Abstract
The present study examined associations between effortful control, a trait marker of self-regulation, adaptive HPA system functioning (as reflected by the CAR), and concurrent and longitudinal depressive problems, in a sample of preadolescent Latino youth (N = 119, mean age = 11.53 years, 59% female). We hypothesized that trait readiness for self-regulation (e.g., effortful control) could be related to physiological state readiness for self-regulation (e.g., CAR), and that both may counter depressive problems. We found that youth’s CAR was positively associated with effortful control, and negatively with youth depressive problems. Effortful control and youth depressive problems were also negatively associated. Longitudinal relations of CAR and effortful control on depressive problems at T2 were not significant in the structural equation model after controlling for T1 depressive problems, although these variables were significant in the bivariate correlations. Results suggest that both trait-regulation and physiological regulation may counter depressive problems in Latino youth.
Keywords: Cortisol Awakening Response (CAR), depressive problems, effortful control, Latinos, adolescents
As youth transition into adolescence, they often face challenges and stressors related to increasing responsibilities and independence, which in turn can be associated with poor mental health and other adjustment problems (Call et al., 2002). Latino youth may be more vulnerable to such problems as they are disproportionately exposed to additional stressors such as poverty, discrimination, racism, and acculturation difficulties, and have higher levels of poor mental health than other ethnic groups (Cauce, Cruz, Corona, & Conger, 2011). The Centers for Disease Control and Prevention (2012) reported that Latino youth were more likely to feel sad or hopeless (32.6%), to seriously consider suicide (16.7%), and to attempt suicide (10.2%) than Caucasian (27.2%, 15.5%, and 6.2%,) and African American (24.7%, 13.2%, and 8.3%) youth.
Such challenges may arise as a function of stressors “getting under the skin” by means of disrupting physiological systems related to stress responding and adaptation (Bendezú, Perzow, & Wadsworth, 2016; Bendezú & Wadsworth, 2018). The hypothalamic-pituitary-adrenal (HPA) axis is one of these physiological systems. Stressors can disrupt HPA axis activity by affecting secretion and regulation of cortisol, the body’s primary stress hormone that is released in response to stressors. Cortisol both regulates, and is regulated by, the HPA axis through a negative feedback loop (Adam, 2006), for which the brain is a primary target organ (Uchoa et al., 2014). Cortisol follows a diurnal rhythm under basal (non-stressed) conditions, but may be altered under conditions of acute or chronic stress (Fries, Dettenborn, & Kirschbaum, 2009).
Researchers have demonstrated that Latino youth exhibit markers of cortisol dysregulation such as flatter diurnal slopes (Cauce et al., 2011; DeSantis et al., 2007; Hajat et al., 2010; Kwak et al., 2017; Martin, Bruce, & Fisher, 2012; Zeiders, Doane, & Roosa, 2012) and lower reactivity (Hostinar, McQuillan, Mirous, Grant, & Adam, 2014); patterns potentially indicative of chronic stress experiences (Miller, Chen, & Zhou, 2007). As a consequence of allostatic load, or the wear and tear on the body and brain that are associated with carrying chronic stressors (Juster, McEwen, & Lupien, 2010), cortisol dysregulation may contribute to the documented health disparities that are evident in Latino youth populations, including depressive problems (Berger & Sarnyai, 2015; Susman, 2007).
In contrast, markers of cortisol regulation including diurnal cortisol rhythms characterized by a steeper slope of decline from morning to evening; a higher morning level; a lower evening level; and a lower average volume (Miller et al., 2007) reflect a well-regulated HPA axis (Bai & Repetti, 2015). Furthermore, embedded within the diurnal rhythm is a distinct morning rhythm known as the Cortisol Awakening Response (CAR; Pruessner et al., 1997), characterized by a rise in basal cortisol levels in response to awakening that peak 30–45 minutes after wake-up and decline thereafter (Stalder et al., 2016). The CAR is a useful indicator of normative HPA axis functioning and capacity for stress-responding due to its unique functionality as a reactivity index with circadian timing (Stalder et al., 2016). A well-functioning HPA axis may potentially act as a physiological resource for coping with stressors (Bai & Repetti, 2015; Obradović, 2012), and thus have positive associations with health and adjustment in adolescents, although researchers have less commonly tested such associations (McEwen, Gray, & Nasca, 2015).
One area of potential interest involves the relations between HPA-axis functioning and markers of self-regulation such as effortful control. Self-regulation is the ongoing, dynamic, and adaptive modulation of internal state or behavior of and by oneself that is mediated by central and peripheral physiology (Nigg, 2017). Self-regulation has been implicated in how individuals pursue goals, engage in social interactions, and navigate the environment; and has particular importance to the development of depression (Nolen-Hoeksema, Gilbert, & Hilt, 2015). It could be the case that trait readiness for self-regulation (e.g., effortful control) is related to physiological state readiness for self-regulation (e.g., CAR), and that both may counter depressive problems. To test this hypothesis, the present study examined the associations between effortful control, a trait marker of self-regulation, adaptive HPA axis functioning (as reflected by the CAR), and concurrent and longitudinal depressive problems, in a sample of preadolescent Latino youth.
