Abstract
The NF-κB family of transcription factors is essential for promoting cell proliferation and preventing cell apoptosis. We have previously shown that Andrographolide (Andro) isolated from an herbal plant, Andrographis paniculata, covalently modifies reduced cysteine62 in the oligonucleotide binding pocket of p50 for inhibition of NF-κB activation. Here we report that Andro, but not its inactive structural analog 4H-Andro, potently suppressed squamous cell carcinogenesis induced by 7,12-dimethyl-1,2-benzanthracene (DMBA) in the hamster model of cheek buccal pouch. Compared with 4H-Andro, Andro reduced phosphorylation of p65 (Ser536) and IκBα (Ser32/36) for inhibiting aberrant NF-κB activation, suppressed c-Myc and cyclin D1 expression and attenuated neoplastic cell proliferation, promoted cancerous cell apoptosis, and mitigated tumor-induced angiogenesis. Consistently, Andro retarded growth, decreased proliferation, and promoted apoptosis of Tb cells, a human tongue squamous cell carcinoma cell line, in time- and dose-dependent manners, with concomitant reduction of the expression of NF-κB targeting molecules in vitro. Our results thus demonstrate that NF-κB activation plays important roles in the pathogenesis of chemically induced squamous cell carcinoma. By inhibition of aberrant NF-κB activation, Andro treats chemically induced oral squamous cell carcinogenesis.
Keywords: NF-κB activation, chemically induced carcinogenesis, Andrographolide, squamous cell carcinoma, proliferation, hamster buccal pouch
Introduction
Oral squamous cell carcinoma is the sixth most common neoplasm worldwide, with more than half a million new cases being diagnosed annually (Parkin et al., 2005; Allen et al., 2007), particularly in certain areas of Asia, the Pacific Islands, Europe, and Brazil, where it constitutes up to one-quarter of all human cancers (Funk et al., 2002; Jemal et al., 2004). Unfortunately, survival of oral squamous cell carcinoma patients has not been significantly improved, despite recent advances in chemotherapy and radiotherapy (Davies and Welch, 2006). It is therefore imperative that we understand the underlying molecular mechanisms during the pathogenesis of oral squamous cell carcinoma and discover novel therapeutic targets for the prevention and treatment of this devastating disease.
The nuclear factor-κB (NF-κB) family of transcription factors includes p105/p50 (NF-κB1), p100/p52 (NF-κB2), p65 (RelA), RelB, and c-Rel, among which the p50/p65 heterodimer is most abundant (Li and Verma, 2002; Hayden and Ghosh, 2008). The p50/p65 heterodimer is localized in the cytoplasm, where it binds to IκB inhibitory proteins, including IκBα, IκBβ, and IκBϵ. Upon stimulation, IκB proteins are rapidly phosphorylated by I-κB kinase α and β (IKKα and IKKβ) and are degraded via the ubiquitin- proteasome pathway (Shishodia and Aggarwal, 2004). Degradation of IκB proteins exposes the nuclear localization signals on the p50/p65 heterodimer, which then translocates to the nucleus for transcriptional up-regulation of a variety of downstream genes that govern innate immunity, inflammation, cell growth, and apoptosis (Gupta et al., 2010). Notably, p65 phosphorylation by both IKKα and β is critically required for its liberation from the IKK complex during NF-κB activation (Sizemore et al., 2002).
Andrographolide (Andro) is a diterpenoid lactone isolated from a plant called Andrographis paniculata, which has been used widely as a regimen of traditional herbal medicine for amelioration of sore throat, diarrhea, and other inflammatory disorders in Asian countries for more than two millennia. Recently, Andro has been shown to inhibit the in vitro cell growth of hepatocellular carcinoma (Zhou et al., 2006), cervical carcinoma (Rajagopal et al., 2003), prostatic adenocarcinoma (Kim et al., 2005), and colorectal carcinoma (Kumar et al., 2004). Mechanistically, Andro is known to induce apoptosis of human cancer cells via the death-receptor-mediated apoptotic pathway (Rajagopal et al., 2003). It also sensitizes tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL)-induced apoptosis in TRAIL-resistant human cancer cells (Zhou et al., 2008). In addition, Andro affects the activity of CDK Pcdc2 and induces the expression of cell-cycle inhibitor P27 for restraining tumor cells at G0/G1 (Zhou et al., 2006). By suppressing the ERK1/2 pathway, it reportedly induces TNF-α synthesis and stimulates production of cytotoxic T-lymphocytes for the inhibition of tumor growth in vivo (Qin et al., 2006). Furthermore, Andro inhibits tumor-induced angiogenesis by decreasing the expression of vascular endothelial growth factor (VEGF), nitric oxide (NO), and various inflammatory cytokines and chemokines while increasing the expression of endogenous anti-angiogenesis factors, such as interleukin (IL)-2 and tissue inhibitors of metalloproteinase-1 (TIMP-1; Sheeja et al., 2007). However, the biochemical mechanisms of how Andro inhibits cancers are still largely unknown. Notably, many chemically diverse substances from natural sources, especially foods, have been shown to target various steps in carcinogenesis, including survival, proliferation, apoptosis, angiogenesis, invasion, and metastasis of tumor cells (Gupta et al., 2010).
