Abstract
Background
Psychological factors (PFs) are known predictors of cardiovascular disease (CVD) in many clinical settings, but data are lacking for human immunodeficiency virus (HIV) infection. We carried out a prospective study to evaluate (1) psychological predictors of preclinical and clinical vascular disease and (2) all-cause mortality (ACM) in HIV patients.
Methods
We conducted a cross-sectional analysis of baseline data to evaluate the predictors of carotid plaques (CPs) and a prospective analysis to explore predictors of vascular events (VEs) and ACM over 10 years. Human immunodeficiency virus patients monitored at the Infectious Disease Units of 6 Italian regions were consecutively enrolled. Traditional CVD risk factors, PFs (depressive symptoms, alexithymia, distress personality), and CPs were investigated. Vascular events and ACM after enrollment were censored at March 2018.
Results
A multicenter cohort of 712 HIV-positive patients (75.3% males, aged 46.1 ± 10.1 years) was recruited. One hundred seventy-five (31.6%) patients had CPs at baseline. At the cross-sectional analysis, alexithymia was independently associated with CPs (odds ratio, 4.93; 95% confidence interval [CI], 2.90–8.50; P < .001), after adjustment for sociodemographic, clinical, and psychological variables. After an average follow-up of 4.4 ± 2.4 years, 54 (7.6%) patients developed a VE, whereas 41 (5.68%) died. Age, current smoking, hypertension, and alexithymia (hazard ratio [HR], 3.66; 95% CI, 1.80–7.44; P < .001) were independent predictors of VE. Likewise, alexithymia was an independent predictor of ACM (HR, 3.93; 95% CI, 1.65–9.0; P = .002), regardless of other clinical predictors.
Conclusions
The present results validate our previous monocentric finding. Alexithymia may be an additional tool for the multifactorial assessment of cardiovascular risk in HIV.
Keywords: alexithymia, atherosclerosis, HIV, mortality, psychological factors
Coronary artery disease is a major cause of morbidity and mortality for patients living with long-term human immunodeficiency virus (HIV) infection [1, 2]. Indeed, epidemiological studies, fully adjusting for numerous cardiovascular disease (CVD) risk factors and investigating validated cardiovascular (CV) events, demonstrate an increased risk of CV events in HIV versus non HIV-infected patients [3]. Between 1999 and 2013, the proportion of mortality attributable to CVD among HIV-infected individuals aged 25 years or older in United States increased from 2.1% to 3.8% in women and from 1.9% to 4.9% in men [4]. After controlling for sex, race/ethnicity, borough of residence, and year, those with HIV had significantly higher CVD mortality than the general population in all age groups through age 65 [5]. There is a critical need to determine efficacious CV risk reduction interventions in HIV-infected patients. So far, preventive strategies have focused on managing of traditional CVD risk factors (such as smoking, lipodystrophy, diabetes, hypertension, and dyslipidemia), effect of highly active antiretroviral therapy (HAART) on CVD risk and immune activation [6]. However, in the field of CV risk prevention on the general and clinical population, epidemiological studies conducted in the last decade also generally demonstrate strong dose-response relationships between an increasing number of psychological risk factors and CVD [7, 8]. This has been poorly considered in the HIV-positive population until now. Psychological factors may be related to atherosclerosis and other vascular events (VEs) through their association with behavioral risk factors [9]. They may also directly affect biological processes by multiple pathways, such as inflammation [10], CV reactivity [11], endothelial dysfunction [12], platelet activation [13], and autonomic dysfunction [14], whereby psychological factors may play both a primary and a secondary pathogenic role, as shown by etiological and prognostic studies [7, 15]. Along these lines, we performed a single-center cohort study to evaluate the role of psychological factors, including alexithymia, distress personality (Type D), and depressive symptoms, in parallel with several traditional predictors of increased carotid intima-media thickness (c-IMT), carotid plaques (CPs), and ensuing VEs in 200 HIV-infected patients. We were able to show that alexithymia, a compromise of affective regulation and emotional cognitive processing, can act as an independent predictor of CPs and VEs in the HIV population [16]. In this study, we present the results of the multicentric validation of our results.
METHODS
Study Design
The study included (1) a cross-sectional analysis of the baseline data to evaluate the predictors of CPs and (2) a prospective cohort analysis to explore the determinants of VE and all-cause mortality (ACM) during the 10-year observation period. For this prospective cohort study, we recruited HIV-infected patients from provincial referral hospitals in several Italian regions including northern Italy, central Italy, southern Italy, and the major islands (Sicily and Sardinia). Patients were enrolled from the following: Infectious Disease Units of Pescara General Hospital (Abruzzo Region) since December 2007; Department of Medical, Surgical and Experimental Sciences, University of Sassari (Sardinia Region) from October 2014; Department of Clinical and Experimental Medicine, Garibaldi Nesima Hospital, University of Catania (Sicily Region) from November 2014; Department of Medicine, Santa Maria Hospital, Perugia (Umbria Region) from June 2015; Santa Maria Annunziata Hospital, Florence (Tuscany Region) from June 2015; and University of Bari (Puglia Region) since July 2016. All recruited patients were followed-up until March 20, 2018. Patients were reassessed every 6 months.
Data Collection
This study involved HIV-1-infected patients aged 18 and over and followed in the context of the CISAI study group (Italian Coordination for Research into Allergy and HIV Infection). Data were collected in real time by clinicians. The clinicians of each center had their own data collection database, which was sent to the referring center at each follow-up time point. The database underwent periodic quality control, including comorbidity data. All patients were included in the cohort after receiving oral information and giving written consent. All patient information was entered into a database using anonymous, coded identification numbers. The present study only included Infectious Diseases Units already collecting psychological and neurocognitive parameters as a part of good clinical practice according to the Italian guidelines for the use of antiretroviral agents and the diagnostic-clinical management of the HIV-1 infection of the Society of Infectious and Tropical Diseases and of the Technical Health Committee, Italian Ministry of Health [17]. Indeed, at participating centers, psychological screening was performed in the context of the usual clinical practice. Furthermore, each participating center was authorized to confer anonymized data for retrospective research purposes by local authorities. Because the present study was set up as a multicentric collection of existing clinical data, and no additional experimental intervention was required for participants, the preliminary opinion of local ethics committees was not required in accordance to local ethical rules. This study was performed in accordance with the principles of the Declaration of Helsinki and current Italian legislation relating to biomedical research. Inclusion criteria were as follows: treated or untreated confirmed chronic HIV infection; age ≥18 years; any CD4 T-cell counts and HIV viremia; absence of acute opportunistic infections, malignancy, or pregnancy at the time of enrollment and subsequent study procedures; sufficient knowledge of the Italian language to undergo psychological profiling; and willingness and ability to provide written informed consent. All consecutive HIV-infected patients aged 18 years or more, attending the Outpatient Clinics of the unit of infectious diseases mentioned above, were offered participation at their first access.
