Abstract
CD146 alternatively called melanoma cell adhesion molecule (MCAM), is a biomarker and therapeutic target of clinical significance. It is found on different cells including the endothelial cells and lymphocytes which participate in heterotypic and homotypic ligand-receptor. This review concentrated on the CD146 expression T cells (or lymphocytes) centering on Treg in lung cancer. Here, we have also considered the vigorous investigation of CD146 mainly acknowledged new roles, essential mechanisms and clinical implications of CD146 in cancer. CD146 has progressively become a significant molecule, particularly recognized as a novel biomarker, prognosis and therapy for cancer. Hence, targeting CD146 expression by utilization of methanol extracts of Calotropis procera leaf may be useful for the treatment of carcinogenesis.
Keywords: CD146, T lymphocytes, Lung cancer, Biomarker, Therapeutic, Calotropis procera leaf
Background
CD146 is a cell adhesion molecule (CAM) which was first discovered in 1987. It is 113,000-daltons membrane glycoprotein that comprises of transmembrane region, five immunoglobulin-like domains and a short cytoplasmic tail [1]. CAMs are utilized in a wide array of pathophysiological processes such as apoptosis, cell cycle, cell migration, cell–cell and cell–matrix interactions, cell signaling and morphogenesis during growth and tissue remodeling. Cell adhesion is an important process necessary for the accurate performance of eukaryotes. Researchers have shown the roles of CAMs in diversity of pathological progressions in cancer, pulmonary hypertension, autoimmune diseases, inflammation and infections [2, 3]. CD146 is known to be a member of the CAM because of its sequence homology analysis. It is a well-known adhesion marker of endothelial cells [4], which has also been recognized on some other cell types such as lymphocytes, pericytes, immune cells, mesenchymal stem cells, human alveolar periosteal sheets, bone marrow fibroblasts etc. [5–9]. It has been studied extensively in circulating endothelial cells [10, 11]. Hence it is called MCAM (melanoma cell adhesion molecule). Emerging researches have shown CD146 is expressed on different types of lung cancer [12–15]. Therefore, CD146 may be a possible biomarker for tumor diagnosis, therapy and prognosis.
Lung cancer remains the noticeable reason for cancer mortality globally [16] and the second most widespread cancer in homo sapiens [17]. Medically, the diagnosis of lung cancer is very dismal. Nevertheless, most cases of the advanced-stage lung cancers are very common, and investigations are going on seriously. However, the prognosis for patients with lung cancer remains unfavorable [18]. Hematologic irregularities, including anemia, thrombocytosis leukocytosis and lymphopenia are frequently observed in lung cancer patients. In the healthy subjects, the expression of CD146+ T cells is between 1 and 3% in the blood. However, the expression in a disease state such as lung cancer is significantly increased compared to the healthy patient [6]. CD146+ T cells have improved the interaction to endothelial monolayers, have effector memory phenotype, T regulatory phenotype, in adhesion, several genes are involved such as galectin 1 (LGAL 1), galectin 3 (LGAL 3), translocation, and inflammation, which may protect apoptosis [19]. These characteristics of the CD146+ T cells in the peripheral blood have steered to the assumption that these may demonstrate a minor pool of cells for homing of activated T cells [19, 20] in retort to inciting stimuli. The expression of CD146+ T cells in lung cancer and autoimmune diseases patients are said to be elevated [21–23]. The significance of CD146 T cells at the site of inflammation in these diseases remains unexplored.
Calotropis procera (CP) is a xerophytic perennial shrub which is found majorly in subtropical and tropical Middle East, Asia and Africa [24]. Various parts of CP had been extensively utilized in alternative medicine because of its pharmacologically active compounds discovered in the plant’s parts, leaves, flowers, roots, and its milky latex [25, 26]. CP had been investigated to contain some important compounds which includes trierpenoids, anthocyanins, norditerpenic esters, organic acid, cysteine protease procerain, alkaloids, phenol, flavonoids cardenolides [27, 28]. Hence, this review focused on the CD146+ expression T cells (or lymphocytes), apoptosis of T cells and lung cancer, binding partners of CD146+ , molecular signaling of CD146+, CD146+ a novel marker of lymphocytes subset population, immunophenotyping and detection of CD146+, CD146+ T cells in cancer and effects of methanol extract of Calotropis procera leaf on CD146 expression. Investigation unfolding the molecular mechanism and regulation of CD146+ expression on the T cells is still limited.
Main text
Apoptosis of T cells and lung cancer
Apoptosis is a biochemical, physiological and pathological process that is involved in the regulation of the homeostasis. It regulates cell number in tissues and also eradicates distinct cells that intimidate animal survival [29]. It is essential in the organism due to the fact that inadequate apoptosis may results in lung cancer. Apoptosis occurring from activation of T cells is believed to help as a feedback mechanism that removes activated T cells [30]. Dissimilar to immature thymocytes and renovated T cell lines, resting T cells are extremely resilient to apoptosis after early activation but become highly vulnerable [31–33]. Therefore, most investigations on activation induced cell death (AICD) have studied mainly the connections between the death receptors, CD95 (Fas) and tumor necrosis factor (TNF)-α receptor, with their agonists CD95L (Fas ligand) [29, 34, 35]. Inactive normal T cells express little or non-measurable levels of CD95 and CD95L, nevertheless mitogenic activation of primary T cells distinctly proliferates their expression [36, 37]. Extra participants of the TNF-α receptor family, such as TRAIL-R1 and TRAIL-R2, can also activate apoptosis in vulnerable cells after binding of their ligands [29]. Notwithstanding the significant function of the death receptors, developing suggestion shows that environmental components, such as nonlymphoid secreted factors and cytokines, can control apoptosis of activated T cells, thus highlighting the implication of the environment in the maintenance of T-cell homeostasis [38, 39]. Any disparity in the apoptotic procedure may lead to some possible diseases state situations, lymphocyte accumulation, lymphocyte depletion, and lung cancer.
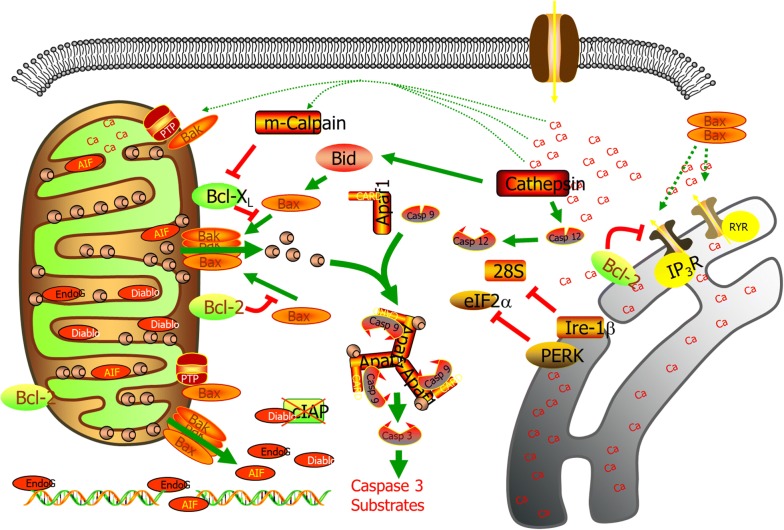
Other causes involved in various forms of apoptosis are reactive oxygen species (ROS). Investigators have studied the connection of ROS in apoptosis of T-cell blasts and hybridomas by utilizing antioxidants such as N-acetyl cysteine and glutathione [40, 41]. Apoptosis in these cells is as a result of changes in mitochondrial permeability and following the release of ROS [42]. Investigations conducted on primary T cells, however, show that the formation of intracellular ROS is essential for T-cell activation and IL-2 secretion but also regulates activation-induced T-cell apoptosis, therefore proposing that intracellular ROS may possibly be involved in peripheral T-cell homeostasis [43–45]. Though, studies with primary T cells are frequently accomplished in cultures deficient of other “nonlymphoid” cells, although activation- induced T-cell apoptosis is assumed to happen in organs and tissues where other cell types, such as red blood cells (RBCs), are present [46]. Furthermore, the principal role is oxygen and CO2 transport [47]. Endoplasmic reticulum (ER) is responsible for intracellular calcium (Ca2+) levels, protein folding, cellular responses to stress, protein synthesis, and trafficking [48]. Variations in Ca2+ regulation and increase of misfolded proteins in the ER lead to ER stress that eventually results in apoptosis (Fig. 1). Actually, the main factors that are responsible for apoptosis are the death receptor and the mitochondrial pathway [49]. The mechanism of ER stress-mediated apoptosis is assumed to utilize mitochondria, protein kinases, pro, and anti-apoptotic proteins heme oxygenase, microtubules, Ca2+, and caspases [48, 50–52]. ER stress-induced apoptosis which has been characterized include brefeldin, tunicamycin and thapsigargin [49]. Nevertheless, the investigation on the main mechanism of ER stress-induced apoptosis is still limited.
Fig. 1.
