EDITORIAL
Transthyretin cardiac amyloidosis (ATTR-CA) was thought to be a rare, untreatable, terminal disease. This bygone refrain is rapidly being proven inaccurate. Non-invasive diagnostic techniques have broadened understanding of affected populations and novel therapies that reduce morbidity and mortality in ATTR-CA are rapidly emerging. Because of these innovations, we are embarking on exciting new horizons for the management of ATTR-CA. However, these strategies have needed further context within the natural history of this disease.
In this issue of Circulation, Lane and colleagues conducted a cohort study aimed at clarifying the natural history of 1,034 individuals with either hereditary (ATTRh) or wild-type (ATTRwt) CA referred to the United Kingdom National Amyloid Centre (UK-NAC) between 2000 and 2017.1 The UK-NAC provides evaluation, diagnosis, monitoring and management services for the national caseload of patients with amyloidosis, thereby affording a unique opportunity to characterize ATTR-CA in a cohort where referral bias may be less substantial than in other series.
The observations from this report highlight the potential for recent advancements in cardiac and amyloid-specific imaging to overcome historical impediments to diagnosis and identify vulnerable individuals that might benefit from ATTR-specific therapies. All diagnoses of ATTR-CA were based on validated diagnostic criteria (biopsy-proven or with technetium-labeled bone scintigraphy coupled with required assessment for monoclonal proteins and genetic testing) and patients were followed on a protocol every 6 months thereafter. These follow-up visits included amino-terminal pro-B-type natriuretic peptide (NTproBNP) levels, electrocardiography, and echocardiography. Functional status via 6-minute walk and quality of life via the Kansas City Cardiomyopathy Questionnaire (KCCQ) were added to each visit in 2010. In a subset of the cohort, hospital service utilization (inpatient, outpatient, and emergency department) was determined using the data from the National Health Service (NHS) in England.
This analysis provides several notable observations informing the types of ATTR-CA and their natural history. First, there was a striking upsurge of new diagnoses of ATTR-CA from 2008 onward, driven largely by an increased recognition of ATTRwt. Increased recognition was likely the direct consequence of more frequent utilization of non-invasive imaging at the UK-NAC that included cardiac magnetic resonance imaging and bone scintigraphy.2, 3 Second, ATTRh CA has a comparatively worse prognosis than ATTRwt. In addition, patients with V122I ATTRh had higher serial NTproBNP in comparison to non-V122I ATTRh or ATTRwt suggesting that it may confer a more malignant myocardial phenotype. Third, the prognosis of ATTRwt individuals diagnosed after 2012 (when bone scintigraphy was implemented as a diagnostic strategy) was better than among individuals with ATTRwt diagnosed in the preceding years. This observation is likely related to a lead-time bias (earlier detection of ATTR-CA) and it underscores the potential for non-invasive diagnostic strategies to recognize ATTRwt earlier in the disease when ATTR-specific therapies might be more beneficial (Figure 1). Fourth, the increasing healthcare utilization prior to establishing the diagnosis of ATTR-CA highlights substantial diagnostic delays. Even once the diagnosis was secured, regardless of genotype, ATTR-CA was associated with progressive declines in functional capacity and quality of life. This observation unfortunately parallels diagnostic delays for light chain amyloidosis (AL)4 and should be a siren call for change.
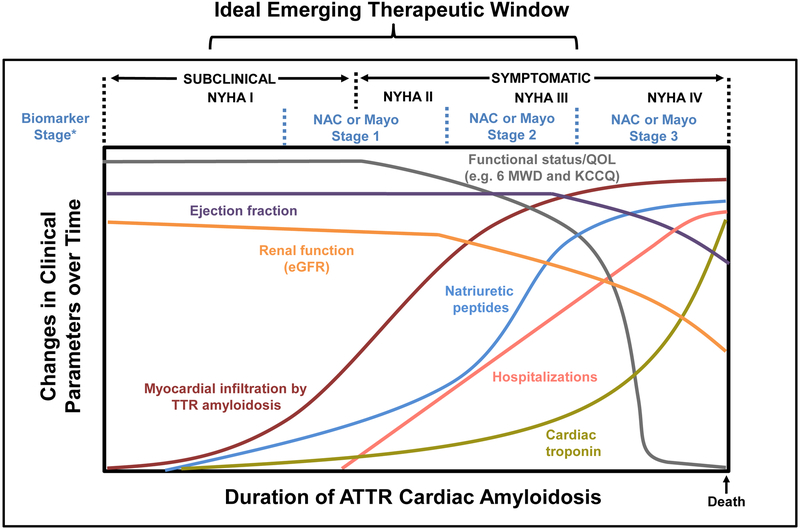
Figure 1. Conceptual model of ATTR cardiac amyloidosis progression over time.
