Abstract
Primary cilia regulate several developmental processes and mediate hedgehog signaling. To study their roles in cranial base development, we created conditional mouse mutants deficient in Polaris, a critical primary cilium component, in cartilage. Mutant post-natal cranial bases were deformed, and their synchondrosis growth plates were disorganized. Expression of Indian hedgehog, Patched-1, collagen X, and MMP-13 was reduced and accompanied by decreases in endochondral bone. Interestingly, there was excessive intramembranous ossification along the perichondrium, accompanied by excessive Patched-1 expression, suggesting that Ihh distribution was wider and responsible for such excessive response. Indeed, expression of heparan sulfate proteoglycans (HS-PGs), normally involved in restricting hedgehog distribution, was barely detectable in mutant synchondroses. Analyses of the data provides further evidence for the essential roles of primary cilia and hedgehog signaling in cranial base development and chondrocyte maturation, and point to a close interdependence between cilia and HS-PGs to delimit targets of hedgehog action in synchondroses.
Keywords: Polaris, primary cilium, cranial base synchondrosis, hedgehog signaling, heparan sulfate proteoglycans
INTRODUCTION
Primary cilia are solitary, immotile, microtubule-based organelles that project from the surfaces of most cells, including chondrocytes (Scherft and Daems, 1967). Cilia are characterized by a system of motor and adaptor proteins that endows them with intraflagellar transport (IFT) and also sustains organelle assembly and growth (Singla and Reiter, 2006). Mutations in the structural and functional components of primary cilia can cause defects in major developmental processes, including limb patterning (Huangfu et al., 2003; Zhang et al., 2003). The findings reflect the fact that primary cilia and their IFT system have turned out to mediate biological signals and action elicited by hedgehog (Hh) proteins (Haycraft et al., 2005; Huangfu and Anderson, 2005). Hh proteins act by binding to the cell-surface receptor Patched1 (Ptch1), and signals are transmitted by the receptor Smoothened via zinc-finger transcription factors Glis. Following hedgehog interaction with Patched-1, Smoothened is activated and translocates to the primary cilium, resulting in production of Gli activator forms that travel to the nucleus and induce expression of hedgehog target genes (Christensen and Ott, 2007). Loss of IFT function deranges Hh signaling at the level of Gli processing and causes developmental defects (Haycraft et al., 2005; Huangfu and Anderson, 2005).
The cranial base and its synchondroses function as growth centers for the neurocranium. At early stages of embryogenesis, a cranial base primordium forms, consisting of three pairs of mesenchymal condensations that differentiate into cartilage and fuse to form the cranial base template (Thorogood, 1988). Primary ossification centers then emerge within this uninterrupted structure at specific times and locations, and the cartilaginous structures remaining between the ossification centers represent the synchondroses (Kjaer, 1990). Each synchondrosis is composed of mirror-image growth plates that share a central resting zone and contain zones of proliferating, pre-hypertrophic, and hypertrophic chondrocytes, followed by endochondral bone and marrow and flanked by intramembranous bone.
We showed previously that Indian hedgehog (Ihh) is expressed by pre-hypertrophic chondrocytes in synchondrosis growth plates, and Ihh itself is present not only in the pre-hypertrophic zone, but also in the preceding proliferating zone and the flanking perichondrium, where it regulates chondrocyte proliferation and intramembranous ossification. In Ihh-null embryos, however, synchondrosis chondrocytes underwent precocious maturation, and both intramembranous and endochondral ossification processes were deranged (Young et al., 2006). In mouse mutants deficient in Kif3a, a component of kinesin-II motor protein complex required for cilium function, we found synchondrosis growth plate malfunction characterized by defective chondrocyte proliferation and maturation (Koyama et al., 2007).
Analysis of the above data, though quite clear, left several questions unanswered. For example, Kif3a has roles not only in cilia but also in the cytoplasm, and thus some of the cranial base defects in Kif3a-mutant mice could theoretically be due to such additional Kif3a functions. To address this issue, we created cartilage-specific conditional mutants deficient in Polaris, a highly specific primary cilium structural component. These studies provided us with the opportunity to address mechanisms by which primary cilia participate in the control of Ihh’s physical and functional topography (both altered in the Kif3a-mutants above). Specifically, we focused on heparan sulfate proteoglycans (HS-PGs), which have been shown to regulate Hh distribution and short- and long-range action in lower organisms, including Drosophila (Takei et al., 2004) and are strongly suspected to have similar roles in higher organisms (Koziel et al., 2004; Koyama et al., 2007).