In previous studies, we have shown that Andro covalently modifies reduced cysteine62 in the oligonucleotide binding pocket of p50 for inhibition of NF-κB activation in inflammation (Xia et al., 2004), arterial restenosis (Wang et al., 2007), and deep vein thrombosis (Li et al., 2009). Importantly, aberrant NF-κB activation, which critically mediates cell proliferation and prevents cell apoptosis (Deveraux et al., 1998), has been detected in a variety of human cancers, such as prostate carcinoma (Paule et al., 2007), esophageal carcinoma (Yamada et al., 2007), breast carcinoma (Ahmed et al., 2006), intestinal carcinoma (Stark et al., 2007), and oral carcinoma (Allen et al., 2007; Garg et al., 2008). Given the observed inhibitory activity of Andro on the NF-κB signal transduction pathway, we speculated whether Andro could inhibit carcinogenesis through targeting p50 and consequently inactivating NF-κB signaling. In this study, we tested this hypothesis using the hamster buccal pouch model of DMBA-induced squamous cell carcinoma and the human cell line of tongue squamous cell carcinoma Tb cells.
Materials & Methods
For complete information, please see the online Appendix.
Results
NF-κB Activation in Squamous Cell Carcinoma
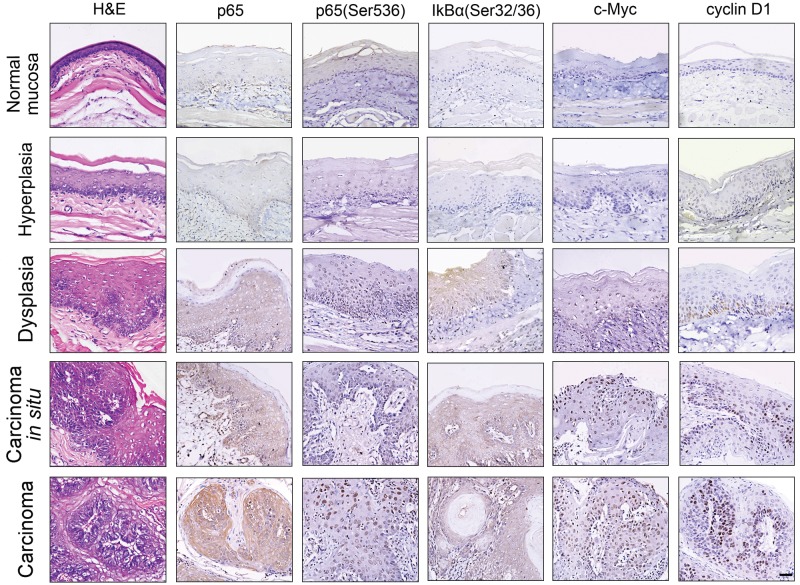
Phosphorylation of p65 (Ser536) and IκBα (Ser32/36) reportedly indicates NF-κB activation, while c-Myc and cyclin D1 are well-known NF-κB downstream targeting molecules (Li and Verma, 2002; Zhang et al., 2005; Allen et al., 2007; Hayden and Ghosh, 2008). To determine whether chemically induced oral squamous cell carcinogenesis could elicit aberrant NF-κB activation, we examined total p65, p65 (Ser536), and IκBα (Ser32/36) phosphorylation and c-Myc and cyclin D1 expression at various stages of DMBA-induced squamous cell carcinogenesis in the hamster buccal pouch model. According to the guidelines of WHO classification, tissue samples stained with H&E were classified into the stages of normal mucosa, hyperplasia, dysplasia, carcinoma in situ, and carcinoma (Fig. 1 and Appendix Table). Total p65, phosphorylated p65 (Ser536), and IκBα (Ser32/36) were not detected at the stages of normal mucosa and hyperplasia. However, total p65, phosphorylated p65 (Ser536), and IκBα (Ser32/36) were clearly visible at the stage of dysplasia, further increased at the stage of carcinoma in situ, and peaked at the stage of carcinoma. Notably, phosphorylated 65 (Ser536) was restricted in the cytoplasm at stages of dysplasia and carcinoma in situ, whereas it was localized in both the cytoplasm and nucleus at the stage of carcinoma. In contrast, phosphorylated IκBα (Ser32/36) was distributed exclusively in the cytoplasm. Consistently, the expression of cytoplasmic and nuclear c-Myc and nuclear cyclin D1 was not detected until the stage of hyperplasia, and gradually intensified at the stages of dysplasia and carcinoma in situ, finally peaking at the stage of carcinoma. These results clearly indicated that DMBA-induced oral squamous cell carcinogenesis triggers aberrant NF-κB activation, especially in the pathological stages of dysplasia, carcinoma in situ, and carcinoma.
