Abstract

Migraine is a debilitating neurological condition that involves the neuropeptide calcitonin gene-related peptide (CGRP). An exciting development is the recent FDA approval of the first in an emerging class of CGRP-targeted drugs designed to prevent migraine. Yet despite this efficacy, there are some fundamental unanswered questions, such as where and how CGRP works in migraine. Preclinical data suggest that CGRP acts via both peripheral and central mechanisms. The relevance of peripheral sites is highlighted by the clinical efficacy of CGRP-blocking antibodies, even though they do not appreciably cross the blood-brain barrier. The most likely sites of action are within the dura and trigeminal ganglia. Furthermore, it would be foolish to ignore perivascular actions in the dura since CGRP is the most potent vasodilatory peptide. Ultimately, the consequence of blocking CGRP or its receptor is reduced peripheral neural sensitization. Underlying their efficacy is the question of why the antibodies have such an excellent safety profile so far. This may be due to the presence of a second CGRP receptor and vesicular release of a large bolus of peptides. Finally, despite the promise of these drugs, there are unmet gaps because they do not work for all patients; so what next? We can expect advances on several fronts, including CGRP receptor structures that may help the development of centrally acting antagonists, combinatorial treatments that integrate other therapies, and development of drugs that target other neuropeptides. This is truly an exciting time for CGRP and the migraine field with many more discoveries on the horizon.
Keywords: migraine, CGRP, therapeutic antibodies, neuropeptides, photophobia, pain
Introduction
Migraine is more than just a bad headache. It is neurological disorder involving sensory abnormalities that occur preceding, during, and following the headache.1−3 The prevalence and socio-economic impact of migraine cannot be overstated. It is estimated that 15% of all people suffer from migraine.4,5 Migraine is the second leading cause of years lived with a disability, and the sixth most common disease globally (>1 billion sufferers).6 The prevalence is 3–4-fold higher in women, such that over 40% of women experience migraine in their lifetime.7 The annual cost of migraine in the US alone is ∼$36 billion.8 Furthermore, headache is in the top five reasons for emergency room visits4 and is associated with the overprescription of opioids.9
Clinical studies over the past 3 decades have demonstrated that migraine involves an increased sensitivity to the neuropeptide calcitonin gene-related peptide (CGRP). CGRP is a multifunctional peptide that has potent vasodilatory activity and has long been implicated in pain pathways.10 Notably, CGRP is both necessary and sufficient to cause migraine for many people (Table 1). In particular, CGRP infusion is able to trigger delayed migraine-like headaches in most migraineurs. Interestingly, the majority of agents in these types of provocation studies are vasodilators. However, this is not a property of all vasodilators since infusion of VIP and adrenomedullin do not trigger a migraine.11,12 This suggests that the vasodilation activity of CGRP is not sufficient to trigger a migraine. Nonetheless, we cannot exclude the possibility that CGRP invokes both vasodilatory and nonvasodilatory actions in the perivasculature,13 or that there are important differences in vascular targets and pharmacokinetics.14,15 On-going preclinical and clinical studies should resolve these possibilities.
Table 1. Clinical Evidence of CGRP Involvement in Migraine.
| 1. Elevation of CGRP in migraineurs | |
| •CGRP levels elevated in plasma,80,81 cerebrospinal fluid,82 and saliva83 during spontaneous migraine attacks. | |
| ◦Also elevated during nitroglycerin-induced migraine.84 | |
| ◦Interictal levels elevated in episodic85 and chronic migraineurs.86 | |
| ◦Reduced by triptans.81,84 | |
| •However, elevation not seen in other episodic87 and chronic88 migraine studies. | |
| 2. Infusion of CGRP causes migraine | |
| •In 66% of migraineurs, the infusion of CGRP is sufficient to induce a migraine-like headache.89,25,90,91 | |
| ◦In contrast, healthy controls have only a mild headache,92 suggesting that migraineurs are more sensitive to CGRP. | |
| •However, CGRP infusion not effective in FHM1 patients93,94 and apparently does not induce aura90 or prodrome symptoms.91 | |
| 3. Efficacy of CGRP-based drugs | |
| •Small molecule CGRP receptor antagonists effective in clinical trials for abortive treatment of migraine.95−97 | |
| ◦Lead antagonist dropped due to liver toxicity after repeated use,98 although new compounds, rimegepant and ubrogepant, look promising as abortive and preventative drugs,73 and are expected to be submitted for FDA approval soon. | |
| •Antibodies that block CGRP or CGRP receptor are effective in clinical trials for prevention of both episodic and chronic migraine.18,19,73,99−101 | |
| ◦FDA approvals of the receptor antibody erenumab (Aimovig) and two ligand antibodies, fremanezumab (AJOVY) and galcanezumab (Emgality), for migraine prevention, with another ligand antibody, eptinezumab, expected to be submitted soon. | |
Perhaps the most persuasive evidence of the importance of CGRP is efficacy of CGRP-based therapeutic antibodies and small molecule receptor antagonists.16 Three monoclonal antibodies have now been approved by the Federal Drug Administration (FDA). Erenumab (Aimovig, Amgen/Novartis), blocks the CGRP receptor and fremanezumab (AJOVY, Teva Pharmaceuticals) and galcanezumab (Emgality, Eli Lilly) block the CGRP ligand. In addition, two receptor antagonists, ubrogepant (Allergan) and rimegepant (Biohaven Pharmaceuticals), and another ligand antibody (eptinezumab, Alder Biopharmaceuticals) are expected to be submitted for FDA approval in 2019. It is especially encouraging that the antibodies are effective for at least 15 months,17 and have minimal adverse effects.18−21 This is a stimulating time in the field because CGRP-based drugs are the first new class of migraine therapeutics in nearly 30 years.
How is CGRP Acting in Migraine?
The role of CGRP and the vasculature in migraine is central to many of the concepts in this article. Historically migraine has been viewed as a vascular disorder,22 yet over the past two decades the vascular theory has been challenged by more neuro-centric theories. This shift was triggered by reports that vascular changes are neither necessary nor sufficient to trigger migraine, and by evidence that brain functions are altered during migraine.3,23 Nonetheless, the debate on vascular contributions continues (see citations in ref (24)).
