Abstract
Background
Candidalysin is a cytolytic peptide toxin secreted by Candida albicans hyphae and has significantly advanced our understanding of fungal pathogenesis. Candidalysin is critical for mucosal C albicans infections and is known to activate epithelial cells to induce downstream innate immune responses that are associated with protection or immunopathology during oral or vaginal infections. Furthermore, candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. However, the role of candidalysin in driving systemic infections is unknown.
Methods
In this study, using candidalysin-producing and candidalysin-deficient C albicans strains, we show that candidalysin activates mitogen-activated protein kinase (MAPK) signaling and chemokine secretion in endothelial cells in vitro.
Results
Candidalysin induces immune activation and neutrophil recruitment in vivo, and it promotes mortality in zebrafish and murine models of systemic fungal infection.
Conclusions
The data demonstrate a key role for candidalysin in neutrophil recruitment and fungal virulence during disseminated systemic C albicans infections.
Keywords: Candida albicans, candidalysin, fungal, systemic, endothelial
Candidalysin is a cytolytic peptide toxin secreted by Candida albicans hyphae. Here we demonstrate a key role for candidalysin in activating endothelial cells, inducing cytokine secretion and neutrophil recruitment, and in promoting fungal virulence during disseminated systemic C albicans infections.
Fungal infections affect one quarter of the world’s population and kill ~1.5 million people each year, surpassing the numbers killed by either malaria, breast cancer, or prostate cancer [1]. One of the most medically important fungi is Candida albicans, which causes millions of mucosal infections and contributes to ~400 000 life-threatening systemic infections annually [1–3]. Despite these alarmingly high figures, it is unclear how C albicans drives mortality during these disseminated systemic infections.
During hematogenously disseminated candidiasis (HDC), C albicans must first cross the endothelial lining of the blood vessels at the site of infection [4]. Once in the bloodstream, C albicans is disseminated throughout the host where the fungus must again cross the endothelial lining before colonizing the major organs. As such, the endothelium functions as an integral component of the host defence system to recognize and respond to C albicans during systemic infections. One potential mechanism by which C albicans crosses the endothelial lining is via induced endocytosis, which is a hypha-dependent process [5]. Candida albicans hyphae are endocytosed by numerous human endothelial cells including umbilical, dermal microvascular, and brain microvascular endothelial cells [6–9]. Endothelial cells respond to C albicans hyphae via the activation of signaling pathways, including the stress-activated p38 mitogen-activated protein kinase (MAPK) pathway [10], and the secretion of proinflammatory mediators such as CXCL8 (interleukin [IL]-8), tumor necrosis factor (TNF)α, IL-1α, and IL-1β [10, 11]. Transcript profiling experiments indicated that overrepresented genes included those for neutrophil recruitment and signal transduction [10]. Hypha formation is critical for the initiation of these endothelial cell responses because hyphal-defective C albicans strains (such as cph1∆/efg1∆) were unable to induce strong proinflammatory responses [12]. Thus, endothelial cells respond to C albicans hyphae through the production of proinflammatory cytokines, which may facilitate clearance through active recruitment of immune cells to the site of infection. Likewise, hyphae are known to trigger pyroptosis and the NLRP3 inflammasome in mononuclear phagocytes [13, 14].
A key pathogenic trait of C albicans is its ability to induce cell damage. Candida albicans-driven damage of epithelial cells and macrophages is mediated by the peptide toxin, candidalysin [13, 15–18]. Candida albicans candidalysin is generated from its parent protein (Ece1p) via sequential enzymatic processing by Kex2/1 and secreted from hyphae [19]. Candidalysin is amphipathic, adopts an α-helical structure, and permeabilizes membranes, resulting in the release of lactate dehydrogenase [15], which is a characteristic of cell damage and membrane destabilization. Candidalysin activates epithelial proinflammatory responses via 2 MAPK signaling pathways (p38/c-Fos and MEK/ERK/MKP1) [20–23], which induce downstream immune responses including neutrophil recruitment and innate Type-17 immunity, which are critical for protection against oral and vaginal candidiasis [15, 16, 24–26]. Furthermore, candidalysin is the hyphal moiety that activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes [13].
Currently, the role of candidalysin in driving systemic C albicans infections is unknown. Given that expression of candidalysin is hypha-associated and induces damage, signaling and proinflammatory responses in epithelial cells [18, 27–30], and the NLRP3 inflammasome in macrophages [13], we investigated whether candidalysin drives endothelial cell activation, immune cell recruitment, and mortality during systemic infections. We found that candidalysin activates MAPK signaling and cytokine responses in endothelial cells and promotes neutrophil recruitment and mortality during zebrafish and murine models of systemic C albicans infection.
METHODS
Candida albicans Strains
Candida albicans strains used in this study are BWP17+CIp30 (wild-type), ece1Δ/Δ (ECE1-deficient), ece1Δ/Δ+ECE1 (ECE1 revertant), and ece1Δ/Δ+ECE1Δ 184–279 (candidalysin-deficient) as previously described [15].
Endothelial Cell Culture and Candida albicans Infection
Human adult dermal microvascular endothelial cells ([HDMECs] PromoCell GmbH) were cultured in supplemented MV endothelial cell growth medium (PromoCell GmbH). The immortalized human microvascular endothelial cell line, HMEC-1 (provided by Ades et al [31]), were cultured as described [32]. Cells were incubated at 37°C in 5% CO2 and transferred to antibiotic and hydrocortisone-free medium. The HDMECs or HMEC-1 (1 × 106) were cultured for 24 hours and then transferred to serum and antibiotic-free media (SFM) for 18 hours. Candida albicans strains were cultured in yeast peptone dextrose (YPD) broth (Oxoid) overnight at 25°C, washed with phosphate-buffered saline (PBS), and resuspended in SFM. For signaling experiments, C albicans was added to the endothelial cells in SFM (multiplicity of infection [MOI] of 10) and incubated for 2 hours, otherwise endothelial cells were incubated with an MOI of 0.1 for 24 hours; in both instances, infections were carried out at 37°C in 5% CO2. Conditioned medium from endothelial cells was collected, centrifuged, and stored at −20°C.
Cell Lysate Preparation and Immunoblotting
The HMEC-1 cells were washed with ice-cold PBS, lysed with 100 μL ice-cold Radio-Immunoprecipitation Assay (Sigma-Aldrich) buffer containing phosphatase and protease inhibitors (Pierce) on ice for 15 minutes, and then centrifuged at 10 000 ×g at 4°C for 10 minutes. Total protein concentration was analyzed by bicinchoninic acid assay (Pierce). Forty micrograms of total protein of each sample was separated by 10% sodium dodecyl sulfate polyacrylamide gel electrophoresis, proteins were transferred to a nitrocellulose membrane (iBlot; Invitrogen), and immunoblot analysis was performed as previously described [15]. Membranes were incubated with primary antibody: rabbit anti-phospho-MEK1/2 (clone 41G9), anti-phospho-p44/42 (ERK1/2) (clone D13.14.4E), anti-phospho-c-Jun (clone 54B3), anti-c-Fos (clone 9F6) (Cell Signaling Technology) at 4°C overnight, followed by horse radish peroxidase-conjugated secondary antibody and developed by enhanced chemiluminescence. β-actin was used as the housekeeping gene (Sigma).
