Abstract
Wound closure in the Drosophila larval epidermis mainly involves nonproliferative, endocyling epithelial cells. Consequently, it is largely mediated by cell growth and migration. We discovered that both cell growth and migration in Drosophila require the cochaperone-encoding gene cdc37. Larvae lacking cdc37 in the epidermis failed to close wounds, and the cells of the epidermis failed to change cell shape and polarize. Likewise, wound-induced cell growth was significantly reduced, and correlated with a reduction in the size of the cell nucleus. The c-Jun N-terminal kinase (JNK) pathway, which is essential for wound closure, was not typically activated in injured cdc37 knockdown larvae. In addition, JNK, Hep, Mkk4, and Tak1 protein levels were reduced, consistent with previous reports showing that Cdc37 is important for the stability of various client kinases. Protein levels of the integrin β subunit and its wound-induced protein expression were also reduced, reflecting the disruption of JNK activation, which is crucial for expression of integrin β during wound closure. These results are consistent with a role of Cdc37 in maintaining the stability of the JNK pathway kinases, thus mediating cell growth and migration during Drosophila wound healing.
INTRODUCTION
The healing of a mammalian skin wound is complex and involves various cellular processes, including blood clotting, inflammation, epithelial cell proliferation and migration, and matrix synthesis and remodeling, which span multiple tissues (Martin, 1997; Gurtner et al., 2008; Shaw and Martin, 2009). In contrast, wound healing in the Drosophila larval epidermis is simple: the epidermis consists mainly of a single, nonproliferative cell layer that underlies the protective cuticle. Thus, wound closure involves primarily cuticle regeneration and cell growth and migration, but not proliferation.
Many signaling pathways are required for wound closure in the Drosophila epidermis (reviewed in Tsai et al., 2018). Of these, c-Jun N-terminal kinase (JNK), which is required for a broad range of wound healing processes, is the most crucial. Without JNK, cells cannot properly polarize, change shape, orient toward the wound center, or migrate to close the wound (Ramet et al., 2002; Galko and Krasnow, 2004; Kwon et al., 2010; Park et al., 2018). Conversely, some proteins acting upstream of JNK appear to be redundant in a pathway that includes both canonical and noncanonical factors in regard to the embryonic dorsal closure process (Lesch et al., 2010; Rios-Barrera and Riesgo-Escovar, 2013). Specifically, both JNK and the AP-1 transcription factors DJun (Jra) and DFos (Kay) are absolutely required for wound closure, and larvae that are lacking any one of these factors cannot repair open wounds. In contrast, the Jun/stress-activated protein (SAP) 2 kinases Hep and Mkk4 are partially redundant, as are the Jun/SAP3 kinases Slpr and Tak1 (Lesch et al., 2010). Although the involvement of the JNK/SAPK pathway in wound healing is well known both in insects and mammals (Angel et al., 2001; Li et al., 2003), the mechanisms underlying the regulation of this pathway are not well understood.
Protein kinases are often associated with the molecular chaperone Hsp90, which helps these client proteins take on their active conformation (Workman et al., 2007; Taipale et al., 2010). Hsp90 interacts with at least 20 other factors, called cochaperones, which either modulate the activity of Hsp90 or affect the specificity of Hsp90 client proteins (Taipale et al., 2010). Cdc37 is one such cochaperone that is known to maintain the function and stability of client kinases (Pearl, 2005; Caplan et al., 2007; Karnitz and Felts, 2007; Taipale et al., 2010), and many kinases are regulated by Cdc37, but the relationship between Cdc37 and the JNK signaling pathway is not clear.
Cdc37 was originally identified as a yeast cell-cycle regulator that was later found to interact with Hsp90 and v-Src (reviewed in Karnitz and Felts, 2007). Hsp90 and Cdc37 are both structurally and functionally conserved in metazoans (Caplan et al., 2007). In Drosophila, cdc37 was initially isolated from a mutagenesis screen based on its involvement in eye development (Simon et al., 1991), and was later found to be essential for Sevenless receptor tyrosine kinase signaling (Cutforth and Rubin, 1994). Null mutations in cdc37 are recessively lethal, indicating it is required for cell viability (Cutforth and Rubin, 1994; Lange et al., 2002). Cdc37 inhibits Hh and Wnt signaling pathways in both flies and mammalian cells (Swarup et al., 2015) and mediates chromosome segregation and cytokinesis by modulating the function of Aurora B kinase (Lange et al., 2002).
In this study, we isolated cdc37 based on its RNA interference (RNAi) knockdown phenotype in larval epidermal wound closure in Drosophila, and found that cdc37 is required not only for reepithelialization but also for cells to change shape, polarize, and grow during epidermal wound closure, and all of these phenotypes are shared by larvae lacking JNK. Molecularly, cdc37 is required for maintaining the protein levels of JNK pathway components.
RESULTS
cdc37 knockdown disrupts epidermal wound closure
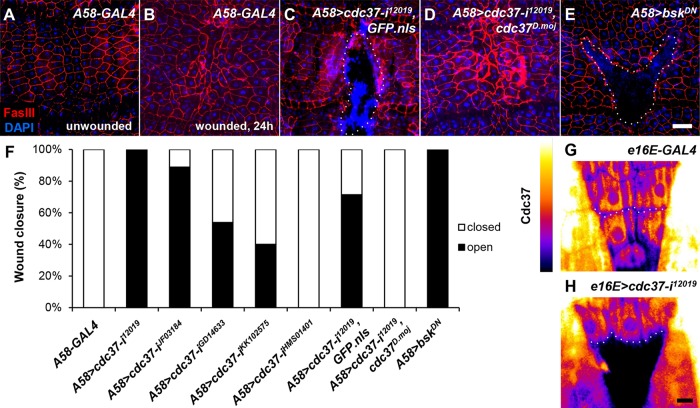
We isolated cdc37 from an RNAi-based genetic screen of essential genes using an epidermal wound closure assay and the larval epidermis-specific A58-GAL4 driver. Whereas wild-type third instar larvae closed a large wound generated by the abrasion of ∼30 epidermal cells within 12–24 h (Figure 1, A and B, and Supplemental Figure S1, A–E; Galko and Krasnow, 2004; Kwon et al., 2010), cdc37-knockdown larvae were unable to close an introduced wound even after 30 h (Figure 1, C and F, and Supplemental Figure S1, F–J). In wild-type larvae, cells located one to three rows distal to the wound margin generally undergo the most dramatic change in cell shape due to cell migration (Supplemental Figure S1, B and C; Kwon et al., 2010), but in the A58-GAL4 UAS-cdc37 RNAi (hereafter, A58>cdc37-i) larvae, these cells displayed a very strong open-wound phenotype. They often retained a pentagonal or hexagonal cell shape even 24–30 h after injury (Figure 1C and Supplemental Figure S1, G–J), which is similar to unwounded epidermis or wounded epidermis expressing a dominant negative Drosophila JNK bsk (bskDN) construct (Figure 1, A and E).