In this Perspective, I suggest that CGRP actions at the vasculature should not be ignored. We have recently reviewed the vascular connections to migraine.24 For example, local changes in vascular tone are difficult to rule out and recent studies support a role for meningeal vasodilation.25,26 Furthermore, a meta-analysis of >1 million people concluded that migraine is associated with elevated risk of cardiovascular and cerebrovascular events,27 and many genes that are associated with migraine are expressed in the vasculature.28 Finally, the high therapeutic efficacy of monoclonal antibodies that do not cross the blood-brain barrier16,29 argues for a peripheral site of CGRP action. On the basis of these observations, a critical reevaluation of vascular contributions to migraine seems justified. Indeed, a neurovascular model of migraine involving peripheral sensitization in the trigeminovasculature was articulated over 25 years ago, although it was limited to peripheral nerves.22 The model I am proposing is similar, with the exceptions that the process can be triggered in both the meninges and CNS, and that it can go in both directions, that is, neural to vascular and vascular to neural.
I propose a model of perivascular CGRP actions that involves a cascade from the trigeminovasculature to the thalamus and eventually the cortex (Figure 1). A key point is that at both sites of action the blood vessels can modulate neural activity. In this model, CGRP can act in both the periphery and CNS to cause migraine symptoms. This hypothesis is based on our unexpected finding from photophobia studies described below. Namely, when mice were sensitized to CGRP by overexpression of a receptor subunit (hRAMP1) in neural tissue, they showed enhanced light aversive behavior following central administration of CGRP, but not following peripheral administration.30 Although this finding does not rule out involvement of the nervous system, it does indicate that the limiting site of peripheral CGRP action is not neuronal. If neurons are not the limiting site in the periphery, then which cells are?
Figure 1.
Model of peripheral and central perivascular CGRP actions. In the periphery, CGRP (green ovals) released from dural trigeminal afferents acts on perivascular cells to cause vasodilation and neurogenic inflammation, with positive feedback loops (blue arrows) leading to peripheral sensitization.33 Nociceptive signals are relayed to the CNS to the thalamus directly and via the parabrachial nucleus. In the CNS, release of CGRP (green ovals) from parabrachial neurons into the posterior thalamic region modulates neural signaling. CGRP in the brain also causes vasodilation,49 which could lead to neural activation by vascular-neural coupling51 and further dilation of vessels in a positive feedback loop (blue arrows) that triggers further CGRP release. CGRP-mediated neuromodulation of glutamatergic synapses results in central sensitization33 and signaling to the cortex that leads to migraine symptoms of photophobia and pain.
In the periphery, it seems likely that a combination of CGRP actions at multiple sites could alter the microenvironment of the trigeminovascular system (Figure 1). Peripheral targets most likely are (1) perivascular cells in the meninges, including mast cells, glia, vessels, primary afferents and (2) trigeminal ganglia cell bodies, including neurons and glia.31 Notably, CGRP is the most potent peptide vasodilator, especially of cranial vessels,10 and CGRP leads to neurogenic inflammation by activating mast cells, which release agents that can sensitize neurons and cause further vasodilation in the dura layer of the meninges.32 This modulation of neural activity could then trigger positive feedback loops that lead to peripheral sensitization of nociceptors.31,33 Dural vessels are fully peripheral, unlike vessels of the pial layer of the meninges. The pial afferents are exposed to cerebrospinal fluid and hence, unlike dural afferents, are within the blood brain barrier.34 CGRP-induced dilation could trigger further dilation by autoregulation of local cerebral blood flow in the pia and parenchyma. In the periphery, CGRP is most likely acting by peripheral sensitization that set up vascular and neural actions in the CNS.
In the CNS, we expect that multiple neural pathways influence photophobia and pain, with the thalamus being a focal point (Figure 1). CGRP and its receptor subunits are widely distributed throughout the CNS, including the retina.35,36 The trigeminal nerve carries nearly all the pain signals from the anterior part of the head31 and is generally thought to be involved in most photophobia pathways.33 The trigeminothalamic tract consists of second order trigeminovascular neurons that directly connect from the trigeminal nucleus to posterior thalamic nuclei (PoT). Note that we use the designation PoT to include all posterior thalamic nuclei, including the ventral posteromedial nucleus (VPM). A second path is via the parabrachial nucleus (PBN), which is known to relay sensory signals, including pain-producing signals, to the forebrain. The role of CGRP-expressing neurons in the PBN in a variety of aversive responses,37 has recently been reviewed.38 In addition, the PBN receives some direct monosynaptic input from the trigeminal nucleus, which contributes to affective pain.39 A key study by Burstein and colleagues identified neurons in the rat PoT (primarily in the dorsal border) that might be involved; they are coordinately activated by dural stimulation of trigeminal afferents and melanopsin-containing retinal ganglion cells.40 A role for CGRP in trigeminal and thalamic pathways is also supported by the presence of CGRP and its receptors in the nociceptive pathway from the meninges to the PoT,41,42 and PoT activation during migraine.43 The presence of CGRP in PoT neurons of the subparafasicular and intralaminar thalamic nuclei is especially intriguing because they receive somatosensory and nociceptive stimuli from ascending pathways.44 In addition, the convergence of auditory and nociception inputs to the CGRP-positive subparafasicular thalamic nucleus are important for conditioned auditory and visual fear responses.45,46
An under-appreciated function of central CGRP is its ability to trigger vasodilation of cerebral blood vessels. While long documented in vitro,47,48 central CGRP-induced vasodilation was only recently shown in vivo,49 ironically, in the thalamus. Furthermore, CGRP could potentially act at vessels distant from its release site by diffusion through interstitial space (volume transmission).50 This would create a second positive feedback loop (Figure 1). In the CNS, this activity would be restricted to perivascular CGRP since it would not pass into the lumen of cerebral vessels. As an aside, CGRP detected in the blood is most likely spillover from peripheral nerves (e.g., from trigeminal afferents),16 although there are also reports of nonneuronal sources of CGRP.10 Dilation of cerebral vessels could then increase neural activity in the CNS by vascular-neural signals. This mechanism is supported by studies with in vitro cortical slices, and might occur either directly or via glial intermediates.51 Coupled with vascular actions, CGRP released from neurons is known to act as a neuromodulator. This activity has been shown to increase glutamatergic transmission, and thus could cause central sensitization.52,53
What Are the Migraine-like Actions of CGRP?