Lactate Dehydrogenase Release and Cytokine and Chemokine Analysis
Endothelial cell damage was analyzed by measuring the release of lactate dehydrogenase (LDH) into the culture medium using a CytoTox96 assay kit (Promega). The concentration of CXCL8 within conditioned medium was measured by CXCL8 enzyme-linked immunosorbent assay ([ELISA]; BD Biosciences). For murine studies, kidney homogenates were clarified by centrifugation, and the concentrations of cytokines and chemokines were measured using a multiplex bead array (R&D Systems) as previously described [33]. Myeloperoxidase (MPO) protein levels in kidney homogenates were determined using ELISA, following the manufacturer’s instructions (Hycult Biotech).
Zebrafish Candida albicans Infection Model
Zebrafish maintenance and experimental work was performed in accordance with UK Home Office regulations and the UK Animals (Scientific Procedures) Act 1986. London wild-type zebrafish larvae were maintained as previously described [34]. For infection, methylcellulose-embedded, tricaine-anesthetized dechorionated larvae were injected systemically by direct inoculation into the common cardinal vein at 2 days postfertilization with 500 colony-forming units of C albicans in PBS or PBS alone as control (n = 20 zebrafish injected per treatment group). Zebrafish viability was assessed by presence of a heartbeat at 24 hours postinfection.
Mouse Model of Hematogenously Disseminated Candidiasis
Animal work was approved by the Institutional Animal Care and Use Committee of the Los Angeles Biomedical Research Institute. Virulence of the C albicans strains was tested in the mouse model of HDC using 6- to 7-week-old male and female BALB/c mice (Taconics) as previously described [35]. In some mice, leukopenia was induced by intravenous administration of 5 mg of 5-fluorouracil (Western Medical) on day −1 relative to infection. Enrofloxacin (Victor Medical) was added to the drinking water of these mice to prevent bacterial infection. Candida albicans strains were serially passaged 3 times in YPD broth, grown at 30°C at 200 rpm for 16–24 hours at each passage. Yeast cells were washed in PBS, renumerated, and injected intravenously via the lateral tail vein. Immunocompetent and leukopenic mice were infected by injecting 2 × 105 and 5 × 104C albicans cells, respectively. Each C albicans strain was inoculated into 10 mice (5 male/5 female). Animals were randomly assigned to the different groups. Researchers were not blinded to the experimental groups because the endpoints (survival, fungal burden, cytokine levels) were objective measures of disease severity. For survival experiments, mice were monitored twice daily and moribund mice were humanely euthanized. To determine organ fungal burden, mice were sacrificed after 1 day and 4 days of infection, after which the kidneys, brain, spleen, and liver were harvested, weighed, homogenized, and quantified on YPD. For histology, mouse kidneys were fixed in 10% buffered formalin and embedded in paraffin. Thin sections were cut and stained with Grocott-Gomori methenamine silver stain and imaged by light microscopy.
Statistics
Data are presented as mean values ± standard deviation of at least 3 independent experiments with each test performed in triplicate unless otherwise stated. Mann-Whitney, log rank, or analysis of variance multiple statistical comparisons were performed with Tukey’s post hoc comparison using GraphPad Prism, version 6.00. Differences between test and control groups were considered significant when P < .05.
RESULTS
Candidalysin Damages and Induces Immune Activation in Endothelial Cells
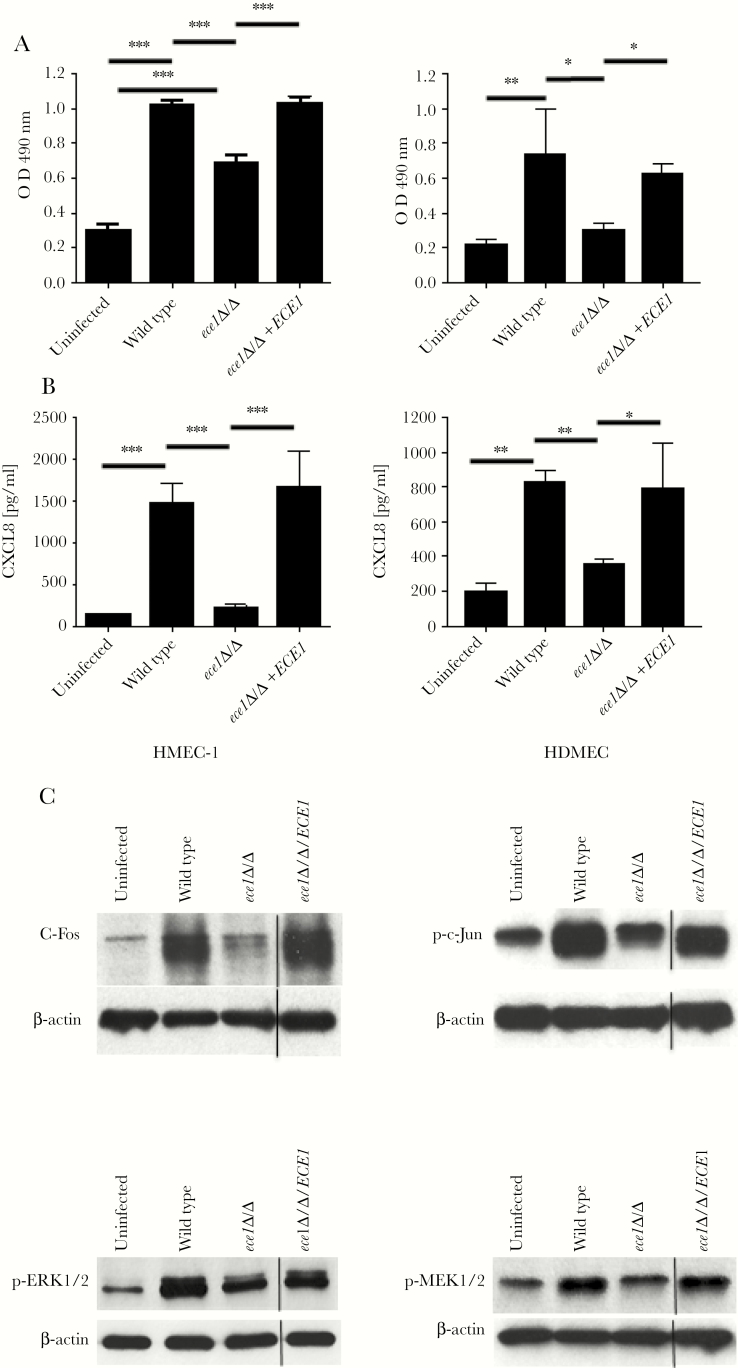
One mechanism by which candidalysin may facilitate C albicans translocation across the endothelial cell lining to initiate disseminated infection is via the induction of damage. Therefore, we determined whether candidalysin was able to induce LDH release in 2 endothelial cell types. The HDMEC and HMEC-1 cells were incubated with candidalysin-producing (BWP17+CIp30 [wild-type], ece1Δ/Δ+ECE1) and candidalysin-deficient (ece1Δ/Δ; encoding candidalysin) C albicans strains, and LDH release was measured after 24 hours. Only candidalysin-producing C albicans strains were able to induce LDH release from both endothelial cell types (Figure 1A), indicating that candidalysin induces damage. However, in the HMEC-1 cell line, the ece1Δ/Δ mutant was still able to induce a modest amount of damage.
Figure 1.