Figure 1.
NF-κB activation during chemically induced squamous cell carcinogenesis. The H&E staining and the immunohistochemical staining of total p65, phosphorylated p65 (Ser536), and IκBα (pSer32/36) or c-Myc and cyclin D1 at the stages of normal, hyperplasia, dysplasia, carcinoma in situ, and carcinoma in DMBA-induced squamous cell carcinogenesis of the hamster buccal pouch. Results are representatives of at least 3 tissue samples from more than 3 hamsters for each group. Scale bars, 50 μm.
Andro Inhibits Oral Squamous Cell Carcinoma
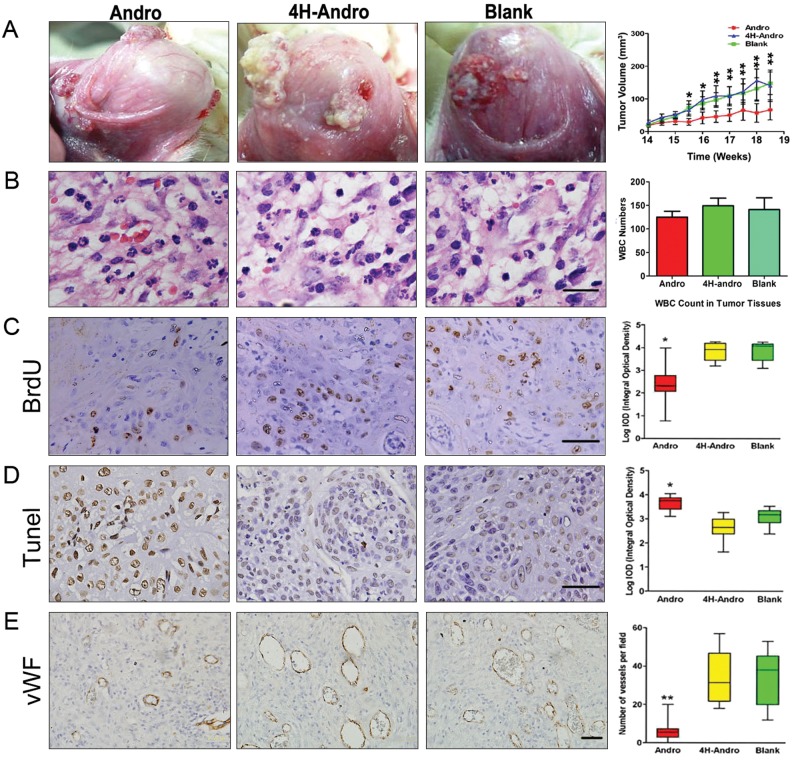
We have previously shown that Andro covalently modifies reduced cysteine62 in the κB binding pocket of p50, which drastically diminishes NF-κB activation (Xia et al., 2004). To elucidate whether NF-κB activation critically participates in the pathogenesis of chemically induced squamous cell carcinoma, we tested whether Andro could inhibit DMBA-induced oral squamous cell carcinogenesis. Intraperitoneal treatment with Andro, but not saline (blank) or 4H-Andro (an inactive structural analog), drastically switched the appearance of almost all tumors from papillary protuberance (ulcerative, erosive, reddish, and fragile with micro-hemorrhage) into smooth, pale, and encapsulated tissue masses, with concomitant amelioration of edema and hyperemia in the neighboring tissues around cancerous lesions (Fig. 2A, left panel). Most importantly, Andro, but not 4H-Andro, potently reduced tumor volumes (Fig. 2A, right panel). Notably, no significant differences in the infiltrated leukocytes, including neutrophils and macrophages, were detected in the serial sections of tumor samples that had been treated with Andro, saline, and 4H-Andro (Fig. 2B), thus eliminating the possibility that Andro might affect leukocyte deposition within these cancerous tissues. These findings attested to the functional significance of NF-κB activation during the pathogenesis of DMBA-induced oral squamous cell carcinoma. Consequently, Andro, but not 4H-Andro, decreased BrdU staining of DNA incorporation (Fig. 2C) and increased Tunel staining of DNA fragmentation (Fig. 2D) of neoplastic tissues, which were markers for cell proliferation and apoptosis, respectively. Using the vWF antigen as a marker for vascular endothelial cells, we found that Andro, but not 4H-Andro, also diminished the formation of neovasculatures within the malignant tissues (Fig. 2E).
