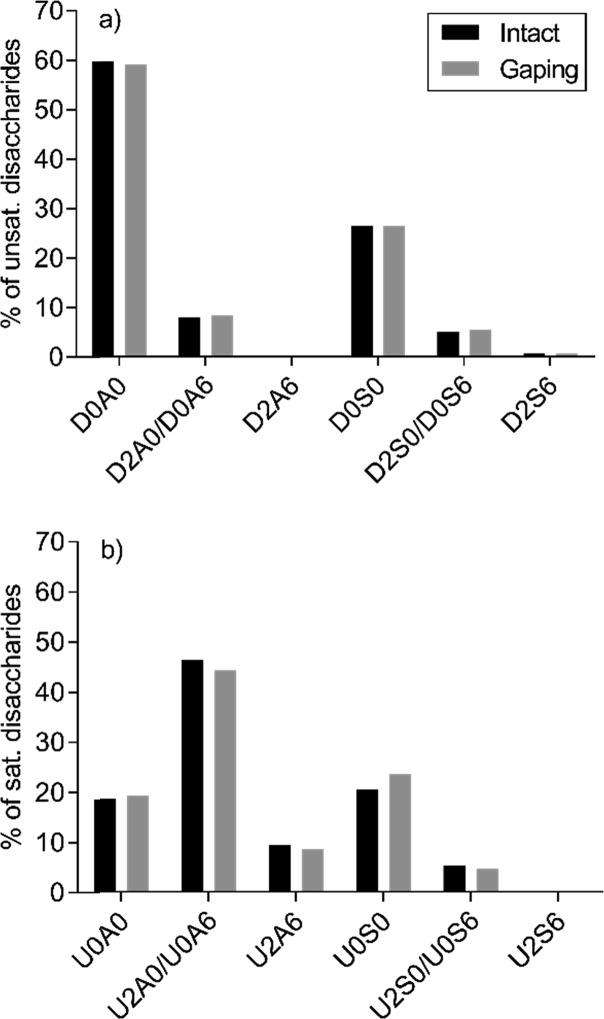
To our knowledge, no comparable quantitative analysis of GAGs in fish muscle has been reported. However, the relative CS dp2 distribution detected in this study was considerably different from that detected in other studies analyzing the CS content in various fish species,37,47−50 although these studies focused on other tissues such as bone, whole embryo or adult, fins, swim bladder, and cartilage. The present CS sulfation degree was much lower as nearly 40% of the CS disaccharides were nonsulfated, while these other studies reported from undetectable amounts to ∼33% at the most of nonsulfated CS disaccharides. On the other hand, the relative content of disulfated CS disaccharides was at a more comparable level to these other studies at around 8 to 10% (Table 2). There were no significant differences detected in the relative distribution of the three dp2 ion species when comparing all gaping and intact samples (Table 2).
Pairwise Comparisons of Intra-Individual Gaping and Intact Tissue Samples
Comparisons of the intact and gaping sample pairs taken from the same individuals (n = 10) showed a significant difference in the relative distribution of the dp2 phenotypes (Table 3). Each dp2 phenotype was tested for discrimination between the two sample types by the paired t-test and by the paired Wilcoxon test (not shown). Both tests showed significant difference between intact samples and gaping samples for all three dp2 phenotypes.
Table 3. Pairwise Comparisons of Intra-Individual Gaping and Intact Samples.
| intact
samples (n = 10) (μg/mg dried tissue) |
Gaping samples (n = 10) (μg/mg dried tissue) |
||||
|---|---|---|---|---|---|
| chondroitin sulfate | average | SD | average | SD | statistics (paired t-test) |
| total dp2 | 1.0161 | 0.362 | 0.8866 | 0.1656 | p = 0.212 |
| 10101 | 0.3438 | 0.1058 | 0.3845 | 0.0928 | p = 0.285 |
| 10111 | 0.5537 | 0.1994 | 0.4336 | 0.0893 | p = 0.027a |
| 10121 | 0.0244 | 0.0646 | 0.0692 | 0.0982 | p = 0.098 |
| dp1 | 0.0296 | 0.0118 | 0.0342 | 0.0104 | p = 0.265 |
| dp3 [0,1,2,3,2] | 0.0012 | 0.0011 | 0.001 | 0.0012 | p = 0.803 |
| dp3 [1,1,1,1,1] | 1.4584 | 0.1257 | 1.537 | 0.2821 | p = 0.507 |
| dp4 | 0.0407 | 0.0264 | 0.045 | 0.0353 | p = 0.636 |
| dp5 | 0.0009 | 0.0011 | 0.0023 | 0.0017 | p = 0.284 |
| dp6 | 0.00033 | 0.00052 | 0.00024 | 0.0002 | p = 0.663 |
| dp8 | 0.00057 | 0.00119 | 0.00091 | 0.00109 | p = 0.578 |
| chain length | 109.6 | 28.75 | 94.64 | 19.91 | p = 0.172 |
| dp2 distribution (%) | |||||
|---|---|---|---|---|---|
| [1,0,1,0,1] | 35.2 | 7.03 | 43.33 | 6.17 | p = 0.002b |
| [1,0,1,1,1] | 55.49 | 5.72 | 49.04 | 5.05 | p = 0.006b |
| [1,0,1,2,1] | 9.3 | 2.52 | 7.63 | 1.5 | p = 0.03a |
Significant at p < 0.05.
Significant at p < 0.01.
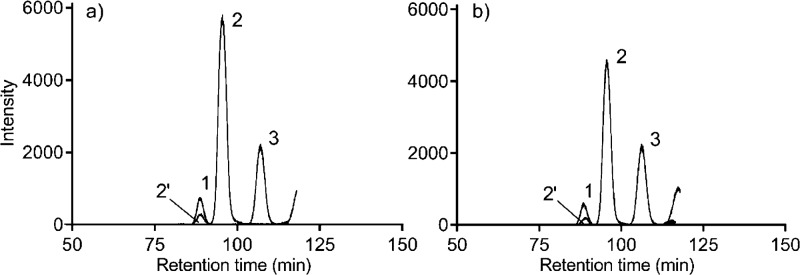
Comparisons of the measured CS amounts showed significant difference in the monosulfated dp2 [1,0,1,1,1] only (Table 3). Representative EICs of the dp2 phenotypes for intact (Figure 2a) and gaping (Figure 2b) samples illustrate the relation between the peak sizes.
Figure 2.