Mitochondria and ER Apoptosis. Variations in Ca2+ regulation and increase of misfolded proteins in the ER lead to ER stress that eventually results in apoptosis
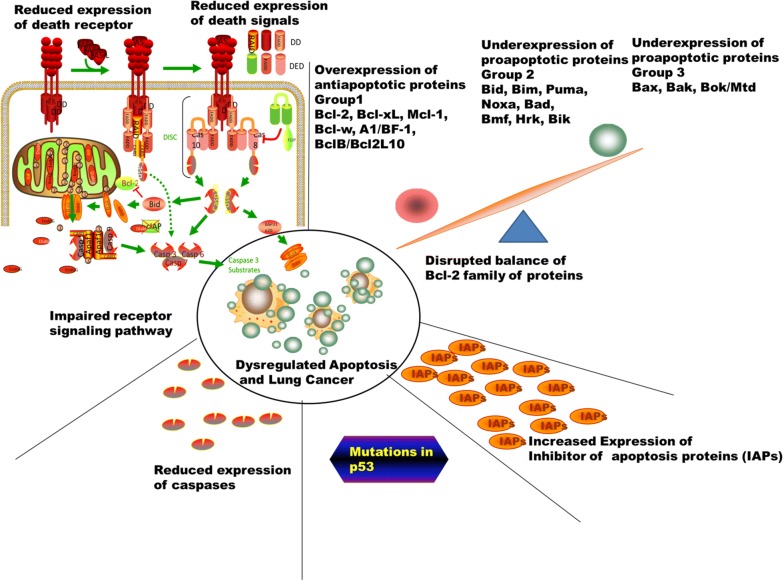
Lung cancer can be regarded as a progression of genetic deviations during which a normal cell is changed into a malignant one whereas avoidance of cell death or apoptosis is one of the important factors in the lung cell that leads to malignant carcinogenesis [53]. Therefore, when there is insufficient or decreased apoptosis, it may lead to carcinogenesis. Lung malignant cell may procure a decrease in apoptosis. There are some major ways in which decrease in apoptosis may occur which includes the imbalance of the anti-apoptotic and pro-apoptotic proteins, decrease in caspase expression and malfunction of the death domain and reduced death receptor signaling. Investigations have been done on different proteins which indicate the presence of pro- or anti-apoptotic effects in the cell. Overexpression and under-expression have vital effects on lung cancer by decreasing apoptosis in the cancer cells. The Bcl-2 (B- cell lymphoma 2) proteins utilized the intrinsic pathway for the control of apoptosis and act in the upstream of molecular damage and take effect in the mitochondria [54]. There are different groups of Bcl 2, which includes group 1 (Bcl-B/Bcl2L10, A1/Bfl-1, Bcl-w, Bcl-2, Bcl-xL, and Mcl-1) which are anti-apoptotic proteins, group 2 (Bik Bid, Noxa, Bim, Bmf, Puma, Bad, Bmf, and Hrk) which are pro-apoptotic and responsible for endoplasmic reticulum stress, DNA damage, growth factor deficiency, group 3 (Bak, Bax, and Bok/Mtd) which are also pro-apoptotic [55]. The anti-apoptotic and pro-apoptotic members of the Bcl-2 family undergo imbalance, which leads to dysregulated apoptosis (Fig. 2). Human lung cancers are associated with a mutation in the p53 gene [56–58]. The Inhibitor apoptosis proteins (IAPs) include X-linked IAP (XIAP, BIRC4), BIRC8, BIRC7, apollon (BRUCE, BIRC6), surviving (BIRC5), c-IAP2 (BIRC3), c-IAP1 (BIRC2) and NAIP (BIRC1). They are characterized by utilizing the baculovirus IAPs domains. They are very useful during apoptosis and signal transduction. IAPs can prevent the caspase from binding to their substrates [59]. Caspases are very vital in the initiation (caspase-2, -8, -9 and -10) and execution (caspase-3, -6 and -7) of apoptosis. Hence, low levels of caspases expression or deficiency in caspase roles may result to reduce in apoptosis and lung carcinogenesis. Currently, there are a lot of drugs and small molecules which are manufactured based on the mechanisms of apoptosis which includes sodium butyrate, oblimersen sodium, depsipetide, HA14-1, fenretinide, ABT-263, flavopiridol gossypol, ABT-737, GX15-070, which direct action on the Bcl 2 proteins [60–64]. Some drugs and small molecules also target p53 and a lot of clinical trials are ongoing for some new drugs. A search at http://www.clinicaltrials.gov will reveal molecules and drugs that target numerous proteins associated with apoptosis such as Bcl 2 family, IAPS and p53.
Fig. 2.
Dysregulated apoptosis and lung cancer. The anti-apoptotic and pro-apoptotic members of the Bcl-2 family undergo imbalance, which leads to dysregulated apoptosis. The overexpression of anti- apoptotic include group 1 (Bcl-2, Bcl-xL, Mcl-1, Bcl-w, A1/BF-1, BclB/Bcl2L10) and under expression of group 2 (Bim, Bid, Puma, Noxa, Bad, Hrk, Bik) and under expression of group 3 (Bak, Bax, and Bok/Mtd) resulted to dysregulated apoptosis and lung cancer. Other factors involved includes reduced expression of caspases, increased expression of inhibitor of apoptosis proteins, mutation on p53, impaired receptor signalling pathway and reduced expression of death receptor
CD146 expression on the CD4+ Treg
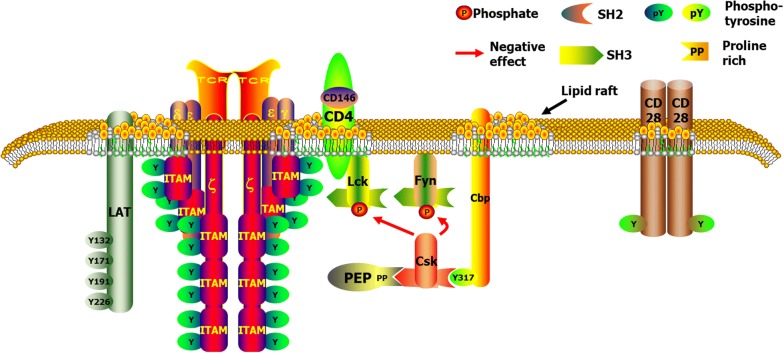
CD146 formerly known as a melanoma marker [65], is an important glycoprotein found on the integral membrane of the cells. It is linked to the immunoglobulin superfamily with a feature V–V–C2–C2–C2 domain structure, and its cytoplasmic tail contains potential protein kinase C recognition sites and PDZ binding sites [66] indicating possible involvements in cell signaling (Fig. 3). CD146 mediates transduction of outside-in signals [67]. However, the precise extracellular ligand for CD146 is unclear. Sendo1 crosslinking with CD146, activate the phosphorylation of FAK through the link with Fyn [68]. Moreover, previous investigations discovered that CD146 mediates tumor secretion-induced p38/IkB kinase/nuclear factor-kB signaling cascade, which is essential in inducing endothelial cell activation, resulting in tumor angiogenesis [69–71]. Intracellular effectors and binding partners are very crucial in completely understanding of CD146 signaling. Treg cells may possibly also inhibit the antitumor immune responses. Predominantly in the environment of cancer, Treg-cell occurrences and roles are significant since increased numbers could results to tumor progression [72]. In most cancer patients, the immunophenotyping of Treg cells have concentrated primarily on co-expression of CD4+ and CD25+, while different types of Treg cell subtypes (Table 1) exist. To better comprehend and exploit Treg-cell biology in association with carcinogenesis and tumorigenesis, it will be very interesting to investigate more specific cell-surface markers.
Fig. 3.
T Cell Receptor Complex and associated components. Showing the possible involvement of CD146 in cell signaling
Table 1.
Characteristics of Subsets of T cells (Treg)
| Subset | Specific marker | Secretory products | Actions | Location |
|---|---|---|---|---|
| nTreg | CD4, CD25, Foxp3 | IL-10, TGF-β | Block T cell proliferation, suppression of DCs, inhibition of effector Th1, Th2, and Th17 cells; suppress mast cells, basophils, and eosinophils; interact with resident tissue cells | Thymus |
| nTeg | CD4, CD25, CD127 | IL-10, TGF-β | Block T cell proliferation, | Neonatal thymus |
| ICOS(+) Treg | CD4, CD25, Foxp3, ICOS | IL-10, IL-17, IFN-γ | Suppress hapten-reactive CD8(+) T cells | Generated from nTregs |
| iTreg | CD4, Foxp3 | IL-10, TGF-β | Similar to nTreg | Periphery |
| Tr1 | CD4, CD25 | IL-10 | Suppress effector Th cell migration and functions suppress mast cells, basophils, and eosinophils | Generated from non-Treg cell precursors and home lungs and draining lymph nodes |
| CD8(+)Treg | CD8, Foxp3, CD25 (not for tonsil origin), CD28 | IL-10, TNF-α, IFN-γ, GB | Block activation of naive or effector T cells; suppress IgG/IgE antibody responses [9], IL-4 expression and the proliferation of CD4(+) T cells | Generated from OT-1 CD8 cells [9] and tonsils |
| IL-17-producing Foxp3 (+) Treg | CD4, Foxp3, CCR6, RORGTF | IL-17 | Inhibit the proliferation of CD4(+) effector T cells | Differentiated from CD4(+)Foxp3(+)CCR6(−) Tregs in peripheral blood and lymphoid tissue |
nTreg natural regulatory T cell, ICOS inducible costimulator, iTreg inducible/adaptive regulatory T cell, Tr1 cell IL-10-producing type 1 regulatory T cell, GB granzyme B, RORGTF ROR gamma transcription factor
Binding partners of CD 146
Previously CD146 was assumed to have a homotypic ligand-receptor interaction, a mechanism which is still unclear [20]. Recent investigations propose a ligand for CD146 is laminin-411 (α4-chain, a β1-chain, and a γ1 chain, also known as laminin-8) [73, 74]. Further investigations were conducted to show that CD146+ cells [75, 76] were self-regulated of very late antigen-4 (VLA-4) and in combination with p-selectin glycoprotein ligand-1 (PSGL-1)-mediated progressing of these cells. Galectin-1 (LGAL-1) and galectin-3 (LGAL3) have been shown to bind to CD146 [77]. Vascular endothelial growth factor receptor-2 (VEGFR2) and CD146 act together unswervingly and that this binding improves VEGFR2 signaling [78]. In endothelial cell and T cells, CD146 is a useful biomarker. It has importance in angiogenesis; nevertheless the molecular mechanism underlying angiogenesis remains unclear. CD146 and Wnt5a are binding partner [79]. The accounts of multiple ligands for CD146 are still debatable due to the mechanisms of adherence and migration of estimate the proportion of immune and cancer cells (EPIC) T cells. However, the investigations unfolding these ligands for CD146 have not been fully explored confirmed by subsequent studies and it is unclear if these ligands are competitive or cooperative. Further researches and explorations are required to explain the binding ligands of CD146 and the precise mechanism of migration and signaling in EPIC T cells.