Figure 2.
Effects of Andro on chemically induced squamous cell carcinogenesis. Hamsters were treated with saline (n = 14), 4H-Andro (n = 16), or Andro (n = 22). Andro, but not 4H-Andro, reduced tumor growth (A). Leukocyte infiltration in cancerous tissue (B). The immunohistochemical staining of BrdU (C), Tunel (D), and vWF (E) following saline, 4H-Andro, and Andro treatment. Scale bars, 20 μm for B and 50 μm for C,D,E. *p < 0.05 and **p < 0.01.
Andro Suppresses Aberrant NF-κB Activation
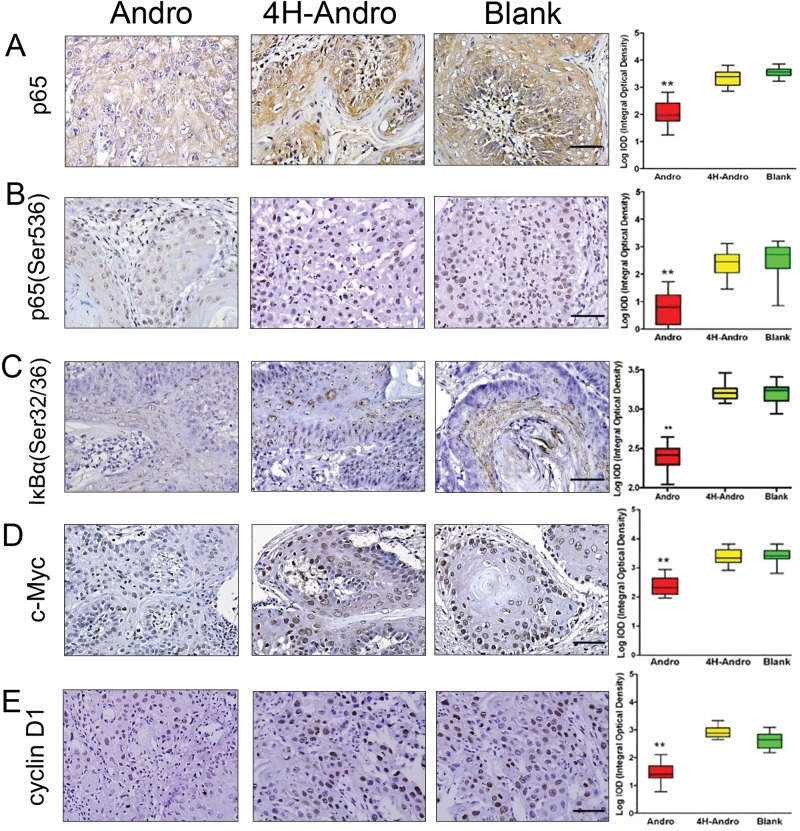
We next tested whether Andro indeed prevented NF-κB activation of oral squamous cell carcinoma through targeting p50. As predicted, Andro significantly reduced the expression of total p65 (Fig. 3A), phosphorylated p65 (Ser536) (Fig. 3B), IκBα (Ser32/36) (Fig. 3C), c-Myc (Fig. 3D), and cyclin D1 (Fig. 3E) in tumor tissues as compared with 4H-Andro. These results convincingly demonstrate that Andro inhibits aberrant NF-κB activation of chemically induced oral squamous cell carcinoma. Analysis of these data, taken together, suggests that Andro exerts its anti-cancer action by attenuating NF-κB activation, suppressing tumor cell proliferation, promoting tumor cell apoptosis, and preventing tumor-induced angiogenesis through targeting NF-κB transcription factor p50.
Figure 3.
