Abstract
Purpose:
To assess molecular targeted therapy (MTT) ‘s ability to affect tumor volume doubling time (TVDT) and disease specific survival (DSS) in patients presenting with lung metastasis from radioactive iodine refractory progressive thyroid cancer.
Methods:
In this retrospective study, we examined the clinical characteristics, average tumor volume doubling times of lung metastasis and disease specific survival of patients with lung metastasis from differentiated thyroid cancer who were treated with MTT.
Results:
The 5-year DSS from the distant metastasis (DM) diagnosis was 72% with median survival of 8 years (95% CI: 6.6–9.5). The median survival was 2.9 years after MTT start (95% CI: 2.1–3.6). On MTT, lung average tumor volume doubling time (midDT) was prolonged to midDT ≥ 3 years in 75% of patients with baseline midDT < 1 year and 100% of patients with midDT 1–3 years. In patients with rapidly progressive thyroid cancer (midDT < 1 year at baseline), the median survival was 4.5 years in those with MTT- achieved midDT ≥ 3 years (95% CI: 2.9–6.2), as opposed to 2.3 years (95% CI: 0.3–4.3) and 0.7 years (95% CI: 0.2–1.3) in those with MTT-achieved midDT of 1–3 years and MTT-achieved midDT < 1year respectively (Log Rank p < 0.001).
Conclusion:
Lung midDT is a useful and important clinical marker of disease specific survival for patients with progressive radioactive iodine refractory (RAIR) metastatic thyroid cancer. In patients with rapidly progressive metastatic RAIR thyroid cancer, molecular targeted therapy prolongs lung tumor volume doubling time and is associated with improved disease specific survival.
Introduction
Molecular targeted therapy (MTT) is often recommended for patients with structurally progressive or symptomatic radioiodine refractory differentiated thyroid cancer (DTC) that is not amenable to localized therapy (1,2). Randomized clinical trials (RCT) demonstrate a significant improvement in progression free survival (PFS) in patients treated with either Sorafenib or Lenvatinib when compared to placebo (3,4). In addition, progression events were significantly decreased in the Lenvatinib-treated patients as opposed to placebo (4). However, documentation of clinically meaningful improvement in overall survival or disease specific survival in response to MTT has not been conclusively demonstrated. Since MTT therapy can be associated with a wide range of side effects, significant financial burdens, more frequent testing and follow-up, and alterations in quality of life (5– 6) , it is important to define the impact of these systemic therapies on the disease specific survival (DSS) to better evaluate the risk-benefit ratio of these treatments.
Response to targeted therapy is traditionally measured using RECIST criteria (3, 4). This allows for determination on treatment response or failure at any given time of the treatment course. However, it does not measure the effect of targeted therapy on the rate of disease progression or the natural history of the disease. Additionally, RECIST criteria have not been correlated to disease specific survival in thyroid cancer. We have previously demonstrated that average tumor volume doubling time (midDT) of pulmonary metastases is an easily measured, reliable prognostic indicator of overall survival in patients with progressive radioiodine refractory (RAIR) differentiated thyroid cancer (7). We defined midDT as the average tumor volume doubling time (TVDT) calculated from the tumor dimensions of two index metastatic pulmonary lesions measured in at least 4 consecutive CT scans. Patients with rapid progression (midDT ≤1 year) demonstrated a 5- year overall survival of only 20% from the time the index pulmonary metastasis was 1 cm in maximal dimension. Five-year overall survival was significantly better in patients with longer doubling times (50% for midDT 1–3 years, 80 % for midDT ≥ 3 years). Interestingly, MTT appeared to prolong the tumor volume doubling time in 3 patients with pretherapy midDT ≤ 1 year and appeared to correlate with response to systemic therapy.
The objective of this study is to more fully evaluate the impact of MTT on the average tumor volume doubling time (midDT) as measured in the pulmonary metastases of a larger cohort of patients RAIR differentiated thyroid cancer. We hypothesized that MTT-induced prolongation of midDT would be associated with an improvement in disease specific survival in patients with rapidly progressive disease (midDT ≤1 year prior to therapy).