Much of what we know about CGRP actions comes from preclinical studies with mice (Figure 2). Admittedly, we will never be sure if a mouse has a migraine; however, we can examine behaviors that are surrogates for migraine symptoms, such as photophobia and pain. Light aversion can be used as a photophobia assay.30,54,55 Grimace and touch sensitivity can be used as a measure of CGRP-induced spontaneous and evoked pain.56,57
Figure 2.

Summary of CGRP-induced migraine-like symptoms. Injection of CGRP into mice can cause the indicated symptoms of light aversion, decreased movement, and spontaneous and evoked pain.
Photophobia and Decreased Movement
Light aversive behavior has been established as a surrogate for photophobia.30,54,55 Photophobia reflects an allodynic response, that is, nonpainful light becomes noxious, and is one of the diagnostic criteria and most common symptoms of migraine.58 Bright light can trigger a migraine, and even dim light causes discomfort and pain during a migraine. Administration of CGRP to mice drove them into the dark zone.30,54,55 While we cannot be certain that mice experience photophobia, we have ruled out that increased anxiety alone drives the behavior. Thus, we presume that aversion to light must reflect an unpleasant sensation that can overcome their strong, innate exploratory drive. Coupled with this behavior, we noticed that the mice also moved less after being given CGRP. Interestingly, the mice only moved less in the dark zone, which may be similar to how migraineurs prefer to rest when they go into the dark.
One important feature of the assay is that either dim or bright light triggers a response. In dim light (55–70 lx), CGRP-sensitized mice, that is, mice in which hRAMP1, the rate-limiting subunit of the CGRP receptor, is overexpressed in the nervous system (nestin/hRAMP1), but not wildtype or littermate control mice, spent less time in the light following intracerebroventricular (icv) injection of CGRP.54 As mentioned above, this sensitized phenotype was not seen in nestin/hRAMP1 mice after peripheral (intraperitoneal, ip) CGRP delivery compared to wildtype mice.30 The finding that nestin/hRAMP1 mice are sensitized to central CGRP but not to peripheral CGRP suggests that neurons are not the rate-limiting step for peripheral CGRP actions. Given that people who do not get migraine are bothered by bright light, it seemed that if the light intensity is great enough, then wildtype mice might also show CGRP-induced light aversion. This proved to be the case for both icv55 and ip30 CGRP delivery to wildtype mice when the light intensity was increased to the equivalent of that on a sunny day (25–27K lux). Antimigraine drugs of the triptan family blocked the effect of bright and dim light in the contexts of both icv and ip CGRP,30,55 consistent with their ability to inhibit CGRP-induced migraine in humans.25
Spontaneous and Evoked Pain
Pain is admittedly a difficult parameter to objectively measure in mice as well as in people. An indicator of spontaneous pain is the mouse grimace scale developed by Mogil.59 Injection of CGRP induces a grimace response that lasts for over an hour.56 The grimace is partially attenuated by sumatriptan, consistent with the pain being at least partially migraine-like. Importantly, given the role of CGRP in light aversive behavior, the grimace is independent of light, being observed both in the dark and light.56 A strength of this assay is that it is translatable to humans, who also grimace when in pain.59
An indicator of evoked pain is tactile mechanical allodynia of the periorbital facial region and hindpaw using von Frey filaments. This tactile sensitivity is considered to be a painful response to ordinarily nonpainful touch. Sensitivity to light touch, predominantly in the cephalic region, is reported by over half of migraineurs.60 We and others have reported that CGRP enhances sensitivity to von Frey filaments used as mechanical stimuli to the hindpaw.57 Therefore, grimace and tactile allodynia assays provide independent assessments of pain in mice following CGRP treatment.
Why Are the CGRP and CGRP Receptor Blocking Antibodies So Safe Thus Far?
To date, the antibodies have a remarkably clean safety profile. This is despite reasonable concerns about cardiovascular complications of blocking CGRP activity,61 especially given its cardioprotective functions.62 Toward this end, it is encouraging that the CGRP receptor antibody did not affect patients with stable angina on a treadmill test.21 While we must remain cautious, why might the antibodies have been so safe thus far? The answer could lie in part because the antibodies knockdown, but do not knockout CGRP signaling (Figure 3).
Figure 3.
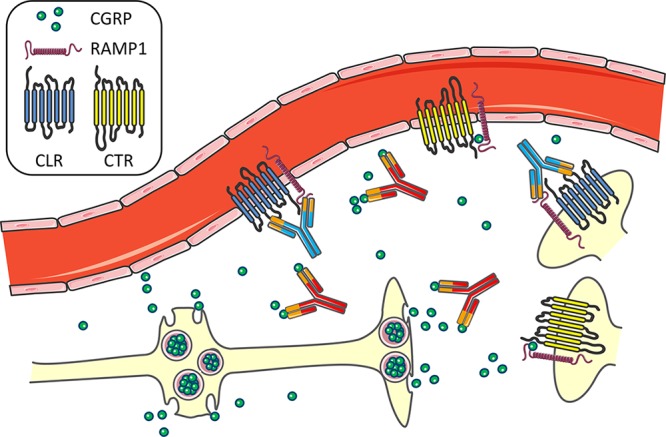
Model of CGRP and CGRP receptor antibody actions in the trigeminovasculature. CGRP is released in a large bolus from dense core vesicles at varicosities and free nerve endings of trigeminal afferents in the meninges. The ligand antibodies are proposed to dampen CGRP signaling by binding some, but not all, of the released CGRP. The receptor antibody blocks the CLR/RAMP1 CGRP receptor, but apparently not the CTR/RAMP1 amylin-1 receptor, which would allow CGRP signaling to still occur at CTR/RAMP1 on vessels and trigeminal nerves.
