 Cyclic (alkyl)(amino)carbene (CAAC) metal complexes can also engage in asymmetric transformations, thereby expanding the toolbox of available chiral carbene ligands.
Cyclic (alkyl)(amino)carbene (CAAC) metal complexes can also engage in asymmetric transformations, thereby expanding the toolbox of available chiral carbene ligands.
Abstract
The popularity of NHCs in transition metal catalysis has prompted the development of chiral versions as electron-rich neutral stereodirecting ancillary ligands for enantioselective transformations. Herein we demonstrate that cyclic (alkyl)(amino)carbene (CAAC) ligands can also engage in asymmetric transformations, thereby expanding the toolbox of available chiral carbenes.
1. Introduction
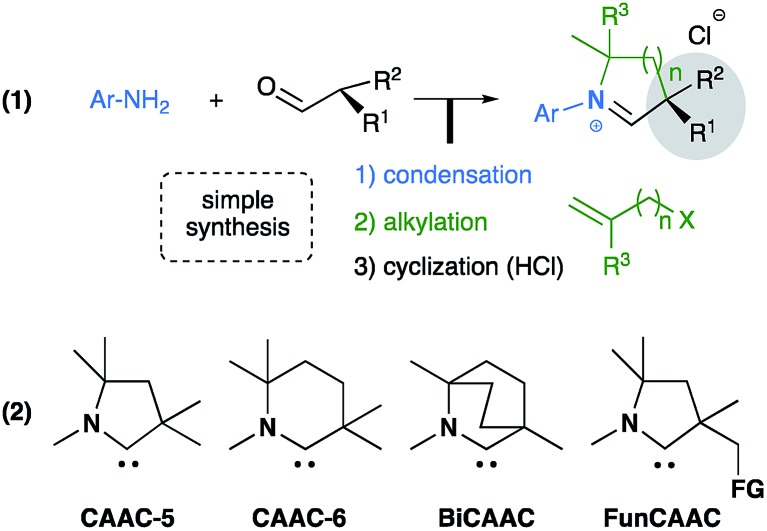
The success of stable N-heterocyclic carbenes (NHCs) as ligands for transition metal catalysts, and as organocatalysts in their own right, has triggered the development of chiral versions.1–3 Surprisingly, as recently noted by Glorius and co-workers,4 despite the existence of a variety of stable heterocyclic carbenes,5 only diaminocarbenes, namely imidazol-2-ylidenes,6 imidazolidin-2-ylidenes7 and 1,2,4-triazol-5-ylidenes8 have been used as ligands for enantioselective transformations. In 2005 our group discovered cyclic (alkyl)(amino)carbenes (CAACs).9,10 We and others have demonstrated that their unique electronic (more σ-donating and π-accepting than NHCs) and steric properties allow for the improvement of known catalytic processes (Ru,11 Pd,9,12 and Rh13) as well as promoting novel reactions with coinage metals (Cu14 and Au15). The direct protonated precursors of CAACs are readily available in one pot from an aldehyde and a primary amine (Scheme 1(1)).16 We have shown that our versatile synthetic methodology facilitates access to a library of 5-membered (CAAC-5), 6-membered (CAAC-6),12 bicylic (BiCAAC)17 and even bifunctional CAACs (FunCAAC)14b (Scheme 1(2)). Of particular importance, the CAAC family features a quaternary carbon adjacent to the carbene carbon, thus allowing the introduction of a chiral center in closer proximity to the active site than NHCs. Herein, we report the first examples of chiral CAAC ligands in asymmetric catalysis.
Scheme 1. Synthetic route to cyclic (alkyl)(amino)carbenes, and existing families of CAACs.
In our initial paper on CAACs,9 we showed that the enantiopure L-MenthCAAC could be prepared without time-consuming enantio- or diastereoselective separation from the inexpensive (–)-menthol (Scheme 2). The key step of the synthesis was based on the well-known propensity of relatively bulky reactants to approach the cyclohexane moiety selectively from the equatorial direction.
Scheme 2. Synthesis of L-MenthCAAC highlighting the key step.