MATERIALS & METHODS
Generation of Conditional Polaris-deficient Mice
We created conditional mutant mice by mating Polaris floxed mice with Col2a1-Cre mice (Haycraft et al., 2007; Song et al., 2007). The genotyping of transgenic mice is described in the Appendix. The Kif3a conditional allele has been previously described (Lin et al., 2003), and both Polaris fl/fl and Kif3a fl/fl mouse lines were maintained on mixed backgrounds. Animal protocols were approved by the IACUC.
Micro-computed Tomography
Post-natal day 14 (P14) skulls from Polaris-deficient mice and control littermates were fixed in buffered 4% paraformaldehyde and were subjected to micro-computed tomography (μCT) in a CT40 SCANCO Medical System (Southeastern, PA, USA), scanned at 45 kV of energy, 12 μm scanning thickness, and medium resolution. Three-dimensional reconstructions were generated with the following parameters: filter width sigma = 0.8, support level = 1.0, and threshold = 173.
Immunohistochemistry and Gene Expression Analysis
For immunofluorescence staining, 10-μm-thick frozen sections were incubated with 1:200 dilution of anti-acetylated α-tubulin monoclonal antibody (Clone 6-11B-1; Sigma, St. Louis, MO, USA), followed by biotinylated secondary antibodies and Cy3-conjugated streptavidin (Vector Laboratories, Burlingame, CA, USA) to yield signal. Sections were counterstained with 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen, Carlsbad, CA, USA) to reveal nuclei. The control was slides without the primary antibody, followed by incubation with secondary antibodies and Cy3-conjugated streptavidin. Serial para-sagittal sections from Polaris-deficient and Kif3a-deficient mice and control littermates were placed on the same slides. Mouse cDNA clones used were described previously (Shibukawa et al., 2007) and in the Appendix.
RESULTS
Cranial Base and Synchondrosis defects in Conditional Polaris-deficient Mice
To create mice deficient in Polaris in cartilage, we mated floxed Polaris mice with Col2a1-Cre mice, and skulls from resulting Polaris fl/fl ;Col2α1-Cre mice (heretofore termed Polaris-deficient) and Polaris fl/wt ;Col2a1-Cre and Polaris fl/fl mice (heretofore termed controls) were processed for micro-computed tomography (μCT). Control cranial bases displayed a typical elongated morphology along the anteroposterior axis, and their intrasphenoidal (is), spheno-occipital (so), and intra-occipital (io) synchondroses were well-defined (Figs. 1A, 1B). In contrast, Polaris-deficient cranial bases (Fig. 1F, double arrow) from littermates were shorter by 10–15% along the anteroposterior axis and also wider laterally, resulting in an overall round neurocranium (Fig. 1F). Mutant synchondroses displayed different levels of abnormalities, with complete fusion in intra-occipital synchondroses (Figs. 1F, 1G, arrows; see also Appendix Fig. 1) and partial fusion in spheno-occipital and intersphenoidal synchondroses (Figs. 1F, 1G, arrowheads). The process of endochondral bone formation was severely altered and was associated with the virtual absence of primary spongiosa (Figs. 1H, 1I, arrow) compared with controls (Figs. 1C, 1D, arrow). However, there was excessive intramembranous ossification along the perichondrial border (Fig. 1H, arrowhead), and the basi-sphenoidal and basi-occipital bones exhibited imperfections and even holes (Fig. 1G). Osterix and collagen I transcripts were detected in control osteoblasts in primary spongiosa and perichondrial intramembranous bone (Fig. 1D and Appendix Fig. 2, arrows and arrowheads, respectively), but were nearly absent in mutant primary spongiosa (Fig. 1I and Appendix Fig. 2, arrow) and were ectopically expressed along the entire perichondrial tissue (Fig. 1I and Appendix Fig. 2, arrowhead).
Figure 1.