FIGURE 1:
cdc37-knockdown larvae display defects in wound closure. (A–E) The epidermis was examined in late third instar larvae of the indicated genotypes, either before wounding (A) or 24 h after injury to the dorsal epidermis (B–E). Cell boundaries were stained red with anti-FasIII antibody, and the nuclei were stained blue with DAPI. The dotted line indicates the wound hole. (A, B) A58-GAL4-only control. (C) A58-GAL4, UAS-cdc37-RNAi12019, UAS-GFP.nls (A58>cdc37-i12019, GFP.nls, hereafter). The strong blue staining patterns show hemocytes attached to the wound site. (D) A58>cdc37-i12019, cdc37D.moj. (E) A58>bskDN. (F) Quantification of the wound closure phenotype. For each genotype, 13 or more larvae were examined. Scale bar: 100 μm (A–E). (G, H) RNAi knockdown of cdc37 was confirmed in the larval epidermis by immunohistochemistry using anti-Cdc37 antibody, as shown in the heat map. cdc37-RNAi was driven using e16E-GAL4 in the posterior half of each segment, leaving the anterior half as an internal control. The dotted line indicates the anterior–posterior compartment boundary. Anterior is up. (G) e16E-only control. (H) e16E>cdc37-i12019. Scale bar: 25 μm (G, H).
We confirmed the open-wound phenotype using three different approaches. First, we tested five different cdc37 RNAi strains. Although phenotypic strength varied, four of the five strains with the A58-GAL4 driver displayed open wounds (Figure 1F and Supplemental Figure S2, A–D). Second, we generated a UAS transgenic line for Drosophila mojavensis cdc37 (UAS-cdc37D.moj) and overexpressed it in the UAS-cdc37-i background, which displayed the strongest phenotype, for a phenotypic rescue without interference from Drosophila melanogaster cdc37 RNAi (Kondo et al., 2009). This experiment assumed the functional conservation of cdc37 between the two species. The sequence identity and similarity of the Cdc37 protein between the two species were 80.6% and 87.9%, respectively. Interestingly, A58>cdc37-i; cdc37D.moj larvae exhibited complete rescue of the open-wound phenotype, whereas the control line, which had the UAS copy number balanced by the addition of UAS-GFP.nls showed only a marginal rescue, presumably due to weaker cdc37 knockdown (Figure 1, D and F). Third, we performed immunohistochemistry experiments in knockdown larvae using anti-Cdc37 antibody to examine Cdc37 expression in the epidermis. The fluorescence intensities of Cdc37 immunostaining correlated well with the phenotypic strengths of the individual RNAi lines (Figure 1, G and H, and Supplemental Figure S2, E–H). Thus, we conclude that Cdc37 is essential for epidermal wound closure in D. melanogaster, and that this function has not diverged between D. melanogaster and D. mojavensis.
cdc37 is required for cell polarization during wound healing
During Drosophila wound healing, cells located near the wound margin polarize toward the wound center, a process that can be monitored by the localization of a GFP-Zip fusion protein (Franke et al., 2005; Baek et al., 2010; Kwon et al., 2010). In wild-type larvae, GFP-Zip proteins localize primarily to the perinuclear region but translocate to the rear side (opposite to the wound) of the cells upon wounding, as was clearly visible 4–10 h after injury in many of the cells in the first to third rows distal to the wound margin (Figure 2A; Kwon et al., 2010). In cdc37-knockdown larvae, GFP-Zip proteins were detected mainly around the perinuclear region and the localization was similar to that of unwounded tissues, as if the cells had not received wound signals (Figure 2B).
FIGURE 2:
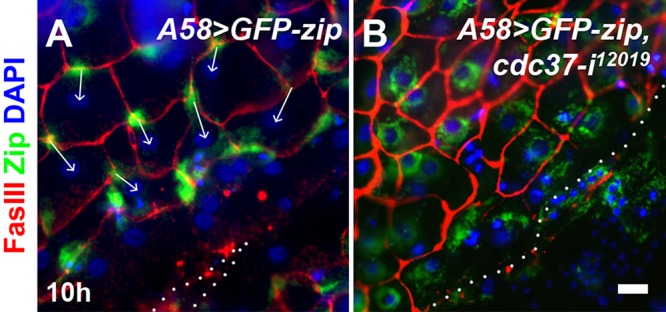
cdc37 is required for cell polarization during wound healing. Localization of the GFP-Zip fusion protein on the rear side of cells was analyzed 10 h after injury. GFP-Zip is shown in green. Cell boundaries were stained red with anti-FasIII antibody, and the cell nuclei were visualized by DAPI staining in blue. The dotted line indicates the wound margin. (A) A58-GFP-zip, control. (B) A58>GFP-zip, cdc37-i12019. The arrows indicate the directionality of the cells, based on the localization of GFP-Zip and the position of the nucleus. Scale bar: 25 μm.
