Abstract
Dietary fat modulates neuronal health and contributes to age-related nervous system disorders. However, the complex interaction between dietary fat and supplementation and its consequences on neurotoxic pathophysiology has been sparsely explored. The indigenous Alaskan bog blueberry (BB), Vaccinum uliginosum, is known to have anti-inflammatory properties, mostly attributed to its rich polyphenolic content. Here, we evaluate the interplay between dietary fat and BB supplementation on sub-chronic manganese (Mn) exposure that inflicts neurotoxicity and behavioral impairments. In both low-fat and normal-fat diets, BB supplementation attenuated the behavioral and the molecular hallmarks of Mn-induced neurotoxicity. On the contrary, a high-fat diet was found to exacerbate these Mn-induced pathological features. Furthermore, BB supplementation failed to recover the behavioral deficits in mice subjected to a high fat diet in Mn-treated mice. Overall, our results demonstrate the importance of including a dietary regimen comprised of polyphenolic rich supplements with low-fat content in combating age-related neurodegenerative disorders.
Keywords: Blueberry, Manganese, High-fat diet, Low-fat diet, Neurotoxicity
1. Introduction
Manganese (Mn) is an essential trace metal which governs physiological processes that support healthy growth, development and brain function (Kwakye, Paoliello, Mukhopadhyay, Bowman, & Aschner, 2015). Particularly, Mn is essential for maintaining redox status, energy balance and metabolism of key biomolecules, including proteins, lipids and carbohydrates (Kwakye et al., 2015). Additionally, Mn functions as a cofactor for crucial enzymes such as pyruvate carboxylase, arginase and Mn-superoxide dismutase, which are required for synthesis of neurotransmitters as well as functioning of neuronal and glial cells in the brain (Erikson & Aschner, 2003). However, excessive and chronic exposure to Mn results in pathological alterations in the brain, particularly in the striatal pallidum and substantia nigra (SN) accompanied by loss of dopaminergic neurons (Liu, Sullivan, Madl, Legare, & Tjalkens, 2006). Mn induced toxicity and degeneration includes motor dysfunctions similar to those observed in Parkinson’s disease (PD) (Stanwood et al., 2009). The symptoms include gait imbalance, rigidity, tremors, dystonia, and bradykinesia (Racette et al., 2017). Studies have elucidated the role of neuroinflammatory factors, such as nitric oxide synthase 2 (iNOS), that precipitate neuronal injury due to Mn exposure via microglial activation (Streifel, Moreno, Hanneman, Legare, & Tjalkens, 2012). Additionally, in vitro studies have demonstrated that such Mn-induced inflammatory microglial activation can result from interactions with signaling pathways involving nuclear factor kappa B (NF-κB) (Streifel et al., 2012).
Since environmental factors play a critical role in age-related neurodegenerative disorders, it is imperative to examine the concerted action diet and supplementation play in the disease etiology and progression. Previous research indicates that long term consumption of high-fat in the diet has been linked to increased inflammation and oxidative stress in the brain (Carey et al., 2017). A high-fat diet (HFD) leads to increased microglial activation (Baufeld, Osterloh, Prokop, Miller, & Heppner, 2016), which in turn can promote pro-inflammatory responses in the brain resulting in impaired neurogenesis and cognitive function, similar to what is seen in aging (Ganguly & Brenhouse, 2015). Studies also associate a HFD and reduced neurogenesis with decreased levels of brain-derived neurotrophic factors (BDNF) (Carey et al., 2017). A low-fat diet (LFD) on the contrary is known to be protective against cognitive decline in older subjects (Uranga et al., 2010) and other age-related chronic diseases (Heilbronn & Ravussin, 2003). LFDs reduce inflammatory microglial activation in the ageing mouse brain (Yin et al., 2018), upregulate BDNF and enhance neurogenesis (Stranahan et al., 2009).
Blueberries (BB) are rich in antioxidant/anti-inflammatory polyphenolic compounds like anthocyanins and proanthocyanidins (Lohachoompol, Srzednicki, & Craske, 2004). Diets supplemented with such phytochemicals, impact the aging brain (Joseph et al., 1999), modulate stress signaling pathways and reduce microglial activation, a key marker of inflammatory response (Carey et al., 2017). Moreover, diets rich in such natural compounds can attenuate age-related behavioral deficits (Joseph et al., 1999) and enhance neurogenesis by elevating neurotrophic growth factors (Park et al., 2010). Indigenous Alaskan botanicals like bog blueberry (BB), Vaccinum uliginosum, contain higher amounts of polyphenolic compounds relative to Vaccinum sp. grown in temperate regions (Dinstel, Cascio, & Koukel, 2013; Grace, Esposito, Dunlap, & Lila, 2014; Maulik et al., 2018; Scerbak, Vayndorf, Hernandez, McGill, & Taylor, 2016). These endemic species of berries primarily have been beneficial in combating age-related cellular symptoms in C. elegans and in in vitro models (Gustafson, Dunlap, McGill, & Kuhn, 2012; Maulik et al., 2018; Scerbak et al., 2016).
The current study aimed to determine and compare the behavioral effects and underlying molecular mechanisms modulated by consumption of different diets in the presence or absence of BB supplementation and after exposure to a neurodegenerative toxic insult. We investigated the hypothesis that a LFD in the presence of BB will be most neuroprotective among all the diet groups in improving behavioral and molecular markers in this metal-induced neurotoxic mouse model. Moreover, we also hypothesized that HFD will cause decline in behavioral measures and result in increased neurotoxic responses in the animals compared to the ones fed with a LFD and a normal-fat diet (NFD). Based on a previous study (Carey et al., 2017), it was also postulated that BB supplementation will improve the deficits induced by a HFD in this metal-induced neurotoxic mouse model.
2. Materials and methods
This project was conducted as per the University of Alaska Fairbanks Institutional Animal Care and Use Committee approved animal care and experimental procedures (IACUC assurance number 1024315).
2.1. Experimental animals
Three month old C57BL6/J male mice were obtained from Jackson Laboratory (Bar Harbor, ME, USA) and were housed in groups of 4 in a 12:12 L:D cycle at 22 ± 1 °C. Mice were given two weeks to acclimatize before introducing them to the experimental regimen. Body weight of the mice was measured at about 7.5 months of age before the first MnCl2 injection.
2.2. Experimental design
Following the two week acclimatization period, mice were placed on one of the six diets and fed ad libitum for a period of four months (n = 24–26 animals per diet). The diets comprised of NFD, LFD, HFD, NFD+5% BB, LFD+5% BB and HFD+5% BB (Table 1). Alaskan BB were collected from interior Alaska and freeze dried. Freeze dried berries were crushed into a powder and shipped to LabDiet (St Louis, MO) where they were added to food pellets at 5% of the total pellet weight. As previously shown, Alaskan BB contain elevated levels of polyphenolic compounds (phenolic, anthocyanin and flavonoids) compared to blueberries grown commercially or at temperate latitudes (Dinstel et al., 2013; Grace et al., 2014).
Table 1.
Summary of statistical effects of individual factors (diet, BB and MnCl2 treatment) and their interactions on behavioral and molecular measures used in the study.