In the case of the CGRP receptor antibody, it blocks only the canonical CGRP receptor (Figure 3). This receptor is an unusual G protein coupled calcitonin-like receptor (CLR) that requires the RAMP1 accessory protein for both trafficking to the cell surface and binding of CGRP.63,64 Our data indicate that RAMP1 is the rate-limiting subunit.65 Interaction of CLR with related RAMPs generates receptors for adrenomedullin, which is involved in nociception66 but not migraine.11 The hRAMP1 subunit is responsible for the species selectivity of the CGRP receptor antagonists used in clinical studies.67 Importantly, RAMP1 can bind other G protein coupled receptors. Most notably, RAMP1 converts the calcitonin receptor (CTR) into an amylin-1 receptor, which can be a second CGRP receptor.68,69 This receptor is also sensitized in the transgenic hRAMP1 mice.70,71 The amylin-1 (CTR/RAMP1) receptor is reportedly not blocked, based on pharmacological data, by the CGRP receptor antibody that was raised against CLR/RAMP1.72 Since both the amylin-1 and CGRP receptors are expressed in vessels, as well as the trigeminal ganglia and trigeminal nucleus,68 this suggests that there could be at least partial compensation when the CGRP (CLR/RAMP1) receptor is blocked. Thus, in the presence of the CGRP receptor antibody, CGRP could still potentially signal at the amylin-1 receptor.
In the case of the ligand antibodies, the blocking antibodies likely only dampen, not completely block, the relatively slow and prolonged actions of neuropeptides over a relatively large area (Figure 3). The vesicular storage of neuropeptides means that when a neuron is activated, it rapidly releases peptides in a huge bolus that could potentially overwhelm the antibodies. In essence there would be a “binding race” driven by the kinetics of CGRP binding its receptor and being bound by an antibody. To appreciate the odds on this race, a relevant consideration is the number of peptides released from a single vesicle. Primarily on the basis of peptide and capacitance measurements of hypothalamic neurons, it can be estimated there are ∼10 000 peptides per dense core vesicle, with ∼103 vesicles released per sec.50 Thus, millions of neuropeptides can be released in a short burst from just a single neuron. As a result, it seems likely that the blocking antibodies will be able to blunt, but are unlikely to completely block, neuropeptide signaling. Rather, I suggest that the amplitude and duration of volumetric transmission (dispersion in extracellular fluid) of the peptides would be decreased, but not abolished. For a more detailed discussion of the relevance of peptide release and transmission in migraine see ref (50). Hence, the inability to completely block CGRP upon release from neurons and the existence of a second CGRP receptor may contribute to the excellent safety profile that has been reported to date for the CGRP- and CGRP receptor-blocking antibodies in clinical trials.
What Next?
The development of CGRP-based drugs is clearly a great beginning;73 however, there are unmet gaps for treatments. For example, a clinically relevant end point of 50% reduction of headache days is achieved in only about half of the subjects in trials.74 To improve existing treatments and develop new drugs, we need to know more about where and how CGRP acts. Along this line, centrally acting CGRP antagonists should be considered. Importantly, given the actions of CGRP in central sensitization of pain,75 it seems likely that CGRP targeted drugs will also be effective for other chronic pain syndromes. However, this potential benefit of a centrally acting antagonist will need to be balanced against the likely risk of greater side effects on CNS function. Likewise, a better understanding of the structures of both CGRP receptors will help direct drug design for both peripheral and central target engagement. In this regard, the recently solved cryo-EM structure of the active canonical CGRP receptor (CGRP:CLR:RAMP1:Gs heterotrimer)76 is likely to provide new perspectives for drug development. In this vein, antibodies and drugs that target the second CGRP receptor should be considered. In addition, combinatorial treatments, in which CGRP targeted therapies may be integrated into other treatment regimens, such as NSAIDs, may prove effective.
Finally, where to after CGRP? New drug targets should include other neuropeptides. Indeed, at least two pharmaceutical companies are moving forward with antibodies that block either PACAP-38 or the PAC1 PACAP receptor. Given the shared activities of CGRP and PACAP,77,78 this is likely to be a fruitful endeavor. But these two peptides are just the very tip of the iceberg. There are hundreds of neuropeptides that can act within the brain as neuromodulators and within the periphery as signaling molecules, which makes them well poised to alter sensory perception in migraine.50 In the periphery, an example of neuropeptide actions is at the cerebrovasculature, which is heavily innervated by sensory, parasympathetic, and sympathetic nerves.79 These nerves release several neuropeptides, including CGRP, PACAP, and NPY, that can either increase or decrease vascular tone. Whether in the perivasculature or deep in the CNS, members of the diverse families of neuropeptides could add to the pathogenesis and complexity of migraine.50 Clearly CGRP will not be the only neuropeptide involved in migraine and given the emerging evidence for other peptides, it seems reasonable that altered neuropeptide actions may be a good target for reducing the heightened sensory state of migraine.
In summary, the advent of new CGRP-based migraine drugs established the significance of CGRP in migraine, yet also highlighted how little we know about the underlying mechanisms. It seems likely that we are heading for a very exciting time in the field.
Acknowledgments
Thanks to members of the Russo lab for their inspiration and comments. This work was supported by grants from the National Institutes of Health (NS075599), Veterans Affairs Medical Center (1I01RX002101), and Department of Defense USAMRAA (W81XWH-16-1-0071 and W81XWH-16-1-0211). AFR is a consultant to Alder BioPharmaceuticals, Pharmnovo, Amgen/Novartis, and Eli Lilly.
The author declares no competing financial interest.
This article is made available for a limited time sponsored by ACS under the ACS Free to Read License, which permits copying and redistribution of the article for non-commercial scholarly purposes.
