Abstract
Background
Clear cell sarcoma (CCS) of soft tissue, or malignant melanoma of soft parts, is a rare disease. We aimed to identify prognostic factors linked to patient survival in CCS by analyzing demographic and clinical features using the Surveillance, Epidemiology, and End Results (SEER) database. This study aimed to identify prognostic factors associated with CCS that would be of clinical value.
Material/Methods
We collected data from patients diagnosed with CCS between 1973 and 2009 from the SEER database. The Kaplan-Meier method and Cox regression analysis were performed to identify prognostic factors for patient survival.
Results
A total of 175 patients with CCS were identified from the SEER database. The 5-year survival rate was 62.9%, and the 10-year survival rate was 51.3%. Patients with CCS with local stage, and with tumor size ≤3 cm were more likely to have good survival rates.
Conclusions
The findings from this study showed that the identifiable prognostic factors in patients with CCS were stage and tumor size. Local stage and tumor size ≤3 cm were favorable prognostic factors for patient survival in CCS.
MeSH Keywords: Sarcoma, Clear Cell; SEER Program; Survival Analysis
Background
The first 21 cases of clear cell sarcoma (CCS) of soft tissue, or malignant melanoma of soft parts, were reported in 1965 [1]. Since then, worldwide, there have been reported individual cases and case series of clear cell sarcoma. CCS is a rare but distinct clinicopathologic entity, accounting for approximately 1% of all soft-tissue sarcomas [2]. In 1983, Chung used the term, malignant melanoma of soft parts [3] because of its clinical and histological similarities with malignant melanoma [4–6].
CCS usually presents in the deep soft tissue of the extremities, often adjacent to tendons, aponeuroses, or fascial structures. Most cases of CCS occur in adolescents and young adults. The histology of CCS shows nests of polygonal or fusiform cells, with groups of cells encased by delicate fibrous septa. Immunohistochemical staining shows positive staining for S-100 and HMB45. Despite their clinical and histological similarities, most CCS have a recurrent chromosomal translocation t(12;22) (q13;q12), which is associated with the EWS gene on chromosome 22q and the ATF1 gene on 12q. Recently, cyclic adenosine monophosphate responsive element binding protein 1 (CREB1) has been found in CCS but is not found in melanoma, which supports the distinction between the two tumor types.
Recently, several studies with small numbers of patients have reported 5-year survival rates ranging from 30–67% [7–13]. Due to the low incidence of the CCS and the limited sample size of previous studies, it is necessary to perform studies with larger study size to enhance the understanding of the behavior of CCS and to help diagnosis and prognosis. Therefore, this study used the Surveillance, Epidemiology, and End Results (SEER) database, which covers 30% of the entire US population, to accumulate a sufficient number of cases for investigation. The SEER database has been used extensively for studying rare cancers [14–17]. This population-based study aimed to identify prognostic factors that were linked to survival from CCS by analyzing demographic and actors associated with CCS that would be of clinical value.
Material and Methods
Patient cohort
The Surveillance, Epidemiology, and End Results (SEER) is a free public cancer database. SEER collects data from 18 geographic registries, representing approximately 30% of the US population [18]. We applied for an account to access data and to determine frequency rates. Inclusion criteria for this study included patients with a diagnosis of clear cell sarcoma (CCS) between 1973 and 2009, and a histological type according to the International Classification of Diseases for Oncology, Third Edition (ICD-O-3) code 9044. The primary site was selected as C49, and the location of the tumor was limited to the limbs and pelvis. There were 175 patients with CCS identified from the SEER database.
Ethical approval and consent to participate
The clinical data used was from the SEER database, which is a public research resource, and patient consent and ethical approval for the study were not required.
Statistical analysis
The incidence rates and clinical trends for CCS were analyzed using SEER*Stat version 8.3.5 (National Cancer Institute, Bethesda, MD, USA). Incidence rates were age-adjusted to the 2000 US standard population. Annual percentage changes were calculated using the weighted least squared method. Demographic and clinical factors were analyzed using descriptive statistics, and the chi-squared test was used to calculate correlations between categorical variables. Cutoff values for tumor size were determined according to the area under the curve (AUC) (Figure 1). Kaplan-Meier curves were generated to assess disease-specific survival, and differences between groups were compared using log-rank analysis. Cox proportional hazard regression was performed on demographic, clinical, and treatment factors to estimate survival differences. Cox regression analysis was used for factors that had statistical significance in univariate analysis. We processed and analyzed the data using statistical software R (version 3.34, http://www.r-project.org). P<0.05 indicated statistical significance.