cdc37 is required for JNK pathway activation
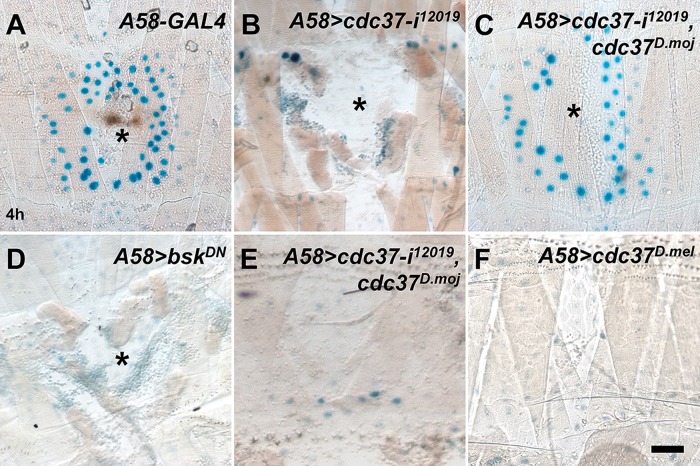
The strong open-wound phenotype observed in the cdc37 knockdown larvae prompted us to evaluate whether the JNK pathway was properly activated in these larvae using the JNK pathway reporter msn-lacZ (Galko and Krasnow, 2004). In wild-type larvae, wound-induced msn-lacZ expression was visible up to five to six cell diameters away from the wound margin (Figure 3A), but in cdc37 knockdown larvae, msn-lacZ expression disappeared almost completely, similar to bskDN-expressing larvae (Figure 3, B and D). We wanted to confirm these results using another JNK pathway reporter puc-lacZ, and obtained essentially the same result (Supplemental Figure S3; Galko and Krasnow, 2004). The defects in msn-lacZ induction were rescued to near wild-type levels by the simultaneous overexpression of cdc37D.moj(Figure 3C). We noticed, however, that msn-lacZ reporter induction was no stronger or broader in the wounded, rescued cdc37 knockdown larvae than in wounded wild-type controls, and that msn-lacZ expression was not induced in unwounded larvae (Figure 3E). These results indicate that cdc37D.moj expression does not generate a gain-of-function phenotype. To investigate this finding further, we generated a UAS transgenic line of D. melanogaster cdc37 (UAS-cdc37D.mel) and expressed cdc37D.mel in the epidermis. Overexpression of cdc37D.mel alone neither induced msn-lacZ expression in unwounded larvae nor increased the induction of msn-lacZ expression in wounded larvae (Figure 3F; unpublished data). Together, these results indicate that cdc37 is necessary, but not sufficient, for activation of the JNK pathway during wound healing, suggesting that cdc37 functions as a permissive factor in JNK signaling.
FIGURE 3:
Activation of the JNK pathway is disrupted in cdc37 knockdown larvae. Activation of the JNK pathway in larvae was monitored using the msn-lacZ reporter at 4 h after injury (A–D) or without wounding (E, F). β-Galactosidase expression was visualized with X-gal staining in blue. The asterisk indicates the wound hole. (A) A58-only control. (B) A58>cdc37-i12019. (C) A58>cdc37-i12019, cdc37D.moj. (D) A58>bskDN. (E) A58>cdc37-i12019, cdc37D.moj. (F) A58>cdc37D.mel. Scale bar: 100 μm.
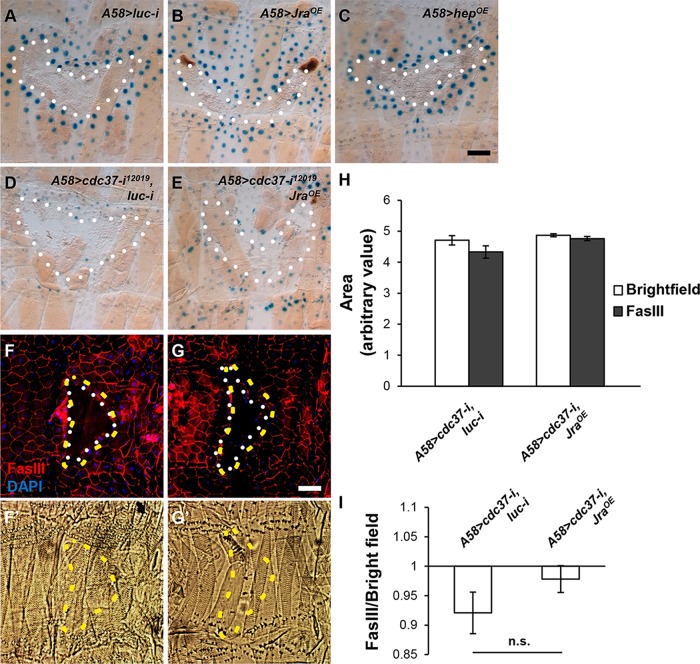
We next examined whether forced activation of the JNK pathway could rescue the wound healing defects of cdc37 knockdown larvae. First of all, overexpression of the constitutively active form of Hep (HepCA) degenerated epidermal tissues, and thus could not be used for wound analysis, while overexpression of Hep or Jra did not interfere with normal wound closure, examined at 24 h after injury. Overexpression of Jra enhanced msn-lacZ induction upon injury, whereas overexpression of Hep did not (Figure 4, A–C), indicating that the latter did not enhance the JNK pathway activation. Thus, we used Jra for further analysis. Overexpression of Jra in the background of cdc37 knockdown did not induce msn-lacZ upon injury (Figure 4, D and E), indicating that Jra still requires cdc37 for msn-lacZ expression. We also examined wound closure in these larvae at 30 h after injury. Wound recovery rates did not differ significantly between cdc37 knockdown larvae and Jra-overexpressing, cdc37-knockdown larvae (Figure 4, F–I). These results indicate that cdc37 is absolutely required for JNK pathway activation.