References
- Charles A. (2013) The evolution of a migraine attack - a review of recent evidence. Headache 53, 413–419. 10.1111/head.12026. [DOI] [PubMed] [Google Scholar]
- de Tommaso M.; Ambrosini A.; Brighina F.; et al. (2014) Altered processing of sensory stimuli in patients with migraine. Nat. Rev. Neurol. 10, 144–155. 10.1038/nrneurol.2014.14. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Holland P. R.; Martins-Oliveira M.; et al. (2017) Pathophysiology of Migraine: A Disorder of Sensory Processing. Physiol. Rev. 97, 553–622. 10.1152/physrev.00034.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burch R.; Rizzoli P.; Loder E. (2018) The Prevalence and Impact of Migraine and Severe Headache in the United States: Figures and Trends From Government Health Studies. Headache 58, 496–505. 10.1111/head.13281. [DOI] [PubMed] [Google Scholar]
- Lipton R. B.; Bigal M. E.; Diamond M.; et al. (2007) Migraine prevalence, disease burden, and the need for preventive therapy. Neurology 68, 343–349. 10.1212/01.wnl.0000252808.97649.21. [DOI] [PubMed] [Google Scholar]
- Vos T.; Abajobir A. A.; Abate K. H.; Abbafati C.; Abbas K. M.; Abd-Allah F.; Abdulkader R. S.; Abdulle A. M.; Abebo T. A.; Abera S. F.; et al. (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390, 1211–1259. 10.1016/S0140-6736(17)32154-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart W. F.; Wood C.; Reed M. L.; et al. (2008) Cumulative lifetime migraine incidence in women and men. Cephalalgia 28, 1170–1178. 10.1111/j.1468-2982.2008.01666.x. [DOI] [PubMed] [Google Scholar]
- Bonafede M.; Sapra S.; Shah N. (2018) Direct and Indirect Healthcare Resource Utilization and Costs Among Migraine Patients in the United States. Headache 58, 700. 10.1111/head.13275. [DOI] [PubMed] [Google Scholar]
- Charleston Iv L.; Burke J. F. (2018) Do racial/ethnic disparities exist in recommended migraine treatments in US ambulatory care?. Cephalalgia 38, 876–882. 10.1177/0333102417716933. [DOI] [PubMed] [Google Scholar]
- Russell F. A.; King R.; Smillie S. J.; et al. (2014) Calcitonin gene-related peptide: physiology and pathophysiology. Physiol. Rev. 94, 1099–1142. 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petersen K. A.; Birk S.; Kitamura K.; et al. (2009) Effect of adrenomedullin on the cerebral circulation: relevance to primary headache disorders. Cephalalgia 29, 23–30. 10.1111/j.1468-2982.2008.01695.x. [DOI] [PubMed] [Google Scholar]
- Rahmann A.; Wienecke T.; Hansen J. M.; et al. (2008) Vasoactive intestinal peptide causes marked cephalic vasodilation, but does not induce migraine. Cephalalgia 28, 226–236. 10.1111/j.1468-2982.2007.01497.x. [DOI] [PubMed] [Google Scholar]
- Jacobs B.; Dussor G. (2016) Neurovascular contributions to migraine: Moving beyond vasodilation. Neuroscience 338, 130–144. 10.1016/j.neuroscience.2016.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amin F. M.; Hougaard A.; Schytz H. W.; et al. (2014) Investigation of the pathophysiological mechanisms of migraine attacks induced by pituitary adenylate cyclase-activating polypeptide-38. Brain 137, 779–794. 10.1093/brain/awt369. [DOI] [PubMed] [Google Scholar]
- Jansen-Olesen I.; Hougaard Pedersen S. (2018) PACAP and its receptors in cranial arteries and mast cells. J. Headache Pain 19, 16. 10.1186/s10194-017-0822-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L.; Haanes K. A.; Warfvinge K.; et al. (2018) CGRP as the target of new migraine therapies - successful translation from bench to clinic. Nat. Rev. Neurol. 14, 338–350. 10.1038/s41582-018-0003-1. [DOI] [PubMed] [Google Scholar]
- Ashina M.; Dodick D.; Goadsby P. J.; et al. (2017) Erenumab (AMG 334) in episodic migraine: Interim analysis of an ongoing open-label study. Neurology 89, 1237–1243. 10.1212/WNL.0000000000004391. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Reuter U.; Hallstrom Y.; et al. (2017) A Controlled Trial of Erenumab for Episodic Migraine. N. Engl. J. Med. 377, 2123–2132. 10.1056/NEJMoa1705848. [DOI] [PubMed] [Google Scholar]
- Silberstein S. D.; Dodick D. W.; Bigal M. E.; et al. (2017) Fremanezumab for the Preventive Treatment of Chronic Migraine. N. Engl. J. Med. 377, 2113–2122. 10.1056/NEJMoa1709038. [DOI] [PubMed] [Google Scholar]
- Maasumi K.; Michael R. L.; Rapoport A. M. (2018) CGRP and Migraine: The Role of Blocking Calcitonin Gene-Related Peptide Ligand and Receptor in the Management of Migraine. Drugs 78, 913–928. 10.1007/s40265-018-0923-5. [DOI] [PubMed] [Google Scholar]
- Depre C.; Antalik L.; Starling A.; et al. (2018) A Randomized, Double-Blind, Placebo-Controlled Study to Evaluate the Effect of Erenumab on Exercise Time During a Treadmill Test in Patients With Stable Angina. Headache 58, 715–723. 10.1111/head.13316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moskowitz M. A. (1993) Neurogenic inflammation in the pathophysiology and treatment of migraine. Neurology 43, S16–20. [PubMed] [Google Scholar]
- Charles A. (2013) Vasodilation out of the picture as a cause of migraine headache. Lancet Neurol. 12, 419–420. 10.1016/S1474-4422(13)70051-6. [DOI] [PubMed] [Google Scholar]
- Mason B. N.; Russo A. F. (2018) Vascular Contributions to Migraine: Time to Revisit?. Front. Cell. Neurosci. 12, 233. 10.3389/fncel.2018.00233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asghar M. S.; Hansen A. E.; Amin F. M.; et al. (2011) Evidence for a vascular factor in migraine. Ann. Neurol. 69, 635–645. 10.1002/ana.22292. [DOI] [PubMed] [Google Scholar]
- Amin F. M.; Asghar M. S.; Hougaard A.; et al. (2013) Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 12, 454–461. 10.1016/S1474-4422(13)70067-X. [DOI] [PubMed] [Google Scholar]
