Abstract
Growing evidence indicates cannabinoid receptors as potential therapeutic targets for chronic pain. Consequently, there is an increasing interest in developing cannabinoid receptor agonists for treating human and veterinary pain. To better understand the actions of a drug, it is of paramount importance to know the cellular distribution of its specific receptor(s). The distribution of canonical and putative cannabinoid receptors in the peripheral and central nervous system of dogs is still in its infancy. In order to help fill this anatomical gap, the present ex vivo study has been designed to identify the cellular sites of cannabinoid and cannabinoid-related receptors in canine spinal ganglia. In particular, the cellular distribution of the cannabinoid receptors type 1 and 2 (CB1 and CB2) and putative cannabinoid receptors G protein-coupled receptor 55 (GPR55), nuclear peroxisome proliferator-activated receptor alpha (PPARα), and transient receptor potential vanilloid type 1 (TRPV1) have been immunohistochemically investigated in the C6–C8 cervical ganglia of dogs. About 50% of the neuronal population displayed weak to moderate CB1 receptor and TRPV1 immunoreactivity, while all of them were CB2-positive and nearly 40% also expressed GPR55 immunolabeling. Schwann cells, blood vessel smooth muscle cells, and pericyte-like cells all expressed CB2 receptor immunoreactivity, endothelial cell being also PPARα-positive. All the satellite glial cells (SGCs) displayed bright GPR55 receptor immunoreactivity. In half of the study dogs, SGCs were also PPARα-positive, and limited to older dogs displayed TRPV1 immunoreactivity. The present study may represent a morphological substrate to consider in order to develop therapeutic strategies against chronic pain.
Keywords: cannabinoid receptor 1, cannabinoid receptor 2, G protein-coupled receptor 55, nuclear peroxisome proliferator-activated receptor alpha, transient receptor potential vanilloid type 1, endocannabinoids, satellite glial cells
Introduction
Spinal ganglia, also referred to as dorsal root ganglia (DRG), contain the cell bodies of pseudounipolar primary sensory neurons, which are surrounded by a layer of satellite glial cells (SGCs), also called amphicytes because of their position around each neuron. Chronic pain, both inflammatory and neuropathic, is associated with hyperexcitability of DRG cellular elements and their down-modulation could thereby decrease pain (1). A growing body of literature suggests that cannabinoid receptors play a critical role in nociception through central and peripheral mechanisms (2–8). Recent studies have shed some light on the expression of cannabinoid receptors on neurons and glial cells of the canine nervous system (9–11). In particular, CB1 receptor was observed in central nervous system (CNS) neurons (9) and in DRG neurons and glial cells (10), whereas CB2 receptor was found in glial cells (astrocytes) of the spinal cord (11).
In addition to the known canonical (i.e., prototypical) cannabinoid receptors CB1 and CB2, other receptors, such as G protein-coupled receptor 55 (GPR55), nuclear peroxisome proliferator-activated receptor alpha (PPARα), and transient receptor potential vanilloid type 1 (TRPV1) are currently considered putative cannabinoid receptors (12–14).
The anti-nociceptive potential of the endocannabinoid system (15) has prompted the development of therapeutic cannabinoid receptors agonists or medical marjiuana to be used in pets in order to treat chronic pain. The clinical/medical properties of botanical and synthetic cannabinoids in the management of neuropathic pain, allodynia, and chronic non-cancer pain have been recently reviewed (16). Methodological challenges (quali-quantitative variability in cannabinoid content of cannabis plant extracts, inconsistent dosing) as well as acute and chronic impacts on cognition, immune and cardiovascular system are still unsolved issues associated with the therapeutic use of phytocannabinoids (17–20). This is why many research efforts are currently focused on body's own cannabinoids (i.e., endocannabinoids) and related physiological compounds, acting through canonical and putative cannabinoid receptors (15, 21).
Although there is a growing interest in the subject, reliable anatomical studies regarding the cellular distribution of cannabinoid receptors in the canine central and peripheral nervous system (PNS) are still lacking. In order to help filling this anatomical gap, the present ex vivo study immunohistochemically investigated the cellular distribution of the cannabinoid and cannabinoid-related receptors CB1, CB2, GPR55, PPARα, and TRPV1 in cervical DRG of pet dogs.
Materials and Methods
Animals
Cervical sensory ganglia and related spinal cord were collected from eight dogs (Table 1). None of them had history of neurological disorders and any gross changes of the spinal cord and vertebral canal. Dogs died spontaneously or were euthanized for human reasons due to different diseases and tissues were collected following owner's permission. According to the Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes, the Italian legislation (D. Lgs. n. 26/2014) does not require any approval by competent authorities or ethics committees, because this research did not influence any therapeutic decisions.
Table 1.
Clinico-pathological data of the dogs included in the present research.
| Dogs | Breed | Gender | Age | Cause of death* |
|---|---|---|---|---|
| Dog 1 | Chihuahua | F | 8 months | Head trauma (T) |
| Dog 2 | Great Dane | M | 2 years | Peritonitis/ intussusception (V) |
| Dog 3 | Pitbull | M | 13 years | Splenic neoplasia, skin neoplasia (N) |
| Dog 4 | Mongrel | M | 11 years | Mast cell tumor (N) |
| Dog 5 | Mongrel | F | 11 years | Mast cell tumor + Cushing's syndrome (N) |
| Dog 6 | Mongrel | M | 14 years | Gastric dilatation-volvulus (V) |
| Dog 7 | Lagotto Romagnolo | F* | 10 years | Thymoma (N) |
| Dog 8 | Cane Corso Italiano | F | 8 years | Gastric tumor (N) |
M, male; F, female; FM, female spayed.
According to the VITAMIND scheme (T, traumatic; V, vascular; N, neoplastic).
Since the suppliers of the antibodies employed in the present study state them to rat-specific (CB2 and TRPV1) or react with rat tissues (CB1, PPARα), rat cervical sensory ganglia were used for comparison purposes (authorization no. 112/2018-PR of 12 February 2018). The distribution of the study receptors in subclasses of rat sensory neurons was out of the scope of the present study, and was not evaluated.
Tissue Collection
Tissue Samples (C6-C8 DRG) were collected within 1 h from death through a dorsal laminectomy. DRG were localized by counting them from the last cervical spinal nerve (C8) located just cranial to the first rib. C6–C8 cervical DRG were selected for the present study because of technical and pathophysiological implications, i.e., large size, involvement in chronic pain (caused by cervical disk herniation and vertebral column instability), presence of all the subsets of sensory neurons activated by mechanical, thermal and nociceptive inputs from the forelegs. Once removed from the spinal cord, DRG were fixed for 12 h in 4% paraformaldehyde in phosphate buffer (0.1 M, pH 7.2) at 4°C. Tissues were subsequently rinsed overnight in phosphate-buffered saline (PBS; 0.15 M NaCl in 0.01 M sodium phosphate buffer, pH 7.2) and stored at 4°C in PBS containing 30% sucrose and sodium azide (0.1%). The following day, the tissues were transferred to a mixture of PBS−30% sucrose–azide and Optimal Cutting Temperature (OCT) compound (Sakura Finetek Europe, Alphen aan den Rijn, The Netherlands) at a ratio of 1:1 for an additional 24 h before being embedded in 100% OCT in Cryomold® (Sakura Finetek Europe). The sections were prepared by freezing the tissues in isopentane cooled in liquid nitrogen. Serial longitudinal sections (14–16 μm thick) of C6–C8 DRG were cut on a cryostat, and mounted on polylysinated slides.
Immunofluorescence
Cryosections were hydrated in phosphate-buffered saline (PBS) and processed for immunostaining. To block non-specific bindings, the sections were incubated in a solution containing 20% normal donkey serum (Colorado Serum Co., Denver, CO, USA), 0.5% Triton X-100 (Sigma Aldrich, Milan, Italy, Europe), and bovine serum albumin (1%) in PBS for 1 h at room temperature (RT). The cryosections were incubated overnight in a humid chamber at RT with a cocktail of primary antibodies (Table 2) diluted in 1.8% NaCl in 0.01 M PBS containing 0.1% sodium azide. After washing in PBS (3 × 10 min), the sections were incubated for 1 h at RT in a humid chamber with the secondary antibodies (Table 3) diluted in PBS. Cryosections, were then washed in PBS (3 × 10 min) and mounted in buffered glycerol at pH 8.6.
Table 2.