Molecular signaling of CD146
Molecular signaling of CD146 commitments have been extensively considered in numerous non-leukocytic cells types; but an inclusive, clear depiction of CD146 is interesting for investigation. Protein tyrosine kinase (PTK)-dependent signaling pathway, with tyrosine phosphorylation of the focal adhesion kinase, p125FAK, paxillin and NF-kB are important molecular signaling of CD146 [68, 80]. Investigations have been conducted on the reciprocal control of CD146 and Akt (serine/threonine specific protein kinase B) in melanoma cell lines. Akt is linked with tumor cell survival, proliferation, and invasiveness. The triggering of Akt is a variation detected in human cancer and tumor cells. Tumor cells that have regularly active Akt may be contingent on Akt for existence [81]. Therefore, Akt pathways may be possible therapies for cancer and tumor cells resulting in inactivation of the Bcl-2-associated death promoter (BAD).
Pervious Investigations [19] suggest utilizing human umbilical cord endothelial cells, as well as zebrafish embryos showed that CD146 binds to Wnt5a with high affinity and is important for endothelial cell migration and activity of c-jun amino-terminal kinase (JNK) via non-canonical signaling. The phosphorylation of Disheveled (Dvl), insulin-like growth factor binding protein 4 (IGFBP4), an opponent of the Wnt/β-catenin signaling, was discovered to trigger Wnt/β-catenin signaling pathway and to encourage the expression of CD146 in renal carcinoma cells. Currently, research is limited in the investigation of human T cells describing the signaling pathways associated with CD146 engagement.
CD146 a novel marker of lymphocyte subset population
Initially, the expression of CD146 on lymphocyte appeared in 1997 [82]. Hence, the expression of CD146 on the leukocytes of healthy donors was not significant. It may be found in CD4+ and CD8+ subpopulation using TCRVβ analysis. The low percentage may be detected on B cells and sometimes in NK population. Moreover, skin specimens from contact dermatitis patients established that 50–80% of the CD3+ cells in tissue sections were CD146+. This initial investigation lay quiescent for nearly a decade until another researcher identified CD146+ T cells in the peripheral blood circulation of healthy donors [6]. CD146 could be upregulated on B cells by mitogen stimulation such as PMA, and by activation with a combination of CD40L and IL-4 [6]. The utilization of the techniques of immunohistochemistry has demonstrated the presence of CD146 on immature cortical thymocytes, confirming the idea that this antigen was expressed on T cells at an early stage [83]. It was also discovered in the peripheral blood of Treg cells in the lung cancer patient which was significantly different from control (healthy subjects).
Immunophenotyping and detection of CD146
Immunophenotyping of CD146 is the exploration of heterogeneous populations of CD146 in the T cells for identifying the presence, expression, and proportions. Nevertheless, CD146 was previously designated as an activation antigen of T cells, the circulation of this antigen is different from other common markers of activation such as, OX-40, CD38, CD25, HLA-Dr and CD69 in freshly isolated cells [19]. However, there are different amount of the expression of CD146 with many of the other activation markers [84]. CD146+ T cells were also discovered to be CCR7−, CD28+, CD45RA−, CD45RO+, designated as effector memory T cells [66]. Markers linked with Th17 cells, CD58 and CD26 were concurrently expressed on CD146 positive cells, but extra markers associated with Th17 cells, including, CCR4 and CCR6 were moderately expressed with CD146 [76]. Markers associated with Treg cells, CD4+, CD25+ and CD127dim/- were simultaneously expressed on the CD146 positive cells in healthy subject Fig. 4 and lung cancer patient Fig. 5 respectively.
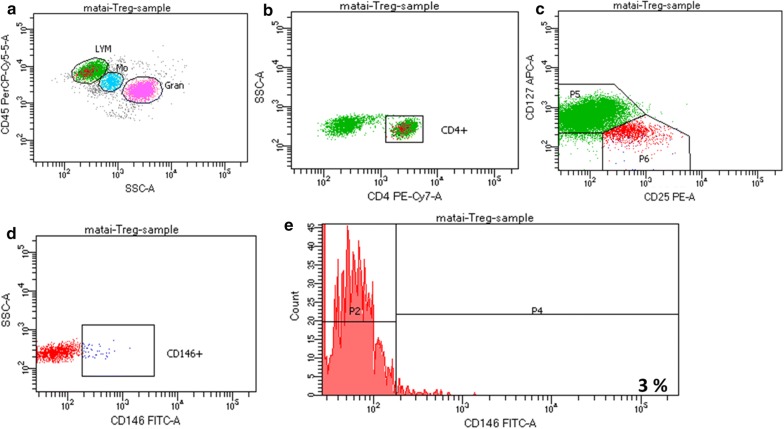
Fig. 4.
Expression of CD 146+ in healthy subject. a CD45PreCP-cy5-5-A vs SSCA. b SSC-A vs CD4PE-Cy7-A. c CD127 APC-A vs CD 25 PE-A. d SSCA vs CD 146 FITC-A. (E) Count vs CD 146 FITC-A = 3%
Fig. 5.
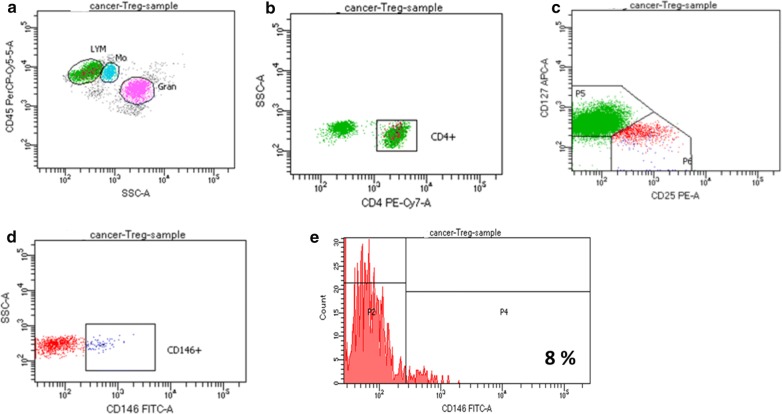
Expression of CD 146+ in lung cancer patient. a CD45PreCP-cy5-5-A vs SSCA. b SSC-A vs CD4PE-Cy7-A. c CD127 APC-A vs CD 25 PE-A. d SSCA vs CD 146 FITC-A. e Count vs CD 146 FITC-A = 8%
CD146 can be detected utilizing the following techniques. These include flow cytometry, immunofluorescence, immunohistochemically staining of paraffin-embedded tumor samples, electron microscopy, confocal microscopy, application of a chemiluminescence detection system [82], immunomagnetic sorting, immunoprecipitation, mass spectrometry, western blot [82, 85], ELISA can be used for the detection of soluble CD146 from serum or plasma Fig. 6 [11], real-time PCR of CD146 mRNA [86] and quantitative real-time PCR can also be utilized for the detection of CD146 [87, 88].
Fig. 6.