Representative combined EICs of the three major CS disaccharide compositions from (a) intact and (b) gaping tissues. Peak 1, disulfated CS dp2 [1,0,1,2,1]; peak 2, monosulfated CS dp2 [1,0,1,1,1]; peak 2′, in source loss of SO3 from monosulfated CS; peak 3, nonsulfated CS dp2 [1,0,1,0,1].
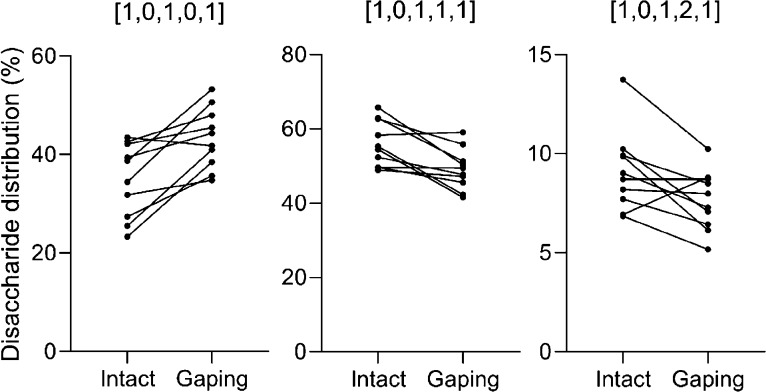
The comparisons of the relative dp2 values of the intact and gaping sample pairs from each individual were illustrated in a dot plot showing connections between the samples taken from the same individual (Figure 3). Intact samples nearly always had lower relative content of nonsulfated CS dp2s compared to gaping samples from the same individual and a higher relative content of both mono- and disulfated CS dp2s.
Figure 3.
Dot plot comparing the relative distribution of the three CS disaccharide compositions in intact (n = 10) and gaping (n = 10) sample pairs from the same individuals. The sample pairs are illustrated with connecting lines.
This means that a difference was found in the CS sulfation degree between intact and gaping samples from the same fillet, while no difference was seen in total dp2 content or any CS saccharide other than the monosulfated disaccharide. This indicates that CS in gaping tissue has been more subject to desulfation. A higher degree of desulfation but not degradation might suggest the action of endosulfatases. Although the endogenous CS catabolism has not been fully established, the most recognized path of CS degradation in vertebrates is by fragmentation of the CS chain into oligosaccharides followed by sequential degradation from the nonreducing end by exoglycosidases and sulfatases working in tandem.51 A study showed that both total CS content and CS4 were significantly reduced by higher galactosamine-4-sulfatase levels in humans.52 Therefore, it might be possible that the different degradation pattern in gaping tissue is caused by bacterial endosulfatase activity.53 Many bacteria in the human intestines are capable of digesting GAGs,54 and chondroitin sulfate 4-O-endosulfatases from marine bacteria have also been identified.55 These bacterial enzymes might potentially display a higher activity or a wider range of CS-degrading pathways. To our knowledge, no eukaryotic CS endosulfatases have yet been reported. The bacterial CS 4-O endosulfatases can remove 4-O-sulfate from CS polysaccharide chains but are inhibited by the 6-O-sulfation of GalNAc.55 This corresponds well with the findings in this study showing that the differences between the singly sulfated and nonsulfated forms in gaping and intact tissues are highly significant, while the difference in relative content of the doubly sulfated CS disaccharides is less significant (Table 3). An earlier study showed a correlation between intestinal fluids and/or blood left in the abdominal cavity post-slaughter and a higher degree of gaping.56 Potentially, bacteria present in salmon intestines are capable of producing endosulfatases acting on chondroitin sulfate. On the other hand, the desulfation of CS polysaccharides by bacterial sulfatases might also promote the digestion of CS by bacterial lyases, which could cause a difference in CS content.
Alternatively, there could be some inherent variation in the sulfation degree in the connective tissue of the salmon fillets, leaving the integrity of some areas more vulnerable to disintegration by the action of sulfatases from the blood and/or intestinal fluids. Further research into the CS desulfation potential of fish blood and intestinal bacteria is needed to clarify any potential effect of these substances on the connective tissue and chondroitin sulfate composition. Comparisons to fresh tissue are also recommended.
Another implication of these findings is that future research might benefit from comparing samples from the same individuals over a time period postmortem instead of comparing different individuals. The results from this study imply that the total content and relative distribution of CS dp2 in the tissue vary from individual to individual and it might be difficult to find a specific threshold amount or dp2 distribution indicative of gaping tissue. On the other hand, the results indicate that changes within the same individual are more informative.
Heparan Sulfate
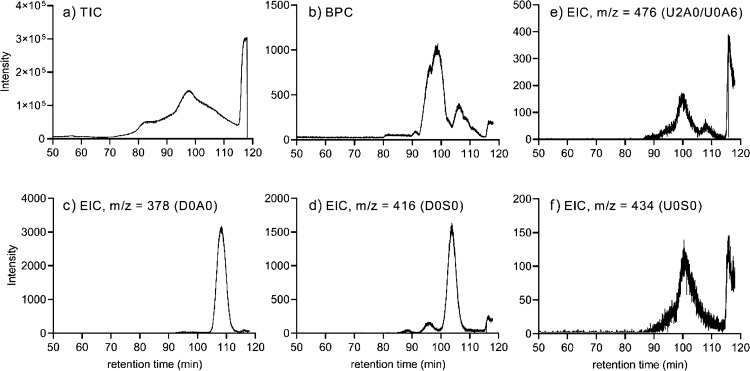
The TICs of the HS samples showed some noise (Figure 4a), but as with CS, the BPC was better resolved (Figure 4b) and it was possible to quantify disaccharides using the SEC-MS data. In addition, Figure 4 illustrates representative EICs of relatively abundant HS Δ-unsaturated disaccharides (Figure 4c,d) and saturated disaccharides (Figure 4e,f).
Figure 4.
Representative chromatograms for heparanase I, II, and III-digested HS extracted from salmon muscle and connective tissues. (a) TIC, (b) BPC, and EICs of the relatively abundant Δ-unsaturated disaccharides (c) D0A0 and (d) D0S0 and the relatively abundant saturated disaccharides (e) U2A0/U0A6 and (f) U0S0.