Materials and Methods
Patients:
Institutional review board (IRB) approval was obtained prior to study start. A dataset was created including 492 patients with lung metastasis from differentiated thyroid cancer who were referred to medical oncology at Memorial Sloan Kettering between January 2006 and March 1, 2017 for MTT consideration. Of these patients, 204 patients (42%) were treated with one or more molecular targeted therapy (ies) during their disease follow-up. We then excluded patients with 1) inadequate follow up, 2) concomitant second active primary cancer, 3) chronic TSH elevation 5) anaplastic or medullary thyroid cancer. Thirteen patients were thus excluded. An additional 34 patients received targeted therapy part of RAI reuptake study with or without additional molecular targeted therapy (ies). These 34 patients were excluded from the study. We excluded patients with non-measurable disease (nodules less than 5 mm, nodules that are not clearly demarcated at diagnosis or with follow up, nodules obscured by effusions), those with miliary distribution of lung nodules that made it difficult to tract a specific nodule over time, and those with insufficient imaging (n = 35) (Figure1).
Figure 1:
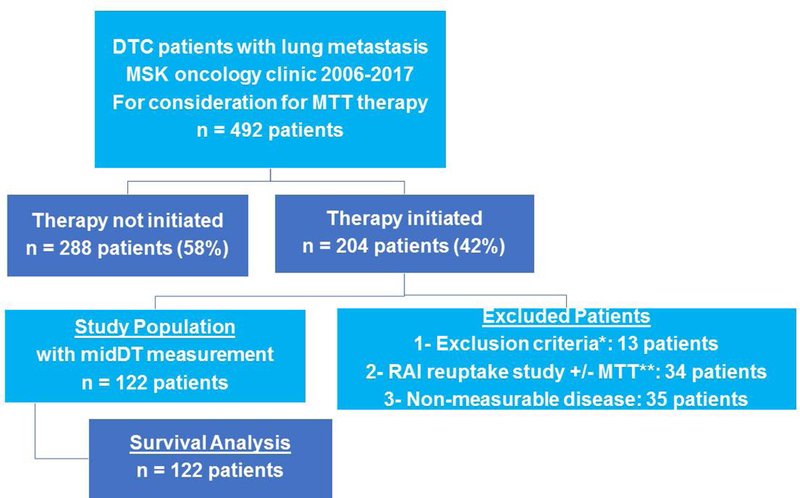
Schematic of study patients’ selection
In total, 122 patients were eligible for the study (Figure 1). As previously described, patients were categorized based on average tumor volume doubling times (midDT) as ≤ 1 year, 1–3 years, or ≥ 3 years (5). In 62 patients, cross sectional imaging (at least 4 chest CT scans prior to initiation of MTT and at least 4 more CT scans on MTT) allowed for calculation of a precise calculation doubling time value before and after initiation of therapy. Precise midDT calculations were not done in patients with interruptions of MTT > 3 months. The doubling time category in the remaining 60 patients was done based on clinical criteria for structural disease progression that corresponded to the doubling time categories without calculating a precise doubling time value. This included patients with TVDT measurement using at least 4 CT before and after MTT start but with drug interruption exceeding 3 months, those with TVDT measurement based on at least 2 CTs before and after MTT start or those with significant tumor volume change over a short period (≥ 40% increase in tumor volume of lung metastasis within 2 months).
All patients were treated with total thyroidectomy and RAI ablation at thyroid cancer diagnosis. Patients received repeat surgery and/or external beam radiation therapy (EBRT) to the neck and/or bone metastasis before or after initiation of targeted therapy and that at the discretion of their treating physician.
