Abstract
Human dental pulp stem cells (hDPSCs) reside in postnatal dental pulp and exhibit the potential to differentiate into odontoblasts as well as neurons. However, the intercellular signaling niches necessary for hDPSC survival and self-renewal remain largely unknown. The objective of this study is to demonstrate the existence of intercellular purinergic signaling in hDPSCs and to assess the impact of purinergic signaling on hDPSC survival and proliferation. hDPSCs were isolated from extracted third molars and cultured in minimum essential medium. To demonstrate responsiveness to ATP application and inhibitions by purinergic receptor antagonists, whole cell patch-clamp recordings of ATP-induced currents were recorded from cultured hDPSCs. Immunofluorescence and enzymatic histochemistry staining were performed to assess purinergic receptor expression and ectonucleotidase activity in hDPSCs, respectively. To determine the effects of purinergic signaling on hDPSC, purinergic receptor antagonists and an ectonucleotidase inhibitor were applied in culture medium, and hDPSC survival and proliferation were assessed with DAPI staining and Ki67 immunofluorescence staining, respectively. We demonstrated that ATP application induced inward currents in hDPSCs. P2X and P2Y receptors are involved in the generation of ATP-induced inward currents. We also detected expression of NTPDase3 and ectonucleotidase activity in hDPSCs. We further demonstrated that purinergic receptors were tonically activated in hDPSCs and that inhibition of ectonucleotidase activity enhanced ATP-induced inward currents. Furthermore, we found that blocking P2Y and P2X receptors reduced—and inhibition of ecto-ATPase activity enhanced—the survival and proliferation of hDPSCs, while blocking P2X receptors alone affected only hDPSC proliferation. Autocrine/paracrine purinergic signaling is essential for hDPSC survival and proliferation. These results reveal potential targets to manipulate hDPSCs to promote tooth/dental pulp repair and regeneration.
Keywords: tooth generation, dental pulp biology, electrophysiology, regeneration, cell differentiation, cell signaling
Introduction
Embryonic neural crest cells migrate into orofacial branchial arches, proliferate and differentiate into postmitotic odontoblasts, and then integrate with adjacent epithelial cells to form the tooth germ (Lumsden 1988). During tooth development, odontoblasts laminate the outermost layer of dental papilla opposing the enamel-forming epithelial layers. Odontoblasts secrete dentin matrix around their apical processes as they gradually retreat toward the center of dental pulp. The dentin matrix is then mineralized to form dentin, which comprises the predominant hard component of teeth (Schmalz and Smith 2014). Odontoblasts continue to form the secondary dentin after tooth eruption. In response to tooth injury or treatment—such as caries, dental pulp near exposure, or direct pulpal capping—newly generated odontoblasts, also called odontoblast-like cells, generate reparative dentin in dental pulp (Schmalz and Smith 2014). In addition, as bacteria approach the dental pulp, odontoblasts act as immune cells to counteract and modulate the impact of inflammation and infection (Farges et al. 2009). Stem cells isolated from human postnatal dental pulp differentiate into odontoblasts and neurons (Ellis et al. 2014; Peng et al. 2016), thus highlighting the potential for using human dental pulp stem cells (hDPSCs) to promote hard tissue/dental pulp and nervous tissue repair and regeneration. However, the exact intercellular signaling mechanisms responsible for hDPSC survival, proliferation, and differentiation within the dental pulp remain enigmatic.
Purinergic signaling is among the most evolutionarily conserved intercellular communication mechanisms utilized across a range of cells and tissues (Burnstock and Verkhratsky 2009). Purinergic signaling is dictated by ATP release, purinergic receptor activation, and extracellular nucleotide hydrolysis. ATP and its metabolites are natural ligands to purinergic receptors. Activation of purinergic receptors regulates a variety of crucial biological processes, including cellular migration, proliferation, and differentiation (Burnstock 2013). Accumulated evidence indicates that purinergic signaling machinery within stem cell niches controls stem cell survival, proliferation, and differentiation (Liu et al. 2008; Liu, Sun, et al. 2012; Suyama et al. 2012; Messemer et al. 2013; Gampe, Stefani, et al. 2015; Kaebisch et al. 2015). For example, ATP via activation of purinergic P2X and/or P2Y receptors stimulates neuronal stem cell migration and neuronal progenitor proliferation while negatively regulating terminal neuronal differentiation (Lin et al. 2007; Grimm et al. 2010; Suyama et al. 2012).
Since odontoblasts are derived from embryonic neural crest cells, hDPSCs are likely the retained neural crest stem cells or progenitors within the dental pulp. Indeed, hDPSCs express neural stem cell/progenitor markers, exhibit neurogenic potentials, and can differentiate into dopaminergic neurons (Ellis et al. 2014; Majumdar et al. 2016). Interestingly, multiple purinergic receptors, including P2X3, P2X4, P2X5, P2X6, P2X7, and all P2Y receptors, are detected in cultured hDPSCs (Peng et al. 2016; Wang et al. 2016). Moreover, activation of these purinergic receptors affects cell migration and differentiation. Furthermore, ATP-permeable mechanical-, chemical-, and thermal-sensitive ion channels (e.g., connexin, pannexin, and TRPA1/4 channels) are found in cultured hDPSCs and postnatal dental pulp odontoblasts, while activation of these channels induces ATP release (Liu, Yu, et al. 2012; Egbuniwe et al. 2014; Shibukawa et al. 2014; Liu et al. 2015). In addition, ectonucleotidases responsible for extracellular ATP degradation are detected within the dental pulp odontoblast layer (Liu, Yu, et al. 2012). These observations suggest that purinergic signaling within the dental pulp may control hDPSC survival, proliferation, and/or differentiation. Here, we demonstrate the presence of functional P2X/P2Y receptors and ectonucleotidase in hDPSCs and explore the potential role of purinergic signaling in hDPSC survival and proliferation.