The Role of Self-Regulation
Effortful Control and Mental Health in Adolescents
Self-regulation includes processes used to change one’s own emotional state and behaviors; to prevent, initiate, or augment emotion responding; to modify the significance of an event for the self; and to modulate the behavioral expression of emotion (Eisenberg, 2015; Nigg, 2017). Effortful control is a key facet of self-regulation, and involves individual differences in shifting and focusing attention as well as modulating emotion and behavior (Rothbart & Bates, 2006). Individuals with high levels of effortful control are expected to have an advantage in regard to adapting effectively in stressful situations (Eisenberg & Valiente, 2004), whereas poor effortful control is associated with social and behavioral problems (King, Lengua, & Monahan, 2013). Although effortful control is most often conceptualized as biologically based, it is increasingly evident that it continues to develop during adolescence (Lengua, 2006; Shiner, Allen, & Masten, 2017) and can be modified by environmental factors (King et al., 2013; Laceulle, Nederhof, Karreman, Ormel, & Aken, 2012).
The importance of self-regulatory processes, including effortful control, for mental health are clear (Nigg, 2017). However, research on effortful control and mental health has largely been conducted with younger children despite evidence that EC may become a stronger predictor of internalizing problems during adolescence (Eisenberg, 2015; King et al., 2013). Effortful control is more often linked with externalizing behaviors in adolescence (i.e., Atherton, Tackett, Ferrer, & Robins, 2017; Eisenberg, 2015; Pérez-Edgar, 2015; Wang, Eisenberg, Valiente, & Spinrad, 2016), but researchers have found relations between internalizing problems and EC in cross-critical for future research onal (Dyson, Robertson, & Wong, 2015; Muris, Meesters, & Blijlevens, 2007; Ormel et al., 2005; Taylor, Jones, Anaya, & Evich, 2017; Yap et al., 2011) and longitudinal studies (Hilt, Armstrong, & Essex, 2012; van Oort, Greaves‐Lord, Ormel, Verhulst, & Huizink, 2011). Youth with higher effortful control may be less likely to become over-aroused when experiencing stress (Eisenberg, 2015; Wang et al., 2016), and effortful control has been found to positively predict youths active coping across time (Taylor, Widaman, & Robins, 2018). Therefore higher effortful control may serve to protect adolescents against internalizing problems. However, relations between effortful control and mental health in Latino adolescents have been less commonly assessed (for exceptions see Loukas & Roalson, 2006; Taylor et al., 2017; Taylor et al., 2018; Valiente, Lemery-Chalfant, & Swanson, 2009).
Effortful Control and Physiological Functioning
Relations between effortful control and physiological markers of stress, such as cortisol, have largely been assessed in early childhood, when it has been assumed that dysregulation of the HPA axis disrupts development of effortful control (e.g. Blair et al., 2011; Lengua, Zalewski, Fisher, & Moran, 2013; Sturge-Apple, Davies, Cicchetti, Hentges, & Coe, 2016; Zalewski et al., 2012). For example, Zalewski and colleagues (2012) reported that blunted diurnal cortisol was related to preschoolers’ lower levels of effortful control six months later, and Sturge-Apple and colleagues (2016) found that children with relatively higher basal morning cortisol levels at ages two and three, had lower effortful control at age four. Low morning cortisol has been associated with smaller relative increases in effortful control across multiple time points (Lengua et al., 2013), as well as classroom regulation difficulties (Lisonbee, Pendry, Mize, & Gwynn, 2010) in preschool age children.
However, other researchers have hypothesized that trait factors such as effortful control can shape group differences in children’s HPA responses to stress (Davis, Bruce, & Gunnar, 2002; Gunnar, Sebanc, Tout, Donzella, & van Dulmen, 2003; Mayer, Abelson, & Lopez-Duran, 2014). For example, Mayer and colleagues (2014) found that lower effortful control at ages 3 and 6 was associated with a greater cortisol response, (steeper reactivity slopes), in the context of a frustration stressor at age 7 and with a flatter blunted response in a fear context. Gunnar and colleagues (2003) also reported that lower effortful control was associated with cortisol dysregulation (in the form of elevated basal levels). Other researchers have argued that coping efforts, or effortful emotional, cognitive and behavioral attempts to manage stress, mitigate stress-linked physiological dysregulation (Bendezú & Wadsworth, 2017).
Associations between effortful control and indices of cortisol dysregulation in adolescents have rarely been addressed, despite calls for integrating contextual and biological components into the study of adolescent health (Granger et al., 2012). Preadolescence is an important developmental period for studying the effects of stress exposure on stress response physiology due to rapid advancements in cognitive development also occurring during this time (Bendezú & Wadsworth, 2017). Although effortful control is assumed to be fairly stable by adolescence, studies have indicated that chronic stress can lead to declines in effortful control across time (e.g., Lengua, 2006). Researchers have found evidence of associations between effortful control, or other types of regulatory skills, and stress physiology. For example, Oldehinkel and colleagues (2011) reported that effortful control was associated with blunted cortisol responses to a stress task. Other researchers have linked emotion-regulation, another aspect of self-regulation that refers to the adjustment of emotional state or expression to meet goals or to maintain homeostatic or allostatic state (Nigg, 2017), and cortisol responses (i.e., Hilt, Sladek, & Stroud, 2017; Kliewer et al., 2016; Poon, Turpyn, Hansen, Jacangelo, & Chaplin, 2016). For example, Kliewer (2016) found that adolescents with lower levels of emotion-regulation had an attenuated cortisol response to a reactivity task, and Poon and colleagues (2016) found that youth with both high physiological reactivity as well as high emotion regulation problems had higher levels of major depressive problems.
Thus, there appears to be evidence that effortful control and cortisol dysregulation are associated, especially in childhood. However, the direction of effects has been mixed or not examined, and few researchers have assessed these associations in adolescence when the relations to onset of depressive problems may be especially relevant.