The amount of HS disaccharides measured after GAG extraction and digestion by heparanase I, II, and III was, on average, 0.209 μg/mg dried tissue. A similar amount of HS, 0.2 μg/mg dried tissue, was detected in swim bladders of groupers (subfamily Epinephelinae).49 For comparison, there was 0.34 μg/mg sulfated HS detected in rat muscle,39 while the amounts detected in various defatted and dried murine tissue types were between 0.120 and 3.30 μg/mg.57 As with the CS analysis, the HS values were compared between all intact and gaping samples as well as between intact and gaping sample pairs from the same individual (n = 10). An overview of all HS dp2 compositions and ion species measured with m/z values and retention times can be found in Table S1. There was no difference detected in the total amount of HS disaccharides recovered from intact and gaping tissues by either comparison method (Table 4). In this study, the amount of CS dp2s detected (Table 2) was 4.7 times more abundant than that of HS dp2s (Table 4). A study of cod and wolffish, the first prone to gaping and the other not,23 reported that the sulfated CS/HS ratios in the fillets were 0.58 and 4.14, respectively.24 However, these measurements were performed using different techniques than applied in this study.
Table 4. HS Measurements and Comparisons of Amounts Detected, Chain Length, and Sulfation Degree.
| all samples (n = 32) |
intact
samples (n = 14) |
gaping samples (n = 18) |
statisticsa |
|||||
|---|---|---|---|---|---|---|---|---|
| parameter | average | SD | average | SD | average | SD | Welch’s t-test | Paired t-test |
| total amount of HS (μg/mg dried tissue) | 0.194 | 0.065 | 0.209 | 0.058 | 0.189 | 0.069 | 0.411 | 0.552 |
| chain length | 14.09 | 4.91 | 14.6 | 4.8 | 13.7 | 5.11 | 0.598 | 0.744 |
| Δ-unsat. sulf. degree | 0.473 | 0.054 | 0.467 | 0.06 | 0.478 | 0.05 | 0.579 | 0.28 |
| Δ-unsat. N-sulf. degree | 0.323 | 0.018 | 0.322 | 0.02 | 0.326 | 0.016 | 0.572 | 0.037b |
| sat. sulf. degree | 0.945 | 0.137 | 0.96 | 0.118 | 0.939 | 0.154 | 0.676 | 0.964 |
| sat. N-sulf. degree | 0.27 | 0.137 | 0.256 | 0.123 | 0.281 | 0.15 | 0.604 | 0.76 |
Welch’s t-test included all samples, while the paired t-test only included those intact (n = 10) and gaping (n = 10) sample pairs retrieved from the same individuals.
Significant at p < 0.05.
The HS chain length, which was calculated by dividing the total amount of disaccharides with the saturated amount, was 14.09 dp2s on average (Table 4), which is short compared to the 25–200 disaccharides considered to be the common range.58 In comparison, various tissue types in rat contained an HS chain length between ∼30 and 60 disaccharide units.27 It is difficult to estimate to what extent the relatively short chain length is caused by postmortem degradation as there are no data of this kind from live or newly slaughtered fish. However, as the salmon was in storage for 7 days postmortem before sampling, it is likely that some degradation has taken place. In addition, no significant differences were detected in the HS chain length between intact and gaping tissues (Table 4). This indicates that gaping is not caused by a difference in rate of the usual form of degradation where HS chains are first cleaved into smaller fragments by endo-β-glucuronidase followed by a well-ordered sequential degradation including several enzymes. Endolytic heparanase activity can cause shorter HS chains59 as they seem to have larger affinity for cleaving glycosidic bonds closest to the nonreducing end.60
The short chain length was most likely also the reason that the HS dp2 distribution detected in this study, as illustrated in Figure 5 using shorthand description,61 was different and that the HS sulfation degree (Table 4) was lower than that detected in other species and organs.27,42,47 HS chains are arranged in domains of high sulfation, low sulfation, and mixed regions. The specific arrangement of domains seems to be important for the function of HS.62 Salmon muscle might contain less sulfated HS chains, or the comparatively low sulfation degree might be a reflection of postmortem desulfation or loss of more densely sulfated regions due to postmortem degradation. However, rat skeletal muscle has also been demonstrated to have a relatively low sulfation degree of HS, both internally in the chain and at the nonreducing end, compared to various other organs.27 No significant difference was detected in the overall sulfation degree of the HS chains between gaping and intact tissues in our samples. However, a significant difference was found in the Δ-unsaturated N-sulfation degree between intact and gaping tissues (p = 0.034) sampled from the same individual (n = 10). The gaping samples generally had a higher Δ-unsaturated N-sulfation degree. No difference was detected when comparing all samples (Table 4).
Figure 5.
Relative distribution of (a) Δ-unsaturated and (b) saturated HS disaccharides in intact and gaping tissues. The HS dp2s are abbreviated using the nomenclature described previously.63
Consistent with the findings of others,42 there was a clear difference in the sulfation pattern of the internal chain and nonreducing end (Figure 5). The unsaturated HS disaccharides had a low level of O-sulfation (Figure 5) as they were mainly nonsulfated (∼59.5%) or just N-sulfated (∼26.5%), while the saturated disaccharides had a much higher sulfation degree with only ∼19% nonsulfated dp2s. Whether this is a reflection of the original chain structure or it is due to postmortem degradation of the HS chains is difficult to estimate. A comparison with fresh salmon tissue samples is needed to clarify that issue.