- Mahmoud A. N.; Mentias A.; Elgendy A. Y.; et al. (2018) Migraine and the risk of cardiovascular and cerebrovascular events: a meta-analysis of 16 cohort studies including 1 152 407 subjects. BMJ. Open 8, e020498. 10.1136/bmjopen-2017-020498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyholt D. R.; Borsook D.; Griffiths L. R. (2017) Migrainomics - identifying brain and genetic markers of migraine. Nat. Rev. Neurol. 13, 725–741. 10.1038/nrneurol.2017.151. [DOI] [PubMed] [Google Scholar]
- Johnson M. P.; Ellis B. B.; Maren D. L. (2016) Peripheral and central nervous system distribution of a CGRP neutralizing antibody [125I]-LY2951742 in male rats. Neurology 78, I3.002. [DOI] [PubMed] [Google Scholar]
- Mason B. N.; Kaiser E. A.; Kuburas A.; et al. (2017) Induction of migraine-like photophobic behavior in mice by both peripheral and central CGRP mechanisms. J. Neurosci. 37, 204–216. 10.1523/JNEUROSCI.2967-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messlinger K.; Russo A. F. (2018) Current understanding of trigeminal ganglion structure and function in headache. Cephalalgia 86261. 10.1177/0333102418786261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raddant A. C.; Russo A. F. (2011) Calcitonin gene-related peptide in migraine: intersection of peripheral inflammation and central modulation. Expert Rev. Mol. Med. 13, e36 10.1017/S1462399411002067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. F. (2015) Calcitonin Gene-Related Peptide (CGRP): A New Target for Migraine. Annu. Rev. Pharmacol. Toxicol. 55, 533–552. 10.1146/annurev-pharmtox-010814-124701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coles J. A.; Myburgh E.; Brewer J. M.; et al. (2017) Where are we? The anatomy of the murine cortical meninges revisited for intravital imaging, immunology, and clearance of waste from the brain. Prog. Neurobiol. 156, 107–148. 10.1016/j.pneurobio.2017.05.002. [DOI] [PubMed] [Google Scholar]
- Warfvinge K.; Edvinsson L. (2017) Distribution of CGRP and CGRP receptor components in the rat brain. Cephalalgia 8873. 10.1177/0333102417728873. [DOI] [PubMed] [Google Scholar]
- Blixt F. W.; Radziwon-Balicka A.; Edvinsson L.; et al. (2017) Distribution of CGRP and its receptor components CLR and RAMP1 in the rat retina. Exp. Eye Res. 161, 124–131. 10.1016/j.exer.2017.06.002. [DOI] [PubMed] [Google Scholar]
- Campos C. A.; Bowen A. J.; Roman C. W.; et al. (2018) Encoding of danger by parabrachial CGRP neurons. Nature 555, 617–622. 10.1038/nature25511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmiter R. D. (2018) The Parabrachial Nucleus: CGRP Neurons Function as a General Alarm. Trends Neurosci. 41, 280–293. 10.1016/j.tins.2018.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez E.; Sakurai K.; Xu J.; et al. (2017) A craniofacial-specific monosynaptic circuit enables heightened affective pain. Nat. Neurosci. 20, 1734–1743. 10.1038/s41593-017-0012-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noseda R.; Kainz V.; Jakubowski M.; et al. (2010) A neural mechanism for exacerbation of headache by light. Nat. Neurosci. 13, 239–245. 10.1038/nn.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimalawansa S. J. (1996) Calcitonin gene-related peptide and its receptors: molecular genetics, physiology, pathophysiology, and therapeutic potentials. Endocr. Rev. 17, 533–585. 10.1210/edrv-17-5-533. [DOI] [PubMed] [Google Scholar]
- Summ O.; Charbit A. R.; Andreou A. P.; et al. (2010) Modulation of nocioceptive transmission with calcitonin gene-related peptide receptor antagonists in the thalamus. Brain 133, 2540–2548. 10.1093/brain/awq224. [DOI] [PubMed] [Google Scholar]
- Afridi S. K.; Giffin N. J.; Kaube H.; et al. (2005) A positron emission tomographic study in spontaneous migraine. Arch. Neurol. 62, 1270–1275. 10.1001/archneur.62.8.1270. [DOI] [PubMed] [Google Scholar]
- de Lacalle S.; Saper C. B. (2000) Calcitonin gene-related peptide-like immunoreactivity marks putative visceral sensory pathways in human brain. Neuroscience 100, 115–130. 10.1016/S0306-4522(00)00245-1. [DOI] [PubMed] [Google Scholar]
- Coolen L. M.; Veening J. G.; Petersen D. W.; et al. (2003) Parvocellular subparafascicular thalamic nucleus in the rat: anatomical and functional compartmentalization. J. Comp. Neurol. 463, 117–131. 10.1002/cne.10740. [DOI] [PubMed] [Google Scholar]
- Coolen L. M.; Veening J. G.; Wells A. B.; et al. (2003) Afferent connections of the parvocellular subparafascicular thalamic nucleus in the rat: evidence for functional subdivisions. J. Comp. Neurol. 463, 132–156. 10.1002/cne.10739. [DOI] [PubMed] [Google Scholar]
- Edwards R. M.; Trizna W. (1991) Calcitonin gene-related peptide stimulates adenylate cyclase and relaxes intracerebral arterioles. J. Pharmacol Exp Ther 257, 1020–1024. [PubMed] [Google Scholar]
- Fergus A.; Jin Y.; Thai Q. A.; et al. (1995) Vasodilatory actions of calcitonin gene-related peptide and nitric oxide in parenchymal microvessels of the rat hippocampus. Brain Res. 694, 78–84. 10.1016/0006-8993(95)00768-L. [DOI] [PubMed] [Google Scholar]
- Desai M.; Slusarczyk A. L.; Chapin A.; et al. (2016) Molecular imaging with engineered physiology. Nat. Commun. 7, 13607. 10.1038/ncomms13607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. F. (2017) Overview of Neuropeptides: Awakening the Senses?. Headache 57 (2), 37–46. 10.1111/head.13084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J.; Ramiro Diaz J.; Iddings J. A.; et al. (2016) Vasculo-Neuronal Coupling: Retrograde Vascular Communication to Brain Neurons. J. Neurosci. 36, 12624–12639. 10.1523/JNEUROSCI.1300-16.2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han J. S.; Li W.; Neugebauer V. (2005) Critical role of calcitonin gene-related peptide 1 receptors in the amygdala in synaptic plasticity and pain behavior. J. Neurosci. 25, 10717–10728. 10.1523/JNEUROSCI.4112-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seybold V. S. (2009) The role of peptides in central sensitization. Handb. Exp. Pharmacol. 194, 451–491. 10.1007/978-3-540-79090-7_13. [DOI] [PubMed] [Google Scholar]