Figure 1.
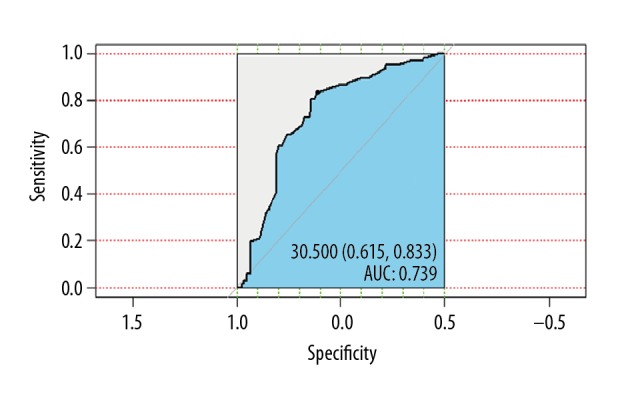
Identification of the optimal cutoff values for tumor size in clear cell sarcoma (CCS) using the area under the curve (AUC).
Results
Patient baseline characteristics
A total of 257 patients with clear cell sarcoma (CCS) were identified from the Surveillance, Epidemiology, and End Results (SEER) database. There were 82 patients who were excluded due to missing data. Finally, 175 patients were included in the study, and their characteristics are summarized in Table 1.
Table 1.
Descriptive demographic and clinical statistics of the study population with clear cell sarcoma (CCS).
| Characteristics | Number of cases | Valid % of total |
|---|---|---|
| Total number of patients | 175 | 100.00 |
| Marital status | ||
| Divorced | 14 | 8.00 |
| Married | 83 | 47.43 |
| Single | 71 | 40.57 |
| Widowed | 7 | 4 |
| Age | ||
| ≤25 | 43 | 24.57 |
| 25 to 60 | 104 | 59.43 |
| >60 | 28 | 16.00 |
| Sex | ||
| Female | 77 | 44.00 |
| Male | 98 | 56.00 |
| Race | ||
| Black | 25 | 14.29 |
| Other | 16 | 9.14 |
| White | 134 | 76.57 |
| Tumor site | ||
| Upper limb | 45 | 25.71 |
| Lower limb | 130 | 74.29 |
| Stage | ||
| Localized | 101 | 57.71 |
| Regional | 59 | 33.71 |
| Distant | 15 | 8.57 |
| Tumor size | ||
| ≤3 | 78 | 44.57 |
| >3 | 97 | 55.43 |
| Surgery | ||
| Yes | 164 | 93.71 |
| No | 11 | 6.29 |
| Radiation | ||
| No | 95 | 54.29 |
| Yes | 80 | 45.71 |
| Chemotherapy | ||
| No | 145 | 82.86 |
| Yes | 30 | 17.14 |
Of the 175 patients with CCS, 98 patients (56.0%) were men, and 77 (44.0%) were women. Among patients with a given stage, 101 patients (57.71%) were diagnosed with localized CCS, 59 patients (33.71%) were diagnosed with regional CCS, and 15 patients (8.57%) were diagnosed with distant or metastatic CCS. The most common tumor location at diagnosis was the lower extremities, in 74.29% of cases. Tumor size was most commonly ≤3 cm at the time of diagnosis (44.57%). A total of 164 patients (93.71%) underwent surgical resection. Eighty patients (45.71%) underwent radiation therapy, and only 30 patients (17.14%) underwent chemotherapy for CCS.
Survival rate
In the 175 patients with CCS, the 5-year disease-specific survival was 62.9%, and the 10-year disease-specific survival was 51.3% (Figure 2). The population-adjusted incidence of CCS during the 35-year study period ranged from 0.006/100,000 in 1973 to 0.014/100,000 in 2009 (Figure 3). There was no significant difference in the annual percent change in incidence during the study period.
Figure 2.