Statistical Methods:
Continuous data are presented as means and standard deviations or median and ranges, as appropriate for each variable. The clinical characteristics of the midDT groups were analyzed using one way Anova and Pearson Chi square as appropriate. The impact of the change in the average tumor volume doubling time (midDT) category associated with MTT was evaluated using Kaplan-Meier survival analysis and log rank testing. In the current study, we elected to use disease specific survival as the study outcome rather than overall survival to accurately detect MTT ‘s effect on thyroid cancer prognosis. We thus excluded all patients who died of undetermined causes or passed away due to other illnesses including other cancers. All analyses were performed using SPSS software (Version 25; SPSS, Inc., Chicago, IL). A p-value of ≤0.05 was considered statistically significant.
Results
Patient Characteristics:
The clinico-pathological characteristics of the 122 patients are summarized in Table 1. The patients were a median of 61 years old at distant metastasis diagnosis, with papillary (32%) or poorly differentiated histology (35%), and predominantly with only pulmonary metastases (75%). MTT was started at a median of 3 years after distant metastasis (DM) diagnosis (range 0.1 – 16.1 years). By then, only 53 patients (43%) had isolated lung metastasis and 57 % had additional metastasis in the bone, liver and/or brain. The FDG-PET avidity in the lungs, measured within 3 months of MTT’s start date, was more likely to be elevated (SUV ≥ 10, 45%), with about a 1/3 of the patients presenting with mild uptake (SUV≤ 5).
Table 1:
Clinical characteristics of the whole cohort
| Age at thyroid cancer diagnosis (years) | 122 | |
| Mean +/− SD | 56 +/−13 | |
| Median | 56 | |
| Range | 28 – 80 | |
| Presence of DM at cancer diagnosis | ||
| M1 | 45 (37%) | |
| Age at DM diagnosis | ||
| Mean +/− SD | 60 +/− 12 | |
| Median | 61 | |
| Range | 30 – 80 | |
| Gender | ||
| Male | 64 (53%) | |
| Histology | ||
| Papillary other than FV-PTC | 39 (32%) | |
| Follicular and FV-PTC | 17 (14%) | |
| Hurthle Cell | 23 (19%) | |
| Poorly Differentiated | 43 (35%) | |
| Distribution of metastasis at DM dx | ||
| Lung only | 92 (75%) | |
| Lung and bone | 13 (11%) | |
| Bone Only | 11 (9%) | |
| Lung and other | 2 (2%) | |
| Lung, bone and other | 4 (3%) | |
| MTT start time from DM dx (years) | ||
| Mean +/−SD | 4.0 +/−3.5 | |
| Median | 3.0 | |
| Range | 0.1–16.1 | |
| Distribution of DM at MTT start | ||
| Lung only | 53 (43%) | |
| Lung and bone | 32 (26%) | |
| Lung and other | 11 (9%) | |
| Lung, bone and other | 26 (22%) | |
| FDG-PET SUV max in lung at MTT start | 80* | |
| SUV 0–5 | 23 (29%) | |
| SUV 5–10 | 21 (26%) | |
| SUV >10 | 36 (45%) | |
| Total time on MTT (years) | ||
| Mean +/− SD | 2.2 +/− 1.9 | |
| Median | 1.7 | |
| Range | 0.0 – 8.9 | |
| Total time on MTT groups | ||
| Less than 6 months | 23 (19%) | |
| 6–12 months | 19 (16%) | |
| More than 12 months | 80 (66%) | |
| Follow up since DM diagnosis (years) | ||
| Mean +/− SD | 6.6 +/− 3.8 | |
| Median | 6.0 | |
| Range | 0.5 – 18.0 | |
| Follow up since MTT start (years) | ||
| Mean +/− SD | 2.5 +/− 1.9 | |
| Median | 2.1 | |
| Range | 0.0 – 8.9 | |
| Status at end of follow up | ||
| Alive | 53 (43%) | |
| Dead | 69 (57%) | |
| Cause of Death | 69** | |
| Thyroid cancer related | 100% | |
DM: distant metastasis; MTT: molecular targeted therapy;
only 80 patients had available FDG-PET scans;
Only 69 patients died , all from thyroid cancer related deaths.