| Measure | Diet effect | Blueberry effect | Mn treatment effect | Interaction effect |
|---|---|---|---|---|
| Total distance | F2,137 = 49.86, p < 0.001 | F1,137 = 11.76, p < 0.0009 | F1,137 = 32.41, p < 0.0001 | Diet and BB F2,137 = 3.85, p < 0.03 |
| Central entries | F1,141 = 90.72, p < 0.001 | F1,141 = 36.44, p < 0.001 | F1,441 = 55.95, p < 0.001 | Diet and Mn F1,141 = 30.45, p < 0.001 Diet and BB F1,141 = 6.04, p < 0.02 |
| Recognition index | F1,141 = 58.35, p < 0.0001 | F1,141 = 32.42, p < 0.0001 | F1,441 = 30.10, p < 0.0001 | BB and Mn F1,141 = 13.01, p < 0.0005 |
| Rears | F1,141 = 41.75, p < 0.0001 | F1,141 = 6.81, p < 0.02 | F1,441 = 66.65, p < 0.0001 | BB and Mn F1,141 = 6.76, p < 0.02 |
| Ataxia | F1,141 = 54.97, p < 0.0001 | F1,141 = 14.98, p < 0.0003 | F1,441 = 68.04, p < 0.0001 | Diet and Mn F1,141 = 5.15, p < 0.03 BB and Mn F1,141 = 20.83, p < 0.0001 |
| BDNF | F1,52 = 26.10, p < 0.0001 | F1,52 = 12.70, p < 0.0009 | F1,52 = 10.20, p < 0.003 | - |
| IBA1 | - | F1,48 = 6.83, p < 0.02 | F1,48 = 11.28, p < 0.002 | BB and Mn F1,48 = 11.21, p < 0.002 Diet and BB F1,48 = 16.26, p < 0.0001 |
| NOS2 | - | F1,52 = 10.36, p < 0.003 | - | - |
| NfkB | - | F1,52 = 5.35, p < 0.03 | F1,52 = 5.75, p < 0.03 | - |
| Total cells | F1,52 = 41.53, p < 0.0001 | - | - | - |
| TH immunoreactivity | F1,52 = 18.55, p < 0.0001 | - | - | - |
| Body weight | F1,164 = 7.831, p < 0.01 | F5,164 = 315.3, p < 0.001 | NA - Injections not yet given | F5, 164 = 2.442, p < 0.04 |
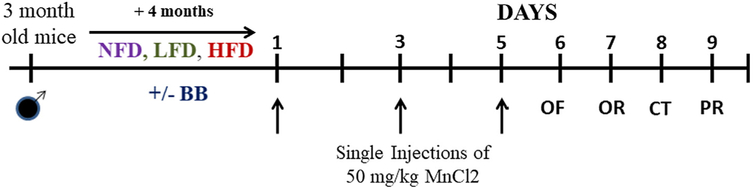
After four months on the diet, the mice were subjected to vehicle and subcutaneous manganese injections (n=13–15 animals per diet and injection group) on every other day for a total of 3 injections (Fig. 1). We used a sub chronic dosage of manganese chloride (MnCl2) that is known to inflict a neurotoxic state which often precedes neurodegeneration (Cordova et al., 2012; Streifel et al., 2012). The dosage was standardized based on previous findings (Dodd, Ward, & Klein, 2005). Twenty-four hours after the last injection, mice were subjected to a series of behavioral tests comprised of the open field test on day 1 to measure anxiety-like behavior and locomotor activity, the novel object recognition test on day 3 to measure short term memory, and the cylinder test on day 5 and parallel rod floor test on day 7 to measure locomotor agility (Fig. 1). All data were collected by experimenters blinded to the study design and hypothesized outcomes.
Fig. 1.
Study design. Three month old male C56/BL7 mice were fed on a Normal-fat diet (NFD), Low-fat diet (LFD) and High-fat diet (HFD) for 4 months in the presence or absence of Alaskan blueberry (BB). All experimental animals were then administered single subcutaneous injections of 0 mg/kg or 50 mg/kg MnCl2 (in saline) on 1st, 3rd and 5th day. Subsequently, the animals were tested for open field (OF), object recognition (OR), cylinder test (CT) and parallel rod floor (PR) test. After the last behavioral assays, the animals were euthanized, and brain samples were collected for the biochemical experiments.
2.3. Drugs
MnCl2 was purchased from Sigma-Aldrich (Catalogue no. 203734) and dissolved in 0.9% sterile physiological saline (pH = 7.4). Sterile saline was used as vehicle for control groups. Animals in the manganese treatment group received subcutaneous injections of 50 mg/kg MnCl2 for three days (day 1, day 3 and day 5). The vehicle group received saline injections on the same days. Injection volume of 0.3 mL per 40 g mouse was adjusted proportionally according to the body weight of the animals. Dosage and treatment regimen were modifications from previously published procedures (Dodd et al., 2005).
2.4. Behavioral tests
2.4.1. Open field
Animals were assessed for anxiety-like behavior and locomotor activity in the open field test (Prut & Belzung, 2003). Animals undergoing testing were transported in home cages and were housed outside the testing room prior to testing. The open field apparatus consisted of an arena (40 cm × 40 cm × 30 cm) with opaque plexiglass walls. Testing was conducted for a 3-min duration (Mitra & Bult-Ito, 2017; Mitra et al., 2016; Mitra, Mucha, Owen, & Bult-Ito, 2017). Animals were individually placed in the center of the field and allowed to explore the arena. The total number of central entries into the central zone was evaluated as an anxiety-like measure (Mitra et al., 2016). Total distance traveled was used to assess locomotor activity (Tatem et al., 2014). All experimental parameters were recorded by the ANYMaze video tracking system (Stoelting Co., Wood Dale, IL, USA). The apparatus was cleaned with a dilute chlorhexidine solution and dried before each test. The experimenter handling the mice was blinded to the diet group being tested.
2.4.2. Novel object recognition
The novel object recognition test was performed to evaluate object recognition memory (Antunes & Biala, 2012). Mice were trained in the open field arena (40 × 40 × 30 cm). The mice were given an opportunity to explore the open field arena without objects present for 3 min. Twenty-four hours later, the training comprised of allowing the mice to explore two identical objects (plastic toys) within a 5 cm distance for 3 min with the ANYMaze video tracking program (Stoelting Co.). Mice were then removed from the apparatus and returned to their home cages for 4 h. After 4 h, one of the objects was replaced with a novel object of different shape and size. In the testing phase mice were reintroduced into the apparatus for 3 min to investigate the familiar and the novel object. Time spent exploring the familiar and novel objects were recorded. The preference of one object over another was assessed through the recognition index (RI), which was determined by the time spent on the novel object relative to the time spent on both novel and familiar objects: [RI = TN/(TN+TF)] where TN is time spent on the novel object and TF is time spent on the familiar object (Mitra, Bastos, Bates, Pereira, & Bult-Ito, 2016). Objects and exploration arena were cleaned with a dilute chlorhexidine solution after each animal. The experimenter handling the mice was blinded to the diet group being tested.
2.4.3. Cylinder test
Forelimb locomotor asymmetry was measured with the cylinder test. Mice were placed in a glass cylinder (8 cm in diameter and 12 cm in height) for 3 min duration. The number of wall contacts with both the forelimbs together was counted manually and a lower number indicated a larger level of forelimb locomotor asymmetry (Fleming, Ekhator, & Ghisays, 2013). The data were recorded by two experimenters blinded to the diet group being tested and the observations from each experimenter were averaged for analysis.
2.4.4. Parallel rod floor
The parallel rod floor apparatus was used to measure motor coordination in the mice (Kamens & Crabbe, 2007). Mice were placed in a 20 × 20 cm acrylic box with a floor made of steel rods spaced 8 mm apart from each other. The floor of the box was raised at a height of 1 cm above a base steel plate. The total testing time was 15 min during which motor coordination deficits were assessed by the total number of paw slip errors normalized to the distance travelled. The parameters were calculated through the AnyMaze behavioral acquisition software. The apparatus was cleaned with dilute chlorhexidine and dried after each animal. The experimenter handling the mice was blinded to the diet group being tested.
2.5. Brain tissue collection and molecular analysis
2.5.1. Perfusion and brain sectioning
Twenty-four hours after the last behavioral assessment, mice (n = 4–5 from each of 12–13 per treatment groups) were subjected to whole body transcardial perfusion with 4% paraformaldehyde in phosphate buffered saline (PBS), following a rinse with PBS to remove the blood (Bult, Kobylk, Micki, & Zee, 2001; Hochstetler, Garland, Swallow, Carter, & Bult-Ito, 2004). Brains collected after perfusion were fixed overnight in 4% paraformaldehyde. Fixed brains were then transferred to 30% sucrose solution in PBS and stored at 4 °C until they submerged completely. Brains were then placed in Tissue-Tek OCT compound in beaker cups (VWR International) and frozen in chilled hexane. Coronal sections of 20 μm between —4.84 Bregma and —5.02 Bregma were obtained from the frozen brains on a freezing microtome (Leica CM1900) as per the mouse brain atlas. Brain slices on gelatin coated slides (VWR Cat. no. 48311-703) were then stored at −20°C until further use.
2.5.2. Nissl staining
Slides containing brain sections were kept on a slide drier for 20 min. The slides were dipped in 100% alcohol (twice for 5 min) followed by 70% alcohol (twice for 5 min). Samples were stained with a 0.1% warm Cresyl Violet solution (37 °C) for 3–10 min. The slides were washed in distilled water and subjected to dehydration in 95% and 100% alcohol (twice for 5 min each). Finally, the slides were cleared with xylene and mounted with vectamount. The slides were visualized and the total number of stained cells in the substantia nigra pars compacta were counted using the ImageJ software (NIH) cell counter.
2.5.3. Immunohistochemistry
Slides containing brain sections were first washed in PBS (five 5-min washes). The slides were then incubated in 30% hydrogen peroxide in PBS for 30 min at room temperature followed by PBS washes (six 5-min washes). Blocking buffer (PBS containing 5% normal goat serum, 2% BSA, and 0.4% Triton X-100) was then added to the slides for 1 h. After 1 h, slides were washed in PBS (three 5-min washes) and incubated with Tyrosine Hydroxylase (TH) primary antibody (Millipore Catalogue AB152; 1:500 in PBS and 0.4% Triton X-100) overnight for 18 h. On day two, slides were washed in PBS (four 5-min washes) and were incubated with secondary biotinylated goat anti-rabbit antibody (1:600, Vector laboratories Cat. no. BA1000; in PBS and 0.04% Triton X-100) for 1 h. Sections were finally processed using the Vectastain Elite ABC immunoperoxidase system (Vector Laboratories) as per the manufacturer instructions and visualized with Ni2+-DAB enzyme substrate. For analysis of TH reactive cells, ImageJ software (NIH) was used. All brain sections were analyzed by measuring the optical density of the neurons positive for TH in the substantia nigra pars compacta by subtracting the background staining.