2. Results and discussion
Years ago, we tested L-MenthCAAC transition metal complexes in a variety of asymmetric catalytic reactions without any success. Given our recent mechanistic work on copper-catalysis, we decided to revive this topic. We considered benchmarking the L-MenthCAAC in the copper-catalysed Asymmetric Conjugate Borylation (ACB) reaction. Over the past decade, this chemical transformation has emerged as a stalwart method for the preparation of chiral organoboron building blocks, which are sought after in organic synthesis.18 Although pioneered by Yun19 and others using chiral phosphine ligands,18d Fernández,20 Hoveyda21 and others18d have successfully shown that carbene ligands, specifically NHCs, could also be utilized in this process.
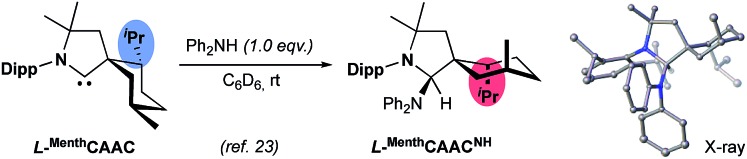
As can be seen in Scheme 3, although the L-MenthCAAC copper complex 1 was found to be highly active in the addition of bis(pinacolato)diboron (B2pin2) to various α,β-unsaturated esters, an almost complete lack of asymmetric induction was observed. In comparison, the known non C2-chiral NHC copper complex 2,22 derived from (S)-1-(naphthalen-1-yl)ethan-1-amine, gave a 55% ee for the borylated adduct P1. Examining the X-ray crystal structure of 1,9 we expected some asymmetric induction since for many years we believed that a conformational inversion of the menthyl ring was energetically inaccessible. However, we recently found that in the solid state the menthyl group of the L-MenthCAAC amine adduct L-MenthCAACNH existed as the other conformer with the methyl and isopropyl substituents in axial position (Scheme 4).23 We were able to confirm with DFT calculations that the inverted menthyl conformer is also readily accessible in complex 1 (Fig. 1). The existence of the two conformers 1 and 1′ readily explains the lack of asymmetric induction since they have antagonistic stereo-inducing effects.
Scheme 3. Comparing L-MenthCAACCuCl 1 and NHC–CuCl 2 in the Asymmetric Conjugate Borylation (ACB) reaction. aDetermined by 1H NMR spectroscopy using mesitylene as an internal standard. bIsolated yield after SiO2 purification. cDetermined by GC on a chiral stationary phase (see ESI for details†).
Scheme 4. Conformational inversion of the menthyl ring.23.
Fig. 1. Proposed justification for the lack of asymmetric induction in catalyst 1.
To circumvent this issue, we sought to prepare a more rigid chiral CAAC. Among the number of readily available molecules derived from the chiral pool, we selected the steroid backbone for its bulk, structural diversity, and tunability. We also reasoned that owing to a three-dimensional skeleton composed of four fused rings (three six-member cyclohexane rings – A, B, C- and one five-member cyclopentane ring D), this steroid motif would provide the desired rigid structural features while keeping the chirality intact during the synthesis (Fig. 2).
Fig. 2. Steroid CAACs as versatile chiral ligand frameworks.
Before proceeding further, we confirmed that our synthetic methodology16 extends to the readily available decahydronaphthalene, a model substrate mimicking the first two fused cyclohexane (A and B) rings of a steroid skeleton (Scheme 5). CAAC-decahydronaphthyl iminium rac-NaphCAAC was obtained in 7 steps and 50% yield as a white powder.
Scheme 5. Preparation of rac-NaphCAAC.