Cortisol Awakening Response and Youth Depressive Problems
Researchers have suggested that alterations or dysregulation of physiological systems during adolescence potentially contribute to poorer adjustment (Berger & Sarnyai, 2015; Susman, 2007). HPA-axis activity is normatively heightened during puberty such that basal cortisol levels and reactivity to stressors tend to increase, and higher CAR estimates (Adam, 2006). Given these developmental changes, there is concern that heightened HPA-axis activity may contribute to the increase of depressive problems during adolescence (Doom & Gunnar, 2013; Klimes-Dougan et al., 2018; Lopez-Duran, Kovacs, & George, 2009). Repeated or sustained exposure to chronic stressors can result in alterations of HPA responses such as hyper activity, protracted recovery, or blunted responses, which in turn contribute to poorer adjustment and coping (Bendezú & Wadsworth, 2017).
Supporting this, HPA hyper-activity has been implicated in vulnerability to depression in adolescents (Lopez-Duran, et al., 2009). Researchers have also substantiated these concerns, specifically in regards to CAR. For example, Rickard and colleagues (2016) found the CAR to be positively related to concurrent depressive symptoms among adolescents, and Adam and colleagues (2010) found the CAR to be related to greater odds of developing major depressive disorder among older adolescents. However, non-supportive evidence also exists. For instance, Adam (2006) found the CAR to be unrelated to concurrent depressive symptoms among adolescents, while the work of Rickard and colleagues (2016) showed that the CAR was not a statistically significant predictor of depressive symptoms over and above significant life events.
Given the mixed support for CAR being a risk factor for depressive problems among adolescents, it may be of merit to consider its potential as a protective factor. Researchers have found support for adolescence as a time of opportunity in terms of adaptive processes given heightened brain plasticity and the continuing development of the HPA axis (Steinberg, 2015). A high CAR may be indicative of preadolescents’ capacity for adaptation. More specifically, while a high CAR could have detrimental effects in the long-term if it becomes chronic, a high CAR in the short-term may be adaptive in terms of mobilizing resources to meet upcoming demands (Adam, 2006; Sladek et al., 2016). This hypothesis is in line with the notion that the CAR is an index of in-the-moment coping reserves for the upcoming day in anticipation of stressors (Fries et al., 2009). Therefore, it is not unreasonable to perceive a high CAR in the short-term during preadolescence as a marker of healthy adaption to stressors and challenge, whereas a low CAR may be a marker of or risk factor for depressive problems. Also important, much of this research has not been tested with higher risk youth populations. Latino youth are of particular concern, given that they often experience both high levels of stressors and high levels of internalizing problems (Cauce et al., 2011; Céspedes & Huey, 2008).
Present Study
In the present study, we hypothesized that Latino adolescents’ trait readiness for self-regulation, (effortful control), contributes to a strong, physiological response to awakening that prepares them to adapt to upcoming demands (Fries et al., 2009), which in turn may thwart emergence of depressive problems. Although there is evidence that dysregulated patterns of cortisol are detrimentally associated with the well-being of adolescents, very few researchers have addressed how adaptive patterns of cortisol may counteract or buffer depressive problems in adolescents. Our study uniquely contributes to the literature by examining associations between effortful control, CAR, and depressive problems in an understudied preadolescent Latino population. Given that prior literature suggests bidirectional relations between effortful control and CAR we tested correlational associations between the main variables in the current study at T1, and assessed whether these variables had long term effects on youth’s depressive problems a year later. Specifically, we hypothesized that (1) effortful control and CAR would be concurrently positively associated; (2) that CAR and depressive problems would be negatively concurrently associated, and that CAR would negatively be associated with depressive problems a year later; (3) Effortful control and depressive problems would be negatively concurrently associated, and that effortful control would negatively predict depressive problems a year later (controlling for prior levels).
Method
Participants and Procedures
Participants at T1 were 48 fifth and 71 sixth grade Latino youths (N = 119, mean age = 11.53 years, 59% female; 85.6% two-parent families) and their parents. Families resided in Indiana and were recruited using community resources after receiving institutional review board approval from (blinded for review). Inclusion criteria were that child and biological mother both participated, and that both self-identified as Latino. Fathers were not required to be in the study, however, 75% of fathers in the two-parent families (n = 76) participated at T1. Families’ mean reported yearly income was between $25,000–30,000. The majority of families were married or cohabiting with their partner (86%). Most parents had not completed high school (69% mothers, 79% of fathers). Mothers (92%) and fathers (90%) were predominantly born outside of the U.S (majority from Mexico), and 96% of children were born in the U.S. Mothers had lived in the U.S. an average of 15.95 years (SD = 6.15).
Eligible families who consented to participate completed two brief home-visits (typically three days apart) by trained bilingual interviewers. During the first visit, interviewers obtained written informed consents in the language of the participants’ choice. Interviewers left paper copies of the survey (in either Spanish or English) for participants to complete, as well as a manila folder to seal the survey in for privacy. Participants were instructed to complete the surveys on their own and then to seal them in the envelope. Surveys were expected to take 90 min for mothers, and 60 min for adolescents to complete. Almost all of the parents completed the survey in Spanish, and all of the adolescents, with one exception, completed their surveys in English. Finally, in the first visit the participants were also given instructions and supplies for collecting their saliva samples. During the second visit, interviewers briefly returned to the families’ homes to collect the interview packets and saliva samples, and to pay the families for their time. Mothers were paid $50 and adolescents $40.