Methods and Materials
Isolation and Culture of hDPSCs
All experiments were approved by the research ethics committee at the University of Rochester Medical Center. Immortalized hDPSCs were isolated from extracted third molars as previously described (Gronthos et al. 2011; Egbuniwe et al. 2013). The hDPSCs were cultured and passaged in minimum essential medium (Gibco) with 10% fetal bovine serum (Hyclone), 2-mmol/L L-glutamine (Sigma-Aldrich), and 1% penicillin/streptomycin (Gibco-BRL) at 37 °C in a 5% CO2 incubator. hDPSCs were routinely plated for experiments at 106/mL (2 mL/35-mm dish, 1 mL/well in 12-well plates, or 100 µL/well in 96-well plates) and allowed to grow for 3 to 7 d before experimentation. Dental pulp stem cell phenotype was confirmed by expression of CD44, Stro-1, and Tuji-1. For immunofluorescence and enzymatic histochemistry study, hDPSCs were cultured in cell culture chamber slides.
Electrophysiologic Recordings
Whole cell voltage-clamp recordings of cultured hDPSCs (3 to 6 d in culture) were performed (22 to 25 °C) with an Axopatch 200B (Axon Instruments) patch-clamp amplifier. The composition of the extracellular solution was as follows (mM): 156 NaCl, 2 KCl, 2 CaCl2, 1 MgCl2, 15 glucose, and 10 HEPES (pH 7.3). ATP and/or purinergic receptor antagonists were applied with a multibarreled perfusion system that permits rapid application of drugs. Patch electrodes were fabricated from borosilicate capillary tubes of 1.0-mm diameter with a pipette puller (Sutter Instrument). The electrode resistance ranged from 6 to 8 MΩ when filled with the pipette internal solution, which contained the following (mM): 140 KCl, 4 MgCl2, 0.25 CaCl2, 5 EGTA, 10 HEPES, 4 K2ATP, and 0.4 Na2GTP (pH 7.4). Only recordings of cells with a membrane potential in current-clamp mode more negative than −40 mV and with access resistance <20 MΩ were used. Currents were filtered at 1.3 kHz (4-pole Bessel filter) and digitized at 5 to 10 kHz with a Digidata 1332A data acquisition system (Axon Instruments). Data were acquired with pCLAMP10.0 software (Molecular Devices) and analyzed offline with Clampfit 9.2 (Molecular Devices). ATP-induced currents (IATP) were recorded at a holding potential (Vh) of −60 mV. The liquid junction potential (−4 mV) was compensated for the voltage values in this study. Current amplitudes induced by 2 to 3 consecutive applications of ATP were averaged. Averaged percentage change in current amplitude after addition of purinergic receptor antagonist was used to quantify blocking effects.
Immunocytochemical Fluorescence Staining
Antibody against Tuj-1, Stro-1, CD44, P2X3, P2X7, P2Y2, Ki67, NTPDase2, or NTPDase3 was used in this study. For more detail, see the Appendix.
Enzymatic Cytochemical Staining
To demonstrate functional ecto-ATPase activity in hDPSCs, the enzymatic cytochemical staining was used. For more detail, see the Appendix.
hDPSC Survival and Proliferation Assay
To test the effect of purinergic signaling on hDPSC proliferation, iso-PPADS tetrasodium salt (100 μM, P2X receptor antagonist; Tocris), suramin sodium salt (100 μM, P2 receptor antagonist; Sigma-Aldrich), or ARL 67156 trisodium salt hydrate (100 µM, ATPase inhibitor; Tocris) was added to 6-well plates with 1 mL of minimum essential medium containing 3 × 106 hDPSCs and then incubated for 1, 2, or 3 d, respectively. Cell density was identified by DAPI staining (1:1,000), and proliferation was determined by Ki67 immunofluorescence. Specifically, under inverted fluorescence microscopy, 4 randomly selected DAPI-stained images were captured for each slide. Average cell count per slide was obtained by counting the number of DAPI-positive cells/mm2 in each quadrant. Ki67 positively stained cells were identified as proliferative cells. For statistical analysis, at least 4 slides at each time point were used for each experimental group.
Statistical Analyses
The data are presented as mean ± SD. For cell culture experiment, 1-way analysis of variance (ANOVA) and least significant difference (LSD) post hoc tests were performed. Throughout this study, P < 0.05 was considered statistically significant.
Results
ATP Induces Inward Currents in hDPSCs
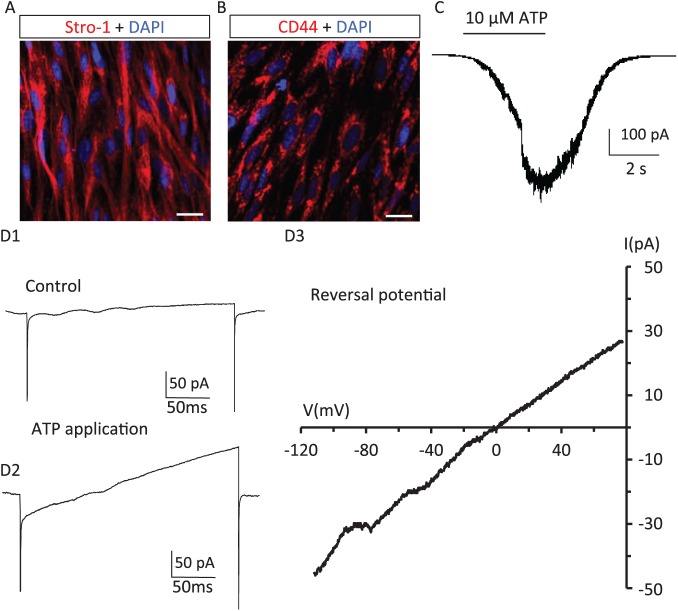
hDPSCs exhibit odontoblast-like stem cell phenotypes (Gronthos et al. 2011; Egbuniwe et al. 2013). To demonstrate the hDPSCs used in this study display similar odontoblast-like stem cell properties, immunofluorescence staining for stem cell markers Stro-1, CD44, and Tuj-1 were performed. As shown in Figure 1A and B, Stro-1 and CD44 were detected in hDPSCs. Expression of Tuj-1 in hDPSCs was also confirmed (Appendix Fig. 1). To demonstrate the presence of functional purinergic receptors in hDPSCs, we used whole cell patch-clamp recordings to detect currents evoked by ATP application. As shown in Figure 1C, focal application of ATP (10 μM) induced inward currents in hDPSCs. To support that the ATP-induced inward current reflected activation of a nonselective cation channel (e.g., P2X receptors), the reversal potential of the ATP-induced current was determined with a voltage ramp from −120 to 80 mV. As expected, the ATP-induced current reversed at −1.67 ± 1.86 mV (n = 3), which was near the cationic equilibrium potential (−1.30 mV) of our intra- and extracellular recording solutions. ATP-induced inward currents in hDPSCs were detectable at nanomolar concentration levels of ATP. As shown in Figure 2, 100nM ATP induced a small but reproducible inward current; the inward currents became progressively larger at higher concentrations of ATP and reached a maximum at 100 μM. The calculated EC50 for ATP-induced inward currents in hDPSCs was 12.6 μM (n = 4 or 6 at each tested concentration). P2X receptors exhibit functional desensitization at higher concentrations of ATP (Giniatullin and Nistri 2013). We found significant desensitization in hDPSCs during sustained applications of ATP at concentrations ≥3 μM. Together, results from Figures 1 and 2 demonstrate the presence of functional ATP-induced, nonselective-cation (Ca2+, Na+, and K+) channel activity in hDPSCs.