Demographic, Clinical, and Viro-Immunological Characterization of the Sample
At the baseline visit, each patient agreeing to participate underwent an in-depth assessment, covering sociodemographic, anthropometric, and clinical characteristics (Table 1). Data on participants’ lifestyle behavior information were obtained through an ad hoc structured self-report questionnaire, following international recommendations [18–20]. The centers participating in the study routinely evaluated the patient’s adherence to antiretroviral therapy at each visit. The method to define the level of adherence adopted spontaneously by all participating centers was to ask patients whether they had taken regular therapy (from the previous visit) based on the prescription. The level of adherence was defined as the division of the pill number that the patient actually used by the pill number that the patient must have used, multiplied by 100. An adherent patient was defined as one who takes >95% of the prescribed doses. Patients with a reported intake of less than 95% of the doses were classified as suboptimal adherents.
Table 1.
Overall Characteristics of the Sample at Baseline
| Variables | Overall Sample (n = 712) |
|---|---|
| Sociodemographic Characteristics | |
| Male gender, % | 75.3 |
| Mean age in years (SD) | 46.1 (10.1) |
| Married, % | 33.8 |
| Low educational attainment ( <13 years), % | 43.3 |
| Stable employed, % | 67.3 |
| Race/Ethnicity | |
| White, % | 97 |
| Black, % | 1.4 |
| Hispanic or Latino, % | 1.6 |
| HIV Transmission Risk Factor | |
| IDU, % | 25.8 |
| MSM, % | 33.6 |
| Heterosexual, % | 40.6 |
| Lifestyle Factors | |
| Smoke, % | 51.7 |
| Alcohol abuse, % | 11.2 |
| Physical activity, % | 29.9 |
| Cardiovascular Risk Factors | |
| Mean BMI (SD) | 24.2 (4) |
| Mean total cholesterol, mg/dL (SD) | 192 (51.9) |
| Mean HDL cholesterol, mg/dL (SD) | 42.7 (13.6) |
| Mean LDL cholesterol, mg/dL (SD) | 117.6 (38.6) |
| Hypertension, % | 20.4 |
| Fasting glucose, mg/dL (SD) | 89.6 (21.5) |
| Diabetes, % | 10.4 |
| Mean carotid intima-media thickness in centimeters (SD) | 0.07 (0.04) |
| Carotid atherosclerotic plaques, % | 31 |
| Previous vascular events (overall), % | 2.6 |
| Previous AMI, angina, or PCI, % | 31.6 |
| Previous stroke or TIA, % | 36.8 |
| Previous cardiac syncope, % | 10.6 |
| Previous intestinal infarction or renal infarction, % | 21 |
| Positive CVD family historya, % | 44 |
| FRS 10-year general CVD risk, mean % of risk (SD) | 7.7 (8.1) |
| FRS% of risk >20, % | 11.3 |
| FRS% of risk 20–10, % | 21.2 |
| FRS% of risk <10, % | 67.5 |
| HIV-Related Variables | |
| AIDS diagnosis, % | 32.4 |
| Nadir LyCD4, cell/mm3 (SD) | 244 (189) |
| Baseline LyCD4, cell/mm3 (SD) | 639.8 (360.3) |
| Baseline LyCD4 cell count <200 cells/mm3, % | 9.3% |
| Baseline HIV-RNA copies/mL (SD) | 13 381.1 (77 065.3) |
| HIV-RNA <50 copies/mL, % | 77.9 |
| Mean duration of HIV infection in years (SD) | 13.1 (8.1) |
| Treated with HAART, % | 91.4 |
| Mean duration of HAART in months (SD) | 103.2 (84.5) |
| Lipodystrophy, % | 25.8 |
| Suboptimal adherence to HAART, % | 7.5 |
| Other Treatment | |
| Antihypertensive medication | 18.3 |
| Antiplatelet/anticoagulant medication | 5.8 |
| Psychiatric medication | 16 |
| Comorbidities | |
| HCV coinfection, % | 25.2 |
| Neoplastic disease, % | 10.7 |
| Psychological Factors | |
| Depressive symptoms, % | 22 |
| Depressive symptom (BDI-II) score (SD) | 11 (10.3) |
| Alexithymia, % | 36.0 |
| Alexithymia (TAS-20) score (SD) | 45.9 (13.2) |
| Distress personality (Type D), %a | 39 |
Abbreviations: AIDS, acquired immune deficiency syndrome; Alexithymia, Toronto Alexithymia Scale (TAS-20) >50; AMI, acute myocardial infarction; BDI-II, Beck Depression Inventory-II; BMI, body mass index; CVD, cardiovascular disease; FRS, Framingham risk score; HAART, highly active antiretroviral therapy; HCV, hepatitis C virus; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IDU, injection drug user; LDL, low-density lipoprotein; LyCD4, CD4 lymphocyte; MSM, men who have sex with men; PCI, percutaneous coronary intervention; RNA, ribonucleic acid; SD, standard deviation; TIA, transient ischemic attack;
aMissing values ≥10%.
Definition of the Main Outcomes
The main outcomes of our investigation were as follows: (1) presence of CPs at baseline; (2) VEs during follow-up (every 6 months), including acute myocardial infarction (AMI), angina or percutaneous coronary intervention (PCI), stroke or transient ischemic attack (TIA), cardiac syncope, organ infarctions (intestinal infarction or renal infarction), and peripheral artery disease (PAD); (3) ACM including acquired immune deficiency syndrome (AIDS) malignancy (diffuse centroblastic lymphoma, plasmoblastic lymphoma), CVD (myocardial infarct, stroke, pulmonary edema, other organ infarct), non-AIDS malignancy (prostate cancer, malignant melanoma, lung cancer, Hodgkin lymphoma, gastric cancer, pancreas cancer), non-AIDS infection (sepsis, infective endocarditis, pneumonia), liver disease, pulmonary disease (chronic obstructive pulmonary disease, pulmonary hypertension), violent or accidental death (suicide, physical assault, trauma). All ultrasonographic scannings of carotid arteries (short and long axis) were performed by certified operators. Vascular events were actively searched in the cohort. They were positively assessed at each follow-up visit by the assisting physicians and recorded in the study dataset when appropriate, after review of clinical, laboratory, and imaging data supporting diagnoses of each VE. Records of all patients dying during follow-up were also reviewed before inclusion in the dataset.