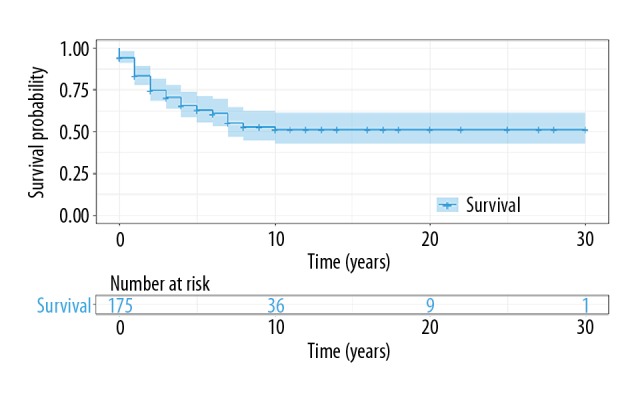
Kaplan-Meier plot for patient survival in the study population with clear cell sarcoma (CCS).
Figure 3.
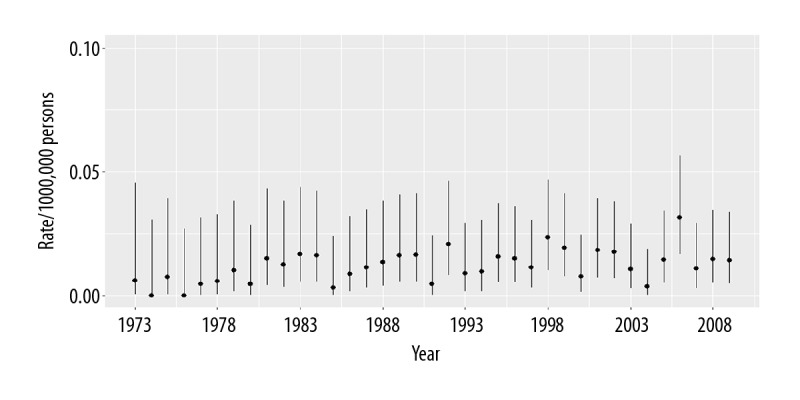
Incidence of clear cell sarcoma (CCS), age-adjusted to the 2000 US standard population.
When stratified according to tumor size, we found that the 5-year disease-specific survival for patients with tumor size >3 cm was lower (42.8%) than for patients with a tumor size ≤3 cm (86.2%; P<0.0001). Also, 10-year disease-specific survival for patients with tumor size >3 cm was much lower (26.4%) than for patients with a tumor size ≤3 cm (80.9%; P<0.0001). The Kaplan-Meier survival curves by tumor size are shown in Figure 4.
Figure 4.
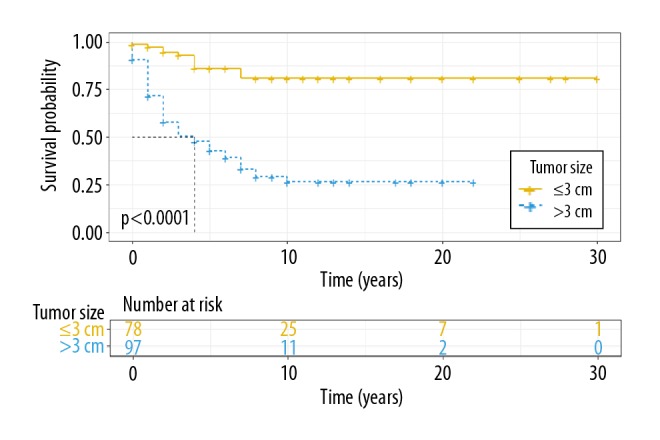
Kaplan-Meier plot of disease-specific survival by tumor size in clear cell sarcoma (CCS).
When stratified according to tumor stage, we found that 5-year disease-specific survival for patients with a localized stage was much higher (82.4%) than for patients diagnosed at a regional stage (44%; P<0.0001). The 10-year disease-specific survival for patients with a localized stage was much higher (68.8%) than for patients diagnosed at a regional stage (32.5%; P<0.0001). None of the patients who were diagnosed at a distant stage survived for 5 years, and 2-year survival was 6.67%. The Kaplan-Meier survival curves by stage are shown in Figure 5.
Figure 5.