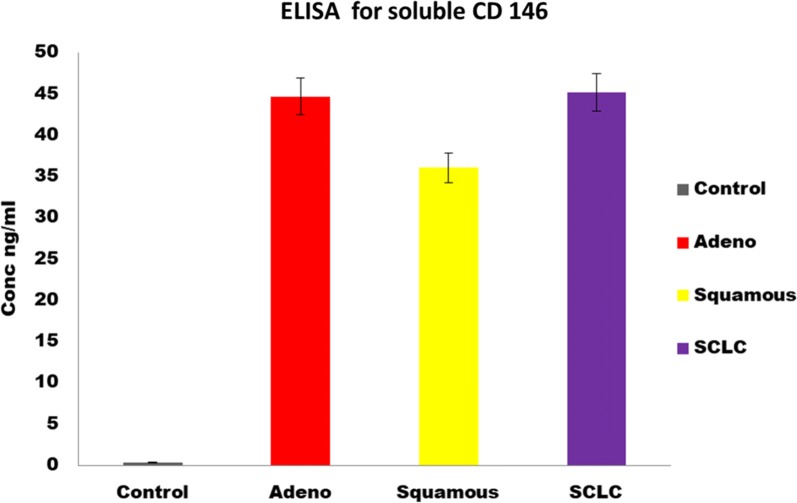
Detection of sCD146 using ELISA. The expression of sCD 146 was significantly higher in patients than in controls (P < 0.05). From the ELISA, the concentration of the sCD146 includes 0.33 ± 0.09 ng/ml in healthy patients (Control), 44.69 ± 0.29 ng/ml, 36.05 ± 0.24 ng/ml, 45.18 ± 0.27 ng/ml in adenocarcinoma, squamous and small cell lung cancer, respectively
CD146 T cells in cancer
CD146 is a multifunctional molecule that contributes in various molecular, biochemical, physiological [89] and pathological processes relating to immunity, signal transduction, stem cell differentiation [75, 90] and angiogenesis [3, 91]. In recent years, various investigations revealed that CD146 overexpression significantly relates with the metastasis, progression and formation of new blood vessels of some malignant tumors which was investigated in melanoma, esophageal cancer, prostate cancer, gallbladder adenocarcinoma, ovarian carcinoma [87, 88, 92–101]. Currently, researchers are conducting investigations on the different kinds of cancer and the output revealed the different correlations of CD146 with cancer. Hence, high number of investigation indicated that CD146 is highly expressed in solid tumors, including lung cancer [12, 13, 102–104], hepatocellular carcinoma [105, 106], epithelial ovarian cancer [107], breast cancer [108, 109], leiomyosarcoma [110], esophageal squamous cell carcinoma [111], gallbladder adenocarcinoma [98], colorectal cancer [112], gastric cancer [113], clear cell renal cell carcinoma [114], melanoma [115], hematological malignancies [116], peripheral nerve tumors [117], parotid carcinoma [118], non-small cell lung cancer [12], infantile haemangioma [119], adenoid cystic carcinoma [120], malignant pleural mesothelioma [121], pancreatic cancer [14], prostate cancer [122], cervical cancer and endometrium cancer [123]. The different kinds of cancer and the implications of CD146 have been summarized in Table 2. These obvious suggestions on the expression of CD146 in cancer indicated that the transmembrane glycoprotein would be further deliberated as a potential biomarker for the diagnosis of cancer patients and therapeutic target.
Table 2.
The implication of increased CD146 in Cancer
| Cancer type | Implications | References |
|---|---|---|
| Lung cancer | Poor prognosis | [12, 13, 102–104] |
| Hepatocellular carcinoma | Promotes metastasis and predicts poor prognosis | [105, 106] |
| Epithelial ovarian cancer | Poor prognosis | [107] |
| Breast cancer | Elevated epithelial-mesenchymal transition | [108, 109] |
| Leiomyosarcoma | Prognostic factor | [110] |
| Esophageal squamous cell carcinoma | Poor prognosis | [111] |
| Gallbladder adenocarcinoma | Progression, metastasis, and poor-prognosis | [98] |
| Colorectal cancer | Poor prognosis | [112] |
| Gastric cancer | Poor prognosis | [113] |
| Clear cell renal cell carcinoma | Elevated reoccurrence | [114] |
| Melanoma | Elevated metastasis and poor prognosis | [115] |
| Hematological malignancies | Elevated tumorigenesis | [116] |
| Peripheral nerve tumors | Modulator of malignant transformation | [117] |
| Parotid carcinoma | Elevated progression and invasion | [118] |
| Non-small cell lung cancer | Poor prognosis | [12] |
| Infantile haemangioma | Elevated progression | [119] |
| Adenoid cystic carcinoma | Elevated progression | [120] |
| Malignant pleural mesothelioma | Poor prognosis | [121] |
| Pancreatic cancer | Poor Prognosis and cancer progression | [14] |
| Cervical cancer | Dissemination and metastasis | [123] |
| Endometrium | Dissemination and metastasis | [123] |
| Prostate cancer | Poor prognosis | [122] |
Effects of methanol extract of Calotropis procera leaf on CD146 expression
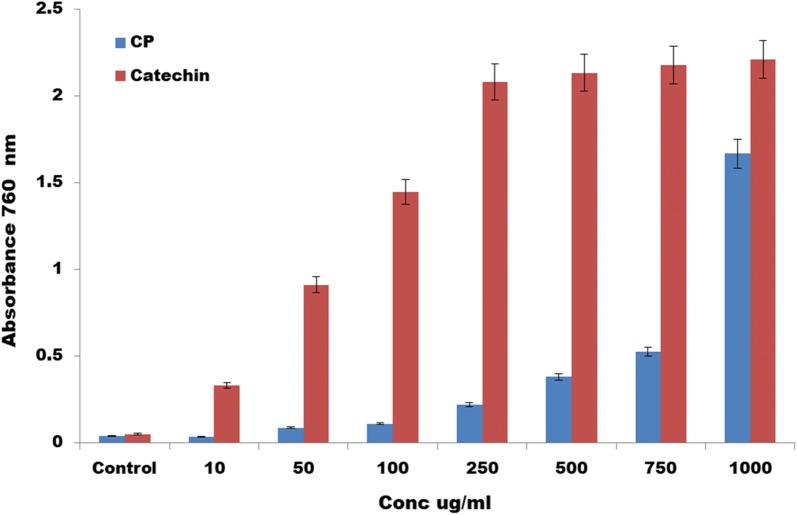
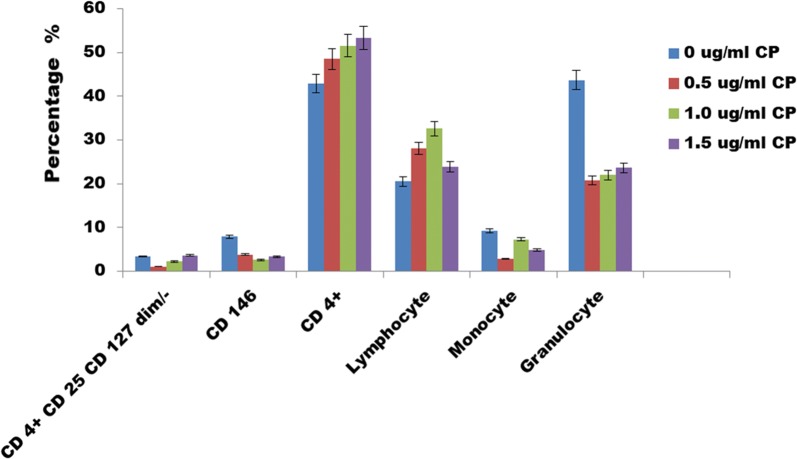
In this review, an effect of methanol extract of Calotropis procera leaf on CD146 expression was explored to discover that it has phenolic contents Fig. 7. It revealed that at 1000 ug/ml, the phenolic content in CP was similar to the standard catechin which indicates that it is an antioxidant. CP was used on the blood cells and we discovered that it reduced the expression of CD146 Fig. 8; hence it may be a potential immunotherapy for the treatment of cancer and various diseases. The CD4+ cells increased and it was dose dependent. Hence it is very interesting to unravel the dose of CP which may be used for the treatment of cancer induced animal models. Further investigations are also required to find out the molecular mechanism that is responsible for the reduction of CD146 using the CP.
Fig. 7.
Total Phenolic content for Calotropis Procera leaf. It revealed that at 1000 ug/ml, the phenolic content was similar to the standard catechin
Fig. 8.
Effects of CP on CD 146 at 12 hours. CP was used on the blood cells and we discovered that it reduced the expression of CD146, increased the expression of CD4+ and dose dependent. It shows that CP may be an immunotherapy
Conclusion
Currently, there is no data to describe the genetic association of CD146 expression and IL-17 secretion. However, some IL-17 secreting T cells can be discovered in healthy individuals without CD146 expression on those cells. T cells may be potential immunotherapy for lung cancer and other types of cancer. Targeting of the T cells and CD146 when the ligand is known through the migration of these cells to sites of injury or tumor cells is interesting. However, investigation unfolding the molecular mechanism and regulation of CD146 expression on the T cells is still limited and is a hot topic for investigations. CD146, apoptosis of the T cells and lung cancer are also of very great significance because these may be excellent target for the treatment of carcinogenesis. Future investigations to unravel the significance of methanol extract of Calotropis procera leaf on CD146 expression will be very interesting. Utilization of cancer cell lines, animal models and using CP as a therapy will be fascinating. Hence the molecular mechanism underlying the process by which CP ameliorate the expression of CD146 will be of unique importance to the investigation. Therefore CD146 is a molecule of significance which can also be studied in other diseases state such as inflammation, COPD, pulmonary arterial hypertension and other respiratory diseases. Animal models of PAH and other diseases model or knockout mouse can be investigated to unravel the expression of CD146 in these models and comparisons with the human samples can also be conducted to unveil the possible diagnosis, therapy and prognosis.
Acknowledgements
We thank Henan Provincial People Hospital and National Natural Science Foundation for financial support and fellowship.
Abbreviations
- VEGF
vascular endothelial growth factor
- IL-10
interleukin 10
- IL-4
interleukin 4
- IL-17
interleukin 17
- NF-κB
nuclear factor-kappa B
- IL-1R
interleukin-1 receptor
- (TNF)-α receptor
tumor necrosis factor -α receptor
- Akt
serine/threonine specific protein kinase B
- CD
cluster of differentiation
- LGAL 1
galectin 1
- LGAL 3
galectin 3
Authors’ contributions
All the authors contributed in the preparation of this paper. AMO was responsible for data collection, analysis and drafting of the article. AKO, ZQW, and ZRZ were responsible for blood sample collections. AMO and XJZ made substantial contributions to manuscript conception and design and participated in its critical review and final editing. All authors read and approved the final manuscript.
Funding
This work was financially supported by institutional fund of Henan Provincial People’s Hospital to AMO, the grant of the National Natural Science Foundation of China (81472835 to XJZ)
Availability of data and materials
Not applicable.
