Abstract
Ultrasound (US) has emerged as a promising noninvasive modality for neuromodulation. Despite previous evidence that US may mediate cellular response by activating mechanosensitive ion channels embedded in the cell membrane, the underlying mechanism is not well understood. In this work, we developed a vertically deployed surface acoustic wave (VD-SAW) platform that generates 30 MHz focused ultrasound wave for mechanical stimulation of single cells. We investigated the role of Piezol in mediating the intracellular calcium response ([Ca2+]i) of HEK293T cells in response to pulsed US operated at a peak pressure of 1.6 MPa with 20% duty cycle, and a total treatment time of 60 seconds. We observed that the elicited calcium response depends critically on the pulse repetition frequency (PRF) or burst duration of the US, as well as the presence of the Piezol. Significantly higher [Ca2+]i increase was produced in the Piezol-transfected (P1TF) than in the Piezol-knockout (P1KO) HEK293T cells. Furthermore, higher calcium response probability, stronger and faster [Ca2+]i increase, and greater cell displacement were produced at 2 Hz PRF with 100 ms burst duration than 200 Hz PRF with 1 ms burst duration. Altogether, we have demonstrated that the VD-SAW platform provides a unique and versatile tool for investigating US-induced mechanotransduction at the single cell level.
Keywords: Ultrasound, Surface Acoustic Wave, Calcium Signaling, Mechanosensitive Ion Channel, Piezo1
Introduction
Ultrasound (US) can be delivered and focused non-invasively within a small region inside the body, producing thermal or mechanical bioeffects depending on the specific pulsing regimen [1]. US neuromodulation has been demonstrated from model organisms to humans since the 1950s [2]. Moreover, recent studies have shown that regulation of neuronal activity by US stimulation may result from the activation of different mechanosensitive (MS) ion channels [3]. Ibsen et al. [4] reported US mediated by microbubbles could evoke behavioral responses in C. elegans transfected with mechanosensitive TRP-4 channel. Kubanek et al. [5] showed that US stimulation could directly modulate the trans-membrane currents through MS sodium and potassium channels expressed in Xenopus oocytes. Pan et al. [6] investigated US-induced transcriptional activities in mammalian cells genetically modified to express MS Piezo1 channels. The capability to elicit cellular response through activation of MS ion channels by US offers a new and potentially powerful tool for targeted neuromodulation, known as “sonogenetics” [4,5].
Despite this, several drawbacks exist in previous efforts to investigate the mechanism of US neuromodulation. When conventional US transducers are used, the setup is often bulky and significantly obstructs the optical path, which prevents concomitant monitoring of cell response by optical microscopy during sonication [6,7]. When ultrasound contrast agents (i.e., microbubbles) are used, the risk of cell injury increases, and the bioeffects produced may vary, depending on the number of bubbles attached or near the cell surface and their mutual interactions during sonication [4,6]. In addition, laser-generated bubbles provide high precision in controlling bubble(s)-cell interaction, which has been used to probe the mechanism of intracellular calcium response at the single cell level [8]. However, laser-generated bubbles are not flexible in delivering repeated simulations and treatment of multiple cells is time consuming.
In this work, we developed a vertically deployed surface acoustic wave (VD-SAW) platform to investigate ultrasound elicited intracellular calcium response in HEK293T cells with Piezo1 either genetically knocked out or re-transfected back to the cell. Although widely used in microfluidics for cell and particle manipulations [9], SAW-based ultrasonic chips have only recently developed to stimulate neurons [10,11]. However, in previous approaches, the SAW has to propagate through PDMS (Polydimethylsiloxane) wall, leading to significant attenuation. Here, we adapt a VD-SAW approach [12] to create an open optical path configuration that allows for highly-localized delivery of 30 MHz acoustic waves to the target cells grown in a glass bottom petri dish with simultaneous real-time fluorescence imaging. We have employed this unique platform to activate Piezo1 ion channels in HEK293T cells, and investigated the effect of burst duration on US-elicited intracellular calcium response.
