Abstract
Therapy to enhance host immune defenses may improve outcomes in serious infections, especially for antibiotic-resistant pathogens. Recombinant human plasma gelsolin (rhu-pGSN), a normally circulating protein, has beneficial effects in diverse preclinical models of inflammation and injury. We evaluated delayed therapy (24–48 hours after challenge) with rhu-pGSN in a mouse model of pneumococcal pneumonia. rhu-pGSN without antibiotics increased survival and reduced morbidity and weight loss after infection with either penicillin-susceptible or penicillin-resistant pneumococci (serotypes 3 and 14, respectively). rhu-pGSN improves outcomes in a highly lethal pneumococcal pneumonia model when given after a clinically relevant delay, even in the setting of antimicrobial resistance.
Keywords: antibiotic-resistance, pneumonia, plasma gelsolin, immunomodulation
Recombinant human plasma gelsolin (rhu-pGSN) was tested as a host immunomodulator to improve outcomes in murine pneumococcal pneumonia. Delayed therapy with rhu-pGSN after infection increased survival and reduced morbidity.
Antibiotic-resistant pathogens represent an escalating threat to public health worldwide. A novel therapeutic strategy is to augment the innate immune response to combat infection, especially if the intervention can also dampen the harmful effects of overly exuberant inflammation. Plasma gelsolin (pGSN) potentially offers such a solution because of 2 attractive features: it has multiple mechanisms of action that can improve infectious outcomes irrespective of antimicrobial susceptibilities, and it is a normal human protein that has already passed many of the required toxicity, safety, and regulatory hurdles between the bench and the bedside.
Gelsolin is a highly conserved, multifunctional protein, initially described in the cytosol of macrophages and subsequently identified in many vertebrate cells [1, 2]. A unique property of gelsolin is that its gene expresses a splice variant coding for a distinct plasma isoform (pGSN), which is secreted into extracellular fluids and differs from its cytoplasm counterpart by expressing an additional sequence of 25 amino acids. pGSN normally circulates in mammalian blood at concentrations of 200–300 μg/mL, placing it among the most abundant plasma proteins.
One of pGSN’s several functions is to dissolve the actin gels (whence its name is derived) that result from cellular disruption to form the protective biofilm inhibiting access of cellular and humoral defenses to embedded pathogenic bacteria. This action sequesters pGSN at sites of organ damage. pGSN’s local tissue actin binding enables a second function, which is to localize inflammation. Actin competes with pGSN’s binding to and inactivation of multiple inflammatory mediators and microbial toxins, such as lysophosphatidic acid, sphingosine-1-phosphate, platelet-activating factor, fibronectin, endotoxin, and lipoteichoic acid. Locally, therefore, these mediators can function to promote host defense [3, 4]. Although pGSN’s local sequestration by exposed actin lowers circulating pGSN levels in proportion to the extent of tissue injury, pGSN’s abundance allows it to inactivate inflammatory mediators entering the systemic circulation, thereby preventing distant organ damage.
pGSN’s most recently elucidated function is its ability to enhance the uptake and killing of gram-positive and gram-negative bacteria by macrophages [5]. It promotes phagocytosis, by stripping actin off macrophage scavenger receptors, and killing, by stimulating the NOS3 synthetic system [5, 6].
The need to lower the level of free pGSN locally to combat infection and address injury can result in excessive systemic depletion and loss of its protective effects in severe infection or trauma, and a strong correlation exists between the magnitude of decline in the pGSN level and the likelihood of mortality. Consistent with these findings, systemic pGSN supplementation has abrogated pathological sequelae and mortality in multiple murine disease models [1, 7, 8].