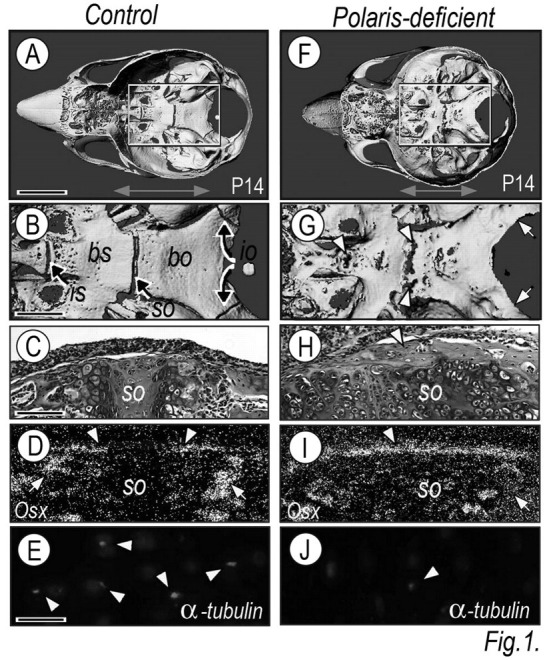
Cranial base organization, synchondrosis development, and ossification patterns are defective in Polaris-deficient mice. Skulls from P14 control (A,B) and Polaris-deficient (F,G) mice were analyzed by μCT and are shown from a ‘bird’s eye’ view. Boxed areas in A and G are shown at higher magnification in B and H, respectively. Intrasphenoidal (is), spheno-occipital (so), intra-occipital (io) synchondroses, and basi-sphenoidal (bs) and basi-occipital (bo) bones are indicated in controls (B). Polaris-deficient cranial bases (F, double arrow) were shorter by 10–15% along the anteroposterior axis than those of control littermates (A, double arrow). Parasagittal H&E–stained sections from control (C) and Polaris-deficient (H) speno-occipital (so) synchondrosis; note that the cellular arrangement in the mutant synchondrosis (H) was significantly altered compared with controls (C). (D, I) Spheno-occipital synchondrosis serial sections were processed for in situ hybridization analysis of bone markers (Osterix) in primary spongiosa and perichondrial bone. (E,J) Synchondrosis growth-plate sections were processed for immunodetection of acetylated tubulin. Scale bars: 0.5 cm in A for A, F; F; 0.2 cm in B for B, G; 150 μm in C for C–D, H–I; and 15 μm in E for E and J.
To verify that Col2a1-Cre had effectively acted on the floxed Polaris gene and led to a reduction in primary cilia, we processed control and mutant sections for immunohistochemistry, using antibodies against primary cilium-associated acetylated α-tubulin. Positively stained primary cilia were readily detectable on controls, but not Polaris-deficient chondrocytes (Figs. 1E, 1J, arrowheads).
Growth Plate Organization and Gene Expression Patterns
Closer analysis showed that control synchondrosis growth plates were rather compact and contained narrow, but still appreciable, chondrocyte zones (Fig. 2A). Resting chondrocytes were ellipsoidal and formed pairs near the perichondrium (Fig. 2A, top inset). Proliferative chondrocytes were flat, and pre-hypertrophic and hypertrophic chondrocytes were round and enlarged. Transcripts of Sox9, aggrecan, and type II collagen were abundant in proliferating and pre-hypertrophic chondrocytes (Figs. 2A, 2B and Appendix Fig. 2), and Collagen X and MMP13 transcripts were prominent in hypertrophic chondrocytes and post-hypertrophic chondrocytes near primary spongiosa, respectively (Figs. 2C, 2D).
Figure 2.
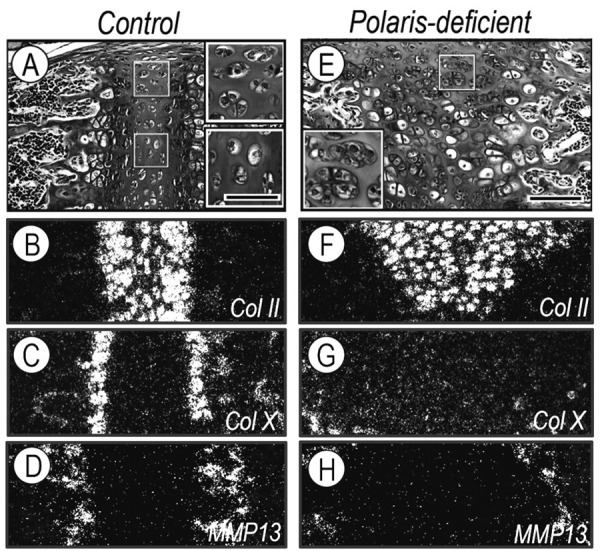
Growth plate organization was abnormal in Polaris-deficient synchondroses. (A,E) Parasagittal sections from P14 control and Polaris-deficient spheno-occipital synchondroses were stained with H&E, and boxed areas in A and E are shown at higher magnification in insets. Note the formation of chondrocyte clusters in mutant synchondroses, while cell doublets are present at the periphery along the chondro- perichondrial border (A, top inset) and individual cells seen at the central zone in controls (A, bottom inset). Serial sections were processed for in situ hybridization analysis of: type II collagen (Col II) (B,F); type X collagen (ColX) (C,G); and matrix metalloprotease 13 (MMP13) (D,H). Scale bars: 35 μm in A for insets in A,E; 150 μm in E for A–H.