Primary antibodies used in the study.
| Primary antibody | Host | Code | Dilution | Source |
|---|---|---|---|---|
| CB1 | Rabbit | Orb10430 | 1:200 | Biorbyt |
| CB2 | Rabbit | ab45942 | 1:200 | Abcam |
| CD31 | Mouse | M0823 Clone JC70A | 1:30 | Dako |
| GFAP | Chicken | ab4674 | 1:800 | Abcam |
| GPR55 | Rabbit | NB110-55498 | 1:200 | Novus Biol. |
| Factor VIII | Rabbit | A0082 | 1:1,000 | Dako |
| PPARα | Rabbit | NB600-636 | 1:200 | Novus Biol. |
| Myelin protein zero (P0) | Chicken | ab39375 | 1:100 | Abcam |
| S100 | Rabbit | 20311 | 1:200 | Dako |
| TRPV1(VR1) | Rabbit | ACC-030 | 1:200 | Alomone |
| VR1 (P-19) | Goat | sc12498 | 1:50 | Santa Cruz |
Primary antibodies Suppliers: Abcam, Cambridge, UK; Alomone, Jerusalem, Israel; Biorbyt Ltd., Cambridge, UK; Dako, Carpinteria, CA, USA; Novus Biologicals, Littleton, CO, USA; Santa Cruz, Biotechnology, CA, USA.
Table 3.
Secondary antibodies used in the study.
| Secondary antibody | Host | Code | Dilution | Source |
|---|---|---|---|---|
| Anti-rabbit 594 | Donkey | ab150076 | 1:100 | Abcam |
| Anti-rabbit 488 | Donkey | ab150073 | 1:800 | Abcam |
| Anti-chicken TRITC | Donkey | 703-025-155 | 1:200 | Jackson |
| Anti-goat 594 | Donkey | ab150132 | 1:600 | Abcam |
| Anti-mouse F(ab')2 fragment TRITC |
Goat | ab51379 | 1:50 | Abcam |
Secondary antibodies Suppliers: Abcam, Cambridge, UK; Jackson Immuno Research Laboratories, Inc. Baltimore Pike, PA, USA.
Cellular nuclei were identified with the DAPI Fluorishield (F6057-20ML, Sigma Aldrich, Milan, Italy, Europe), DRG neurons were identified with the blue fluorescent Nissl staining solution (NeuroTrace®, # N-21479, Molecular Probes, Eugene, OR, USA; dilution 1:200). Satellite glial cells were identified with a polyclonal chicken anti-glial fibrillary acid protein (GFAP) antiserum. Schwann cells were identified with a polyclonal chicken anti-myelin Protein Zero (P0) antiserum. Since CB2 receptor may also be expressed by blood vessels (22–24), the endothelial cells were recognized with two different antibodies, i.e., the mouse anti-CD31 antibody (25, 26), and the rabbit anti-Factor VIII-related antigen/von Willebrand factor (27), herein referred to as FVIII-Rag.
In order to determine the proportion of neurons immunoreactive for each of the marker, sections subjected to single immunohistochemistry for cannabinoid receptors were counterstained with blue fluorescent Nissl stain solution (NeuroTrace®, see above) following the manufacturer's instructions. At least one hundred Nissl stained neurons were counted for each marker. Data were collected from preparations obtained from at least three animals (n = 3). The percentage of immunopositive neurons was expressed as mean ± standard deviation.
Specificity of the Primary Antibodies
The specificity of the anti-cannabinoid receptors CB1, CB2, and PPARα antibodies in dog tissues has been recently tested by Western blot (Wb) analysis on canine intestinal tissues (24). In the present study we used the antibody anti-human GPR55 (NB110-55498; Novus Bio) which, based on sequence identity (85%), is predicted to cross-react also with canine tissues. However, we tested its specificity on canine tissue by Wb analysis.
To identify TRPV1 immunoreactive neurons, we utilized two different antisera raised in rabbit (Alomone, ACC-030) and goat (Santa Cruz, c12498), directed against two different portions of the rat TRPV1. The immunogen of the rabbit anti-TRPV1 (Alomone) was the peptide [(C)EDAEVFK DSMVPGEK (824–838) of rat TRPV1. The immunogen of the goat anti-VR1 antibody (Santa Cruz) was a synthetic peptide [PHIFTTRSRTRLFGKGDSE(C)] (28–47) from N-terminus of the rat TRPV1. The manufacturer's datasheets for both the anti-TRPV1 antibodies state that the antibodies are specific only for rodents (mouse and rat) and human DRG neurons. The specificity of the goat anti-VR1 antibody has been tested on canine tissues with Wb (48). Thus, we tested the specificity of the two antibodies on rat and canine DRG cryosections beforehand, by using a double-staining protocol. On rat DRG cryosections, the anti-TRPV1 antibody raised in rabbit (Alomone) and the anti-VR1 antibody raised in goat, showed full correspondence within the same neurons, which appeared brightly labeled, providing additional value to the specificity of both the anti-TRPV1 antibodies (data not shown). As observed in porcine DRG (49), only the rabbit anti-TRPV1 antibody identified TRPV1-immunoreactivity in the canine ganglia. However, the specificity of the rabbit anti-TRPV1 antibody was not tested on canine tissues by Wb.
The specificity of the endothelial markers antibodies (anti-CD31 and anti FVIII-Rag) was tested by using a double-staining protocol. Both antibodies recognized the same endothelial cells; however, the antibody anti-CD31 showed a sharper and more delicate immunolabeling of the cells (data not shown). For this reason, the anti-CD31 antibody was used as endothelial marker.
The specificity of the anti-myelin marker protein zero (P0) antiserum was tested by using a double-staining protocol. The anti-P0 antiserum was co-localized with the anti-S100 antiserum; both the myelin markers were co-localized in all the Schwann cells (data not shown).
Fluorescence Microscopy
Preparations were examined on a Nikon Eclipse Ni microscope equipped with the appropriate filter cubes to distinguish the fluorochromes employed. The images were recorded with a Nikon DS-Qi1Nc digital camera and NIS Elements software BR 4.20.01 (Nikon Instruments Europe BV, Amsterdam, Netherlands). Slight adjustments to contrast and brightness were made using Corel Photo Paint, whereas the figure panels were prepared using Corel Draw (Corel Photo Paint and Corel Draw, Ottawa, ON, Canada).
Western Blot
Tissue sample (small intestine/jejunum) was collected, frozen in liquid nitrogen and stored at −80°C until sample processing. Hundred milligram of tissue was homogenized in 1 ml of SDS buffer (Tris-HCl, 62.5 mM; pH 6.8; SDS, 2%; and glycerol, 20%) supplemented with a protease inhibitor cocktail (Sigma-Aldrich, Co, St. Louis, MO, USA). Total protein content was determined by Peterson's Modification of Lowry Method using a Protein Assay Kit. 20 μg of total proteins were separated on NuPage4–12% bis-Tris Gel (Life Technologies Ltd, Paisley, UK) for 30 min at 200 V. The proteins were then electrophoretically transferred onto a nitrocellulose membrane by a semi-dry system (Trans Turbo Blot Bio -Rad). Non-specific binding on nitrocellulose membranes was blocked with 5% milk powder in PBS-T20 (Phosphate Buffer Saline-0.1% Tween-20) for 1 h at room temperature. After blocking treatment, the membrane was incubated overnight at 4°C with the primary antibodies (GPR55 NB110-55498), 1:500 diluted in PBS added with 1.5% of milk. After washes, the blot was incubated with a goat anti rabbit biotin-conjugate antibody (1:50,000 dilution in TBS-T20, 1 h at RT) and then with a 1:1,000 dilution of an anti-biotin horseradish peroxidase (HRP)-linked antibody (40 min at RT). Immunoreactive bands were visualized using chemiluminescent substrate (Clarity Western ECL Substrate Bio Rad), according to the manufacturer's instructions. The intensity of the luminescent signal was acquired by Chemidoc Instrument (Bio Rad) and the apparent molecular weight of the resultant bands was analyzed by Quantity One Software (Bio-Rad). Western blot analysis of GPR55 revealed a single band of expected molecular weight (~40 kDa) (Figure 1).
Figure 1.
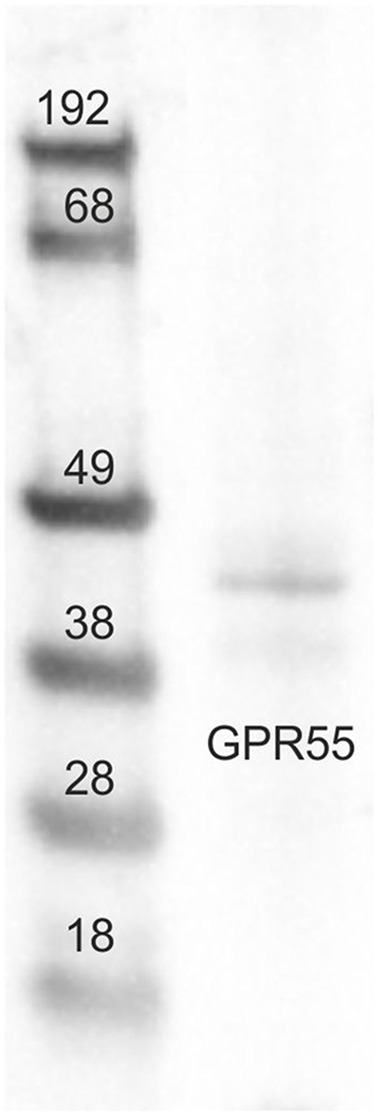
Representative image of Western blots (WB) analysis showing the specificity of the primary antibody rabbit anti-G protein-coupled receptor 55 (GPR55). The antibody revealed a single band of expected molecular weight (~40 kDa). The images of the different immunoblots were slightly adjusted in brightness and contrast to match their backgrounds.