Patients were treated with one or more MTT regimen during their follow up. This included therapy with either Aflibercept, Sorafenib, Pazopanib, Lenvatinib, Sorafenib/Everolimus, Sorafenib/Temerolimus, Everolimus alone, Temsirolimus alone, Dabrafenib alone or in combination with Lapatinib, Vemurafenib, Fostamatinib, Axitinib, Sunitinib, Vandetanib or Cabozantinib. The median total time on MTT (including interruption time between drugs) is 1.7 years (range: 0.3 – 8.9). Most of the patients received therapy for more than a year (66%), with 16% treated for 6–12 months and 23 patients treated less than 6 months (19%).
Patients were followed a median of 6 years (range: 0.5 −18.0) from DM diagnosis and a median of 2.1 years (range 0.0 – 8.9) after MTT start. All deaths were thyroid cancer related. More than half of the patients succumbed to their disease by the end of the follow up period. Deaths occurred a median 1.7 years (range: 0.0 – 7.0) after MTT start.
Despite molecular targeted therapy, more than half of our study population of patients with RAIR progressive metastatic thyroid cancer succumbed to their disease. The 5-year DSS from DM diagnosis was 72% with median survival of 8 years (95% CI: 6.6–9.5). The median survival was 2.9 years after MTT start (95% CI: 2.1–3.6) with 2-year and 5-year DSS of 64% and 31% respectively.
TVDT of lung metastasis before and after MTT
Figure 2 depicts the change in lung midDT before and after MTT initiation in the 62 patients with precise midDT measurement. Before the initiation of molecular targeted therapy, 32 (52%) patients had midDT ≤ 1 year, 17 (27%) patients had midDT 1–3 years and 13 (21%) patients had midDT ≥ 3 years in lung metastasis. All patients received systemic therapy for more than a year (median range 1.2–2.5 years).
Figure 2: Alluvial graph depicting MTT- induced change in lung midDT.

The figure depicts the change in midDT from baseline pre-drug midDT (on left hand side) to achieved on-drug midDT (right hand side). In the left side of the graph, the midDT groups (midDT≤ 1 year, midDT 1−3 years and midDT ≥ 3 years) are divided into 3 cluster nodes (vertical bolded lines). Similarly, the achieved on-drug midDT groups (midDT ≤ 1 year, midDT 1−3 years, midDT ≥ 3 years) are divided in 3 clustered nodes (vertical bolded lines) . The height of nodes represents the proportion of patients in each midDT group. The color stream fields represent the change in midDT from before the drug was started to after drug is instituted
In the 32 patients with baseline midDT ≤ 1 year, systemic therapy significantly prolonged the lung metastasis tumor volume doubling time in 24 (75%) patients who achieved midDT ≥ 3 years. An additional 2 (6%) patients achieved midDT 1–3 years. Systemic therapy did not change the midDT classification of 6 out of these 32 patients (19%). Their median baseline doubling time was 0.29 years (0.1–1.0) and became 0.40 years (range: 0.17–0.82) after MTT start. Two out of the 6 patients succumbed to thyroid cancer by end of the follow up.
Seventeen out of 62 patients (27%) had lung midDT 1–3 years prior to MTT start. All patients (100%) had prolongation of lung midDT with systemic therapy, achieving midDT ≥3 years.
Thirteen out of 62 (21%) patients had stable or slowly progressive lung metastasis from DTC as documented by baseline midDT ≥ 3 years. After MTT start, 1 (8%) patient midDT ≥ 1 year, 3 (23%) had midDT 1–3 years and 9 (69%) patients continued to be classified with midDT ≥ 3 years.
Disease specific survival in patients with rapidly progressive thyroid cancer treated with MTT.