Definitions and Measures of the Psychological Constructs
Three psychological parameters—depressive symptoms, distress personality [Type D], and alexithymia—were evaluated for their relevance in the CV risk area [7, 16, 21–23]. Depressive symptoms were evaluated with Beck Depression Inventory-II (BDI-II) [24] using a specific cutoff for the HIV-infected population (BDI-II score ≥17) [25]. Type D is a relatively new construct characterized by 2 global personality traits: negative affectivity (NA) and social inhibition (SI) [26]. Type D was evaluated with Italian-validated [27] DS-14 (NA score ≥9; SI score ≥9, as opposed to the original DS-14 cutoff of 10). Alexithymia refers to the psychological dysfunctional trait of having no words to express emotions or feelings [28]. It is a multidimensional construct comprising emotional and cognitive components: difficulties in identifying and describing feelings as well as in differentiating somatic sensations and feelings, lack of fantasy and imagination, and an externally oriented cognitive style [28]. It is interesting to note that the relevance of alexithymia has grown exponentially in the last decades; it is currently considered a relevant concept for a range of psychological and physical disorders [28]. We used the Italian version of the Toronto Alexithymia Scale-20 item (TAS-20), which is universally used for the alexithymia construct worldwide [29]. The TAS-20 cutoff point >50 (including borderline alexithymia + high alexithymia), a threshold previously used in studies on large populations [21, 22], was chosen because it proved to be more sensitive in predicting CV risk in the general and clinical population [16, 21, 22].
Statistical Analysis
Cross-Sectional Analysis of Baseline Data
Multivariable logistic regression analyses were used to evaluate potential independent predictors of the presence of CPs, treated as a dichotomic variable. We defined the regression model including a priori several potential confounders (age, gender, body mass index [BMI], smoking, hypertension, diabetes, one among total cholesterol, physical activity, alcohol/drug abuse, educational level, and infection duration), because of their known association with CPs, and including other eventually significant variables, which were selected using a stepwise forward process (including in model fitting all variables that were significant at a 0.10 level in univariate analyses). Each covariate was tested in its original form or transformed if needed. In addition, each included variable was tested for multicollinearity, for potential interaction and/or quadratic/cubic terms. We found only total and low-density lipoprotein (LDL) cholesterol to be collinear and chose to include total cholesterol. For continuous covariates for which a defined threshold has been indicated in the literature (ie, TAS-20 score >50), we tested the inclusion of both the continuous and categorical form, and we selected the one that was included in the model with highest pseudo or adjusted R-squared values. All variables that were not included into the final models were not significant. In addition, we tried to limit, as much as possible, the number of covariates included in the final model to avoid overfitting; among the nonsignificant variables, inside the final model, we left those that were associated with the highest increase in model pseudo or adjusted R-squared, or those that changed the significant coefficients by more than 10%. The results of the logistic analysis are presented as odds ratio (OR) and 95% confidence limits.
Survival Analysis of Follow-up Data
Cox proportional hazards analysis was used to calculate the adjusted relative hazards of VEs and ACM by each variable. Stochastic level of entry into the model was set at 0.10, and interaction terms were explored for all variables in the final model. A minimum events-to-variable ratio of 10 was maintained in multivariate modeling to avoid overfitting, and Schoenfeld’s test was performed to check the validity of proportional hazards assumption. Kaplan-Meier survival analysis was used to examine the association between a TAS-20 score >50 and VEs or ACM. Statistical significance was defined as a 2-sided P value <.05 for all analyses, which were performed using STATA 10.1 (StataCorp, College Station, TX).
RESULTS
Study Sample
The initial cohort screened for eligibility to participate in the study consisted of 743 patients. Twelve HIV-infected patients visiting occasionally were not offered participation. Nineteen patients were excluded due to linguistic barriers. Nineteen VEs were not computed before the start of the study. The final sample was 712 patients, all of whom gave informed consent to participate. Of them, 553 (77.7%) attended CPs evaluation, 660 (92.7%) had a psychological assessment, and 508 (71.3%) had both procedures and could thus be included in the multivariate analyses. The clinical, behavioral, and sociodemographic characteristics of the final sample (n = 712) are described in Table 1. The mean age was 46.1 years, 75% were males, the mean BMI was 24.2, 20% had hypertension, 10% had diabetes, 51% were active smokers, and the mean 10-year Framingham risk score was 7.7%. Seventy-seven percent of patients were virologically suppressed and 91.4% were treated with HAART. Additional information is detailed in the Supplemental Material.
Predictors of Carotid Plaques: Cross-Sectional Analysis
One hundred seventy patients (31.6%) were found to have at least 1 vascular carotid lesion that met the primary (≥1.5 mm) definition of CPs. As shown in Table 2, at univariate analyses, presence of CPs was significantly associated with older age, low education, stable employment, smoking, HIV transmission risk factor (injection drug user [IDU]), total cholesterol and LDL, hypertension, diabetes, Framingham risk score, AIDS diagnosis, nadir CD4 T-cell counts, duration of HIV infection, treatment with HAART and its duration, lipodystrophy, depressive symptoms, and alexithymia. At multivariate analyses, only male gender, increasing age, total cholesterol, hypertension, years lived with HIV, and alexithymia remained significantly associated with CPs (Table 2). In particular, subjects with a TAS-20 score >50 (presence of alexithymia) consistently showed an OR of 4.9 (95% confidence interval [CI], 2.9–8.5; P < .001) of having CPs.
Table 2.