From the saturated and unsaturated dp2 distribution (Figure 5), it is also possible to estimate the probability of where the various disaccharide phenotypes are positioned. U2A6 dp2s are much more likely to be at the NRE than internally in the chain. This is in stark contrast to various bovine and murine organs,42 while some rat organs also show this tendency, albeit to a lesser degree.27 In humans, the HS nonreducing end seems to be very important for binding affinity of HS to fibroblast growth factor-2 (FGF2), which regulates, among other things, cell adhesion.63
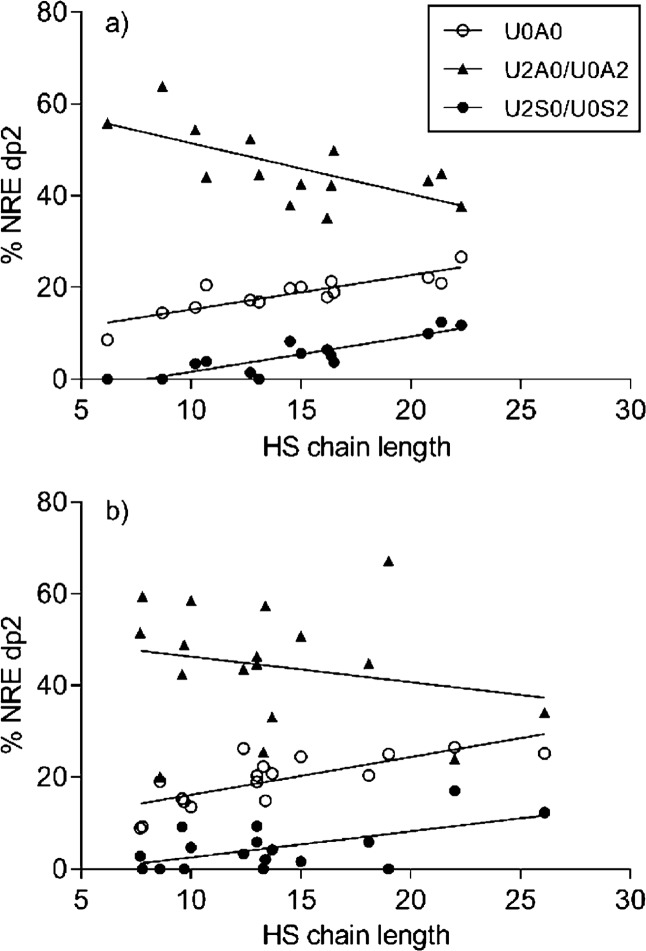
Comparisons between NRE dp2 distribution and chain length showed a gradual change in the composition as the chain length increased or decreased (Figure 6). The proportion of N-sulfated disaccharides at the nonreducing end increases with increasing chain length, consistent with findings of others.42 The three NRE dp2 phenotypes, U0A0, U2A0/U0A2, and U2S0/U0S2, seem to have a correlated pattern, while the other dp2 phenotypes do not seem to have a structured pattern (data not shown). Interestingly, there is a difference between intact and gaping samples in the stringency of this structure. While the intact samples can be regressed to a trend line with R2 values between 0.44 and 0.77 (Figure 6a), the gaping samples are less uniform, and the trend lines are less valid with R2 values between 0.05 and 0.56 (Figure 6b). Whether a larger variation in the HS chain structure means a less organized and weaker connective tissue is uncertain but might be worth the attention.
Figure 6.
Distribution of HS disaccharides at the NRE compared to chain length for (a) intact and (b) gaping samples. Linear regression values for (a) intact tissue were as follows: U0A0, R2 = 0.73, p < 0.0001; U2A0/U0A2, R2 = 0.44, p = 0.0094; U2S0/U0S2, R2 = 0.77, p < 0.0001. Linear regression values for (b) gaping tissue were as follows: U0A0, R2 = 0.56, p < 0.0005; U2A0/U0A2, R2 = 0.05, p = 0.4103; U2S0/U0S2, R2 = 0.35, p < 0.0128.
Conclusions
There were significant differences detected in the relative content of CS dp2 phenotypes in gaping and intact tissue samples when comparing samples taken from the same individual. The CS dp2s in gaping tissue had a lower sulfation degree. In addition, the amount of singly sulfated CS dp2 was significantly lower in gaping tissue. This difference in CS sulfation degree could be due either to an inherent difference in the tissue or endosulfatases in which bacteria are known to produce because there was no difference in degradation of CS otherwise. The HS analysis revealed overall short chain lengths potentially caused by the action of endogenous heparanases. The only significant difference in the HS structure between intact and gaping samples was that gaping samples had a higher Δ-N-sulfation degree in terms of the internal chain. Gaping samples also had a less uniform chain structure. Further elucidation of reasons and implications of these differences requires additional research including samples of fresh tissue for comparison. Differences in the GAG structure between intact and gaping tissues were mainly detected when comparing intact and gaping samples taken from the same individuals. Potential threshold values for GAG function failure in relation to gaping might thus be difficult to ascertain for a species in general. Instead, it might be beneficial for future research to compare postmortem time series of samples from the same individuals.
Experimental Section
Samples
Thirty farmed Atlantic salmon were kindly provided by the farming company Luna (Faroe Islands). The salmon, taken at an aquaculture facility during normal slaughtering procedures, were ∼2.5 years of age and had an average weight of 4.2 kg. All salmon were from the same net pen and had experienced the same conditions from fry to slaughter. None of the salmon had reached maturity. The 30 salmon were used in a postmortem experimental setup (detailed in ref (64)) for analyzing the effect of cleaning of the abdomen and initial storage temperature in the presence of gaping. After 7 days in cold storage, all 30 salmon were evaluated for the presence of gaping. At the same time, one gaping and/or one intact tissue sample was taken from each of the 30 salmon (n = 43). The samples consisted of connective tissue, myocommata, and the adjacent muscle tissue. Illustrations of intact and gaping fillets as well as sampling area are provided in Figure S1. The samples were stored at −18 °C and later homogenized into powder while frozen by sterile mortar and pestle. The samples were subsequently lyophilized.
Reagents
Heparin lyases I, II, and III from Flavobacterium heparinum were purchased from Ibex Pharmaceuticals (Montreal, Canada). Chondroitinase ABC from Proteus vulgaris and benzonase were purchased from Sigma-Aldrich (St. Louis, MO, USA). Pronase was purchased from Roche Applied Science.
GAG Extraction
GAGs were extracted from dry tissue (∼60 mg) of the 43 samples by a slightly modified version of the extraction procedure previously described.27 The tissue was suspended in 0.75 mL of protein digest buffer (50 mM Tris/HCl (pH ≈ 8), 1 mM CaCl2, 1% Triton X-100) and incubated with end-over-end mixing for 48 h at 55 °C. Pronase (1 mg/sample) was added initially and again after 24 h. Following heat inactivation of pronase by suspending the sample tubes into boiling water for 5 min, the buffer was adjusted to 2 mM MgCl2. Benzonase (400 mg/sample) was added, and samples were incubated at 37 °C for 3 h. Then, the samples were adjusted to 0.5 M NaOH and incubated overnight with end-over-end mixing at 4 °C. Thereafter, the samples were acidified with acetic acid to pH ≈ 5.5 and centrifuged at 15.000g for 15 min. The supernatants were transferred to new tubes, and 3 mL of MilliQ water was added. DEAE-Sephacel columns were prepared for GAG purification by adding 2.0 mL of slurry (Sigma-Aldrich) to the 10 mL columns (Bio-Rad) with frits only at the bottom. The resins were flushed with 10 mL of 0.1 M NaCl and 20 mM NaOAc (pH = 6.0) and eluted by gravity. The samples were added and washed with 25 mL of the same solution. The samples were since eluted, and GAGs were recovered by eluting with 2.5 mL of 1 M NaCl and 29 mM NaOAc (pH = 6.0). For desalting, PD-10 (GE Healthcare) columns and buffer reservoirs were washed with 30 mL of MilliQ water before the samples were added and eluted by gravity with 3.5 mL of MilliQ water. Each of the eluted samples was divided into two tubes and freeze-dried overnight for further analysis.
