Abstract
Colorectal cancer (CRC) is the third most common cancer and the fourth leading cause of cancer deaths worldwide. Integrin α6 is overexpressed in all stages of CRC which makes it a potential diagnostic biomarker for CRC. Previously, we identified an integrin α6-targeted peptide CRWYDENAC (dubbed RWY) using phage display technology and employed it for nasopharyngeal carcinoma specific nanotherapeutics. In this study, we developed a radiotracer, 18F-RWY, based on this integrin α6-targeted RWY peptide for positron emission tomography (PET) imaging of CRC. Integrin α6 was overexpressed on several CRC cells including HT29 cells where the biotin-labeled RWY peptide colocalized with integrin α6. 18F-RWY PET imaging was performed on subcutaneous, chemically induced, and genetically engineered CRC mice. 18F-RWY generated high PET signals in subcutaneous HT29 tumors, and the tumor uptake of 18F-RWY was reduced by a blocking study using nonradio-labeled RWY. Moreover, 18F-RWY PET imaging enabled detection of CRC in chemically induced and genetically engineered CRC mice. The overexpression of integrin α6 in tumor tissues isolated from chemically induced and genetically engineered CRC mice was confirmed. These results demonstrate the potential clinical application of 18F-RWY for PET imaging of CRC.
Introduction
Colorectal cancer (CRC), which accounted for 1.7 million new cases and more than 800 000 deaths in 2018, is the third most common cancer worldwide and the fourth leading cause of cancer deaths, with its incidence increasing each year.1,2 Surgical resection is an effective treatment for patients with localized stage CRC, with a 5-year survival rate of up to 90% being observed.2 Early diagnosis of CRC is critical for the choice of follow-up treatment and prognosis of patients. Currently, the gold standard for the diagnosis of CRC relies on the pathological report of the biopsy samples taken during endoscopy. Complete colonoscopy or computed tomography (CT) colonoscopy is necessary for the staging of CRC. As a noninvasive method of tumor detection, positron emission tomography/CT (PET/CT) is crucial for the performance of presurgical staging and identification of metastatic or recurrent lesions.3 PET/CT has been shown to alter the therapy of nearly one-third of patients with advanced primary rectal cancer.4
18F-Fluorodeoxyglucose (18F-FDG) is the most widely clinically used PET radiotracer for the detection of cancers. The growth and proliferation of malignant cells are active and accelerated, and glucose utilization and glycolysis are significantly increased. Therefore, the concentration of deoxyglucose in tumor cells is significantly higher than that in normal cells.5 As an important organ for nutrient absorption, the colon is adjacent to the small intestine, where glucose uptake is active, and physiological glucose uptake in the gastrointestinal tracts may lead to misinterpretation of PET/CT results of 18F-FDG.4 In addition, some organs with high glucose metabolism, such as the brain and the heart, often show a high standardized uptake value (SUV) in 18F-FDG based PET images, which makes it difficult to distinguish the metastases of these organs.6
Integrin α6 is a subtype of the integrin family, which is expressed on the surface of cells and acts as a regulator of a variety of cellular functions that are critical to the occurrence, development, and metastasis of solid tumors.7 Several studies have reported the prognostic significance of integrin α6 in several tumors including hepatocellular carcinoma,8 breast cancer,9 and bladder cancer.10 Previously, we identified a tumor-targeted peptide CRWYDENAC (dubbed RWY) using phage display technology and further confirmed its target as integrin α6.11 Many studies have suggested that integrin α6 is overexpressed in CRC, and its overexpression is associated with the development of more aggressive and metastatic phenotypes in CRC cells.12,13 The notably high expression level of integrin α6 in CRC makes it a potential target for molecular imaging of CRC. In this study, we develop an integrin α6-targeted RWY-based PET radiotracer 18F-ALF-NOTA-RWY (dubbed 18F-RWY for short) for PET imaging of CRC in subcutaneous, genetically engineered and chemically induced CRC mice.
Results
Binding of RWY to CRC Cells
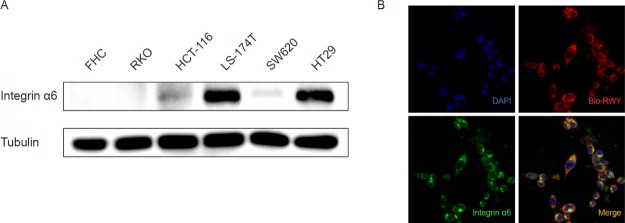
The expression levels of integrin α6 were examined on several CRC cells including RKO, HCT116, LS-174T, SW620 and HT29, and normal colorectal epithelial FHC cells. High expression of integrin α6 was observed in LS-174T and HT29 cells; medium expression in HCT116 and SW620 cells; and low expression in FHC and RKO cells (Figure 1A). The integrin α6-overexpressing CRC cell HT29 was used in our following studies. Confocal imaging showed that biotin-labeled RWY peptide colocalized with integrin α6 in HT29 cells (Figure 1B).
Figure 1.