Univariate and Multivariate Analyses Evaluating the Potential Predictors of Each Recorded Outcome
| Variables | Carotid Plaques at Baseline | P a | OR (95% CI) | P b | Vascular Events | P a | HR (95% CI) | P c | Overall Mortality | P a | HR (95% CI) | P c |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (n = 175) | (n = 54) | (n = 41) | ||||||||||
| Sociodemographic Characteristics | ||||||||||||
| Male gender | 80.5% | .3 | 1.84 (.99–3.42) | .05 | 90.7% | .006 | 2.31 (.85–6.24) | .09 | 87.8% | .06 | -- | -- |
| Mean age in years (SD)d | 51.8 (9.5) | <.001 | 1.08 (1.05–1.12) | <.001 | 53.8 (9.9) | <.001 | 1.07 (1.02–1.1) | .002 | 49.1 (10.6) | .04 | 1.05 (1–1.08) | .023 |
| Married | 41.6% | .08 | -- | -- | 44% | .1 | -- | -- | 51.1% | .015 | -- | -- |
| Low educational attainment (<13 years) | 50.4% | .006 | -- | -- | 44.9% | .8 | -- | -- | 61.5 | .017 | -- | -- |
| Stable employed | 58.8% | .004 | 0.83 (.47–1.84) | .5 | 50.9% | .027 | -- | -- | 43.2% | .005 | -- | -- |
| Race/Ethnicity | .412 | -- | -- | .9 | -- | -- | .739 | -- | -- | |||
| White | 32.1% | 96.3% | 95% | |||||||||
| Black | 12.5% | 1.85% | 2.5% | |||||||||
| Hispanic or Latino | 22.2% | 1.85% | 2.5% | |||||||||
| HIV transmission risk factor | .038 | 1.19 (0.85–1.66) | .3 | .031 | .92 (.63–1.33) | .6 | .061 | -- | -- | |||
| IDU | 26.6% | 37.74% | 39% | |||||||||
| MSM | 28.4% | 18.86% | 19.5% | |||||||||
| Heterosexual | 45% | 43.4% | 41.5% | |||||||||
| Lifestyle Factors | ||||||||||||
| Smoke | 58.1% | .008 | 1.64 (0.97–2.77) | .06 | 69.8% | .001 | 2.02 (1.01–4.04) | .044 | 70.7% | .03 | -- | -- |
| Alcohol abuse | 8.8% | .3 | -- | -- | 15.7% | .2 | -- | -- | 40.5% | <.001 | 2.52 (1.09–5.81) | .03 |
| Physical activity | 25.9% | .2 | -- | -- | 24.5% | .3 | -- | -- | 14.6% | .028 | -- | -- |
| Cardiovascular Risk Factors | ||||||||||||
| Mean BMI (SD)e | 24.1 (3.9) | .6 | -- | -- | 25.4 (4.5) | .02 | 1.01 (0.94–1.09) | .6 | 25.6 (4.7) | .03 | -- | -- |
| Mean total cholesterol, mg/dL (SD)f | 199 (55.5) | .03 | 1.05 (1–1.01) | .042 | 195.6 (54.4) | .5 | -- | -- | 165 (52.5) | .001 | -- | -- |
| Mean HDL, mg/dL (SD)e | 41.3 (13.6) | .13 | -- | -- | 40.4 (11.7) | .046 | -- | -- | 32.5 (13.2) | <.001 | -- | -- |
| Mean LDL, mg/dL (SD) | 125.2 (46.9) | .004 | -- | -- | 118.2 (43.4) | .9 | -- | -- | 103.04 (33.5) | .06 | -- | -- |
| Hypertension, % | 32% | <.001 | 1.75 (1–3.06) | .045 | 51.8% | <.001 | 2.23 (1.15–4.32) | .017 | 29.3% | .2 | -- | -- |
| Fasting glucose, mg/dL (SD) | 95.7 (20.1) | .0007 | -- | -- | 99.4 (28.1) | .0009 | -- | -- | 103.8 (24.9) | .0001 | -- | -- |
| Diabetes | 16.4% | .003 | 1.04 (.48-2.26) | .9 | 25% | <.001 | 0.77 (0.35–1.68) | .4 | 34.2% | <.001 | 1.84 (.88–3.82) | .09 |
| Mean carotid intima-media thickness in centimeters (SD) | 0.10 (0.06) | <.001 | -- | -- | 0.08 (0.07) | .2 | -- | -- | 0.06 (0.02) | .11 | -- | -- |
| Previous vascular event | 4.57% | .23 | -- | -- | 0% | .2 | 4.8% | .35 | ||||
| Positive CVD family history | 48% | .29 | -- | -- | 10% | .9 | -- | -- | 36.4% | .28 | -- | -- |
| FRS 10-year general CVD, mean% of risk (SD)e | 12.3 (9.3) | <.001 | -- | -- | 16.1 (10.7) | <.001 | 1.01 (.98–1.) | .3 | 12.8 (10.1) | .005 | ||
| HIV-Related Variables | ||||||||||||
| AIDS diagnosis | 41.4% | .002 | 1.38 (0.83–2.29) | .2 | 49.1% | .002 | 1.31 (0.73–2.33) | .3 | 60% | <.001 | 1.96 (.98–3.93) | .05 |
| Nadir LyCD4, cell/mm3 (SD) | 208 (192) | .006 | -- | -- | 227.5 (201.7) | .5 | -- | -- | 183 (146) | .05 | -- | -- |
| Baseline LyCD4, cell/mm3 (SD) | 599.7 (331.9) | .95 | -- | -- | 600.1 (325.2) | .4 | -- | -- | 384.9 (245.6) | <.001 | -- | -- |
| Baseline LyCD4 cell count <200 cells/mm3 | 11.6% | .5 | -- | -- | 12% | .5 | -- | -- | 22.2% | .006 | ||
| Baseline HIV-RNA copies/mL (SD) | 13 601.4 (83 453.8) | .72 | -- | -- | 11 817 (40 281) | .8 | -- | -- | 9619.1 (34 667.3) | .78 | -- | -- |
| HIV-RNA <50 copies/mL | 77% | .93 | -- | -- | 6.6% | .1 | -- | -- | 66.6% | .12 | -- | -- |
| Duration of HIV infection in years (SD)d | 15.8 (7.2) | <.001 | 1.05 (1.01–1.08) | .003 | 14.4 (7.7) | .2 | -- | -- | 13.6 (7.5) | .07 | -- | -- |
| Treated with HAART | 94.3% | .02 | -- | -- | 90.6% | .8 | -- | -- | 83.7% | .06 | -- | -- |
| Mean duration of HAART in months (SD) | 121 (82.3) | <.001 | ||||||||||
| Lipodystrophy | 40.1% | <.001 | -- | -- | 35.1% | .1 | -- | -- | 53.5% | <.001 | ||
| Suboptimal adherence to HAART | 10.6% | 0.3 | -- | -- | 10.2% | .4 | -- | -- | 31.4% | <.001 | 2.62 (1.14–6) | .022 |
| Other Treatment | ||||||||||||
| Antihypertensive | 47.5% | <.001 | -- | -- | 40.7% | <.001 | -- | -- | 29.3% | .06 | -- | -- |
| Antiplatelet/anticoagulant | 87% | <.001 | -- | -- | 38% | <.001 | -- | -- | 4.8% | .8 | -- | -- |
| Psychiatric medication | 38.5% | .1 | -- | -- | 26.5% | .027 | -- | -- | 31.8% | .004 | -- | -- |
| Comorbidities | ||||||||||||
| HCV coinfection | 28% | .2 | -- | -- | 27.8% | .6 | -- | -- | 43.9% | .005 | -- | -- |
| Cancer | 12.6% | .4 | -- | -- | 15.7% | .2 | -- | -- | 48.8% | <.001 | -- | -- |
| Psychological Factors | ||||||||||||
| Depressive symptoms | 27.7% | .017 | .84 (0.46–1.55) | .5 | 44.2% | <.001 | 1.37 (0.72–2.58) | .3 | 40.5% | .005 | 0.62 (0.28–1.4) | .2 |
| Alexithymia | 61.4% | <.001 | 4.93 (2.9–8.5) | <.001 | 74.5% | <.001 | 3.66 (1.8–7.44) | <.001 | 77.8% | <.001 | 3.93 (1.64–9) | .002 |
| Distress personality (Type D)g | 33.6% | .6 | -- | -- | 42.5% | .6 | -- | -- | 34.3% | .5 | -- | -- |
Abbreviations: aHR, adjusted hazards ratio; AIDS, acquired immune deficiency syndrome; Alexithymia, Toronto Alexithymia Scale (TAS-20) >50; aOR, adjusted odds ratio; BMI, body mass index; CI, confidence interval; CVD, cardiovascular disease; FRS, Framingham risk score; HAART, highly active antiretroviral therapy; HCV, high-density lipoprotein; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IDU, injection drug user; LDL, low-density lipoprotein; LyCD4, CD4 lymphocyte; MSM, men who have sex with men; RNA, ribonucleic acid; SD, standard deviation.