Materials and Methods
1. Cell culture and transfection
The HEK-P1KO cell line (Piezol knockout human embryonic kidney cells) and plasmid Mouse Piezol-pIRES-EGFP in pcDNA3.1 were gifts from Dr. Jorg Grandl of Duke Neurobiology. HEK-P1KO cells were maintained in DMEM (high glucose) with 10% heat-inactivated fetal bovine serum (FBS) and penicillin / streptomycin antibiotics (DMEM complete medium) in a cell culture incubator at 37 °C with 5% CO2 as previously described [13]. P1KO cells were seeded in a 6-well plate and transiently transfected in the presence of 10 μM ruthenium red (RR) with Mouse Piezo1 (3 μg) using Fugene6 (Promega, Madison, WI) following the manufacturer’s protocol. About 20–30% of cells showed positive GFP expression indicating successful transfection of Piezo1. After 48 hours, cells were reseeded in 35 mm glass-bottomed petri dishes (81158, ibidi), which were pre-wetted by 1× PBS and coated with 50 μg/mL Fibronectin (33010018; ThermoFisher Scientific). Cells were then incubated in DMEM complete medium at 37 °C for 3 hours before US treatment.
2. Measurement of intracellular calcium response and membrane poration
Fluorescence imaging of [Ca2+]i was performed using the indicator dye fura-2 AM (F1221; ThermoFisher Scientific) [14]. After the cells were fully adhered on the fibronectin-coated glass surface, the culture medium in the petri-dish was replaced with fura-2 AM working solution: 6 μM in OptiMEM (11058–021; ThermoFisher Scientific), and incubated at 37 °C in the dark for 30 min to load fura-2 into the cells. Subsequently, the petri-dish was washed 3–5 times with 1× PBS to remove RR and extra fura-2 AM. OptiMEM was used as the final medium during US treatment and recording. Propidium Iodide (PI) was added into the final medium at a concentration of 100 μg/mL to monitor membrane permeability change and cell necrosis after sonication and a monochromator (DELTARAM X; PTI) was used for calcium and PI imaging as described in our previous study [8]. Intracellular calcium response was measured by ratiometric imaging with fura-2 at 340 and 380 nm excitation, and the fluorescent emission signal was recorded at 510 nm, using a sCMOS camera (EDGE 5.5 CL; PCO) at a frame rate of 10 Hz for a total recording time up to 300 s. Thereafter, the ratio (F) between the fluorescence intensity from 340 and 380 nm excitation over time from each cell of interest was calculated by the EasyRatio imaging processing software (HORIBA Scientific). The normalized ratio change over time [(F – F0)/F0] where F0 is the baseline value of the cell before sonication was further analyzed using MATLAB (MathWorks) to calculate the peak amplitude and rise time of the calcium response. During sonication, the cells might move along the acoustic streaming direction, leading to some fluctuations in the fluorescence intensity, up to 10% of the (F – F0)/F0 value. Therefore, in our data analysis, only the cells with the normalized ratio change over time greater than 10% were considered to be “responsive” above the background noise level.
3. Ultrasound transducer fabrication and operation
Surface acoustic wave (SAW)-based chip design [9,12] with interdigital transducers (IDTs) were used to generate 30 MHz ultrasound. The fabrication of IDTs was carried out using conventional photolithography and lift-off processes [9]. The double-layer metal electrodes (90 nm Au and 10 nm Cr) were deposited on a 0.5 mm thick 128° Y-cut lithium niobate substrate. Each IDT (L × W = 12 × 8 mm, see Fig. 1a) was shaped as a series of concentric circular arcs having a 40° opening angle and 20 fingers with 32.5 μm in width and finger spacing to match with the quarter wavelength of the SAW. In comparison, the corresponding wavelength of the VD-SAW generated leaky pressure wave propagating in the fluid medium is 50 μm, which is comparable to the size of single cells. The IDT was driven by using a function generator (Model 4065, B&K Precision) connected to a 55 dB power amplifier (Model A150, ENI). The temperature elevation during sonication was monitored using a thermocouple and found to be less than 1° C.
Figure 1: VD-SAW experimental system used for cell study.