Integrin α6-target peptide binds to CRC cells. (A) Expression of integrin α6 in the normal intestinal epithelial cell line (FHC) and CRC cell lines (HT29, LS-174T, RKO, HCT116, and SW620) were examined by western blotting. (B) Cellular fluorescent imaging of HT29 cells incubated with biotin-labeled RWY peptide at 37 °C for 2 h. Blue, DAPI; red, bio-RWY; green, integrin α6.
Development of an Integrin α6-Targeted PET Radiotracer
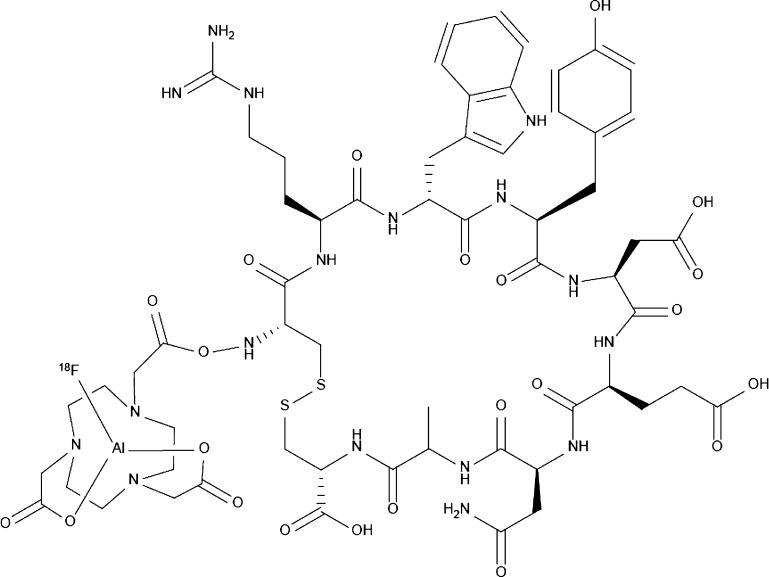
We synthesized a 1,4,7-triazacyclononanetriacetic acid (NOTA)-conjugated RWY peptide that was radiolabeled with radionuclide fluorine-18 (18F) to form a PET radiotracer 18F-ALF-NOTA-RWY (dubbed 18F-RWY for short) (Figure 2), according to a previous report.14 The stability of 18F-RWY was determined by high-performance liquid chromatography (HPLC). A unique radioactive peak was observed by HPLC (Figure S1), indicating that 18F-RWY was stable.
Figure 2.
Chemical structure of 18F-AlF-NOTA-RWY (18F-RWY). The 1,4,7-triazacyclononanetriacetic acid (NOTA)-conjugated RWY peptide was radiolabeled with radionuclide fluorine-18 (18F).
Biodistribution in a Subcutaneous CRC Mouse Model
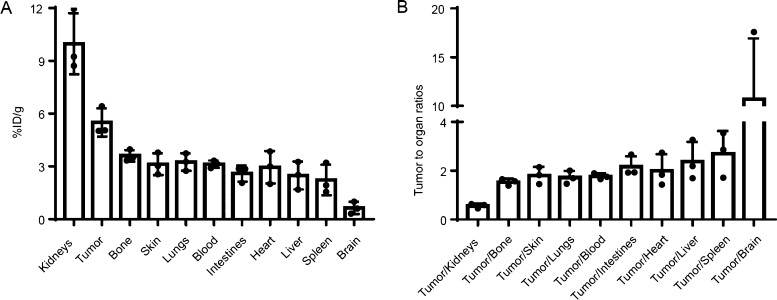
Mice-bearing HT29 subcutaneous xenograft tumors were intravenously injected with 3.7 MBq (100 μCi) of 18F-RWY. Mice were sacrificed 60 min after intravenous injection. Tumor tissues and normal organs were harvested, weighed, and measured by a γ counter. Quantification indicated the highest radioactive accumulation of 18F-RWY in the kidneys, suggesting it was predominantly cleared by the urinary system (Figure 3A). Tissue uptake in tumor tissues was higher than in other normal tissues and organs (including the bone, skin, lungs, blood, intestines, heart, liver, spleen, and brain) except for the kidneys (Figure 3B).
Figure 3.
In vivo biodistribution of 18F-RWY in subcutaneous HT29 tumor-bearing mice. (A) A γ counter was used to quantify the 18F-RWY uptakes in tumor tissues and normal tissues and organs (n = 3). (B) Tumor-to-organ ratios of 18F-RWY at 60 min postinjection (n = 3).