FIGURE 4:
Overexpression of Jra does not rescue the wound healing defects displayed by cdc37-knockdown larvae. (A–E) Examination of JNK pathway activation using the msn-lacZ reporter in the larvae of the indicated genotypes, 4 h after injury. (A) A58>luc-i (control). (B) A58>Jra. (C) A58>hep. (D) A58>cdc37-i, luc-i (control). (E) A58>cdc37-i, Jra. β-Galactosidase expression was visualized with X-gal staining in blue. The white-dotted line indicates the wound margin. (F–G′) Wound closure analysis in the larvae of the indicated genotypes, 30 h after injury. (F, F′) A58>cdc37-i, luc-i (control). (G, G′) A58>cdc37-i, Jra. (F, G) Cell boundaries were stained red with anti-FasIII antibody, and the nuclei were stained blue with DAPI. The white-dotted line indicates the epidermal wound margin, 30 h after injury, and the yellow-dashed line indicates the original wound margin, left as impressions on the cuticle. (F′, G′) Bright-field micrographs of the cuticle layer. Scale bar: 100 μm. (H) Quantification of the size of the wound hole measured in the bright-field micrograph (original) or in the fluorescent micrograph (recovered). (I) Ratios of the area of the epidermal wound hole (white-dotted) to the area of the cuticle impression (yellow-dotted), which may indicate wound recovery. The difference is not significant. The error bars represent SEM.
cdc37 knockdown disrupts the wound-induced growth of cells and nuclei
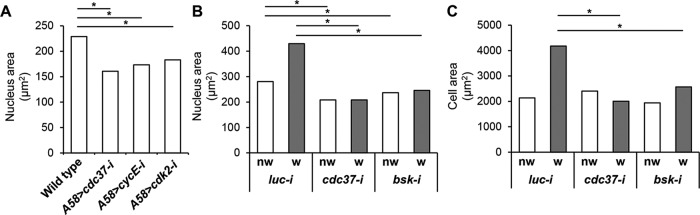
Wounding induces endoreplication, and consequently cell growth, in the Drosophila adult epidermis (Losick et al., 2013; Zielke et al., 2013). We noted that the epidermal cells in cdc37-knockdown larvae remained small even 24 h after injury. Thus, we wanted to determine whether cdc37 is required for endoreplication. In unwounded epidermis, cdc37 knockdown via A58-GAL4 significantly reduced nuclear width (p < 0.01; similar to that observed following knockdown of cycE or cdk2, the genes that encode the cyclin and cyclin-dependent kinase required for S phase entry, respectively), consistent with the argument that cdc37 is required for the developmental endocycle of epidermal cells (Figure 5A). Knockdown of cycE or cdk2 did not noticeably interfere with wound closure or wound-induced msn-lacZ expression (Supplemental Figure S4, A–D), indicating that other factors may compensate for retarded cell growth, including cell migration, cell–cell fusion (Losick et al., 2013; Lee et al., 2017), and possibly the thinning of epidermal tissues, which would increase the two-dimensional width.
FIGURE 5:
cdc37 knockdown disrupts the wound-induced growth of cells and the nuclei. Nuclear width was assessed based on DAPI fluorescence. Nuclei were ranked by size, and those in the top 15% were chosen for additional analysis. (A) The average width of a nucleus that was within the top 15% in unwounded epidermis. (B) The average width of a nucleus that was within the top 15% selected from nonwounded (nw) and wounded (w) segments of the epidermis 7 h after injury. (C) The average width of a cell that was chosen for the analysis indicated in B. In B and C, luc-i was used as a control, and all the RNAi constructs were driven using A58-GAL4. At least eight larval epidermal samples were analyzed for each genotype. *, p < 0.01; Wilcoxon rank-sum test.
Next, we attempted to measure the growth of the cells and nuclei in the wounded epidermis. It is generally true that cells proximal to the wound tend to undergo the most prominent changes. However, we also noticed occasionally that cells away from the wound were enlarged greatly, while some cells of the wound margin shrank. These factors interfered with simple size measurement of wound-proximal cells. Therefore, we first measured the width of all cell nuclei in a defined region and then chose those with the largest nuclear areas (top 15%) for further analysis, by marking the cell boundaries for those cells. First, although the average size of the cell nuclei (top 15%) in wounded versus unwounded wild-type larvae increased significantly (p > 0.01), this increase was disrupted in both cdc37-knockdown larvae and bskDN-expressing larvae (Figure 5B). Second, the average size of the cells containing the largest nucleus (top 15%) also increased after wounding in wild-type larvae, but not in cdc37-knockdown or bskDN-expressing larvae (Figure 5C). These results suggest that cdc37 is required for endoreplication and cell growth during wound healing, which also depends on Cdk2, and is consistent with a previous report showing that Cdk2 interacts with Cdc37 (Prince et al., 2005).
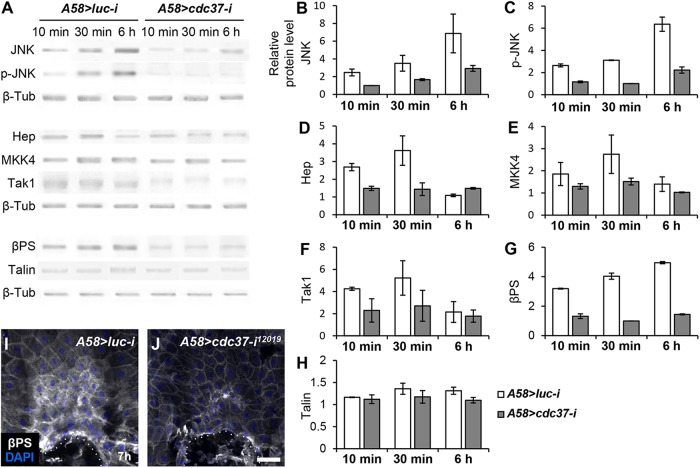
JNK and integrin β subunit protein levels are reduced in cdc37-knockdown larvae
As a way to examine whether Cdc37 might influence protein stability, we measured global protein levels of the JNK pathway signaling components in the epidermis of cdc37-knockdown larvae compared with wild-type larvae by Western blotting. JNK protein levels were reduced in the epidermis of knockdown larvae, even before wound incubation (Figure 6, A and B; unpublished data). Activated JNK, assessed using an anti–phospho-JNK antibody, was also reduced, potentially reflecting a reduction in total protein levels (Figure 6, A and C), as were the levels of Hep, Mkk4, and Tak1, kinases acting upstream of JNK (Figure 6, A and D–F). These results are consistent with previous reports showing that Cdc37 increases the stability of client kinases (Caplan et al., 2007; Karnitz and Felts, 2007), although our results cannot exclude the potential involvement of transcriptional or translational regulation.