Effects of Andro on NF-κB activation, proliferation, apoptosis, and angiogenesis. The immunohistochemical staining of total p65 (A), phosphorylated p65 (Ser536) (B), and IκBα (Ser32/36) (C) or c-Myc (D) and cyclin D1 (E) following saline, 4H-Andro, and Andro treatment. The statistical analysis was performed for least 5 tissue samples from more than 3 hamsters for each group, with ImageTool software. Scale bars, 50 μm. *p < 0.05 and **p < 0.01.
Effects of Andro on NF-κB Activation and Survival of Tb Cells
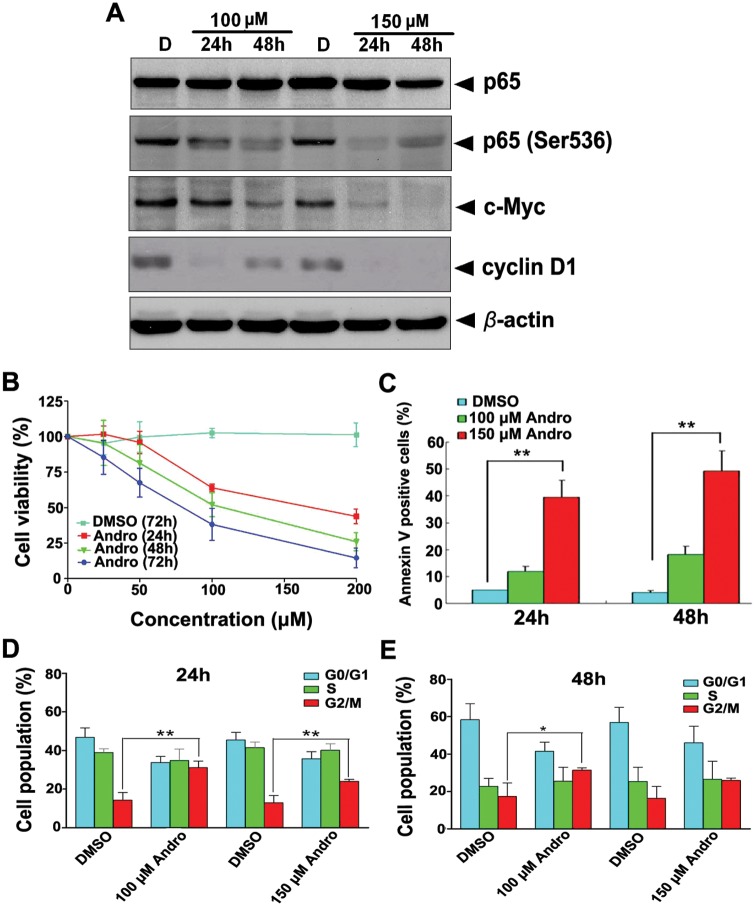
In an effort to correlate our findings obtained from using the hamster model of chemically induced squamous cell carcinogenesis with human cancers, we tested the actions of Andro on human tongue squamous cell carcinoma Tb cells. Indeed, treatment with Andro, but not DMSO, reduced the expression of total p65, phosphorylated p65 (Ser536), c-Myc, and cyclin D1 in a concentration-dependent manner (Fig. 4A). Compared with DMSO, Andro also inhibited growth and proliferation of Tb cells while inducing apoptosis in a dose- and time-dependent manner (Figs. 4B-4E). Our results thus provide evidence for the inhibition of growth and the promotion of apoptosis of human squamous cell carcinoma through inactivation of NF-κB transcription factor p50.
Figure 4.
Effects of Andro on NF-κB activation and survival of Tb cells. Time and dose courses of Andro on the expression of total p65, phosphorylated p65 (Ser536), c-Myc, and cyclin D1 (A) and growth (B), apoptosis (C), and G2/M arrest (D,E). The viability of cells was measured with the MTT assay. Results are the mean (± SD) values of 3 separate experiments. *p <0.05 and **p < 0.01.
Discussion
Chemical induction of epithelial carcinogenesis in the buccal pouch of the Syrian hamster is a multistage model, which reiterates many features observed in human oral squamous cell carcinoma. This model has been widely used to test the efficacy of therapeutic agents, due mainly to easy accessibility for examination and follow-up of the oral lesions (Salley, 1954; Gimenez-Conti, 1993; Li et al., 2002). In the present study, we used this model and demonstrated that DMBA-induced squamous cell carcinogenesis induces NF-κB activation. Importantly, Andro abolished NF-κB activation and potently inhibited tumor growth, attesting to the causal roles of aberrant NF-κB activation during thepathogenesis of chemically induced oral squamous cell carcinoma. Our results indicate a potential clinical correlation of our experimental findings obtained in the chemically induced squamous cell carcinogenesis in the buccal pouch of hamsters. Consistently, NF-κB activation is known to be closely associated with tumorigenesis and metastasis (Loercher et al., 2004; Zhang et al., 2005; Allen et al., 2007) and sensitivity to chemotherapyand radiotherapy (Wang et al., 1999; Kato et al., 2000) and papilloma virus infection (Mishra et al., 2006), and mastication of tobacco (Sawhney et al., 2007) triggers NF-κB activation in human oral squamous cell carcinoma.