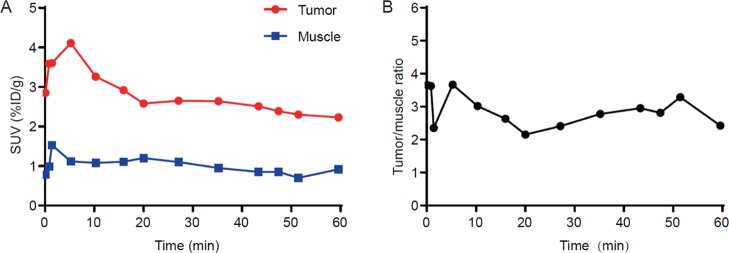
To analyze the dynamic distribution of 18F-RWY in vivo, a dynamic PET/CT imaging was performed. The time activity curves for tumor and muscle were calculated. As shown in (Figure 4A), the accumulation of 18F-RWY in the tumor reached peak in approximately 5 min and remained stable during 60 min postinjection. Similar trends were observed in muscle. Comparatively, the radiotracer uptake in the tumor was approximately three folds higher than that observed in muscle during 60 min postinjection (Figure 4B).
Figure 4.
Dynamic PET imaging in subcutaneous HT29 tumor-bearing mice. (A) Dynamic PET imaging was performed for a period of 60 min (n = 3). Representative time activity of 18F-RWY uptake in tumor and muscle tissue. (B) The representative tumor-to-muscle ratio was calculated (n = 3).
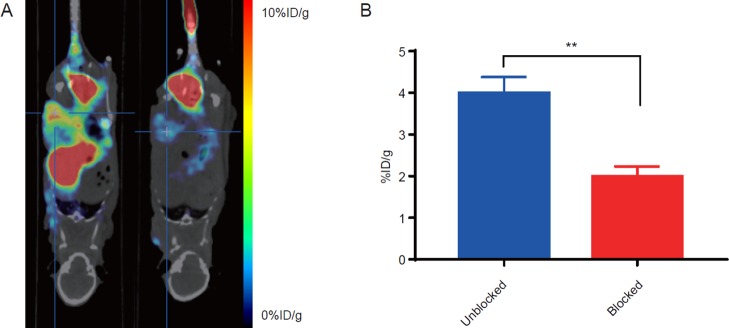
To further confirm the binding specificity of 18F-RWY, an in vivo blocking assay was performed by tail vein injection of 1 mg of nonradiolabeled RWY peptide before injection of 18F-RWY (Figure 5A). The PET signal in the tumor was dramatically reduced from 3.99 ± 0.22 to 1.99 ± 0.14 % ID/g (Figure 5B), suggesting that the tumor accumulation of 18F-RWY was mediated by the RWY peptide.
Figure 5.
PET/CT imaging of the blocking study in subcutaneous HT29 tumor-bearing mice. (A) Representative PET/CT images of HT29 tumor-bearing mice after injection of 18F-RWY only (left) or accompanied with a blocking dose of 1 mg of nonradiolabeled RWY peptide (right). (B) Quantification of radiotracer uptake in the tumor. The statistical analysis was performed using Student’s t-test, n = 3, **p < 0.01.
PET/CT Imaging on Chemically Induced and Genetically Engineered CRC mice
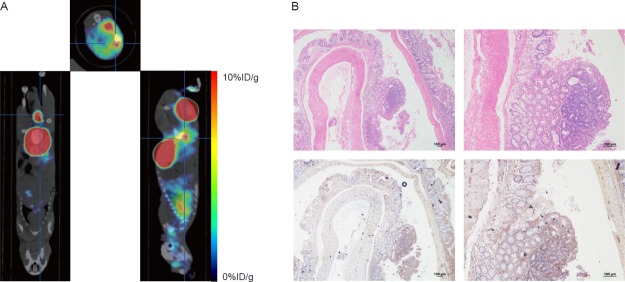
In chemically induced CRC mice, 18F-RWY showed high tumor uptake and low background uptake. Tumor uptake of 18F-RWY was quantified as 5.87 ± 0.99 % ID/g (Figure 6A). In the 3D model image, the 18F-RWY displayed excellent tumor enrichment (Figure S2). To further confirm the presence of the tumor, mice were sacrificed 24 h after PET/CT imaging until the radioactivity decayed to negligible amounts. The colon and rectum were collected for observations, including H&E staining as well as immunohistochemistry. Gross and microscopic observations showed that the tumor was successfully induced in the colon, which was also confirmed by H&E staining results. High integrin α6 expression in the tumor tissues was observed in the image of immunohistochemistry compared with that in the intestine adjacent to the tumor (Figure 6B).
Figure 6.
PET/CT imaging with 18F-RWY in chemically induced CRC mice. (A) Representative PET/CT imaging with 18F-RWY in a chemically induced CRC mouse. (B) H&E and immunohistochemistry staining of tumor isolated from a chemically induced CRC mouse. Integrin α6 expression was analyzed using immunohistochemistry. Scale bar, 100 μm.