In Polaris-deficient synchondroses, the resting zone was larger and disorganized, and displayed chondrocyte pairs and clusters scattered throughout the tissue (Fig. 2E, inset). The other zones were ill-defined, and the chondro-bone/marrow border was deformed (Fig. 2E). Sox-9 and aggrecan were low and scattered (Appendix Fig. 2), strong collagen II expression was maintained, but was topographically deranged (Fig. 2F), and collagen X and MMP-13 transcripts were quite low (Figs. 2G, 2H), implying that chondrocyte maturation and hypertrophy were severely delayed.
Chondrocyte Proliferation, Hedgehog Signaling, and Heparan Sulfate Proteoglycans
Primary cilia mediate hedgehog signaling (Haycraft et al., 2005; Huangfu and Anderson, 2005), and Ihh in the growth plate regulates proliferation and ossification (Koyama et al., 1996b; Lanske et al., 1996; Nakamura et al., 1997; St-Jacques et al., 1999). Thus, we investigated whether and how deficiencies in Polaris and primary cilia would affect these and related parameters in synchondroses. Ihh was expressed by pre-hypertrophic chondrocytes (Figs. 3A, 3B), while Ptch-1 and the hedgehog target/mediator factor PTHrP were expressed in preceding resting and proliferative zones (Fig. 3C and Appendix Fig. 2). Proliferating chondrocytes were identified by the expression of histone H4C (Fig. 3D); proliferating cells were also visible in bone marrow. In Polaris-deficient synchondrosis, Ihh was low, but still appreciable (Figs. 3G, 3H, arrowhead), and Ptch-1, PTHrP, and H4C transcripts were barely detectable (Figs. 3I, 3J and Appendix Fig. 2). Interestingly, Ptch-1 was ectopically expressed all along the perichondrial tissue (Fig. 3I, arrow), where excessive intramembranous bone formation was taking place (see Figs. 1G–1I above).
Figure 3.
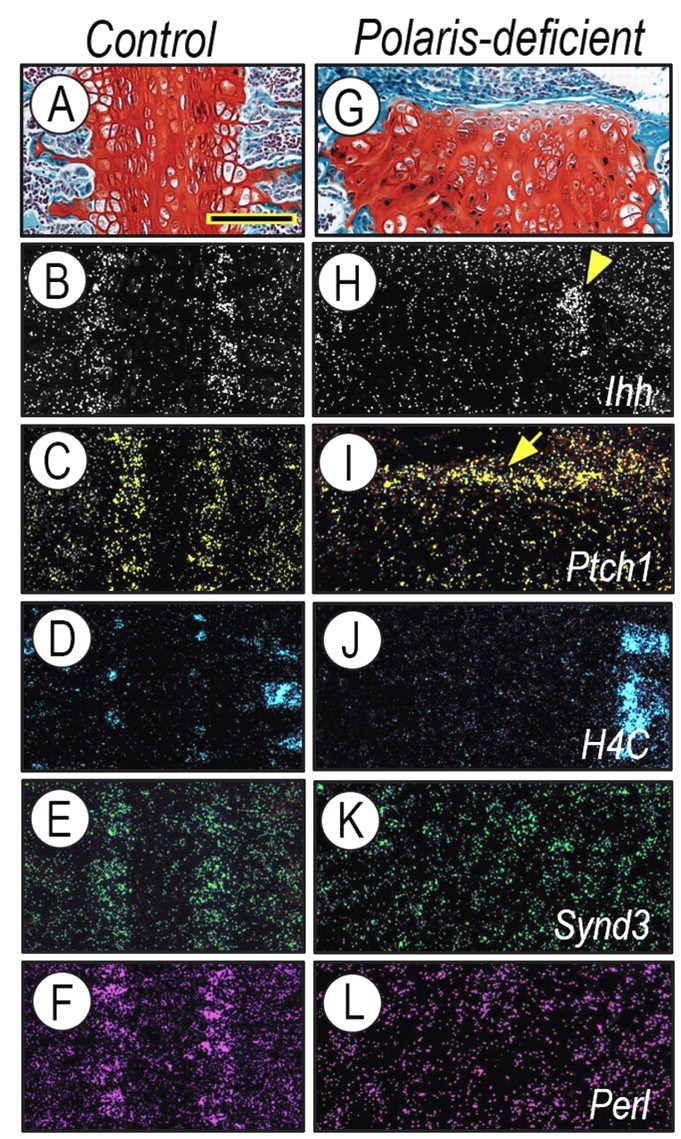
Polaris-deficient synchondroses display alterations in cellular organization and expression for Hedgehog-related and HS-PG genes. Serial sections of control (A–F) and Polaris-deficient (G–L) P14 spheno-occipital synchondroses were processed for safranin O-fast green (A,G) staining. In situ hybridization analysis shows that, in controls, Ihh was expressed in the pre-hypertrophic zone (B), while Ptch1(C), Syndecan-3 (E), and Perlecan (F) were all prominent in proliferating H4C-positive (D) chondrocytes. In Polaris-deficient synchondroses, residual Ihh expression (H, arrowhead) was accompanied by a marked reduction in the expression of Ptch-1 (I), H4C (J), Syndecan-3 (K), and Perlecan (L) in chondrocytes, while there was ectopic Ptch-1 expression all along the perichondrium (I, arrow). Scale bars: 150 μm in A for A–L.