- Recober A.; Kuburas A.; Zhang Z.; et al. (2009) Role of calcitonin gene-related peptide in light-aversive behavior: implications for migraine. J. Neurosci. 29, 8798–8804. 10.1523/JNEUROSCI.1727-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E. A.; Kuburas A.; Recober A.; et al. (2012) Modulation of CGRP-induced light aversion in wild-type mice by a 5-HT(1B/D) agonist. J. Neurosci. 32, 15439–15449. 10.1523/JNEUROSCI.3265-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rea B. J.; Wattiez A. S.; Waite J. S.; et al. (2018) Peripherally administered calcitonin gene-related peptide induces spontaneous pain in mice: implications for migraine. Pain 159, 2306–2317. 10.1097/j.pain.0000000000001337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marquez de Prado B.; Hammond D. L.; Russo A. F. (2009) Genetic enhancement of calcitonin gene-related peptide-induced central sensitization to mechanical stimuli in mice. J. Pain 10, 992–1000. 10.1016/j.jpain.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo A. F.; Recober A. (2013) Unanswered questions in headache: so what is photophobia, anyway?. Headache 53, 1677–1678. 10.1111/head.12231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langford D. J.; Bailey A. L.; Chanda M. L.; et al. (2010) Coding of facial expressions of pain in the laboratory mouse. Nat. Methods 7, 447–449. 10.1038/nmeth.1455. [DOI] [PubMed] [Google Scholar]
- Burstein R.; Yarnitsky D.; Goor-Aryeh I.; et al. (2000) An association between migraine and cutaneous allodynia. Ann. Neurol. 47, 614–624. . [DOI] [PubMed] [Google Scholar]
- MaassenVanDenBrink A.; Meijer J.; Villalon C. M.; et al. (2016) Wiping Out CGRP: Potential Cardiovascular Risks. Trends Pharmacol. Sci. 37, 779–788. 10.1016/j.tips.2016.06.002. [DOI] [PubMed] [Google Scholar]
- Kee Z.; Kodji X.; Brain S. D. (2018) The Role of Calcitonin Gene Related Peptide (CGRP) in Neurogenic Vasodilation and Its Cardioprotective Effects. Front. Physiol. 9, 1249. 10.3389/fphys.2018.01249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLatchie L. M.; Fraser N. J.; Main M. J.; et al. (1998) RAMPs regulate the transport and ligand specificity of the calcitonin-receptor-like receptor. Nature 393, 333–339. 10.1038/30666. [DOI] [PubMed] [Google Scholar]
- Hay D. L.; Walker C. S. (2017) CGRP and its receptors. Headache 57, 625–636. 10.1111/head.13064. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Winborn C. S.; Marquez de Prado B.; et al. (2007) Sensitization of calcitonin gene-related peptide receptors by receptor activity-modifying protein-1 in the trigeminal ganglion. J. Neurosci. 27, 2693–2703. 10.1523/JNEUROSCI.4542-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma W.; Chabot J. G.; Quirion R. (2006) A role for adrenomedullin as a pain-related peptide in the rat. Proc. Natl. Acad. Sci. U. S. A. 103, 16027–16032. 10.1073/pnas.0602488103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallee J. J.; Salvatore C. A.; LeBourdelles B.; et al. (2002) Receptor activity-modifying protein 1 determines the species selectivity of non-peptide CGRP receptor antagonists. J. Biol. Chem. 277, 14294–14298. 10.1074/jbc.M109661200. [DOI] [PubMed] [Google Scholar]
- Walker C. S.; Eftekhari S.; Bower R. L.; et al. (2015) A second trigeminal CGRP receptor: function and expression of the AMY1 receptor. Ann. Clin. Transl. Neurol. 2, 595–608. 10.1002/acn3.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hendrikse E. R.; Bower R. L.; Hay D. L.; Walker C. S. (2018) Molecular studies of CGRP and the CGRP family of peptides in the central nervous system. Cephalalgia 5787. 10.1177/0333102418765787. [DOI] [PubMed] [Google Scholar]
- Zhang Z.; Liu X.; Morgan D. A.; et al. (2011) Neuronal receptor activity-modifying protein 1 promotes energy expenditure in mice. Diabetes 60, 1063–1071. 10.2337/db10-0692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohn K. J.; Li B.; Huang X.; et al. (2017) CGRP receptor activity in mice with global expression of human receptor activity modifying protein 1. Br. J. Pharmacol. 174, 1826–1840. 10.1111/bph.13783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L.; Lehto S. G.; Zhu D. X.; et al. (2016) Pharmacologic Characterization of AMG 334, a Potent and Selective Human Monoclonal Antibody against the Calcitonin Gene-Related Peptide Receptor. J. Pharmacol. Exp. Ther. 356, 223–231. 10.1124/jpet.115.227793. [DOI] [PubMed] [Google Scholar]
- Reuter U. (2018) A Review of Monoclonal Antibody Therapies and Other Preventative Treatments in Migraine. Headache 58 (1), 48–59. 10.1111/head.13302. [DOI] [PubMed] [Google Scholar]
- Loder E. W.; Robbins M. S. (2018) Monoclonal Antibodies for Migraine Prevention: Progress, but Not a Panacea. JAMA 319, 1985–1987. 10.1001/jama.2018.4852. [DOI] [PubMed] [Google Scholar]