Changes in various parameters are shown. The relative scale specific to each factor and time course are not proportional. Myocardial amyloid infiltration occurs before clinically manifest changes in ejection fraction, cardiac biomarkers and renal function. Thus, most patients with ATTR-CA likely have a long latency period prior to declines in functional capacity, which can occur rapidly in the context of multiple hospitalizations for acute decompensated heart failure and arrhythmias. The ideal emerging therapeutic window for novel therapies is hypothesized to be before significant organ dysfunction has occurred and prior to rapid and potentially irreversible declines in functional capacity. * Biomarker Stage defined by the Mayo Clinic for ATTRwt as cardiac troponin T (< 0.05 ng/mL) and NTproBNP (< 3,000 pg/mL) with Stages I, II, and III are defined as having both, one, or neither of the markers below the threshold, with a median survival of 66, 40 and 20 months, respectively or by the UK-NAC for both ATTRwt and ATTRh with estimated glomerular filtration rate (eGFR) of 45 mL/min instead of troponin. Median survival was 69, 47, and 24 months in Stages I, II, and III, respectively, with longer survival in ATTRwt compared to ATTRh-CA.
Studies comparing outcomes of patients with the most common heritable form of CA(V122I ATTRh) versus those with ATTRwt have been conflicting, but have generally shown a worse prognosis with the former, as shown in the present study.5, 6 Although this prognostic difference had hypothetical biological underpinnings, residual confounding from differential access to care and socioeconomic factors between V122I subjects and ATTRwt remained a concern. At the UK-NAC, the median time from symptom onset to diagnosis was shorter (25 months) in ATTRh than in ATTRwt (39 months), which when coupled with the more rapid increase in NTproBNP and decrements in quality of life, provides substantive evidence that the V122I genotype is more aggressive than ATTRwt. These observations highlight the role that more widespread use of genetic testing can play in identifying allele carriers who are at risk for ATTRh-CA, facilitating emerging therapies at a time earlier in the course of their disease, before they enter the phase of rapid progression.
In the subset of patients with available data from the NHS in England, Lane et al observed steady increased health care usage in the years leading up to a formal diagnosis of ATTR-CA. This increasing use of hospital-based healthcare highlights the sobering number of missed opportunities to secure an accurate diagnosis of ATTR-CA. Once diagnosed, the hospital-based healthcare utilization appears to stabilize. However, quality of life observations from individuals with KCCQ data within their first 12 months after diagnosis highlight ongoing burdens of disease characterized by significant physical impairments with concomitant limited social interactions despite high self-efficacy. Over the years that follow, there remains a progressive decline in quality of life mirrored by a progressive decline in exertional capacity.
We are entering an era with a rapid emergence of therapeutic options for ATTR that have the potential to change the natural history of the disease. Two gene-silencing therapies reduce circulating TTR, and halt or slow the progression of ATTRh polyneuropathy: inotersen, an antisense oligonucleotide; and patisiran, a small interfering RNA.7, 8 These treatments may have favorable cardiac effects in ATTRh and ATTRwt-CA as well.9, 10 Other treatments stabilize the TTR tetrameric structure preventing tetramer dissociation, which is the rate limiting step in TTR amyloid fibril formation. Tafamidis is a small molecule engineered to bind to the thyroxine binding pocket of TTR and stabilizes the TTR tetramer. The Transthyretin Amyloidosis Cardiomyopathy Clinical Trial demonstrated that tafamidis reduced mortality and cardiovascular hospitalizations in both ATTRwt-CA and ATTRh-CA along with a slowing of the decline in functional capacity and quality of life.11 Preliminary studies with AG10, another TTR stabilizing molecule, have been promising and a Phase 3 trial is underway (ClinicalTrials.gov: ).12 Therefore, observations in the present study provide important context in how these therapies might influence the natural history of ATTR-CA. Both types of therapies work by inhibiting amyloid fibril formation and may, therefore, be more efficacious if administered earlier in the course of the disease when there is less cardiac dysfunction from amyloid deposits (Figure 1).