Figure 3 depicts the disease specific survival of the 122 studied patients classified based on MTT- achieved midDT group. As expected, the disease specific survival was significantly longer in patients who achieved midDT > 3 years as opposed to patients with MTT- achieved midDT ≤ 1 year. Specifically, the median survival was 7.1 years in those with MTT- achieved midDT ≥ 3 years (95% CI: 3.3–10.9), as opposed to 5.6 years (95% CI: 2.5–8.7) and 2.8 years (95% CI: 1.9–3.7) in those with MTT-achieved midDT of 1–3 years and MTT-achieved midDT ≤ 1year respectively (Log Rank p = 0.002). We compared the clinical characteristics of these 122 patients based on MTT achieved midDT groups. As expected, patients with persistent midDT ≤ 1 year were more likely to succumb to their disease than those who responded to therapy. The patients did not otherwise differ based on age, gender, histology, distribution of distant metastasis or the time of systemic therapy start from distant metastasis diagnosis.
Figure 3:
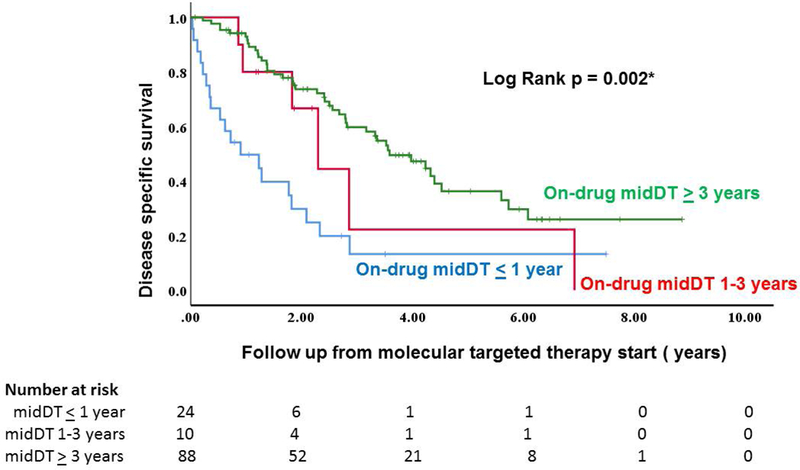
Disease specific survival of patients with rapidly progressive thyroid cancer (baseline midDT <1 year) based on MTT-achieved midDT
In patients with rapidly progressive thyroid cancer (midDT ≤ 1 year, n= 69 patients), disease specific survival is significantly improved in patients who achieved prolongation of lung midDT with molecular targeted therapy. Specifically, the median survival was 4.5 years in those with MTT- achieved midDT ≥ 3 years (95% CI: 2.9–6.2), as opposed to 2.3 years (95% CI: 0.3–4.3) and 0.7 years (95% CI: 0.2–1.3) in those with MTT-achieved midDT of 1–3 years and MTT-achieved midDT ≤ 1year respectively (Log Rank p < 0.001). The patients did not differ based on age, gender, histology, distribution of distant metastasis or the time of systemic therapy start from distant metastasis diagnosis.
Twenty six out of 122 patients (21%) had a baseline midDT 1–3 years. Since all patients achieved on-drug midDT ≥ 3 years, we could not assess a MTT- induced change in survival in that group
Twenty seven out of 122 patients (22%) had midDT ≥ 3 years at baseline. All but 5 patients (85%) had stable midDT, while 1 patients had midDT ≥ 1 year and 4 patients had midDT 1–3 years. Given the small number of patients in the on-drug midDT ≤ 1 years and 1–3 years groups, difference in survival with MTT could not be assessed.
Discussion
In our prior study of patients with RAIR lung metastasis from differentiated thyroid cancer, we introduced lung midDT (average TVDT) as important predictor of disease specific survival. Furthermore, our preliminary analysis of 3 patients suggested a clinical utility of TVDT measurement in assessing response to molecular targeted therapy (5). In this retrospective analysis, we confirmed in a larger cohort of patients that MTT can prolong tumor volume doubling time of lung metastasis from thyroid cancer. Furthermore, midDT prolongation of lung metastasis is now shown to be associated with a statistically significant improvement in disease specific survival of patients with rapidly progressive metastatic RAIR metastatic thyroid cancer.