aχ 2 test for categorical variables; t test and Kruskal-Wallis test for normally distributed and nonnormally distributed continuous variables, respectively. In univariate analyses, all P values refer to the comparison between the subjects with and those without the selected outcome: eg, those with versus those without a baseline diagnosis of carotid plaques.
bLogistic regression analysis including 450 observations (see text for covariates selection); Wald χ 2 = 96.8; Hosmer-Lemeshow goodness of fit P = .62; area under the receiver operating characteristic curve = 0.84.
cCox proportional hazard analyses including 586 observations (vascular events) and 610 observations (overall mortality), respectively; see text for covariates selection.
NOTE: In all multivariate analyses: d = 1-year increase; e = 1-unit increase; f = 10-mg/dL increase; g = Missing values ≥10%.
Predictors of Vascular Events: Follow-up Survival Analysis
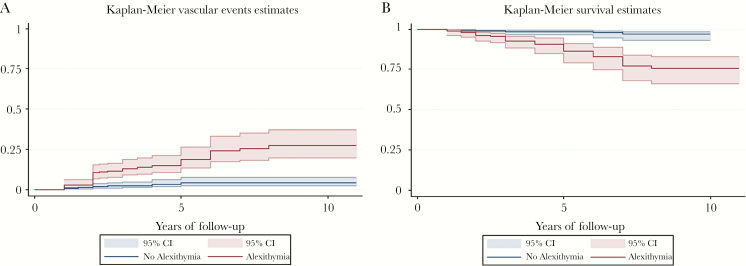
The mean follow-up of the 712 patients was 4.4 ± 2.4 years (for a total of 3133 person-years). Fifty-four patients (7.6%) experienced a VE during the follow-up: 23 (42.6%) experienced AMI, angina, or PCI; 15 (27.8%) experienced stroke or TIA; 3 experienced cardiac syncope (5.6%); 10 (18.5%) experienced intestinal infarction or renal infarction; and 3 (5.6%) experienced PAD. Eleven patients (7 males, 4 females, mean age 41 years) were lost-to-follow up. At univariate analyses, age, male gender, employment status, HIV transmission risk factor (IDU), smoking, BMI, lower high-density lipoprotein (HDL), hypertension, diabetes, Framingham risk score, AIDS diagnosis, depressive symptoms, and alexithymia were significantly associated with VEs. All other factors were not significantly associated with VEs. The incidence rate ratio of VEs was 5.8 (95% CI, 2.9–11.7) per 100 person-years among patients with alexithymia versus nonalexithymic. In the Cox proportional hazard model, age (hazard ratio [HR], 1.07; 95% CI, 1.02–1.10; P = .002), current smoking (HR, 2.02; 95% CI, 1.01–4.04; P = .044), hypertension (HR, 2.23; 95% CI, 1.15–4.32; P = .017), and alexithymia (HR, 3.66; 95% CI, 1.80–7.44; P < .001) were the only factors significantly associated with VEs (Table 2). The relationship between VEs and alexithymia is also apparent from the shape variation of the Kaplan-Meier estimates of time to event (Figure 1A).
Figure 1.
(A) Kaplan-Meier estimates of time to vascular events by alexithymia. (B) Kaplan-Meier estimates of time to all-cause mortality by alexithymia. CI, confidence interval.
Predictors of All-Cause Mortality: Follow-up Survival Analysis
The mean follow-up of the 712 patients was 4.3 ± 2.6 years (for a total of 3062 person-years). Forty-one patients (5.68%) died during the follow-up. Causes of death are detailed in the Supplemental Material. At univariate analyses, age, being married or unemployed, low education, smoking status, alcohol abuse, physical inactivity, BMI, total cholesterol and lower HDL, diabetes, AIDS diagnosis, Framingham risk score, low CD4 count, nadir CD4 cell, lipodystrophy, suboptimal adherence to HAART, HCV coinfection, neoplastic disease, depressive symptoms, and alexithymia were significantly associated with mortality (Table 2). The overall incidence of ACM was 1.32 per 100 person-years. The incidence rate of ACM was 6.2 (95% CI, 2.7–15.7) per 100 person-years in patients with alexithymia. In the Cox proportional hazard model, age (HR, 1.05; 95% CI, 1.0–1.08; P = .023), alcohol abuse (HR, 2.52; 95% CI, 1.09–5.81; P = .030), AIDS diagnosis (HR, 1.96; 95% CI, 0.98–3.93; P = .05), suboptimal HAART adherence (HR, 2.62; 95% CI, 1.14–6.0; P = .022), and alexithymia (HR, 4.93; 95% CI, 1.64–9.00; P = .002) were the only factors significantly associated with ACM (Table 2). The relationship between ACM and alexithymia is also apparent from the shape variation of the Kaplan-Meier estimates of time to mortality (Figure 1B).
DISCUSSION
This is the first study to examine the prospective association between alexithymia and incident CV events and ACM among a multisite cohort of well characterized HIV-positive patients. In this setting, we established that alexithymia predicts CV events and ACM independently of demographic and other psychosocial, viro-immunological, and CV risk factors. Case-control studies on global large populations have documented that psychosocial factors are independent predictors for CV and cerebrovascular disease [8]. In the context of psychological factors, alexithymia is emerging as a potential CV risk indicator, with several studies suggesting a relationship between alexithymia, CVD, and CV mortality in the general population [21, 22]. Indeed, several CV risk factors, such as sympathetic overreactivity [30], inflammation [31], diabetes [32], obesity/metabolic syndrome [33, 34], and atherosclerosis [16, 21], have been associated with a higher prevalence of alexithymia. A large prospective study on a cohort of over 2000 middle-aged persons showed that alexithymia is an independent predictor of both ACM and CV mortality [35, 36], with the risk of CV death increasing by 1.2% for each 1-point increase in Toronto Alexithymia Scale [22]. Consistently, in this multicentric cohort, alexithymic HIV patients had a higher prevalence of traditional CV risk factors at baseline compared with nonalexithymic HIV patients. Indeed, among alexithymics, we found a higher percentage of smokers and subjects with diabetes and less compliance to physical activity (Table 3). Nevertheless, when analyses were stratified by the presence of diabetes, alexithymia remained significantly and strongly associated with the presence of plaques, VEs, and ACM in nondiabetic subjects. Alexithymia also remained strongly associated with these 3 outcomes in the group of nonsmoking HIV alexithymic patients and in the group of alexithymic patients who performed regular physical activity.