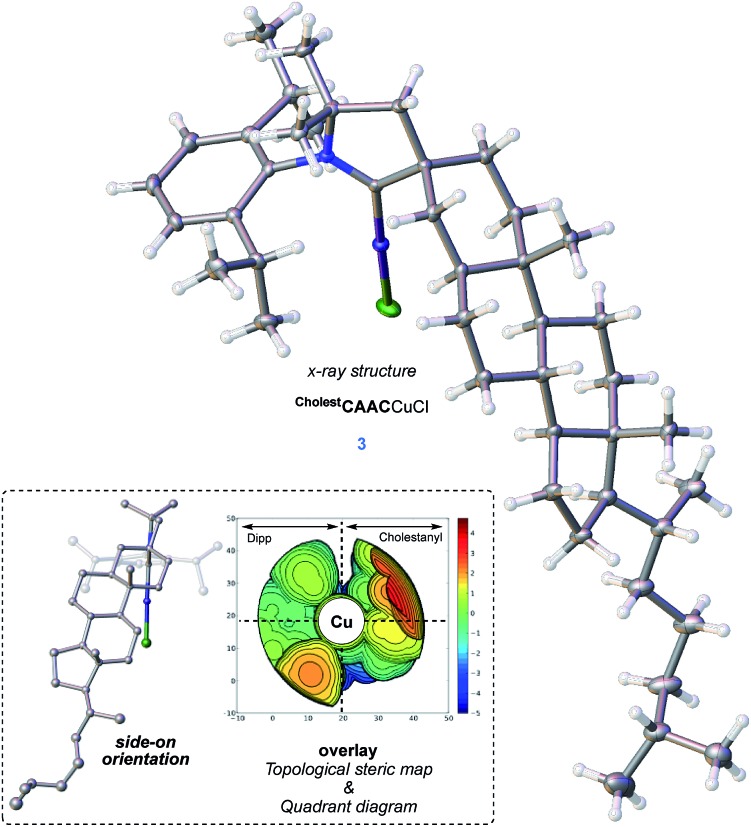
Reassured by these results, we applied the same synthetic strategy to 5α-cholestan-3-one, an inexpensive enantiopure steroid derived from coprostanol (Scheme 6). The CAAC-cholestanyl iminium CholestCAAC was obtained in 6 steps and 63% yield as a white fluffy powder.24 The corresponding copper complex 3 was prepared by deprotonation of the CholestCAAC iminium with KHDMS followed by addition of copper chloride in THF. Single crystals were obtained by slow diffusion of diethyl ether in acetonitrile, and an X-ray diffraction study confirmed the formation of CholestCAACCuCl complex 3 (Fig. 3). It crystallized in the P21 space group with one molecule in the asymmetric unit cell.
Scheme 6. Preparation of CholestCAAC and complex 3.
Fig. 3. X-ray crystal structure of 3 (top) with the side-on orientation (omitting hydrogen for clarity) and an overlaid topological steric map with a quadrant diagram.
The absolute stereochemistry was conclusively established using anomalous dispersion with a Flack parameter of 0.002(7) from refinement. The X-ray data of 3 show a distinctive orientation of the cholestanyl backbone, which gives rise to the topographic steric map showed in Fig. 3.25,26 As can be seen, the corresponding quadrant diagram could support facial stereoselectivity in catalyst-substrate adducts.
We next evaluated complex 3 in the ACB reaction (Scheme 7). As with L-MenthCAACCuCl 1, the steroid copper complex CholestCAACCuCl 3 efficiently catalyzes the addition of B2pin2 to various β-substituted α,β-unsaturated esters, affording the corresponding 1,4-adducts in moderate to good isolated yields (47 to 77%). More importantly, we were pleased to observe enantio-inductions with enantioselectivities reaching 55%.
Scheme 7. Evaluation of CholestCAACCuCl complex 3 in ACB reaction. aDetermined by 1H NMR spectroscopy using mesitylene as an internal standard. bIsolated yield after SiO2 purification. cDetermined by GC on a chiral stationary phase (see ESI for details†). dAnthracene was used as internal standard.
3. Conclusions
This work rationalizes the inability of L-MenthCAAC to induce enantiomeric excess, and indicates that upon providing the right steric environment, the use of chiral CAACs should not be overlooked in metal-catalyzed asymmetric transformations. This new family of stereo-directing chiral carbene ligands are readily available from inexpensive precursors belonging to the chiral pool, and it is noteworthy that the chirality is not restricted to chiral amines (as is the case of NHCs), but extends to chiral aldehydes, a much larger feedstock.