FIGURE 6:
The JNK pathway kinase and βPS integrin protein levels are reduced in cdc37-knockdown larvae. (A) Western blotting was performed on epidermal samples from control (luc knockdown) and cdc37-knockdown late third instar larvae at the indicated times after wounding. β-Tub was used as a loading control. (B–H) Quantification of three independent Western blotting results. Error bars represent the SEM. (I, J) Protein levels of βPS integrin were analyzed by immunostaining using an anti-βPS antibody 7 h after wounding. Cell nuclei were stained blue using DAPI. The dotted line indicates the wound margin. Scale bar: 100 μm.
Because JNK up-regulates protein levels of the integrin β subunit βPS and integrins are essential for wound closure in Drosophila (Lee et al., 2017; Park et al., 2018), we also measured βPS and talin protein levels. βPS levels were greatly reduced in the epidermis of cdc37-knockdown larvae compared with wild-type controls, whereas talin levels did not differ between the two groups of larvae (Figure 6, A, G, and H). These results were confirmed by immunostaining the wounded larval epidermis with anti-βPS antibody (Figure 6, I and J).
DISCUSSION
Our results indicate that cdc37 is an essential factor in the activation of the JNK pathway during Drosophila wound healing. Without cdc37, the levels of many proteins involved in JNK pathway signaling, including JNK itself, were severely reduced, suggesting that the stability of these proteins is compromised in the absence of cdc37.
In Drosophila, JNK mediates diverse wound healing responses, including gene expression, cell shape change and polarization, reepithelialization, and cell fusion (Ramet et al., 2002; Galko and Krasnow, 2004; Bosch et al., 2005; Mattila et al., 2005; Campos et al., 2010; Kwon et al., 2010; Lesch et al., 2010; Brock et al., 2012; Losick et al., 2016; Lee et al., 2017; Park et al., 2018). The present study suggests that the JNK pathway also mediates wound-induced endoreplication and cell growth. However, prior reports have indicated that JNK suppresses wound-induced endoreplication in adult stages (Losick et al., 2016), which is a discrepancy that requires further investigation.
Larvae lacking cdc37 displayed disrupted activation of the JNK pathway and displayed phenotypes similar to those of larvae lacking active JNK. Thus, we conclude that most of the cdc37-knockdown phenotypes we analyzed were likely caused by the disruption of JNK activation during wound healing. It should be noted, however, that cell nucleus size and JNK protein levels were also reduced in the unwounded epidermis of cdc37 knockdown larvae, indicating that loss of cdc37 expression also causes developmental defects. This was not unexpected, given that cdc37 null mutations are cell lethal (Lange et al., 2002). Considering that A58-GAL4 is only active after early larval stages (Galko and Krasnow, 2004), and that endoreplicating cells are resistant to apoptosis (Mehrotra et al., 2008; Hassel et al., 2014; Zhang et al., 2014), the wound healing defects in cdc37-knockdown larvae may have been uncovered luckily due to cell stress caused by wounding in the apoptosis-resistant epidermal cells.
Cdc37 is best known as a cochaperone that confers client kinase specificity to Hsp90 (Karnitz and Felts, 2007; Taipale et al., 2010). The client kinases requiring the Hsp90-Cdc37 complex for activity and stability are diverse and include Cdk2, Cdk4, Src, Aurora B, Raf1, and RIP3 (Stepanova et al., 1996; Lange et al., 2002; Prince et al., 2005; Li et al., 2015; and reviewed in Hunter and Poon, 1997). However, Cdc37 may also function as an independent molecular chaperone alone, similar to Hsp90 (Kimura et al., 1997). Our investigation into the possible involvement of Hsp90 (also known as Hsp83 in Drosophila) in wound healing using multiple Hsp90 RNAi lines did not yield any definitive answer. We also assessed whether aurora B, cdc2, or ckII were involved in wound healing, as these factors reportedly interact with cdc37 in various contexts (Cutforth and Rubin, 1994; Kimura et al., 1997; Lange et al., 2002; Miyata and Nishida, 2004). Larvae deficient of each of these factors closed wounds normally (Supplemental Figure S4, E–G). Finally, we did not observe any noticeable changes in the protein level or localization of Cdc37 during wound healing (Supplemental Figure S5). Thus, the requirement of cdc37 for JNK activation is a novel finding. Nonetheless, defining the detailed molecular mechanisms underlying Cdc37 functions requires further investigation.
MATERIALS AND METHODS
Fly stocks
The following stocks were obtained from the Bloomington Stock Center: Oregon R, w1118, msn-lacZ (msn06946), puc-lacZ, UAS-Jra, UAS-hep, e16E-GAL4, hs-GAL4, UAS-cdc37-RNAi (JF03184, GD14633, HMS01401), UAS-bskDN, UAS-luciferase RNAi, UAS-GFP.nls, UAS-Dcr-2, UAS-cdk2-RNAi (HMS00174; Sopko et al., 2014), UAS-cycE-RNAi (HMS00060), and UAS-ckIIαDN (Lin et al., 2002). The following stocks were obtained from the Vienna Drosophila RNAi Center in Austria: UAS-cdc37-RNAi (KK102575), UAS-aurora B-RNAi (GD11982, KK112558; Bell et al., 2015), and UAS-cdc2-RNAi (106130). UAS-cdc37-RNAi (12019R-2) was obtained from the National Institute of Genetics in Japan. D. mojavensis was obtained from The National Drosophila Species Stock Center.
Generating cdc37D.mel and cdc37D.moj transgenic flies
Total RNA was isolated from mid- to late-third instar larvae of D. melanogaster or D. mojavensis. The corresponding cDNAs were synthesized and the cdc37 open reading frame (ORF) was amplified from the resulting cDNA pools using the following primer sets: 5′-AGCGGCCGCATGGTGGACTACAGCAAG-3′ (forward) and 5′-AGACTCGAGTCAGTCAACGTCCTCGGT-3′ (reverse) for D. melanogaster and 5′-AGCGGCCGCATGGTTGACTACAGCAAG-3′ (forward) and 5′-AGACTCGAGTCAGTCGAGGTCATTGGT-3′ (reverse) for D. mojavensis. These ORFs were subcloned into a pUAST vector at the NotI-XhoI sites and injected into w1118 embryos to produce transgenic flies.