Figure 1.
ATP induces inward currents in hDPSCs. Expression of stem cell markers in cultured hDPSCs: (A) Stro-1 and (B) CD44. Scale bar: 20 μm. (C) ATP induces an inward current in hDPSCs. Representative whole cell voltage clamp recording performed in an hDPSC. ATP application (10 μM, 5 s) induced an inward current at a holding potential of −60 mV. (D) Identification of the reversal potential of ATP-induced inward currents in hDPSCs. D1: Current change in response to a voltage ramp (from −80 to 120 mV, 200 ms) recorded from an hDPSC under control conditions. D2: Current change in response to the same voltage ramp during application of 10μM ATP. D3: The reversal potential of the ATP-induced inward current is calculated by subtracting D1 from D2. The ATP-induced inward current reversed at −3.0 mV, which is near the cation equilibrium potential (−1.3 mV) with the recording solutions used in these experiments. hDPSC, human dental pulp stem cell.
Figure 2.
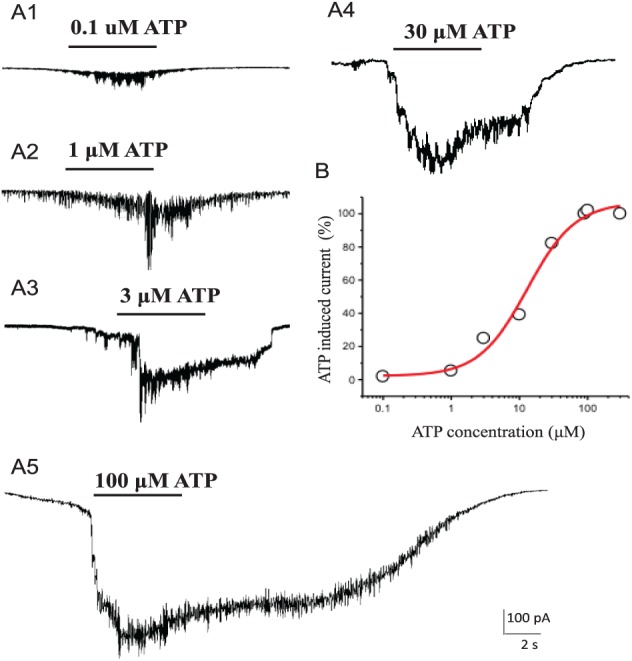
Concentration dependence of ATP-induced inward currents in hDPSCs. (A) A1: 100nM ATP induced a detectable inward current in hDPSCs. A2: 1μM ATP induced a larger inward current in hDPSCs. A3: 3μM ATP application induced a large inward current that exhibits desensitization during sustained ATP applications. A4: 30μM ATP induced an inward current that approximates a maximum response. A5: 100μM ATP application induced a maximal inward current. (B) The concentration response curve of ATP-induced inward currents in hDPSCs (EC50, 12.6 μM). hDPSC, human dental pulp stem cell.
Detection of Functional Ionotropic P2X Receptors in hDPSCs
Previous studies had shown mRNA expression for P2X receptors in cultured hDPSCs (Wang et al. 2011; Peng et al. 2016). In this study, immunofluorescence staining was used to determine if hDPSCs express P2X nonselective cation channels. As showed Figure 3A and B, P2X3 and P2X7 receptors were detected in cultured hDPSCs. To demonstrate that ATP-induced inward currents in hDPSCs were mediated via inotropic P2X receptors, we evaluated the effect of a P2X receptor antagonist. We found that application of P2X receptor antagonist (iso-PPADS tetrasodium salt, 100 μM; Tocris) abolished ATP-induced inward currents in most hDPSCs recorded (Fig. 3C1). Importantly, ATP-induced inward currents were observed in the same cells following washout of the P2X receptor antagonist (Fig. 3C2, C3). The mean percentage changes after blocking P2X receptors and washout were 18.5% ± 23.5% (n = 6) and 92.8% ± 62.1% (n = 6), respectively. Interestingly, we also noted that in some hDPSCs, 100μM iso-PPADS did not completely abolish ATP-induced currents (see Fig. 4). These results suggest that there exist non–P2X receptor mechanisms contributing to ATP-induced inward currents in some hDPSCs.
Figure 3.