Conflicts of interest
There are no conflicts to declare.
Supplementary Material
Acknowledgments
This work was supported by the U.S. Department of Energy, Office of Science, Basic Energy Sciences, Catalysis Science Program, under Award # DE-SC0009376, and the Agence Nationale de la Recherche (ANR-16-CE07-0019 – Hel-NHC) Thanks are due to the Alfred P. Sloan Foundation's University Centre for Exemplary Mentoring and the National Science Foundation Graduate Research Fellowship Program under Grant No. DGE-1650112 (G. P. J.), and to the National Science Foundation (V. L. – Grant # CHE-1455348). Any opinions, findings, and conclusions or recommendations expressed in this material are those of the author(s) and do not necessarily reflect the views of the National Science Foundation. We also greatly acknowledge the Region Bretagne for the ARED 2018, (“Biometa”, N° 601), and Rennes-Metropole for supporting international PhD mobility (D. P.). MM and TV thank Shimadzu and Chiral Technology for their support in the separation of chiral molecules by Supercritical Fluid Chromatography (SFC) technology.
Footnotes
†Electronic supplementary information (ESI) available: Experimental procedures, NMR spectra. CCDC 1919315. For ESI and crystallographic data in CIF or other electronic format see DOI: 10.1039/c9sc02810b
References
- (a) Sheehan J. C., Hunneman D. H. J. Am. Chem. Soc. 1966;88:3666–3667. [Google Scholar]; (b) Enders D., Gielen H., Raabe G., Runsink J., Teles J. H. Chem. Ber. 1996;129:1483–1488. [Google Scholar]; (c) Herrmann W. A., Goossen L. J., Köcher C., Artus G. R. J. Angew. Chem., Int. Ed. 1996;35:2805–2807. [Google Scholar]; (d) Powell M. T., Hou D. R., Perry M. C., Cui X., Burgess K. J. Am. Chem. Soc. 2001;123:8878–8879. doi: 10.1021/ja016011p. [DOI] [PubMed] [Google Scholar]; (e) Seiders T. J., Ward D. W., Grubbs R. H. Org. Lett. 2001;3:3225–3228. doi: 10.1021/ol0165692. [DOI] [PubMed] [Google Scholar]
- For reviews: ; (a) Wang F., Liu L.-J., Wang W., Li S., Shi M. Coord. Chem. Rev. 2012;256:804–853. [Google Scholar]; (b) Zhao M., Zhang Y.-T., Chen J., Zhou L. Asian J. Org. Chem. 2018;7:54–69. [Google Scholar]; (c) Ouyang J., Crassous J. Coord. Chem. Rev. 2018;376:533–547. [Google Scholar]; (d) Flanigan D. M., Romanov-Michailidis F., White N. A., Rovis T. Chem. Rev. 2015;115:9307–9387. doi: 10.1021/acs.chemrev.5b00060. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Grossmann A., Enders D. Angew. Chem., Int. Ed. 2012;51:314–325. doi: 10.1002/anie.201105415. [DOI] [PubMed] [Google Scholar]
- Selected examples of chiral NHC in: – Au catalysis – ; (a) Wang Y.-M., Kuzniewski C. N., Rauniyar V., Hoong C., Toste F. D. J. Am. Chem. Soc. 2011;133:12972–12975. doi: 10.1021/ja205068j. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Matsumoto Y., Selim K. B., Nakanishi H., Yamada K., Yamamoto Y., Tomioka K. Tetrahedron Lett. 2010;51:404–406. [Google Scholar]; (c) Bartolome C., García-Cuadrado D., Ramiro Z., Espinet P. Inorg. Chem. 2010;49:9758–9764. doi: 10.1021/ic101059c. [DOI] [PubMed] [Google Scholar]; (d) Handa S., Slaughter L. M., Angew. Chem., Int. Ed., 2012, 51 , 2912 –2915 , ; – Cu catalysis – . [DOI] [PubMed] [Google Scholar]; (e) Drissi-Amraoui S., Morin M., Crévisy C., Baslé O., Marcia de Figueiredo R., Mauduit M., Campagne J.