Candidalysin induces cell damage, cytokine release, and intracellular signaling in human endothelial cells. Cell damage (A) and CXCL8 (B) chemokine release quantified by lactate dehydrogenase assay and enzyme-linked immunosorbent assay, respectively, for human microvascular endothelial cell line (HMEC-1) and human adult dermal microvascular endothelial cells (HDMECs) after exposure to wild-type, ece1Δ/Δ , or ece1Δ/Δ+ECE1 Candida albicans (multiplicity of infection [MOI] of 0.1) for 24 hours; medium alone was used as uninfected control. Data are presented as mean ± standard deviation (n = 3); statistical analysis was achieved using one-way analysis of variance with Tukey’s post hoc multiple comparison test. *, P < .05; **, P < .01; and ***, P < .001. (C) Immunoblot analysis of HMEC-1 intracellular signaling responses to wild-type, ece1Δ/Δ, ece1Δ/Δ+ECE1 C albicans (MOI of 10) or control for 2 hours. The HMEC-1 cell lysates (40 μg of total protein) were probed with c-Fos, phospho-c-Jun, phospho-ERK1/2, or phospho-MEK1/2, and β-actin was used as a loading control.
Candidalysin induces cytokine release in epithelial cells and macrophages [13, 15–19, 24, 28, 30]. Therefore, we tested whether candidalysin could also induce cytokine release in HDMEC and HMEC-1 cells. CXCL8 was used as a marker for immune activation because this chemokine is released from endothelial cells in response to C albicans [10, 11]. We found that only candidalysin-producing C albicans strains were able to induce CXCL8 release (Figure 1B), demonstrating that candidalysin is a critical activator of immune responses in endothelial cells.
Epithelial cells respond to candidalysin activity via the induction of MAPK signaling. Two key proteins that are activated are the AP-1 transcription factor c-Fos (via p38) and the MAPK phosphatase MKP1 (via MEK1/2-ERK1/2) [20–23]. To determine whether candidalysin induces MAPK signaling in endothelial cells, we incubated HMEC-1 cells with C albicans strains and tested for c-Fos upregulation and phosphorylation of MEK1/2 and ERK1/2 at 2 hours (previously determined as the optimum time [20]). We additionally tested for the phosphorylation of another AP-1 transcription factor, c-Jun, because this can be activated by JNK (third arm of the MAPK pathway) and can form a heterodimer with c-Fos to activate gene expression. Only candidalysin-producing C albicans strains strongly induced c-Fos, phospho-c-Jun, phospho-MEK1/2, and phospho-ERK1/2 in HMEC-1 cells (Figure 1C), indicating that candidalysin is critical for MAPK activation in endothelial cells.
Candidalysin Is Required for Virulence in a Zebrafish Model of Candida albicans Systemic Infection
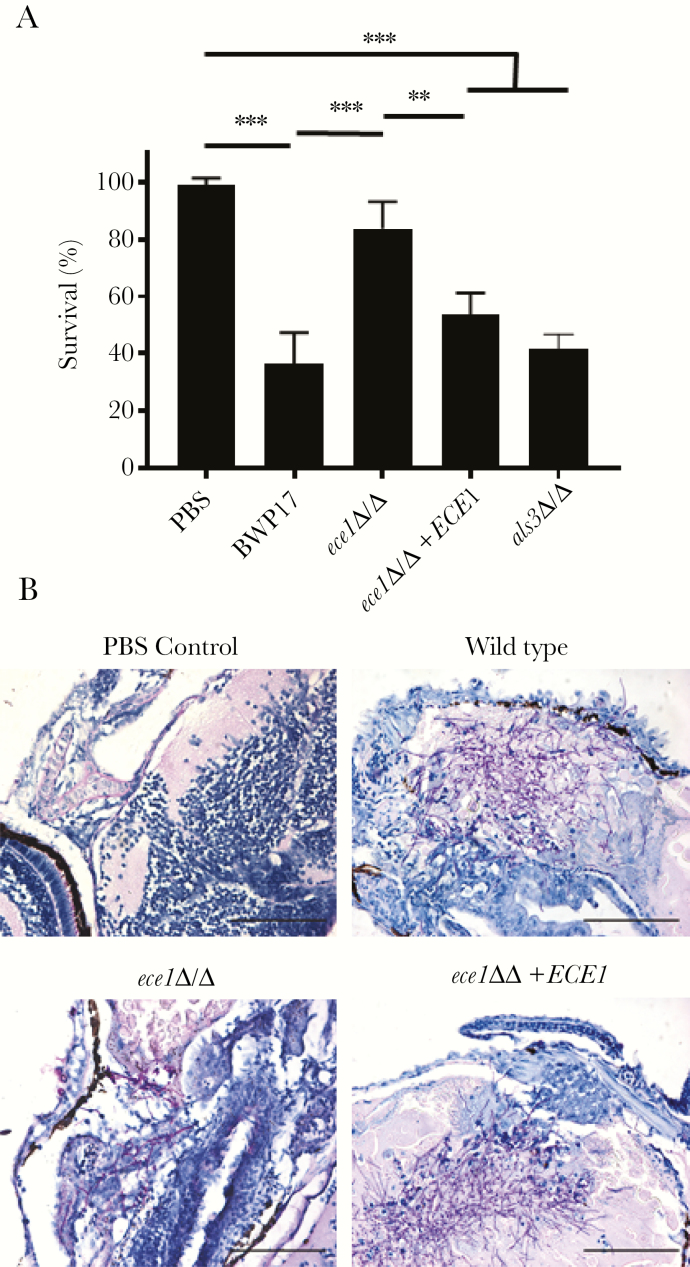
The ability of candidalysin to damage and induce MAPK-mediated immune responses in endothelial cells may enhance the ability of C albicans to translocate across the endothelial lining and promote systemic infection, similar to C albicans translocation across epithelial layers [17]. Furthermore, these activities may be associated with protective or detrimental immune responses [16, 24]. To determine whether candidalysin was required for systemic infection, we first utilized a zebrafish model to screen the virulence potential of the C albicans strains. Zebrafish larvae were infected with C albicans strains in the common cardinal vein, and survival was monitored for 24 hours. Although ~35% of fish survived when infected with candidalysin-producing strains, significantly more (~80%) fish survived when infected with a candidalysin-deficient (ece1Δ/Δ) strain (Figure 2A). Histological analysis showed extensive hyphal network formation in fish infected with the candidalysin-producing strains along with widespread tissue destruction and edema. In contrast, in fish infected with the candidalysin-deficient strain, fewer hyphae were present with limited associated tissue damage (Figure 2B). In a control experiment, a C albicans strain deficient in the hypha-associated adhesin/invasion ALS3 (als3 Δ/Δ) was equally as virulent as the wild-type (Figure 2A). The data indicate that candidalysin promotes infection and a deleterious phenotype in this zebrafish systemic model.
Figure 2.
Systemic infection of zebrafish larvae with ECE1-deficient Candida albicans show improved survival compared with wild-type or ece1Δ/Δ+ECE1-infected zebrafish. (A) Zebrafish survival 24 hours after injection of 500 colony-forming unit of wild-type, ece1Δ/Δ, ece1Δ/Δ+ECE1, als3Δ/Δ or phosphate-buffered saline (PBS) as control directly into the cardinal vein. Data are presented as mean ± standard deviation (n = 4 independent experiments with at least 20 zebrafish per treatment group); statistical analysis was achieved using one-way analysis of variance with Tukey’s post hoc multiple comparison test. **, P < .01; and ***, P < .001. (B) Sagittal histological sections of periodic acid-Schiff-stained infected zebrafish showing the hyphal form of C albicans penetrating zebrafish tissue for wild-type, ece1Δ/Δ and ece1Δ/Δ+ECE1 strains compared with PBS-injected controls. Scale bars = 100 μm.