The aberrant activation of NF-κB transcription factors is known to regulate diverse pathological responses of carcinogenesis, including cell-cycle progression and proliferation, apoptosis, and angiogenesis (Deveraux et al., 1998; Li and Verma, 2002; Hayden and Ghosh, 2008). Consistently, we found that Andro suppressed NF-κB activation for reduced expression of cyclin D1 and c-Myc and decreased BrdU incorporation, leading to inhibition of cell proliferation in vivo. In addition, Andro increased Tunel staining of DNA fragmentation for the induction of cell apoptosis in vivo through targeting p50 and abrogating NF-κB activation. Notably, Andro treatment also reduced microvessel density (MVD) in the cancerous tissues in vivo, suggesting that Andro inhibits angiogenesis during the pathogenesis of chemically induced oral squamous cell carcinoma. Notably, Andro is widely used in China as an over-the-counter medicine, which apparently has no serious complications or toxic effects reported in patients thus far. Taken together, the in vivo experimental findings that Andro significantly inhibits chemically induced oral squamous cell carcinogenesis suggest that the clinical merit for Andro treatment of squamous cell carcinoma warrants further investigation.
To dissect the molecular mechanisms of how inhibition of aberrant NF-κB activation translates into attenuation of chemically induced oral squamous cell carcinoma, we conducted in vitro experiments using the human tongue squamous cell carcinoma Tb cells. Consistent with a previous report (Yao et al., 2006) and our in vivo findings, treatment of Tb cells with Andro significantly decreased the phosphorylation of p65 (S536) and the expression of c-Myc and cyclin D1. Interestingly, Andro inhibited cell growth and induced cell apoptosis by arresting these squamous carcinoma cells at the G2/M stages in vitro. Importantly, our findings that Andro inhibits NF-κB activation for reduced p65 expression and phosphorylation and mitigated IκBα phosphorylation are fully consistent with those of previous reports (Jin et al., 2011; Kim et al., 2011). Taken together, our results thus confirm that Andro inhibits tumor growth in vivo by the reduction of tumor cell proliferation, induction of tumor cell apoptosis, and suppression of tumor-induced angiogenesis through attenuation of aberrant NF-κB activation.
In summary, analysis of our experimental data suggests that NF-κB signaling plays a critical role during carcinogenesis of chemically induced hamster buccal pouch squamous cell carcinoma. Importantly, by targeting p50 and suppressing NF-κB activation, Andro potently attenuates chemically induced tumor growth for treating oral squamous cell carcinoma.
Supplementary Material
Acknowledgments
We thank Li Yan, Yu-Yun Jiang, Yu-Ying Zhang, and Xiao-Ming Zhou for preliminary studies.
Footnotes
A supplemental appendix to this article is published electronically only at http://jdr.sagepub.com/supplemental.
This study was funded by grants from the National Basic Research Program of China (973 Project, 2010CB529702 to Li-Jing Wang and Xuesong Yang); and by the Natural Science Foundation of China (30871304 to Li-Jing Wang; 30971493 to Xuesong Yang; and 30900764 to Jin-Tao Wang).