Specifically regarding pneumonia, recombinant human pGSN (rhu-pGSN) administration improved survival in mouse models of primary or secondary (ie, postinfluenza) pneumococcal pneumonia, even in the absence of antibiotic treatment [5]. However, these experiments used rhu-pGSN pretreatment, which can establish proof of principle but is inconsistent with clinical circumstances. Hence, we evaluated effects of delayed treatment with rhu-pGSN on mortality and morbidity in murine pneumococcal pneumonia. To model potential use of pGSN supplementation in antibiotic-resistant infections, we used both antibiotic-susceptible and antibiotic-resistant pneumococcal strains and studied the effects of rhu-pGSN alone or in combination with antibiotic treatment.
METHODS
All protocols were approved by the Harvard Medical Area Biosafety and Animal Care and Use Committees.
Bacterial Strains and Culture
S. pneumoniae serotype 3 and 14 (catalog nos. 6303 and 700 677, respectively) were obtained from the American Type Culture Collection (Rockville, MD). Serotype 3 bacteria were cultured overnight on 5% sheep blood–supplemented agar in petri dishes (catalog no. 90001-282; VWR, West Chester, PA) and prepared and quantified as previously reported [5]. Serotype 14 required a more detailed protocol to achieve consistent results. We followed the growth protocol reported by Restrepo et al [9], which uses 2 sequential expansions in liquid broth culture before centrifugation and adjustment of bacterial concentration by OD600 for in vivo administration.
Mouse Models of Pneumococcal Pneumonia
Healthy 6– 8-week-old male CD1 mice were obtained from Charles River Laboratories (Wilmington, MA). Primary pneumococcal pneumonia was induced as previously reported [5]. For antibiotic-susceptible pneumonia, 1.5 × 106–2 × 106 colony-forming units (CFU) of Streptococcus pneumoniae type 3 was instilled intranasally into mice anesthetized with ketamine (72 mg/kg administered intraperitoneally) plus xylazine (9.6 mg/kg administered intraperitoneally). To model antibiotic-resistant pneumonia, we used Streptococcus pneumoniae type 14, which is resistant to penicillin (minimum inhibitory concentration, 8 μg/mL) and other antibiotics [10]. For this pathogen, range-finding experiments identified a highly lethal inoculum of approximately 300 × 106 colony-forming units (CFU), which was instilled into mice anesthetized as described above. Most trials used 10 mice per group for the vehicle, penicillin (PEN), pGSN, or penicillin plus pGSN (PEN + pGSN) groups.
Treatments and Outcomes
rhu-pGSN was synthesized in Escherichia coli and purified by Fujifilm Biosynth (Billingham, United Kingdom). rhu-pGSN was administered to mice by intraperitoneal injection at doses ranging from 5 to 10 mg as detailed in “Results.” In some experiments, penicillin (G Procaine Injectable Suspension [NDC 57319-485-05]; Phoenix Pharmaceuticals) was administered by intramuscular injection of 0.1–2 mg. We monitored the mice for 10 days, measuring survival, changes in weight, and overall morbidity by using a composite index (ie, 1 point each for hunched appearance, ruffled fur, or partly closed eyes, 1.5 points each for a prolapsed penis or splayed hind quarter, and 2 points for listlessness; the assessment could have a maximum score of 8 and was performed without blinding to treatment group) adapted from guidelines published elsewhere [11]. The weight and morbidity score for the last day each mouse was alive were carried forward for animals that died. To assess lung inflammation by quantifying neutrophil influx, one cohort of animals was euthanized and underwent lung lavage 48 hours following infection, as previously described [5, 12]. After centrifugation, resuspended lavage samples were counted by a hemocytometer, and differential cell counts were determined on Wright-Giemsa–stained cytocentrifuge preparations.
Statistical Analysis
Data were analyzed using Prism (GraphPad Software) or SAS (SAS Institute) software. Differences in Kaplan-Meier survival curves were analyzed using a log-rank test with the Sidak adjustment for multiple comparisons. For other measurements, differences between groups were examined by analysis of variance.