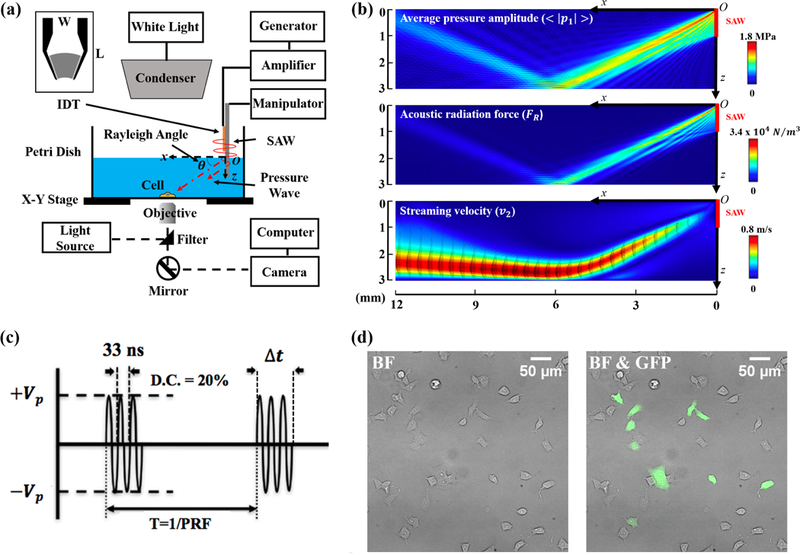
(a) Schematic illustration of the VD-SAW experimental platform. A modulated sinusoidal signal is generated by a function generator, amplified by a 55-dB power amplifier to drive the IDT (L × W = 12 × 8 mm), mounted on a holder. The position of the IDT is manipulated by a three-axis micromanipulator. (b) Calculation results of the average pressure amplitude , acoustic radiation force density (FR) and streaming velocity (v2) with black arrow representing velocity vector obtained by numerical simulation in COMSOL. (c) Modulated input signals at 30 MHz center frequency (33 ns period) with different parameters used in the experiments (Vp: peak voltage, T: pulse repetition period, PRF: pulse repetition frequency, D.C.: duty cycle, At: burst duration). (d) Bright field (BF) image of a typical group of HEK-P1KO cells (left) with overlaid fluorescent image of HEK-P1TF cells expressing Piezol-IRES-GFP in green (right).
4. Cell displacement in response to US
To quantify the cell movement during sonication, TrackMate [15], an open-source Fiji plugin in ImageJ, was used to track the displacement of the maximum fluorescent intensity (380 nm) point over the entire cytosol. In our analysis, Laplacian of Gaussian (LoG) segmentation method was used to detect the local maxima of an image convoluted by a Gaussian kernel, corresponding to notably bright spots based on intensity contrast. After applying the LoG detector on each frame of the TIFF file, each spot between frames was linked together using a simplified Linear Assignment Problem (LAP) tracking method. The simplified LAP tracking method disregards any occurrences of splitting or merging, while linking together spots from different frames based on both minimal square distance and similar intensities [15]. The central locations of these bright spots were then downloaded into an Excel file and analyzed using MATLAB.
Results
1. VD-SAW experimental system and numerical simulation of the acoustic and fluid fields
We have integrated the VD-SAW transducer seamlessly with an inverted microscope (Axio Observer Z1; Zeiss) to deliver pulsed ultrasound to target cells grown on the 35-mm glass-bottom petri dish (Fig. 1a). The 30 MHz focused IDT was deployed vertically using a 3D translational stage to immerse the leading edge of the IDT substrate 1 mm into the culture medium and positioned 2 mm above the glass surface. The SAW generated by the IDT, propagating originally along the substrate surface in air, would convert part of its energy into a leaky pressure wave in the culture medium irradiated at the Rayleigh angle (23°) toward the target cells. Fig. 1(b) shows the numerical simulation results performed in COMSOL Multiphysics 5.3 (Burlington, MA). The average pressure amplitude, acoustic radiation force, and streaming velocity were calculated under the continuous wave condition of the IDT. For the target cells (located within an area with a radius about 300 mm centered around x = 6 mm and z = 3 mm), the model estimated a peak pressure of 1.6 MPa, an acoustic radiation force (per unit volume) of 2.6×104 N/m3, and a streaming velocity of 0.8 m/s, corresponding to a shear stress of 50 dyne/cm2. During the experiments, the IDT was operated in pulsed mode with a peak-peak input voltage of about 40 V, a duty cycle (DC) of 20%, a pulse repetition frequency (PRF) of either 2 or 200 Hz, and a total treatment time of 60 seconds (Fig. 1c and Table 1). By varying the PRF, we could adjust the ultrasound burst duration (Δt) from 1 to 100 ms while maintaining the same total acoustic energy delivered to the cells. Fig. 2(d) shows examples of the bright filed images of the HEK-P1KO cells seeded on fibronectin-coated glass-bottom petri dish and overlaid with fluorescent images of Piezo1-transfected cells expressing Piezo1-IRES-GFP (green).
Table 1: Summary of the ultrasound parameters used in the study.
(PRF: pulse repetition frequency, Vp: peak voltage, Ttotal: total US treatment time, D.C.: duty cycle, Teff. effective US exposure time, Δt: burst duration, T: pulse repetition period).
| PRF (Hz) | Vp (V) | Ttotal (s) | D.C. (%) | Teff (s) | Δt (ms) | T (ms) |
|---|---|---|---|---|---|---|
| 2 | 20 | 60 | 20 | 12 | 100 | 500 |
| 200 | 20 | 60 | 20 | 12 | 1 | 5 |
Figure 2: Characterization of intracellular calcium response induced by the VD-SAW platform.