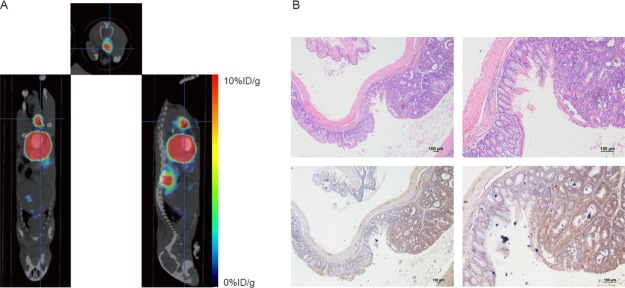
In an Apcmin/+ genetically engineered CRC mouse, representative PET/CT and maximum intensity projection imaging displayed an excellent targeting effect of the 18F-RWY. The maximum value of calculated tumor uptake was 5.74 ± 0.71 % ID/g (Figure 7A). In the 3D model image, the 18F-RWY also showed excellent tumor targeting (Figure S3). Compared with 18F-RWY, 18F-FDG did not show ideal tumor enrichment. Instead, it was mainly enriched in some nonspecific sites, such as the heart, the brain, and the subcutaneous adipose tissue (Figure S4). H&E staining confirmed the presence of the tumor in the colon. Immunohistochemistry showed that tumor tissues showed relatively high expression of integrin α6 compared to the intestine tissues adjacent to the tumor (Figure 7B).
Figure 7.
PET/CT imaging with 18F-RWY in genetically engineered CRC mice. (A) Representative PET/CT imaging with 18F-RWY in an Apcmin/+ genetically engineered CRC mouse. (B) H&E and immunohistochemistry staining of a tumor resected from an Apcmin/+ genetically engineered CRC mouse. Integrin α6 expression was analyzed using immunohistochemistry. Scale bar, 100 μm.
Discussion
Previously, we identified a tumor-targeted peptide RWY using phage display technology and further confirmed its target as integrin α6.11 Herein, we developed an integrin α6-targeted PET radiotracer, 18F-RWY, and focused its application on CRC. The integrin α6-overexpressing CRC cells HT29 were used to establish subcutaneous CRC tumors. We confirmed that integrin α6 is the primary cellular target of the RWY peptide, and the RWY peptide binds to integrin α6 CRC cells in vitro. In the blocking assay, we further confirmed the specificity of 18F-RWY binding to the subcutaneous tumor of CRC, thereby suggesting that 18F-RWY can effectively and specifically accumulate in CRC tumors. We further validated the feasibility of 18F-RWY for the detection of CRC in chemically induced and genetically engineered CRC mice as well. We finally confirmed the overexpression of integrin α6 in tumor tissues isolated from chemically induced and genetically engineered CRC mice.
Diagnosis and monitoring of CRC rely heavily on colonoscopy. Nevertheless, as an invasive technique, colonoscopy requires a long period of bowel preparation and anesthesia to reduce pain, which is intolerable for some patients.15 Colonoscopy, on the other hand, is highly dependent on the experience of the operator, and detection of neoplasms in the occult site is often difficult because of blind spots.16 Approximately 26% of neoplastic polyps was missed during single colonoscopy, according to a meta-analysis.17 For verdant operators, unskilled manipulation is likely to lead to bleeding, perforation, and other severe complications. Therefore, more noninvasive approaches are still urgently needed to be explored for better diagnosis of CRC.
With the introduction and rapid development of precision medicine, it is necessary to explore tumor biomarkers that can reflect changes on the tumor cell and subcell level before changes in the anatomic structure.18,19 Because of the noninvasiveness, high sensitivity, and lack of blind spots, the PET imaging modality is widely accepted in oncological imaging. PET imaging based on tumor-targeting molecules has been rapidly developed and has achieved good results in many cancers.1918F-FDG, a PET radiotracer based on glucose metabolism, is the most widely used for oncological imaging. However, because of the abnormal absorbability of inflammatory lesions, metabolism-based imaging often confuses malignant lesions with chronic inflammatory lesions.20 Elevation of glucose metabolism is common in inflammatory lesions such as ulcerative colitis and Crohn’s disease.21 Even in the normal colon and rectum, there is a certain amount of physiological glucose uptake, which hampers the application of 18F-FDG as a PET radiotracer in colorectal oncological imaging.4 In addition, it is also difficult to distinguish the metastases of organs with high glucose metabolism, such as the brain.6
In this study, we provided a noninvasive oncological imaging modality for molecular imaging of CRC via targeting to integrin α6. Early CRC with a localized stage can be successfully treated before distant metastasis occurs.22 The ability to detect early colorectal lesions at a curable stage is the most important criterion for CRC screening. The overexpression of integrin α6 in a majority of CRCs has been reported by several publications.23−25 Elevation of this molecule occurs long before the changes in the anatomical structure when the tumor is visible to the naked eye. Most notably, integrin α6, which plays an important role in proliferation of tumor cells, was found to be upregulated in more than 80% of CRCs in transcription levels.24,25 The extremely high positive ratio of integrin α6 in CRC makes it a good molecular imaging target for the sensitive detection of CRC. Moreover, Beaulieu et al.26 found that the integrin α6 subunit transcript (ITGA6) levels were significantly increased at all stages of CRC and could serve as a part of a stool assay to detect early colorectal lesions. The overexpression of integrin α6 in the early stage of CRC enlightened us that PET imaging with 18F-RWY may serve as a new approach for the detection of early CRC. On the other hand, PET/CT can determine whether there is early metastasis, thereby eliminating unnecessary examination in clinical practice.