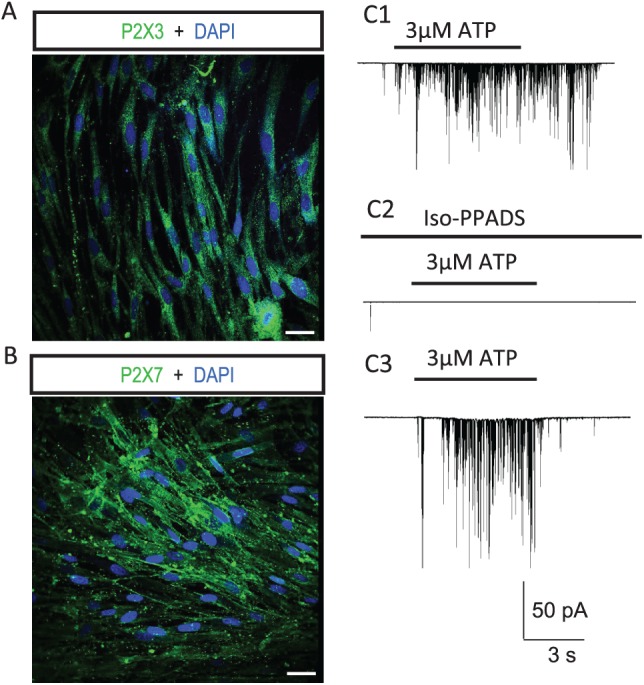
P2X receptors are required to mediate ATP-induced inward currents in most hDPSCs. Immunocytochemical detection of (A) P2X3 and (B) P2X7 receptors in hDPSCs. Scale bars: 20 μm. (C) P2X receptor blockade abolished ATP-induced inward currents in most hDPSCs (72%). C1: Inward current in an hDPSC induced by 3μM ATP. C2: Application of P2X receptor blocker iso-PPADS (100 μM) abolished the ATP-induced inward current. C3: After washout of P2X receptor blocker, the ATP-induced inward was reappeared. hDPSC, human dental pulp stem cell.
Figure 4.
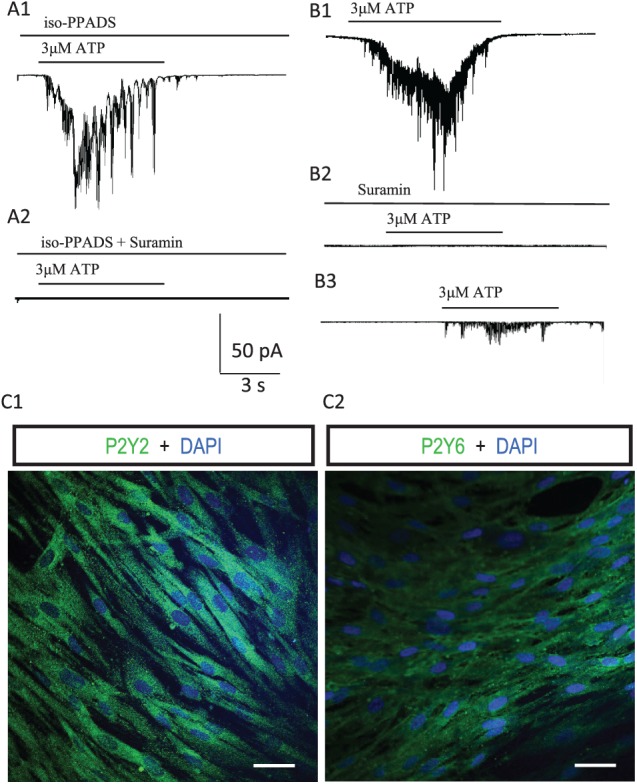
P2Y receptor activation participates in ATP-induced inward currents in hDPSCs. (A) P2X and P2Y receptors contribute to ATP-induced inward current in some hDPSCs. A1: ATP application (3 μM) induced an inward current in an hDPSC, even in the presence of blocking P2X receptors with iso-PPADS. A2: Further blockade of P2Y receptors with coapplication with suramin (100 μM) abolished the ATP-induced inward current. (B) Blocking P2X and P2Y receptors with suramin abolished ATP-induced inward currents in hDPSCs. B1: ATP-induced inward current in an hDPSC. B2: Blockade of P2X and P2Y receptors with suramin (100 μM) abolished the ATP-induced inward current. B3: After washout of suramin, the ATP-induced inward current was partially restored. (C) Immunocytochemical detection of P2Y receptors in hDPSCs: (C1) P2Y2 and (C2) P2Y6. Scale bars: 20 μm. hDPSC, human dental pulp stem cell.
Metabotropic P2Y Receptors Contribute to ATP-Induced Currents in hDPSCs
Previous studies reported that P2Y receptor mRNAs are also detected in hDPSCs (Peng et al. 2016). Therefore, we tested if P2Y receptors contribute to ATP-induced inward currents in hDPSCs. In hDPSCs that still exhibited ATP-induced currents in the presence of P2X receptor antagonist (Fig. 4A1), coapplication of a nonspecific P2X/P2Y receptor inhibitor (suramin, 100 µM) totally abolished ATP-induced inward currents (Fig. 4A2). As expected, blocking P2X and P2Y receptors with suramin alone completely abolished ATP-induced inward currents in hDPSCs (Fig. 4B1, B2). After washout, partial restoration of ATP-induced currents was observed (Fig. 4B3). These results suggest that P2Y receptors work synergistically with P2X receptors to promote ATP-induced inward currents in some hDPSCs. The mean percentage changes after blocking P2X/P2Y receptors and washout were 9.9% ± 7.1% (n = 4) and 83.4% ± 16.6% (n = 4), respectively. To confirm the expression of P2Y receptors in hDPSCs, immunofluorescence staining for P2Y receptors was performed (Fig. 4C). As expected, P2Y2 and P2Y6 receptors were detected in hDPSCs (Fig. 4C1, C2). These results indicate that P2X and P2Y receptors are expressed in hDPSCs and participate in ATP-induced inward currents.