RESULTS
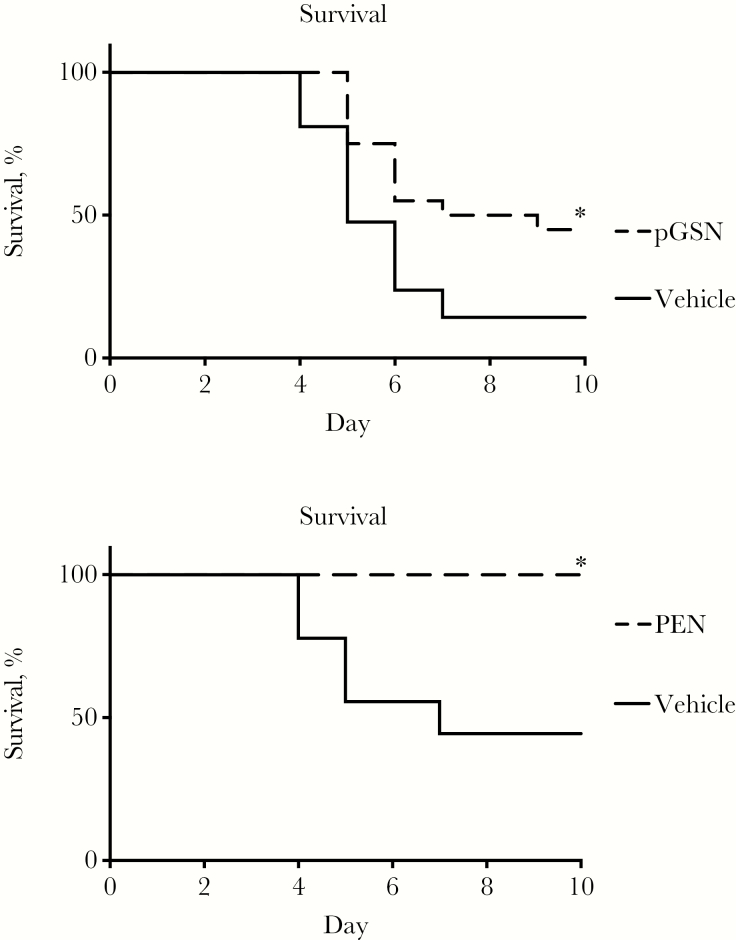
We tested delayed treatment with rhu-pGSN in the same murine model used previously to demonstrate improved survival with pretreatment [5]. As shown in Figure 1A, pGSN treatment given only on days 2 and 3 after infection with serotype 3 pneumococci led to substantially improved survival from a highly lethal inoculum as compared to vehicle controls, even in the absence of antibiotic treatment. To contrast with subsequent experiments using serotype 14, we confirmed that serotype 3 was highly susceptible to penicillin, as shown by 100% survival of mice treated with antibiotic (Figure 1B).
Figure 1.
Antibiotic-susceptible pneumococcal pneumonia. A, Treatment with plasma gelsolin (pGSN; 5 mg intraperitoneally on days 2 and 3 after infection) improves survival in mice infected with serotype 3 pneumococci. Data are a summary of 2 trials, with 10 mice per group per trial. *P = .01. B, Treatment with penicillin (PEN; 100 μg intramuscularly on days 2 and 3 after infection) improves survival in mice infected with serotype 3 pneumococci. Data are from a single trial, with 8–9 mice/group. *P = .02.