Generating anti-Cdc37 antibody
Total RNA was isolated from third instar larvae and cDNAs were synthesized. A fragment of the cdc37 gene corresponding to amino acids 29–183 was amplified from the cDNA pool by PCR using the following primer sets: 5′-AAGGATCCTTCCGCTGGCGGCACCAG-3′ (forward) and 5′-AACTCGAGTTATTACGTCTCCTCGCCCACCAA-3′ (reverse) for N-terminal His tagging and 5′-AACCATGGTTTTCCGCTGGCGGCACCAG-3′ (forward) and 5′-AAGCGGCCGCCGTCTCCTCGCCCACCAA-3′ (reverse) for C-terminal His tagging. The resulting fragments were subcloned into the vector pET28a for expression in the Escherichia coli (BL21). His-tagged proteins were purified using a Ni-bead column. Polyclonal antibodies against the purified Cdc37 peptide were raised in rabbits by Young In Frontier (Seoul, Korea). In this study, we primarily used antiserum obtained using the C-terminal His-tagged fusion (Supplemental Figure S5, A and B).
Wounding and dissection
Mid- to late-third instar larvae were wounded using a pair of forceps (Fine Science Tools; Cat. No. 11295-00) to pinch the outer integument to abrade ∼30 epidermal cells on the dorsal side of segment A2 or A3. The larvae were then placed on cornmeal–agar media for recovery. Epidermal tissues were dissected in phosphate-buffered saline (PBS), as described in Park et al. (2018). Samples were fixed in 4% paraformaldehyde for 15 min. For Western blotting and coimmunoprecipitation, five different dorsal segments were wounded to maximize wound healing responses. Unless otherwise specified, at least six larvae were examined for each experiment.
Immunohistochemistry
Fixed samples were washed three times in PBS and incubated with primary antibodies diluted in PBS plus 0.5% Triton X-100 (PBST) supplemented with 1% normal goat serum for 2 h at room temperature. The following primary antibodies were used: mouse anti–Fasciclin III (1:50 dilution; Developmental Studies Hybridoma Bank [DSHB]; Cat. No. 7G10) and rat anti-Cdc37 (1:200 dilution; obtained from M. Therrien at the University of Montreal in Canada; Cutforth and Rubin, 1994). Samples were washed in PBST plus 5% normal goat serum three times for 10 min each and incubated with secondary antibodies diluted in PBST plus 1% normal goat serum overnight at 4°C. The following secondary antibodies were used: Cy3-conjugated goat anti-mouse immunoglobulin G (IgG) (1:100; Jackson ImmunoResearch; Cat. No. 75512), Alexa Fluor 488–conjugated goat anti-mouse IgG (1:200; Molecular Probes; Cat. No. A11001), and Alexa Fluor 546–conjugated goat anti-rat IgG (1:200; Molecular Probes; Cat. No. A11081). After washing in PBST three times for 10 min each, the samples were mounted in VECTASHIELD (Vector Laboratories; Cat. No. H-1000). Cell nuclei were counterstained in a 1:500 dilution of 4′,6-diamidino-2-phenylindole (DAPI; Molecular Probes; Cat. No. D1306).
β-Galactosidase staining
Mid- to late-third instar larvae were dissected in PBS and fixed in 2% glutaraldehyde for 15 min at room temperature. The samples were washed three times in PBS and incubated in 10 mM NaPO4, 150 mM NaCl, 1 mM MgCl2, 3.1 mM K4[FeII(CN)6], 3.1 mM K3[FeIII(CN)6], 0.3% Triton X-100, and 0.2% X-Gal for 30 min at 37°C.
Measurement of the cell and nucleus
Micrographs of DAPI-stained epidermal tissues were analyzed using ImageJ. Background signals were eliminated using the Threshold tool, and the widths of all of the cell nuclei in the trapezoidal area of a dorsal segment (Kwon et al., 2010) were measured. Cells containing the widest nuclei (widest 15%) were selected for further analysis. The cell boundaries of these cells were marked using the Freehand Selection tool and the width of the cell was measured. At least eight animals per genotype were analyzed and a Wilcoxon rank-sum test was used to perform the statistical analysis in Figure 5.
Western blot analysis and coimmunoprecipitation
For Western blotting, epidermal filets were boiled in SDS sample buffer (250 mM Tris-HCl, pH 6.8, 0.5M dithiothreitol, 10% SDS, 0.25% bromophenol blue, and 50% glycerol) at 100°C for 5 min, subjected to 8% or 10% SDS–PAGE, and transferred to nitrocellulose membranes. The membranes were then blocked with 5% skim milk in TBST (10 mM Tris, pH 8.0, 150 mM NaCl, and 0.05% Tween 20) for 2 h and probed with primary antibodies at 4°C overnight. The following antibodies were used: rabbit anti-JNK (1:1000 dilution; Santa Cruz Biotechnology; Cat. No. sc-571), rabbit anti-pJNK (1:1000 dilution; Promega; Cat. No. V793A), rat anti-Cdc37 (1:400 dilution; Figure 1; Supplemental Figures S2 and S5, C–F′; Cutforth and Rubin, 1994), rabbit anti-Cdc37 (1:1000 dilution; Supplemental Figure S5, A and B), rabbit anti-Hep (1:1000 dilution; Rallis et al., 2010), guinea pig anti-Mkk4 (1:1000 dilution; Rallis et al., 2010), rabbit anti-Tak1 (1:1000 dilution; Paquette et al., 2010), rabbit anti-Slpr (1:500 dilution; Polaski et al., 2006), mouse anti-βPS (1:1 dilution; DSHB; Cat. No. CF.6G11), mouse anti-talin (1:1 dilution; DSHB; Cat. No. A22A), and goat anti–β-tubulin (1:1000 dilution; Santa Cruz Biotechnology). The membranes were then washed three times with TBST and incubated with horseradish peroxidase–conjugated secondary antibodies (1:1000 dilution; anti-rabbit [Cat. No. sc-2004], anti-rat [Cat. No. sc-2006], anti-mouse [Cat. No. sc-2005], anti-goat [Cat. No. sc-2056], and anti-guinea pig [Cat. No. sc-2438], all purchased from Santa Cruz Biotechnology) in TBST plus 1% skim milk for 1 h. After washing in TBST three times, the membranes were visualized using the WEST-ZOL Plus Western blot detection system (iNtRon; Cat. No. 16024).