Candidalysin Is Required for Neutrophil Recruitment and Virulence in a Murine Model of Disseminated Candida albicans Infection
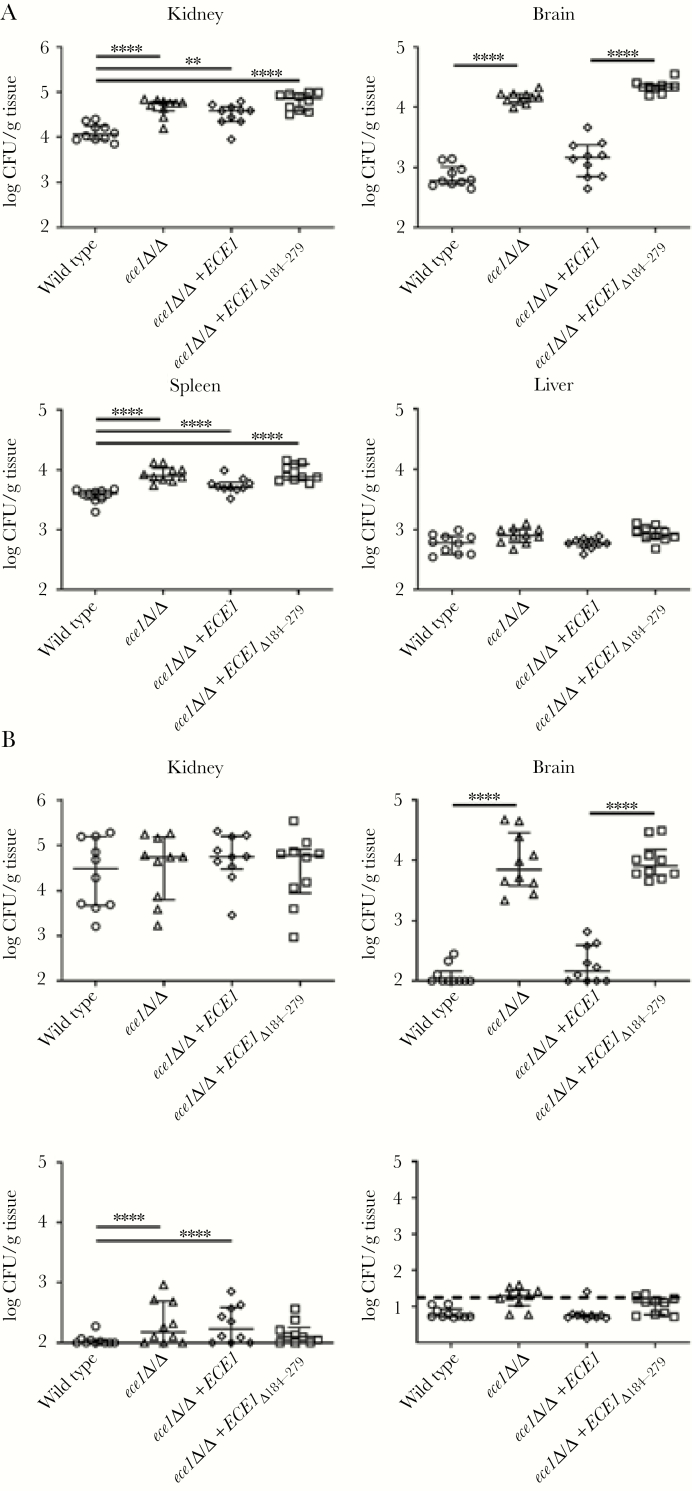
The zebrafish screen demonstrated the importance of candidalysin in promoting C albicans-mediated infection. Therefore, we progressed to determine whether candidalysin was also required for systemic infection in a murine model of disseminated candidiasis. For these experiments, we included an additional C albicans strain deficient solely in the candidalysin-encoding region of ECE1 (ece1Δ/Δ+ECE1Δ184–279). Animals were infected with C albicans strains, and fungal burden, tissue invasion, cytokine production, and survival were monitored over time. Fungal burdens were monitored at day 1 and 4 postinfection in the spleen, brain, kidney, and liver (Figure 3). Also of note, at day 1, there was a significant increase in burdens in the spleen, brain, and kidney of animals infected with both candidalysin-deficient C albicans strains (ece1Δ/Δ, ece1Δ/Δ+ECE1Δ184–279) in comparison with the wild-type strain (BWP17+CIp30). A significant increase in burdens was also observed in the spleen and kidney of animals infected with the ECE1 revertant strain (ece1Δ/Δ+ECE1). By day 4, there were no differences in burdens between the C albicans strains, except for in the brain, where burdens remained high in animals infected with candidalysin-deficient C albicans strains (2 logs greater than wild-type). There was no difference in burdens between C albicans strains on day 1 or 4 in the liver.
Figure 3.
Absence of candidalysin increases organ-specific tropism during hematogenously disseminated candidiasis. Fungal burden of the spleen, brain, kidney, and liver of mice at 1 day (A) and 4 days (B) postinfection after inoculation with 2 × 105Candida albicans cells of the indicated strains. Results are median ± interquartile range of 2 independent experiments with 10 mice per strain at each time point. Statistical significance is indicated by **, P < .01 and ****, P < .0001 (Mann-Whitney test with Bonferroni correction for multiple comparisons). Dashed line indicates limit of detection in the liver. CFU, colony-forming units.
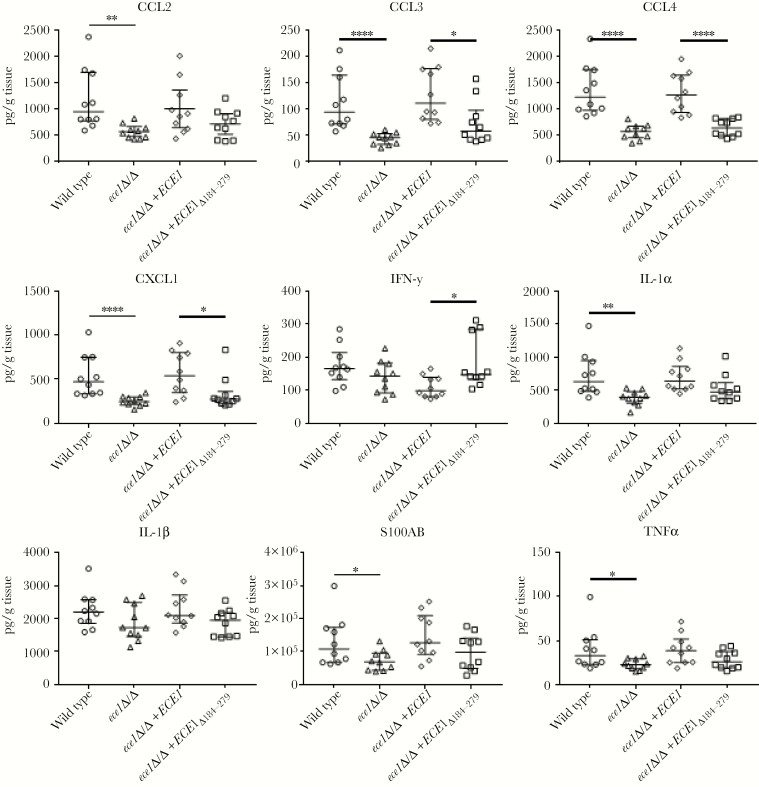
Given that the main differences in fungal burdens between the C albicans strains was at day 1, we assessed the proinflammatory response in kidney homogenates at this time point (Figure 4). In comparison to wild-type C albicans, the ECE1-deficient strain (ece1Δ/Δ) induced significantly decreased levels of CCL2, CCL3, CCL4, CXCL1, IL-1α, S100A8, and TNFα, which was mirrored by the candidalysin-deficient strain (ece1Δ/Δ+ECE1Δ184–279) for CCL3, CCL4, and CXCL1. However, although not always statistically significant, there was a clear trend of reduced secretion of all cytokines, except interferon-γ, with ece1Δ/Δ+ECE1Δ184–279. Also of note, all cytokine responses recovered to wild-type levels with the ece1Δ/Δ+ECE1 revertant strain. The data indicate that candidalysin is a key driver of proinflammatory responses during systemic infection.