Ethics approval and consent to participate
Compliance with ethical guidelines.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Ayobami Matthew Olajuyin, Phone: +8615611636897, Email: doctorayobami@gmail.com.
Xiaoju Zhang, Phone: +8615837101166, Email: 15837101166@163.com, Email: zhangxiaoju1010@henu.edu.cn.
References
- 1.Lehmann JM, Holzmann B, Breitbart EW, Schmiegelow P, Riethmüller G, Johnson JP. Discrimination between benign and malignant cells of melanocytic lineage by two novel antigens, a glycoprotein with a molecular weight of 113,000 and a protein with a molecular weight of 76,000. Cancer Res. 1987;47(3):841–845. [PubMed] [Google Scholar]
- 2.Trzpis M, McLaughlin PM, de Leij LM, Harmsen MC. Epithelial cell adhesion molecule: more than a carcinoma marker and adhesion molecule. Am J Pathol. 2007;171(2):386–395. doi: 10.2353/ajpath.2007.070152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Z, Yan X. CD146, a multi-functional molecule beyond adhesion. Cancer Lett. 2013;330(2):150–162. doi: 10.1016/j.canlet.2012.11.049. [DOI] [PubMed] [Google Scholar]
- 4.Kratzer A, Chu HW, Salys J, Moumen Z, Leberl M, Bowler R, et al. Endothelial cell adhesion molecule CD146: implications for its role in the pathogenesis of COPD. J Pathol. 2013;230(4):388–398. doi: 10.1002/path.4197. [DOI] [PubMed] [Google Scholar]
- 5.Uematsu K, Kawase T, Nagata M, Suzuki K, Okuda K, Yoshie H, et al. Tissue culture of human alveolar periosteal sheets using a stem-cell culture medium (MesenPRO-RS™): in vitro expansion of CD146-positive cells and concomitant upregulation of osteogenic potential in vivo. Stem cell Res. 2013;10(1):1–19. doi: 10.1016/j.scr.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Elshal MF, Khan SS, Takahashi Y, Solomon MA, McCoy JP. CD146 (Mel-CAM), an adhesion marker of endothelial cells, is a novel marker of lymphocyte subset activation in normal peripheral blood. Blood. 2005;106(8):2923–2924. doi: 10.1182/blood-2005-06-2307. [DOI] [PubMed] [Google Scholar]
- 7.Khan SS, Solomon MA, McCoy JP., Jr Detection of circulating endothelial cells and endothelial progenitor cells by flow cytometry. Cytom Part B. 2005;64(1):1–8. doi: 10.1002/cyto.b.20040. [DOI] [PubMed] [Google Scholar]
- 8.Stalin J, Nollet M, Dignat-George F, Bardin N, Blot-Chabaud M. Therapeutic and diagnostic antibodies to CD146: thirty years of research on its potential for detection and treatment of tumors. Antibodies. 2017;6(4):17. doi: 10.3390/antib6040017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Espagnolle N, Guilloton F, Deschaseaux F, Gadelorge M, Sensébé L, Bourin P. CD 146 expression on mesenchymal stem cells is associated with their vascular smooth muscle commitment. J Cell Mol Med. 2014;18(1):104–114. doi: 10.1111/jcmm.12168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bardin N, George F, Mutin M, Brisson C, Horschowski N, Frances V, et al. S-Endo 1, a pan-endothelial monoclonal antibody recognizing a novel human endothelial antigen. Tissue Antigens. 1996;48(5):531–539. doi: 10.1111/j.1399-0039.1996.tb02666.x. [DOI] [PubMed] [Google Scholar]
- 11.Bardin N, Moal V, Anfosso F, Daniel L, Brunet P, Sampol J, et al. Soluble CD146, a novel endothelial marker, is increased in physiopathological settings linked to endothelial junctional alteration. Thromb Haemost. 2003;90(11):915–920. doi: 10.1160/TH02-11-0285. [DOI] [PubMed] [Google Scholar]
- 12.Kristiansen G, Yu Y, Schlüns K, Sers C, Dietel M, Petersen I. Expression of the cell adhesion molecule CD146/MCAM in non-small cell lung cancer. Anal Cell Pathol. 2003;25(2):77–81. doi: 10.1155/2003/574829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ilie M, Long E, Hofman V, Selva E, Bonnetaud C, Boyer J, et al. Clinical value of circulating endothelial cells and of soluble CD146 levels in patients undergoing surgery for non-small cell lung cancer. Br J Cancer. 2014;110(5):1236. doi: 10.1038/bjc.2014.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stalin J, Nollet M, Garigue P, Fernandez S, Vivancos L, Essaadi A, et al. Targeting soluble CD146 with a neutralizing antibody inhibits vascularization, growth and survival of CD146-positive tumors. Oncogene. 2016;35(42):5489. doi: 10.1038/onc.2016.83. [DOI] [PubMed] [Google Scholar]
- 15.England CG, Jiang D, Hernandez R, Sun H, Valdovinos HF, Ehlerding EB, et al. ImmunoPET imaging of CD146 in murine models of intrapulmonary metastasis of non-small cell lung cancer. Mol Pharm. 2017;14(10):3239–3247. doi: 10.1021/acs.molpharmaceut.7b00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Arrieta O, Guzmán-de EA, Alba-Lopez L, Acosta-Espinoza A, Alatorre-Alexander J, Alexander-Meza J, et al. National consensus of diagnosis and treatment of non-small cell lung cancer. Rev Invest Clin. 2013;65:S5–S84. [PubMed] [Google Scholar]
- 17.Fitzmaurice C, Akinyemiju TF, Al Lami FH, Alam T, Alizadeh-Navaei R, Allen C, et al. Global, regional, and national cancer incidence, mortality, years of life lost, years lived with disability, and disability-adjusted life-years for 29 cancer groups, 1990 to 2016: a systematic analysis for the global burden of disease study. JAMA Oncol. 2018;4(11):1553–1568. doi: 10.1001/jamaoncol.2018.2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arrieta O, Cardona AF, Corrales L, Campos-Parra AD, Sánchez-Reyes R, Amieva-Rivera E, et al. The impact of common and rare EGFR mutations in response to EGFR tyrosine kinase inhibitors and platinum-based chemotherapy in patients with non-small cell lung cancer. Lung Cancer. 2015;87(2):169–175. doi: 10.1016/j.lungcan.2014.12.009. [DOI] [PubMed] [Google Scholar]
- 19.Elshal MF, Khan SS, Raghavachari N, Takahashi Y, Barb J, Bailey JJ, et al. A unique population of effector memory lymphocytes identified by CD146 having a distinct immunophenotypic and genomic profile. BMC immunol. 2007;8(1):29. doi: 10.1186/1471-2172-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Guezguez B, Vigneron P, Lamerant N, Kieda C, Jaffredo T, Dunon D. Dual role of melanoma cell adhesion molecule (MCAM)/CD146 in lymphocyte endothelium interaction: MCAM/CD146 promotes rolling via microvilli induction in lymphocyte and is an endothelial adhesion receptor. J Immunol. 2007;179(10):6673–6685. doi: 10.4049/jimmunol.179.10.6673. [DOI] [PubMed] [Google Scholar]
- 21.Dagur PK, Biancotto A, Wei L, Sen HN, Yao M, Strober W, et al. MCAM-expressing CD4+ T cells in peripheral blood secrete IL-17A and are significantly elevated in inflammatory autoimmune diseases. J Autoimmun. 2011;37(4):319–327. doi: 10.1016/j.jaut.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dagur PK, Biancotto A, Stansky E, Sen HN, Nussenblatt RB, McCoy JP. Secretion of interleukin-17 by CD8+ T cells expressing CD146 (MCAM) Clin immunol. 2014;152(1–2):36–47. doi: 10.1016/j.clim.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dagur PK, McCoy JP., Jr Endothelial-binding, proinflammatory T cells identified by MCAM (CD146) expression: characterization and role in human autoimmune diseases. Autoimmun Rev. 2015;14(5):415–422. doi: 10.1016/j.autrev.2015.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hassan LM, Galal TM, Farahat EA, El-Midany MM. The biology of Calotropis procera (Aiton) WT. Trees. 2015;29(2):311–320. doi: 10.1007/s00468-015-1158-7. [DOI] [Google Scholar]
- 25.Sharma R, Thakur GS, Sanodiya BS, Savita A, Pandey M, Sharma A, et al. Therapeutic potential of Calotropis procera: a giant milkweed. ISOR J Pharm Bio Sci. 2012;4(2):42–57. [Google Scholar]
- 26.Pandey A, Swarnkar V, Pandey T, Srivastava P, Kanojiya S, Mishra DK, et al. Transcriptome and metabolite analysis reveal candidate genes of the cardiac glycoside biosynthetic pathway from Calotropis procera. Sci Rep. 2016;6:34464. doi: 10.1038/srep34464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascolo N, Sharma R, Jain S, Capasso F. Ethnopharmacology of Calotropis procera flowers. J Ethnopharmacol. 1988;22(2):211–221. doi: 10.1016/0378-8741(88)90129-8. [DOI] [PubMed] [Google Scholar]
- 28.Juncker T, Cerella C, Teiten M-H, Morceau F, Schumacher M, Ghelfi J, et al. UNBS1450, a steroid cardiac glycoside inducing apoptotic cell death in human leukemia cells. Biochem Pharmacol. 2011;81(1):13–23. doi: 10.1016/j.bcp.2010.08.025. [DOI] [PubMed] [Google Scholar]