Results
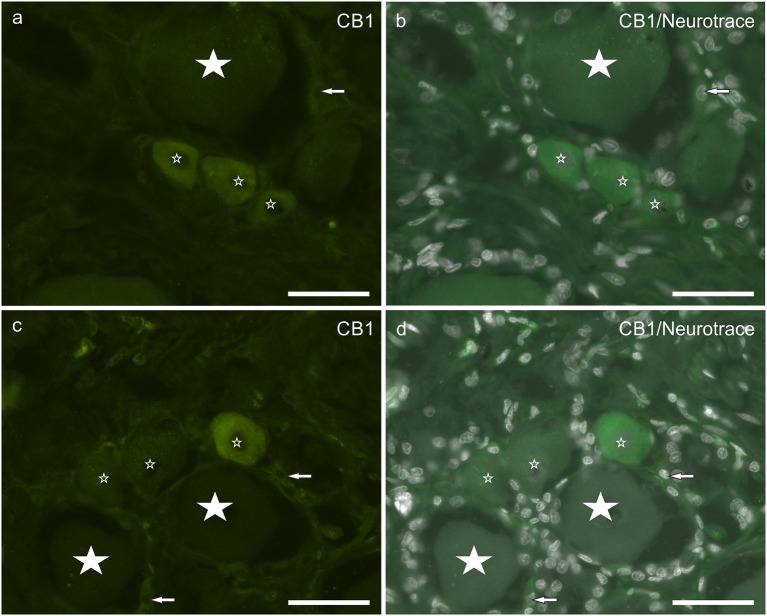
CB1 Receptor Immunoreactivity
About half neuronal population (55 ± 6%; 278/507 counted sensory neurons, n = 4) displayed weak to moderate cytoplasmic CB1 receptor immunoreactivity (Figures 2a–d). CB1 receptor immunoreactivity was occasionally observed in SGCs, although it could be confused with background. This finding is partially consistent with observation in the rat DRG, in which neurons and SGCs expressed CB1 receptor immunoreactivity also in the nuclei (neurons > SGCs) (Supplementary Figures 1a–c).
Figure 2.
(a–d) Photomicrographs of cryosections of canine cervical (C8) dorsal root ganglion showing cannabinoid receptor 1 (CB1) immunoreactivity. Small stars indicate small neurons showing CB1 receptor weak to moderate immunoreactivity. Large stars indicate CB1 receptor negative. Arrows indicate satellite glial cells showing weak CB1 receptor immunoreactivity. Bar: a–d = 50 μm.
CB2 Receptor Immunoreactivity
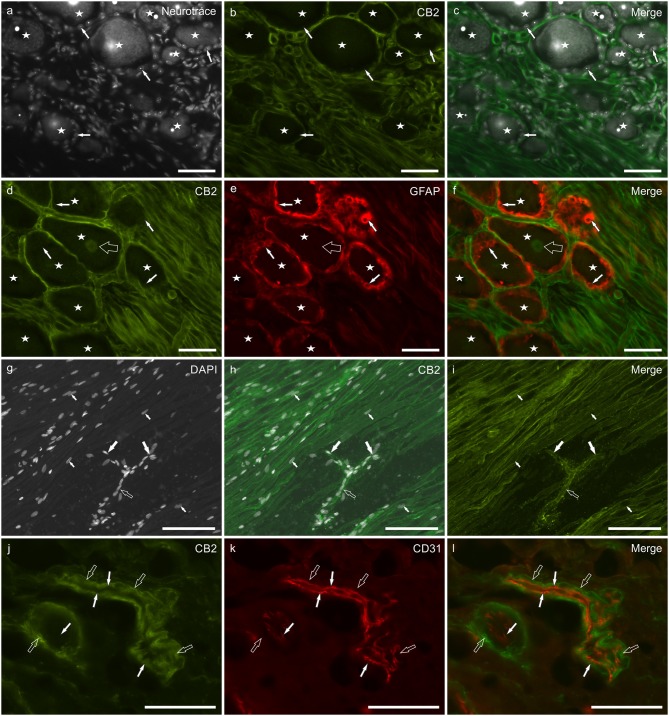
CB2 receptor immunoreactivity was brightly expressed by Schwann cells and cells surrounding blood capillaries (most likely pericytes) (Figures 3a–l), while smooth muscle cells of blood vessels showed moderate CB2 receptor immunolabeling (Supplementary Figure 2). SGCs did not display CB2 receptor immunolabeling (Figures 3a–f). Faint CB2 immunolabeling was expressed by the nuclei of all the DRG neurons (Figures 3d,f). GFAP immunostaining was stronger at the periphery of the ganglia, while CB2 receptor immunoreactivity was stronger in the central portion of the ganglia (data not shown). The expression of the CB2 receptor on Schwann cells depicted the path of nerve fibers, rolling between neurons before abandoning the ganglion at its central and peripheral pole (Figures 3g–i). In the oldest subjects, the CB2 receptor immunolabeling was less intense than in the younger dogs (data not shown). The co-localization of CB2 receptor with the myelin marker P0 showed that both the markers were expressed by all Schwann cells (Supplementary Figures 3a–d). CB2 receptor immunoreactivity was brightly expressed by pericyte-like cells (Figures 3j–l). The co-localization study between CB2 receptor and the endothelial marker CD31 showed that the endothelium was CB2 receptor negative whereas the vascular smooth muscle cells showed faint CB2 receptor immunoreactivity (Figures 3j–l). The CB2 receptor immunolabeling was also observed within the neuronal nuclei of the rat DRG, whereas Schwann cells and blood vessels were CB2 receptor negative (Supplementary Figures 1d–f).
Figure 3.
Photomicrographs of cryosections of canine cervical (C8) dorsal root ganglion showing cannabinoid receptor 2- (CB2), glial fibrillary acidic protein- (GFAP), and CD31-immunoreactivity. (a–c) Stars indicate NeuroTrace labeled (a) dorsal root ganglion sensory neurons which were CB2 receptor negative (b), as well as the satellite glial cells (white arrows). (d–f) Stars indicate sensory neurons encircled by satellite glial cells (white arrows) which were GFAP-immunoreactive (e) and CB2 receptor negative. CB2 receptor immunoreactivity was expressed by Schwann cells and neuronal nuclei (open arrow). (g–i) The empty arrow indicates one neuronal axon that bifurcates (T-junction) in its central and peripheral portions (large white arrows). The small arrows indicate the nuclei of Schwann cells. (j–l) Open arrows indicate smooth muscle cells (vessel on the left) and pericyte-like cells (elongated and thin blood vessel on the right) showing CB2 receptor immunoreactivity (j). White arrows indicate endothelial cells showing CD31 immunoreactivity (k). Bar: a–f, j–l = 50 μm; g–i = 100 μm.
GPR55 Immunoreactivity
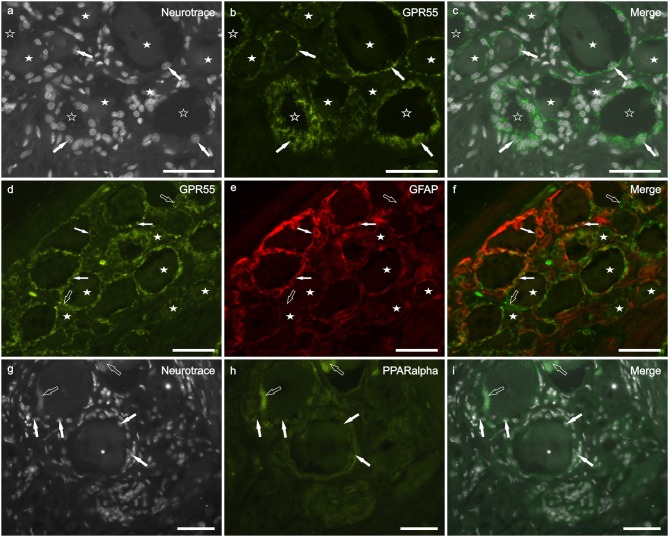
Bright GPR55 immunoreactivity, with grainy appearance, was expressed by all (GFAP positive and GFAP negative) SGCs (Figures 4a–f). Also a percentage of different size sensory neurons (38 ± 14%; 214/542 cells counted, n = 3) showed faint to moderate GPR55 immunolabeling (Figures 4d–f). This finding is consistent with that obtained in neurons and SGCs of the rat DRG (Supplementary Figures 1g–i).