Table 3.
Overall Characteristics of the Sample at Baseline Stratified According to the Presence of Alexithymia
| Variables | No Alexithymia N = 416 (64%) | Alexithymia N = 234 (36%) | P a |
|---|---|---|---|
| Sociodemographic Characteristics | |||
| Male gender, % | 75.7 | 75.2 | .8 |
| Mean age in years (SD) | 45.3 (9.87) | 47.8 (10) | .003 |
| Married, % | 33.3 | 34.9 | .6 |
| Low educational attainment (<13 years) | 37 | 50.3 | .003 |
| Stable employed, % | 72.2 | 53.1 | <.001 |
| Race/Ethnicity | .5 | ||
| White, % | 98.5 | 99.1 | |
| Black, %b | -- | -- | |
| Hispanic or Latino, % | 1.5 | 0.9 | |
| HIV Transmission Risk Factor | .027 | ||
| IDU, % | 22.9 | 30.9 | |
| MSM, % | 36.7 | 27.8 | |
| Heterosexual, % | 40.4 | 41.3 | |
| Lifestyle Factors | |||
| Smoke, % | 42.1 | 58.8 | <.001 |
| Alcohol abuse, % | 7.7 | 17 | <.001 |
| Regular physical activity, % | 37.5 | 20.2 | <.001 |
| Cardiovascular Risk Factors | |||
| Mean BMI (SD) | 23.9 (3.98) | 24.5 (4.02) | .09 |
| Mean total cholesterol, mg/dL (SD) | 195 (48.25) | 186 (50.38) | .036 |
| Mean HDL cholesterol, mg/dL (SD) | 44.9 (14.3) | 42.1 (12.55) | .017 |
| Mean LDL cholesterol, mg/dL (SD) | 119.2 (37.26) | 115.5 (40.55) | .2 |
| Hypertension, % | 21.9 | 24.4 | .4 |
| Fasting glucose, mg/dL (SD) | 87.9 (19.82) | 92.4 (23.87) | .013 |
| Diabetes, % | 6.7 | 13.9 | .003 |
| Mean carotid intima-media thickness in centimeters (SD) | .071 (.053) | .080 (.042) | .045 |
| Carotid atherosclerotic plaques, % | 20 | 55.6 | <.001 |
| Previous vascular events, % | 2.4 | 2.5 | .8 |
| Positive CVD family historyΨ, % | 51.8 | 37.2 | .004 |
| FRS 10-year general CVD risk, mean% (SD) | 8.3 (9.37) | 9.7 (8.74) | .09 |
| FRS 10-year general CVD risk stratification | .021 | ||
| FRS% of risk >20, % | 11 | 12.9 | |
| FRS% of risk 20–10, % | 18.7 | 27.6 | |
| FRS% of risk <10, % | 70.3 | 59.5 | |
| HIV-Related Variables | |||
| AIDS diagnosis, % | 33.3 | 33 | .4 |
| Nadir LyCD4, cell/mm3 (SD) | 247.5 (180.77) | 236.7 (197.66) | .4 |
| Baseline LyCD4 cell count <200 cells/mm3, % | 7.4 | 13.3 | .015 |
| Baseline LyCD4, cell/mm3 (SD) | 670.4 (364.56) | 592 (359.43) | .009 |
| Baseline HIV-RNA copies/mL (SD) | 11 779.4 (70 883.35) | 18 210.6 (92 237.13) | .3 |
| HIV-RNA <50 copies/mL, % | 79.5 | 73.2 | .07 |
| Mean duration of HIV infection in years (SD) | 12.8 (8.05) | 13.6 (8.24) | .2 |
| Treated with HAART, % | 91.8 | 89.3 | .3 |
| Mean duration of HAART in months (SD) | 103.8 (83.8) | 106.4 (87.3) | .7 |
| Lipodystrophy, % | 22.4 | 32.5 | .005 |
| Suboptimal adherence to HAART, % | 4.5 | 12.4 | .001 |
| Other Treatment | |||
| Antihypertensive medication, % | 18.7 | 20.5 | .5 |
| Antiplatelet/anticoagulant medication, % | 5 | 7 | .2 |
| Psychiatric medication, % | 12.2 | 22.4 | .001 |
| Comorbidities | |||
| HCV coinfection, % | 22.9 | 29.9 | .049 |
| Neoplastic disease, % | 11 | 11.7 | .7 |
| Psychological Factors | |||
| Depressive symptoms, % | 10.4 | 44 | <.001 |
| Distress personality (Type D),% Ψ | 29.3 | 55.5 | <.001 |
Abbreviations: AIDS, acquired immune deficiency syndrome; Alexithymia, Toronto Alexithymia Scale (TAS-20) >50; BMI, body mass index; CVD, cardiovascular disease; FRS, Framingham risk score; HAART, highly active antiretroviral therapy; HCV, high-density lipoprotein; HDL, high-density lipoprotein; HIV, human immunodeficiency virus; IDU, injection drug user; LDL, low-density lipoprotein; LyCD4, CD4 lymphocyte; MSM, men who have sex with men; RNA, ribonucleic acid; SD, standard deviation.
aχ 2 test for categorical variables; t test and Kruskal-Wallis test for normally distributed and nonnormally distributed continuous variables, respectively. All P values refer to the comparison between the subjects with and those without alexithymia.
bTen HIV-positive South African patients did not compile the Toronto Alexithymia Scale due to difficulties in understanding the Italian language.
To explain the strong association of alexithymia with CV outcomes and death in HIV, we can speculate that alexithymia may reflect a clustering of different risk factors (psychosocial and biological) that converges in a single patient with synergistic effects on health-related outcomes. Thus, the alexithymic component in people with HIV may be considered as a proxy for frailty, in that HIV-positive people with alexithymia tend to have a greater age, a higher percentage of smokers, and heavy alcohol drinkers and a higher percentage of individuals with diabetes, less compliance to physical activity, lower HDL, lower number of CD4 lymphocytes, a higher c-IMT, lower adherence to antiretroviral therapy, more frequent life-threatening habits that are more harmful for health, and marked psychopathological problems (Table 3).