Ectonucleotidases Affect ATP-Induced Currents in hDPSCs
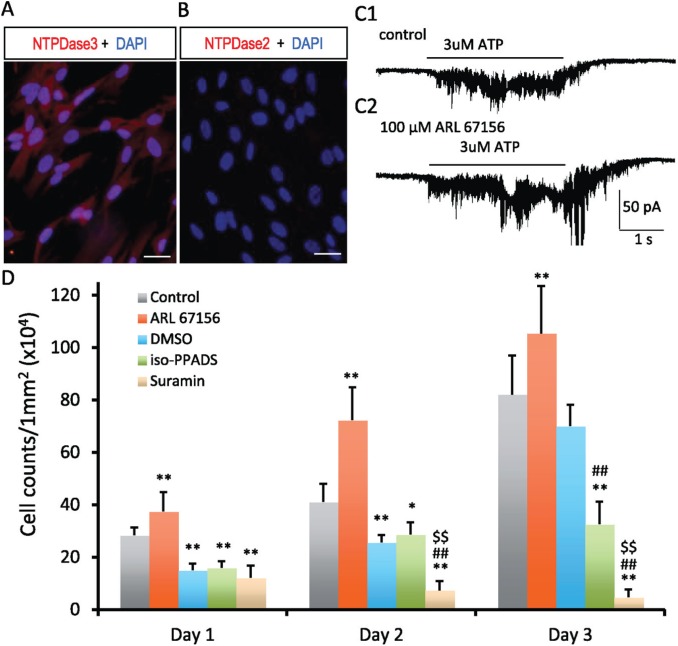
Purinergic signaling is affected by the presence of ecto-NTPDases, the major enzymes responsible for extracellular ATP degradation. Our previous studies demonstrated the presence of functional ecto-ATPase activity and the expression of ecto-NTPDases within the dental pulp odontoblast layer (Liu, Yu, et al. 2012; Liu et al. 2015; Ma et al. 2016). Since NTPDases had been detected in neuronal progenitors in the neurogenesis subventricular zone of postnatal brain, we thus tested if ecto-NTPDases are also expressed in hDPSCs. As shown in Figure 5A and B, NTPDase3 but not NTPDase2 was detected in hDPSCs. We further tested if there exists functional ecto-ATPase activity in hDPSCs. As expected, ecto-ATPase activity was detected in cultured hDPSCs (Appendix Fig. 2). We then tested if ecto-NTPDases affect purinergic signaling in hDPSCs by controlling the degradation of extracellular ATP. We found that application of NTPDase inhibitor (ARL 67156 trisodium salt hydrate, 100 μM) enhanced ATP-induced inward currents in hDPSCs (Fig. 5C1, C2). Specifically, the enhancement of the inward current was more prominent toward the end of ATP application, which is consistent with a reduction in ATP degradation. In addition, the enhancement of ATP-induced inward current coincided with slowly recovering current following the stop of ATP application. These results suggest the existence of functional ectonucleotidases in hDPSCs that affects purinergic signaling by control of extracellular ATP degradation.
Figure 5.
Expression of ectonucleotidases in hDPSCs and effects of purinergic signaling on hDPSC survival and proliferation. Immunofluorescence staining for ecto-NTPDases in hDPSCs. Expression of NTPDase3 was detected in hDPSCs (A). NTPDase2 was not detected in hDPSCs (B). Scale bars 20 μm. (C) Inhibition of ecto-NTPDases enhances purinergic signaling in hDPSCs. C1: ATP-induced (3 μM) inward current in hDPSCs. C2: Application of ecto-NTPDase inhibitor (100μM ARL 67156) enhanced the ATP-induced inward current in hDPSCs. D: Effects of purinergic signaling on the survival and proliferation of hDPSCs. DMSO exhibited deteriorating effects on survival and proliferation of hDPSCs. As compared with the control group, the cell number was significantly reduced at days 1, 2, and 3 in DMSO vehicle control group. Interestingly, inhibition of ectonucleotidase activity with 100mM ARL 67156 significantly increased the number of hDPSCs in culture from day 1 to day 3, which suggest trophic effects of ATP signaling on hDPSC survival and proliferation. As compared with the DMSO group (vehicle control), blocking P2X receptors with 100μM iso-PPADS had little effect on hDPSCs at days 1 and 2; an inhibition effect on hDPSCs was observed at day 3. Blocking P2X/P2Y receptors with suramin (100 μM) significantly reduced the number of hDPSCs in culture at days 1, 2, and 3 (cell proliferation). The data are presented as mean ± SD, n = 7 in each group (analysis of variance); least significant difference post hoc tests were performed at day 1, 2, or 3; and P < 0.05 was considered statistically significant. *P < 0.05, **P < 0.01, vs. control. ##P < 0.01, vs. DMSO. $$P < 0.01, vs. iso-PPADS. hDPSC, human dental pulp stem cell.
Effects of Purinergic Signaling on hDPSC Survival and Proliferation
Purinergic signaling plays an essential role in stem cell survival, self-renewal, and differentiation (Kaebisch et al. 2015). The cell growth curve of hDPSCs in culture is displayed in Appendix Figure 3A. To demonstrate how the cell-counting number of hDPSCs in culture represents cellular proliferation, we performed immunofluorescence staining for cellular proliferation marker Ki67. As illustrated in Appendix Figure 3B, almost all cultured hDPSCs were positive for Ki67 at days 3, 5, and 7; the percentages of Ki67-positive cells were 96.8%, 98.1%, and 93.7%, respectively (n = 5 in each group). To support the existence of autocrine/paracrine purinergic signaling in hDPSCs and demonstrate the role of purinergic signaling in cell survival and proliferation, we further tested the effects of blocking purinergic signaling on the cell-counting number of hDPSCs. As shown in Figure 5C, as compared with the vehicle control group (DMSO), blocking P2X/P2Y receptors with suramin (100 μM) significantly reduced the number of hDPSCs at days 1, 2, and 3. In contrast, blocking P2X receptors alone with iso-PPADS (100 μM) did not significantly alter the number of hDPSCs at days 1 and 2 but reduced the number of hDPScs at day 3. These results suggest that purinergic P2Y signaling plays an essential trophic role for the survival and proliferation of hDPSCs, while P2X signaling may participate in the proliferation. To support that ecto-NTPDases participate in purinergic signaling in hDPSCs, we evaluated the effect of ectonucleotidase inhibitor ARL 67156 (100 μM) on the hDPSC number in culture. As shown in Figure 5C, when compared with the control, inhibition of extracellular ATP degradation significantly enhanced the number of hDPSCs at days 1, 2, and 3. Together, these results suggest that there exists autocrine/paracrine purinergic signaling responsible for hDPSC survival and proliferation.