Figure 4.
Photomicrographs of cryosections of canine cervical (C8) dorsal root ganglion showing GPR55 (a–f) and PPARalpha (g–i) immunolabeling. (a–c) Arrows indicate the Neurotrace-labeled nuclei of satellite glial cells (a) which showed bright GPR55 immunolabelling (b). White stars indicate unlabeled sensory neurons; open stars indicate empty spaces in which sensory neurons were no more evident. (d–f) White arrows indicate satellite glial cells which co-expressed bright GPR55- (d) and glial fibrillary acidic protein (GFAP) immunoreactivity; open arrows indicate SGCs which were GPR55 immunoreactive and GFAP negative (e). Stars indicate sensory neurons of different dimension, which expressed faint –to-moderate GPR55 immunoreactivity. (g–i) White arrows indicate the Neurotrace labeled nuclei of SGCs which showed PPARalpha immunoreactivity (h). Open arrow indicate autofluorescent pigment. Bar: a–i = 50 μm.
PPARα Immunoreactivity
PPARα immunoreactivity was expressed by SGCs (Figures 4g–i) and endothelial cells of blood vessels (data not shown). Quite surprisingly, four out of eight dogs did not show PPARα immunoreactivity. In the remainders, all the SGC were PPARα-positive. These data are partially consistent with those obtained in rat DRG, in which also the neuronal cytoplasm showed faint PPARα immunoreactivity (Supplementary Figures 1j–l).
TRPV1 Immunoreactivity
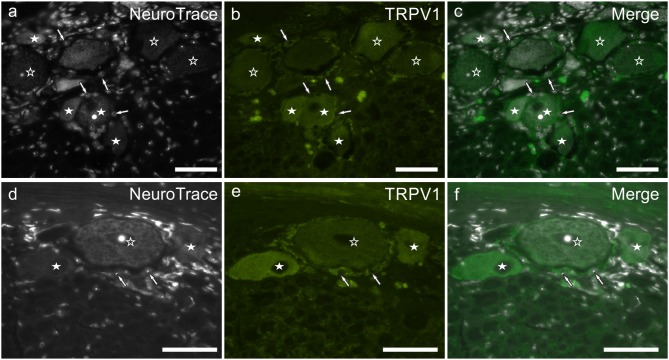
TRPV1 immunoreactivity was unevenly distributed and highly variable within the study cases. In the younger subjects, it was limited to different size neurons (and neuronal processes) while in older dogs, TRPV1 immunolabeling was expressed also by SGCs (Figures 5a–f). In all the subjects, the brightest TRPV1 immunolabeling was displayed by small neurons. The percentage of TRPV1 immunoreactive neurons was 55 ± 11% (563/1,017 cells counted, n = 4). In the rat DRG, TRPV1 immunolabeling was expressed only by the cytoplasm of a subset of sensory neurons and nerve fibers (Supplementary Figures 1m–o).
Figure 5.
(a–f) Photomicrographs of cryosections of the C8 cervical dorsal root ganglia belonging to two aged dogs showing transient receptor potential vanilloid type 1 (TRPV1) immunoreactivity. White stars indicate neurons showing bright TRPV1 immunoreactivity, while open stars indicate larger neurons showing weaker TRPV1 immunoreactivity. Arrows indicate the Neurotrace labeled nuclei of satellite glial cells showing bright TRPV1 immunolabeling (b,e). Bar: a–f = 50 μm.
The results of the cellular distribution and intensity of the immunolabeling in the canine DRG are summarized in Table 4.
Table 4.
Semiquantitative evaluation of the density of CB1, CB2, GPR55, PPARα, and TRPV1 receptors immunoreactivity in different cellular elements (neurons, satellite glial cells, Schwann cells, blood vessels) of the canine C8 cervical dorsal root ganglia.
| Canine cervical dorsal root ganglion | |||||
|---|---|---|---|---|---|
| CB1 | CB2 | GPR55 | PPARα | TRPV1 | |
| Neurons | CD ++ | ND + | CD + | – | CD ++/+++ |
| Satellite glial cells | CD + | – | CD +++ | CD ++ | CD +++ |
| Schwann cells | – | CM +++ | – | – | – |
| Blood vessels | – | ED +++ SMCD ++ |
– | ED ++ | – |
Immunoreactive cells are graded as: –, negative; +, weakly stained; ++, moderately stained; +++, strongly stained.
C, cytoplasmic; D, diffuse labeling; E, endothelium; M, membranes; N, nuclear; SMC, smooth muscle cells.
Discussion
The present study showed the expression of canonical and putative cannabinoid receptors in different cellular elements of canine cervical DRG, such as neurons (CB1 and GPR55), SGCs (GPR55 and CB1), Schwann cells and muscle cells of blood vessels (CB2). These findings further substantiate the hypothesis that endogenous ligands, e.g., endocannabinoids and related compounds, may play important roles in modulating the responses associated with hyperexcitability of DRG, such as chronic pain (1). While the role of DRG in pain physiology (i.e., on the crossroads between PNS and CNS) is well-established (50), much less is known about its active involvement in processing chronic pain (1, 51). Given the involvement of the endocannabinoid system in pain modulation (15, 50, 52), our findings may help to shed new light on this challenging issue.
CB1 and CB2 Receptors
The expression of CB1 receptor in DRG neurons and SGCs is in agreement with previous studies in laboratory rodents (53), humans (54) and dogs (11). However, the neuronal subpopulation expressing CB1 receptors (i.e., small sensory neurons) was different from a previous in situ hybridization study by Hohmann et al. (55) who found medium-and large-sized cells in rat DRG to predominantly express CB1 receptor mRNA. Although, in the present study, the area of DRG neurons was not measured, it is possible to state with some confidence that, in the rat DRG, CB1 receptor immunoreactivity was expressed also by large-sized neurons.
The expression of faint CB2 receptor immunolabeling in neurons and its absence in SGCs of canine DRG, partially agrees with previous findings in laboratory rodents, where only very weak immunoflorescence was found in basal conditions (56). Although CB2 receptor was considered lacking in neurons and glial cells, recent literature highlights its expression in these cell types (57, 58), even in humans (54) and dogs (11, 59). Similarly to CB1 (28), CB2 receptor is upregulated in a variety of PNS and CNS diseases and is suggested as a promising pharmacological target in the management of chronic pain and neuroinflammation (29–31, 56). At present we are not able to explain the presence of the CB receptors in neuronal nuclei of canine (CB2 receptor) and rat (CB1 and CB2 receptors) DRG. The study on the subcellular distribution and function of cannabinoid receptors is still expanding. The nuclear envelope, which is a part of the endoplasmic reticulum, may be one of the sources of nuclear Ca2+; Curry et al. (60) identified the expression of CB1 and CB2 receptors on the nuclear membrane of cardiac muscle cells and demonstrated that these receptors, when activated by anandamide, can (negatively) modulate nuclear Ca2+ release and, very likely, gene transcription.
To the best of our knowledge, this is the first time that CB2 receptor immunoreactivity in Schwann cells has been reported. Up to now, endocannabinoid receptor immunolabeling of Schwann cells was limited to CB1, which was shown in about 100% of this cell type in the canine sciatic nerve (10). Besides forming the myelin sheath, Schwann cells orchestrate much of the regenerative response that occurs after nerve injury in order to restore nerve function (32). The expression of CB1 (10) and CB2 receptors (present study) in Schwann cells could thus support the neuroprotective and/or neuroreparative role suggested for cannabinoids and related compounds in the PNS (33, 56).
The presence of thin interneuronal GFAP-negative cellular processes expressing CB2 receptor-immunoreactivity is at present not easy to interpret. These CB2 receptor immunoreactive slender evaginations might belong to GFAP-negative SGCs (34) or to a different type of DRG glial cells, i.e., pericyte-like satellite cells (35, 36). Also the presence of different cell types with elongated cellular processes immunoreactive for CB2 receptor, such as fibroblasts and histiocytes (34, 36), cannot be excluded.
Some considerations are needed when dealing with DRG blood vessels. First, little information is available and it mainly refers to laboratory rodents. Second, blood-nerve barrier is lacking in intact DRG (37) and fenestrations together with open intercellular junctions characterize ganglionic vessels (38, 39). Although the sheath of SGCs is considered to control the traffic of substances from blood to ganglionic neurons—thus functionally substituting for the vascular barrier (40)—circulating signaling molecules are allowed to diffuse into the microenvironment of DRG. This was recently confirmed by Svíženská et al. (56), who demonstrated that sciatic nerve injury induces bilateral increase of CB2 receptor (both protein and mRNA) in lumbar L4–L5 as well as cervical C7–C8 DRG.