- 29.Ashkenazi A, Dixit VM. Death receptors: signaling and modulation. Science. 1998;281(5381):1305–1308. doi: 10.1126/science.281.5381.1305. [DOI] [PubMed] [Google Scholar]
- 30.Green DR, Ware CF. Fas-ligand: privilege and peril. Proc Natl Acad Sci. 1997;94(12):5986–5990. doi: 10.1073/pnas.94.12.5986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Russell JH, White CL, Loh DY, Meleedy-Rey P. Receptor-stimulated death pathway is opened by antigen in mature T cells. Proc Natl Acad Sci. 1991;88(6):2151. doi: 10.1073/pnas.88.6.2151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wesselborg S, Janssen O, Kabelitz D. Induction of activation-driven death (apoptosis) in activated but not resting peripheral blood T cells. J Immunol. 1993;150(10):4338–4345. [PubMed] [Google Scholar]
- 33.Salmon M, Pilling D, Borthwick NJ, Viner N, Janossy G, Bacon PA, et al. The progressive differentiation of primed T cells is associated with an increasing susceptibility to apoptosis. Eur J immunol. 1994;24(4):892–899. doi: 10.1002/eji.1830240417. [DOI] [PubMed] [Google Scholar]
- 34.Dhein J, Walczak H, Bäumler C, Debatin K-M, Krammer PH. Autocrine T-cell suicide mediated by APO-1/(Fas/CD95) Nature. 1995;373(6513):438. doi: 10.1038/373438a0. [DOI] [PubMed] [Google Scholar]
- 35.Zheng L, Fisher G, Miller RE, Peschon J, Lynch DH, Lenardo MJ. Induction of apoptosis in mature T cells by tumour necrosis factor. Nature. 1995;377(6547):348. doi: 10.1038/377348a0. [DOI] [PubMed] [Google Scholar]
- 36.Suda T, Okazaki T, Naito Y, Yokota T, Arai N, Ozaki S, et al. Expression of the Fas ligand in cells of T cell lineage. J Immunol. 1995;154(8):3806–3813. [PubMed] [Google Scholar]
- 37.Lynch DH, Ramsdell F, Alderson MR. Fas and FasL in the homeostatic regulation of immune responses. Immunol Today. 1995;16(12):569–574. doi: 10.1016/0167-5699(95)80079-4. [DOI] [PubMed] [Google Scholar]
- 38.Hyde H, Borthwick NJ, Janossy G, Salmon M, Akbar AN. Upregulation of intracellular glutathione by fibroblast-derived factor (s): enhanced survival of activated T cells in the presence of low Bcl-2. Blood. 1997;89(7):2453–2460. [PubMed] [Google Scholar]
- 39.Ayroldi E, Zollo O, Cannarile L, D’Adamio F, Grohmann U, Delfino DV, et al. Interleukin-6 (IL-6) prevents activation-induced cell death: IL-2-independent inhibition of Fas/fasL expression and cell death. Blood. 1998;92(11):4212–4219. [PubMed] [Google Scholar]
- 40.Sandstrom PA, Mannie MD, Buttke TM. Inhibition of activation-induced death in T cell hybridomas by thiol antioxidants: oxidative stress as a mediator of apoptosis. J Leukoc Biol. 1994;55(2):221–226. doi: 10.1002/jlb.55.2.221. [DOI] [PubMed] [Google Scholar]
- 41.Williams MS, Henkart PA. Role of reactive oxygen intermediates in TCR-induced death of T cell blasts and hybridomas. J Immunol. 1996;157(6):2395–2402. [PubMed] [Google Scholar]
- 42.Marchetti P, Hirsch T, Zamzami N, Castedo M, Decaudin D, Susin SA, et al. Mitochondrial permeability transition triggers lymphocyte apoptosis. J Immunol. 1996;157(11):4830–4836. [PubMed] [Google Scholar]
- 43.Los M, Schenk H, Hexel K, Baeuerle PA, Dröge W, Schulze-Osthoff K. IL-2 gene expression and NF-kappa B activation through CD28 requires reactive oxygen production by 5-lipoxygenase. EMBO J. 1995;14(15):3731–3740. doi: 10.1002/j.1460-2075.1995.tb00043.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tatla S, Woodhead V, Foreman JC, Chain BM. The role of reactive oxygen species in triggering proliferation and IL-2 secretion in T cells. Free Radic Biol Med. 1999;26(1–2):14–24. doi: 10.1016/S0891-5849(98)00133-6. [DOI] [PubMed] [Google Scholar]
- 45.Hildeman DA, Mitchell T, Teague TK, Henson P, Day BJ, Kappler J, et al. Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity. 1999;10(6):735–744. doi: 10.1016/S1074-7613(00)80072-2. [DOI] [PubMed] [Google Scholar]
- 46.Westermann J, Bode U. Distribution of activated T cells migrating through the body: a matter of life and death. Immunol Today. 1999;20(7):302–306. doi: 10.1016/S0167-5699(99)01474-7. [DOI] [PubMed] [Google Scholar]
- 47.Kessel RG. Basic medical histology: the biology of cells, tissues, and organs. Oxford: Oxford University Press; 1998. [Google Scholar]
- 48.Rao RV, Ellerby H, Bredesen DE. Coupling endoplasmic reticulum stress to the cell death program. Cell Death Diff. 2004;11(4):372. doi: 10.1038/sj.cdd.4401378. [DOI] [PubMed] [Google Scholar]
- 49.Chae H-J, Kim H-R, Xu C, Bailly-Maitre B, Krajewska M, Krajewski S, et al. BI-1 regulates an apoptosis pathway linked to endoplasmic reticulum stress. Mol Cell. 2004;15(3):355–366. doi: 10.1016/j.molcel.2004.06.038. [DOI] [PubMed] [Google Scholar]
- 50.Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3(11):E255. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- 51.Breckenridge DG, Germain M, Mathai JP, Nguyen M, Shore GC. Regulation of apoptosis by endoplasmic reticulum pathways. Oncogene. 2003;22(53):8608. doi: 10.1038/sj.onc.1207108. [DOI] [PubMed] [Google Scholar]
- 52.Bakhshi J, Weinstein L, Poksay KS, Nishinaga B, Bredesen DE, Rao RV. Coupling endoplasmic reticulum stress to the cell death program in mouse melanoma cells: effect of curcumin. Apoptosis. 2008;13(7):904. doi: 10.1007/s10495-008-0221-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Weinberg R, Hanahan D. The hallmarks of cancer. Cell. 2000;100(1):57–70. doi: 10.1016/S0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 54.Gross A, McDonnell JM, Korsmeyer SJ. BCL-2 family members and the mitochondria in apoptosis. Genes Dev. 1999;13(15):1899–1911. doi: 10.1101/gad.13.15.1899. [DOI] [PubMed] [Google Scholar]
- 55.Dewson G, Kluck R. Bcl-2 family-regulated apoptosis in health and disease. Cell Health Cytoskelet. 2010;2(9):22. [Google Scholar]
- 56.Quinlan DC, Davidson AG, Summers CL, Warden HE, Doshi HM. Accumulation of p53 protein correlates with a poor prognosis in human lung cancer. Cancer Res. 1992;52(17):4828–4831. [PubMed] [Google Scholar]
- 57.Denissenko MF, Pao A, Tang M-S, Pfeifer GP. Preferential formation of benzo [a] pyrene adducts at lung cancer mutational hotspots in P53. Science. 1996;274(5286):430–432. doi: 10.1126/science.274.5286.430. [DOI] [PubMed] [Google Scholar]
- 58.Zhang E, Yin D, Sun M, Kong R, Liu X, You L, et al. P53-regulated long non-coding RNA TUG1 affects cell proliferation in human non-small cell lung cancer, partly through epigenetically regulating HOXB7 expression. Cell Death Dis. 2014;5(5):e1243. doi: 10.1038/cddis.2014.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Wei Y, Fan T, Yu M. Inhibitor of apoptosis proteins and apoptosis. Acta Biochim Biophys Sin. 2008;40(4):278–288. doi: 10.1111/j.1745-7270.2008.00407.x. [DOI] [PubMed] [Google Scholar]
- 60.Rai K, Moore J, Wu J, Novick S, O’Brien S. Effect of the addition of oblimersen (Bcl-2 antisense) to fludarabine/cyclophosphamide for relapsed/refractory chronic lymphocytic leukemia (CLL) on survival in patients who achieve CR/nPR: five-year follow-up from a randomized phase III study. J Clin Oncol. 2008;26(15_suppl):7008. doi: 10.1200/jco.2008.26.15_suppl.7008. [DOI] [Google Scholar]
- 61.Wong RS. Apoptosis in cancer: from pathogenesis to treatment. J Exp Clin Cancer Res. 2011;30(1):87. doi: 10.1186/1756-9966-30-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Russo M, Spagnuolo C, Volpe S, Tedesco I, Bilotto S, Russo GL. ABT-737 resistance in B-cells isolated from chronic lymphocytic leukemia patients and leukemia cell lines is overcome by the pleiotropic kinase inhibitor quercetin through Mcl-1 down-regulation. Biochem Pharmacol. 2013;85(7):927–936. doi: 10.1016/j.bcp.2013.01.011. [DOI] [PubMed] [Google Scholar]
- 63.Kang MH, Reynolds CP. Bcl-2 inhibitors: targeting mitochondrial apoptotic pathways in cancer therapy. Clin Cancer Res. 2009;15(4):1126–1132. doi: 10.1158/1078-0432.CCR-08-0144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Chipuk JE, Moldoveanu T, Llambi F, Parsons MJ, Green DR. The BCL-2 family reunion. Mol Cell. 2010;37(3):299–310. doi: 10.1016/j.molcel.2010.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Luca M, Hunt B, Bucana C, Johnson J, Fidler I, Bar-Eli M. Direct correlation between MUC18 expression and metastatic potential of human melanoma cells. Melanoma Res. 1993;3(1):35–41. doi: 10.1097/00008390-199304000-00006. [DOI] [PubMed] [Google Scholar]