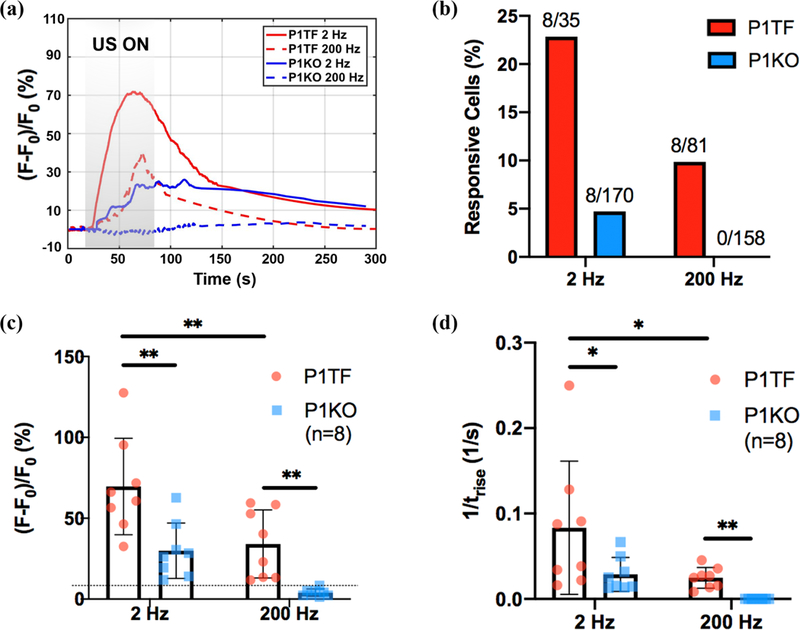
(a) Representative normalized 340 nm/380 nm ratio change [(F–F0)/F0] of P1TF and P1KO cells in response to 30 MHz pulsed US at different PRF (2 Hz and 200 Hz). (b) Responsive percentage of P1TF and P1KO cell populations (defined by normalized ratio change exceeding 10% of the baseline value). (c) Average peak (F – F0)/F0 of P1TF and P1KO cell populations. Dashed line indicates the noise level (i.e., 10% ratio change above the baseline level). (d) Average rise rate (1/trise, where trise is the time that ΔF/F0 increases from 10% to 90% of the peak value) of P1TF and P1KO cell populations. Two-tailed student’s t test, *, p<0.05: **, p<0.01.
2. Activation of Piezo1 ion channels and the effect of PRF (or burst duration) on intracellular calcium response induced by US
Pulsed ultrasound generated by the VD-SAW platform can elicit calcium response in the HEK 293T cells in a PRF and Piezo1 dependent manner. No membrane poration (assessed by PI uptake) or cell detachment or injury were observed immediately following the experiment. Figure 2 shows representative calcium response over time curves in different cell groups and treatment conditions. Overall, the calcium response was initiated almost immediately from the beginning of sonication (20 s), increased at various rates to reach the maximum either before or after the cessation of the sonication (80 s), except in P1KO cells treated at 200 Hz PRF, before gradually returning to the baseline level (Fig. 2a). At 2 Hz PRF (i.e., Δt = 100 ms), calcium response was observed in 22% (8 out of 35) of the P1TF cells compared to 5% (8 out of 170) of the P1KO cells (Fig. 2b). In contrast, at 200 Hz PRF (i.e., Δt = 1 ms), calcium response was only observed in 9% (8 out of 81) of the P1TF cells and none of the P1KO cell (0 out of 158 cells). It should be noted that the calcium response was measured by the global spreading of the calcium signaling, with the normalized ratio change (F – F0)/F0 exceeding 10% of the baseline level. Quantitatively, the contribution of Piezo1 to the global calcium response elicited by the pulsed ultrasound is also discernible. At 2 Hz PRF, the average amplitude of the normalized ratio change in the responsive P1TF cells is 69.6(±26.3)% compared to 30.0(±15.1)% in the responsive P1KO cells, and the difference is statistically significant (Fig. 2c). In addition, the rise time of the responsive P1TF cells is significantly shorter than the corresponding value in their counterpart P1KO cells (Fig 2d). Similar differences were further observed at 200 Hz PRF between the two cell groups where the normalized ratio change in the P1KO cells fell below 10% of the baseline value, and thus deemed non-responsive. Overall, HEK 293T cells with Piezo1 showed higher percentage in calcium response, normalized ratio change and faster rise time than its knockout counterpart. Moreover, stronger calcium response was observed at 2 Hz than 200 Hz PRF, indicating a vital role of the US burst duration in eliciting the calcium signaling.