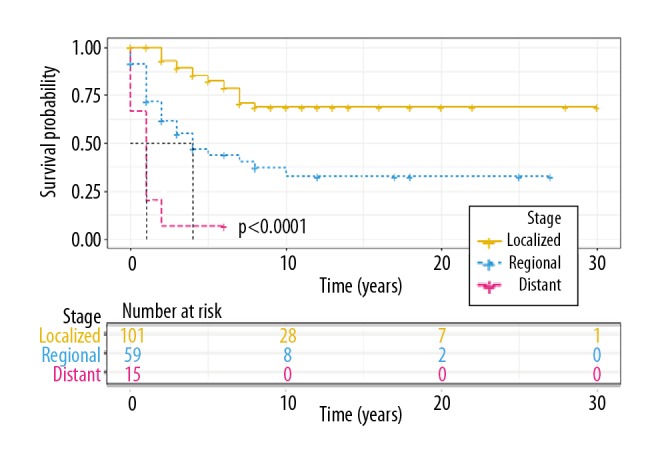
Kaplan-Meier plot of disease-specific survival by stage in clear cell sarcoma (CCS).
Risk factors for survival
Data regarding age, race, gender, marital status, stage, tumor site, tumor size, surgery, radiation, and chemotherapy were included in univariate Cox regression analysis. We found that statistically significant prognostic factors for patients with CCS were race, gender, tumor stage, tumor size, chemotherapy, and surgery. Stage and tumor size were independent prognostic factors of survival in multivariate Cox regression analysis, but race, surgery, gender, and chemotherapy were not (Table 2). Multivariate analysis showed a higher risk of death among patients with distant stage and regional stage (HR=22.22; 95% CI, 8.32–59.35; HR=3.25, 95% CI, 1.8–5.86, respectively), and patients with tumor size >3 cm (HR=5.82; 95% CI, 2.83–12).
Table 2.
Multivariable analysis results.
| Characteristics | Hazard ratio (95% CI) | P Value |
|---|---|---|
| Sex | ||
| Female | Reference group | NA |
| Male | 0.76 (0.42–1.36) | 0.352 |
| Race | ||
| Black | Reference group | NA |
| Other | 1.48 (0.56–3.95) | 0.432 |
| White | 0.59 (0.33–1.06) | 0.079 |
| Stage | ||
| Localized | Reference group | NA |
| Regional | 3.25 (1.8–5.86) | <0.001 |
| Distant | 22.22 (8.32–59.35) | <0.001 |
| Tumor size | ||
| ≤3 | Reference group | NA |
| >3 | 5.82 (2.83–12) | <0.001 |
| Surgery | ||
| Yes | Reference group | NA |
| No | 0.99 (0.4–2.41) | 0.979 |
| Chemotherapy | ||
| No | Reference group | NA |
| Yes | 1.23 (0.63–2.3) | 0.551 |
NA – not applicable. CI – confidence interval.
Discussion
Clear cell sarcoma (CCS) of soft tissue, or malignant melanoma of soft parts, is a rare malignant sarcoma that accounts for approximately 1% of all soft tissue sarcomas [2]. Although previous studies have reported general clinical characteristics of CCS [7,9–12,19,20], because of the rarity of the tumor, some of these previous studies have used the same cases. There are no studies with large sample sizes to verify these earlier findings. Currently, the most extensive study included 75 patients with CCS [19]. To estimate the influence of various prognostic factors on survival, we used a large population-based sample. The population base of the Surveillance, Epidemiology, and End Results (SEER) database covers 30% of the entire US population and standardizes both classification and outcome criteria. These characteristics of SEER were was crucial to avoid potential selection bias and to collect sufficient numbers of patients for the study.
Previous studies have shown that the prognostic factors associated with CCS are tumor size [7,9–13,19], tumors site [12], treatment [7,13], and stage [13,21]. However, only tumor size and stage were prognostic factors in the present study. However, to our knowledge, this study was the largest reported cohort of patients with CCS.
Similar to Blazer et al. [21], we found that the majority of patients were diagnosed at an early or localized stage. The 5-year disease-specific survival was 62.9%, and 10-year disease-specific survival was 51.3%. More significantly, compared with patients with a localized stage, patients who were diagnosed with a regional stage had a 3.2-fold higher risk of death, and patients who were diagnosed with metastatic disease had a 22-fold higher risk of death.
CCS has been diagnosed mainly in young adults, and the age range at diagnosis has been reported to be between 20–40 years [22,23], In a few rare cases, CCS may be diagnosed at extremes of age. The median age of the patients in our study was 41.28 years (range, 6–91 years). Although Kawai et al. [19] reported that gender was an independent prognostic factor, previous studies found that gender was not an independent factor for prognosis of CCS [3,12,21]. The findings of these previous studies are consistent with those of the present study.