In the prior study of 88 patients with RAIR thyroid cancer with lung metastasis, we used the 5-year overall survival from the time the lung metastasis was 1 cm as the main outcome of the study. In the design of this study, we elected to use disease specific survival from the distant metastasis diagnosis as the main outcome of the study. This was done to accurately measure the effect of molecular targeted therapy on patient’s survival. In the initial cohort of the study, two patients died of myelodysplastic syndrome and pancreatic cancer. They were excluded from the final study group (n = 122). To ensure uniformity of our results, we reanalyzed the overall survival after MTT based on midDT groups (n = 124) and found similar results whether we used overall survival or disease specific survival as the main study outcome.
The results of this study are a continuation of the risk adaptive approach model to the management of patients presenting with differentiated thyroid cancer, a model that is now extended to those patients presenting with metastatic RAIR thyroid cancer. We previously demonstrated that midDT is an important tool in risk assessment of patients with RAIR metastatic thyroid cancer. Thus, among these patients with RAIR metastatic DTC, those with midDT ≤ 1 year are at highest risk for mortality form their disease, while patients with lung metastasis midDT ≥ 3 years are at lowest risk for mortality. Furthermore, we have now demonstrated a significant improvement in disease- specific survival in patients with rapidly progressive thyroid cancer who achieved a significant MTT-induced midDT prolongation. With that in mind, the decision to start systemic therapy should be balanced against potential adverse effects, quality of life issues, financial burden and overall patient preferences and values.
This study further underscores the importance of measuring midDT in lung metastasis from thyroid cancer to properly select patients that are appropriate for consideration for systemic therapy and to assess response to these therapies. It is thus essential that serial computer tomography examinations (CT) are obtained at short interval before and after MTT start to allow for precise TVDT and midDT measurement when appropriate. In the current study, precise TVDT was consistently measured on patients who were treated for more than 6 months. For the survival analysis, to ensure that our study population is representative of all patients with rapidly progressive thyroid cancer that would be eligible for MTT therapy, we expanded our criterion for midDT measurement to include those with clinical features of structural progression.
This study was not specifically designed to address the efficacy of one drug regimen over another. We however believe that the risk assessment and response to therapy model can be applied to any MTT used. Thus, any drug that can significantly prolong midDT is expected to prolong the disease specific survival of patients with RAIR metastatic thyroid cancer. Furthermore, while MTT induced prolongation can be achieved, we believe that duration of therapy and duration of midDT prolongation are crucial to influence survival. Thus, patients who can have sustained midDT prolongation for more than a year fared better than those who lasted on drug less than 6 months.
This study focused on TVDT of lung metastasis in patients with RAIR thyroid cancer. However, in our clinical practice, we recognized that thyroglobulin doubling time and structural doubling measurement are complementary in the risk assessment, proper selection of patients who are eligible for further therapy and in determining response to therapy (8). This is especially true when dealing with patients with extrapulmonary metastasis. While lung metastasis TVDT reflects response to therapy in the lung, it does not take into consideration TVDT assessment or response to therapy in extra- pulmonary metastatic lesions which may or may not demonstrate a concordant response to therapy. In our prior study, we found that Tg DT and midDT showed a moderate correlation (Pearson correlation coefficient r = 0.50, r2 = 0.25, p < 0.001) (5). When not concordant, Tg DT was shorter than lung metastasis TVDT, reflecting rapid disease progression in extrapulmonary metastasis. In the current study, thyroglobulin levels were not consistently measured in our patients. Thus, we could not use Tg DT to assess response to therapy.