- 66.Guezguez B, Vigneron P, Alais S, Jaffredo T, Gavard J, Mège R-M, et al. A dileucine motif targets MCAM-l cell adhesion molecule to the basolateral membrane in MDCK cells. FEBS Lett. 2006;580(15):3649–3656. doi: 10.1016/j.febslet.2006.05.048. [DOI] [PubMed] [Google Scholar]
- 67.Ouhtit A, Gaur RL, Elmageed ZYA, Fernando A, Thouta R, Trappey AK, et al. Towards understanding the mode of action of the multifaceted cell adhesion receptor CD146. Biochim Biophys Acta. 2009;1795(2):130–136. doi: 10.1016/j.bbcan.2009.01.002. [DOI] [PubMed] [Google Scholar]
- 68.Anfosso F, Bardin N, Francès V, Vivier E, Camoin-Jau L, Sampol J, et al. Activation of human endothelial cells via S-Endo-1 antigen (CD146) stimulates the tyrosine phosphorylation of focal adhesion kinase p125FAK. J Biol Chem. 1998;273(41):26852–26856. doi: 10.1074/jbc.273.41.26852. [DOI] [PubMed] [Google Scholar]
- 69.Yan X, Lin Y, Yang D, Shen Y, Yuan M, Zhang Z, et al. A novel anti-CD146 monoclonal antibody, AA98, inhibits angiogenesis and tumor growth. Blood. 2003;102(1):184–191. doi: 10.1182/blood-2002-04-1004. [DOI] [PubMed] [Google Scholar]
- 70.Zheng C, Qiu Y, Zeng Q, Zhang Y, Lu D, Yang D, et al. Endothelial CD146 is required for in vitro tumor-induced angiogenesis: the role of a disulfide bond in signaling and dimerization. Int J Biochem Cell Biol. 2009;41(11):2163–2172. doi: 10.1016/j.biocel.2009.03.014. [DOI] [PubMed] [Google Scholar]
- 71.Bu P, Gao L, Zhuang J, Feng J, Yang D, Yan X. Anti-CD146 monoclonal antibody AA98 inhibits angiogenesis via suppression of nuclear factor-κB activation. Mol Cancer Ther. 2006;5(11):2872–2878. doi: 10.1158/1535-7163.MCT-06-0260. [DOI] [PubMed] [Google Scholar]
- 72.Dranoff G. The therapeutic implications of intratumoral regulatory T cells. Clin Cancer Res. 2005;11(23):8226–8229. doi: 10.1158/1078-0432.CCR-05-2035. [DOI] [PubMed] [Google Scholar]
- 73.Flanagan K, Fitzgerald K, Baker J, Regnstrom K, Gardai S, Bard F, et al. Laminin-411 is a vascular ligand for MCAM and facilitates TH17 cell entry into the CNS. PLoS ONE. 2012;7(7):e40443. doi: 10.1371/journal.pone.0040443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ishikawa T, Wondimu Z, Oikawa Y, Ingerpuu S, Virtanen I, Patarroyo M. Monoclonal antibodies to human laminin α4 chain globular domain inhibit tumor cell adhesion and migration on laminins 411 and 421, and binding of α6β1 integrin and MCAM to α4-laminins. Matrix Biol. 2014;36:5–14. doi: 10.1016/j.matbio.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 75.Schneider-Hohendorf T, Rossaint J, Mohan H, Böning D, Breuer J, Kuhlmann T, et al. VLA-4 blockade promotes differential routes into human CNS involving PSGL-1 rolling of T cells and MCAM-adhesion of TH17 cells. J Exp Med. 2014;211(9):1833–1846. doi: 10.1084/jem.20140540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Breuer J, Korpos E, Hannocks M-J, Schneider-Hohendorf T, Song J, Zondler L, et al. Blockade of MCAM/CD146 impedes CNS infiltration of T cells over the choroid plexus. J Neuroinflamm. 2018;15(1):236. doi: 10.1186/s12974-018-1276-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Jouve N, Despoix N, Espeli M, Gauthier L, Cypowyj S, Fallague K, et al. The involvement of CD146 and its novel ligand Galectin-1 in apoptotic regulation of endothelial cells. J Biol Chem. 2013;288(4):2571–2579. doi: 10.1074/jbc.M112.418848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jiang T, Zhuang J, Duan H, Luo Y, Zeng Q, Fan K, et al. CD146 is a coreceptor for VEGFR-2 in tumor angiogenesis. Blood. 2012;120(11):2330–2339. doi: 10.1182/blood-2012-01-406108. [DOI] [PubMed] [Google Scholar]
- 79.Ye Z, Zhang C, Tu T, Sun M, Liu D, Lu D, et al. Wnt5a uses CD146 as a receptor to regulate cell motility and convergent extension. Nature Commun. 2013;4:2803. doi: 10.1038/ncomms3803. [DOI] [PubMed] [Google Scholar]
- 80.Solovey A, Gui L, Chang L, Enenstein J, Browne P, Hebbel RP. Identification and functional assessment of endothelial P1H12. J Lab Clin Med. 2001;138(5):322–331. doi: 10.1067/mlc.2001.118519. [DOI] [PubMed] [Google Scholar]
- 81.Li G, Kalabis J, Xu X, Meier F, Oka M, Bogenrieder T, et al. Reciprocal regulation of MelCAM and AKT in human melanoma. Oncogene. 2003;22(44):6891. doi: 10.1038/sj.onc.1206819. [DOI] [PubMed] [Google Scholar]
- 82.Pickl WF, Majdic O, Fischer GF, Petzelbauer P, Faé I, Waclavicek M, et al. MUC18/MCAM (CD146), an activation antigen of human T lymphocytes. J Immunol. 1997;158(5):2107–2115. [PubMed] [Google Scholar]
- 83.Şeftalioğlu A, Karakoç L. Expression of CD146 adhesion molecules (MUC18 or MCAM) in the thymic microenvironment. Acta Histochem. 2000;102(1):69–83. doi: 10.1078/0065-1281-00544. [DOI] [PubMed] [Google Scholar]
- 84.Hadjinicolaou A, Wu L, Fang B, Watson P, Hall F, Busch R. Relationship of CD 146 expression to activation of circulating T cells: exploratory studies in healthy donors and patients with connective tissue diseases. Clin Exp Immunol. 2013;174(1):73–88. doi: 10.1111/cei.12151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Schrage A, Loddenkemper C, Erben U, Lauer U, Hausdorf G, Jungblut PR, et al. Murine CD146 is widely expressed on endothelial cells and is recognized by the monoclonal antibody ME-9F1. Histochem Cell Biol. 2008;129(4):441–451. doi: 10.1007/s00418-008-0379-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Fürstenberger G, Von Moos R, Senn H, Boneberg E. Real-time PCR of CD146 mRNA in peripheral blood enables the relative quantification of circulating endothelial cells and is an indicator of angiogenesis. Br J Cancer. 2005;93(7):793. doi: 10.1038/sj.bjc.6602782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Loges S, Clausen H, Reichelt U, Bubenheim M, Erbersdobler A, Schurr P, et al. Determination of microvessel density by quantitative real-time PCR in esophageal cancer: correlation with histologic methods, angiogenic growth factor expression, and lymph node metastasis. Clin Cancer Res. 2007;13(1):76–80. doi: 10.1158/1078-0432.CCR-06-1324. [DOI] [PubMed] [Google Scholar]
- 88.Wu Z, Wu Z, Li J, Yang X, Wang Y, Yu Y, et al. MCAM is a novel metastasis marker and regulates spreading, apoptosis and invasion of ovarian cancer cells. Tumor Biol. 2012;33(5):1619–1628. doi: 10.1007/s13277-012-0417-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Luo Y, Duan H, Qian Y, Feng L, Wu Z, Wang F, et al. Macrophagic CD146 promotes foam cell formation and retention during atherosclerosis. Cell Res. 2017;27(3):352. doi: 10.1038/cr.2017.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Corselli M, Crisan M, Murray IR, West CC, Scholes J, Codrea F, et al. Identification of perivascular mesenchymal stromal/stem cells by flow cytometry. Cytom Part A. 2013;83(8):714–720. doi: 10.1002/cyto.a.22313. [DOI] [PubMed] [Google Scholar]
- 91.Tu T, Zhang C, Yan H, Luo Y, Kong R, Wen P, et al. CD146 acts as a novel receptor for netrin-1 in promoting angiogenesis and vascular development. Cell Res. 2015;25(3):275. doi: 10.1038/cr.2015.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lei X, Guan C-W, Song Y, Wang H. The multifaceted role of CD146/MCAM in the promotion of melanoma progression. Cancer Cell Int. 2015;15(1):3. doi: 10.1186/s12935-014-0147-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wu G-J, Dickerson EB. Frequent and increased expression of human METCAM/MUC18 in cancer tissues and metastatic lesions is associated with the clinical progression of human ovarian carcinoma. Taiwanese J Obs Gynecol. 2014;53(4):509–517. doi: 10.1016/j.tjog.2014.03.003. [DOI] [PubMed] [Google Scholar]
- 94.Wu G-J, Zeng G-F. METCAM/MUC18 is a novel tumor and metastasis suppressor for the human ovarian cancer SKOV3 cells. BMC Cancer. 2016;16(1):136. doi: 10.1186/s12885-016-2181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zeng P, Li H, Lu P-H, Zhou L-N, Tang M, Liu C-Y, et al. Prognostic value of CD146 in solid tumor: a systematic review and meta-analysis. Sci Rep. 2017;7(1):4223. doi: 10.1038/s41598-017-01061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Wu G-J. Resolving the controversial role of METCAM/MUC18 in the progression of human breast cancer. Breast, cervical and prostate cancer. Brisbane: Hard Cover; iConcept Press Ltd; 2014. pp. 87–100. [Google Scholar]
- 97.Wu G-J. Dual Role of METCAM/MUC18 expression in the progression of cancer cells. In: Gene expression and regulation in mammalian cells-transcription from general aspects. IntechOpen; 2018.