In the present study we detected CD31 and FVIII-RAg immunoreactivity in a small proportion of DRG vessels, mostly confined to the periphery of the ganglion rather than among sensory neurons. The finding is quite unexpected, since the endothelial marker CD31 allowed to trace an extensive network of blood vessels in the mouse L4 DRG, that was found to encapsulate and encircle sensory neurons (41). The paucity of vascularization of canine DRG did not seem to depend on methodological issues since the antibody anti-CD31 was recently found to perfectly label the endothelium of canine blood vessels, at least in the intestinal mucosa (24).
In the present study CB2 receptor immunoreactivity was limited to smooth muscle cells of blood vessels, being absent from CD31-positive endothelium, differently from what observed in canine intestinal (24) and skin blood vessels (42), or human brain endothelium (43). One possible explanation for this discrepancy might be the well-known regional distribution of the cannabinoid receptors in blood vessels (44). Indeed, CB2 receptor immunoreactivity of vascular smooth vessels was recently detected in bovine pancreas (45) and mice skin (46). Endocannabinoids exert a prohomeostatic function on vascular biology through complex mechanisms often involving canonical as well as putative cannabinoid receptors [e.g., TRPV1 and GPR55 (47)]. In particular, vasodilating effect occurs at different cellular site, i.e., nerves, endothelial cells, vascular smooth muscle cells, perycites (61), employing different receptors and leading to nitric oxide release (47).
GPR55
The GPR55 represents a novel target for various cannabinoids (62). Strong expression of GPR55 immunoreactivity in of different size neurons and SGCs was found in the present study. GPR55 immunoreactivity was expressed also by GFAP negative SGCs; a recent study showed that GFAP recognizes up to 89% of all SGCs of the canine DRG (34). This finding indicates that GPR55 might be utilized as canine SGCs marker. In the present study, a similar pattern of GPR55 immunoreactivity has been observed also in the neurons and SGCc of rat DRG. This is a relatively new finding, since up to now GRP55 immunoreactivity has been detected only in the neuronal component of DRG (63). Consistently, the GPR55 immunoreactivity in medium- and large-sized DRG neurons as detected here agrees with the finding of Lauckner et al. (63), who observed strong GPR55 signal in mice DRG large neurons. Interestingly, large sensory neurons may mediate inflammatory and neuropathic pain hypersensitivity by switching their phenotype and expressing the nociceptive neurotransmitter Substance P (64, 65). It is noteworthy to recall that some phytocannabinoids, e.g., Δ9-tetrahydrocannabinol (THC), cannabidiol, synthetic cannabinoids (AM251 and O-1602), as well as palmitoylethanolamide (PEA) have been described as GPR55 ligands (7, 66).
Although further functional investigations are necessary, GPR55 immunoreactivity in both SGCs and neurons as detected in the present study likely may suggest a relevant role of this receptor in neuron-SGCs crosstalk, which is currently considered a critical component of neuroinflammatory changes eventually leading to chronic pain (67–70).
PPARα
The PPARα is a ligand-activated transcription factor belonging to the superfamily of nuclear hormone receptors. By modulating gene expression, it plays key roles in maintaining glucose and lipid homeostasis and inhibiting inflammation (71). The PPARα activation has also been shown to induce rapid, cellular changes without requiring transcription (72). In the present study PPARα immunoreactivity has been detected in the canine SGCs and endothelial cells. In the comparative study on rat DRG, we observed bright PPARα immunoreactive SGCs, whereas neurons wear faintly immunolabeled. These findings are in line with previous data on the expression of PPARα in mice DRG (73–75) and canine gastrointestinal tract (24). The ganglia of four out of eight dogs did not show PPARα immunoreactivity. At present we do not have any clear explanation for this discrepancy. No apparent correlation with any particular factor (e.g., age or cause of death) was found. Nonetheless, we cannot exclude that it was due to an undetected subclinical state, given that metabolic disorder, for example, is associated with significantly decreased spinal PPARα expression (76).
TRPV1
The TRPV1 is a ligand-gated non-selective cation channel usually expressed by peptidergic nociceptors of rodents (77, 78) and large mammals (49) as well as non-peptidergic nociceptors (79, 80). The TRPV1 is activated by heat (>43°C), low pH and capsaicin (81) and desensitized by endocannabinoids (82, 83).
In accordance with previous studies in rodent and human DRG (54, 81, 84, 85) we have observed diffuse TRPV1 immunoreactivity in neurons of canine DRG, with the brightest immunolabeling being displayed by small size neurons. This latter finding agreed with the study of Binzen et al. (86), who found TRPV1 to be mainly expressed in small-sized neurons of rat DRG, the vast majority of which co-expressed CB1 receptors. Our comparative study on rat DRG confirmed that the brightest TRPV1 immunoreactivity was mainly expressed by small neurons. Moreover, SGCs from two old dogs were also brightly immunolabeled, in accordance with TRPV1 expression by DRG glial cells (87).
To the best of our knowledge no information is yet available about the influence of age on neuronal and/or glial expression of TRPV1, however one could tentatively speculate that aging itself has an impact on pain pathophysiology through changes in the pain involved receptor TRPV1. Actually, increased expression of TRPV1 was recently observed in rat DRG after neuropathic pain induction (88). Marrone et al. (89) reported TRPV1 immunoreactivity in microglial cells rather than neurons of the mice brain areas. Moreover, they showed that in mice suffering from neuropathic pain, TRPV1 was also functionally expressed in cortical neurons. Together with the present morphological data, the findings by Marrone et al. (89) indicate that TRPV1 might be a key player of glia-neuron communication.
Recent studies have shown that TRPV1 is desensitized by a number of cannabinoids, including THC, cannabinol, synthetic cannabinoid WIN 55,212-2, AEA, rimonabant (7) as well as PEA (83, 90–92). This ability is very important as TRPV1 channel desensitization is considered to be responsible for analgesic and anti-inflammatory effects (89).
A limitation of the study is the lack of unquestionable specificity test of the employed TRPV1 antibody in dog tissue. The TRPV1 has been cloned and functionally characterized from different species, including dogs. Peptide alignment of the dog TRPV1 ortholog with other species of the TRPV1 family revealed a high degree of sequence homology (human, 89.1%; rat, 87.5%; mouse, 83.3%) (93). Actually, the antibody performs well in an optimized IHC assay, binding the indicated target, not only in dog tissue (TRPV1 immunolabeled SGCs were observed also in cat and horse cervical DRG, while in small rodents and guinea-pig the TRPV1 immunoreactivity was always limited to DRG neurons—RC personal observation). Thus, since the dog was proposed as a good model for studying the role of TRPV1 in inflammatory diseases and nociception and the effects of TRPV1 antagonists in humans (93), additional molecular analysis, such as knockout cell lines and Western blot (assuming the IHC-based antibody also works in Western blots), might be necessary to strength the results of TRPV1 immunolabeling, and to increase confidence for the validity in the dog.
Conclusion
The present study highlighted the expression of canonical and putative cannabinoid receptors on different DRG cell types, in particular neurons and glial cells (SGCs and Schwann cells). Given the key role of DRG elements and cannabinoid receptors in the pathophysiology of chronic pain, targeting and modulating these receptors, possibly through a multifaceted approach, may become a novel way to manage pain in veterinary patients.
Data Availability
All datasets generated for this study are included in the manuscript/Supplementary Files.
Author Contributions
RC, LM, and GM: study concept and design. Western blot analysis was carried out by CB and MF. The immunohistochemical experiments were carried out by FG, GG, AS, MM, and CT. RC and GG: acquisition of data. All authors interpreted the data. RC: drafting of the manuscript and study supervision. All authors contributed to revision of the article for critical intellectual content and have approved the final version.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
The excellent technical assistance of Dr. Giacomo Lazzari and Dr. Margherita De Silva is gratefully acknowledged.
Footnotes
Funding. This research received a grant from Innovet Italia S.r.l. (2017).