2.5.4. Brain tissue harvesting
After 24 h of the last behavioral test, mice from each treatment group were sacrificed through cervical dislocation and brains were extracted and snap frozen in liquid nitrogen at 80 to −80 °C until further processing. Hippocampal and substantia nigra regions were obtained using a micropuncher and homogenized in chilled RIPA buffer (with added Proteinase/Phosphatase inhibitors cocktail, Roche). Homogenates were then centrifuged at 10,000g for 10 min at 4 °C. The supernatant was collected in fresh Eppendorf© tubes and stored at −80 °C until further analysis. Total protein was measured by Pierce BCA kit.
2.5.5. ELISA
Total BDNF protein was measured in hippocampal homogenates using a commercially available ELISA kit following the manufacturer's protocol (BDNF Emax ImmunoAssay System, Promega Southampton, UK, Cat No. G7611), NOS2 (iNOS) and NF-κB levels were measured in the homogenates from substantia nigra region using kits from Cloud-Clone Corporation (Catalog No. ABIN415396 and ABIN425131, respectively). Finally, Iba-1 levels were measured in the substantia nigra region using a kit from Lifespan BioSciences Inc. (Catalog No. LS-F16598). All samples were assayed in duplicate (n = 5 per group). Data collection was performed at 450 nm using a Biotek EL808 spectrophotometric plate reader.
2.6. Statistical analysis
All statistical analyses were performed in the Statistical Analysis Software (SAS version 9.4, Cary, NC). Behavioral assessments were tested in a general linear model (GLM) analysis of variance (ANOVA) for the main effects of diet, BB, Mn treatment and interaction effects (diet × BB, diet × Mn treatment, BB × Mn treatment and diet × blueberry × Mn treatment). Wherever significance was found appropriate post hoc pair-wise comparisons were conducted using the studentized range test.
3. Results
3.1. Alaskan BB improved OF locomotor activity in Mn treated animals when supplemented with a LFD
All HFD groups had significantly higher body weights compared to NFD and LFD groups (Tables 2 and 3), while the NFD and LFD had similar body weights at 7.5 months of age, which explains the significant diet effect. The significant blueberry effect was due to the HFD groups with BB and BB + Mn and the LFD with tending to have lower body weights than the diets groups without BB. The significant diet by blueberry interaction effect on the body weight of the mice was due to the NFD group with BB + Mn tending to have higher body weights compared to the other NFD groups, while this tended to be in the opposite direction in the LFD and HFD groups.
Table 2.
Body weights of the animals (g) fed a NFD, LFD and HFD in the presence of Alaskan blueberry (BB) at about 7.5 months of age before the first injection of Mn. Data presented as mean ± standard error of the mean (SEM).
| Diet Groups | Weights (in g) |
|---|---|
| NFD | 32.72 ± 0.58 |
| NFD + Mn | 32.05 ± 0.74 |
| NFD + BB | 32.05 ± 0.57 |
| NFD + BB + Mn | 33.44 ± 1.0 |
| LFD | 34.83 ± 0.93 |
| LFD + Mn | 33.79 ± 1.02 |
| Low + BB | 33.93 ± 0.54 |
| LFD + BB + Mn | 31.08 ± 0.67 |
| HFD | 52.77 ± 0.88 |
| HFD + Mn | 53.77 ± 0.58 |
| HFD + BB | 51.68 ± 0.55 |
| HFD + BB + Mn | 50.15 ± 0.77 |
Table 3.
The diet composition (from LabDiet®) in the study was used as per Carey et al. (2014). The freeze- dried Alaska blueberries were added to the pellet at 5% of the pellet weight. The pellets were prepared by PMI Nutrition International, St. Louis, MO, (http://www.labdiet.com/Products/CertifiedDiets/).
| % Kcal from * | 26 Kcal% fat diet (Normal-fat diet) |
10 Kcal% fat diet (Low-fat diet) |
60 Kcal% fat diet (High-fat diet) |
|---|---|---|---|
| Protein | 19. 7 | 20.5 | 18.4 |
| carbohydrate | 54.1 | 69.1 | 21.3 |
| Fat (Lard) | 26.1 | 10.4 | 60.3 |
| Kcal/g | 3.5 | 3.6 | 5.1 |
The values of the fat, protein and carbohydrate contents in the diets containing 5% dried BB were corrected so that they had the same percentages as the diets without BB.
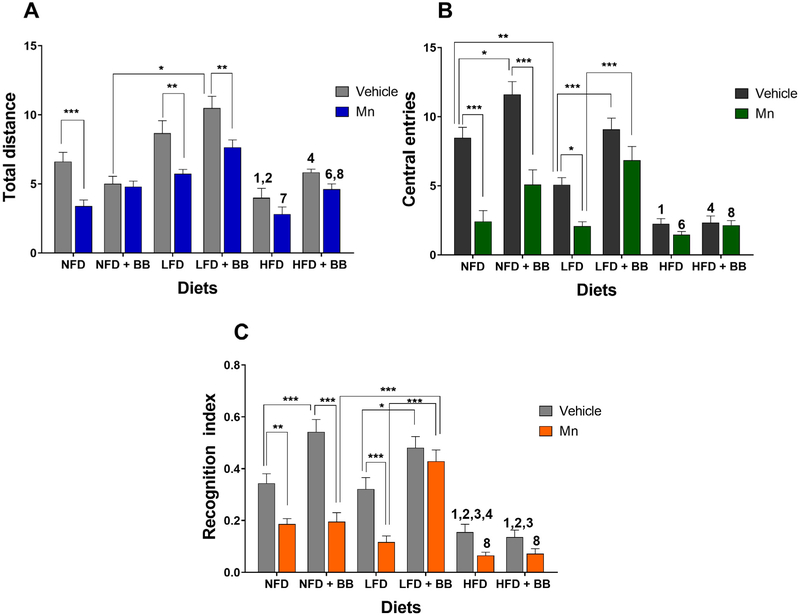
There were significant main effects of diet, BB and Mn treatment on the total distance travelled by the mice in the open field (Table 1 and Fig. 2A). The interaction effect between diet and BB on the assessed behavior was also significant (Table 1 and Fig. 2A). In the vehicle groups, mice consuming the HFD travelled shorter distances compared to ones in the NFD (t23 = 3.174, p < 0.03) and the LFD (t23 = 5.664, p < 0.0001) groups. In the vehicle groups, animals consuming the LFD with BB travelled greater distances compared to the ones fed the NFD with BB (t24 = 6.756, p < 0.0001) and the HFD with BB (t22 = 2.194, p < 0.0002). All animals on a NFD (t23 = 3.88, p < 0.002), LFD (t23 = 3.542, p < 0.004) and LFD with BB (t23 = 3.432, p < 0.005) groups displayed reduced locomotor activity after administration of Mn injections when compared to the vehicle groups. Mice in the NFD with BB group (t23 = 0.2482, p > 0.1) and the HFD with (t23 = 1.468, p > 0.1) and without BB (t22 = 1.387, p > 0.1) groups when exposed to Mn were not significantly different from the vehicle control groups and showed comparatively reduced locomotor deficits. Also, when exposed to Mn, the LFD group without BB was significantly different from the HFD group without BB (t22 = 3.468, p < 0.02). In the presence of BB, animals treated with Mn in the LFD group travelled greater distances compared to the NFD (t22 = 3.369, p < 0.02) and HFD (t22 = 3.651, p < 0.006) groups. The significant diet and BB interaction effect was due to the mice on the LFD and HFD, both in the vehicle and MN treatment groups, tending to travel longer distances with BB treatment compared to without BB, while no BB effect was observed in the NFD groups (Fig. 2A).
Fig. 2.
(A) Locomotor activity in open field test was measured as the total distance travelled in the open field test, (B) anxiogenic behavior was assessed as the number of central entries in the open field test and (C) memory function was measured as the recognition index in the object recognition test. *p < 0.05, **p < 0.01 and ***p < 0.001 represents significant differences between vehicle and Mn treated or BB and non BB treated groups. 1, 2, 3 and 4 represents significant difference between HFD with other diet groups in vehicle condition, 6, 7 and 8 represents significant difference between HFD with other diet groups in MnCl2 condition, where 1 = NFD, 2/6 = NFD + BB, 3/7 = LFD and 4/8 = LFD + BB (n = 12–13 animals per group, p < 0.05, Bonferroni multiple comparison test). Data are shown as the mean ± standard error of the mean (SEM).