Given that HS-PGs control short- and far-range diffusion of hedgehog proteins and are required for hedgehog signaling (Koyama et al., 1996a; Bernfield et al., 1999; Gritli-Linde et al., 2001; Yin et al., 2002), we hypothesized that HS-PG gene expression might be altered in mutant synchondroses. To test this, we first analyzed the expression of Syndecan-3 and Perlecan. We selected these HS-PGs based on our previous work on Syndecan-3 (Shimo et al., 2004) and the severe growth plate phenotype and deranged Ihh gene expression previously described in Perlecan-null mice (Arikawa-Hirasawa et al., 1999). Interestingly, Syndecan-3 and Perlecan were co-expressed in proliferating chondrocytes (Figs. 3E, 3F) that are the target of Ihh action reflected by strong Ptch-1 expression (Fig. 3C). Strikingly, both Syndecan-3 and Perlecan were minimally expressed in Polaris-deficient synchondroses (Figs. 3K, 3L).
For completeness, we directly compared Polaris-deficient synchondroses with Kif3a-deficient synchondroses (Appendix Fig. 3, Appendix Table). In general, Kif3a-deficient synchondroses were much more severely disorganized. There were only remnants of the growth plate, subdivided into peculiarly shaped regions of collagen II-expressing chondrocytes and surrounded in every spatial direction by bone-like and Osteopontin-expressing cells. The remnant cartilaginous tissues were composed of unusual chondrocyte pairs and clusters, with a significantly expanded inter-territorial matrix, that were post-mitotic. Syndecan-3 and Perlecan expression was also reduced. For more insight into the nature of synchondrosis defects, see the wild-type and mutant specimens schematically depicted in Fig. 4.
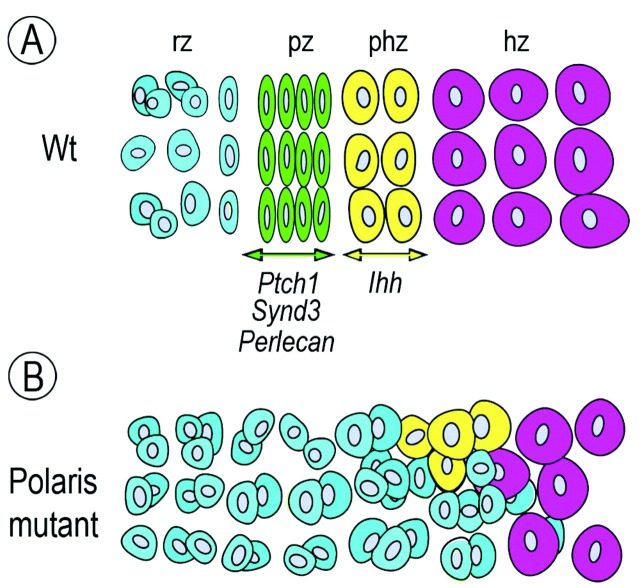
Figure 4.
Schematic of phenotypic consequences in Polaris-deficient synchondroses. (A) In controls, growth plate zonal organization and gene expression patterns were well-defined and typical. The strong expression of Ihh in the pre-hypertrophic zone is accompanied by equally strong and distinct expression of Patched-1 (Ptch1), Syndecan-3 ( Synd3 ), and Perlecan in the proliferative zone. As discussed in the text, such restricted HS-PG expression would be instrumental in directing and delimiting Ihh action in the proliferative zone. (B) In Polaris-deficient synchondroses, the growth plates are entirely disorganized, lack typical zones, and display a seemingly enlarged and widespread resting zone made of chondrocyte doublets or clusters. The latter are present only at the chondro-perichondral border in controls (see A). The growth plate disorganization in mutants is accompanied by reduced Ihh expression and very low expression of Patched-1 and HS-PGs. The latter may have caused wider distribution of residual Ihh, ectopic action on perichondrial tissue, and excessive intramembranous ossification. rz/pz/phz/hz, resting/proliferating/pre-hypertrophic/hypertrophic growth plate zones.