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Ahmed KM, Cao N, Li JJ. (2006). HER-2 and NF-κB as the targets for therapy-resistant breast cancer. Anticancer Res 26:4235-4243. [PMC free article] [PubMed] [Google Scholar]
- Allen CT, Ricker JL, Chen Z, Van Waes C. (2007). Role of activated nuclear factor-κB in the pathogenesis and therapy of squamous cell carcinoma of the head and neck. Head Neck 29:959-971. [DOI] [PubMed] [Google Scholar]
- Davies L, Welch HG. (2006). Epidemiology of head and neck cancer in the United States. Otolaryngol Head Neck Surg 135:451-457. [DOI] [PubMed] [Google Scholar]
- Deveraux QL, Roy N, Stennicke HR, Van Arsdale T, Zhou Q, Srinivasula SM, et al. (1998). IAPs block apoptotic events induced by caspase-8 and cytochrome c by direct inhibition of distinct caspases. EMBO J 17:2215-2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funk GF, Karnell LH, Robinson RA, Zhen WK, Trask DK, Hoffman HT. (2002). Presentation, treatment, and outcome of oral cavity cancer: a National Cancer Data Base report. Head Neck 24:165-180. [DOI] [PubMed] [Google Scholar]
- Garg R, Ingle A, Maru G. (2008). Dietary turmeric modulates DMBA-induced p21ras, MAP kinases and AP-1/NF-κB pathway to alter cellular responses during hamster buccal pouch carcinogenesis. Toxicol Appl Pharmacol 232:428-439. [DOI] [PubMed] [Google Scholar]
- Gimenez-Conti I. (1993). The hamster cheek pouch carcinogenesis model. Acta Odontol Latinoam 7:3-12. [PubMed] [Google Scholar]
- Gupta SC, Sundaram C, Reuter S, Aggarwal BB. (2010). Inhibiting NF-κB activation by small molecules as a therapeutic strategy. Biochim Biophys Acta 1799:775-787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. (2008). Shared principles in NF-κB signaling. Cell 132:344-362. [DOI] [PubMed] [Google Scholar]
- Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. (2004). Cancer statistics, 2004. CA Cancer J Clin 54:8-29. [DOI] [PubMed] [Google Scholar]
- Jin HR, Jin X, Dat NT, Lee JJ. (2011). Cucurbitacin B suppresses the transactivation activity of RelA/p65. J Cell Biochem 112:1643-1650. [DOI] [PubMed] [Google Scholar]
- Kato T, Duffey DC, Ondrey FG, Dong G, Chen Z, Cook JA, et al. (2000). Cisplatin and radiation sensitivity in human head and neck squamous carcinomas are independently modulated by glutathione and transcription factor NF-κB. Head Neck 22:748-759. [DOI] [PubMed] [Google Scholar]
- Kim JM, Heo HS, Ha YM, Ye BH, Lee EK, Choi YJ, et al. (2011). Mechanism of Ang II involvement in activation of NF-κB through phosphorylation of p65 during aging. Age (Dordr) [Epub ahead of print, February 12, 2011] (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim TG, Hwi KK, Hung CS. (2005). Morphological and biochemical changes of andrographolide-induced cell death in human prostatic adenocarcinoma PC-3 cells. In Vivo 19:551-557. [PubMed] [Google Scholar]
- Kumar RA, Sridevi K, Kumar NV, Nanduri S, Rajagopal S. (2004). Anticancer and immunostimulatory compounds from Andrographis paniculata. J Ethnopharmacol 92:291-295. [DOI] [PubMed] [Google Scholar]
- Li N, Chen X, Liao J, Yang G, Wang S, Josephson Y, et al. (2002). Inhibition of 7, 12-dimethylbenz[a]anthracene (DMBA)-induced oral carcinogenesis in hamsters by tea and curcumin. Carcinogenesis 23:1307-1313. [DOI] [PubMed] [Google Scholar]
- Li Q, Verma IM. (2002). NF-κB regulation in the immune system. Nat Rev Immunol 2:725-734. [DOI] [PubMed] [Google Scholar]
- Li YD, Ye BQ, Zheng SX, Wang JT, Wang JG, Chen M, et al. (2009). NF-κB transcription factor p50 critically regulates tissue factor in deep vein thrombosis. J Biol Chem 284:4473-4483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loercher A, Lee TL, Ricker JL, Howard A, Geoghegen J, Chen Z, et al. (2004). Nuclear factor-κB is an important modulator of the altered gene expression profile and malignant phenotype in squamous cell carcinoma. Cancer Res 64:6511-6523. [DOI] [PubMed] [Google Scholar]
- Mishra A, Bharti AC, Varghese P, Saluja D, Das BC. (2006). Differential expression and activation of NF-κB family proteins during oral carcinogenesis: role of high risk human papillomavirus infection. Int J Cancer 119:2840-2850. [DOI] [PubMed] [Google Scholar]