3.2. Alaskan BB with a LFD reduced anxiogenic OF behavior inflicted by Mn insult.
Significant main effects of the diet, BB and Mn treatment on the anxiety-like OF behavior, i.e., number of central entries, were found (Table 1 and Fig. 2B). The interaction effects between diet and Mn and diet and BB on the number of central entries were also significant (Table 1 and Fig. 2B).
HFD animals were most anxiogenic in comparison to the NFD vehicle group (t23 = 6.342, p < 0.0001). Vehicle animals consuming BB with a NFD (t24 = 3.286, p < 0.02) and a LFD (t24 = 4.168, p < 0.0009) were less anxiogenic compared to their controls without BB. Mice fed with a NFD (t23 = 6.15, p < 0.0001), a NFD with BB (t23 = 6.646, p < 0.0001) and a LFD (t23 = 3.046, p < 0.02) scored lower numbers of central entries and hence were more anxious-like after administration of Mn injections when compared to their vehicle groups. Animals on a LFD with BB when exposed to Mn were not significantly different from the control group (t23 = 0.2482, p > 0.1) and displayed similar anxiolytic behaviors as the latter. Moreover, in the presence of Mn mice on a LFD with BB performed better than the mice on a LFD without BB (t22 = 4.756, p < 0.0001). The mice on a HFD with or without BB had the lowest number of central entries compared to the mice on the NFD or LFD. In addition, Mn did not affect this anxiety-like behavior any further. The significant diet and Mn interaction effect was due to the mice on the NFD with and without BB and on the LFD significantly decreasing the number of central entries after Mn treatment, while the mice on the LFD with BB and on the HFD with and without BB did not show a Mn effect. The significant diet and BB interaction effect was due to the mice on the NFD and LFD with BB performing better in the OF than the mice on the NFD and LFD without BB, while BB treatment had no effect on the mice on a HFD (Fig. 2B).
3.3. A LFD in combination with Alaskan BB improved novel object recognition memory.
Significant main effects of the diet, BB and Mn on the recognition index in OR behavior was found (Table 1 and Fig. 2C). There was also a significant interaction effect between BB and Mn treatment (Table 1 and Fig. 2C) for the recognition index.
HFD mice had the lowest recognition index in comparison with NFD (t23 = 3.971, p < 0.002) and LFD (t23 = 3.5, p < 0.001) groups. Animals consuming BB with a NFD (t24 = 4.272, p < 0.0006) and a LFD (t24 = 3.427, p < 0.02) showed enhanced memory compared to their controls without BB. Mice on a NFD (t23 = 3.244, p < 0.009), a NFD with BB (t23 = 7.146, p < 0.0001) and a LFD (t23 = 4.208, p < 0.0004) had a significantly lower recognition index and had cognitive impairment after administration of Mn injections when compared to their vehicle groups. Mice on a LFD with BB (t23 = 1.061, p > 0.1) when exposed to Mn were not significant different and displayed similar recognition index as the vehicle control mice. Also, when exposed to Mn, animals on the LFD with BB group overcame the memory deficits and performed better than both mice on a NFD with BB (t22 = 5.36, p < 0.0001) and a LFD with BB (t22 = 6.451, p < 0.0001). The significant BB and Mn interaction effect was due to the mice on the LFD with BB and the HFD with and without BB showing no significant effect of Mn treatment. On the contrary, the mice fed on NFD with and without BB and NFD alone had significantly reduced recognition index after Mn treatment compared to their vehicle controls (Fig. 2C).
3.4. Alaskan BB ameliorated Mn-induced sensory motor deficits in the cylinder test
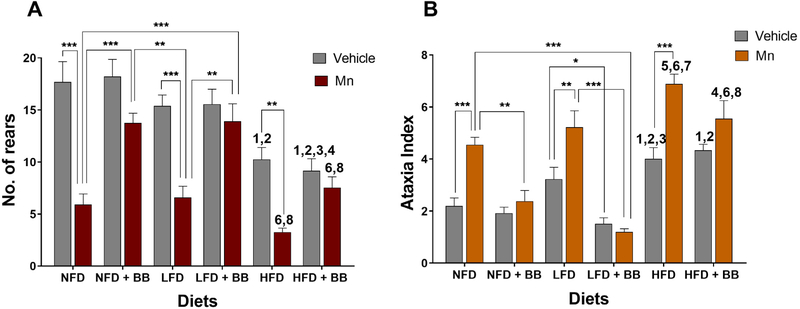
Significant main effects of the diet, BB and Mn treatment on the sensory motor function in the cylinder test, i.e., number of rears, were observed (Table 1 and Fig. 3A). A significant BB and Mn interaction effect was also found (Table 1 and Fig. 3A) for the number of rears.
Fig. 3.
(A) Sensory function was assessed by measuring 'number of rears' in the cylinder test and (B) motor co-ordination was measured in form of 'ataxia index' in parallel rod floor test. *p < 0.05, **p < 0.01 and ***p < 0.001 represents significant differences between vehicle and Mn treated or BB and non BB treated groups. 1, 2, 3 and 4 represents significant difference between HFD with other diet groups in vehicle condition, 6 and 8 represents significant difference between HFD with other diet groups in MnCl2 condition, where 1 = NFD, 2/6 = NFD + BB, 3 = LFD and 4/8 = LFD + BB (n = 12–13 animals per group, p < 0.05, Bonferroni multiple comparison test). Data are shown as the mean ± SEM.
Mice on a HFD had a significantly higher sensory motor deficit score, i.e., ataxia index, compared to mice on a NFD (t23 = 4.088, p < 0.002), with the mice on a LFD having an intermediate rearing score. Mn induced sensory defects in mice on a NFD (t23 = 6.468, p < 0.0001), a LFD (t23 = 4.834, p < 0.0001) and a HFD (t22 = 3.77, p < 0.001) compared to their vehicle controls. These Mn-induced deficits were rescued by BB supplementation for mice on a NFD (t22 = 4.219, p < 0.0008) and a LFD (t22 = 3.95, p < 0.002) compared to the mice not receiving BB. The Mn-induced deficit of mice on a HFD was also ameliorated by BB supplementation as the sensory defects of mice on a HFD with BB were not significantly different between the Mn and vehicle groups (t22 = 2.355, p > 0.1). The significant BB and Mn interaction effect was due to Mn treatment significantly reducing the number of rears of the mice on the NFD, LFD, and HFD without BB, while Mn had no effect on these mice on these diets with BB (Fig. 3A).
3.5. Alaskan BB mitigated Mn-induced motor coordination defects in the parallel rod floor test.
Significant main effects of the diet, BB and Mn treatment on the motor coordination function, i.e., the ataxia index, in the parallel rod floor test (Table 1 and Fig. 3B). The interaction effects between the diet and Mn treatment and between BB and Mn treatment (Table 1 and Fig. 3B) for the ataxia index were also significant.
Overall, vehicle treated mice consuming a HFD scored higher ataxia index scores compared to mice on a NFD (t23 = 3.184, p < 0.004) and on a LFD (t23 = 1.384, p < 0.04). After administration of Mn, all three diets groups: NFD (t23 = 4.417, p < 0.005), LFD (t23 = 3.528, p < 0.004) and HFD (t23 = 4.991, p < 0.0001) showed impaired motor co-ordination with higher ataxia index scores compared to their vehicle treated groups. BB improved motor coordination because no significant differences were found between vehicle and Mn treated mice for all the BB consuming diet groups (NFD: t23 = 0.8201, p > 0.1; LFD: t23 = 0.5526, p > 0.1, HFD t23 = 0.5526, p > 0.1). Consumption of BB ameliorated motor dysfunction in the Mn-treated NFD groups (t22 = 3.737, p < 0.005). Similarly, mice on a LFD with BB displayed improved motor functions in both vehicle (t24 = 3.073, p < 0.04) and Mn (t22 = 6.94, p < 0.0001) treated groups compared to mice on a LFD.
The significant Diet and Mn interaction effect was due to the mice on the NFD and LFD with BB significantly reducing their ataxia index after Mn treatment compared to their diet without BB groups after Mn treatment, while the mice on the HFD with BB after Mn treatment did not significantly differ from their HFD without BB group after Mn treatment (Fig. 3B). The significant BB and Mn interaction effects were due to all the diet groups without BB exhibiting significantly higher ataxia index scores following Mn treatment compared to their vehicle groups, while all the diet groups with BB after Mn treatment were not significantly different from their vehicle groups (Fig. 3B).