For coimmunoprecipitation, epidermal filets were briefly lysed in NP-40 buffer (50 mM Tris-HCl, pH 8.0, 150 mM NaCl, 1% NP-40) at 4°C and incubated with protein G–sepharose beads (Sigma Aldrich; Cat. No. P3296) plus antibodies for coupling at 4°C overnight. The beads were then washed with NP-40 buffer and used for Western blot analysis.
Supplementary Material
Acknowledgments
We thank J. Ng, Y. Miyata, N. Silverman, B. Stronach, M. Therrien, The Bloomington Stock Center, The National Institute of Genetics in Japan, and The Vienna Drosophila Resource Center for fly stocks and antibodies. We are also very grateful to our colleagues in the laboratory for helpful discussions. This work was supported by a National Research Foundation of Korea grant funded by the Korean Government, Ministry of Science and ICT (Grant no. 2015R1A2A2A01006660) to K.-M.C.
Abbreviations used:
- JNK
c-Jun N-terminal kinase
- SAP
stress-activated protein
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E18-12-0822) on September 4, 2019.
REFERENCES
- Angel P, Szabowski A, Schorpp-Kistner M. (2001). Function and regulation of AP-1 subunits in skin physiology and pathology. Oncogene , 2413–2423. [DOI] [PubMed] [Google Scholar]
- Baek SH, Kwon YC, Lee H, Choe KM. (2010). Rho-family small GTPases are required for cell polarization and directional sensing in Drosophila wound healing. Biochem Biophys Res Commun , 488–492. [DOI] [PubMed] [Google Scholar]
- Bell GP, Fletcher GC, Brain R, Thompson BJ. (2015). Aurora kinases phosphorylate Lgl to induce mitotic spindle orientation in Drosophila epithelia. Curr Biol , 61–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bosch M, Serras F, Martin-Blanco E, Baguna J. (2005). JNK signaling pathway required for wound healing in regenerating Drosophila wing imaginal discs. Dev Biol , 73–86. [DOI] [PubMed] [Google Scholar]
- Brock AR, Wang Y, Berger S, Renkawitz-Pohl R, Han VC, Wu Y, Galko MJ. (2012). Transcriptional regulation of Profilin during wound closure in Drosophila larvae. J Cell Sci , 5667–5676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos I, Geiger JA, Santos AC, Carlos V, Jacinto A. (2010). Genetic screen in Drosophila melanogaster uncovers a novel set of genes required for embryonic epithelial repair. Genetics , 129–140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caplan AJ, Mandal AK, Theodoraki MA. (2007). Molecular chaperones and protein kinase quality control. Trends Cell Biol , 87–92. [DOI] [PubMed] [Google Scholar]
- Cutforth T, Rubin GM. (1994). Mutations in Hsp83 and cdc37 impair signaling by the sevenless receptor tyrosine kinase in Drosophila. Cell , 1027–1036. [DOI] [PubMed] [Google Scholar]
- Franke JD, Montague RA, Kiehart DP. (2005). Nonmuscle myosin II generates forces that transmit tension and drive contraction in multiple tissues during dorsal closure. Curr Biol , 2208–2221. [DOI] [PubMed] [Google Scholar]
- Galko MJ, Krasnow MA. (2004). Cellular and genetic analysis of wound healing in Drosophila larvae. PLoS Biol , E239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gurtner GC, Werner S, Barrandon Y, Longaker MT. (2008). Wound repair and regeneration. Nature , 314–321. [DOI] [PubMed] [Google Scholar]
- Hassel C, Zhang B, Dixon M, Calvi BR. (2014). Induction of endocycles represses apoptosis independently of differentiation and predisposes cells to genome instability. Development , 112–123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T, Poon RY. (1997). Cdc37: a protein kinase chaperone? Trends Cell Biol , 157–161. [DOI] [PubMed] [Google Scholar]
- Karnitz LM, Felts SJ. (2007). Cdc37 regulation of the kinome: when to hold ’em and when to fold ’em. Sci STKE , pe22. [DOI] [PubMed] [Google Scholar]
- Kimura Y, Rutherford SL, Miyata Y, Yahara I, Freeman BC, Yue L, Morimoto RI, Lindquist S. (1997). Cdc37 is a molecular chaperone with specific functions in signal transduction. Genes Dev , 1775–1785. [DOI] [PubMed] [Google Scholar]
- Kondo S, Booker M, Perrimon N. (2009). Cross-species RNAi rescue platform in Drosophila melanogaster. Genetics , 1165–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon YC, Baek SH, Lee H, Choe KM. (2010). Nonmuscle myosin II localization is regulated by JNK during Drosophila larval wound healing. Biochem Biophys Res Commun , 656–661. [DOI] [PubMed] [Google Scholar]
- Lange BM, Rebollo E, Herold A, Gonzalez C. (2002). Cdc37 is essential for chromosome segregation and cytokinesis in higher eukaryotes. EMBO J , 5364–5374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Lee CW, Park SH, Choe KM. (2017). Spatiotemporal regulation of cell fusion by JNK and JAK/STAT signaling during Drosophila wound healing. J Cell Sci , 1917–1928. [DOI] [PubMed] [Google Scholar]
- Lesch C, Jo J, Wu Y, Fish GS, Galko MJ. (2010). A targeted UAS-RNAi screen in Drosophila larvae identifies wound closure genes regulating distinct cellular processes. Genetics , 943–957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Xu T, Cao Y, Wang H, Li L, Chen S, Wang X, Shen Z. (2015). A cytosolic heat shock protein 90 and cochaperone CDC37 complex is required for RIP3 activation during necroptosis. Proc Natl Acad Sci USA , 5017–5022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Gustafson-Brown C, Hanks SK, Nason K, Arbeit JM, Pogliano K, Wisdom RM, Johnson RS. (2003). c-Jun is essential for organization of the epidermal leading edge. Dev Cell , 865–877. [DOI] [PubMed] [Google Scholar]