The survival rate for CCS is not favorable in most studies. Takahira et al. reported that 5-year survival was 33.3% [24]. Bianchi et al. reported that the 5-year survival rate was 56% [20]. Deenik et al. reported that 5-year survival was 54% [7]. Hocar et al. reported that 5-year survival was 59% [13]. However, the 10-year survival rate dropped to 41%, and the 5-year survival rate was 70% for tumor size ≤5 cm, 46.8% for tumor size >5 cm and 48.9% and 32% at 10 years, respectively [13]. Lucas et al. reported that the 5-year survival rate was 67%, but the 10-year survival rate was 33%, and the 20-year survival rate was 10% [11]. Ferrari et al. reported that the survival rate was 68.9% at 5 years and 66.4% at 10 years [12]. In our study, the disease-specific survival was 62.9% at 5 years and 51.3% at 10 years. According to tumor size, disease-specific survivals were 86.2% for tumor size ≤3 cm and 42.8% for tumor size >3 cm at 5 years and 80.9% for tumor size ≤3 cm and 26.4% for tumor size >3 cm at 10 years, respectively. Therefore, most studies showed that the 5-year survival rate was >50%, but the 10-year survival rate was relatively low. The findings of these studies are somewhat consistent with ours. The 5-year survival rate in previous studies was similar to our findings, but the 10-year survival rate in our study was much higher than previous findings. On multivariate analysis, tumor size was an independent risk factor (P<0.01), which suggests that tumor size is negatively correlated with survival in CCS. These findings were consistent with those of previous studies.
In the present study, of 175 patients with CCS, 78 had a tumor size less than or equal to 3 cm, with a mean size of 4.6 cm (range 0.4–28 cm). Some studies found that tumor size was an independent prognostic factor for patients with CCS [7,11–13,19]. Kawai et al. found that tumor size >5 cm had a poor prognosis and an increased incidence of local recurrence [19]. Ferrari et al. reported that overall survival was substantially worse in patients with a tumor size >5 cm [12]. Finley et al. reported that patients with tumor size >5 cm had a higher risk of metastasis [9]. This finding may be partly explained by the fact that larger tumors take longer to develop and invade surrounding tissues such as blood vessels and lymph vessels to a greater extent.
Distant metastasis is regarded as a predictor of worse prognosis. Our findings support that patients with late-stage metastatic CCS have worse survival than those who are diagnosed at a local and regional stage. Patients with regional stage CCS had worse survival than those diagnosed at a local stage. Multivariate analysis showed that tumor stage was an independent prognostic factor in CCS.
Karita et al. reported five clear cell sarcoma patients who received treatment with doxorubicin, cisplatin, and caffeine, but only one patient had metastasis, and all five patients survived more than 5 years [25]. Other studies reported that patients with CCS who received chemotherapy had a reduced risk of recurrence and metastases [26,27]. However, most studies found that chemotherapy was ineffective [7,9,12,28]. We also showed that chemotherapy appeared to be ineffective in CCS.
Univariate analysis showed that surgical treatment was a significant prognostic factor, while it was not an independent prognostic factor in multivariate analysis. This finding may be partly explained by the fact that patients with non-surgical treatment all had distant metastases, and the prognosis of these patients was even worse in our study.
This study had several limitations. The SEER database is a retrospective patient cohort that did not provide specific surgical information. Therefore, we could not study the impact of specific surgical treatments on patient prognosis. The current edition of the American Joint Committee on Cancer (AJCC) staging system does not include a code for patients with CCS diagnosed before 2004, and most patients did not have information on lymph node metastasis status. Therefore, we used the terms local, regional, and distant for staging CCS. Potential prognostic factors, such as biologic markers and tumor necrosis, were not included in the SEER information. Finally, the specifics of local recurrence, distant metastasis, and comorbidities that could influence treatment and outcomes were not available in SEER. Despite these limitations, we identified independent prognostic factors for survival for a rare soft tissue sarcoma that may assist clinicians in the assessment of prognosis and patient survival.