Supplementary Material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fvets.2019.00313/full#supplementary-material
References
- 1.Krames ES. The dorsal root ganglion in chronic pain and as a target for neuromodulation: a review. Neuromodulation. (2015) 18:24–32. 10.1111/ner.12247 [DOI] [PubMed] [Google Scholar]
- 2.Hohmann AG, Martin WJ, Tsou K, Walker JM. Inhibition of noxious stimulus-evoked activity of spinal cord dorsal horn neurons by the cannabinoid WIN 55,212–2. Life Sci. (1995) 56:2111–8. 10.1016/0024-3205(95)00196-D [DOI] [PubMed] [Google Scholar]
- 3.Martin WJ, Hohmann AG, Walker JM. Suppression of noxious stimulus-evoked activity in the ventral posterolateral nucleus of the thalamus by a cannabinoid agonist: correlation between electrophysiological and antinociceptive effects. J Neurosci. (1996) 16:6601–11. 10.1523/JNEUROSCI.16-20-06601.1996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsou K, Lowitz KA, Hohmann AG, Martin WJ, Hathaway CB, Bereiter DA, et al. Suppression of noxious stimulus-evoked expression of Fos protein-like immunoreactivity in rat spinal cord by a selective cannabinoid agonist. Neuroscience. (1996) 70:791–8. 10.1016/S0306-4522(96)83015-6 [DOI] [PubMed] [Google Scholar]
- 5.Calignano A, La Rana G, Giuffrida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. (1998) 394:277–81. 10.1038/28393 [DOI] [PubMed] [Google Scholar]
- 6.Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. (1998) 75:111–9. 10.1016/S0304-3959(97)00213-3 [DOI] [PubMed] [Google Scholar]
- 7.Stella N. Cannabinoid and cannabinoid-like receptors in microglia, astrocytes, and astrocytomas. Glia. (2010) 58:1017–30. 10.1002/glia.20983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Davis MP. Cannabinoids in pain management: CB1, CB2 and non-classic receptor ligands. Expert Opin Investig Drugs. (2014) 23:1123–40. 10.1517/13543784.2014.918603 [DOI] [PubMed] [Google Scholar]
- 9.Pirone A, Cantile C, Miragliotta V, Lenzi C, Giannessi E, Cozzi B. Immunohistochemical distribution of the cannabinoid receptor 1 and fatty acid amide hydrolase in the dog claustrum. J Chem Neuroanat. (2016) 74:21–7. 10.1016/j.jchemneu.2016.02.002 [DOI] [PubMed] [Google Scholar]
- 10.Freundt-Revilla J, Kegler K, Baumgärtner W, Tipold A. Spatial distribution of cannabinoid receptor type 1 (CB1) in normal canine central and peripheral nervous system. PLoS ONE. (2017) 12:e0181064. 10.1371/journal.pone.0181064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Freundt-Revilla J, Heinrich F, Zoerner A, Gesell F, Beyerbach M, Shamir M, et al. The endocannabinoid system in canine steroid-responsive meningitis-arteritis and intraspinal spirocercosis. PLoS ONE. (2018) 13:e0187197. 10.1371/journal.pone.0187197 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Petrosino S, Di Marzo V. The pharmacology of palmitoylethanolamide and first data on the therapeutic efficacy of some of its new formulations. Br J Pharmacol. (2017) 174:1349–65. 10.1111/bph.13580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. (2010) 62:588–631. 10.1124/pr.110.003004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yang H, Zhou J, Lehmann C. GPR55–a putative “type 3” cannabinoid receptor in inflammation. J Basic Clin Physiol Pharmacol. (2016) 27:297–302. 10.1515/jbcpp-2015-0080 [DOI] [PubMed] [Google Scholar]
- 15.Donvito G, Nass SR, Wilkerson JL, Curry ZA, Schurman LD, Kinsey SG, et al. The endogenous cannabinoid system: a budding source of targets for treating inflammatory and neuropathic pain. Neuropsychopharmacology. (2018) 43:52–79. 10.1038/npp.2017.204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pergolizzi JV, Jr, Lequang JA, Taylor R., Jr, Raffa RB, Colucci D, NEMA Research Group The role of cannabinoids in pain control: the good, the bad, and the ugly. Minerva Anestesiol. (2018) 84:955–69. 10.23736/S0375-9393.18.12287-5 [DOI] [PubMed] [Google Scholar]
- 17.Sachs J, McGlade E, Yurgelun-Todd D. Safety and toxicology of cannabinoids. Neurotherapeutics. (2015) 12:735–46. 10.1007/s13311-015-0380-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bonn-Miller MO, Loflin MJE, Thomas BF, Marcu JP, Hyke T, Vandrey R. Labeling accuracy of cannabidiol extracts sold online. JAMA. (2017) 318:1708–9. 10.1001/jama.2017.11909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Pavlovic R, Nenna G, Calvi L, Panseri S, Borgonovo G, Giupponi L, et al. Quality traits of “Cannabidiol Oils”: cannabinoids content, terpene fingerprint and oxidation stability of European commercially available preparations. Molecules. (2018) 23:1230 10.3390/molecules23051230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Carcieri C, Tomasello C, Simiele M, De Nicolò A, Avataneo V, Canzoneri L, et al. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J Pharm Pharmacol. (2018) 70:143–9. 10.1111/jphp.12845 [DOI] [PubMed] [Google Scholar]
- 21.Skaper SD, Facci L, Barbierato M, Zusso M, Bruschetta G, Impellizzeri D, et al. N-palmitoylethanolamine and neuroinflammation: a novel therapeutic strategy of resolution. Mol Neurobiol. (2015) 52:1034–42. 10.1007/s12035-015-9253-8 [DOI] [PubMed] [Google Scholar]
- 22.Kunos G, Bátkai S, Offertáler L, Mo F, Liu J, Karcher J, et al. The quest for a vascular endothelial cannabinoid receptor. Chem Phys Lipids. (2002) 121:45–56. 10.1016/S0009-3084(02)00145-7 [DOI] [PubMed] [Google Scholar]
- 23.Lípez-Miranda V, Herradón E, Martín MI. Vasorelaxation caused by cannabinoids: mechanisms in different vascular beds. Curr Vasc Pharmacol. (2008) 6:335–46. 10.2174/157016108785909706 [DOI] [PubMed] [Google Scholar]
- 24.Galiazzo G, Giancola F, Stanzani A, Fracassi F, Bernardini C, Forni M, et al. Localization of cannabinoid receptors CB1, CB2, GPR55, and PPARα in the canine gastrointestinal tract. Histochem Cell Biol. (2018) 150:187–205. 10.1007/s00418-018-1684-7 [DOI] [PubMed] [Google Scholar]
- 25.Kader KN, Moore LR, Saul JM, Zborowski M, Ziats NP, Bellamkonda RV. Isolation and purification of canine adipose microvascular endothelial cells. Microvasc Res. (2001) 61:220–6. 10.1006/mvre.2001.2296 [DOI] [PubMed] [Google Scholar]
- 26.Ren G, Michael LH, Entman ML, Frangogiannis NG. Morphological characteristics of the microvasculature in healing myocardial infarcts. J Histochem Cytochem. (2002) 50:71–9. 10.1177/002215540205000108 [DOI] [PubMed] [Google Scholar]
- 27.Preziosi R, Sarli G, Paltrinieri M. Prognostic value of intratumoral vessel density in cutaneous mast cell tumors of the dog. J Comp Pathol. (2004) 130:143–51. 10.1016/j.jcpa.2003.10.003 [DOI] [PubMed] [Google Scholar]
- 28.Mitrirattanakul S, Ramakul N, Guerrero AV, Matsuka Y, Ono T, Iwase H, et al. Site-specific increases in peripheral cannabinoid receptors and their endogenous ligands in a model of neuropathic pain. Pain. (2006) 126:102–14. 10.1016/j.pain.2006.06.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Skaper SD, Facci L, Giusti P. Glia and mast cells as targets for palmitoylethanolamide, an anti-inflammatory and neuroprotective lipid mediator. Mol Neurobiol. (2013) 48:340–52. 10.1007/s12035-013-8487-6 [DOI] [PubMed] [Google Scholar]
- 30.Navarro G, Morales P, Rodríguez-Cueto C, Fernández-Ruiz J, Jagerovic N, Franco R. Targeting cannabinoid CB2 receptors in the central nervous system. Medicinal chemistry approaches with focus on neurodegenerative disorders. Front Neurosci. (2016) 10:406. 10.3389/fnins.2016.00406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cassano T, Calcagnini S, Pace L, De Marco F, Romano A, Gaetani S. Cannabinoid receptor 2 signaling in neurodegenerative disorders: from pathogenesis to a promising therapeutic target. Front Neurosci. (2017) 11:30. 10.3389/fnins.2017.00030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Glenn TD, Talbot WS. Signals regulating myelination in peripheral nerves and the Schwann cell response to injury. Curr Opin Neurobiol. (2013) 23:1041–8. 10.1016/j.conb.2013.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Truini A, Galeotti F, Cruccu G. Treating pain in multiple sclerosis. Expert Opin Pharmacother. (2011) 12:2355–68. 10.1517/14656566.2011.607162 [DOI] [PubMed] [Google Scholar]