GAG Digestion
CS and HS were chosen for MS analysis as these are the most abundant GAGs in the muscle connective tissue that otherwise has a low concentration of GAGs. Forty percent of the total extracted GAG pool was digested with heparin lyases I, II, and III, targeting heparan sulfate, while 15% was digested with chondroitinase ABC, targeting chondroitin sulfate. Each of the two tubes with extracted and dried GAG pool samples was rehydrated with 5.0 μL of H2O. For HS digestion, 4.0 μL of GAG pool was incubated at 37 °C for 24 h with 0.5 μL of 100 mM Ca(OAc)2, 2.0 μL of 100 mM Tris/HCl (pH = 7.4), and 7.0 μL of a mixture of HS lyases I, II, and III. For CS digestion, 1.5 μL of GAG pool was incubated at 37 °C for 24 h with 1.0 μL of 100 mM NH4OAc, 3.0 μL of 100 mM Tris/HCl (pH = 8.0), and 6.5 μL of CH ABC. Because digested GAGs can be separated from GAG polysaccharides with size exclusion chromatography used in connection with LC–MS, no further treatment was needed. A simplified overview of GAG extraction and digestion is provided in Figure S2. For optimal use of laboratory facilities, some of the extracted GAG samples were pooled in equivalent amounts, resulting in a final sample size of n = 32 for SEC-LC/MS analysis (Table 5). Pooled samples were of either gaping or intact tissue and from fish within the same experimental group and with the same gaping classification.64 Pooling of samples was performed without interfering with the possibility of comparing intact and gaping tissue samples from the same individual.
Table 5. Tissue Samples for Glycosaminoglycan Extraction and Mass Spectrometry Analysisa.
| salmon no. | gaping tissue | intact tissue | number of samples |
|---|---|---|---|
| 1 | X | 1 | |
| 2 and 9 (pooled) | X | 1 | |
| 3 | X | 1 | |
| 4 | X | 1 | |
| 5 | X | X | 2 |
| 6 | X | 1 | |
| 7 | X | X | 2 |
| 8 and 10 (pooled) | X | 1 | |
| 11 | X | 1 | |
| 12 and 15 (pooled) | X | 1 | |
| 13 | X | X | 2 |
| 14 | X | X | 2 |
| 16 and 18 (pooled) | X | X | 2 |
| 17 | X | X | 2 |
| 19 and 20 (pooled) | X | 1 | |
| 21 | X | X | 2 |
| 22 and 25 (pooled) | X | 1 | |
| 23 and 24 (pooled) | X | X | 2 |
| 26 | X | X | 2 |
| 27 and 28 (pooled) | X | 1 | |
| 29 | X | X | 2 |
| 30 | X | 1 | |
| total | 18 | 14 | 32 |
Some samples were pooled in equivalent amounts following GAG extraction. Pooled samples were of same tissue type, gaping or intact, from two individuals, as indicated.
Liquid Chromatography–Mass Spectrometry Analysis
For the disaccharide analysis of HS and CS, the technical design of the SEC-LC/MS system used was the same as reported by Shi and Zaia.27 The mobile phase (12.5 mM formic acid, pH adjusted to 4.4 by ammonia, in 10% acetonitrile) was delivered isocratically at 0.015 mL/min. Disaccharides and oligosaccharides eluting from the SEC column were analyzed using the Applied Biosystems QSTAR Pulsar-I (Q-ToF) mass spectrometer operating in negative polarity using the enhanced mode. The MS analyses were performed with an internal standard (ΔHexA2S-GlcNS6S), making quantification possible. HS and CS saccharides were identified according to m/z values and retention times, as described.27 Throughout the HS and CS analyses, 10 randomly chosen samples were run in triplicate to confirm consistent technical performance.
Statistical Analysis
All statistical analyses were performed using R (http://www.r-project.org). The significance level was set at p-values below 0.05. Welch’s t-test65 was used when comparing all gaping and intact tissue samples (n = 32). The paired t-test66 and paired Wilcoxon test,67 a distribution-free method that replaces the data with corresponding order statistics, were applied to all pairwise comparisons of HS and CS measurements from gaping (n = 10) and intact (n = 10) sample pairs taken from the same 10 individuals.
Acknowledgments
We thank Hóraldur Joensen for constructive discussion.
Glossary
Abbreviations used
- CS
Cchondroitin sulfate
- dp2
disaccharide
- ECM
Eextracellular matrix
- EIC
Eextracted ion chromatogram
- GAGs
Gglycosaminoglycans
- HPLC
Hhigh- performance liquid chromatography
- HS
Hheparan sulfate
- MS
Mmass spectrometry
- PGs
Pproteoglycans
- SEC
Ssize exclusion chromatography
- TIC
Ttotal ion chromatogram
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01136.
This work was funded by NIH grant P41GM104603, Faroese Research Council (www.gransking.fo) grant 0421, and Fisheries Research Fund of the Faroe Islands (www.fvg.fo/) grant 201000728.
The authors declare no competing financial interest.