Figure 4.
Candidalysin is required to induce an early antifungal immune response in the kidney. Level of chemokines and cytokines in kidney homogenates of immunocompetent mice with hematogenously disseminated candidiasis after 1 day of infection with 2 × 105Candida albicans cells of the indicated strains. Results are median ± interquartile range of 2 independent experiments with 10 mice per strain. Statistical significance is indicated by *, P < .05, **, P < .01, and ****, P < .0001 (Mann-Whitney test with Bonferroni correction for multiple comparisons).
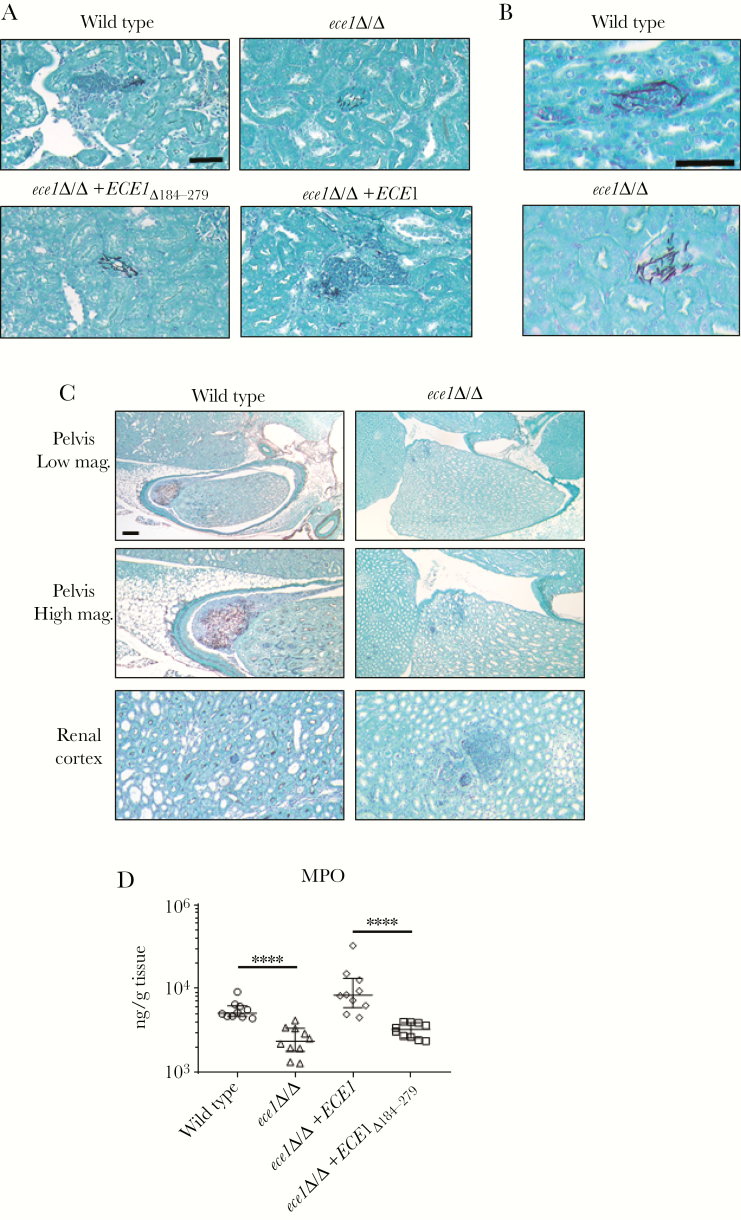
The key role of candidalysin in damaging and inducing neutrophil-recruiting chemokines (eg, CXCL1, CXCL8) in mice and human endothelial cells prompted us to analyze the invasion and neutrophil recruitment capabilities of the C albicans strains by histology. All C albicans strains were able to form hyphae and invaded the kidney at day 1 postinfection (Figure 5A and B). However, only the candidalysin-producing strains induced strong neutrophil accumulation to the infection site. At later time points during hematogenously disseminated candidiasis, inflammatory processes spread to the renal tubules and pelvis with marked neutrophil accumulation, which can mediate tissue damage [36, 37]. Therefore, we compared the ability of the wild-type and ece1Δ/Δ C albicans strains to extend to the pelvis region of the kidney and cause renal pelvis injury at day 4 postinfection (Figure 5C) when similar fungal burdens were observed (Figure 3B). Only wild-type C albicans was able to invade the pelvis region, with concomitant neutrophil accumulation and associated local immunopathology, whereas ece1Δ/Δ was unable to invade the pelvis region and remained in the renal cortex (Figure 5C). The increased ability of candidalysin-producing C albicans strains to recruit neutrophils was confirmed by the increased production of MPO in kidney homogenates (Figure 5D). The data indicate that during systemic infection, candidalysin promotes C albicans invasion and is a potent mediator of neutrophil recruitment to the infection site.
Figure 5.
Candidalysin is required for renal neutrophil recruitment and immunopathology. (A and B) Histopathology of the kidney of mice after 1 day of infection with 2 × 105Candida albicans cells of the indicated strains. Sections (A, low magnification; B, high magnification) were stained with Gomori methenamine silver (GMS). Fungal cells are filamentous and stained black, and polymorphonuclear (PMN) cells are round and dark purple. Scale bar = 50 µm. (C) Histopathology of the pelvis region (top and middle) and renal cortex (bottom) of the mouse kidney 4 days postinfection with 2 × 105C albicans cells of the indicated strains. Sections were stained with GMS. Fungal cells are filamentous and stained black, and PMN cells are round and purple surrounding fungal filaments. Only wild-type C albicans invades the pelvis region with local immunopathology, whereas the ece1Δ/Δ remains in the renal cortex. Scale bar = 200 µm. (D) Myeloperoxidase (MPO) expression in kidney homogenates after 1 day of infection with 2 × 105C albicans cells of the indicated strains. Results are median ± interquartile range of 2 independent experiments with 10 mice per strain. Statistical significance is indicated by ****, P < .0001 (Mann-Whitney test with Bonferroni correction for multiple comparisons).