A second wave of data was collected from the same families approximately one year later when youth were in sixth and seventh grade, and following the same procedures described previously. Attrition tests, which assessed whether those who did not participate at T2 (n = 22) differed from those who were assessed at T2 in regards to our variables of interest and covariates, did not find any significant differences between the two groups.
Saliva protocol.
Youth collected three saliva samples at T1 for two consecutive weekdays (upon wake-up, 30 minutes after wake-up, and prior to bedtime) using a commercially available passive-drool collection technique. Bilingual research assistants demonstrated how to collect and store saliva and answered any questions during the first home visit. Participants were given a form to record the date and time of each saliva collection, as well as any caffeine or nicotine use and were informed of the importance of strict adherence to the saliva protocols, especially the importance of collecting the first sample immediately upon waking. Written bilingual directions about collection were also left with families (Stalder et al., 2016), which included instructions about their behavior: no dairy products, no acidic or high sugar foods for 20 minutes prior to sample collection; no major meals for an hour before sample collection; and no teeth brushing 45 minutes prior to sample collection (Granger et al., 2012). Families were additionally provided a phone number which they could call if they had questions. Saliva samples were taken on weekdays when children’s schedules were more consistent. However, a small number could have participated during their school’s spring break, thus waking time was used as a control. Participants were instructed to immediately store the samples in their freezer. Saliva vials were collected during the second visit and transported in a cooler to a laboratory at (blinded for review) where they were stored in freezers at −80°C.
At the conclusion of T1 data collection, saliva samples were shipped on dry ice to the Biochemisches Laboratory at the University of Trier, Germany, for processing. After thawing, saliva samples were centrifuged at 2000g for 10 minutes, and 100ul of saliva were used for duplicate analysis. Cortisol concentrations in the saliva samples were measured with a time-resolved fluorescence immunoassay. The intra-assay coefficient of variation was between 4.0% and 6.7%, and the corresponding inter-assay coefficients of variation were between 7.1% - 9.0% (Dressendörfer, Kirschbaum, Rohde, Stahl, & Strasburger, 1992). Results were then sent to the project PI’s in an Excel document.
Measures
Cortisol Awakening Response (CAR).
Cortisol was collected at T1. Cortisol values below the assay detection limit (0.43 nmol/l) were winsorized to the lower detection limit (< 5 individuals per collection, a total of 15 child samples). Cortisol values above 3SD of the sample mean for each collection were winsorized to 3SD (< 3 individuals per collection, a total of 8 child samples). Waking and 30-minute post-waking samples were averaged across the two days of collection and again high values were winsorized to 3SD of the sample mean (1 morning and 1 30-minute sample for children). Next, data were cleaned for timing. All morning samples occurred between 4:30am and 10:40am for children. Time-since-waking values for individual 30-minute samples from each day that were less than 15 minutes or more than 45 minutes later than the waking sample were set to missing to exclude the samples from analysis (2 children each day). Then, an average time-since-waking variable was created across the two days of collection for use in cortisol awakening response calculation (Stalder et al., 2016). Waking cortisol values on day 1 and 2 were significantly correlated r = .37, p < .01, as well as 30-minute post-waking r = .30, p < .01.
Consistent with recommendations (Chida & Steptoe, 2009; Stalder et al., 2016), CAR was calculated using Area Under the Curve with respect to increase (AUCi; (Pruessner, Kirschbaum, Meinlschmid, & Hellhammer, 2003) using only the waking and 30-minute post-waking samples with the following equation:
AUCi is considered more appropriate to assess specifically the cortisol response to awakening, as opposed to AUC with respect to ground, which also depends on the cortisol levels present at waking, is more highly associated with diurnal patterns, and confounds total cortisol production with change in levels (Chida & Steptoe, 2009; Stalder et al., 2016). In the current sample, 115 children (97%) had non-missing AUCi scores after cleaning and computation, and AUCi scores were normatively distributed (children: range = −167.03 to 223.25; mean = 32.14, SD = 73.58).
Effortful Control.
Youth effortful control was reported by children and their mothers at T1 using the Effortful Control Scale from the Early Adolescent Temperament Questionnaire-Revised (EAT-QR, 16-items for youth, 18-items for mothers; Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001), which includes a validated Spanish translation. This scale consists of three subscales: Attentional control, the capacity to focus and shift attention when desired, included six items for children and seven for parents, i.e. “When trying to study, you/your child have/has difficulty tuning out background noise and concentrating.” Activation control, the capacity to perform an action when there is a strong tendency to avoid it, included 5 items for children and six for parents, i.e. “You/your child put/s off working on projects until right before they’re due.” Inhibitory control, the capacity to plan and to suppress inappropriate responses, included five items for both children and parents, i.e., “It’s hard for you/your child not to open presents before you’re/they’re supposed to.” Scale responses ranged from 1 = almost always false, to 5 = almost always true. Child and mom reports were significantly correlated (full scale: r = .31, p < .01; attentional control: r = .25, p < .01; activation control: r = .31, p < .01; inhibitory control: r = .18, p < .05). Alphas for the full effortful control scale were α = .77 for youth, and α = .79 for mothers. A latent variable was constructed using the three subscales as indicators, with each subscale representing the mean across mother and child reports.
Depressive Problems.