Supplementary Material
References
- Pittman K.; Grigory V. M.; Brandebourg T. Bridging the gap to sustainable salmon farming: overcoming the gaping problem. J. Fisheries Livest. Prod. 2013, 01, 104. 10.4172/2332-2608.1000104. [DOI] [Google Scholar]
- Bremner H. A.; Hallett I. C. Muscle Fiber–Connective tissue junctions in the fish blue grenadier (macruronus novaezelandiae). A scanning electron microscope study. J. Food Sci. 1985, 50, 975–980. 10.1111/j.1365-2621.1985.tb12993.x. [DOI] [Google Scholar]
- Hallett I. C.; Bremer H. A. Fine structure of the myocommata-muscle fibre junction in hoki (Macruronus novaezelandiae). J. Sci. Food Agric. 1988, 44, 245–261. 10.1002/jsfa.2740440306. [DOI] [Google Scholar]
- Ofstad R.; Egelandsdal B.; Kidman S.; Myklebust R.; Olsen R. L.; Hermansson A.-R. Liquid loss as effected by Post mortem ultrastructural changes in fish muscle: cod (Gadus morhua L) and salmon (Salmo salar). J. Sci. Food Agric. 1996, 71, 301–312. . [DOI] [Google Scholar]
- Fletcher G. C.; Hallett I. C.; Jerrett A. R.; Holland A. J. Changes in the fine structure of the myocommata-muscle Fibre junction related to gaping in rested and exercised muscle from king salmon (Oncorhynchus tshawytscha). LWT–Food Sci. Technol. 1997, 30, 246–252. 10.1006/fstl.1996.0175. [DOI] [Google Scholar]
- Mouw J. K.; Ou G.; Weaver V. M. Extracellular matrix assembly: a multiscale deconstruction. Nat. Rev. Mol. Cell Biol. 2014, 15, 771–785. 10.1038/nrm3902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iozzo R. V.; Schaefer L. Proteoglycan form and function: A comprehensive nomenclature of proteoglycans. Matrix Biol. 2015, 42, 11–55. 10.1016/j.matbio.2015.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjørnevik M.; Espe M.; Beattie C.; Nortvedt R.; Kiessling A. Temporal variation in muscle fibre area, gaping, texture, colour and collagen in triploid and diploid Atlantic salmon(Salmo salar L). J. Sci. Food Agric. 2004, 84, 530–540. 10.1002/jsfa.1656. [DOI] [Google Scholar]
- Espe M.; Ruohonen K.; Bjørnevik M.; Frøyland L.; Nordtvedt R.; Kiessling A. Interactions between ice storage time, collagen composition, gaping and textural properties in farmed salmon muscle harvested at different times of the year. Aquaculture 2004, 240, 489–504. 10.1016/j.aquaculture.2004.04.023. [DOI] [Google Scholar]
- Hagen Ø.; Johnsen C. A. Flesh quality and biochemistry of light-manipulated Atlantic cod (Gadus morhua) and the significance of collagen cross-links on fillet firmness and gaping. Food Chem. 2016, 190, 786–792. 10.1016/j.foodchem.2015.06.007. [DOI] [PubMed] [Google Scholar]
- Kim K.; Haard N. F. Degradation of proteoglycans in the skeletal muscle of pacific rockfish (Sebastes Sp.) during ice storage. J. Muscle Foods 1992, 3, 103–121. 10.1111/j.1745-4573.1992.tb00467.x. [DOI] [Google Scholar]
- Billinghurst R. C.; Dahlberg L.; Ionescu M.; Reiner A.; Bourne R.; Rorabeck C.; Mitchell P.; Hambor J.; Diekmann O.; Tschesche H.; Chen J.; Van Wart H.; Poole A. R. Enhanced cleavage of type II collagen by collagenases in osteoarthritic articular cartilage. J. Clin. Invest. 1997, 99, 1534–1545. 10.1172/JCI119316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danielson K. G.; Baribault H.; Holmes D. F.; Graham H.; Kadler K. E.; Iozzo R. V. Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J. Cell Biol. 1997, 136, 729–743. 10.1083/jcb.136.3.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costell M.; Gustafsson E.; Aszódi A.; Mörgelin M.; Bloch W.; Hunziker E.; Addicks K.; Timpl R.; Fässler R. Perlecan maintains the integrity of cartilage and some basement membranes. J. Cell Biol. 1999, 147, 1109–1122. 10.1083/jcb.147.5.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ly M.; Laremore T. N.; Linhardt R. J. Proteoglycomics: Recent Progress and Future Challenges. OMICS 2010, 14, 389–399. 10.1089/omi.2009.0123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farach-Carson M. C.; Carson D. D. Perlecan – a multifunctional extracellular proteoglycan scaffold. Glycobiology 2007, 17, 897–905. 10.1093/glycob/cwm043. [DOI] [PubMed] [Google Scholar]
- Prydz K Determinants of glycosaminoglycan (GAG) structure. Biomolecules 2015, 5, 2003–2022. 10.3390/biom5032003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bastiaansen-Jenniskens Y. M.; Koevoet W.; Jansen K. M. B.; Verhaar J. A. N.; DeGroot J.; VanOsch G. J. V. M. Inhibition of glycosaminoglycan incorporation influences collagen network formation during cartilage matrix production. Biochem. Biophys. Res. Commun. 2009, 379, 222–226. 10.1016/j.bbrc.2008.12.028. [DOI] [PubMed] [Google Scholar]
- Chakravarti S.; Magnuson T.; Lass J. H.; Jepsen K. J.; LaMantia C.; Carroll H. Lumican regulates collagen fibril assembly: Skin fragility and corneal opacity in the absence of lumican. J. Cell Biol. 1998, 141, 1277–1286. 10.1083/jcb.141.5.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al Jamal R.; Roughley P. J.; Ludwig M. S. Effect of glycosaminoglycan degradation on lung tissue viscoelasticity. Am. J. Physiol. Lung Cell. Mol. Physiol. 2001, 280, L306–L315. 10.1152/ajplung.2001.280.2.L306. [DOI] [PubMed] [Google Scholar]
- Tingbø M. G.; Kolset S. O.; Ofstad R.; Enersen G.; Hannesson K. O. Identification and distribution of heparan sulfate proteoglycans in the white muscle of Atlantic cod (Gadus morhua) and spotted wolffish (Anarhichas minor). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2006, 143, 441–452. 10.1016/j.cbpb.2005.12.022. [DOI] [PubMed] [Google Scholar]
- Kalamajski S.; Oldberg Å. The role of small leucine-rich proteoglycans in collagen fibrillogenesis. Matrix Biol. 2010, 29, 248–253. 10.1016/j.matbio.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Ofstad R.; Olsen R. L.; Taylor R.; Hannesson K. O. Breakdown of intramuscular connective tissue in cod (Gadus morhua L.) and spotted wolffish (Anarhichas minor O.) related to gaping. LWT–Food Sci. Technol. 2006, 39, 1143–1154. 10.1016/j.lwt.2005.06.019. [DOI] [Google Scholar]