3. Different patterns of the US-elicited intracellular calcium response
Figure 3 illustrates several examples of the distinctly different patterns in the calcium response elicited in different cell groups and under various treatment conditions. At 2 Hz PRF, the responsive P1TF cell often showed a strong calcium signaling that was initiated locally on the boundary and then spread out rapidly and converged toward the central region of the cell to produce a global response (Fig. 3a). In contrast, the non-responsive P1TF (NR-P1TF) cell mainly showed transient and discrete spots of calcium response confined to the cell boundary, but didn’t spread throughout the cytosol (Fig. 3b). In comparison, the responsive P1KO cell frequently revealed a diffused pattern of calcium response that was originated from multiple spots randomly distributed across the cell surface without a clear converging pattern or propagation direction (Fig. 3c). Moreover, the non-responsive P1KO (NR-P1KO) cell occasionally showed narrow bands of calcium response confined to the cell boundary along the direction of US-induced streaming flow (Fig. 3d). The calcium signal was transient and disappeared quickly without propagating to other regions of the cell.
Figure 3: Representative time-elapsed images of intracellular calcium response elicited in different cell types and under various treatment conditions.
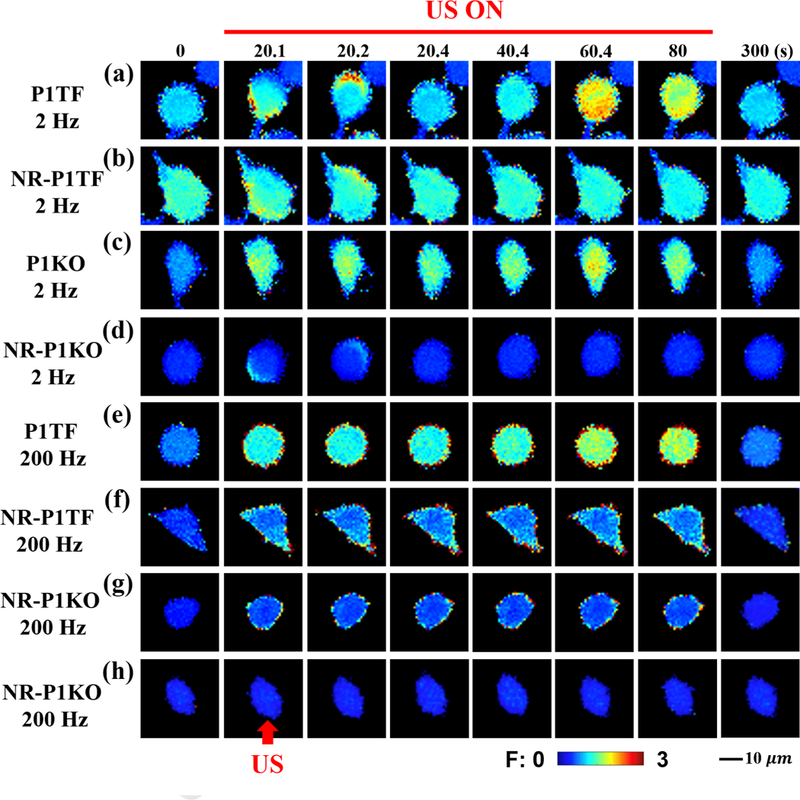
(a) responsive P1TF cell treated at 2 Hz PRF, (b) non-responsive P1TF (NR-P1TF) cell treated at 2 Hz PRF, (c) responsive P1KO cell treated at 2 Hz PRF, (d) non-responsive P1KO (NR-P1KO) cell treated at 2 Hz PRF, (e) responsive P1TF cell treated at 200 Hz PRF, (f) non-responsive P1TF (NR-P1TF) cell treated at 200 Hz PRF, (g) and (h) non-responsive P1KO (NR-P1KO) cell treated at 200 Hz PRF. The red arrow indicates the direction of the incident pulsed US. All images share the same length and ratio change scale bars.
At 200 Hz PRF, overall calcium response was weaker than its counterpart at 2 Hz PRF. In comparison with other cohorts at 200 Hz PRF, the responsive P1TF cell showed a strong calcium response around the boundary, superimposed with a diffused pattern of calcium signaling across the cell surface (Fig. 3e). In contrast, the NR-P1TF cell showed a weaker and diffused calcium response across the cell surface, with slightly stronger signals near the boundary (Fig. 3f). In comparison, the NR-P1KO cell occasionally showed a weak and discrete calcium response ring confined to the cell boundary. In most cases, however, the NR-P1KO showed no detectable change in the calcium response during the sonication period (Fig. 3h).