3.6. A LFD diet in presence of Alaskan BB elevated BDNF levels in Mn exposed animals
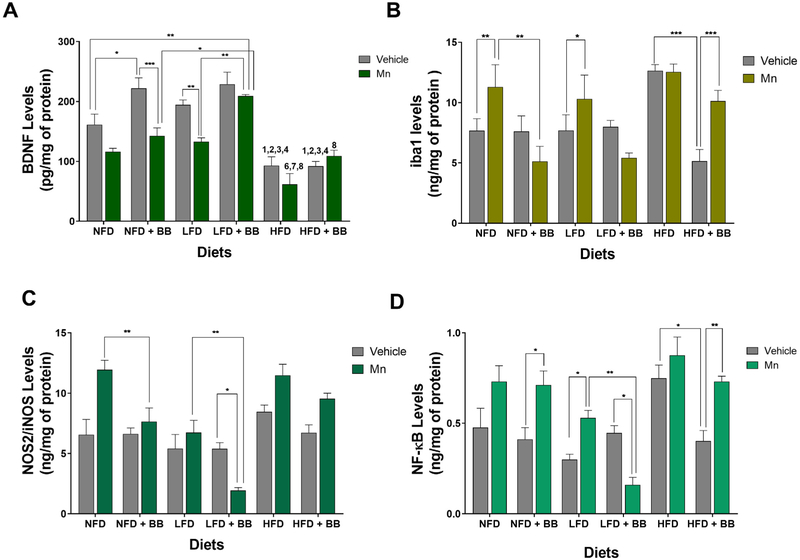
Significant main diet, BB and Mn treatment effects were found for the total BDNF levels in the hippocampal region (Table 1 and Fig. 4A). No significant interaction effects were found.
Fig. 4.
(A) Brain homogenates from hippocampus were measured using ELISA for BDNF levels. Brain homogenates from substantia nigra region were measured using ELISA for (B) iba1 levels (C) NOS2 levels and (D) NF-ΚB levels. *p < 0.05, **p < 0.01 and ***p < 0.001 represents significant differences between vehicle and Mn treated or BB and non BB treated groups. 1, 2, 3 and 4 represents significant difference between HFD with other diet groups in vehicle condition; 6, 7 and 8 represents significant difference between HFD with other diet groups in MnCl2 condition, where 1 = NFD, 2/6 = NFD + BB, 3/7 = LFD and 4/8 = LFD + BB (n = 5 animals per group, p < 0.05, Bonferroni multiple comparison test). Data are shown as the mean ± SEM.
Mice on a NFD with BB had elevated hippocampal BDNF levels compared to mice on a NFD without BB (t8 = 3.314, p < 0.03) and a similar trend was observed in the LFD vehicle groups. Mn administration reduced the BDNF levels in the LFD without BB group (t8 = 3.36, p < 0.001) and showed a similar trend in the NFD without BB group (t8 = 2.447, p < 0.1). There was a significant difference between control and Mn treated NFD with BB group (t8 = 4.319, p < 0.0006). However, after Mn treatment the LFD group with BB showed significantly higher BDNF levels than the LFD group without BB (t8 = 4.18, p < 0.002). There were no significant differences between control and Mn treated LFD with BB groups (t8 = 1.066, p > 0.1). Animals on a HFD had very low levels of BDNF compared to mice on a NFD (t8 = 1.81, p < 0.008) and a LFD (t8 = 5.542, p < 0.0001). But there was no significant difference observed between either BB consuming HFD groups (t8 = 0.035, p > 0.5) or between Mn treated HFD without BB and Mn treated HFD with BB group (t8 = 2.569, p > 0.1).
3.7. Attenuation of inflammatory markers by Alaskan BB in Mn treated animals
3.7.1. Iba1
Significant main BB and Mn treatment effects were found for the total iba1 levels in the SN region (Table 1 and Fig. 3B). There was also an interaction effect between BB and diet and between BB and Mn treatment on this inflammatory marker (Table 1 and Fig. 4B). BB decreased the iba1 levels in mice on a HFD (t8 = 4.561, p < 0.0006) only in the vehicle groups. After Mn treatment, there was a significant increase in iba1 levels for mice fed NFD (t8 = 3.624, p < 0.005), LFD (t8 = 2.814, p < 0.05) and HFD with BB (t8 = 4.357, p < 0.0005). The level of iba1 in the NFD with BB group (t8 = 3.755, p < 0.008) after Mn treatment was significantly lower compared to the NFD without BB group. A similar trend was shown in mice on a LFD with BB (t8 = 2.959, p < 0.08) after Mn treatment compared to the non BB treated LFD group.
3.7.2. NOS2/iNOS
A significant main BB effect was found for the total NOS2/iNOS levels in the substantia nigra region (Table 1 and Fig. 4C). No significant interaction effects were found. After Mn treatment, mice on a NFD (t8 = 3.681, p < 0.009) and LFD (t8 = 4.106, p < 0.003) with BB had lower levels of NOS2/iNOS levels compared to their respective diet groups without BB. Mn administration significant decreased NOS2/iNOS levels in the mice on a LFD with BB compared to the LFD with BB vehicle group (t8 = 2.948, p < 0.03).
3.7.3. NF-κB
Significant main BB and Mn treatment effects were found for the total NF-κB levels in the SN region (Table 1 and Fig. 4D). No significant interaction effects were found. The level of NF-κB in the SN region of mice on a HFD without BB was significantly higher compared to the mice on the HFD with BB (t8 = 3.613, p < 0.02) only for the vehicle group. In the NFD and LFD vehicle groups, BB did not change NF-κB levels compared to their respective without BB groups. Mn treatment tended to increase NF-κB levels in the NFD and HFD without BB, while these differences were significant for the other comparisons compared to their respective vehicle controls; Mn treatment significantly increased NF-κB levels in mice on a NFD with BB (t8 = 3.104, p < 0.02), LFD without BB (t8 = 2.793, p < 0.05), and HFD with BB (t8 = 3.416, p < 0.008), while it decreased NF-κB levels in mice on a LFD with BB (t8 = 2.956, p < 0.008). BB supplementation significantly reduced NF-κB levels in mice on a LFD compared to the LFD without BB group after Mn treatment (t8 = 3.844, p < 0.006).
3.8. A HFD resulted in neuronal loss in the substantia nigra
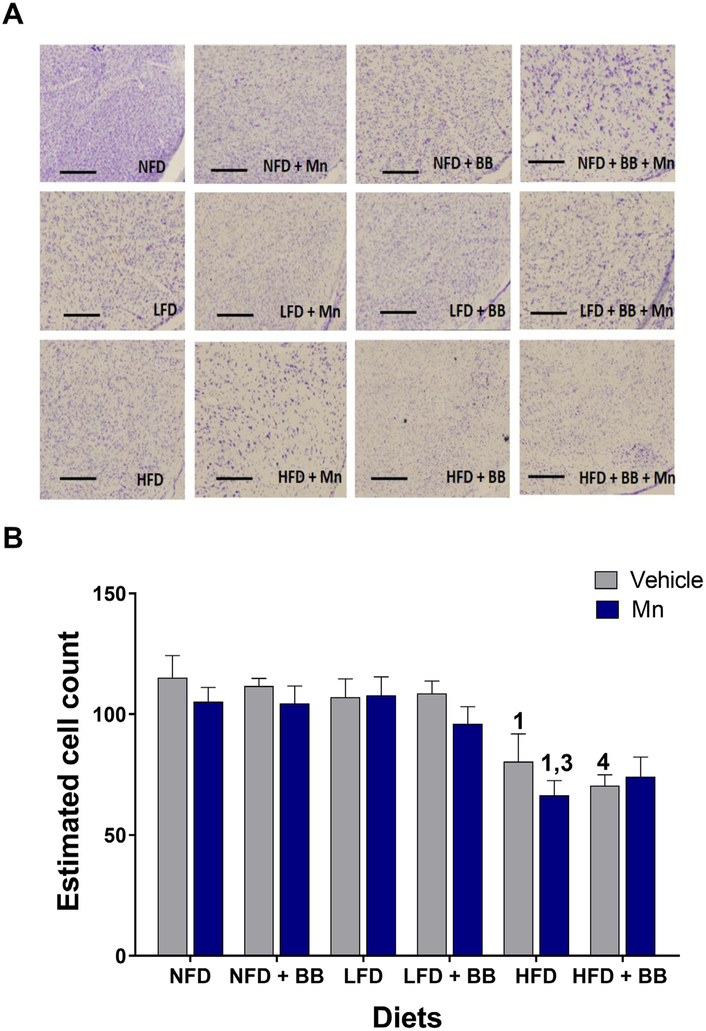
3.8.1. Nissl staining
A significant main diet effect (Table 1 and Figs. 5A and B) was found on the total number of neurons estimated through Nissl staining in the substantia nigra pars compacta (SNPc) region of the mid brain. No significant interaction effects were found.
Fig. 5.