- 98.Wang W, Yang Z-L, Liu J-Q, Jiang S, Miao X-Y. Identification of CD146 expression, angiogenesis, and lymphangiogenesis as progression, metastasis, and poor-prognosis related markers for gallbladder adenocarcinoma. Tumor Biol. 2012;33(1):173–182. doi: 10.1007/s13277-011-0260-8. [DOI] [PubMed] [Google Scholar]
- 99.Wu G-J, Wu M-WH, Wang S-W, Liu Z, Qu P, Peng Q, et al. Isolation and characterization of the major form of human MUC18 cDNA gene and correlation of MUC18 over-expression in prostate cancer cell lines and tissues with malignant progression. Gene. 2001;279(1):17–31. doi: 10.1016/S0378-1119(01)00736-3. [DOI] [PubMed] [Google Scholar]
- 100.Watson-Hurst K, Becker D. The role of N-Cadherin, MCAM, and β3 integrin in melanoma progression, proliferation, migration and invasion. Cancer Biol Therapy. 2006;5(10):1375–1382. doi: 10.4161/cbt.5.10.3241. [DOI] [PubMed] [Google Scholar]
- 101.Garcia S, Dalès J-P, Charafe-Jauffret E, Carpentier-Meunier S, Andrac-Meyer L, Jacquemier J, et al. Poor prognosis in breast carcinomas correlates with increased expression of targetable CD146 and c-Met and with proteomic basal-like phenotype. Hum Pathol. 2007;38(6):830–841. doi: 10.1016/j.humpath.2006.11.015. [DOI] [PubMed] [Google Scholar]
- 102.Zhang X, Wang Z, Kang Y, Li X, Ma X, Ma L. MCAM expression is associated with poor prognosis in non-small cell lung cancer. Clin Transl Oncol. 2014;16(2):178–183. doi: 10.1007/s12094-013-1057-6. [DOI] [PubMed] [Google Scholar]
- 103.Oka S, Uramoto H, Chikaishi Y, Tanaka F. The expression of CD146 predicts a poor overall survival in patients with adenocarcinoma of the lung. Anticancer Res. 2012;32(3):861–864. [PubMed] [Google Scholar]
- 104.Alama A, Truini A, Coco S, Genova C, Grossi F. Prognostic and predictive relevance of circulating tumor cells in patients with non-small-cell lung cancer. Drug Dis Today. 2014;10(19):1671–1676. doi: 10.1016/j.drudis.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 105.Jiang G, Zhang L, Zhu Q, Bai D, Zhang C, Wang X. CD146 promotes metastasis and predicts poor prognosis of hepatocellular carcinoma. J Exp Clin Cancer Res. 2016;35(1):38. doi: 10.1186/s13046-016-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Hernandez R, Sun H, England CG, Valdovinos HF, Ehlerding EB, Barnhart TE, et al. CD146-targeted immunoPET and NIRF imaging of hepatocellular carcinoma with a dual-labeled monoclonal antibody. Theranostics. 2016;6(11):1918. doi: 10.7150/thno.15568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Aldovini D, Demichelis F, Doglioni C, Di Vizio D, Galligioni E, Brugnara S, et al. M-CAM expression as marker of poor prognosis in epithelial ovarian cancer. Int J Cancer. 2006;119(8):1920–1926. doi: 10.1002/ijc.22082. [DOI] [PubMed] [Google Scholar]
- 108.Zeng Q, Li W, Lu D, Wu Z, Duan H, Luo Y, et al. CD146, an epithelial-mesenchymal transition inducer, is associated with triple-negative breast cancer. Proc Natl Acad Sci. 2012;109(4):1127–1132. doi: 10.1073/pnas.1111053108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zabouo G, Imbert A-M, Jacquemier J, Finetti P, Moreau T, Esterni B, et al. CD146 expression is associated with a poor prognosis in human breast tumors and with enhanced motility in breast cancer cell lines. Breast Cancer Res. 2009;11(1):R1. doi: 10.1186/bcr2215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Zhou Y, Huang H, Yuan L-J, Xiong Y, Huang X, Lin J-X, et al. CD146 as an adverse prognostic factor in uterine sarcoma. Eur J Med Res. 2015;20(1):67. doi: 10.1186/s40001-015-0160-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Li Y, Yu J-M, Zhan X-M, Liu L-L, Jin N, Zhang Y-X. Correlation of CD146 expression and clinicopathological characteristics in esophageal squamous cell carcinoma. Oncol Lett. 2014;8(2):859–863. doi: 10.3892/ol.2014.2227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Tian B, Zhang Y, Li N. CD146 protein as a marker to predict postoperative liver metastasis in colorectal cancer. Cancer Biotherapy Radiopharm. 2013;28(6):466–470. doi: 10.1089/cbr.2012.1426. [DOI] [PubMed] [Google Scholar]
- 113.Liu W-F, Ji S-R, Sun J-J, Zhang Y, Liu Z-Y, Liang A-B, et al. CD146 expression correlates with epithelial-mesenchymal transition markers and a poor prognosis in gastric cancer. Int J Mol Sci. 2012;13(5):6399–6406. doi: 10.3390/ijms13056399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Feng G, Fang F, Liu C, Zhang F, Huang H, Pu C. CD146 gene expression in clear cell renal cell carcinoma: a potential marker for prediction of early recurrence after nephrectomy. Int Urol Nephrol. 2012;44(6):1663–1669. doi: 10.1007/s11255-012-0255-4. [DOI] [PubMed] [Google Scholar]
- 115.Sers C, Riethmüller G, Johnson JP. MUC18, a melanoma-progression associated molecule, and its potential role in tumor vascularization and hematogenous spread. Cancer Res. 1994;54(21):5689–5694. [PubMed] [Google Scholar]
- 116.Filshie R, Zannettino A, Makrynikola V, Gronthos S, Henniker A, Bendall L, et al. MUC18, a member of the immunoglobulin superfamily, is expressed on bone marrow fibroblasts and a subset of hematological malignancies. Leu. 1998;12(3):414. doi: 10.1038/sj.leu.2400922. [DOI] [PubMed] [Google Scholar]
- 117.Shih I-M, Wang T-L, Westra WH. Diagnostic and biological implications of mel-CAM expression in mesenchymal neoplasms. Clin Cancer Res. 1996;2(3):569–575. [PubMed] [Google Scholar]
- 118.Pires FR, Shih I-M, da Cruz Perez DE, De Almeida OP, Kowalski LP. Mel-CAM (CD146) expression in parotid mucoepidermoid carcinoma. Oral Oncol. 2003;39(3):277–281. doi: 10.1016/S1368-8375(02)00115-X. [DOI] [PubMed] [Google Scholar]
- 119.Li Q, Yu Y, Bischoff J, Mulliken JB, Olsen BR. Differential expression of CD146 in tissues and endothelial cells derived from infantile haemangioma and normal human skin. J Pathol. 2003;201(2):296–302. doi: 10.1002/path.1443. [DOI] [PubMed] [Google Scholar]
- 120.Chen W, Zhang H, Jiang Y, Li J, Liu B, Sun M. Inhibition of CD146 gene expression via RNA interference reduces in vitro perineural invasion on ACC-M cell. J Oral Pathol Med. 2009;38(2):198–205. doi: 10.1111/j.1600-0714.2008.00706.x. [DOI] [PubMed] [Google Scholar]
- 121.Sato A, Torii I, Okamura Y, Yamamoto T, Nishigami T, Kataoka TR, et al. Immunocytochemistry of CD146 is useful to discriminate between malignant pleural mesothelioma and reactive mesothelium. Mod Pathol. 2010;23(11):1458. doi: 10.1038/modpathol.2010.134. [DOI] [PubMed] [Google Scholar]
- 122.Wu GJ, Varma VA, Wu MWH, Wang SW, Qu P, Yang H, et al. Expression of a human cell adhesion molecule, MUC18, in prostate cancer cell lines and tissues. Prostate. 2001;48(4):305–315. doi: 10.1002/pros.1111. [DOI] [PubMed] [Google Scholar]
- 123.Zhang H, Zhang J, Wang Z, Lu D, Feng J, Yang D, et al. CD146 is a potential marker for the diagnosis of malignancy in cervical and endometrial cancer. Oncol Lett. 2013;5(4):1189–1194. doi: 10.3892/ol.2013.1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.