-M., Angew. Chem., Int. Ed., 2015, 54 , 11830 –11834 , ; – Pd catalysis – . [DOI] [PubMed] [Google Scholar]; (f) Kündig E. P., Seidel T. M., Jia Y. X., Bernardinelli G., Angew. Chem., Int. Ed., 2007, 46 , 8484 –8487 , ; – Rh catalysis – . [DOI] [PubMed] [Google Scholar]; (g) Kim J. H., Greßies S., Boultadakis-Arapinis M., Daniliuc C., Glorius F., ACS Catal., 2016, 6 , 7652 –7656 , . – Ru catalysis – . [Google Scholar]; (h) Kannenberg A., Rost D., Eibauer S., Tiede S., Blechert S. Angew. Chem., Int. Ed. 2011;50:3299–3302. doi: 10.1002/anie.201007673. [DOI] [PubMed] [Google Scholar]
- Janssen-Mueller D., Schlepphortst C., Glorius F. Chem. Soc. Rev. 2017;46:4845–4854. doi: 10.1039/c7cs00200a. [DOI] [PubMed] [Google Scholar]
- For reviews, see: ; (a) Vivancos A., Segarra C., Albrecht M. Chem. Rev. 2018;118:9493–9586. doi: 10.1021/acs.chemrev.8b00148. [DOI] [PubMed] [Google Scholar]; (b) Guisado-Barrios G., Soleilhavoup M., Bertrand G. Acc. Chem. Res. 2018;51:3236–3244. doi: 10.1021/acs.accounts.8b00480. [DOI] [PubMed] [Google Scholar]; (c) Melaimi M., Soleilhavoup M., Bertrand G. Angew. Chem., Int. Ed. 2010;49:8810–8849. doi: 10.1002/anie.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arduengo III A. J., Harlow R. L., Kline M. J. Am. Chem. Soc. 1991;113:361–363. [Google Scholar]
- Arduengo III A. J., Goerlich J. R., Marshall W. J. J. Am. Chem. Soc. 1995;117:11027–11028. [Google Scholar]
- Enders D., Breuer K., Raabe G., Runsink J., Teles J. H., Melder J. P., Ebel K., Brode S. Angew. Chem., Int. Ed. 1995;34:1021–1023. [Google Scholar]
- Lavallo V., Canac Y., Präsang C., Donnadieu B., Bertrand G. Angew. Chem., Int. Ed. 2005;44:5705–5709. doi: 10.1002/anie.200501841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- For reviews on CAACs see: ; (a) Melaimi M., Jazzar R., Soleihavoup M., Bertrand G. Angew. Chem., Int. Ed. 2017;56:10046–10068. doi: 10.1002/anie.201702148. [DOI] [PubMed] [Google Scholar]; (b) Soleilhavoup M., Bertrand G. Acc. Chem. Res. 2015;48:256–266. doi: 10.1021/ar5003494. [DOI] [PubMed] [Google Scholar]; (c) Melaimi M., Soleilhavoup M., Bertrand G. Angew. Chem., Int. Ed. 2010;49:8810–8849. doi: 10.1002/anie.201000165. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Roy S., Mondal K. C., Roesky H. W. Acc. Chem. Res. 2016;49:357–369. doi: 10.1021/acs.accounts.5b00381. [DOI] [PubMed] [Google Scholar]; (e) Paul U. S. D., Radius U. Eur. J. Inorg. Chem. 2017:3362–3375. [Google Scholar]