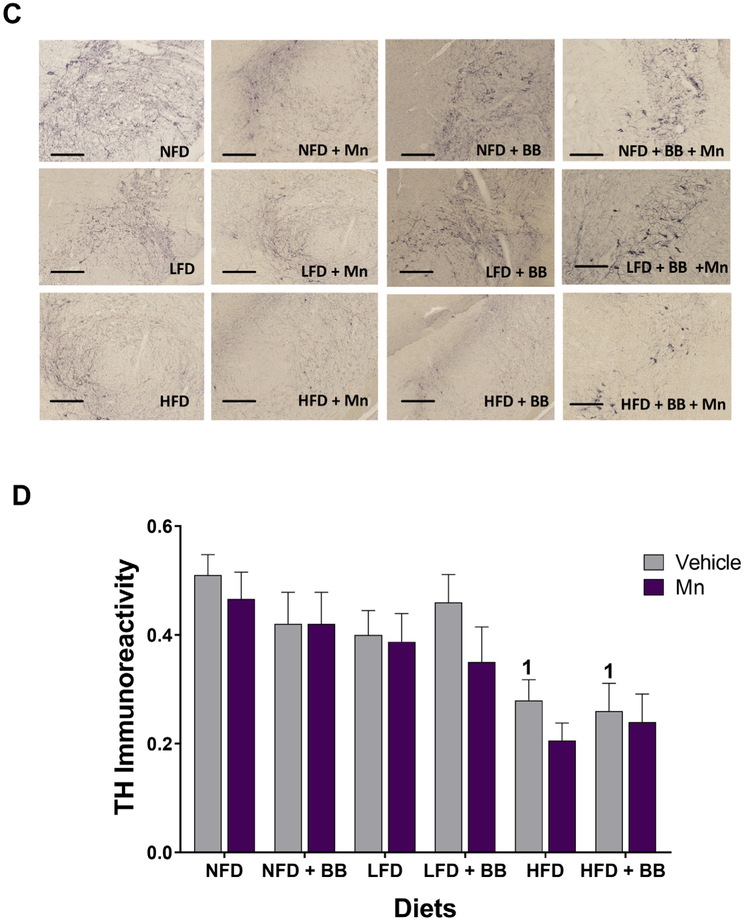
(A) Bright field images of Nissl staining of coronal brain sections in the SNPc region of the mid brain area. Scale bar 200 μm. (B) Graphical representation revealed the significant effect of HFD on reduced cell counts in the SNPc region of the mid brain, 1, 3 and 4 represents significant diet differences from NFD, LFD and LFD + BB respectively (n = 5, p < 0.05, Bonferroni multiple comparison test). (C) Bright field images of DAB-TH staining of coronal brain sections in the SNPc region of the mid brain area. Scale bar 200 μm. (D) Graphical representation revealed the significant effect of HFD on reduced TH immunoreactivity in the SNPc region of the mid brain. 1 represents significant differences from NFD (n = 4–5, p < 0.05, Bonferroni multiple comparison test). Data are shown as the mean ± SEM.
The HFD vehicle group had significantly smaller numbers of stained neurons compared to the NFD (t8 = 3.401, p < 0.03) vehicle group. After Mn administration, the HFD group showed a significant reduction in number of cells compared to the NFD (t8 = 3.791, p < 0.007) and the LFD (t8 = 4.045, p < 0.003) groups. In presence of vehicle, HFD with BB group was also significantly different from LFD with BB group (t8 = 3.733, p < 0.008).
3.8.2. TH staining
A significant main diet effect (Table 1 and Figs. 5C and D) was found for the total number of dopaminergic neurons assessed through tyrosine hydroxylase staining in the substantia nigra pars compacta (SNPc) region. No significant interaction effects were found.
Overall, the HFD groups tended to have smaller numbers of TH positive neurons in the SNPc compared to the NFD and LFD groups. The HFD vehicle group had a significant smaller number of TH neurons compared to NFD vehicle group (t8 = 3.106, p < 0.05). Moreover, HFD with BB vehicle group had lesser number of neurons compared to NFD vehicle groups (t8 = 3.376, p < 0.03).
4. Discussion
Results from our study reveal that endemic Alaskan BB consumption significantly improved anxiety-like (open field), motor coordination (parallel rod floor test), sensory (cylinder test) and cognitive (novel object recognition) behaviors for mice on a LFD and NFD diet when introduced to neurotoxic challenge with Mn. Among all the diet groups, mice fed on HFD performed the poorest in all behavioral tasks. These animals were more anxiogenic and had impaired memory, and sensory and motor co-ordination, possibly due to a metabolic burden or increased body weight as examined by others (Carey, Gomes, & Shukitt-Hale, 2014; Heyward et al., 2012). Hence, interference in behavioral performance due to increased body weight cannot be ruled out in the HFD animals. Mn treatment triggered reduced locomotor, memory and motor functions in tested behaviors for all the three diet groups and this effect was most pronounced in mice fed on a HFD. Interestingly, Mn treated HFD animals that consumed BB performed better in the cylinder test (sensory motor) when compared to HFD animals that were not fed with BB but received Mn insult. This effect of BB was not seen for other motor coordination (parallel rod floor), locomotory (total distance in the open field) and affective (anxiety-like number of central entries in the open field) behaviors in the HFD animals. Overall, this suggests that when combined with a toxic insult a HFD can dampen the beneficial effect of BB significantly for multiple behavioral domains. We also observed that BB was not able to reverse the memory impairment in the HFD animals as shown by Carey and colleagues (Carey et al., 2017) who used a temperate species of BB, Vaccinium ashei, for their experiments. This difference in findings could have resulted from using a different Vaccinum sp. growing under the influence of varying environmental conditions and thereby having alternative polyphenolic content.
BDNF is abundant in brain regions that modulate neuroplasticity underlying learning and memory (Cunha, Brambilla, & Thomas, 2010). The hippocampus serves as an important hub for controlling memory acquisition, storage and retrieval (Jarrard, 1993). Prior studies have indicated that the levels of BDNF in the hippocampus directly correlate to performance in memory tasks for rodents (Carey et al., 2017, 2014). There is also evidence that obesity triggered by diet can lead to memory impairments in adult rodents (Stranahan, Cutler, Button, Telljohann, & Mattson, 2011). Furthermore, polyphenols are shown to enhance cognition by regulating extracellular signal-regulated kinase pathways (Gomez-Pinilla & Nguyen, 2012). In our study, BB improved the BDNF levels either in combination with a NFD or LFD. However, this effect was dependent on the diet of the animals and the type of treatments they received (Mn or Vehicle). These results show a novel interaction where a certain diet (a NFD) in combination with a supplement (BB) can be beneficial in the normal physiological state. The same supplement (BB) when combined with a different diet formulation (a LFD) can improve molecular markers in a neurotoxic condition. Consumption of a HFD has been linked with reduced BDNF levels in the cortex and hippocampus of rodent brains (Park et al., 2010; Pistell et al., 2010). Contrary to prior investigations, which have revealed elevated BDNF levels in presence of BB and a HFD (Carey et al., 2017), we found increased BDNF in the hippocampus of animals fed with BB and LFD. This is not surprising because the mechanistic action of BB is linked to the complex interactions between BB anthocyanins with molecular pathway intermediates (Carey et al., 2017). Because our study utilizes an endemic BB species with a very different anthocyanin profile that is largely influenced by environmental conditions (Dinstel et al., 2013; Grace et al., 2014) the current results corroborates the hypothesis of differential regulation of BDNF. Though, our experiments did not investigate any specific BDNF pathway, such complex associations between BB polyphenols and BDNF expression coupled with selection of diet type warrant further scrutiny.
Microglial cells are key players of neuroinflammation and their activation is often associated with aging and neuronal dysfunction (B. Liu & Hong, 2003). Such changes further cause nitrosative stress causing NOS2 production and elevated levels of the stress-sensitive protein, NF-κB (Streifel et al., 2012). A HFD is notable for increased expression of microglia and associated factors (Carey et al., 2017). Previous research elucidates the role of blueberries in attenuating microglial activation and levels of NF-κB in aged mice and rats (Goyarzu et al., 2004). Studies also elaborate on the mechanistic actions of BB in reducing microglial mediated release of nitric oxide (Carey et al., 2017; Choi, Lee, & Lee, 2012). Our study showed increases in microglial and nitrosative stress markers (NOS2/iNOS and NF-κB) in the HFD group compared to other diets (Carey et al., 2017). Interestingly, our finding highlights the novel interactions of Alaskan BB with different diets in combating neurotoxicity. In the case of Mn treatment, BB in combination with a NFD conferred protection by reducing only levels of NOS2/iNOS and not NF-κB. On the other hand, BB in presence of a LFD was beneficial in reducing both NOS2/iNOS and NF-κB and thereby improving the neurotoxic pathology. This preventative action of BB on Mn-toxicity was not reflected in the HFD group. Expression of NOS2 in response to Mn necessitates the upregulation of NF-κB (Streifel et al., 2012). Research also shows mechanisms of nitric oxide synthase (NOS) expression occurring independent of NF-κB activation (McAdam et al., 2012). However, it is unknown whether such independent actions occur during Mn-induced neurotoxicity.