DISCUSSION
This study provides clear evidence that conditional Polaris deficiency and the accompanying loss of primary cilia in chondrocytes are incompatible with normal neurocranial and bone development and normal synchondrosis function. Also, Polaris deficiency severely affects the synchondrosis growth plates that lack distinct growth-plate zones and characteristic gene expression patterns and fail to sustain a seamless transition from hypertrophic cartilage into endochondral bone and marrow. At the same time, mutant growth-plate chondrocytes appear to be unable to respond to residual Ihh expression, as indicated by the near-absence of Patched-1 and PTHrP gene expression; this finding reaffirms the essential nature of primary cilia in hedgehog responsiveness. The data greatly extend those from previous studies on conditional Kif3a mutants from this and other groups (Haycraft et al., 2007; Koyama et al., 2007; Song et al., 2007). Our direct side-by-side comparison of Polaris- and Kif3a-deficient synchondroses at identical post-natal ages shows that the Kif3a phenotype is more severe than the Polaris phenotype. There are several possible explanations for such differences, including possible gene function redundancies or differential susceptibility of floxed genes to Cre action. A more plausible explanation is that the more severe phenotype of Kif3a mutant mice reflects this protein’s involvement in multiple cellular mechanisms such as mitosis, in addition to its role in primary cilia (Cole et al., 1998). Kif3a deficiency could thus have led to broader cellular disturbances.
While the Polaris-deficient growth-plate chondrocytes are unable to mature properly and secure a normal endochondral bone formation process, the perichondrial tissues flanking the synchondroses exhibit excessive intramembranous ossification. A clue to explain this phenomenon is our observation that perichondrial tissue adjacent to the Polaris-deficient growth plates exhibits excessive and ectopic Patched-1 gene expression. Because Patched-1 is a direct target of hedgehog signaling, its excessive expression must reflect an equally excessive presence and broad distribution of Ihh. It is thus plausible that residual amounts of Ihh produced by mutant chondrocytes may have diffused and migrated more broadly than normal, and may have reached the entire length of the perichondrium, triggering an equal length-wise response and ossification. As pointed out above, work in Drosophila originally revealed that HS-PGs are necessary for Hh movement away from its site of synthesis, and also for establishing the outer limits of its diffusion and action (Takei et al., 2004). Our own work and that of another group suggested that HS-PGs would have equivalent roles in Ihh diffusion and action in chick and mouse skeletal elements (Koziel et al., 2004; Shimo et al., 2004). The data we present here add another element to the story and point to the possible roles of Syndecan-3 and Perlecan. By being strongly and specifically expressed in the proliferative zone, these HS- PGs could be well-positioned to attract Ihh away from the pre-hypertrophic zone where it is made, and could help Ihh to migrate toward, and act on, the proliferative zone; they would also establish its outer diffusion limit. The significant down-regulation of both HS-PGs in Polaris-deficient growth plates could thus have led to, or even caused, wider and abnormal Ihh diffusion and ectopic action.
An interesting outcome of the present study relates to the synchondrosis resting zone. As analysis of our data now reveals, the distribution and configuration of resting chondrocytes in control synchondroses are specific, with cell doublets present at the very periphery along the chondro-perichondrial border and individual cells seen through the bulk of the zone. The cellular configuration and distribution are altered in both Polaris- and Kif3a-deficient synchondroses, with the appearance of numerous doublets or clusters of round non-proliferating chondrocytes occupying much of the very enlarged resting zone. A similar accumulation of immature chondrocytes was observed in our previous study on Wnt/β-catenin-deficient cranial base synchondroses, whereas a sharp reduction and near absence of immature chondrocytes (and concomitant widespread maturation) were seen in the Ihh-deficient and constitutive active Wnt/β-catenin pathway (Nagayama et al., 2008). In long-bone growth plates, pacing of maturation rates is regulated by the Ihh-PTHrP loop (Lanske et al., 1996; Tamamura et al., 2005; Maeda et al., 2007). Clearly, then, negative and positive regulators control the resting immature status of chondrocytes and their ingression and progression through maturation. Given recent evidence that cilia may regulate Wnt signaling in addition to Hh signaling (Cano et al., 2004; Ross et al., 2005), analysis of our Polaris and Kif3a data indicates that chondrocytes cannot obey either positive or negative regulatory cues in the absence of primary cilia. It is tempting to speculate also that chondrocyte clustering could have consequences on its own and could have contributed to the accumulation of immature cells. If so, primary cilia would also be needed by chondrocytes to establish physiologic cell arrangements and configurations, and such parameters could in turn influence the transition from resting to proliferating/maturing chondrocytes.