Discussion
By activation of purinergic receptors, extracellular ATP and its metabolites participate in a wide diversity of biological actions, such as neurotransmission, sensory transduction, secretion, and vasodilatation, as well as cellular proliferation, differentiation, and apoptosis (Burnstock 2013). Purinergic receptors are divided into ligand-gated ionotropic P2X receptors and G protein–coupled metabotropic receptors (P2Y and P1 receptors). Interestingly, expression of P2X and P2Y receptors was detected in dental pulp odontoblasts (Lee et al. 2017). In addition, various purinergic receptors were detected in cultured hDPSCs (Peng et al. 2016; Wang et al. 2016). In this study, we demonstrated that ATP application activated P2X and P2Y receptors to induce an inward cation current in hDPSCs. It is well established that P2X receptor activation results in opening of nonselective cation channels, consistent with our observation that the reversal potential of ATP-induced currents in hDPSCs is equal to the equilibrium potential of Na+, K+, and Ca2+ under our recording conditions. Interestingly, we also found that ATP-induced inward currents in a portion of hDPSCs are affected by activation of metabotropic P2Y receptors, which could be related to the changes of intracellular Ca2+ signaling. Intracellular Ca2+-dependent potassium or calcium channels had been observed in a variety of cells (Sugasawa et al. 1996). Specifically, P2Y receptor activation triggering an increase in intracellular Ca2+ had been demonstrated in hDPSCs (Peng et al. 2016). However, the underlying mechanism by which P2Y receptor activation promotes ATP-induced currents in hDPSCs will require further investigation.
Purinergic signaling plays an essential role in stem cell migration, proliferation, and differentiation (Burnstock and Ulrich 2011; Glaser et al. 2012). For example, P2Y1 receptor activation promotes proliferation of neuronal stem cells in the postnatal subventricular zone and thus is important for the maintenance of neuronal stem cells in the adult brain (Tsumura et al. 2012). It had been shown that adenine nucleotides through the P2Y1 receptor stimulated retinal progenitor cell proliferation by modulating expression of p57KIP2, a cell cycle regulator (de Almeida-Pereira et al. 2017). Interestingly, it was shown that ATP concentration dependently stimulates hDPSC migration, enhances hDPSC proliferation at a lower concentration, and promotes hDPSC differentiation at high concentration (Peng et al. 2016; Wang et al. 2016). In this study, we demonstrated that there exists autocrine/paracrine purinergic signaling in cultured hDPSCs. P2X and P2Y receptors are expressed on hDPSCs, and purinergic receptor activation is required for the survival and proliferation of hDPSCs. Specifically, we found that blocking P2X receptors alone had no or little effect on hDPSC survival and proliferation, while blocking P2Y and P2X receptors significantly reduced the survival and proliferation of hDPSCs. Regarding the potential differentiation of hDPSCs to odontoblasts as well as neurons (Ellis et al. 2014), elucidating the underlying molecular mechanisms responsible for hDPSC survival, proliferation, and differentiation could lead to significant progress toward the application of hDPSCs in dental hard or pulpal tissue repair and regeneration and in neurologic degenerative disorders.
The magnitude and duration of purinergic signaling are influenced by ectonucleotidases that promote ATP degradation to ADP, AMP, or adenosine and thus shift the purinergic signaling from P2X receptor activation to P2Y and/or P1 receptor activation (Liu et al. 2017). Extracellular nucleotide hydrolysis mediated by ectonucleotidases that affect cellular signaling cascades is essential for the development and maintenance of mesenchymal stem cells (Iser et al. 2014). Recently, purinergic receptors and nucleotide-processing ectoenzymes were found to regulate stem cell fate differentiation. For example, intravitreal injection of E-NTPDase inhibitor increased the proliferation of retinal progenitor cells in vivo (de Almeida-Pereira et al. 2017). In addition, it was shown that NTPDase2 is expressed in neural stem/progenitor cells and that NTPDase2/purinergic signaling controls progenitor cell proliferation in neurogenic niches of the postnatal brain (Shukla et al. 2005; Langer et al. 2007; Gampe, Haverkamp, et al. 2015). Interestingly, in this study, we demonstrated that NTPDase3 and neural stem cell/progenitor marker Tuj-1 are expressed in hDPSCs. Our previous work had shown the presence of NTPDses2/3 immunostaining signals and ecto-ATPase activity within the odontoblast layer of human dental pulp (Liu, Yu, et al. 2012). Since we have demonstrated that NTPDase2 is expressed in Schwann’s cells and satellite cells while NTPDase3 is expressed in trigeminal ganglion nociceptive neurons in the trigeminal nervous system (Ma et al. 2016; Liu et al. 2017), the detected NTPDase2 signal in the dental pulp odontoblast layer is likely from the Schwann’s cells that encapsulate the nerve fibers projecting into the odontoblast layer. These results suggest that ectonucleotidases may affect the fate of hDPSCs by regulating the strength and durations of intercellular purinergic signaling within dental pulp. Indeed, we showed that inhibition of ectonucleotidases enhanced ATP-induced inward currents in hDPSCs. Moreover, consistent with the notion that ectonucleotidases modulate hDPSC survival/proliferation in dental pulp, we found that hDPSC counting number in culture was increased by ectonucleotidase inhibition. Thus, manipulation of ectonucleotidase activity within the dental pulp may provide an alternative mean of promoting cell-based stem cell therapies in dentistry.