Depressive problems were child-reported at T1 and a year later at T2 using 6-items from the depressive mood subscale from EAT-QR (Capaldi & Rothbart, 1992; Ellis & Rothbart, 2001). Items included: “I get sad more than other people realize” and “I feel pretty happy most of the day.” Item responses ranged from 1 = almost always false, to 5 = almost always true (T1 α = .77, T2 α = .79). Items were randomly parceled onto 3 indicators to create a latent variable. Use of parcels also allows testing of hypotheses at the error-free latent variable level, avoiding biasing effects of unreliability that would occur if single indicators (e.g., scale score that is sum of all items) were used for each construct (Kishton & Widaman, 1994). Only child report was used to measure depressive problems as mother and child reports of child depressive problems were not significantly correlated, and youth report of their own depressive problems are expected to be more accurate than their parents (Fisher et al., 2006; Gartstein, Bridgett, Dishion, & Kaufman, 2009).
Covariates.
Covariates were reported at T1 and included: two-parent family (1 = single parent, 2 = two-parents); annual household income (1 = less than $10,000 to 14 = more than $100,000; range = 1–13, mean = 5.07); mothers’ education (1 = less than 7th grade to 7 = graduate or professional degree); child sex (0 = girls, 1 = boys); rurality (0 = non-rural, 1= rural); child grade (0 = 5th grade, 1 = 6th grade); and Body Mass Index (BMI) scores (obese [≥ 95th percentile for kg/m2] = 3, overweight [≥ 85th percentile for kg/m2] = 2, normal weight < 85th percentile for kg/m2 = 1) which has been linked to biased CAR estimates (Stalder et al., 2016). In regards to BMI, 39.5% of children were normal weight, 20.2% were overweight, and 40.3% were obese in this sample.
Analytic Strategy
We first ran descriptive statistics and correlations for our study variables and examined variables for skewness and kurtosis, and tested whether our data was missing at random. We then tested our hypotheses using a structural equation model in Mplus program Version 7.11. We used full information maximum likelihood (FIML) estimation to evaluate predictions from the conceptual model to address any missing data. FIML involves the fitting of covariance structure models directly to the raw data from each participant rather than to covariances among manifest variables which avoids deleting persons with missing data (such as in listwise deletion). FIML estimation has been found to be efficient and unbiased when data are missing completely or are missing at random and appears to be less biased than other approaches (Arbuckle, 1996). Model fit was assessed using the standard chi-square index of statistical fit that is routinely provided under maximum likelihood estimation of parameters, as well as the root mean square error of approximation (RMSEA), the Tucker-Lewis index (TLI), and the comparative fit index (CFI) using standard cut-offs for practical fit: RMSEA < .05, and TLI and CFI values > .95.
Results
Preliminary Analyses
Correlations (Table 1) provided initial support for our hypotheses. Effortful control was significantly positively correlated with youth’s CAR, and was negatively associated with T1 and T2 depressive problems. T1 CAR was significantly negatively associated with T1 depressive problems, and marginally with T2 depressive problems. CAR was marginally negatively associated with child sex, and significantly with wake time. Two-parent family was marginally negatively correlated with T1 and T2 depressive problems. Child sex was significantly negatively correlated with T2 depressive problems, (but not T1). Child BMI at T1 was marginally positively correlated with T1 depressive problems, but not with child CAR. Data was missing completely at random using Little’s MCAR test in SPSS: χ2 (52, N = 119) = 50.004, p = .55. Preliminary analyses also revealed that although the majority of CAR values were positive (reflecting the expected increase in cortisol from wake-up to 30-minutes post-wake), 30% of adolescents (n = 34), had negative CARs.
Table 1.
Correlations among variables and covariates (N = 119)
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. Effortful Control T1 | 1.00 | |||||||||||
| 2. Depressive Problems T1 | −.59** | 1.00 | ||||||||||
| 3. Depressive Problems T2 | −.42** | .67** | 1.00 | |||||||||
| 4. CAR T1 | .21* | −.24* | −.16† | 1.00 | ||||||||
| 5. Household Income T1 | .01 | −.05 | −.07 | −.02 | 1.00 | |||||||
| 6. Two-Parent T1 | .11 | −.17† | −.15† | −.06 | .28** | 1.00 | ||||||
| 7. Child Sex T1 | .02 | −.02 | −.24* | −.16† | .13 | .14 | 1.00 | |||||
| 8. Mother Education T1 | .03 | .00 | −.15† | .03 | .28** | .00 | .00 | 1.00 | ||||
| 9. Child Wake Time T1 | .04 | −.03 | .02 | −.18* | .00 | .19* | .00 | −.17† | 1.00 | |||
| 10. Rural T1 | −.20* | −.00 | .13 | −.02 | .08 | .00 | .00 | −.15† | −.10 | 1.00 | ||
| 11. Child Grade T1 | −.01 | .02 | .14 | −.03 | .12 | −.10 | .00 | .00 | .15† | .00 | 1.00 | |
| 12. Child BMI | −.03 | .16† | .05 | −.02 | −.02 | −.08 | .28** | −.05 | −.04 | −.11 | .08 | 1.00 |
| Mean | 3.44 | 2.44 | 2.49 | 31.70 | 5.02 | .86 | .41 | 2.48 | 6.72 | .78 | .60 | 2.01 |
| Standard Deviation | (.55) | (.76) | (.75) | (73.87) | (2.76) | (.35) | (.49) | (1.48) | (.88) | (.42) | (.49) | (.90) |
Note:
p <.01
p <.05
p <.10
(two-tailed). CAR = Cortisol Awakening Response. Child sex (boys = 1, girls = 0); Marital status (two-parent = 1, single-parent = 0); Rural (0 = no, 1 = yes); Grade (0 = 5th, 1 = 6th).