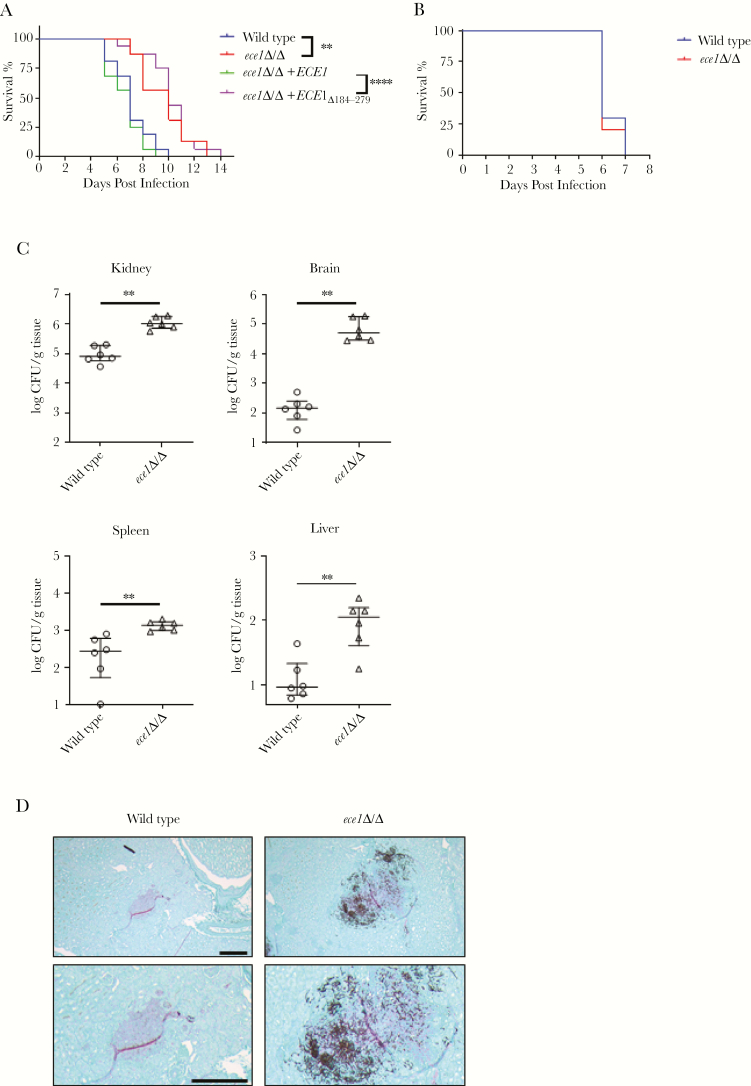
The differences in the capacity of candidalysin-producing and -deficient C albicans strains to induce cytokines and neutrophil accumulation suggest that candidalysin plays a key role in driving systemic infection. Therefore, we undertook survival experiments and confirmed that candidalysin-producing strains were more pathogenic than candidalysin-deficient strains. Animals infected with candidalysin-producing strains all succumbed by day 10 (25% survival at day 7), whereas animals infected with candidalysin-deficient strains succumbed by day 14 (100% survival at day 7) (Figure 6A). Given the important function of candidalysin in mediating neutrophil recruitment, we next depleted animals of neutrophils using 5-fluorouracil and assessed the virulence capacity of the wild-type and ece1Δ/Δ C albicans strains. Under these conditions, both C albicans strains became equally virulent and all mice succumbed to infection by day 7 (Figure 6B), despite fungal burdens being significantly higher in all organs infected with C albicans ece1Δ/Δ (Figure 6C). As previously shown, C albicans ece1Δ/Δ was unable to invade the pelvis region and remained in the renal cortex (Figure 6D). These data, taken together, suggest that candidalysin production during systemic infections induces local proinflammatory and chemotactic responses, which function to recruit neutrophils to the infection site, thereby promoting pathogenicity and disease.
Figure 6.
Candidalysin-induced immunopathology is required for virulence during hematogenously disseminated candidiasis. (A) Survival of immunocompetent mice after intravenous inoculation with 2 × 105 yeast phase cells of the indicated strains of Candida albicans (n = 10). (B) Survival of leukopenic mice infected with 5 × 104 wild-type or ece1Δ/Δ C albicans yeast. Statistical significance is indicated by **, P < .01 (wild-type vs ece1Δ/Δ) and ****, P < .0001 (ece1Δ/Δ+ECE1 vs ece1Δ/Δ+ECE1Δ184–279) (Wilcoxon rank-sum test). (C) Fungal burden of the kidney, brain, spleen, and liver of leukopenic mice at 4 days postinfection after inoculation with 5 × 104 wild-type or ece1Δ/Δ C albicans yeast. Results are median ± interquartile range with 6 mice per strain. Statistical significance is indicated by **, P < .01 (Mann-Whitney test). (D) Histopathology of the pelvis region (top, lower magnification; bottom, higher magnification) of the kidney of leukopenic mice after 4 days of infection with 5 × 104C albicans cells of the indicated strains. Sections were stained with Gomori methenamine silver. Scale bars = 200 µm. CFU, colony-forming units.
DISCUSSION
The discovery of candidalysin, the first peptide toxin identified in any human fungal pathogen, has provided a significant advance in our understanding of C albicans pathogenesis [15, 18, 27–30]. Candidalysin is produced by hyphae and is critically important for promoting mucosal C albicans infections [15–17, 19, 24, 25]. Although candidalysin activates the inflammasome in macrophages [13], its role in promoting systemic C albicans infection was hitherto unknown. In this study, we demonstrate that candidalysin promotes virulence during systemic C albicans infections with a key role in mediating neutrophil accumulation in infected renal tissue.
We first investigated the ability of candidalysin to damage and activate endothelial cells, which may facilitate C albicans translocation across the blood vessel lining to establish a hematogenous disseminated infection. Candidalysin damaged endothelial cells and induced MAPK signaling via MEK1/2 and ERK1/2, resulting in the activation of the AP-1 transcription factors, c-Fos and c-jun, and the secretion of the neutrophil-recruiting chemokine CXCL8. Although candidalysin activates similar MAPK/c-Fos-mediated pathways in oral [15, 19, 24] and vaginal [16] epithelial cells, the induction of the MAPK/c-jun pathway appears specific to endothelial cells. Given that CXCL8 expression is mediated by c-jun/AP-1 in endothelial cells [38], the data indicate that candidalysin activates c-jun/AP-1 to upregulate CXCL8 secretion. Therefore, although both endothelial and epithelial cells respond to candidalysin in a similar fashion, some cell-specific differences are observable. However, we note that these experiments were undertaken in vitro and acknowledge that evidence for a direct effect of candidalysin on endothelial cell activation events in vivo is limited from these studies.
In previous studies, 2 other fungal proteins, Ssa1p and Als3p, were shown to mediate C albicans endocytosis in endothelial cells via N-cadherin [6, 7, 39]. Although immune activation mechanisms by Ssa1 has not been investigated, Als3p does not induce MAPK/c-Fos signaling or cytokine release in oral epithelial cells [40] and is dispensable for virulence in murine [41] and zebrafish (this work) models of disseminated candidiasis. Therefore, although C albicans possesses multiple hypha-associated factors to promote virulence, candidalysin appears to be the critical factor inducing endothelial cell damage and proinflammatory cytokines that recruit and activate phagocytes, such as neutrophils.