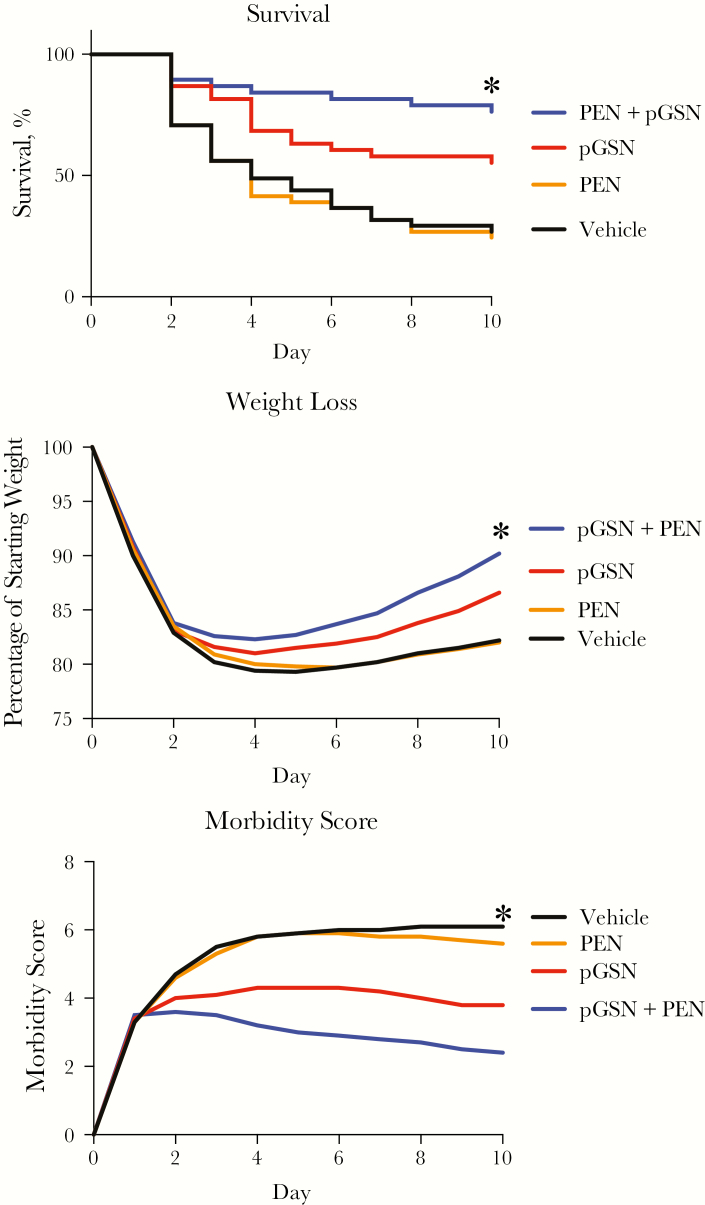
To determine whether these findings extended to antibiotic-resistant pneumonia, we developed a similar model using highly virulent serotype 14 pneumococci. Treatments were begun 24 hours after infection and continued daily for 9 days. Mice treated with only the diluent vehicle experienced high mortality (Figure 2A). Penicillin treatment alone had no benefit, consistent with the reported in vitro high-level resistance of this bacterial strain [10]. During the 24 hours before treatment, all mice experienced identical deterioration, evidenced by equivalent weight loss and morbidity scores. Neutrophil influx 48 hours after infection was decreased in animals treated with a single dose of pGSN with or without penicillin (mean total number [±SD] of neutrophils in lavage, 186 × 104 ± 54 × 104 in the vehicle group, 153 × 104 ± 74 × 104 in the PEN group, 111 × 104 ± 16 × 104 in the pGSN group, and 104 × 104 ± 20 × 104 in the PEN + pGSN group; P < .03; n = 5–6/group). rhu-pGSN treatment alone caused substantial improvement in overall survival, recovery from weight loss, and improvement in morbidity score (Figure 2A–C). In vitro, penicillin treatment alone or in combination with pGSN had no effect on bacterial growth (increase in bacterial CFU after 1 hour of culture, 88 000 for the vehicle group, 105 000 for the PEN [16 μg/mL] group, and 88 000 for the PEN + pGSN [250 μg/mL] group; 2 replicates on average). In vivo, treatment with a combination of penicillin and pGSN resulted in higher survival than with pGSN alone (Figure 2A), but the difference was not was not statistically different when adjusted for multiple comparisons (P = 0.47; Supplementary Materials). Results of all survival experiments are presented in the Supplementary Materials and show that, for each of the 9 experiments, survival was highest in the PEN + pGSN group, followed by the pGSN group, compared with either the PEN group or the vehicle group. In addition, survival for the PEN + pGSN group was significantly better than for the pGSN group.