Several significant unmet needs and unanswered questions remain, however. These include defining the optimal timing of initiating ATTR-CA therapies, determining comparative effectiveness between TTR silencers and TTR stabilizers, and clarifying the role of combination therapies. With the dramatically changing therapeutic landscape, early recognition of ATTR-CA will be critical. Approaches to identify the presence of ATTR-CA in “at-risk” populations have identified affected individuals in cohorts with tendonopathies,13 heart failure with preserved ejection fraction,14 and aortic stenosis.15 Given the potential to diagnose ATTR earlier in the course of the disease, more data will be needed to guide the decision on when to initiate treatment and which emerging treatment(s) to employ at each stage of disease. Although early recognition is key to leveraging emerging therapies, these uncertainties remain, underscoring the necessity of studies like the present report by Lane et al to unfold the truth about ATTR-CA.
Acknowledgments
FUNDING
J.L.G. has grant support from the Texas Health Resources Clinical Scholarship.
M.S.M has grant support from NIH R01HL139671–01, R21AG058348 and K24AG036778.
Footnotes
CONFLICT OF INTEREST DISCLOSURES
J.L.G. has consulting income from Pfizer.
M.S.M. has consulting income from Pfizer, GSK, EIdos, Prothena, Akcea and Alnylam, and institution received clinical trial funding from Pfizer, Prothena, Eidos and Alnylam
REFERENCES
- 1.Lane T, Fontana M, Martinez-Naharro A, Cristina Quarta C, Whelan CJ, Petrie A, Rowczenio DM, Gilbertson JA, Hutt DF, Rezk T, Strehina SG, Caringal-Galima J, Manwani R, Sharpley FA, Wechalekar AD, Lachmann HJ, Mahmood S, Sachchithanantham S, Drage EPS, Jenner HD, McDonald R, Bertolli O, Calleja A, Hawkins PN, Gillmore JD. Natural history, quality of life and outcome in cardiac ATTR amyloidosis. Circulation. 2019. [In Press]. [DOI] [PubMed] [Google Scholar]
- 2.Bokhari S, Castano A, Pozniakoff T, Deslisle S, Latif F and Maurer MS. (99m)Tc-pyrophosphate scintigraphy for differentiating light-chain cardiac amyloidosis from the transthyretin-related familial and senile cardiac amyloidoses. Circ Cardiovasc Imag. 2013;6:195–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syed IS, Glockner JF, Feng D, Araoz PA, Martinez MW, Edwards WD, Gertz MA, Dispenzieri A, Oh JK, Bellavia D, Tajik AJ and Grogan M. Role of cardiac magnetic resonance imaging in the detection of cardiac amyloidosis. JACC Cardiovasc Imag. 2010;3:155–164. [DOI] [PubMed] [Google Scholar]
- 4.Lousada I, Comenzo RL, Landau H, Guthrie S and Merlini G. Light Chain Amyloidosis: Patient Experience Survey from the Amyloidosis Research Consortium. Adv Ther. 2015;32:920–928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ruberg FL, Maurer MS, Judge DP, Zeldenrust S, Skinner M, Kim AY, Falk RH, Cheung KN, Patel AR, Pano A, Packman J and Grogan DR. Prospective evaluation of the morbidity and mortality of wild-type and V122I mutant transthyretin amyloid cardiomyopathy: the Transthyretin Amyloidosis Cardiac Study (TRACS). Am Heart J. 2012;164:222–228 e1. [DOI] [PubMed] [Google Scholar]