In both zebrafish and murine infection models, candidalysin-producing strains were significantly more lethal than candidalysin-deficient C albicans strains. The increase in mortality directly correlated with immune activation, because only candidalysin-producing strains induced strong cytokine/chemokine secretion, particularly those associated with immune cell (notably neutrophil) recruitment (CCL2/3/4, CXCL1, S100A8). Histological and ELISA (MPO) analyses confirmed that only candidalysin-producing strains were able to induce prominent neutrophil accumulation to the kidney at day 1 postinfection, at a time point when neutrophils are protective [42, 43]. In agreement with the early protective effect of neutrophils, mice infected with wild-type C albicans had reduced fungal burdens at this time point. In contrast, neutrophils exert detrimental effects in this model at later stages of infection, and part of this immunopathogenic response is driven by the CCL3-CCR1 chemokine axis [37, 44]. Also of note, only wild-type C albicans invaded the pelvic region of the kidney at day 4, which correlated with significant neutrophil accumulation and associated tissue injury, despite the presence of similar fungal burdens in the mouse kidneys infected with the ECE1-deficient C albicans strain. Instead, infection with candidalysin-deficient strains resulted in decreased CCL3 levels, the ligand for CCR1, and decreased renal pelvis damage and neutrophil-mediated immunopathology. Given that candidalysin is known to promote C albicans translocation across the gut barrier [17], the data support the notion that candidalysin may also facilitate C albicans invasion through parenchymal organs during systemic infections. Our data correlate (1) with previous data in the systemic candidiasis model indicating that CCR1-mediated neutrophil recruitment at later infection time points can be detrimental [37, 44] and (2) with mucosal infection studies demonstrating that neutrophil recruitment to the site of infection is candidalysin-dependent [15, 16, 24].
The dual role of candidalysin in promoting infection while concurrently inducing neutrophil responses was confirmed in neutrophil-depletion experiments, where both candidalysin-producing and -deficient C albicans strains became equally virulent. It is interesting to note that the significantly higher fungal burdens in all organs of leukopenic animals infected with C albicans ece1Δ/Δ suggest that candidalysin activity and immune cell activation, but not fungal burdens, are the critical factors in determining morbidity and mortality during systemic infections. The findings are clinically relevant because neutropenic patients are highly susceptible to disseminated candidiasis [3, 45]. As such, the data inextricably link (1) candidalysin activity with C albicans pathogenicity and (2) neutrophil activation with disease outcome.
As mentioned above, it is intriguing that mortality and immune activation did not correlate with increased fungal burdens. This was most notable in the brain, where significantly more fungal burdens were observed with candidalysin-deficient strains. These striking differences in fungal burdens were recently found to be due to candidalysin-dependent induction of IL-1β and CXCL1 secretion from CARD9+ microglial cells, which function to recruit CXCR2-expressing neutrophils to the brain to control the infection and hence reduce fungal burdens [46]. This finding, together with the fact that candidalysin-deficient strains produce normal hyphae [15], may challenge the long-held view that C albicans pathogenicity in systemic infections directly correlates with increased fungal burdens and hypha formation. Indeed, in certain tissues (ie, brain), the data appear to uncouple pathogenicity from hypha formation and correlate pathogenicity with the ability of C albicans to damage and induce strong local immune responses, predominantly through candidalysin activity and neutrophil recruitment.
CONCLUSIONS
The data, taken together, suggest that during disseminated candidiasis, candidalysin production by C albicans hyphae stimulates a strong proinflammatory response with neutrophil recruitment, which reduces fungal burdens during early infection but later hastens mortality likely related to immunopathogenic effects. In contrast, in the absence of candidalysin, there is a reduced inflammatory response with a lack of neutrophil recruitment, which promotes C albicans proliferation during early infection but also prolongs survival, enabling the fungus to traffic to and survive in the brain. In summary, the data demonstrate that candidalysin production during disseminated systemic infections promotes virulence, neutrophil recruitment, and disease and may identify candidalysin-associated damage and immune activation pathways as novel targets to combat disseminated fungal infections.
Notes
Financial support. This work was funded by grants from the Wellcome Trust (214229_Z_18_Z), Biotechnology and Biological Sciences Research Council (BB/N014677/1), National Institutes of Health (NIH) (R37-DE022550), King’s Health Partners Challenge Fund (R170501), and the NIH Research at Guys and St. Thomas’s NHS Foundation Trust and the King’s College London Biomedical Research Centre (IS-BRC-1215–20006; to J. R. N.); National Institutes of Health Grants K99DE026856 (to M. S.) and R01DE022600 and R01AI124566 (to S. G. F.). This work was also funded in part by the Division of Intramural Research of the National Institute of Allergy and Infectious Diseases, NIH (to M. S. L.) and the Deutsche Forschungsgemeinschaft under Germany’s Excellence Strategy (EXC 22167-390884018 and the CRC/TR 124 FungiNet project C1; to B. H.).
Potential conflicts of interest. All authors: No reported conflicts of interest. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest.
References
- 1. Brown GD, Denning DW, Gow NA, Levitz SM, Netea MG, White TC. Hidden killers: human fungal infections. Sci Trans Med 2012; 4:165rv13. [DOI] [PubMed] [Google Scholar]
- 2. Brown GD, Denning DW, Levitz SM. Tackling human fungal infections. Science 2012; 336:647. [DOI] [PubMed] [Google Scholar]
- 3. Pappas PG, Lionakis MS, Arendrup MC, Ostrosky-Zeichner L, Kullberg BJ. Invasive candidiasis. Nat Rev Dis Primers 2018; 4:18026. [DOI] [PubMed] [Google Scholar]
- 4. Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Candida albicans-endothelial cell interactions: a key step in the pathogenesis of systemic candidiasis. Infect Immun 2008; 76:4370–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Filler SG, Sheppard DC. Fungal invasion of normally non-phagocytic host cells. PLoS Pathog 2006; 2:e129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Phan QT, Myers CL, Fu Y, et al. Als3 is a Candida albicans invasin that binds to cadherins and induces endocytosis by host cells. PLoS Biol 2007; 5:e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Sun JN, Solis NV, Phan QT, et al. Host cell invasion and virulence mediated by Candida albicans Ssa1. PLoS Pathog 2010; 6:e1001181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Liu Y, Filler SG. Candida albicans Als3, a multifunctional adhesin and invasin. Eukaryot Cell 2011; 10:168–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Seidl K, Solis NV, Bayer AS, et al. Divergent responses of different endothelial cell types to infection with Candida albicans and Staphylococcus aureus. PLoS One 2012; 7:e39633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Müller V, Viemann D, Schmidt M, et al. Candida albicans triggers activation of distinct signaling pathways to establish a proinflammatory gene expression program in primary human endothelial cells. J Immunol 2007; 179:8435–45. [DOI] [PubMed] [Google Scholar]