It is worth noting that this is the first evaluation of the prevalence of alexithymia in an unselected multisite sample of HIV patients. We unraveled that HIV-infected patients have a higher prevalence of alexithymia compared with the general population, regardless of the TAS-20 threshold used. Indeed, the prevalence of severe alexithymia (TAS-20 >61) in our cohort was 15% against 9.9% of the general population [37], and the prevalence of both borderline and severe alexithymia (TAS-20 >50) was 36% versus 20.7% [21]. Moreover, among patients with carotid atherosclerosis, alexithymia was almost 3 times as frequent in HIV-positive subjects compared with a large seronegative population (23.8% vs 61.3%) [21]. The higher prevalence of alexithymia in people with HIV may suggest an effect of HIV on the central nervous system (CNS). Indeed, the alexithymic tract was associated at baseline with indicators of disease severity such as low CD4 T-cell counts (<200 cell/mm). The neurologic sequelae of HIV infection arise as a direct result of viral replication in the CNS as well as from the subsequent neuroinflammatory processes. Alexithymia, which is often associated with HIV, may reflect effects of the virus on brain areas such as the prefrontal and anterior cingulate cortex (ACC) [38]. In fact, it has previously been documented that lower CD4 is associated HIV-related ACC atrophy and decreased ability to recognize emotions [39]. Preliminary data on non-HIV people have documented in the alexithymics a decreased neuronal activity mediated by increased GABA concentration in ACC [40]. A remarkable property of this ACC tissue is that it has more extensive projections than any other cortical region to autonomic regulatory structures, consistent with stimulation studies showing effects of cingulate electrical stimulation on virtually all autonomic and many endocrine functions [41]. These mechanisms may mediate the role of alexithymia on central regulation of CV functioning. Moreover, in our population, the increased risk of alexithymics for adverse health outcomes can be mediated by higher likelihood of alexithymics to harmful lifestyle habits (in an attempt to compensate for the reduced capacity for affective self-regulation with external regulators) and by a suboptimal adherence to HAART. This aspect is likely to be the basis of a looping process in which all patients with alexithymic disease not exposing themselves to therapeutic HAART concentrations are more at risk of having neurocognitive sequelae with the further worsening of the alexithymic condition.
CONCLUSIONS
Some limitations should also be acknowledged. First, alexithymia was measured at only 1 time point, and changes in the TAS-20 score could not be captured on the total sample. However, data collected on a fraction (n = 105, 16.5%) of patients in our sample, who were retested for psychological traits after an average of 18 months since their first evaluation, revealed that alexithymic scores were only minimally modified (data not shown), indicating a remarkable stability of this psychological phenotype over time, in line with the main prospective studies on alexithymia [42, 43]. Second, information about abdominal obesity, diet, and previous smoking, variables potentially associated with CV outcomes, is missing. The major strengths of our study are the accurate consecutive multisite enrollment of the study sample, the contextual evaluation of both psychological traits and CPs, psychological traits being evaluated by a certified investigator, and the active follow-up for VEs and ACM, which allowed limiting data censoring to a minimum in the final Cox’s multivariate models. This result requires confirmation from studies with larger samples and longer follow-up. Indeed, further research is strongly justified: our results, if confirmed, can open the way for a new wave of preventive strategies targeting alexithymia for the control of CV disorders in the HIV population.
Supplementary Data
Supplementary materials are available at Open Forum Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Acknowledgments
We are greatly indebted to the nurses in the wards of the Infectious Disease Unit of Pescara, particularly Angela Pisciella and Teresa Moschiano, who helped with patients enrolled in the study, and the staff at the “Fondazione onlus Camillo De Lellis Per l’Innovazione e la Ricerca in Medicina,” Pescara, Italy for their invaluable and relentless help.
Author contributions. F. Va., F. Sa., and G. P. supervised the study. F. Va. and G. P. designed the study. F. Va., F. So., P. D. S., E. T., G. D. S., P. M., G. N., B. Z., V. L., F. V., C. B., E. P., and A. S. enrolled patients and collected data at the study sites. F. Va., F. So., P. D. S., and E. P. created and managed the database. F. Va., G. P., L. M., M. E. F., F. So., and F. Sa. analyzed and/or interpreted the data. L. M., M. E. F., G. P., and F. Va. acted as supervisor for data analysis and/or interpretation. F. Va., G. P., and F. Sa. drafted the initial manuscript. F. Va., G. P., F. Sa., G. M., G. D. S., P. M., L. M., M. E. F., and P. B. reviewed and/or revised the manuscript. F. Va., F. So., G. M., G. D. S., P. M., G. N., F. Vi., P. D. S., E. T., E. P., A. S., B. Z., V. L., C. B., M. E. F., P. B., F. So., L. M., and G. P. approved the final manuscript.
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Triant VA, Lee H, Hadigan C, Grinspoon SK. Increased acute myocardial infarction rates and cardiovascular risk factors among patients with human immunodeficiency virus disease. J Clin Endocrinol Metab 2007; 92:2506–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Obel N, Thomsen HF, Kronborg G, et al. . Ischemic heart disease in HIV-infected and HIV-uninfected individuals: a population-based cohort study. Clin Infect Dis 2007; 44:1625–31. [DOI] [PubMed] [Google Scholar]
- 3. Freiberg MS, Chang CC, Kuller LH, et al. . HIV infection and the risk of acute myocardial infarction. JAMA Intern Med 2013; 173:614–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Feinstein MJ, Bahiru E, Achenbach C, et al. . Patterns of cardiovascular mortality for HIV-infected adults in the United States: 1999 to 2013. Am J Cardiol 2016; 117:214–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Hanna DB, Ramaswamy C, Kaplan RC, et al. . Trends in cardiovascular disease mortality among persons with HIV in New York City, 2001–2012. Clin Infect Dis 2016; 63:1122–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Nou E, Lo J, Hadigan C, et al. . Pathophysiology and management of cardiovascular disease in HIV-infected patients. Lancet Diabetes Endocrinol 2016; 4:598–610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Nicholson A, Kuper H, Hemingway H. Depression as an aetiologic and prognostic factor in coronary heart disease: a meta-analysis of 6362 events among 146,538 participants in 54 observational studies. Eur Heart J 2006; 27:2763–74. [DOI] [PubMed] [Google Scholar]
- 8. Rosengren A, Hawken S, Ounpuu S, et al. . Association of psychosocial risk factors with risk of acute myocardial infarction in 11119 cases and 13648 controls from 52 countries (the INTERHEART study): case-control study. Lancet 2004; 364:953–62. [DOI] [PubMed] [Google Scholar]
- 9. Needham BL, Epel ES, Adler NE, et al. . Trajectories of change in obesity and symptoms of depression: the CARDIA study. Am J Public Health 2010; 100:1040–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kivimäki M, Steptoe A. Effects of stress on the development and progression of cardiovascular disease. Nat Rev Cardiol 2018; 15:215–29. [DOI] [PubMed] [Google Scholar]
- 11. Brindle RC, Ginty AT, Phillips AC, et al. . A tale of two mechanisms: a meta-analytic approach toward understanding the autonomic basis of cardiovascular reactivity to acute psychological stress. Psychophysiology 2014; 51:964–76. [DOI] [PubMed] [Google Scholar]
- 12. Kershaw KN, Lane-Cordova AD, Carnethon MR, et al. . Chronic stress and endothelial dysfunction: the multi-ethnic study of atherosclerosis (MESA). Am J Hypertens 2017; 30:75–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Aschbacher K, Roepke SK, von Känel R, et al. . Persistent versus transient depressive symptoms in relation to platelet hyperactivation: a longitudinal analysis of dementia caregivers. J Affect Disord 2009; 116:80–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Carney RM, Freedland KE, Veith RC. Depression, the autonomic nervous system, and coronary heart disease. Psychosom Med 2005; 67:29–33. [DOI] [PubMed] [Google Scholar]
- 15. Hare DL, Toukhsati SR, Johansson P, et al. . Depression and cardiovascular disease: a clinical review. Eur Heart J 2014; 35:1365–72. [DOI] [PubMed] [Google Scholar]
- 16. Parruti G, Vadini F, Sozio F. Psychological factors, including alexithymia, in the prediction of cardiovascular risk in HIV infected patients: results of a cohort study. PLoS One 2013; 8:e54555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ammassari A, Andreoni M, Angarano G, et al. Italian guidelines for the use of antiretroviral agents and the diagnostic -clinical management of HIV-1 infected persons Available at: http://www.salute.gov.it/imgs/C_17_pubblicazioni_2696_allegato.pdf. Accessed 24 March 2018. [PubMed]
- 18. Palmieri L, Rielli R, Dematte` L, et al. . CUORE project: implementation of the 10-year risk score. Eur J Cardiovasc Prev Rehabil 2011; 18:642–9. [DOI] [PubMed] [Google Scholar]
- 19. U.S. Department of Health and Human Services. 2008 Physical Activity Guidelines for the Americans Available at: https://health.gov/paguidelines/2008/pdf/paguide.pdf. Accessed 22 September 2008.