Nevertheless, there are still several limitations of our study. First, as we can see in the results, the uptakes of the PET radiotracer 18F-RWY in tumor and other tissues reach a peak in only a few minutes, and the rapid clearance of 18F-RWY in vivo may reduce the sensitivity of CRC detection. The addition of modified chemical groups to alter its metabolism may prolong its half-life in vivo. Second, the binding affinity between RWY and integrin α6 still need improvement. The affinity analysis between the synthesized integrin α6 protein and RWY by surface plasmon resonance (SPR) is a direct method. One of our on-going studies is to improve the binding affinity via optimizing the peptide structure. Third, endoscopy is still the most commonly used method for the diagnosis of CRC currently. The gold standard for the diagnosis of CRC still depends on the pathological examination of tissue collected by endoscopy. Moreover, the high price of PET/CT imaging hampers its application as an early screening approach in clinical practice. However, from another point of view, this imaging will be beneficial for patients who need PET/CT examination during the perioperative period because the performance of PET/CT with our new radiopharmaceuticals can directly be used to diagnose and evaluate metastasis without the requirement of endoscopic examination anymore. Only a limited number of mice has been used for the detection of CRC. A series of clinical trials are needed to assess the effectiveness of this method in humans before it can be used clinically.
Conclusions
Integrin α6 was overexpressed on several CRC cells and tumor tissues isolated from chemically induced and genetically engineered CRC mice. Integrin α6 was the primary cellular target of RWY peptide, and the RWY peptide binds to CRC cells in vitro and in vivo. The integrin α6-targeted peptide-based radiotracer 18F-RWY is feasible for PET imaging of CRC in subcutaneous, chemically induced, and genetically engineered CRC mouse models, suggesting its future clinical applications.
Experimental Section
Cells and Animal Models
All the cell lines used in the experiment were obtained from the American Type Culture Collection (ATCC). Normal intestinal epithelial cell line FHC was cultured in Dulbecco’s modified Eagle’s medium: F-12 medium (ATCC 30-2006), and human colorectal adenocarcinoma cell line (HT29, LS-174T, RKO, HCT116, and SW620) was cultured in RPMI-1640 medium supplemented with 10% (v/v) fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The cells were maintained in an incubator with a humid atmosphere of 5% CO2 at 37 °C. All mice were purchased from Vital River, Charles River Laboratories China (Beijing, China). The animal experiments were reviewed and approved by the animal welfare and ethics committees of Sun Yat-sen University Cancer Center. Approximately, 1 × 107 cells in a suspension with 20% Matrigel (Corning 354234, USA) were subcutaneously injected into the lateral thigh of BALB/c nude mice to create a subcutaneous tumor. To obtain a chemical-induced CRC mouse model, azoxymethane and dextran sulfate sodium were purchased from Sigma (St. Louis, MO) and administrated to C57BL6 mice based on the previous literature.27 Mice were scanned by PET 80 days after drugs’ induction. Apcmin/+ mice were purchased from The Jackson Laboratory (Bar Harbor, ME) and maintained under specific pathogen-free conditions in the Guangdong Pharmaceutical University Animal Experimental Center. Genotyping was tested by PCR with toe DNA using primers as follows: P1, 5′-TTCTGAGAAAGACAGAAGTTA-3′, together with P2, 5′-TTCCACTTTGGCATAAGGC-3′, was used to detect the mutant Apc allele (313 bp), which is only present in heterozygous Apcmin/+ mice. P3, 5′-GCCATCCCTTCACGTTAG-3′, together with the Apc-common primer, was used to detect the wild-type allele (619 bp).28
Biodistribution
To investigate the biodistribution of 18F-RWY in vivo, the mice bearing HT29 were euthanized 60 min after intravenous injection of 18F-RWY. Tumor and normal organs were collected, and the radioactivity was measured by a γ counter (Wallac Wizard 1470, PerkinElmer Inc.). The organs were weighed to calculate their mean organ distribution. Radioactivity was expressed as a percentage of the injected dose per gram [% ID/g, mean ± standard deviation (SD), n = 3/group].
PET/CT Imaging
The peptide powder previously freeze-dried was dissolved in saline and added to with 5 μL of acetic acid, 324 μL of ethanol and 370 MBq of fluoride ion (approximately 10 mCi). The mixture was boiled for 10 min and cooled to room temperature. The 18F-RWY was captured by a C18 plus column (Waters, Sep-Pak, USA) because the free fluoride ion can pass through the column. After being washed with saline twice, the peptide was eluted with 400 μL of ethanol, and the obtained solution was blown in nitrogen until the liquid was almost evaporated. A volume of 200 μL saline was added after the radioactivity was measured. Mice were anesthetized with 2.5% tribromoethanol (T48402, Sigma-Aldrich, Germany) and intravenously injected with approximately 3.7 MBq (100 μCi) of 18F-RWY or 18F-FDG. PET/CT was performed after 1 h in vivo circulation with the imaging agent. To analyze the enrichment of the tracer in the tumor, regions of interest were drawn on tumors in three dimensions, and the maximum uptake value was calculated. The tumor and tissue densities were assumed as 1 g/cm3, and its uptake is presented as the % ID/g and was calculated by measuring the tumor and tissue radioactivity. To perform dynamic imaging, the images were acquired as follows: 1 × 5, 1 × 25, 9 × 30, 5 × 60, 5 × 120, and 9 × 240 s. Time activity curves were obtained according to the pharmacokinetics of 18F-RWY in the tumors and other organs. The uptake of the tracer was quantified as SUV.