4. Different cell displacement patterns produced by pulsed US of different PRF
To understand the distinct calcium response patterns elicited at various PRFs in different cell groups, we further analyzed the global cell displacement during sonication (Fig. 4a). The results summarized in Fig. 4(b) demonstrate that cell displacement is primarily determined by the PRF (or Δt), but independent of the presence of Piezol or whether a calcium response was elicited. Furthermore, greater cell displacement (and presumably larger membrane deformation) was produced at 2 Hz than 200 Hz PRF, which is consistent with the stronger calcium response elicited at 2 Hz PRF (see Fig. 2 and Fig. 3). Importantly, at 2 Hz PRF it can be noted that the pitch in cell displacement was in synchrony with the repetition frequency of the pulsed US (Fig. 4(c) inset, 10 cycles within 5s). Under this driving condition, the cell was displaced significantly and rapidly to reach the maximum deformation (solid line) before the cessation of the sonication, leading to a stronger and faster calcium response (shown in the normalized ratio change in the dashed line) in P1TF cell (red) than in the P1KO (blue) cell, which showed a weaker and slower response. In comparison, at 200 Hz PRF the cell displacement could not be fully resolved due to the 10 Hz frame rate used in calcium imaging. However, the cell displacement was found to be gradually increased to a much lower peak value during the sonication and the resultant calcium response was much weaker in the P1TF cell with non-detectable response observed in the P1KO cell (Fig. 4d). It is also worth noting that after sonication most of the cells didn’t recover fully back to its original position and shape within the 300-s observation window, suggesting that significant re-arrangement of the cell cytoskeleton might have occurred.
Figure 4: Cell displacement produced by pulsed US of different PRF.
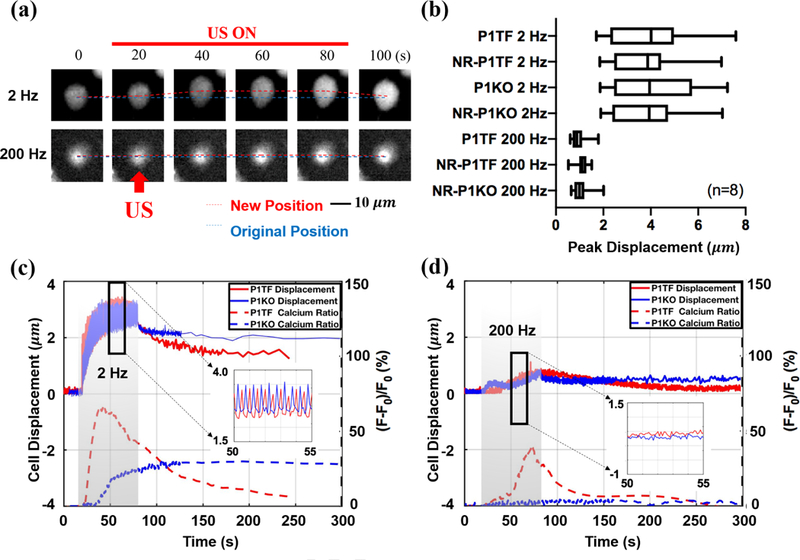
(a) Illustration of the cell displacement in response to pulsed US of 2 Hz and 200 Hz PRF. The red dashed line indicates the time varying positions of the brightest spots (380 nm intensity) on the cell surface tracked by TrackMate, and the blue dashed line indicates its original position. The red arrow indicates the direction of the incident pulsed US. (b) Peak cell displacement in different cell groups treated by the pulsed US at 2 Hz and 200 Hz PRF. (c) and (d) Typical curves of cell displacement over time and the associated normalized 340 nm/380 nm ratio change [(F–F0)/F0] induced by pulsed US at 2 Hz and 200 Hz PRF in different cell groups.