- (a) Marx V. M., Sullivan A. H., Melaimi M., Virgil S. C., Keitz B. K., Weinberger D. S., Bertrand G., Grubbs R. H. Angew. Chem., Int. Ed. 2014;54:1919–1923. doi: 10.1002/anie.201410797. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Anderson D. R., Lavallo V., O'leary D. J., Bertrand G., Grubbs R. H. Angew. Chem., Int. Ed. 2007;46:7262–7265. doi: 10.1002/anie.200702085. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Anderson D. R., Ung T., Mkrtumyan G., Bertrand G., Grubbs R. H., Schrodi Y. Organometallics. 2008;27:563–566. doi: 10.1021/om7008028. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Marx V. M., Sullivan A. H., Melaimi M., Virgil S. C., Keitz B. K., Weinberger D. S., Bertrand G., Grubbs R. H. Angew. Chem., Int. Ed. 2015;54:1919–1923. doi: 10.1002/anie.201410797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weinstein C. M., Junor G. P., Tolentino D. R., Jazzar R., Melaimi M., Bertrand G. J. Am. Chem. Soc. 2018;140:9255–9260. doi: 10.1021/jacs.8b05518. [DOI] [PubMed] [Google Scholar]
- (a) Wiesenfeldt M. P., Nairoukh Z., Li W., Glorius F. Science. 2017;357:908–912. doi: 10.1126/science.aao0270. [DOI] [PubMed] [Google Scholar]; (b) Wei Y., Rao B., Cong X., Zeng X. J. Am. Chem. Soc. 2015;137:9250–9253. doi: 10.1021/jacs.5b05868. [DOI] [PubMed] [Google Scholar]; (c) Tran L., Fulton J. L., Linehan J. C., Balasubramanian M., Lercher J. A., Bullock R. M. ACS Catal. 2019;9:4106–4114. [Google Scholar]; (d) Nairoukh Z., Wollenburg M., Schlepphorst C., Bergander K., Glorius F. Nat. Chem. 2019;11:264–270. doi: 10.1038/s41557-018-0197-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Romero E. A., Jazzar R., Bertrand G. Chem. Sci. 2017;8:165–168. doi: 10.1039/c6sc02668k. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Chu J., Munz D., Jazzar R., Melaimi M., Bertrand G. J. Am. Chem. Soc. 2016;138:7884–7887. doi: 10.1021/jacs.6b05221. [DOI] [PubMed] [Google Scholar]
- (a) Hu X., Martin D., Melaimi M., Bertrand G. J. Am. Chem. Soc. 2014;136:13594–13597. doi: 10.1021/ja507788r. [DOI] [PubMed] [Google Scholar]; (b) Kinjo R., Donnadieu B., Bertrand G. Angew. Chem., Int. Ed. 2011;50:5560–5563. doi: 10.1002/anie.201100740. [DOI] [PubMed] [Google Scholar]; (c) Zeng X., Kinjo R., Donnadieu B., Bertrand G. Angew. Chem., Int. Ed. 2010;49:942–945. doi: 10.1002/anie.200905341. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Lavallo V., Frey G. D., Donnadieu B., Soleilhavoup M., Bertrand G. Angew. Chem., Int. Ed. 2008;47:5224–5228. doi: 10.1002/anie.200801136. [DOI] [PMC free article] [PubMed] [Google Scholar]; (e) Jin L., Weinberger D. S., Melaimi M., Moore C. E., Rheingold A. L., Bertrand G. Angew. Chem., Int. Ed. 2014;53:9059–9063. doi: 10.1002/anie.201404665. [DOI] [PubMed] [Google Scholar]
- (a) Jazzar R., Liang H., Donnadieu B., Bertrand G. J. Organomet. Chem. 2006;691:3201–3205. doi: 10.1016/j.jorganchem.2006.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jazzar R., Dewhurst R. D., Bourg J.-B., Donnadieu B., Canac Y., Bertrand G. Angew. Chem., Int. Ed. 2007;46:2899–2902. doi: 10.1002/anie.200605083. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Jazzar R., Bourg J.-B., Dewhurst R. D., Donnadieu B., Bertrand G. J. Org. Chem. 2007;72:3492–3499. doi: 10.1021/jo0703909. [DOI] [PMC free article] [PubMed] [Google Scholar]; (d) Zeng X., Frey G. D., Kinjo R., Donnadieu B., Bertrand G. J. Am. Chem. Soc. 2009;131:8690–8696. doi: 10.1021/ja902051m. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomás-Mendivil E., Hansmann M. M., Weinstein C. M., Jazzar R., Melaimi M., Bertrand G. J. Am. Chem. Soc. 2017;139:7753–7756. doi: 10.1021/jacs.7b04640. [DOI] [PubMed] [Google Scholar]