Purinergic signaling is well preserved across a range of cell types, including stem cells, which play an essential role in cellular migration, proliferation, and differentiation. Demonstrating the existence of autocrine/paracrine purinergic signaling in hDPSCs and its role in hDPSC survival/proliferation would provide insight to accelerate stem cell therapy in tooth tissue repair and regeneration and in treatment of neurologic disorders.
Author Contributions
S. Zhang, D. Ye, L. Ma, Y. Ren, R.T. Dirksen, contributed to data acquisition and analysis, critically revised the manuscript; X. Liu, contributed to conception, design, data acquisition, analysis, and interpretation, drafted and critically revised the manuscript. All authors gave final approval and agree to be accountable for all aspects of the work.
Supplemental Material
Supplemental material, DS_10.1177_0022034518807920 for Purinergic Signaling Modulates Survival/Proliferation of Human Dental Pulp Stem Cells by S. Zhang, D. Ye, L. Ma, Y. Ren, R.T. Dirksen and X. Liu in Journal of Dental Research
Footnotes
A supplemental appendix to this article is available online.
This research was supported by a University of Rochester Clinical and Translational Science Award from the National Center for Advancing Translational Sciences of the National Institutes of Health (KL2 TR000095 to X.L.) and a research grant from the National Institutes of Health (AR059646 to R.T.D.). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors are grateful for the technical support and help from Dr. Lan Wei-LaPierre in Dr. Dirksen’s laboratory and Donna Hoak in Dr. Eliav’s laboratory.
The authors declare no potential conflicts of interest with respect to the authorship and/or publication of this article.
References
- Burnstock G. 2013. Purinergic signalling: pathophysiology and therapeutic potential. Keio J Med. 62(3):63–73. [DOI] [PubMed] [Google Scholar]
- Burnstock G, Ulrich H. 2011. Purinergic signaling in embryonic and stem cell development. Cell Mol Life Sci. 68(8):1369–1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G, Verkhratsky A. 2009. Evolutionary origins of the purinergic signalling system. Acta Physiol (Oxf). 195(4):415–447. [DOI] [PubMed] [Google Scholar]
- de Almeida-Pereira L, Magalhaes CF, Repossi MG, Thorstenberg MLP, Sholl-Franco A, Coutinho-Silva R, Ventura ALM, Fragel-Madeira L. 2017. Adenine nucleotides control proliferation in vivo of rat retinal progenitors by P2Y1 receptor. Mol Neurobiol. 54(7):5142–5155. [DOI] [PubMed] [Google Scholar]
- Egbuniwe O, Grant AD, Renton T, Di Silvio L. 2013. Phenotype-independent effects of retroviral transduction in human dental pulp stem cells. Macromol Biosci. 13(7):851–859. [DOI] [PubMed] [Google Scholar]
- Egbuniwe O, Grover S, Duggal AK, Mavroudis A, Yazdi M, Renton T, Di Silvio L, Grant AD. 2014. TRPA1 and TRPV4 activation in human odontoblasts stimulates ATP release. J Dent Res. 93(9):911–917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis KM, O’Carroll DC, Lewis MD, Rychkov GY, Koblar SA. 2014. Neurogenic potential of dental pulp stem cells isolated from murine incisors. Stem Cell Res Ther. 5(1):30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farges JC, Keller JF, Carrouel F, Durand SH, Romeas A, Bleicher F, Lebecque S, Staquet MJ. 2009. Odontoblasts in the dental pulp immune response. J Exp Zool B Mol Dev Evol. 312B(5):425–436. [DOI] [PubMed] [Google Scholar]
- Gampe K, Haverkamp S, Robson SC, Gachet C, Huser L, Acker-Palmer A, Zimmermann H. 2015. NTPDase2 and the P2Y1 receptor are not required for mammalian eye formation. Purinergic Signal. 11(1):155–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gampe K, Stefani J, Hammer K, Brendel P, Potzsch A, Enikolopov G, Enjyoji K, Acker-Palmer A, Robson SC, Zimmermann H. 2015. NTPDase2 and purinergic signaling control progenitor cell proliferation in neurogenic niches of the adult mouse brain. Stem Cells. 33(1):253–264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giniatullin R, Nistri A. 2013. Desensitization properties of P2X3 receptors shaping pain signaling. Front Cell Neurosci. 7:245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glaser T, Cappellari AR, Pillat MM, Iser IC, Wink MR, Battastini AM, Ulrich H. 2012. Perspectives of purinergic signaling in stem cell differentiation and tissue regeneration. Purinergic Signal. 8(3):523–537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm I, Ullsperger SN, Zimmermann H. 2010. Nucleotides and epidermal growth factor induce parallel cytoskeletal rearrangements and migration in cultured adult murine neural stem cells. Acta Physiol (Oxf). 199(2):181–189. [DOI] [PubMed] [Google Scholar]
- Gronthos S, Arthur A, Bartold PM, Shi S. 2011. A method to isolate and culture expand human dental pulp stem cells. Methods Mol Biol. 698:107–121. [DOI] [PubMed] [Google Scholar]
- Iser IC, Bracco PA, Goncalves CE, Zanin RF, Nardi NB, Lenz G, Battastini AM, Wink MR. 2014. Mesenchymal stem cells from different murine tissues have differential capacity to metabolize extracellular nucleotides. J Cell Biochem. 115(10):1673–1682. [DOI] [PubMed] [Google Scholar]
- Kaebisch C, Schipper D, Babczyk P, Tobiasch E. 2015. The role of purinergic receptors in stem cell differentiation. Comput Struct Biotechnol J. 13:75–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer D, Ikehara Y, Takebayashi H, Hawkes R, Zimmermann H. 2007. The ectonucleotidases alkaline phosphatase and nucleoside triphosphate diphosphohydrolase 2 are associated with subsets of progenitor cell populations in the mouse embryonic, postnatal and adult neurogenic zones. Neuroscience. 150(4):863–879. [DOI] [PubMed] [Google Scholar]