- 20.Baldissera N, Binkin N, De Mei B. et al. Abitudini di Consumo alcolico nelle ASL partecipanti al Sistema di Sorveglianza PASSI 2007. Not Ist Super Sanità 2008; 21:1–2. Article in Italian. Available at: https://www.epicentro.iss.it/passi/rapporto2010/R2010IndicatoriAlcol. Accessed January 2010.
- 21. Gabre HJ, Schwahn C, Barnow S. Alexithymia, hypertension, and subclinical atherosclerosis in the general population. J Psychosom Res 2010; 68:139–47. [DOI] [PubMed] [Google Scholar]
- 22. Tolmunen T, Lehto SM, Heliste M, et al. . Alexithymia is associated with increased cardiovascular mortality in middle-aged finnish men. Psychosom Med 2010; 72:187–91. [DOI] [PubMed] [Google Scholar]
- 23. Denollet J, Sys SU, Stroobant N, et al. . Personality as independent predictor of long-term mortality in patients with coronary heart disease. Lancet 1996; 347:417–21. [DOI] [PubMed] [Google Scholar]
- 24. Beck AT, Steer RA, Brown GK.. Manual for the Beck Depression Inventory-II. San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 25. Hobkirk AL, Starosta AJ, De Leo JA, et al. . Psychometric validation of the BDI-II among HIV-positive CHARTER study participants. Psychol Assess 2015; 27:457–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Denollet J. Type D personality. A potential risk factor refined. J Psychosom Res 2000; 49:255–66. [DOI] [PubMed] [Google Scholar]
- 27. Gremigni P, Sommaruga M. [Type D personality, a relevant construct in cardiology. Preliminary validation study of the Italian questionnaire]. Psicoterapia Cognitiva e Comportamentale 2005; 11:7–18. [Google Scholar]
- 28. Taylor GJ, Bagby RM, Parker JD. The alexithymia construct. A potential paradigm for psychosomatic medicine. Psychosomatics 1991; 32:153–64. [DOI] [PubMed] [Google Scholar]
- 29. Taylor GJ, Bagby RM, Parker JD. The 20-item Toronto alexithymia scale. IV. Reliability and factorial validity in different languages and cultures. J Psychosom Res 2003; 55:277–83. [DOI] [PubMed] [Google Scholar]
- 30. Bermond B, Bierman DJ, Cladder MA, et al. . The cognitive and affective alexithymia dimensions in the regulation of sympathetic responses. Int J Psychophysiol 2010; 75:227–33. [DOI] [PubMed] [Google Scholar]
- 31. Honkalampi K, Lehto SM, Koivumaa-Honkanen H, et al. . Alexithymia and tissue inflammation. Psychother Psychosom 2011; 80:359–64. [DOI] [PubMed] [Google Scholar]
- 32. Housiaux M, Luminet O, Van Broeck N, Dorchy H. Alexithymia is associated with glycaemic control of children with type 1 diabetes. Diabetes Metab 2010; 36:455–62. [DOI] [PubMed] [Google Scholar]
- 33. Melin EO, Svensson R, Thunander M, et al. . Gender, alexithymia and physical inactivity associated with abdominal obesity in type 1 diabetes mellitus: a cross sectional study at a secondary care hospital diabetes clinic. BMC Obes 2017; 2:4–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Karukivi M, Jula A, Hutri-Kähönen N, et al. . Is alexithymia associated with metabolic syndrome? A study in a healthy adult population. Psychiatry Res 2016; 236:58–63. [DOI] [PubMed] [Google Scholar]
- 35. Kauhanen J, Kaplan GA, Julkunen J, et al. . The association of alexithymia with all-cause mortality: prospective epidemiologic evidence. Psychosom Med 1994; 56:149. [Google Scholar]
- 36. Kauhanen J, Kaplan GA, Cohen RD, et al. . Alexithymia and risk of death in middle-aged men. J Psychosom Res 1996; 41:541–9. [DOI] [PubMed] [Google Scholar]
- 37. Mattila AK, Saarni SI, Salminen JK, et al. . Alexithymia and health-related quality of life in a general population. Psychosomatics 2009; 50:59–68. [DOI] [PubMed] [Google Scholar]
- 38. Bogdanova Y, Díaz-Santos M, Cronin-Golomb A. Neurocognitive correlates of alexithymia in asymptomatic individuals with HIV. Neuropsychologia 2010; 48:1295–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Clark US, Walker KA, Cohen RA, et al. . Facial emotion recognition impairments are associated with brain volume abnormalities in individuals with HIV. Neuropsychologia 2015; 70:263–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ernst J, Böker H, Hättenschwiler J, et al. . The association of interoceptive awareness and alexithymia with neurotransmitter concentrations in insula and anterior cingulate. Soc Cogn Affect Neurosci 2014; 9:857–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Pessoa L. Emergent processes in cognitive-emotional interactions. Dialogues Clin Neurosci 2010; 12:433–48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Tolmunen T, Heliste M, Lehto SM, et al. . Stability of alexithymia in the general population: an 11-year follow-up. Compr Psychiatry 2011; 52:536–41. [DOI] [PubMed] [Google Scholar]
- 43. Hiirola A, Pirkola S, Karukivi M, et al. . An evaluation of the absolute and relative stability of alexithymia over 11 years in a Finnish general population. J Psychosom Res 2017; 95:81–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.