Dysregulation of neuronal function due to a disease pathology can result in synaptic loss, impaired signaling and progressive change of neuronal structures (Bredesen, Rao, & Mehlen, 2006). These transitions often occur before the actual neuronal cell loss or cell death in chronic neurodegenerative diseases (Bredesen et al., 2006). Mn vulnerability is often linked with changes in cytoskeletal structures which cause damage to the neuronal circuits in the long axons of the dopaminergic neurons of the SN (Stanwood et al., 2009). The neuropathological alterations observed with such exposure also indicate that Mn toxicity is not just restricted to dopaminergic neurons in the SN, but also can occur in the globus pallidus and striatum (Perl & Olanow, 2007; Stanwood et al., 2009). Few studies with rare human post mortem tissue samples also show that modified morphology resulting from excessive environmental exposure to Mn treatment can vary from no obvious changes to massive atrophy of neurons and glial structures in brain regions (Perl & Olanow, 2007). This could possibly explain our results which demonstrated no differences in total cell number / TH positive neurons between the manganese and vehicle treated NFD and LFD groups in the SN region. Furthermore, only HFD group showed significant loss of neurons in comparisons with the other diet groups. Studies have suggested that HFD can possibly lead to dysfunction of dopamine and opioid neurotransmitter systems and related variations in gene expression levels (Reyes, 2012). The animals for this study were euthanized for brain sample collection within a 7–10 day of the subchronic Mn administration. Hence, the experimental period post exposure may be enough to present remarkable modifications in behaviors, biochemical markers and possibly neurite length structure rather than complete neuronal loss. Though there has been research to characterize the dosage and neurotoxicity of Mn (Moreno, Sullivan, Carbone, Hanneman, & Tjalkens, 2008; Suzuki, Mouri, Suzuki, Nishiyama, & Fujii, 1975), little work has been done to study the interaction of diet and environmental toxins. Further research should target investigation of neuronal structure changes occurring due to different diets. Future investigations should also focus on whether such cytoskeletal changes are limited to neurite structure or cause overall circuit level disruption.
In summary, our study illustrates a complex interaction between dietary fat and BB supplementation in mitigating metal induced neurotoxicity and concomitant behavioral impairments. The data reiterates the deleterious effects of consuming a HFD, bolstering the association of this diet with metabolic overload and neurotoxic pathophysiology. Importantly, the results reflect that consuming a HFD can mask the health promoting effects of BB, indicating that a balanced dietary restriction is imperative for good health and supplements alone cannot lessen the effects of a HFD and metabolic overload.
Acknowledgments
We thank the Biological Research and Diagnostics (BIRD) Facility animal quarters staff for excellent routine animal care and Timothy Howe from Alaska Stable Isotope Facility for Blueberry lyophilization. The authors would also like to thank Abigail Moran, Tandi Marth and Sequoia Tyadalma for their support with the study.
Funding
Research reported in this publication was supported by an Institutional Development Award (IDeA) from the National Institute of General Medical Sciences of the National Institutes of Health under grant number P20GM103395 and by the National Institute of General Medical Sciences of the National Institutes of Health under Award Numbers UL1GM118991, TL4GM118992, and RL5GM118990. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. UA is an AA/EO employer and educational institution and prohibits illegal discrimination against any individual: www.alaska.edu/titleIXcompliance/nondiscrimination.
Footnotes
Conflict of interests
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethics statement
This project was conducted as per the University of Alaska Fairbanks Institutional Animal Care and Use Committee approved animal care and experimental procedures (IACUC assurance number 1024315) following the guidelines of National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978).
References
- Antunes M, & Biala G (2012). The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cognitive Processing, 13(2), 93–110. 10.1007/s10339-011-0430-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baufeld C, Osterloh A, Prokop S, Miller KR, & Heppner FL (2016). High-fat dietinduced brain region-specific phenotypic spectrum of CNS resident microglia. Acta Neuropathologica, 132, 361–375. 10.1007/s00401-016-1595-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bredesen DE, Rao RV, & Mehlen P (2006). Cell death in the nervous system. Nature, 443, 796 10.1038/nature05293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bult A, Kobylk, Micki E, & Zee EAVD (2001). Differential expression of protein kinase C βI (PKCβI) but not PKCα and PKCβII in the suprachiasmatic nucleus of selected house mouse lines, and the relationship to arginine-vasopressin. Brain Research, 914(1), 123–133. 10.1016/S0006-8993(01)02821-9. [DOI] [PubMed] [Google Scholar]
- Carey AN, Gildawie KR, Rovnak A, Thangthaeng N, Fisher DR, & Shukitt-Hale B (2017). Blueberry supplementation attenuates microglia activation and increases neuroplasticity in mice consuming a high-fat diet. Nutritional Neuroscience, 1–11. 10.1080/1028415X.2017.1376472. [DOI] [PubMed] [Google Scholar]
- Carey AN, Gomes SM, & Shukitt-Hale B (2014). Blueberry supplementation improves memory in middle-aged mice fed a high-fat diet. Journal of Agricultural and Food Chemistry, 62(18), 3972–3978. 10.1021/jf404565s. [DOI] [PubMed] [Google Scholar]
- Choi SS, Lee DH, & Lee SH (2012). Blueberry protects LPS-stimulated BV-2 microglia through inhibiting activities of p38 MAPK and ERK1/2. Food Science and Biotechnology, 21(4), 1195–1201. 10.1007/s10068-012-0156-4. [DOI] [Google Scholar]
- Cordova FM, Aguiar AS Jr., Peres TV, Lopes MW, Gonçalves FM, Remor AP, … Leal RB (2012). In vivo manganese exposure modulates erk, Akt and Darpp-32 in the striatum of developing rats, and impairs their motor function. PLoS ONE, 7(3), e33057 10.1371/journal.pone.0033057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha C, Brambilla R, & Thomas KL (2010). A simple role for BDNF in learning and memory? Frontiers in Molecular Neuroscience, 3, 1 10.3389/neuro.02.001.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dinstel RR, Cascio J, & Koukel S (2013). The antioxidant level of Alaska's wild berries: High, higher and highest. International Journal of Circumpolar Health, 72 10.3402/ijch.v72i0.21188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodd CA, Ward DL, & Klein BG (2005). Basal ganglia accumulation and motor assessment following manganese chloride exposure in the C57BL/6 mouse. International Journal of Toxicology, 24(6), 389–397. 10.1080/10915810500366500. [DOI] [PubMed] [Google Scholar]
- Erikson KM, & Aschner M (2003). Manganese neurotoxicity and glutamate-GABA interaction. Neurochemistry International 43(4), 475–480. 10.1016/S0197-0186(03)00037-8. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Ekhator OR, & Ghisays V (2013). Assessment of sensorimotor function in mouse models of Parkinson's disease. Journal of Visualized Experiments, 76 10.3791/50303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ganguly P, & Brenhouse HC (2015). Broken or maladaptive? Altered trajectories in neuroinflammation and behavior after early life adversity. Developmental Cognitive Neuroscience, 11, 18–30. 10.1016/j.dcn.2014.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez-Pinilla F, & Nguyen TTJ (2012). Natural mood foods: The actions of polyphenols against psychiatric and cognitive disorders. Nutritional Neuroscience, 15(3), 127–133. 10.1179/1476830511Y.0000000035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goyarzu P, Malin DH, Lau FC, Taglialatela G, Moon WD, Jennings R, … Joseph JA (2004). Blueberry supplemented diet: Effects on object recognition memory and nuclear factor-kappa B levels in aged rats. Nutritional Neuroscience, 7(2), 75–83. 10.1080/10284150410001710410. [DOI] [PubMed] [Google Scholar]
- Grace MH, Esposito D, Dunlap KL, & Lila MA (2014). Comparative analysis of phenolic content and profile, antioxidant capacity, and anti-inflammatory bioactivity in wild Alaskan and commercial Vaccinium berries. Journal of Agricultural and Food Chemistry, 62(18), 4007–4017. 10.1021/jf403810y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafson SJ, Dunlap KL, McGill CM, & Kuhn TB (2012). A nonpolar blueberry fraction blunts NADPH oxidase activation in neuronal cells exposed to tumor necrosis factor-α. Oxidative Medicine and Cellular Longevity, 2012, 12 10.1155/2012/768101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilbronn LK, & Ravussin E (2003). Calorie restriction and aging: Review of the literature and implications for studies in humans. The American Journal of Clinical Nutrition, 78(3), 361–369. 10.1093/ajcn/78.3.361. [DOI] [PubMed] [Google Scholar]