- Lee BM, Jo H, Park G, Kim YH, Park CK, Jung SJ, Chung G, Oh SB. 2017. Extracellular ATP induces calcium signaling in odontoblasts. J Dent Res. 96(2):200–207. [DOI] [PubMed] [Google Scholar]
- Lin JH, Takano T, Arcuino G, Wang X, Hu F, Darzynkiewicz Z, Nunes M, Goldman SA, Nedergaard M. 2007. Purinergic signaling regulates neural progenitor cell expansion and neurogenesis. Dev Biol. 302(1):356–366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Hashimoto-Torii K, Torii M, Haydar TF, Rakic P. 2008. The role of ATP signaling in the migration of intermediate neuronal progenitors to the neocortical subventricular zone. Proc Natl Acad Sci U S A. 105(33):11802–11807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Ma L, Zhang S, Ren Y, Dirksen RT. 2017. CD73 controls extracellular adenosine generation in the trigeminal nociceptive nerves. J Dent Res. 96(6):671–677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Sun L, Torii M, Rakic P. 2012. Connexin 43 controls the multipolar phase of neuronal migration to the cerebral cortex. Proc Natl Acad Sci U S A. 109(21):8280–8285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Wang C, Fujita T, Malmstrom HS, Nedergaard M, Ren YF, Dirksen RT. 2015. External dentin stimulation induces ATP release in human teeth. J Dent Res. 94(9):1259–1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Yu L, Wang Q, Pelletier J, Fausther M, Sevigny J, Malmstrom HS, Dirksen RT, Ren YF. 2012. Expression of ecto-ATPase NTPDase2 in human dental pulp. J Dent Res. 91(3):261–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lumsden AG. 1988. Spatial organization of the epithelium and the role of neural crest cells in the initiation of the mammalian tooth germ. Development. 103 Suppl: 155–169. [DOI] [PubMed] [Google Scholar]
- Ma L, Trinh T, Ren Y, Dirksen RT, Liu X. 2016. Neuronal NTPDase3 mediates extracellular ATP degradation in trigeminal nociceptive pathway. PloS One. 11(10):e0164028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majumdar D, Kanafi M, Bhonde R, Gupta P, Datta I. 2016. Differential neuronal plasticity of dental pulp stem cells from exfoliated deciduous and permanent teeth towards dopaminergic neurons. J Cell Physiol. 231(9):2048–2063. [DOI] [PubMed] [Google Scholar]
- Messemer N, Kunert C, Grohmann M, Sobottka H, Nieber K, Zimmermann H, Franke H, Norenberg W, Straub I, Schaefer M, et al. 2013. P2X7 receptors at adult neural progenitor cells of the mouse subventricular zone. Neuropharmacology. 73:122–137. [DOI] [PubMed] [Google Scholar]
- Peng H, Hao Y, Mousawi F, Roger S, Li J, Sim JA, Ponnambalam S, Yang X, Jiang LH. 2016. Purinergic and store-operated Ca(2+) signaling mechanisms in mesenchymal stem cells and their roles in ATP-induced stimulation of cell migration. Stem Cells. 34(8):2102–2114. [DOI] [PubMed] [Google Scholar]
- Schmalz G, Smith AJ. 2014. Pulp development, repair, and regeneration: challenges of the transition from traditional dentistry to biologically based therapies. J Endod. 40(4):S2–S5. [DOI] [PubMed] [Google Scholar]
- Shibukawa Y, Sato M, Kimura M, Sobhan U, Shimada M, Nishiyama A, Kawaguchi A, Soya M, Kuroda H, Katakura A, et al. 2014. Odontoblasts as sensory receptors: transient receptor potential channels, pannexin-1, and ionotropic ATP receptors mediate intercellular odontoblast-neuron signal transduction. Pflugers Arch. 467(4):843–863. [DOI] [PubMed] [Google Scholar]
- Shukla V, Zimmermann H, Wang L, Kettenmann H, Raab S, Hammer K, Sevigny J, Robson SC, Braun N. 2005. Functional expression of the ecto-ATPase NTPDase2 and of nucleotide receptors by neuronal progenitor cells in the adult murine hippocampus. J Neurosci Res. 80(5):600–610. [DOI] [PubMed] [Google Scholar]
- Sugasawa M, Erostegui C, Blanchet C, Dulon D. 1996. ATP activates non-selective cation channels and calcium release in inner hair cells of the guinea-pig cochlea. J Physiol. 491:707–718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suyama S, Sunabori T, Kanki H, Sawamoto K, Gachet C, Koizumi S, Okano H. 2012. Purinergic signaling promotes proliferation of adult mouse subventricular zone cells. J Neurosci. 32(27):9238–9247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsumura M, Sobhan U, Muramatsu T, Sato M, Ichikawa H, Sahara Y, Tazaki M, Shibukawa Y. 2012. TRPV1-mediated calcium signal couples with cannabinoid receptors and sodium-calcium exchangers in rat odontoblasts. Cell Calcium. 52(2):124–136. [DOI] [PubMed] [Google Scholar]
- Wang W, Yi X, Ren Y, Xie Q. 2016. Effects of adenosine triphosphate on proliferation and odontoblastic differentiation of human dental pulp cells. J Endod. 42(10):1483–1489. [DOI] [PubMed] [Google Scholar]
- Wang XY, He ZC, Song LY, Spencer S, Yang LX, Peng F, Liu GM, Hu MH, Li HB, Wu XM, et al. 2011. Chemotherapeutic effects of bioassay-guided extracts of the American cockroach, Periplaneta americana. Integr Cancer Ther. 10(3):NP12–NP23. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, DS_10.1177_0022034518807920 for Purinergic Signaling Modulates Survival/Proliferation of Human Dental Pulp Stem Cells by S. Zhang, D. Ye, L. Ma, Y. Ren, R.T. Dirksen and X. Liu in Journal of Dental Research