- Lin JM, Kilman VL, Keegan K, Paddock B, Emery-Le M, Rosbash M, Allada R. (2002). A role for casein kinase 2α in the Drosophila circadian clock. Nature , 816–820. [DOI] [PubMed] [Google Scholar]
- Losick VP, Fox DT, Spradling AC. (2013). Polyploidization and cell fusion contribute to wound healing in the adult Drosophila epithelium. Curr Biol , 2224–2232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Losick VP, Jun AS, Spradling AC. (2016). Wound-induced polyploidization: regulation by Hippo and JNK signaling and conservation in mammals. PLoS One , e0151251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin P. (1997). Wound healing—aiming for perfect skin regeneration. Science , 75–81. [DOI] [PubMed] [Google Scholar]
- Mattila J, Omelyanchuk L, Kyttala S, Turunen H, Nokkala S. (2005). Role of Jun N-terminal kinase (JNK) signaling in the wound healing and regeneration of a Drosophila melanogaster wing imaginal disc. Int J Dev Biol , 391–399. [DOI] [PubMed] [Google Scholar]
- Mehrotra S, Maqbool SB, Kolpakas A, Murnen K, Calvi BR. (2008). Endocycling cells do not apoptose in response to DNA rereplication genotoxic stress. Genes Dev , 3158–3171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyata Y, Nishida E. (2004). CK2 controls multiple protein kinases by phosphorylating a kinase-targeting molecular chaperone, Cdc37. Mol Cell Biol , 4065–4074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paquette N, Broemer M, Aggarwal K, Chen L, Husson M, Erturk-Hasdemir D, Reichhart JM, Meier P, Silverman N. (2010). Caspase-mediated cleavage, IAP binding, and ubiquitination: linking three mechanisms crucial for Drosophila NF-κB signaling. Mol Cell , 172–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park SH, Lee CW, Lee JH, Park JY, Roshandell M, Brennan CA, Choe KM. (2018). Requirement for and polarized localization of integrin proteins during Drosophila wound closure. Mol Biol Cell , 2137–2147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearl LH. (2005). Hsp90 and Cdc37—a chaperone cancer conspiracy. Curr Opin Genet Dev , 55–61. [DOI] [PubMed] [Google Scholar]
- Polaski S, Whitney L, Barker BW, Stronach B. (2006). Genetic analysis of slipper/mixed lineage kinase reveals requirements in multiple Jun-N-terminal kinase-dependent morphogenetic events during Drosophila development. Genetics , 719–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prince T, Sun L, Matts RL. (2005). Cdk2: a genuine protein kinase client of Hsp90 and Cdc37. Biochemistry , 15287–15295. [DOI] [PubMed] [Google Scholar]
- Rallis A, Moore C, Ng J. (2010). Signal strength and signal duration define two distinct aspects of JNK-regulated axon stability. Dev Biol , 65–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramet M, Lanot R, Zachary D, Manfruelli P. (2002). JNK signaling pathway is required for efficient wound healing in Drosophila. Dev Biol , 145–156. [DOI] [PubMed] [Google Scholar]
- Rios-Barrera LD, Riesgo-Escovar JR. (2013). Regulating cell morphogenesis: the Drosophila Jun N-terminal kinase pathway. Genesis , 147–162. [DOI] [PubMed] [Google Scholar]
- Shaw TJ, Martin P. (2009). Wound repair at a glance. J Cell Sci , 3209–3213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simon MA, Bowtell DD, Dodson GS, Laverty TR, Rubin GM. (1991). Ras1 and a putative guanine nucleotide exchange factor perform crucial steps in signaling by the sevenless protein tyrosine kinase. Cell , 701–716. [DOI] [PubMed] [Google Scholar]
- Sopko R, Foos M, Vinayagam A, Zhai B, Binari R, Hu Y, Randklev S, Perkins LA, Gygi SP, Perrimon N. (2014). Combining genetic perturbations and proteomics to examine kinase-phosphatase networks in Drosophila embryos. Dev Cell , 114–127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepanova L, Leng X, Parker SB, Harper JW. (1996). Mammalian p50Cdc37 is a protein kinase-targeting subunit of Hsp90 that binds and stabilizes Cdk4. Genes Dev , 1491–1502. [DOI] [PubMed] [Google Scholar]
- Swarup S, Pradhan-Sundd T, Verheyen EM. (2015). Genome-wide identification of phospho-regulators of Wnt signaling in Drosophila. Development , 1502–1515. [DOI] [PubMed] [Google Scholar]
- Taipale M, Jarosz DF, Lindquist S. (2010). HSP90 at the hub of protein homeostasis: emerging mechanistic insights. Nat Rev Mol Cell Biol , 515–528. [DOI] [PubMed] [Google Scholar]
- Tsai CR, Wang Y, Galko MJ. (2018). Crawling wounded: molecular genetic insights into wound healing from Drosophila larvae. Int J Dev Biol , 479–489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Workman P, Burrows F, Neckers L, Rosen N. (2007). Drugging the cancer chaperone HSP90: combinatorial therapeutic exploitation of oncogene addiction and tumor stress. Ann NY Acad Sci , 202–216. [DOI] [PubMed] [Google Scholar]
- Zhang B, Mehrotra S, Ng WL, Calvi BR. (2014). Low levels of p53 protein and chromatin silencing of p53 target genes repress apoptosis in Drosophila endocycling cells. PLoS Genet , e1004581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zielke N, Edgar BA, DePamphilis ML. (2013). Endoreplication. Cold Spring Harb Perspect Biol , a012948. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.