- Heyward FD, Walton RG, Carle MS, Coleman MA, Garvey WT, & Sweatt JD (2012). Adult mice maintained on a high-fat diet exhibit object location memory deficits and reduced hippocampal SIRT1 gene expression. Neurobiology of Learning and Memory, 98(1), 25–32. 10.1016/j.nlm.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hochstetler KJ, Garland T, Swallow JG, Carter PA, & Bult-Ito A (2004). Number of arginine-vasopressin neurons in the suprachiasmatic nuclei is not related to level or circadian characteristics of wheel-running activity in house mice. Behav Genetics, 34, 131 10.1023/B:BEGE.0000009482.91124.52. [DOI] [PubMed] [Google Scholar]
- Jarrard LE (1993). On the role of the hippocampus in learning and memory in the rat. Behavioral and Neural Biology, 60(1), 9–26. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Denisova NA, Bielinski D, Martin A, McEwen JJ, & Bickford PC (1999). Reversals of age-related declines in neuronal signal transduction, cognitive, and motor behavioral deficits with blueberry, spinach, or strawberry dietary supplementation. The Journal of Neuroscience, 19(18), 8114–8121. 10.1523/jneurosci.19-18-08114.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamens HM, & Crabbe JC (2007). The parallel rod floor test: A measure of ataxia in mice. Nature Protocols, 2, 277 10.1038/nprot.2007.19. [DOI] [PubMed] [Google Scholar]
- Kwakye GF, Paoliello MM, Mukhopadhyay S, Bowman AB, & Aschner M (2015). Manganese-induced Parkinsonism and Parkinson’s disease: shared and distinguishable features. International Journal of Environmental Research and Public Health, 12(7), 7519–7540. 10.3390/ijerph120707519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu B, & Hong JS (2003). Role of microglia in inflammation-mediated neurodegenerative diseases: Mechanisms and strategies for therapeutic intervention. Journal of Pharmacology and Experimental Therapeutics, 304(1), 1–7. 10.1124/jpet.102.035048. [DOI] [PubMed] [Google Scholar]
- Liu X, Sullivan KA, Madl JE, Legare M, & Tjalkens RB (2006). Manganeseinduced neurotoxicity: The role of astroglial-derived nitric oxide in striatal interneuron degeneration. Toxicological Sciences, 91(2), 521–531. 10.1093/toxsci/kfj150. [DOI] [PubMed] [Google Scholar]
- Lohachoompol V, Srzednicki G, & Craske J (2004). The change of total anthocyanins in blueberries and their antioxidant effect after drying and freezing. Journal of Biomedicine and Biotechnology, 2004(5), 248–252. 10.1155/s1110724304406123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maulik M, Mitra S, Hunter S, Hunstiger M, Oliver SR, Bult-Ito A, & Taylor BE (2018). Sir-2.1 mediated attenuation of alpha-synuclein expression by Alaskan bog blueberry polyphenols in a transgenic model of Caenorhabditis elegans. Scientific Reports, 8(1), 10216 10.1038/s41598-018-26905-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McAdam E, Haboubi HN, Forrester G, Eltahir Z, Spencer-Harty S, Davies C, … Jenkins GJS (2012). Inducible nitric oxide synthase (iNOS) and nitric oxide (NO) are important mediators of reflux-induced cell signalling in esophageal cells. Carcinogenesis, 33(11), 2035–2043. 10.1093/carcin/bgs241. [DOI] [PubMed] [Google Scholar]
- Mitra S, Bastos CP, Bates K, Pereira GS, & Bult-Ito A (2016). Ovarian sex hormones modulate compulsive, affective and cognitive functions in a non-induced mouse model of obsessive-compulsive disorder. Frontiers in Behavioral Neuroscience, 10, 215 10.3389/fnbeh.2016.00215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, & Bult-Ito A (2017). Attenuation of compulsive-like behavior by fluvoxamine in a non-induced mouse model of obsessive-compulsive disorder. Behavioural Pharmacology. 10.1097/FBP.0000000000000348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Mucha M, Khatri SN, Glenon R, Schulte MK, & Bult-Ito A (2016). Attenuation of compulsive-like behavior through positive allosteric modulation of α4β2 nicotinic acetylcholine receptors in non-induced compulsive-like mice. Frontiers in Behavioral Neuroscience, 10, 244 10.3389/fnbeh.2016.00244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitra S, Mucha M, Owen S, & Bult-Ito A (2017). Postpartum lactation-mediated behavioral outcomes and drug responses in a spontaneous mouse model of obsessive-compulsive disorder. ACS Chemical Neuroscience, 8(12), 2683–2697. 10.1021/acschemneuro.7b00231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreno JA, Sullivan KA, Carbone DL, Hanneman WH, & Tjalkens RB (2008). Manganese potentiates nuclear factor-κB-dependent expression of nitric oxide synthase 2 in astrocytes by activating soluble guanylate cyclase and extracellular responsive kinase signaling pathways. Journal of neuroscience research, 86(9), 2028–2038. 10.1002/jnr.21640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park HR, Park M, Choi J, Park K-Y, Chung HY, & Lee J (2010). A high-fat diet impairs neurogenesis: Involvement of lipid peroxidation and brain-derived neurotrophic factor. Neuroscience Letters, 482(3), 235–239. 10.1016/j.neulet.2010.07.046. [DOI] [PubMed] [Google Scholar]
- Perl DP, & Olanow CW (2007). The neuropathology of manganese-induced Parkinsonism. Journal of Neuropathology & Experimental Neurology, 66(8), 675–682. 10.1097/nen.0b013e31812503cf. [DOI] [PubMed] [Google Scholar]
- Pistell PJ, Morrison CD, Gupta S, Knight AG, Keller JN, Ingram DK, & Bruce-Keller AJ (2010). Cognitive impairment following high fat diet consumption is associated with brain inflammation. Journal of Neuroimmunology, 219(1), 25–32. 10.1016/j.jneuroim.2009.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prut L, & Belzung C (2003). The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: A review. European Journal of Pharmacology, 463(1), 3–33. 10.1016/S0014-2999(03)01272-X. [DOI] [PubMed] [Google Scholar]
- Racette BA, Searles Nielsen S, Criswell SR, Sheppard L, Seixas N, Warden MN, & Checkoway H (2017). Dose-dependent progression of parkinsonism in manganese-exposed welders. Neurology, 88(4), 344–351. 10.1212/WNL.0000000000003533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes TM (2012). High-fat diet alters the dopamine and opioid systems: Effects across development. International Journal of Obesity Supplements, 2(Suppl 2), S25–S28. 10.1038/ijosup.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scerbak C, Vayndorf EM, Hernandez A, McGill C, & Taylor BE (2016). Mechanosensory neuron aging: Differential trajectories with lifespan-extending alaskan berry and fungal treatments in Caenorhabditis elegans. Frontiers in Aging Neuroscience, 8 10.3389/fnagi.2016.00173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanwood GD, Leitch DB, Savchenko V, Wu J, Fitsanakis VA, Anderson DJ,… McLaughlin B. (2009). Manganese exposure is cytotoxic and alters dopaminergic and gabaergic neurons within the basal ganglia. Journal of neurochemistry, 110(1), 378–389. 10.1111/j.1471-4159.2009.06145.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Cutler RG, Button C, Telljohann R, & Mattson MP (2011). Dietinduced elevations in serum cholesterol are associated with alterations in hippocampal lipid metabolism and increased oxidative stress. Journal of Neurochemistry, 118(4), 611–615. 10.1111/j.1471-4159.2011.07351.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stranahan AM, Lee K, Martin B, Maudsley S, Golden E, Cutler RG, & Mattson MP (2009). Voluntary exercise and caloric restriction enhance hippocampal dendritic spine density and BDNF levels in diabetic mice. Hippocampus, 19(10), 951–961. 10.1002/hipo.20577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streifel KM, Moreno JA, Hanneman WH, Legare ME, & Tjalkens RB (2012). Gene deletion of nos2 protects against manganese-induced neurological dysfunction in juvenile mice. Toxicological Sciences, 126(1), 183–192. 10.1093/toxsci/kfr335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki Y, Mouri T, Suzuki Y, Nishiyama K, & Fujii N (1975). Study of subacute toxicity of manganese dioxide in monkeys. Tokushima Journal of Experimental Medicine, 22, 5–10. [PubMed] [Google Scholar]
- Tatem KS, Quinn JL, Phadke A, Yu Q, Gordish-Dressman H, & Nagaraju K (2014). Behavioral and locomotor measurements using an open field activity monitoring system for skeletal muscle diseases. Journal of Visualized Experiments (JoVE), 91, 51785 10.3791/51785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uranga RM, Bruce-Keller AJ, Morrison CD, Fernandez-Kim SO, Ebenezer PJ, Zhang L, … Keller JN (2010). Intersection between metabolic dysfunction, high fat diet consumption, and brain aging. Journal of Neurochemistry, 114(2), 344–361. 10.1111/j.1471-4159.2010.06803.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Z, Raj DD, Schaafsma W, van der Heijden RA, Kooistra SM, Reijne AC,… Eggen BJL (2018). Low-fat diet with caloric restriction reduces white matter microglia activation during aging. Frontiers in Molecular Neuroscience, 11, 65 10.3389/fnmol.2018.00065. [DOI] [PMC free article] [PubMed] [Google Scholar]