Supplementary Material
Acknowledgments
We express our gratitude to Dr. Y. Yamada for the Perlecan cDNA. This work was supported by Institutional funds.
The authors provide further evidence for the essential roles of primary cilia and hedgehog signaling in cranial base development and chondrocyte maturation, and point to a close interdependence between cilia and HS-PGs to delimit targets of hedgehog action in synchondroses.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
REFERENCES
- Arikawa-Hirasawa E, Watanabe H, Takami H, Hassell JR, Yamada Y -1999-. Perlecan is essential for cartilage and cephalic development. Nat Genet 23:354–358. [DOI] [PubMed] [Google Scholar]
- Bernfield M, Gotte M, Park PW, Reizes O, Fitzgerald ML, Lincecum J, et al -1999-. Functions of cell surface heparan sulfate proteoglycans. Annu Rev Biochem 68:729–777. [DOI] [PubMed] [Google Scholar]
- Cano DA, Murcia NS, Pazour GJ, Hebrok M -2004-. Orpk mouse model of polycystic kidney disease reveals essential role of primary cilia in pancreatic tissue organization. Development 131:3457–3467. [DOI] [PubMed] [Google Scholar]
- Christensen ST, Ott CM -2007-. Cell signaling. A ciliary signaling switch. Science 317:330–331. [DOI] [PubMed] [Google Scholar]
- Cole DG, Diener DR, Himelblau AL, Beech PL, Fuster JC, Rosenbaum JL -1998-. Chlamydomonas kinesin-II-dependent intraflagellar transport -IFT-: IFT particles contain proteins required for ciliary assembly in Caenorhabditis elegans sensory neurons. J Cell Biol 141:993–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gritli-Linde A, Lewis P, McMahon AP, Linde A -2001-. The whereabouts of a morphogen: direct evidence for short- and graded long-range activity of hedgehog signaling peptides. Dev Biol 236:364–386. [DOI] [PubMed] [Google Scholar]
- Haycraft CJ, Banizs B, Aydin-Son Y, Zhang Q, Michaud EJ, Yoder BK -2005-. Gli2 and Gli3 localize to cilia and require the intraflagellar transport protein polaris for processing and function. PLoS Genet 1-4-:e53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haycraft CJ, Zhang Q, Song B, Jackson WS, Detloff PJ, Serra R, et al -2007-. Intraflagellar transport is essential for endochondral bone formation. Development 134:307–316. [DOI] [PubMed] [Google Scholar]
- Huangfu D, Anderson KV -2005-. Cilia and Hedgehog responsiveness in the mouse. Proc Natl Acad Sci USA 102:11325–11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huangfu D, Liu A, Rakeman AS, Murcia NS, Niswander L, Anderson KV -2003-. Hedgehog signalling in the mouse requires intraflagellar transport proteins. Nature 426:83–87. [DOI] [PubMed] [Google Scholar]
- Kjaer I -1990-. Radiographic determination of prenatal basicranial ossification. J Craniofac Genet Dev Biol 10:113–123. [PubMed] [Google Scholar]
- Koyama E, Yamaai T, Iseki S, Ohuchi H, Nohno T, Yoshioka H, et al -1996a-. Polarizing activity, Sonic hedgehog, and tooth development in embryonic and postnatal mouse. Dev Dyn 206:59–72. [DOI] [PubMed] [Google Scholar]
- Koyama E, Leatherman JL, Noji S, Pacifici M -1996b-. Early chick limb cartilaginous elements possess polarizing activity and express hedgehog-related morphogenetic factors. Dev Dyn 207:344–354. [DOI] [PubMed] [Google Scholar]
- Koyama E, Young B, Nagayama M, Shibukawa Y, Enomoto-Iwamoto M, Iwamoto M, et al. -2007-. Conditional Kif3a ablation causes abnormal hedgehog signaling topography, growth plate dysfunction, and excessive bone and cartilage formation during mouse skeletogenesis. Development 134:2159–2169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koziel L, Kunath M, Kelly OG, Vortkamp A -2004-. Ext1-dependent heparan sulfate regulates the range of Ihh signaling during endochondral ossification. Dev Cell 6:801–813. [DOI] [PubMed] [Google Scholar]