- Tingbø M. G.; Kolset S. O.; Ofstad R.; Enersen G.; Hannesson K. O. Sulfated glycosaminoglycans in the extracellular matrix of muscle tissue in Atlantic cod (Gadus morhua) and spotted wolffish (Anarhichas minor). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2005, 140, 349–357. 10.1016/j.cbpc.2004.09.032. [DOI] [PubMed] [Google Scholar]
- Hannessson K. O.; Tingø M. G.; Olsen R. L.; Enersen G.; Bævre A. B.; Ofstad R. An immunological study of glycosaminoglycans in the connective tissue of bovine and cod skeletal muscle. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2007, 146, 512–520. 10.1016/j.cbpb.2006.11.024. [DOI] [PubMed] [Google Scholar]
- Torgersen J. S.; Koppang E. O.; Stien L. H.; Kohler A.; Pedersen M. E.; Mørkøre T. Soft texture of Atlantic salmon fillets is associated with glycogen accumulation. Plos One 2014, 9, e85551 10.1371/journal.pone.0085551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi X.; Zaia J. Organ-specific heparan sulfate structural phenotypes. J. Biol. Chem. 2009, 284, 11806–11814. 10.1074/jbc.M809637200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zaia J. Glycosaminoglycan glycomics using mass spectrometry. Mol. Cell. Proteomics 2013, 12, 885–892. 10.1074/mcp.R112.026294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandra R.; Namburi R. B.; Ortega-Martinez O.; Shi X.; Zaia J.; Dupont S. T.; Thorndyke M. C.; Lindahl U.; Spillmann D. Brittlestars Contain Highly Sulfated Chondroitin Sulfates/Dermatan Sulfates that Promote fibroblast growth factor 2-induced Cell Signaling. Glycobiology 2014, 24, 195–207. 10.1093/glycob/cwt100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao C.; Shi X.; White M.; Huang Y.; Hartshorn K.; Zaia J. Comparative glycomics of leukocyte glycosaminoglycans. Rev. Geophys. 2013, 280, 2447–2461. 10.1111/febs.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias D. K.; Pastrana-Mena R.; Ranucci E.; Tao D.; Ferruti P.; Ortega C.; Staples G. O.; Zaia J.; Takashima E.; Tsuboi T.; Borg N. A.; Verotta L.; Dinglasan R. R. A Small Molecule Glycosaminoglycan Mimetic Blocks Plasmodium Invasion of the Mosquito Midgut. PLoS Pathog. 2013, 9, e1003757 10.1371/journal.ppat.1003757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thelin M. A.; Svensson K. J.; Shi X.; Bagher M.; Axelsson J.; Isinger-Ekstrand A.; van Kuppevelt T. H.; Johansson J.; Nilbert M.; Zaia J.; Belting M.; Maccarana M.; Malmström A. Dermatan sulfate is involved in the tumorigenic properties of Esophagus Squamous Cell Carcinoma. Cancer Res. 2012, 72, 1943–1952. 10.1158/0008-5472.CAN-11-1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staples G. O.; Shi X.; Zaia J. Glycomics analysis of mammalian heparan sulfates modified by the human extracellular sulfatase HSulf2. PLoS One 2011, 6, e16689 10.1371/journal.pone.0016689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schumacher V. A.; Schlötzer-Schrehardt U.; Karumanchi S. A.; Shi X.; Zaia J.; Jeruschke S.; Zhang D.; Pavenstädt H.; Drenckhan A.; Amann K.; Ng C.; Hartwig S.; Ng K.-H.; Ho J.; Kreidberg J. A.; Taglienti M.; Royer-Pokora B.; Ai X. WT1-Dependent Sulfatase Expression Maintains the Normal Glomerular Filtration Barrier. J. Am. Soc. Nephrol. 2011, 22, 1286–1296. 10.1681/ASN.2010080860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langsdorf A.; Schumacher V.; Shi X.; Tran T.; Zaia J.; Jain S.; Taglienti M.; Kreidberg J. A.; Fine A.; Ai X. Expression regulation and function of heparan sulfate 6-O-endosulfatases in the spermatogonial stem cell niche. Glycobiology 2011, 21, 152–161. 10.1093/glycob/cwq133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stine J. J.; Wu T. H.; Oliveira A. C. M.; Smiley S.; Bechtel P. J.. Extraction and determination of chondroitin sulfate from fish processing byproducts. In A sustainable future: Fish byprocessing byproducts; Bechtel P. J.; Smiley S. Eds.; Alaska Sea Grant, University of Alaska Fairbanks: Fairbanks, Alaska, 2010; pp. 41–53. 10.4027/sffpb.2010.04. [DOI] [Google Scholar]
- Maccari F.; Galeotti F.; Volpi N. Isolation and structural characterization of chondroitin sulfate from bony fishes. Carbohydr. Polym. 2015, 129, 143–147. 10.1016/j.carbpol.2015.04.059. [DOI] [PubMed] [Google Scholar]
- Tamura J.-i.; Arima K.; Imazu A.; Tsutsumishita N.; Fujita H.; Yamane M.; Matsumi Y. Sulfation patterns and the amounts of chondroitin sulfate in the diamond squid, Thysanoteuthis rhombus. Biosci., Biotechnol., Biochem. 2009, 73, 1387–1391. 10.1271/bbb.90037. [DOI] [PubMed] [Google Scholar]
- Barbosa I.; Garcia S.; Barbier-Chassefière V.; Caruelle J. P.; Martelly I.; Papy-García D. Improved and simple micro assay for sulfated glycosaminoglycans quantification in biological extracts and its use in skin and muscle tissue studies. Glycobiology 2003, 13, 647–653. 10.1093/glycob/cwg082. [DOI] [PubMed] [Google Scholar]
- Stefansson M.; Sjöberg P. J. R.; Markides K. E. Regulation of multimer formation in electrospray mass spectrometry. Anal. Chem. 1996, 68, 1792–1797. 10.1021/ac950980j. [DOI] [Google Scholar]
- Domalain V.; Tognetti V.; Hubert-Roux M.; Lange C. M.; Joubert L.; Baudoux J.; Rouden J.; Afonso C. Role of cationization and multimers formation for diastereomers differentiation by ion mobility-mass spectrometry. J. Am. Soc. Mass Spectrom. 2013, 24, 1437–1445. 10.1007/s13361-013-0690-1. [DOI] [PubMed] [Google Scholar]
- Staples G. O.; Shi X.; Zaia J. ExtendedN-Sulfated domains reside at the Nonreducing end of Heparan sulfate chains. J. Biol. Chem. 2010, 285, 18336–18343. 10.1074/jbc.M110.101592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little P. J.; Ballinger M. L.; Burch M. L.; Osman N. Biosynthesis of natural and hyperelongated chondroitin sulfate glycosaminoglycans: New insights into an elusive process. Open Biochem. J. 2008, 2, 135–142. 10.2174/1874091X00802010135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Igarashi N.; Takeguchi A.; Sakai S.; Akiyama H.; Hagashi K.; Toida T. Effect of molecular sizes of chondroitin sulfate on interaction with L-selectin. Int. J. Carbohydr. Chem. 2013, 856142. 10.1155/2013/856142. [DOI] [Google Scholar]