Discussion
We have developed a new VD-SAW platform to deliver 30 MHz focused US to individual cells cultured within a small region (300 μm radius) inside a petri dish while allowing for simultaneous fluorescent calcium imaging and bioeffects assessment. Using this unique experimental system, we have demonstrated the pivotal role that Piezo1 (or other MS ion channels) may play in mediating US-elicited calcium signaling in a PRF-dependent manner. Under the experimental conditions (30 MHz, 20% DC), the contribution of either cavitation or heating is negligible. Moreover, the contribution of acoustic radiation force may also be insignificant due to the long distance between the target area and the VD-SAW transducer (Fig. 1a). In contrast, cells in the target area were exposed to the maximum acoustic streaming produced by the VD-SAW transducer (Fig. 1b), confirmed by their significant displacement during sonication (see Fig. 4). It should be noted that no calcium response was detected outside the target region. Altogether, the elicited calcium response was most likely mediated by the flow shear stress associated with the acoustic streaming. The shear stress (50 dyne/cm2) generated by our VD-SAW device is within the range reported to activate Piezo1 in HEK293T cells [16]. Future work to characterize the flow field produced by the VD-SAW platform will help to clarify the underlying mechanism.
The burst duration of the pulsed US was found to be critical in determining the cell displacement (Fig. 4) and associated calcium response (Fig. 2). While it is well known that the activation of Piezo1 ion channel depends on membrane tension under quasi-static loading conditions [17,18], the characteristic of Piezo 1 activation under cyclic dynamic loading has not been thoroughly investigated. The observation that the P1TF cells showed a much stronger calcium response at PRF of 2 Hz (Δt = 100 ms) than 200 Hz (Δt = 1 ms) suggests that not only the amplitude but also the duration of the membrane deformation may play a critical role in US-elicited calcium response. Such a stretch duration dependency has been observed in cell membrane deformation and poration produced by impulsive shear-induced stretch of individual cells in cavitation fields [8,19,20]. In addition, significantly higher calcium response elicited in the P1TF cells compared to the P1KO cells also indicates that Piezo1 might be more sensitive to US stimulation than other endogenous MS ion channels in the HEK293T cells in our experiments. Fine-tuning of the US exposure parameters may lead to the optimal condition for exclusive activation of the Piezo1, which will be valuable for sonogenetics applications [4,5].
The low percentage in the elicited calcium response (Fig. 2b) may be attributed to multiple factors, including heterogeneity of the Piezo1 (or other MS ion channel) distribution on the cell membrane [18,21] and varying cell size, shape and orientation to the streaming field. Future work is warrantied to determine the optimal sonication strategy to elicit a robust calcium response in neurons without cellular injuries.
Supplementary Material
Highlights.
A vertically deployed surface acoustic wave (VD-SAW) platform is developed to deliver focused ultrasound to single cells cultured in a petri dish under a microscope.
30 MHz pulsed ultrasound generated by the VD-SAW platform produces sufficient acoustic streaming/shear stress to elicit calcium signaling in adherent HEK293T cells.
Stronger calcium response is elicited at pulse repetition frequency (PRF) of 2 Hz than 200 Hz, and in HEK293T cells with Piezo1 than without Piezo1.
Higher cell displacement (and presumably larger cell membrane deformation) is produced during sonication at PRF of 2 Hz than 200 Hz.
Acknowledgement
The authors would like to express their gratitude to Dr. Jorg Grandl of Duke Neurobiology for providing the P1KO cell line and Piezol plasmid and to Eric Stach for technical support. This work was supported by National Institutes of Health through Grant 5R37-DK052985–22.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- [1].ter Haar G, Ultrasound bio-effects and safety considerations, Front Neurol Neurosci 36 (2015) 23–30. 10.1159/000366233. [DOI] [PubMed] [Google Scholar]
- [2].Fry FJ, Ades HW, Fry WJ, Production of reversible changes in the central nervous system by ultrasound, Science 127 (1958) 83–84. 10.1126/science.127.3289.83. [DOI] [PubMed] [Google Scholar]
- [3].Bystritsky A, Korb AS, Douglas PK, Cohen MS, Melega WP, Mulgaonkar AP, DeSalles A, Min BK, Yoo SS, A review of low-intensity focused ultrasound pulsation, Brain Stimul 4 (2011) 125–136. 10.1016/j.brs.2011.03.007. [DOI] [PubMed] [Google Scholar]