- Iyengar S.; Ossipov M. H.; Johnson K. W. (2017) The role of calcitonin gene-related peptide in peripheral and central pain mechanisms including migraine. Pain 158, 543–559. 10.1097/j.pain.0000000000000831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang Y. L.; Khoshouei M.; Deganutti G.; et al. (2018) Cryo-EM structure of the active, Gs-protein complexed, human CGRP receptor. Nature 561, 492–497. 10.1038/s41586-018-0535-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaiser E. A.; Russo A. F. (2013) CGRP and migraine: could PACAP play a role too?. Neuropeptides 47, 451–461. 10.1016/j.npep.2013.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L.; Tajti J.; Szalardy L.; et al. (2018) PACAP and its role in primary headaches. J. Headache Pain 19, 21. 10.1186/s10194-018-0852-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edvinsson L.; Uddman R. (2005) Neurobiology in primary headaches. Brain Res. Rev. 48, 438–456. 10.1016/j.brainresrev.2004.09.007. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L.; Ekman R. (1990) Vasoactive peptide release in the extracerebral circulation of humans during migraine headache. Ann. Neurol. 28, 183–187. 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- Goadsby P. J.; Edvinsson L. (1993) The trigeminovascular system and migraine: studies characterizing cerebrovascular and neuropeptide changes seen in humans and cats. Ann. Neurol. 33, 48–56. 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- van Dongen R. M.; Zielman R.; Noga M.; et al. (2017) Migraine biomarkers in cerebrospinal fluid: A systematic review and meta-analysis. Cephalalgia 37, 49–63. 10.1177/0333102415625614. [DOI] [PubMed] [Google Scholar]
- Cady R. K.; Vause C. V.; Ho T. W.; et al. (2009) Elevated saliva calcitonin gene-related peptide levels during acute migraine predict therapeutic response to rizatriptan. Headache 49, 1258–1266. 10.1111/j.1526-4610.2009.01523.x. [DOI] [PubMed] [Google Scholar]
- Juhasz G.; Zsombok T.; Jakab B.; et al. (2005) Sumatriptan causes parallel decrease in plasma calcitonin gene-related peptide (CGRP) concentration and migraine headache during nitroglycerin induced migraine attack. Cephalalgia 25, 179–183. 10.1111/j.1468-2982.2005.00836.x. [DOI] [PubMed] [Google Scholar]
- Ashina M.; Bendtsen L.; Jensen R.; et al. (2000) Evidence for increased plasma levels of calcitonin gene-related peptide in migraine outside of attacks. Pain 86, 133–138. 10.1016/S0304-3959(00)00232-3. [DOI] [PubMed] [Google Scholar]
- Cernuda-Morollon E.; Larrosa D.; Ramon C.; et al. (2013) Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology 81, 1191–1196. 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- Tvedskov J. F.; Lipka K.; Ashina M.; et al. (2005) No increase of calcitonin gene-related peptide in jugular blood during migraine. Ann. Neurol. 58, 561–568. 10.1002/ana.20605. [DOI] [PubMed] [Google Scholar]
- Lee M. J.; Lee S. Y.; Cho S.; et al. (2018) Feasibility of serum CGRP measurement as a biomarker of chronic migraine: a critical reappraisal. J. Headache Pain 19, 53. 10.1186/s10194-018-0883-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lassen L. H.; Haderslev P. A.; Jacobsen V. B.; et al. (2002) CGRP may play a causative role in migraine. Cephalalgia 22, 54–61. 10.1046/j.1468-2982.2002.00310.x. [DOI] [PubMed] [Google Scholar]
- Hansen J. M.; Hauge A. W.; Olesen J.; et al. (2010) Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia 30, 1179–1186. 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- Guo S.; Vollesen A. L. H.; Olesen J.; Ashina M. (2016) Premonitory and non-headache symptoms induced by CGRP and PACAP38 in migraine patients. Pain 157, 2773. 10.1097/j.pain.0000000000000702. [DOI] [PubMed] [Google Scholar]
- Petersen K. A.; Lassen L. H.; Birk S.; et al. (2005) BIBN4096BS antagonizes human alpha-calcitonin gene related peptide-induced headache and extracerebral artery dilatation. Clin. Pharmacol. Ther. 77, 202–213. 10.1016/j.clpt.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Hansen J. M.; Thomsen L. L.; Olesen J.; et al. (2008) Calcitonin gene-related peptide does not cause the familial hemiplegic migraine phenotype. Neurology 71, 841–847. 10.1212/01.wnl.0000325482.64106.3f. [DOI] [PubMed] [Google Scholar]
- Hansen J. M.; Thomsen L. L.; Olesen J.; et al. (2011) Calcitonin gene-related peptide does not cause migraine attacks in patients with familial hemiplegic migraine. Headache 51, 544–553. 10.1111/j.1526-4610.2011.01861.x. [DOI] [PubMed] [Google Scholar]
- Olesen J.; Diener H. C.; Husstedt I. W.; et al. (2004) Calcitonin gene-related peptide receptor antagonist BIBN 4096 BS for the acute treatment of migraine. N. Engl. J. Med. 350, 1104–1110. 10.1056/NEJMoa030505. [DOI] [PubMed] [Google Scholar]
- Ho T. W.; Ferrari M. D.; Dodick D. W.; et al. (2008) Efficacy and tolerability of MK-0974 (telcagepant), a new oral antagonist of calcitonin gene-related peptide receptor, compared with zolmitriptan for acute migraine: a randomised, placebo-controlled, parallel-treatment trial. Lancet 372, 2115–2123. 10.1016/S0140-6736(08)61626-8. [DOI] [PubMed] [Google Scholar]
- Connor K. M.; Shapiro R. E.; Diener H. C.; et al. (2009) Randomized, controlled trial of telcagepant for the acute treatment of migraine. Neurology 73, 970–977. 10.1212/WNL.0b013e3181b87942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho T. W.; Connor K. M.; Zhang Y.; et al. (2014) Randomized controlled trial of the CGRP receptor antagonist telcagepant for migraine prevention. Neurology 83, 958–966. 10.1212/WNL.0000000000000771. [DOI] [PubMed] [Google Scholar]
- Dodick D. W.; Silberstein S. D.; Bigal M. E.; et al. (2018) Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA 319, 1999–2008. 10.1001/jama.2018.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stauffer V. L.; Dodick D. W.; Zhang Q. (2018) Evaluation of Galcanezumab for the Prevention of Episodic Migraine: The EVOLVE-1 Randomized Clinical Trial. JAMA Neurol. 75, 1080. 10.1001/jamaneurol.2018.1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skljarevski V.; Matharu M.; Millen B. A.; Ossipov M. H.; Kim B.-K.; Yang J. Y. (2018) Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia 38, 9543. 10.1177/0333102418779543. [DOI] [PubMed] [Google Scholar]