Structural Equation Model Analysis
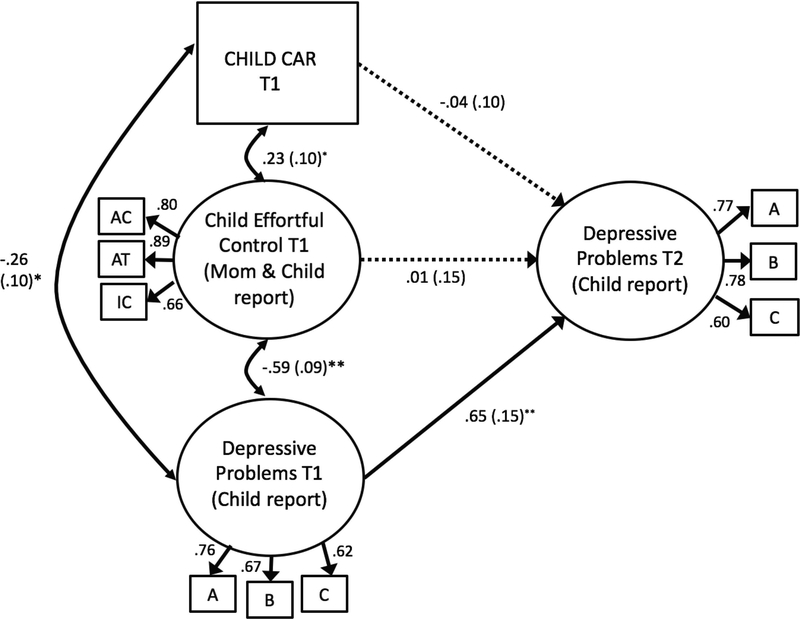
We then tested a structural equation model (Figure 1) that included the following covariates: two-parent family, mothers’ education, child sex, rurality, grade, Child BMI, and wake time. BMI was not significantly associated with any of our main variables in the model and was removed. This final model demonstrated close fit to the data: χ2 (101, N = 119) = 109.837, p > .05; Comparative Fit Index (CFI) = 0.977; Tucker-Lewis Index (TLI) = 0.972; and the Root Mean Square Error of Approximation (RMSEA) = .027. (see Figure 1). Factor loadings for the latent variables were all significant and ranged from 0.60 to 0.89. This final model also included constraints on depressive problems by setting loadings to be equal across the two-time points which produced a nonsignificant change in fit, Δχ2 (2) = 2.272, p > .05.
Figure 1.
Results from the structural equation model. (N = 119)
Note: **p < .01, *p < .05. χ2 (101, N = 119) = 109.837, p > .05; Comparative Fit Index (CFI) = 0.977; Tucker-Lewis Index (TLI) = 0.972; and the Root Mean Square Error of Approximation (RMSEA) = .027. Results are standardized (with standard errors in parentheses). Dotted lines are non-significant. CAR = Cortisol Awakening Response; ACC = activation control; ATT = attention; INH = inhibitory control; A/B/C = parcel indicators of depressive problems. Covariates included: mothers’ education, child sex, rurality, grade, and wake time. Factor loadings for Depressive Problems at T1 and T2 were constrained to equality.
As expected in hypothesis 1, child CAR and EC were concurrently positively associated (ß = .23, SE = .10, p < .05). Supporting hypothesis 2, CAR and T1 depressive problems were also significantly negatively associated (ß = −.26, SE = .10, p < .05); however, CAR and T2 depressive problems were not significant. Support for hypothesis 3 was mixed. Effortful control and T1 depressive problems were significantly negatively related (ß = .59, SE = .09, p < .01), however there was not an effect of EC at T1 on T2 depressive problems when controlling for prior depressive problems at T1. Depressive problems were strongly associated across T1 and at T2 (ß = .65, SE = .15, p < .01).
In regard to significant covariates, child sex (coded for boys) was negatively associated with depressive problems at T2 (ß = −.23, SE = .10, p < .05) and marginally with child CAR (ß = −.16, SE = .09, p = .07). Wake time was significantly negatively associated with CAR (ß = −.18, SE = .09, p < .05) as well as two-parent family (ß = .19, SE = .08, p < .05) and mother’s education (ß = −.17, SE = .08, p < .05). Rurality was significantly negatively associated with effortful control (ß = −.20, SE = .09, p < .05).
Discussion
We examined the associations between effortful control, a trait marker of self-regulation, adaptive HPA system functioning (as reflected by the CAR), and concurrent and longitudinal depressive problems, in a sample of preadolescent Latino youth. Overall, we found support for our study hypotheses.
CAR and Effortful Control
Our first hypothesis, examining whether CAR and effortful control were concurrently positively associated, was supported. Although there is a dearth of research on the links between CAR and effortful control in youth, the CAR is considered a discrete, but important, aspect of normative HPA axis functioning in humans (Stalder et al., 2016). Therefore, our finding that child CAR was positively associated with effortful control is consistent with related work of other researchers whom have highlighted the importance of robust HPA axis functioning for the development of self-regulatory abilities in youth (Davis et al., 2002; McEwen et al., 2015). A neurobiological perspective may provide an explanation for this as both regulation of the HPA axis and effortful control of behavior involve shared neural structures (Whittle et al., 2008). There is also preliminary evidence that a higher CAR is related to greater hippocampal volume (Pruessner, Pruessner, Hellhammer, Bruce Pike, & Lupien, 2007), which in turn has been linked to improved effortful control (Whittle et al., 2008). Though an adequate test of this proposition is beyond the scope of the available data, it is possible that Latino adolescents’ trait readiness for self-regulation contributes to a strong, physiological response to awakening that prepares them to adapt to upcoming demands (Fries et al., 2009), which in turn may thwart against the emergence of depressive symptomatology.