Conclusions
This study used the Surveillance, Epidemiology, and End Results (SEER) database to study the largest reported cohort of patients with clear cell sarcoma (CCS). The findings indicated that prognostic factors were stage and tumor size. Local stage and tumor size ≤3 cm were favorable prognostic factors for survival in patients with CCS. Our findings may assist clinicians to evaluate patient prognosis for this rare form of soft tissue sarcoma.
Footnotes
Source of support: Departmental sources
Conflict of interest
None.
References
- 1.Enzinger FM. Clear-cell sarcoma of tendons and aponeuroses. An analysis of 21 cases. Cancer. 1965;18:1163–74. doi: 10.1002/1097-0142(196509)18:9<1163::aid-cncr2820180916>3.0.co;2-0. [DOI] [PubMed] [Google Scholar]
- 2.Graadt van Roggen JF, Mooi WJ, Hogendoorn PC. Clear cell sarcoma of tendons and aponeuroses (malignant melanoma of soft parts) and cutaneous melanoma: Exploring the histogenetic relationship between these two clinicopathological entities. J Pathol. 1998;186:3–7. doi: 10.1002/(SICI)1096-9896(199809)186:1<3::AID-PATH153>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 3.Chung EB, Enzinger FM. Malignant melanoma of soft parts. A reassessment of clear cell sarcoma. Am J Surg Pathol. 1983;7:405–13. doi: 10.1097/00000478-198307000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Hasegawa T, Hirose T, Kudo E, Hizawa K. Clear cell sarcoma. An immunohistochemical and ultrastructural study. Acta Pathologica Japonica. 1989;39:321–27. doi: 10.1111/j.1440-1827.1989.tb02442.x. [DOI] [PubMed] [Google Scholar]
- 5.Swanson PE, Wick MR. Clear cell sarcoma. An immunohistochemical analysis of six cases and comparison with other epithelioid neoplasms of soft tissue. Arch Pathol Lab Med. 1989;113:55–60. [PubMed] [Google Scholar]
- 6.Zucman J, Delattre O, Desmaze C, et al. EWS and ATF-1 gene fusion induced by t(12;22) translocation in malignant melanoma of soft parts. Nat Genet. 1993;4:341–45. doi: 10.1038/ng0893-341. [DOI] [PubMed] [Google Scholar]
- 7.Deenik W, Mooi WJ, Rutgers EJ, et al. Clear cell sarcoma (malignant melanoma) of soft parts: A clinicopathologic study of 30 cases. Cancer. 1999;86:969–75. [PubMed] [Google Scholar]
- 8.Eckardt JJ, Pritchard DJ, Soule EH. Clear cell sarcoma. A clinicopathologic study of 27 cases. Cancer. 1983;52:1482–88. doi: 10.1002/1097-0142(19831015)52:8<1482::aid-cncr2820520825>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 9.Finley JW, Hanypsiak B, McGrath B, et al. Clear cell sarcoma: The Roswell Park experience. J Surg Oncol. 2001;77:16–20. doi: 10.1002/jso.1057. [DOI] [PubMed] [Google Scholar]
- 10.Sara AS, Evans HL, Benjamin RS. Malignant melanoma of soft parts (clear cell sarcoma). A study of 17 cases, with emphasis on prognostic factors. Cancer. 1990;65:367–74. doi: 10.1002/1097-0142(19900115)65:2<367::aid-cncr2820650232>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 11.Lucas DR, Nascimento AG, Sim FH. Clear cell sarcoma of soft tissues. Mayo Clinic experience with 35 cases. Am J Surg Pathol. 1992;16:1197–204. doi: 10.1097/00000478-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Ferrari A, Casanova M, Bisogno G, et al. Clear cell sarcoma of tendons and aponeuroses in pediatric patients: A report from the Italian and German Soft Tissue Sarcoma Cooperative Group. Cancer. 2002;94:3269–76. doi: 10.1002/cncr.10597. [DOI] [PubMed] [Google Scholar]
- 13.Hocar O, Le Cesne A, Berissi S, et al. Clear cell sarcoma (malignant melanoma) of soft parts: a clinicopathologic study of 52 cases. Dermatol Res Pract. 2012;2012 doi: 10.1155/2012/984096. 984096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Giuffrida AY, Burgueno JE, Koniaris LG, et al. Chondrosarcoma in the United States (1973 to 2003): An analysis of 2890 cases from the SEER database. J Bone Joint Surg Am. 2009;91:1063–72. doi: 10.2106/JBJS.H.00416. [DOI] [PubMed] [Google Scholar]