Figure 2.
Antibiotic-resistant pneumococcal pneumonia. A, Treatment with plasma gelsolin (pGSN; 5 mg intraperitoneally daily, starting on day 1 after infection) improves survival in mice infected with serotype 14 pneumococci, compared with vehicle or penicillin (PEN; 1 mg intramuscularly daily; *P = .02 and .04, respectively, by the log-rank test after Sidak adjustment for multiple comparisons). Combined treatment with penicillin and pGSN (PEN + pGSN) also resulted in higher survival, compared with vehicle or penicillin (*P = .0001 for both comparisons, by the log-rank test after Sidak adjustment for multiple comparisons). Survival of the pGSN versus PEN + pGSN groups was not statistically significant after Sidak adjustment for multiple comparisons (P = .47). Data are a summary of 4 trials (n = 38–41 mice/group), with 8–11 mice/group per trial. See also the Supplementary Materials. B and C, Assessment of weight loss (B) and morbidity (C) showed more-rapid recovery of weight and a lower morbidity index in the pGSN and PEN + pGSN groups (compared to vehicle, *P = .05 for weight loss; P = .01 and .001 respectively for morbidity by analysis of variance). Mean values for each day are shown. Data are a summary of 4 trials (n = 38–41 mice/group; B) and 3 trials (n = 30 mice/group; C). The last observation for any mouse was carried forward for animals that died.
DISCUSSION
Benefits in animal models are frequently not confirmed in later clinical trials, likely because the experiments are configured to maximize the benefits of the intervention, rather than to mimic the circumstances experienced by patients. We sought to evaluate the potential of pGSN to improve outcomes following the more clinically relevant scenario of delaying administration until the mice had become visibly ill, rather than administering pretreatment or concurrent treatment, as in prior studies [5]. The key findings were that delayed pGSN treatment improved survival, either when used alone without an antibiotic or in combination with a suboptimal antibiotic to which the bacterial strain is highly resistant. The observed lowered bronchoalveolar neutrophil counts in infected pGSN-treated animals may reflect accelerated bacterial clearance by pGSN-stimulated resident macrophages, pGSN’s inflammation-modulating activity, or both.
Some limitations merit discussion. Our findings are from only 1 time point involving delayed treatment, using only 1 strain of antibiotic-resistant bacteria in a study of only 1 strain of 1 model species. With serotype 14, our ability to study longer delays before therapy was limited in pilot trials by the relatively high number of deaths by day 2 or 3 without treatment. This outcome translates into a need for an even higher number of mice at the start than what we established as an optimal maximum (ie, 40, in 4 groups of 10). Use of more mice in a single experiment would likely lead to increased variability due to operator fatigue and other factors, resulting in confounded, noisy results. This conundrum may be resolved in future studies that address some of the other concerns cited above. Specifically, it would be desirable to extend this proof-of-principle study to other antibiotic-resistant organisms in other model systems. Previous findings that pGSN enhances the microbicidal function of macrophages against other bacteria (eg, E. coli and Francisella tularensis live vaccine strain [5]) are encouraging in this regard, but direct testing is needed. Another potential limitation is that the assessments of weights and morbidity were not performed by observers blinded to treatment category, allowing for the possibility of observer bias.
The totality of the data suggests a synergistic interaction of pGSN with penicillin treatment that had no effects by itself. However, this conclusion relies on pooled analysis of all the range-finding as well as final trials performed. When only the final 4 replicate studies (Figure 2) are analyzed, the comparison is in the same direction but does not achieve statistical significance. Potentiation of penicillin effects on bacterial growth in vitro by concomitant rhu-pGSN was not observed. We speculate that antibacterial defenses enhanced by pGSN are even more effective against bacteria that are slightly perturbed (but not killed) by penicillin. The mechanism merits future attention, especially if similar results are observed in other infections with resistant bacteria.