CAR and Depressive Problems
Our second hypothesis, that CAR and depressive problems would be negatively concurrently associated concurrently and across time was partially supported. As with effortful control, we found that child CAR at T1 was significantly negatively associated with depressive problems at T1, but was not significant across time to T2 depressive problems. These findings are consistent with the idea that a normative CAR response signifies a well-functioning HPA axis that has the capacity for responding adaptively to stress (Bai & Repetti, 2015). However, it is in contrast to studies that have found a greater-than-average CAR to be predictive of adolescent depressive problems (Adam et al., 2010; Nelemans et al., 2014; Rickard et al., 2016). It is unclear as to why these findings are disparate. It could be the case that these mixed findings result from variations in collection methods (Stalder et al., 2016) as well as study design. For example, Stalder and colleagues (2016) assessed CAR research between 2013 and 2014 and found 32% of the studies only assessed CAR across one day, about 47% assessed CAR across 2 days and only 21% assessed CAR for 3 or more days. As with our data, the majority (92%) did not have an objective control of awakening time, with most (66%) relying on a diary log method. Additionally, other methodological factors such as differing covariates, the number and nature of study days and timing, as well as compliance can affect CAR data and results (Stalder et al., 2016).
Context or chronicity of CAR levels may also be relevant. Although the present study found that CAR was negatively concurrently associated with depressive problems, longitudinal research has indicated that youth with consistently high CARs across adolescence have an increased risk of developing depressive problems over time (Adam et al., 2010; Nelemans et al., 2014). Our sample were preadolescent youth, and CAR may have a differing effect as youth more fully move into adolescence. It is also possible that for youth populations with higher levels of stressors a higher CAR is protective, although this hypothesis should be explored more stringently in future studies. Given that prior studies with Latino youth have found that higher levels of stressors are associated with blunted cortisol responses such as flatter diurnal slopes (i.e. DeSantis et al., 2007; Hajat et al., 2010; Kwak et al., 2017; Zeiders, Doane, & Roosa, 2012), for at-risk youth (such as in our sample), higher CAR levels may reflect resiliency to contextual stressors.
Effortful Control and Depressive Problems
Our final hypothesis, that effortful control would be negatively associated with depressive problems was supported concurrently, but not across time. The relations between effortful control and internalizing problems has been well established (Eisenberg, 2015). However, these associations have less frequently been examined in Latino populations. Our work was consistent with prior research finding effortful control serves to protect Latino youth from depressive problems (Loukas & Roalson, 2006; Taylor et al., 2017; Taylor et al., 2018; Valiente et al., 2009), although given that our findings were only significant for T1, we cannot show directionality. Researchers have found that effortful control and coping strategies are linked in Latino children in middle childhood and early adolescence (Taylor et al., 2018; Valiente et al., 2009), and that higher effortful control negatively predicts Latino children’s internalizing and externalizing problems (Loukas & Roalson, 2006; Taylor et al., 2017; Valiente et al., 2009). Researchers have also hypothesized that regulatory traits such as effortful control could provide a promising index of adolescent resilience (Dishion & Connell, 2006). This idea has been bolstered by recent evidence that coping and self-regulation strategies get “underneath the skin,” protecting against dysregulation in physiologic systems (e.g., HPA) known to confer risk for internalizing psychopathology (e.g., Bendezú et al, 2016; Wadsworth et al., 2016). Given this, our study is consistent with prior work that suggests that individual differences in effortful control relate to higher levels of resilience and adjustment in Latino youth, which is likely due to positive effects on coping behaviors.
Study Limitations
Findings should be considered within the context of study limitations. Although we were able to assess multiple time points for depressive problems, our study was limited to one time point of CAR. Therefore, we were not able to assess either changes or patterns in HPA system functioning, which are theorized to be risk factors for development of depressive problems (Adam et al., 2010). The single time point for CAR also restricted our ability to assess changes in the CAR over time in relation to effortful control and depressive problems. This also limited our ability to stringently determine the direction of effects between CAR and effortful control.
Another limitation was that we did not use a time stamp to verify when the saliva samples were taken, and therefore some of our participants could have been noncompliant to collection times which would affect the CAR results. However, all participants who provided saliva samples completed the collection form, and values where participants were non-compliant (i.e. the second time was not at the correct time) were marked as missing (as discussed in the CAR measure). Also, researchers have found that inaccurate sampling was associated with an underestimation of CAR (Stalder et al., 2016), therefore our findings may have been stronger using objective methods of assessing participant compliance. The sample was additionally drawn from predominantly rural and semi-urban areas in the Midwest, and thus may not be generalizable to other Latino populations. However, youth in rural areas remain woefully understudied in the literature (Conger, 2013), and this is especially true for Latino populations despite their significantly growing populations in the Southeastern and Midwestern United States (Stein, Gonzales, Coll, & Prandoni, 2016).