- Parkin DM, Bray F, Ferlay J, Pisani P. (2005). Global cancer statistics, 2002. CA Cancer J Clin 55:74-108. [DOI] [PubMed] [Google Scholar]
- Paule B, Terry S, Kheuang L, Soyeux P, Vacherot F, de la Taille A. (2007). The NF-κB/IL-6 pathway in metastatic androgen-independent prostate cancer: new therapeutic approaches? World J Urol 25:477-489. [DOI] [PubMed] [Google Scholar]
- Qin LH, Kong L, Shi GJ, Wang ZT, Ge BX. (2006). Andrographolide inhibits the production of TNF-α and interleukin-12 in lipopolysaccharide-stimulated macrophages: role of mitogen-activated protein kinases. Biol Pharm Bull 29:220-224. [DOI] [PubMed] [Google Scholar]
- Rajagopal S, Kumar RA, Deevi DS, Satyanarayana C, Rajagopalan R. (2003). Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. J Exp Ther Oncol 3:147-158. [DOI] [PubMed] [Google Scholar]
- Salley JJ. (1954). Experimental carcinogenesis in the cheek pouch of the Syrian hamster. J Dent Res 33:253-262. [DOI] [PubMed] [Google Scholar]
- Sawhney M, Rohatgi N, Kaur J, Shishodia S, Sethi G, Gupta SD, et al. (2007). Expression of NF-κB parallels COX-2 expression in oral precancer and cancer: association with smokeless tobacco. Int J Cancer 120:2545-2556. [DOI] [PubMed] [Google Scholar]
- Sheeja K, Guruvayoorappan C, Kuttan G. (2007). Antiangiogenic activity of Andrographis paniculata extract and andrographolide. Int Immunopharmacol 7:211-221. [DOI] [PubMed] [Google Scholar]
- Shishodia S, Aggarwal BB. (2004). Nuclear factor-κB: a friend or a foe in cancer? Biochem Pharmacol 68:1071-1080. [DOI] [PubMed] [Google Scholar]
- Sizemore N, Lerner N, Dombrowski N, Sakurai H, Stark GR. (2002). Distinct roles of the IκB kinase α and β subunits in liberating nuclear factor κB and in phosphorylating the p65 subunit of NF-κB. J Biol Chem 277:3863-3869. [DOI] [PubMed] [Google Scholar]
- Stark LA, Reid K, Sansom OJ, Din FV, Guichard S, Mayer I, et al. (2007). Aspirin activates the NF-κB signalling pathway and induces apoptosis in intestinal neoplasia in two in vivo models of human colorectal cancer. Carcinogenesis 28:968-976. [DOI] [PubMed] [Google Scholar]
- Wang CY, Cusack JC, Liu R, Baldwin AS. (1999). Control of inducible chemoresistance: enhanced anti-tumor therapy through increased apoptosis by inhibition of NF-κB. Nat Med 5:412-417. [DOI] [PubMed] [Google Scholar]
- Wang YJ, Wang JT, Fan QX, Geng JG. (2007). Andrographolide inhibits NF-κB activation and attenuates neointimal hyperplasia in arterial restenosis. Cell Res 17:933-941. [DOI] [PubMed] [Google Scholar]
- Xia YF, Ye BQ, Li YD, Wang JG, He XJ, Lin X, et al. (2004). Andrographolide attenuates inflammation by inhibition of NF-κB activation through covalent modification of reduced cysteine 62 of p50. J Immunol 173:4207-4217. [DOI] [PubMed] [Google Scholar]
- Yamada K, Moriyama H, Yasuda H, Hara K, Maniwa Y, Hamada H, et al. (2007). Modification of the Rb-binding domain of replication-competent adenoviral vector enhances cytotoxicity against human esophageal cancers via NF-κB activity. Hum Gene Ther 18:389-400. [DOI] [PubMed] [Google Scholar]
- Yao J, Duan L, Fan M, Wu X. (2006). NF-κB signaling pathway is involved in growth inhibition, G2/M arrest and apoptosis induced by Trichostatin A in human tongue carcinoma cells. Pharmacol Res 54:406-413. [DOI] [PubMed] [Google Scholar]
- Zhang PL, Pellitteri PK, Law A, Gilroy PA, Wood GC, Kennedy TL, et al. (2005). Overexpression of phosphorylated nuclear factor-κB in tonsillar squamous cell carcinoma and high-grade dysplasia is associated with poor prognosis. Mod Pathol 18:924-932. [DOI] [PubMed] [Google Scholar]
- Zhou J, Zhang S, Ong CN, Shen HM. (2006). Critical role of pro-apoptotic Bcl-2 family members in andrographolide-induced apoptosis in human cancer cells. Biochem Pharmacol 72:132-144. [DOI] [PubMed] [Google Scholar]
- Zhou J, Lu GD, Ong CS, Ong CN, Shen HM. (2008). Andrographolide sensitizes cancer cells to TRAIL-induced apoptosis via p53- mediated death receptor 4 up-regulation. Mol Cancer Ther 7:2170-2180. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.