Immunohistochemistry
Tumors from mouse models were carefully dissected, fixed with formalin, and embedded with paraffin. Paraffin blocks were cut into 3 mm sections and some were stained with hematoxylin and eosin (H&E). The remaining sections were deparaffinized in xylene and dehydrated by serial immersion in 100, 90, 80, and 70% ethanol and distilled water. Antigen retrieval was performed under high pressure with boiling citrate buffer (10 mM, pH 9.0) for 3 min. All samples were blocked with 5% bovine serum albumin and incubated with anti-integrin α6 antibody (Abcam, ab181551) at a dilution of 1:150 overnight at 4 °C. After washing with PBST three times, the samples were incubated with an HRP-conjugated rabbit anti-mouse monoclonal secondary antibody at a dilution of 1:200 at 25 °C for 30 min. Finally, the chromogenic reaction was performed using the DAB Kit (Zhongshan Jinqiao, ZLI-9017, China). Images were visualized and captured by a microscope (Nikon Eclipse, Japan) at 10× and 20× magnification.
Immunofluorescence
Approximately 1 × 105 HT29 cells were plated on coverslips and added to 80 μM biotin-labeled peptide. After a 4 h incubation at 37 °C, the coverslips were washed with PBST five times, fixed with 4% paraformaldehyde, incubated with PBS containing 0.25% Triton X-100 (Sigma-Aldrich, Germany), and blocked with 5% BSA for half an hour. Antibody incubation was performed with anti-integrin α6 antibody (Abcam, ab181551) at a dilution of 1:1000 overnight at 4 °C. To detect the fluorescence, the samples were incubated with both streptavidin-Cy3 (Thermo Fisher 434315, USA) and goat anti-mouse Alexa Fluor 488 secondary antibody (Abcam, ab150113) at a dilution of 1:1000 in 1% BSA for 1 h at room temperature in the dark. The samples were incubated with 1 μg/mL DAPI, and the coverslips were mounted with ProLong Gold antifade (Invitrogen P26930, USA). Fluorescence images were visualized and captured by a confocal microscopy confocal laser-scanning system (Olympus FV1000, Japan) at 40× and 100× magnification. Colocalization was analyzed with ImageJ (http://rsbweb.nih.gov/ij/) and the colocalization finder plug-in.
Data Analysis and Statistics
Statistical analyses were performed using Prism 7.0 (GraphPad, San Diego, USA). All data were presented as the mean ± SD. The P-values were tested for significance using the two-sided Student t-test. P-values less than 0.05 were considered to be statistically significant (*p < 0.05, **p < 0.01).
Acknowledgments
This work was supported by grants from the National Key R&D Program of China (2017YFA0505600 and 2016YFA0502100), the National Natural Science Foundation of China (NSFC) (projects 81602364, 81621004, 81520108022, 81572403) and the Science and Technology Project of Guangdong Province (2014B020210002 and 2017A020211010), the Health and Medical Collaborative Innovation Project of Guangzhou City, China (201400000001 and 20150802024), and the Sci-Tech Project Foundation of Guangzhou City (201607020038 and 201803040015).
Supporting Information Available
The Supporting Information is available free of charge on the ACS Publications website at DOI: 10.1021/acsomega.9b01920.
Stability in vitro of 18F-RWY analyzed by HPLC, 3D model PET/CT image with 18F-RWY in a chemically induced CRC mouse, 3D model PET/CT image with 18F-RWY in a genetically engineered CRC mouse, and PET/CT imaging with 18F-FDG in a genetically engineered CRC mouse (PDF)
Author Contributions
∥ Y.-T.X., C.Z., and J.-C.Y. contributed equally to this work.
The authors declare no competing financial interest.
Notes
The authenticity of this article has been validated by uploading the key raw data onto the Research Data Deposit public platform (www.researchdata.org.cn), with the approval RDD number as RDDB2019000585.