- Lanske B, Karaplis AC, Lee K, Luz A, Vortkamp A, Pirro A, et al. -1996-. PTH/PTHrP receptor in early development and Indian hedgehog- regulated bone growth. Science 273:663–666. [DOI] [PubMed] [Google Scholar]
- Lin F, Hiesberger T, Cordes K, Sinclair AM, Goldstein LS, Somlo S, et al. -2003-. Kidney-specific inactivation of the KIF3A subunit of kinesin-II inhibits renal ciliogenesis and produces polycystic kidney disease. Proc Natl Acad Sci USA 100:5286–5291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeda Y, Nakamura E, Nguyen MT, Suva LJ, Swain FL, Razzaque MS, et al -2007-. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci USA 104:6382–6387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagayama M, Iwamoto M, Hargett A, Kamiya N, Tamamura Y, Young B, et al. -2008-. Wnt/β-catenin signaling regulates cranial base development and growth. J Dent Res 87:244–249. [DOI] [PubMed] [Google Scholar]
- Nakamura T, Aikawa T, Iwamoto-Enomoto M, Iwamoto M, Higuchi Y, Pacifici M, et al -1997-. Induction of osteogenic differentiation by hedgehog proteins. Biochem Biophys Res Commun 237:465–469. [DOI] [PubMed] [Google Scholar]
- Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, et al. -2005-. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet 37:1135–1140; erratum in Nat Genet 37:1381, 2005. [DOI] [PubMed] [Google Scholar]
- Scherft JP, Daems WT -1967-. Single cilia in chondrocytes. J Ultrastruct Res 19:546–555. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Young B, Wu C, Yamada S, Long F, Pacifici M, et al. -2007-. Temporomandibular joint formation and condyle growth require Indian hedgehog signaling. Dev Dyn 236:426–434. [DOI] [PubMed] [Google Scholar]
- Shimo T, Gentili C, Iwamoto M, Wu C, Koyama E, Pacifici M -2004-. Indian hedgehog and syndecans-3 coregulate chondrocyte proliferation and function during chick limb skeletogenesis. Dev Dyn 229:607–617. [DOI] [PubMed] [Google Scholar]
- Singla V, Reiter JF -2006-. The primary cilium as the cell’s antenna: signaling at a sensory organelle. Science 313:629–633. [DOI] [PubMed] [Google Scholar]
- Song B, Haycraft CJ, Seo HS, Yoder BK, Serra R -2007-. Development of the post-natal growth plate requires intraflagellar transport proteins. Dev Biol 305:202–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St-Jacques B, Hammerschmidt M, McMahon AP -1999-. Indian hedgehog signaling regulates proliferation and differentiation of chondrocytes and is essential for bone formation. Genes Dev 13:2072–2086; erratum in Genes Dev 13:2617, 1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takei Y, Ozawa Y, Sato M, Watanabe A, Tabata T -2004-. Three Drosophila EXT genes shape morphogen gradients through synthesis of heparan sulfate proteoglycans. Development 131:73–82. [DOI] [PubMed] [Google Scholar]
- Tamamura Y, Otani T, Kanatani N, Koyama E, Kitagaki J, Komori T, et al. -2005-. Developmental regulation of Wnt/beta-catenin signals is required for growth plate assembly, cartilage integrity, and endochondral ossification. J Biol Chem 280:19185–19195. [DOI] [PubMed] [Google Scholar]
- Thorogood P -1988-. The developmental specification of the vertebrate skull. Development 103-Suppl-:141–153. [DOI] [PubMed] [Google Scholar]
- Yin M, Gentili C, Koyama E, Zasloff M, Pacifici M -2002-. Antiangiogenic treatment delays chondrocyte maturation and bone formation during limb skeletogenesis. J Bone Miner Res 17:56–65. [DOI] [PubMed] [Google Scholar]
- Young B, Minugh-Purvis N, Shimo T, St-Jacques B, Iwamoto M, Enomoto-Iwamoto M, et al. -2006-. Indian and sonic hedgehogs regulate synchondrosis growth plate and cranial base development and function. Dev Biol 299:272–282. [DOI] [PubMed] [Google Scholar]
- Zhang Q, Murcia NS, Chittenden LR, Richards WG, Michaud EJ, Woychik RP, et al. -2003-. Loss of the Tg737 protein results in skeletal patterning defects. Dev Dyn 227:78–90. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.