In summary, rhu-pGSN can improve outcomes in a highly lethal pneumococcal pneumonia model when given after a clinically relevant delay, even in the setting of antimicrobial resistance. These findings support further evaluation of pGSN as an adjunctive therapy for serious antibiotic-resistant infections.
Supplementary Data
Supplementary materials are available at The Journal of Infectious Diseases online. Consisting of data provided by the authors to benefit the reader, the posted materials are not copyedited and are the sole responsibility of the authors, so questions or comments should be addressed to the corresponding author.
Notes
Acknowledgment. We thank Dr James Bolognese for consultation on statistical analysis.
Financial support. This work was supported by the National Institutes of Health (grant AI125152).
Potential conflicts of interest. S. L. and M. D. are employees and shareholders of BioAegis Therapeutics. T. S. is a founder of, shareholder of, and consultant to BioAegis Therapeutics. All other authors report no potential conflicts.
All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
Presented in part: IDWeek, San Diego, California, 4–8 October 2017.
References
- 1. Piktel E, Levental I, Durnas B, Janmey PA, Bucki R. Plasma gelsolin: indicator of inflammation and its potential as a diagnostic tool and therapeutic target. Int J Mol Sci 2018; 19:E2516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Silacci P, Mazzolai L, Gauci C, Stergiopulos N, Yin HL, Hayoz D. Gelsolin superfamily proteins: key regulators of cellular functions. Cell Mol Life Sci 2004; 61:2614–23. [DOI] [PubMed] [Google Scholar]
- 3. Bucki R, Kulakowska A, Byfield FJ, et al. Plasma gelsolin modulates cellular response to sphingosine 1-phosphate. Am J Physiol Cell Physiol 2010; 299:C1516–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Bucki R, Georges PC, Espinassous Q, et al. Inactivation of endotoxin by human plasma gelsolin. Biochemistry 2005; 44:9590–7. [DOI] [PubMed] [Google Scholar]
- 5. Yang Z, Chiou TT, Stossel TP, Kobzik L. Plasma gelsolin improves lung host defense against pneumonia by enhancing macrophage NOS3 function. Am J Physiol Lung Cell Mol Physiol 2015; 309:L11–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Ordija CM, Chiou TT, Yang Z, et al. Free actin impairs macrophage bacterial defenses via scavenger receptor MARCO interaction with reversal by plasma gelsolin. Am J Physiol Lung Cell Mol Physiol 2017; 312:L1018–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cohen TS, Bucki R, Byfield FJ, et al. Therapeutic potential of plasma gelsolin administration in a rat model of sepsis. Cytokine 2011; 54:235–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Christofidou-Solomidou M, Scherpereel A, Solomides CC, et al. Recombinant plasma gelsolin diminishes the acute inflammatory response to hyperoxia in mice. J Investig Med 2002; 50:54–60. [DOI] [PubMed] [Google Scholar]
- 9. Restrepo AV, Salazar BE, Agudelo M, Rodriguez CA, Zuluaga AF, Vesga O. Optimization of culture conditions to obtain maximal growth of penicillin-resistant Streptococcus pneumoniae. BMC Microbiol 2005; 5:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Jabes D, Nachman S, Tomasz A. Penicillin-binding protein families: evidence for the clonal nature of penicillin resistance in clinical isolates of pneumococci. J Infect Dis 1989; 159:16–25. [DOI] [PubMed] [Google Scholar]
- 11. Burkholder T, Foltz C, Karlsson E, Linton CG, Smith JM. Health evaluation of experimental laboratory mice. Curr Protoc Mouse Biol 2012; 2:145–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Yang Z, Huang YC, Koziel H, et al. Female resistance to pneumonia identifies lung macrophage nitric oxide synthase-3 as a therapeutic target. ELife 2014; 3:e03711. [DOI] [PMC free article] [PubMed] [Google Scholar]