- 34.Tongtako W, Lehmbecker A, Wang Y, Hahn K, Baumgärtner W, Gerhauser I. Canine dorsal root ganglia satellite glial cells represent an exceptional cell population with astrocytic and oligodendrocytic properties. Sci Rep. (2017) 7:13915. 10.1038/s41598-017-14246-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wyburn GM. The capsule of spinal ganglion cells. J Anat. (1958) 92:528–33. [PMC free article] [PubMed] [Google Scholar]
- 36.Bunge MB, Bunge RP, Peterson ER, Murray MR. A light and electron microscope study of long-term organized cultures of rat dorsal root ganglia. J Cell Biol. (1967) 32:439–66. 10.1083/jcb.32.2.439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jacobs JM. Vascular permeability and neurotoxicity. Environ Health Perspect. (1978) 26:107–16. 10.1289/ehp.7826107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anzil AP, Blinzinger K, Herrlinger H. Fenestrated blood capillaries in rat cranio-spinal sensory ganglia. Cell Tissue Res. (1976) 167:563–7. 10.1007/BF00215185 [DOI] [PubMed] [Google Scholar]
- 39.Bush MS, Reid AR, Allt G. Blood-nerve barrier: distribution of anionic sites on the endothelial plasma membrane and basal lamina of dorsal root ganglia. J Neurocytol. (1991) 20:759–68. 10.1007/BF01187849 [DOI] [PubMed] [Google Scholar]
- 40.Pannese E. The structure of the perineuronal sheath of satellite glial cells (SGCs) in sensory ganglia. Neuron Glia Biol. (2010) 6:3–10. 10.1017/S1740925X10000037 [DOI] [PubMed] [Google Scholar]
- 41.Jimenez-Andrade JM, Herrera MB, Ghilardi JR, Vardanyan M, Melemedjian OK, Mantyh PW. Vascularization of the dorsal root ganglia and peripheral nerve of the mouse: implications for chemical-induced peripheral sensory neuropathies. Mol Pain. (2008) 4:10. 10.1186/1744-8069-4-10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campora L, Miragliotta V, Ricci E, Cristino L, Di Marzo V, Albanese F, et al. Cannabinoid receptor type 1 and 2 expression in the skin of healthy dogs and dogs with atopic dermatitis. Am J Vet Res. (2012) 73:988–95. 10.2460/ajvr.73.7.988 [DOI] [PubMed] [Google Scholar]
- 43.Zhang X, Chen Y, Wang C, Huang LY. Neuronal somatic ATP release triggers neuron-satellite glial cell communication in dorsal root ganglia. Proc Natl Acad Sci USA. (2007) 104:9864–9. 10.1073/pnas.0611048104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stanley C, O'Sullivan SE. Vascular targets for cannabinoids: animal and human studies. Br J Pharmacol. (2014) 171:1361–78. 10.1111/bph.12560 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Dall'Aglio C, Polisca A, Cappai MG, Mercati F, Troisi A, Pirino C, et al. (2017). Immunohistochemistry detected and localized cannabinoid receptor type 2 in bovine fetal pancreas at late gestation. Eur J Histochem. 61:2761. 10.4081/ejh.2017.2761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zheng JL, Yu TS, Li XN, Fan YY, Ma WX, Du Y, et al. Cannabinoid receptor type 2 is time-dependently expressed during skin wound healing in mice. Int J Legal Med. (2012) 126:807–14. 10.1007/s00414-012-0741-3 [DOI] [PubMed] [Google Scholar]
- 47.Ho WSV, Kelly MEM. Cannabinoids in the cardiovascular system. Adv Pharmacol. (2017) 80:329–66. 10.1016/bs.apha.2017.05.002 [DOI] [PubMed] [Google Scholar]
- 48.Vercelli C, Barbero R, Cuniberti B, Odore R, Re G. Expression and functionality of TRPV1 receptor in human MCF-7 and canine CF.41 cells. Vet Comp Oncol. (2015) 13:133–42. 10.1111/vco.12028 [DOI] [PubMed] [Google Scholar]
- 49.Russo D, Clavenzani P, Sorteni C, Bo Minelli L, Botti M, Gazza F, et al. Neurochemical features of boar lumbosacral dorsal root ganglion neurons and characterization of sensory neurons innervating the urinary bladder trigone. J Comp Neurol. (2013) 521:342–66. 10.1002/cne.23177 [DOI] [PubMed] [Google Scholar]
- 50.Woodhams SG, Chapman V, Finn DP, Hohmann AG, Neugebauer V. The cannabinoid system and pain. Neuropharmacology. (2017) 124:105–20. 10.1016/j.neuropharm.2017.06.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Berta T, Qadri Y, Tan PH, Ji RR. Targeting dorsal root ganglia and primary sensory neurons for the treatment of chronic pain. Expert Opin Ther Targets. (2017) 21:695–703. 10.1080/14728222.2017.1328057 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Guerrero-Alba R, Barragán-Iglesias P, González-Hernández A, Valdez-Moráles EE, Granados-Soto V, Condés-Lara M, et al. Some prospective alternatives for treating pain: the endocannabinoid system and its putative receptors GPR18 and GPR55. Front Pharmacol. (2019) 9:1496. 10.3389/fphar.2018.01496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sanudo-Pena MC, Strangman NM, Mackie K, Walker JM, Tsou K. CB1 receptor localization in rat spinal cord and roots, dorsal root ganglion, and peripheral nerve. Zhongguo Yao Li Xue Bao. (1999) 20:1115–20. [PubMed] [Google Scholar]
- 54.Anand U, Otto WR, Sanchez-Herrera D, Facer P, Yiangou Y, Korchev Y, et al. Cannabinoid receptor CB2 localisation and agonist-mediated inhibition of capsaicin responses in human sensory neurons. Pain. (2008) 138:667–80. 10.1016/j.pain.2008.06.007 [DOI] [PubMed] [Google Scholar]
- 55.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. (1999) 90:923–31. 10.1016/S0306-4522(98)00524-7 [DOI] [PubMed] [Google Scholar]
- 56.Svíženská IH, Brázda V, Klusáková I, Dubový P. Bilateral changes of cannabinoid receptor type 2 protein and mRNA in the dorsal root ganglia of a rat neuropathic pain model. J Histochem Cytochem. (2013) 61:529–47. 10.1369/0022155413491269 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sánchez-Zavaleta R, Cortés H, Avalos-Fuentes JA, García U, Segovia Vila J, Erlij D, et al. Presynaptic cannabinoid CB2 receptors modulate [3 H]-Glutamate release at subthalamo-nigral terminals of the rat. Synapse. (2018) 72:e22061. 10.1002/syn.22061 [DOI] [PubMed] [Google Scholar]
- 58.Stella N. Endocannabinoid signaling in microglial cells. Neuropharmacology. (2009) 56:244–53. 10.1016/j.neuropharm.2008.07.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fernández-Trapero M, Espejo-Porras F, Rodríguez-Cueto C, Coates JR, Pérez-Díaz C, De Lago E, et al. Upregulation of CB2 receptors in reactive astrocytes in canine degenerative myelopathy, a disease model of amyotrophic lateral sclerosis. Dis Model Mech. (2017) 10:551–8. 10.1242/dmm.028373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Currie S, Rainbow RD, Ewart MA, Kitson S, Pliego EH, Kane KA, et al. IP(3)R-mediated Ca(2+) release is modulated by anandamide in isolated cardiac nuclei. J Mol Cell Cardiol. (2008) 45:804–11. 10.1016/j.yjmcc.2008.07.005 [DOI] [PubMed] [Google Scholar]
- 61.Benyó Z, Ruisanchez É, Leszl-Ishiguro M, Sándor P, Pacher P. Endocannabinoids in cerebrovascular regulation. Am J Physiol Heart Circ Physiol. (2016) 310:785–801. 10.1152/ajpheart.00571.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Morales P, Reggio PH. An update on non-CB1, non-CB2 cannabinoid related G-protein-coupled receptors. Cannabis Cannabinoid Res. (2017) 2:265–73. 10.1089/can.2017.0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lauckner JE, Jensen JB, Chen HY, Lu HC, Hille B, Mackie K. GPR55 is a cannabinoid receptor that increases intracellular calcium and inhibits M current. Proc Natl Acad Sci USA. (2008) 105:2699–704. 10.1073/pnas.0711278105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Neumann S, Doubell TP, Leslie T, Woolf CJ. Inflammatory pain hypersensitivity mediated by phenotypic switch in myelinated primary sensory neurons. Nature. (1996) 384:360–4. 10.1038/384360a0 [DOI] [PubMed] [Google Scholar]
- 65.Ruscheweyh R, Forsthuber L, Schoffnegger D, Sandkuhler J. Modification of classical neurochemical markers in identified primary afferent neurons with Abeta-, Adelta-, and C-fibers after chronic constriction injury in mice. J Comp Neurol. (2007) 502:325–36. 10.1002/cne.21311 [DOI] [PubMed] [Google Scholar]