- Sugahara K.; Takemura Y.; Sugiura M.; Kohno Y.; Yoshida K.; Takeda K.; Khoo K. H.; Morris H. R.; Dell A. Chondroitinase ABC-resistant sulfated trisaccharides isolated from digests of chondroitin/dermatan sulfate chains. Carbohydr. Res. 1994, 255, 165–182. 10.1016/S0008-6215(00)90977-7. [DOI] [PubMed] [Google Scholar]
- Lauder R. M.; Huckerby T. N.; Nieduszynski I. A. A fingerprinting method for chondroitin/dermatan sulfate and hyaluronan oligosaccharides. Glycobiology 2000, 10, 393–401. 10.1093/glycob/10.4.393. [DOI] [PubMed] [Google Scholar]
- Zhang F.; Zhang Z.; Thistle R.; McKeen L.; Hosoyama S.; Toida T.; Linhardt R. J.; Page-McCaw P. Structural characterization of glycosaminoglycans from zebrafish in different ages. Glycoconj. J. 2009, 26, 211–218. 10.1007/s10719-008-9177-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higashi K.; Takeuchi Y.; Mukuno A.; Tomitori H.; Miya M.; Linhardt R. J.; Toida T. Composition of glycosaminoglycans in Elasmobranchs including several deep-sea sharks: Identification of chondroitin/dermatan sulfate from the dried fins of Isurus oxyrinchus and Prionace glauca. PLoS One 2015, 10, e0120860 10.1371/journal.pone.0120860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan Y.; Wang P.; Zhang F.; Yu Y.; Zhang X.; Lin L.; Linhardt R. J. Glycosaminoglycans from fish swim bladder: isolation, structural characterization and bioactive potential. Glycoconj. J. 2018, 35, 87–94. 10.1007/s10719-017-9804-5. [DOI] [PubMed] [Google Scholar]
- Vázguez J. A.; Fraguas J.; Novosa-Carvallal R.; Reis R. L.; Antelo L. T.; Pérez-Martín R. I.; Valcarcel J. Isolation and chemical characterization of chondroitin sulfate from cartilage by-products of blackmouth catshark (Galeus melastomus). Mar. Drugs 2018, 16, 344. 10.3390/md16100344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneiwa T.; Mizumoto S.; Sugahara K.; Yamada S. Identification of human hyaluronidase-4 as a novel chondroitin sulfate hydrolase that preferentially cleaves the galactosaminidic linkage in the trisulfated tetrasaccharide sequence. Glycobiology 2010, 20, 300–309. 10.1093/glycob/cwp174. [DOI] [PubMed] [Google Scholar]
- Bhattacharyya S.; Kotlo K.; Shukla S.; Danziger R. S.; Tobacman J. K. Distinct effects ofN-Acetylgalactosamine-4-sulfatase and galactose-6-sulfatase expression on chondroitin sulfates. J. Biol. Chem. 2008, 283, 9523–9530. 10.1074/jbc.M707967200. [DOI] [PubMed] [Google Scholar]
- Ulmer J. E.; Vilén E. M.; Namburi R. B.; Benjdia A.; Beneteau J.; Malleron A; Bonaffé D.; Driguez P.-A.; Descroix K.; Lassalle G.; Le Narvor C.; Sandström C.; Spillmann D.; Berteau O. Characterization of glycosaminoglycan (GAG) sulfatases from the human gut SymbiontBacteroides thetaiotaomicronReveals the first GAG-specific bacterial endosulfatase. J. Biol. Chem. 2014, 289, 24289–24303. 10.1074/jbc.M114.573303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawai K.; Kamochi R.; Oiki S.; Murata K.; Hashimoto W. Probiotics in human gut microbiota can degrade host glycosaminoglycans. Sci. Rep. 2018, 8, 10674. 10.1038/s41598-018-28886-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.; Han W.; Cai X.; Zheng X.; Sugahara K.; Li F. Cloning and characterization of a novel chondroitin sulfate/dermatan sulfate 4-O-endosulfatase from a marine bacterium. J. Biol. Chem. 2015, 290, 7823–7832. 10.1074/jbc.M114.629154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han W.; Wang W.; Zhao M.; Sugahara K.; Li F. A novel eliminase from a marine bacterium that degrades hyaluronan and chondroitin sulfate. J. Biol. Chem. 2014, 289, 27886–27898. 10.1074/jbc.M114.590752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warda M.; Toida T.; Zhang F.; Sun P.; Munoz E.; Xie J.; Linhardt R. J. Isolation and characterization of heparan sulfate from various murine tissues. Glycoconj. J. 2006, 23, 555–563. 10.1007/s10719-006-7668-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kreuger J.; Spillman D.; Li J.-p.; Lindahl U. Interactions between heparan sulfate and proteins: the concept of specificity. J. Cell Biol. 2006, 174, 323–327. 10.1083/jcb.200604035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindahl U.; Kjellén L. Pathophysiology of heparan sulphate: many diseases, few drugs. J. Intern. Med. 2013, 273, 555–571. 10.1111/joim.12061. [DOI] [PubMed] [Google Scholar]
- Peterson S.; Liu J. Deciphering mode of action of heparanase using structurally defined oligosaccharides. J. Biol. Chem. 2012, 287, 34836–34843. 10.1074/jbc.M112.390161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawrence R.; Lu H.; Rosenberg R. D.; Esko J. D.; Zhang L. Disaccharide structure code for the easy representation of constituent oligosaccharides from glycosaminoglycans. Nat. Methods 2008, 5, 291–292. 10.1038/nmeth0408-291. [DOI] [PubMed] [Google Scholar]
- Nugent M. A.; Zaia J.; Spencer J. L. Heparan sulfate-protein binding specificity. Biochemistry (Moscow) 2013, 78, 726–735. 10.1134/S0006297913070055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naimy H.; Buczek-Thomas J. A.; Nugent M. A.; Leymarie N.; Zaia J. Highly sulfated nonreducing end-derived heparan sulfate domains bind fibroblast growth factor-2 with high affinity and are enriched in biologically active fractions. J. Biol. Chem. 2011, 286, 19311–19319. 10.1074/jbc.M110.204693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobsen Á.; Joensen H.; Eysturskarδ J. Gaping and loss of fillet firmness in farmed salmon (Salmo salarL.) closely correlated with post-slaughter cleaning of the abdominal cavity. Aquac. Res. 2017, 48, 321–331. 10.1111/are.12884. [DOI] [Google Scholar]
- Moser B. K.; Stevens G. R. Homogeneity of variance in the two-sample means test. Am. Stat. 1992, 46, 19–21. 10.2307/2684403. [DOI] [Google Scholar]
- Altman D. G.Practical statistics for medical research, 1st edition; Chapman and Hall/CRC: London, England, 1991. [Google Scholar]
- Wilcoxon F Individual comparisons by ranking methods. Biom. Bull. 1945, 1, 80–83. 10.2307/3001968. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.