- 11. Filler SG, Pfunder AS, Spellberg BJ, Spellberg JP, Edwards JE Jr. Candida albicans stimulates cytokine production and leukocyte adhesion molecule expression by endothelial cells. Infect Immun 1996; 64:2609–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barker KS, Park H, Phan QT, et al. Transcriptome profile of the vascular endothelial cell response to Candida albicans. J Infect Dis 2008; 198:193–202. [DOI] [PubMed] [Google Scholar]
- 13. Kasper L, König A, Koenig PA, et al. The fungal peptide toxin Candidalysin activates the NLRP3 inflammasome and causes cytolysis in mononuclear phagocytes. Nat Commun 2018; 9:4260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Krysan DJ, Sutterwala FS, Wellington M. Catching fire: Candida albicans, macrophages, and pyroptosis. PLoS Pathog 2014; 10:e1004139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Moyes DL, Wilson D, Richardson JP, et al. Candidalysin is a fungal peptide toxin critical for mucosal infection. Nature 2016; 532:64–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Richardson JP, Willems HME, Moyes DL, et al. Candidalysin drives epithelial signaling, neutrophil recruitment, and immunopathology at the vaginal mucosa. Infect Immun 2018; 86:e00645–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Allert S, Forster TM, Svensson CM, et al. Candida albicans-induced epithelial damage mediates translocation through intestinal barriers. mBio 2018; 9:e00915–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wilson D, Naglik JR, Hube B. The missing link between Candida albicans hyphal morphogenesis and host cell damage. PLoS Pathog 2016; 12:e1005867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Richardson JP, Mogavero S, Moyes DL, et al. Processing of Candida albicans Ece1p is critical for candidalysin maturation and fungal virulence. mBio 2018; 9:e02178–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Moyes DL, Runglall M, Murciano C, et al. A biphasic innate immune MAPK response discriminates between the yeast and hyphal forms of Candida albicans in epithelial cells. Cell Host Microbe 2010; 8:225–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moyes DL, Murciano C, Runglall M, Islam A, Thavaraj S, Naglik JR. Candida albicans yeast and hyphae are discriminated by MAPK signaling in vaginal epithelial cells. PLoS One 2011; 6:e26580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Moyes DL, Murciano C, Runglall M, Kohli A, Islam A, Naglik JR. Activation of MAPK/c-Fos induced responses in oral epithelial cells is specific to Candida albicans and Candida dubliniensis hyphae. Med Microbiol Immunol 2012; 201:93–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Murciano C, Moyes DL, Runglall M, et al. Candida albicans cell wall glycosylation may be indirectly required for activation of epithelial cell proinflammatory responses. Infect Immun 2011; 79:4902–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Verma AH, Richardson JP, Zhou C, et al. Oral epithelial cells orchestrate innate type 17 responses to Candida albicans through the virulence factor Candidalysin. Sci Immunol 2017; 2:eaam8834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Verma AH, Zafar H, Ponde NO, et al. IL-36 and IL-1/IL-17 drive immunity to oral candidiasis via parallel mechanisms. J Immunol 2018; 201:627–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Gladiator A, Wangler N, Trautwein-Weidner K, LeibundGut-Landmann S. Cutting edge: IL-17-secreting innate lymphoid cells are essential for host defense against fungal infection. J Immunol 2013; 190:521–5. [DOI] [PubMed] [Google Scholar]
- 27. Tang SX, Moyes DL, Richardson JP, Blagojevic M, Naglik JR. Epithelial discrimination of commensal and pathogenic Candida albicans. Oral Dis 2016; 22(Suppl 1):114–9. [DOI] [PubMed] [Google Scholar]
- 28. Naglik JR, König A, Hube B, Gaffen SL. Candida albicans-epithelial interactions and induction of mucosal innate immunity. Curr Opin Microbiol 2017; 40:104–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Richardson JP, Ho J, Naglik JR. Candida-epithelial interactions. J Fungi 2018; 4:22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Richardson JP, Moyes DL, Ho J, Naglik JR. Candida innate immunity at the mucosa. Semin Cell Dev Biol 2019; 89:58–70. [DOI] [PubMed] [Google Scholar]
- 31. Ades EW, Candal FJ, Swerlick RA, et al. HMEC-1: establishment of an immortalized human microvascular endothelial cell line. J Invest Dermatol 1992; 99:683–90. [DOI] [PubMed] [Google Scholar]
- 32. Grubb SE, Murdoch C, Sudbery PE, Saville SP, Lopez-Ribot JL, Thornhill MH. Adhesion of Candida albicans to endothelial cells under physiological conditions of flow. Infect Immun 2009; 77:3872–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Swidergall M, Solis NV, Lionakis MS, Filler SG. EphA2 is an epithelial cell pattern recognition receptor for fungal β-glucans. Nat Microbiol 2018; 3:53–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Widziolek M, Prajsnar TK, Tazzyman S, Stafford GP, Potempa J, Murdoch C. Zebrafish as a new model to study effects of periodontal pathogens on cardiovascular diseases. Sci Rep 2016; 6:36023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Sanchez AA, Johnston DA, Myers C, Edwards JE Jr, Mitchell AP, Filler SG. Relationship between Candida albicans virulence during experimental hematogenously disseminated infection and endothelial cell damage in vitro. Infect Immun 2004; 72:598–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lionakis MS, Lim JK, Lee CC, Murphy PM. Organ-specific innate immune responses in a mouse model of invasive candidiasis. J Innate Immun 2011; 3:180–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lionakis MS, Fischer BG, Lim JK, et al. Chemokine receptor Ccr1 drives neutrophil-mediated kidney immunopathology and mortality in invasive candidiasis. PLoS Pathog 2012; 8:e1002865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Umebashi K, Tokito A, Yamamoto M, Jougasaki M. Interleukin-33 induces interleukin-8 expression via JNK/c-Jun/AP-1 pathway in human umbilical vein endothelial cells. PLoS One 2018; 13:e0191659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Phan QT, Fratti RA, Prasadarao NV, Edwards JE Jr, Filler SG. N-cadherin mediates endocytosis of Candida albicans by endothelial cells. J Biol Chem 2005; 280:10455–61. [DOI] [PubMed] [Google Scholar]
- 40. Murciano C, Moyes DL, Runglall M, et al. Evaluation of the role of Candida albicans agglutinin-like sequence (Als) proteins in human oral epithelial cell interactions. PLoS One 2012; 7:e33362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Cleary IA, Reinhard SM, Miller CL, et al. Candida albicans adhesin Als3p is dispensable for virulence in the mouse model of disseminated candidiasis. Microbiology 2011; 157:1806–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Lionakis MS, Levitz SM. Host control of fungal infections: lessons from basic studies and human cohorts. Annu Rev Immunol 2018; 36:157–91. [DOI] [PubMed] [Google Scholar]
- 43. Romani L, Mencacci A, Cenci E, Del Sero G, Bistoni F, Puccetti P. An immunoregulatory role for neutrophils in CD4+ T helper subset selection in mice with candidiasis. J Immunol 1997; 158:2356–62. [PubMed] [Google Scholar]
- 44. Lionakis MS, Albert ND, Swamydas M, Lee CR, Loetscher P, Kontoyiannis DP. Pharmacological blockade of the chemokine receptor CCR1 protects mice from systemic candidiasis of hematogenous origin. Antimicrob Agents Chemother 2017; 61:e02365–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Swerdloff JN, Filler SG, Edwards JE Jr. Severe candidal infections in neutropenic patients. Clin Infect Dis 1993; 17(Suppl 2):S457–67. [DOI] [PubMed] [Google Scholar]
- 46. Drummond RA, Swamydas M, Oikonomou V, et al. CARD9+ microglia promote antifungal immunity via IL-1β- and CXCL1-mediated neutrophil recruitment. Nat Immunol 2019; 20:559–70. [DOI] [PMC free article] [PubMed] [Google Scholar]