Supplementary Material
References
- Bray F.; Ferlay J.; Soerjomataram I.; Siegel R. L.; Torre L. A.; Jemal A. Global Cancer Statistics 2018: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. Ca-Cancer J. Clin. 2018, 68, 394–424. 10.3322/caac.21492. [DOI] [PubMed] [Google Scholar]
- Brenner H.; Kloor M.; Pox C. P. Colorectal Cancer. Lancet 2014, 383, 1490–1502. 10.1016/s0140-6736(13)61649-9. [DOI] [PubMed] [Google Scholar]
- Higashikawa K.; Akada N.; Yagi K.; Watanabe K.; Kamino S.; Kanayama Y.; Hiromura M.; Enomoto S. Exploration of Target Molecules for Molecular Imaging of Inflammatory Bowel Disease. Biochem. Biophys. Res. Commun. 2011, 410, 416–421. 10.1016/j.bbrc.2011.05.146. [DOI] [PubMed] [Google Scholar]
- Heriot A. G.; Hicks R. J.; Drummond E. G. P.; Keck J.; Mackay J.; Chen F.; Kalff V. Does Positron Emission Tomography Change Management in Primary Rectal Cancer? A Prospective Assessment. Dis. Colon Rectum 2004, 47, 451–458. 10.1007/s10350-003-0089-3. [DOI] [PubMed] [Google Scholar]
- Turker N. S.; Heidari P.; Kucherlapati R.; Kucherlapati M.; Mahmood U. An EGFR Targeted PET Imaging Probe for the Detection of Colonic Adenocarcinomas in the Setting of Colitis. Theranostics 2014, 4, 893–903. 10.7150/thno.9425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purandare N. C.; Puranik A.; Shah S.; Agrawal A.; Gupta T.; Moiyadi A.; Shetty P.; Shridhar E.; Jalali R.; Rangarajan V. Common malignant brain tumors. Nucl. Med. Commun. 2017, 38, 1109–1116. 10.1097/mnm.0000000000000753. [DOI] [PubMed] [Google Scholar]
- Desgrosellier J. S.; Cheresh D. A. Integrins in Cancer: Biological Implications and Therapeutic Opportunities. Nat. Rev. Cancer 2010, 10, 9–22. 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Begum N. A.; Mori M.; Matsumata T.; Takenaka K.; Sugimachi K.; Barnard G. F. Differential Display and Integrin Alpha 6 Messenger RNA Overexpression in Hepatocellular Carcinoma. Hepatology 1995, 22, 1447–1455. 10.1002/hep.1840220518. [DOI] [PubMed] [Google Scholar]
- Wang Y.; Shenouda S.; Baranwal S.; Rathinam R.; Jain P.; Bao L.; Hazari S.; Dash S.; Alahari S. K. Integrin Subunits Alpha5 and Alpha6 Regulate Cell Cycle by Modulating the Chk1 and Rb/E2F Pathways to Affect Breast Cancer Metastasis. Mol. Cancer 2011, 10, 84. 10.1186/1476-4598-10-84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman H. B.; Lee C.; Bromberg J.; Liebert M. Expression of the Alpha6beta4 Integrin Provides Prognostic Information in Bladder Cancer. Oncol. Rep. 2000, 7, 13–19. 10.3892/or.7.1.13. [DOI] [PubMed] [Google Scholar]
- Feng G.-K.; Zhang M. Q.; Wang H. X.; Cai J.; Chen S. P.; Wang Q.; Gong J.; Leong K. W.; Wang J.; Zhang X.; Zeng M. S. Identification of an Integrin α6-Targeted Peptide for Nasopharyngeal Carcinoma-Specific Nanotherapeutics. Adv. Ther. 2019, 2, 1900018. 10.1002/adtp.201900018. [DOI] [Google Scholar]
- Ramos D. M.; Dang D.; Sadler S. The Role of the Integrin Alpha v Beta6 in Regulating the Epithelial to Mesenchymal Transition in Oral Cancer. Anticancer Res. 2009, 29, 125–130. 10.1159/000151428. [DOI] [PubMed] [Google Scholar]
- Agrez M.; Chen A.; Cone R. I.; Pytela R.; Sheppard D. The Alpha v Beta 6 Integrin Promotes Proliferation of Colon Carcinoma Cells through a Unique Region of the Beta 6 Cytoplasmic Domain. J. Cell Biol. 1994, 127, 547–556. 10.1083/jcb.127.2.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Z.; Niu G.; Wang F.; Chen X. 68Ga-labeled NOTA-RGD-BBN peptide for dual integrin and GRPR-targeted tumor imaging. Eur. J. Nucl. Med. Mol. Imaging 2009, 36, 1483–1494. 10.1007/s00259-009-1123-z. [DOI] [PubMed] [Google Scholar]