- 6.Maurer MS, Hanna M, Grogan M, Dispenzieri A, Witteles R, Drachman B, Judge DP, Lenihan DJ, Gottlieb SS, Shah SJ, Steidley DE, Ventura H, Murali S, Silver MA, Jacoby D, Fedson S, Hummel SL, Kristen AV, Damy T, Plante-Bordeneuve V, Coelho T, Mundayat R, Suhr OB, Waddington Cruz M, Rapezzi C and Investigators T. Genotype and Phenotype of Transthyretin Cardiac Amyloidosis: THAOS (Transthyretin Amyloid Outcome Survey). J Am Coll Cardiol. 2016;68:161–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Benson MD, Waddington-Cruz M, Berk JL, Polydefkis M, Dyck PJ, Wang AK, Plante-Bordeneuve V, Barroso FA, Merlini G, Obici L, Scheinberg M, Brannagan TH 3rd, Litchy WJ, Whelan C, Drachman BM, Adams D, Heitner SB, Conceicao I, Schmidt HH, Vita G, Campistol JM, Gamez J, Gorevic PD, Gane E, Shah AM, Solomon SD, Monia BP, Hughes SG, Kwoh TJ, McEvoy BW, Jung SW, Baker BF, Ackermann EJ, Gertz MA and Coelho T. Inotersen Treatment for Patients with Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:22–31. [DOI] [PubMed] [Google Scholar]
- 8.Adams D, Gonzalez-Duarte A, O'Riordan WD, Yang CC, Ueda M, Kristen AV, Tournev I, Schmidt HH, Coelho T, Berk JL, Lin KP, Vita G, Attarian S, Plante-Bordeneuve V, Mezei MM, Campistol JM, Buades J, Brannagan TH 3rd, Kim BJ, Oh J, Parman Y, Sekijima Y, Hawkins PN, Solomon SD, Polydefkis M, Dyck PJ, Gandhi PJ, Goyal S, Chen J, Strahs AL, Nochur SV, Sweetser MT, Garg PP, Vaishnaw AK, Gollob JA and Suhr OB. Patisiran, an RNAi Therapeutic, for Hereditary Transthyretin Amyloidosis. N Engl J Med. 2018;379:11–21. [DOI] [PubMed] [Google Scholar]
- 9.Solomon SD, Adams D, Kristen A, Grogan M, Gonzalez-Duarte A, Maurer MS, Merlini G, Damy T, Slama MS, Brannagan TH 3rd, Dispenzieri A, Berk JL, Shah AM, Garg P, Vaishnaw A, Karsten V, Chen J, Gollob J, Vest J and Suhr O. Effects of Patisiran, an RNA Interference Therapeutic, on Cardiac Parameters in Patients With Hereditary Transthyretin-Mediated Amyloidosis. Circulation. 2019;139:431–443. [DOI] [PubMed] [Google Scholar]
- 10.Dasgupta NR and Benson MD. POTENTIAL REVERSAL OF TRANSTHYRETIN AMYLOID CARDIOMYOPATHY WITH TTR SPECIFIC ANTISENSE OLIGONUCLEOTIDE THERAPY. J Am Coll Cardiol. 2018;71:A660. [Google Scholar]
- 11.Maurer MS, Schwartz JH, Gundapaneni B, Elliott PM, Merlini G, Waddington-Cruz M, Kristen AV, Grogan M, Witteles R, Damy T, Drachman BM, Shah SJ, Hanna M, Judge DP, Barsdorf AI, Huber P, Patterson TA, Riley S, Schumacher J, Stewart M, Sultan MB, Rapezzi C and Investigators A-AS. Tafamidis Treatment for Patients with Transthyretin Amyloid Cardiomyopathy. N Engl J Med. 2018;379:1007–1016. [DOI] [PubMed] [Google Scholar]
- 12.Judge DP, Falk RH, Maurer MS, Shah SJ, Witteles RM, Grogan M, Selby VN, Jacoby D, Hanna M, Nativi-Nicolau J, Patel J, Rao S, Sinha U, Turtle CW, Fox JC and Heitner SB. Transthyretin Stabilization by AG10 in Symptomatic Transthyretin Amyloid Cardiomyopathy. J Am Coll Cardiol. 2019. [DOI] [PubMed] [Google Scholar]
- 13.Sperry BW, Reyes BA, Ikram A, Donnelly JP, Phelan D, Jaber WA, Shapiro D, Evans PJ, Maschke S, Kilpatrick SE, Tan CD, Rodriguez ER, Monteiro C, Tang WHW, Kelly JW, Seitz WH Jr. and Hanna M. Tenosynovial and Cardiac Amyloidosis in Patients Undergoing Carpal Tunnel Release. J Am Coll Cardiol. 2018;72:2040–2050. [DOI] [PubMed] [Google Scholar]
- 14.Gonzalez-Lopez E, Gallego-Delgado M, Guzzo-Merello G, de Haro-Del Moral FJ, Cobo-Marcos M, Robles C, Bornstein B, Salas C, Lara-Pezzi E, Alonso-Pulpon L and Garcia-Pavia P. Wild-type transthyretin amyloidosis as a cause of heart failure with preserved ejection fraction. Eur Heart J. 2015;36:2585–2594. [DOI] [PubMed] [Google Scholar]
- 15.Longhi S, Lorenzini M, Gagliardi C, Milandri A, Marzocchi A, Marrozzini C, Saia F, Ortolani P, Biagini E, Guidalotti PL, Leone O and Rapezzi C. Coexistence of Degenerative Aortic Stenosis and Wild-Type Transthyretin-Related Cardiac Amyloidosis. JACC Cardiovasc Imag. 2016;9:325–327. [DOI] [PubMed] [Google Scholar]