- 15.Jacobs AJ, Michels R, Stein J, Levin AS. Socioeconomic and demographic factors contributing to outcomes in patients with primary lymphoma of bone. J Bone Oncol. 2015;4:32–36. doi: 10.1016/j.jbo.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Miller BJ, Cram P, Lynch CF, Buckwalter JA. Risk factors for metastatic disease at presentation with osteosarcoma: An analysis of the SEER database. J Bone Joint Surg Am. 2013;95:e89. doi: 10.2106/JBJS.L.01189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mukherjee D, Chaichana KL, Parker SL, et al. Association of surgical resection and survival in patients with malignant primary osseous spinal neoplasms from the Surveillance, Epidemiology, and End Results (SEER) database. Eur Spine J. 2013;22:1375–82. doi: 10.1007/s00586-012-2621-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Song K, Shi X, Liang X, et al. Risk factors for metastasis at presentation with conventional chondrosarcoma: A population-based study. Int Orthop. 2018;42(12):2941–48. doi: 10.1007/s00264-018-3942-7. [DOI] [PubMed] [Google Scholar]
- 19.Kawai A, Hosono A, Nakayama R, et al. Clear cell sarcoma of tendons and aponeuroses: A study of 75 patients. Cancer. 2007;109:109–16. doi: 10.1002/cncr.22380. [DOI] [PubMed] [Google Scholar]
- 20.Bianchi G, Charoenlap C, Cocchi S, et al. Clear cell sarcoma of soft tissue: A retrospective review and analysis of 31 cases treated at Istituto Ortopedico Rizzoli. Eur J Surg Oncol. 2014;40(5):505–10. doi: 10.1016/j.ejso.2014.01.016. [DOI] [PubMed] [Google Scholar]
- 21.Blazer DG, 3rd, Lazar AJ, Xing Y, et al. Clinical outcomes of molecularly confirmed clear cell sarcoma from a single institution and in comparison with data from the Surveillance, Epidemiology, and End Results registry. Cancer. 2009;115(13):2971–79. doi: 10.1002/cncr.24322. [DOI] [PubMed] [Google Scholar]
- 22.Panagopoulos I, Mertens F, Dêbiec-Rychter M, et al. Molecular genetic characterization of the EWS/ATF1 fusion gene in clear cell sarcoma of tendons and aponeuroses. Int J Cancer. 2002;99(4):560–67. doi: 10.1002/ijc.10404. [DOI] [PubMed] [Google Scholar]
- 23.Patel RM, Downs-Kelly E, Weiss SW, et al. Dual-color, break-apart fluorescence in situ hybridization for EWS gene rearrangement distinguishes clear cell sarcoma of soft tissue from malignant melanoma. Mod Pathol. 2005;18(12):1585–90. doi: 10.1038/modpathol.3800503. [DOI] [PubMed] [Google Scholar]
- 24.Takahira T, Oda Y, Tamiya S, et al. Alterations of the p16INK4a/p14ARF pathway in clear cell sarcoma. Cancer Sci. 2004;95(8):651–55. doi: 10.1111/j.1349-7006.2004.tb03324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Karita M, Tsuchiya H, Yamamoto N, et al. Caffeine-potentiated chemotherapy for clear cell sarcoma: A report of five cases. Int J Clin Oncol. 2013;18(1):33–37. doi: 10.1007/s10147-011-0337-9. [DOI] [PubMed] [Google Scholar]
- 26.Fujimoto M, Hiraga M, Kiyosawa T, et al. Complete remission of metastatic clear cell sarcoma with DAV chemotherapy. Clin Exp Dermatol. 2003;28(1):22–24. doi: 10.1046/j.1365-2230.2003.01109.x. [DOI] [PubMed] [Google Scholar]
- 27.Mankin H, Hornicek FJ. Diagnosis, classification, and management of soft tissue sarcomas. Cancer Control. 2005;12(1):5–21. doi: 10.1177/107327480501200102. [DOI] [PubMed] [Google Scholar]
- 28.Kuiper DR, Hoekstra HJ, Veth RPH, Wobbesa T. The management of clear cell sarcoma. Eur J Surg Oncol. 2003;29(7):568–70. doi: 10.1016/s0748-7983(03)00115-x. [DOI] [PubMed] [Google Scholar]