- Dmochowska N.; Wardill H. R.; Hughes P. A. Advances in Imaging Specific Mediators of Inflammatory Bowel Disease. Int. J. Mol. Sci. 2018, 19, 2471. 10.3390/ijms19092471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Misawa M.; Kudo S.-E.; Mori Y.; Cho T.; Kataoka S.; Yamauchi A.; Ogawa Y.; Maeda Y.; Takeda K.; Ichimasa K.; Nakamura H.; Yagawa Y.; Toyoshima N.; Ogata N.; Kudo T.; Hisayuki T.; Hayashi T.; Wakamura K.; Baba T.; Ishida F.; Itoh H.; Roth H.; Oda M.; Mori K. Artificial Intelligence-Assisted Polyp Detection for Colonoscopy: Initial Experience. Gastroenterology 2018, 154, 2027–2029.e3. 10.1053/j.gastro.2018.04.003. [DOI] [PubMed] [Google Scholar]
- van Rijn J. C.; Reitsma J. B.; Stoker J.; Bossuyt P. M.; van Deventer S. J.; Dekker E. Polyp Miss Rate Determined by Tandem Colonoscopy: A Systematic Review. Am. J. Gastroenterol. 2006, 101, 343–350. 10.1111/j.1572-0241.2006.00390.x. [DOI] [PubMed] [Google Scholar]
- Lee S. Y.; Jeon S. I.; Jung S.; Chung I. J.; Ahn C.-H. Targeted Multimodal Imaging Modalities. Adv. Drug Delivery Rev. 2014, 76, 60–78. 10.1016/j.addr.2014.07.009. [DOI] [PubMed] [Google Scholar]
- Wang Q.; Li S.-B.; Zhao Y.-Y.; Dai D.-N.; Du H.; Lin Y.-Z.; Ye J.-C.; Zhao J.; Xiao W.; Mei Y.; Xiao Y.-T.; Liu S.-C.; Li Y.; Xia Y.-F.; Song E.-W.; Tang G.-H.; Zhang W.-G.; Li Z.-J.; Zheng X.-B.; Cao D.-H.; Li M.-Z.; Zhong Q.; Chen Z.-P.; Qian C.-N.; Fan W.; Feng G.-K.; Zeng M.-S. Identification of a sodium pump Na + /K + ATPase α1-targeted peptide for PET imaging of breast cancer. J. Controlled Release 2018, 281, 178–188. 10.1016/j.jconrel.2018.05.019. [DOI] [PubMed] [Google Scholar]
- Strauss L. G. Fluorine-18 Deoxyglucose and False-Positive Results: A Major Problem in the Diagnostics of Oncological Patients. Eur. J. Nucl. Med. 1996, 23, 1409–1415. 10.1007/bf01367602. [DOI] [PubMed] [Google Scholar]
- Halpenny D. F.; Burke J. P.; Lawlor G. O.; OʼConnell M. Role of PET and Combination PET/CT in the Evaluation of Patients with Inflammatory Bowel Disease. Inflammatory Bowel Dis. 2009, 15, 951–958. 10.1002/ibd.20817. [DOI] [PubMed] [Google Scholar]
- Maratt J. K.; Saini S. D. Colorectal cancer screening in the 21st century: where do we go from here?. Am J Manag Care 2015, 21, e447–9. [PubMed] [Google Scholar]
- Ni H.; Dydensborg A. B.; Herring F. E.; Basora N.; Gagné D.; Vachon P. H.; Beaulieu J.-F. Upregulation of a functional form of the β4 integrin subunit in colorectal cancers correlates with c-Myc expression. Oncogene 2005, 24, 6820–6829. 10.1038/sj.onc.1208848. [DOI] [PubMed] [Google Scholar]
- Dydensborg A. B.; Teller I. C.; Groulx J.-F.; Basora N.; Pare F.; Herring E.; Gauthier R.; Jean D.; Beaulieu J.-F. Integrin Alpha6Bbeta4 Inhibits Colon Cancer Cell Proliferation and C-Myc Activity. BMC Cancer 2009, 9, 223. 10.1186/1471-2407-9-223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groulx J.-F.; Giroux V.; Beauséjour M.; Boudjadi S.; Basora N.; Carrier J. C.; Beaulieu J.-F. Integrin α6A splice variant regulates proliferation and the Wnt/β-catenin pathway in human colorectal cancer cells. Carcinogenesis 2014, 35, 1217–1227. 10.1093/carcin/bgu006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beaulieu J.-F.; Herring E.; Kanaoka S.; Tremblay É. Use of Integrin Alpha 6 Transcripts in a Stool MRNA Assay for the Detection of Colorectal Cancers at Curable Stages. Oncotarget 2016, 7, 14684–14692. 10.18632/oncotarget.7407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neufert C.; Becker C.; Neurath M. F. An inducible mouse model of colon carcinogenesis for the analysis of sporadic and inflammation-driven tumor progression. Nat. Protoc. 2007, 2, 1998–2004. 10.1038/nprot.2007.279. [DOI] [PubMed] [Google Scholar]
- Ju J.; Hong J.; Zhou J.-n.; Pan Z.; Bose M.; Liao J.; Yang G.-y.; Liu Y. Y.; Hou Z.; Lin Y.; Ma J.; Shih W. J.; Carothers A. M.; Yang C. S. Inhibition of Intestinal Tumorigenesis inApcmin/+Mice by (−)-Epigallocatechin-3-Gallate, the Major Catechin in Green Tea. Cancer Res. 2005, 65, 10623–10631. 10.1158/0008-5472.can-05-1949. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.