- [4].Ibsen S, Tong A, Schutt C, Esener S, Chalasani SH, Sonogenetics is a non-invasive approach to activating neurons in Caenorhabditis elegans, Nat Commun 6 (2015) 8264 10.1038/ncomms9264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Kubanek J, Shi J, Marsh J, Chen D, Deng C, Cui J, Ultrasound modulates ion channel currents, Sci Rep 6 (2016) 24170 10.1038/srep24170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Pan Y, Yoon S, Sun J, Huang Z, Lee C, Allen M, Wu Y, Chang YJ, Sadelain M, Shung KK, Chien S, Wang Y, Mechanogenetics for the remote and noninvasive control of cancer immunotherapy, Proc Natl Acad Sci U S A 115 (2018) 992–997. 10.1073/pnas.1714900115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Prieto ML, Firouzi K, Khuri-Yakub BT, Maduke M, Activation of Piezo1 but Not NaV1.2 Channels by Ultrasound at 43 MHz, Ultrasound Med Biol 44 (2018) 1217–1232. 10.1016/j.ultrasmedbio.2017.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Li F, Yang C, Yuan F, Liao D, Li T, Guilak F, Zhong P, Dynamics and mechanisms of intracellular calcium waves elicited by tandem bubble-induced jetting flow, Proc Natl Acad Sci U S A 115 (2018) E353–E362. 10.1073/pnas.1713905115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ding X, Li P, Lin SC, Stratton ZS, Nama N, Guo F, Slotcavage D, Mao X, Shi J, Costanzo F, Huang TJ, Surface acoustic wave microfluidics, Lab Chip 13 (2013) 3626–3649. 10.1039/c31c50361e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Lin ZR, Zhou W, Huang XW, Wang KY, Tang J, Niu LL, Meng L, Zheng HR, On-Chip Ultrasound Modulation of Pyramidal Neuronal Activity in Hippocampal Slices, Advanced Biosystems 2 (2018). ARTN 1800041 10.1002/adbi.201800041. [DOI] [Google Scholar]
- [11].Ye J, Tang S, Meng L, Li X, Wen X, Chen S, Niu L, Li X, Qiu W, Hu H, Jiang M, Shang S, Shu Q, Zheng H, Duan S, Li Y, Ultrasonic Control of Neural Activity through Activation of the Mechanosensitive Channel MscL, Nano Lett 18 (2018) 4148–4155. 10.1021/acs.nanolett.8b00935. [DOI] [PubMed] [Google Scholar]
- [12].Dentry MB, Yeo LY, Friend JR, Frequency effects on the scale and behavior of acoustic streaming, Phys Rev E Stat Nonlin Soft Matter Phys 89 (2014) 013203. 10.1103/PhysRevE.89.013203. [DOI] [PubMed] [Google Scholar]
- [13].Dubin AE, Murthy S, Lewis AH, Brosse L, Cahalan SM, Grandl J, Coste B, Patapoutian A, Endogenous Piezol Can Confound Mechanically Activated Channel Identification and Characterization, Neuron 94 (2017) 266–270 e263 10.1016/j.neuron.2017.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Grynkiewicz G, Poenie M, Tsien RY, A new generation of Ca2+ indicators with greatly improved fluorescence properties, J Biol Chem 260 (1985) 3440–3450. [PubMed] [Google Scholar]
- [15].Tinevez JY, Perry N, Schindelin J, Hoopes GM, Reynolds GD, Laplantine E, Bednarek SY, Shorte SL, Eliceiri KW, TrackMate: An open and extensible platform for single-particle tracking, Methods 115 (2017) 80–90. 10.1016/j.ymeth.2016.09.016. [DOI] [PubMed] [Google Scholar]
- [16].Ranade SS, Qiu Z, Woo SH, Hur SS, Murthy SE, Cahalan SM, Xu J, Mathur J, Bandell M, Coste B, Li YS, Chien S, Patapoutian A, Piezo1, a mechanically activated ion channel, is required for vascular development in mice, Proc Natl Acad Sci U S A 111 (2014) 10347–10352. 10.1073/pnas.1409233111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Lewis AH, Grandl J, Mechanical sensitivity of Piezo1 ion channels can be tuned by cellular membrane tension, Elife 4 (2015). 10.7554/eLife.12088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Wu J, Lewis AH, Grandl J, Touch, Tension, and Transduction-The Function and Regulation of Piezo Ion Channels, Trends Biochem Sci 42 (2017) 57–71. 10.1016/j.tibs.2016.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Li F, Chan CU, Ohl CD, Yield strength of human erythrocyte membranes to impulsive stretching, Biophys J 105 (2013) 872–879. 10.1016/j.bpj.2013.06.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan F, Yang C, Zhong P, Cell membrane deformation and bioeffects produced by tandem bubble-induced jetting flow, Proc Natl Acad Sci U S A 112 (2015) E7039–7047. 10.1073/pnas.1518679112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Amarouch MY, Syam N, Abriel H, Biochemical, single-channel, whole-cell patch clamp, and pharmacological analyses of endogenous TRPM4 channels in HEK293 cells, Neurosci Lett 541 (2013) 105–110. 10.1016/j.neulet.2013.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