- (a) Schiffner J. A., Muther K., Oestreich M. Angew. Chem., Int. Ed. 2010;49:1194–1196. doi: 10.1002/anie.200906521. [DOI] [PubMed] [Google Scholar]; (b) Neeve E. C., Geier S. J., Mkhalid I. A., Westcott S. A., Marder T. B. Chem. Rev. 2016;116:9091–9161. doi: 10.1021/acs.chemrev.6b00193. [DOI] [PubMed] [Google Scholar]; (c) Liu Q., Tian B., Tian P., Tong X., Lin G.-Q. Chin. J. Org. Chem. 2015;35:1–14. [Google Scholar]; (d) Zheng K., Liu X., Feng X. Chem. Rev. 2018;118:7586–7656. doi: 10.1021/acs.chemrev.7b00692. [DOI] [PubMed] [Google Scholar]
- (a) Lee J. E., Yun J. Angew. Chem., Int. Ed. 2008;47:145–147. doi: 10.1002/anie.200703699. [DOI] [PubMed] [Google Scholar]; (b) Chea H., Sim H.-S., Yun J. Adv. Synth. Catal. 2009;351:855–858. [Google Scholar]; (c) Sim H. S., Feng X., Yun J. Chem.–Eur. J. 2009;15:1939–1943. doi: 10.1002/chem.200802150. [DOI] [PubMed] [Google Scholar]; (d) Feng X., Yun J. Chem. Commun. 2009:6577–6579. doi: 10.1039/b914207j. [DOI] [PubMed] [Google Scholar]
- Lillo V., Prieto A., Bonet A., Díaz-Requejo M. M., Ramírez J., Peŕez P. J., Fernańdez E. Organometallics. 2009;28:659–662. [Google Scholar]
- O'Brien J. M., Lee K. S., Hoveyda A. H. J. Am. Chem. Soc. 2010;132:10630–10633. doi: 10.1021/ja104777u. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (a) Jahier-Diallo C., Morin M. S. T., Queval P., Rouen M., Artur I., Querard P., Toupet L., Crévisy C., Baslé O., Mauduit M., Chem.–Eur. J., 2015, 21 , 993 –997 , . For a higher ee, see: . [DOI] [PubMed] [Google Scholar]; (b) Park J. K., Lackey H. H., Rexford M. D., Kovnir K., Shatruk M., McQuade D. T. Org. Lett. 2010;12:5008–5011. doi: 10.1021/ol1021756. [DOI] [PubMed] [Google Scholar]
- Tolentino D., Neale S., Isaac C., Macgregor S., Whittlesey M., Jazzar R., Bertrand G. J. Am. Chem. Soc. 2019;141:9823–9826. doi: 10.1021/jacs.9b04957. [DOI] [PubMed] [Google Scholar]
- Cholesterol derived NHCs have been reported: ; (a) Rakers L., Martínez-Prieto L. M., López-Vinasco A. M., Philippot K., van Leeuwen P. W. N. M., Chaudret B., Glorius F. Chem. Commun. 2018;54:7070–7073. doi: 10.1039/c8cc02833h. [DOI] [PubMed] [Google Scholar]; (b) Rakers L., Grill D., Matos A. L. L., Wulff S., Wang D., Börgel J., Körsgen M., Arlinghaus H. F., Galla H.-J., Gerke V., Glorius F. Cell Chem. Biol. 2018;25:952–961. doi: 10.1016/j.chembiol.2018.04.015. [DOI] [PubMed] [Google Scholar]
- Clavier H., Nolan S. P. Chem. Commun. 2010;46:841–861. doi: 10.1039/b922984a. [DOI] [PubMed] [Google Scholar]
- Steric maps were extrapolated using the SambVca 2 web-based program developed by Cavallo and co-workers. Falivene F., Credendino R., Poater A., Petta A., Serra L., Oliva R., Scarano V., Cavallo L., Organometallics, 2016, 35 , 2286 –2293 . [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.