This study is a retrospective analysis of institutional experience with molecular targeted therapy. Such analyses are subject to all weaknesses of retrospective studies including selection biases. As such, patients were selected for systemic therapy based on a host of clinical factors, some of which may not have been captured in this study. This study does not take into consideration progression in extra-pulmonary metastasis as main reason for drug initiation, nor does it study the response to therapy in extra-pulmonary metastasis and its effects on disease specific survival. In fact, almost all patients with baseline lung midDT ≥ 3 years (26 out of 27 patients) were started on MTT due to progression of disease in extrapulmonary sites (neck, mediastinum or extrapulmonary distant metastasis). Only one patient with baseline midDT ≥ 3 years was started based on rising thyroglobulin level with slow disease progression in the lungs. Also, patients selected for systemic therapy have generally better performance status and predicted to have better overall survival than the general cohort of patients with rapidly progressive metastatic thyroid cancer.
In conclusion, lung midDT is a useful and important clinical marker of disease specific survival for patients with progressive RAIR metastatic thyroid cancer. In patients with rapidly progressive metastatic RAIR thyroid cancer, molecular targeted therapy prolongs lung tumor volume doubling time and is associated with improved disease specific survival.
Acknowledgments
Disclosure of funding received for this work: This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Disclosure Summary Statement: Drs Sabra, Sherman and Tuttle are consultants for ESAI Pharmaceutical. Drs Sherman and Tuttle are also consultants for Bayer Inc.
References
- 1.Haugen BR, Alexander EK, Bible KC, Doherty GM, Mandel SJ, Nikiforov YE, Pacini F, Randolph GW, Sawka AM, Schlumberger M, Schuff KG, Sherman SI, Sosa JA, Steward DL, Tuttle RM, Wartofsky L 2016. 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Network NCC. NCCN Clinical Practice Guidelines in Oncology: Thyroid Carcinoma version 1.2018 (last accessed September 14, 2018)
- 3.Brose MS, Nutting CM, Jarzab B, Elisei R, Siena S, Bastholt L, de la Fouchardiere C, Pacini F, Paschke R, Shong YK, Sherman SI, Smit JW, Chung J, Kappeler C, Pena C, Molnar I, Schlumberger MJ 2014. Sorafenib in radioactive iodine-refractory, locally advanced or metastatic differentiated thyroid cancer: a randomised, double-blind, phase 3 trial. Lancet 384:319–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schlumberger M, Tahara M, Wirth LJ, Robinson B, Brose MS, Elisei R, Habra MA, Newbold K, Shah MH, Hoff AO, Gianoukakis AG, Kiyota N, Taylor MH, Kim SB, Krzyzanowska MK, Dutcus CE, de las Heras B, Zhu J, Sherman SI 2015. Lenvatinib versus placebo in radioiodine-refractory thyroid cancer. N Engl J Med 372:621–630. [DOI] [PubMed] [Google Scholar]
- 5.Cabanillas ME, Patel A, Danysh BP, Dadu R, Kopetz S, Falchook G 2015BRAF Inhibitors: Experience in Thyroid Cancer and General Review of Toxicity Horm Cancer 6:21–36 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bible KC, Ain KB, Rosenthal MS 2014. Protein kinase inhibitor therapy in advanced thyroid cancer: ethical challenges and potential solutions International Journal of Endocrine Oncology: Int J Endo Oncol : 145–151 [Google Scholar]
- 7.Sabra MM, Sherman EJ, Tuttle RM 2017. Tumor volume doubling time of pulmonary metastases predicts overall survival and can guide the initiation of multikinase inhibitor therapy in patients with metastatic, follicular cell-derived thyroid carcinoma. Cancer 123:2955–2964 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miyauchi A, Kudo T, Miya A, Kobayashi K, Ito Y, Shimoyamate-dori C, Takamura Y, Higashiyama T, Fukushima M, Kihara M, Tomoda C, Inoue H, Yabuta T, Masuoka H 2011. Prognostic impact of serum thyroglobulin doubling-time under TSH suppression in patients with papillary thyroid carcinoma who underwent total thyroidectomy. Thyroid 7:707–716 [DOI] [PubMed] [Google Scholar]