- 66.Kramar C, Loureiro M, Renard J, Laviolette SR. Palmitoylethanolamide modulates GPR55 receptor signaling in the ventral hippocampus to regulate mesolimbic dopamine activity, social interaction, and memory processing. Cannabis Cannabinoid Res. (2017) 2:8–20. 10.1089/can.2016.0030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Cairns BE, Arendt-Nielsen L, Sacerdote P. Perspectives in pain research 2014: neuroinflammation and glial cell activation: the cause of transition from acute to chronic pain? Scand J Pain. (2015) 6:3–6. 10.1016/j.sjpain.2014.10.002 [DOI] [PubMed] [Google Scholar]
- 68.Hanani M. Intercellular communication in sensory ganglia by purinergic receptors and gap junctions: implications for chronic pain. Brain Res. (2012) 1487:183–91. 10.1016/j.brainres.2012.03.070 [DOI] [PubMed] [Google Scholar]
- 69.Iwata K, Katagiri A, Shinoda M. Neuron-glia interaction is a key mechanism underlying persistent orofacial pain. J Oral Sci. (2017) 59:173–5. 10.2334/josnusd.16-0858 [DOI] [PubMed] [Google Scholar]
- 70.Skaper SD, Facci L, Zusso M, Giusti P. An inflammation-centric view of neurological disease: beyond the neuron. Front Cell Neurosci. (2018) 12:72. 10.3389/fncel.2018.00072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Naidenow J, Hrgovic I, Doll M, Hailemariam-Jahn T, Lang V, Kleemann J, et al. Peroxisome proliferator-activated receptor (PPAR) α and δ activators induce ICAM-1 expression in quiescent non stimulated endothelial cells. J Inflamm (Lond). (2016) 13:27. 10.1186/s12950-016-0135-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lo Verme J, Fu J, Astarita G, La Rana G, Russo R, Calignano A, et al. The nuclear receptor peroxisome proliferator-activated receptor-alpha mediates the anti-inflammatory actions of palmitoylethanolamide. Mol Pharmacol. (2005) 67:15–9. 10.1124/mol.104.006353 [DOI] [PubMed] [Google Scholar]
- 73.LoVerme J, Russo R, La Rana G, Fu J, Farthing J, Raso G, et al. Rapid broad-spectrum analgesia through activation of peroxisome proliferator-activated receptor-alpha. J Pharmacol Exp Ther. (2006) 319:1051–61. 10.1124/jpet.106.111385 [DOI] [PubMed] [Google Scholar]
- 74.D'Agostino G, La Rana G, Russo R, Sasso O, Iacono A, Esposito E, et al. Central administration of palmitoylethanolamide reduces hyperalgesia in mice via inhibition of NF-kappaB nuclear signalling in dorsal root ganglia. Eur J Pharmacol. (2009) 613:54–9. 10.1016/j.ejphar.2009.04.022 [DOI] [PubMed] [Google Scholar]
- 75.Khasabova IA, Xiong Y, Coicou LG, Piomelli D, Seybold V. Peroxisome proliferator-activated receptor α mediates acute effects of palmitoylethanolamide on sensory neurons. J Neurosci. (2012) 32:12735–43. 10.1523/JNEUROSCI.0130-12.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Wang J, Zhang Q, Zhao L, Li D, Fu Z, Liang L. Down-regulation of PPARa in the spinal cord contributes to augmented peripheral inflammation and inflammatory hyperalgesia in diet-induced obese rats. Neuroscience. (2014) 278:165–78. 10.1016/j.neuroscience.2014.07.071 [DOI] [PubMed] [Google Scholar]
- 77.Zwick M, Davis BM, Woodbury J, Burkett JN, Koerber HR, Simpson JF, et al. Glial cell line-derived neurotrophic factor is a survival factor for isolectin B4-positive, but not vanilloid receptor 1-positive, neurons in the mouse. J Neurosci. (2002) 22:4057–65. 10.1523/JNEUROSCI.22-10-04057.2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yu L, Yang F, Luo H, Liu FY, Han JS, Xing GG, et al. The role of TRPV1 in different subtypes of dorsal root ganglion neurons in rat chronic inflammatory nociception induced by complete Freund's adjuvant. Mol Pain. (2008) 4:61. 10.1186/1744-8069-4-61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, et al. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. (1998). 21:531–43. 10.1016/S0896-6273(00)80564-4 [DOI] [PubMed] [Google Scholar]
- 80.Breese NM, George AC, Pauers LE, Stucky CL. Peripheral inflammation selectively increases TRPV1 function in IB4-positive sensory neurons from adult mouse. Pain. (2005) 115:37–49. 10.1016/j.pain.2005.02.010 [DOI] [PubMed] [Google Scholar]
- 81.Caterina MJ, Schumacher MA, Tominaga M, Rosen TA, Levine JD, Julius D. The capsaicin receptor: a heat-activated ion channel in the pain pathway. Nature. (1997) 389:816–24. 10.1038/39807 [DOI] [PubMed] [Google Scholar]
- 82.Zygmunt PM, Petersson J, Andersson DA, Chuang H-H, Sørgard M, Di Marzo V, et al. Vanilloid receptors on sensory nerves mediate the vasodilator action of anandamide. Nature. (1999) 400:452–7. 10.1038/22761 [DOI] [PubMed] [Google Scholar]
- 83.Ambrosino P, Soldovieri MV, Russo C, Taglialatela M. Activation and desensitization of TRPV1 channels in sensory neurons by the PPARα agonist palmitoylethanolamide. Br J Pharmacol. (2013) 168:1430–44. 10.1111/bph.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Helliwell RJ, McLatchie LM, Clarke M, Winter J, Bevan S, McIntyre P. Capsaicin sensitivity is associated with the expression of the vanilloid (capsaicin) receptor (VR1) mRNA in adult rat sensory ganglia. Neurosci Lett. (1998) 250:177–80. 10.1016/S0304-3940(98)00475-3 [DOI] [PubMed] [Google Scholar]
- 85.Hoffman EM, Schechter R, Miller K. E. Fixative composition alters distributions of immunoreactivity for glutaminase and two markers of nociceptive neurons, Nav1.8 and TRPV1, in the rat dorsal root ganglion. J Histochem Cytochem. (2010) 58:329–44. 10.1369/jhc.2009.954008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Binzen U, Greffrath W, Hennessy S, Bausen M, Saaler-Reinhardt S, Treede RD. Co-expression of the voltage-gated potassium channel Kv1.4 with transient receptor potential channels (TRPV1 and TRPV2) and the cannabinoid receptor CB1 in rat dorsal root ganglion neurons. Neuroscience. (2006) 142:527–39. 10.1016/j.neuroscience.2006.06.020 [DOI] [PubMed] [Google Scholar]
- 87.Doly S, Fischer J, Salio C, Conrath M. The vanilloid receptor-1 is expressed in rat spinal dorsal horn astrocytes. Neurosci Lett. (2004) 357:123–6. 10.1016/j.neulet.2003.12.051 [DOI] [PubMed] [Google Scholar]
- 88.Chukyo A, Chiba T, Kambe T, Yamamoto K, Kawakami K, Taguchi K, et al. Oxaliplatin-induced changes in expression of transient receptor potential channels in the dorsal root ganglion as a neuropathic mechanism for cold hypersensitivity. Neuropeptides. (2018) 67:95–101. 10.1016/j.npep.2017.12.002 [DOI] [PubMed] [Google Scholar]
- 89.Marrone MC, Morabito A, Giustizieri M, Chiurchiù V, Leuti A, Mattioli M, et al. TRPV1 channels are critical brain inflammation detectors and neuropathic pain biomarkers in mice. Nat Commun. (2017) 8:15292. 10.1038/ncomms15292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ho WS, Barrett DA, Randall MD. ‘Entourage' effects of N-palmitoylethanolamide and N-oleoylethanolamide on vasorelaxation to anandamide occur through TRPV1 receptors. Br J Pharmacol. (2008) 155:837–46. 10.1038/bjp.2008.324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.De Petrocellis L, Di Marzo V. Non-CB1, non-CB2 receptors for endocannabinoids, plant cannabinoids, and synthetic cannabimimetics: focus on G-protein-coupled receptors and transient receptor potential channels. J Neuroimmune Pharmacol. (2010) 5:103–21. 10.1007/s11481-009-9177-z [DOI] [PubMed] [Google Scholar]
- 92.Aldossary SA, Alsalem M, Kalbouneh H, Haddad M, Azab B, Al-Shboul O, et al. The role of transient receptor potential vanilloid receptor 1 and peroxisome proliferator-activated receptors-α in mediating the antinociceptive effects of palmitoylethanolamine in rats. Neuroreport. (2019) 30:32–7. 10.1097/WNR.0000000000001161 [DOI] [PubMed] [Google Scholar]
- 93.Phelps PT, Anthes JC, Correll CC. Cloning and functional characterization of dog transient receptor potential vanilloid receptor-1 (TRPV1). Eur J Pharmacol. (2005) 513:57–66. 10.1016/j.ejphar.2005.02.045 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All datasets generated for this study are included in the manuscript/Supplementary Files.