Implications and Future Directions
Overall, our study suggests that a higher CAR may be adaptive in the short-term for Latino preadolescents perhaps by promoting flexible responding to their environments through effortful control, an aspect of temperament known to facilitate positive adjustment and lower levels of internalizing problems in this population (Loukas & Roalson, 2006; Taylor et al., 2017; Taylor et al., 2018). Importantly, our study focused on factors that promote well-being in this population, rather than solely documenting vulnerabilities and risks. Other notable strengths of the current study include data from multiple reporters, a relatively large sample size for biological data, and a homogenous Midwestern Latino sample.
Future studies could be strengthened by including objective or clinical measures comparable to those included in other studies on CAR and depressive problems in adolescents (e.g., Adam et al., 2010). As for measuring the CAR, since only two saliva samples per subject were included in CAR estimates, we likely captured CAR magnitude (i.e., a general estimation of CAR) as opposed to the true CAR peak (Stalder et al., 2016). Accuracy of the CAR was dependent on our participant’s willingness and ability to collect their saliva at the appropriate times (i.e., at wake-up and thirty minutes after), as well as the accuracy of their self-reported collection times. Non-adherence to collection protocols and/or inaccurate/falsified collection times could have implications for validity of CAR estimates (Stalder et al., 2016). Furthermore, there may have been confounding variables related to the CAR-adjustment link that were not accounted for in the present study. For example, the experience of stressors or significant life events have been found to have interactive effects on the CAR (Yu et al., 2016), which has been conceptualized as a biomarker of sensitivity or responsivity to one’s environment (Ellis, Essex, & Boyce, 2005). Therefore, it is possible that youth with higher CARs in the short-term are more vulnerable to maladjustment in high-stress environments while also being more capable of reaping the benefits of low-stress environments, thereby resulting in better adjustment. Consequently, considering youth’s experiences with stressors in future studies on CAR, effortful control, and depressive problems may give us more confidence in high CAR as being adaptive in the short-term for Latino adolescents.
Given the expanding U.S. Latino population, improving the health of Latino youth is a critical goal for health professionals, researchers, and policy-makers. Better understanding how healthy physiological processes are associated with potential resilience processes such as effortful control during early adolescence may have concomitant adjustment benefits for youth such as improved mental health, adjustment, and coping skills. Although regulatory traits are most often conceptualized as biologically based, it is increasingly evident that these characteristics continue to develop during early adolescence (Lengua, 2006; Shiner et al., 2017) and can be modified by environmental factors (King et al., 2013; Laceulle et al., 2012). The present study suggests that CAR and effortful control are associated in early adolescence, and that both are linked with depressive problems. Future research should examine these associations longitudinally in order to assess how effortful control, CAR, and depressive symptoms are associated across adolescence and would allow for testing of directional pathways. For example, a mediational model in which effortful control is associated with depressive problems by means of CAR activity could be evaluated. A model in which effortful control is related to CAR indirectly via depressive problems is also plausible. However, longitudinal research is needed to determine such effects.
The role of persistent psychosocial stress on physiology and, in turn, development during preadolescence and beyond is limited with Latino populations (McClure et al., 2013). Our study suggests that effortful control and physiological functioning are associated, which has important implications for interventions aimed at both effortful control and physiological health of adolescents, and Latino youth in particular. Researchers have hypothesized that physiological stress, and allostatic load in particular, may be one of the mechanisms that link stressors such as discrimination to disparate health outcomes in minority populations, most notably poor mental health, obesity, and hypertension (Berger & Sarnyai, 2015). Thus, efforts to elucidate factors that contribute to a well-functioning HPA system may enhance a child’s capacity to avoid, or limit, the deleterious effects of adversity (Bai & Repetti, 2015).
Also, critical for future research is to determine factors that contribute to effortful control during adolescence. Effortful control and other self-regulatory processes have unparalleled importance for mental health, with poor self-regulation associated with depressive problems, addiction, attention deficit/hyperactive disorder, impulsivity and risk-taking, obsessive compulsive and habit disorders, and suicidal ideation (Nigg, 2017). Latino youth are more likely to report feeling sad or hopeless, anxious, or even attempt suicide than youth of other ethnic groups, with increased internalizing problems linked to individual, family, and cultural stressors (Cauce et al., 2011; Céspedes & Huey, 2008; Potochnick & Perreira, 2010). Therefore, interventions aimed at promoting and improving self-regulatory processes such as effortful control in Latino youth may have concomitant health benefits during a developmental period when risk for mental and physical health disparities and disease increases. Ultimately, such work could contribute to assessing how Latino youth adapt and thrive despite challenging circumstances, and contribute to improving the life chances, health, and adjustment of Latino youth in the United States.
Acknowledgments.
The authors wish to thank the youth and their families who participated in this study, as well as the research assistants who assisted us in data collection and managing the data.
Note: This research was funded by Purdue University start-up funds from Dr. Jones and Dr. Taylor, as well as awards from the Ingestive Behavior Research Center at Purdue University and the Purdue Research Foundation. Dr. Marceau was funded by the National Institute on Drug Abuse, K01 DA039288.
References
- Adam EK (2006). Transactions among adolescent trait and state emotion and diurnal and momentary cortisol activity in naturalistic settings. Psychoneuroendocrinology, 31(5), 664–679. doi: 10.1016/j.psyneuen.2006.01.010 [DOI] [PubMed] [Google Scholar]
- Adam EK, Doane LD, Zinbarg RE, Mineka S, Craske MG, & Griffith JW (2010). Prospective prediction of major depressive disorder from cortisol awakening responses in adolescence. Psychoneuroendocrinology, 35(6), 921–931. doi: 10.1016/j.psyneuen.2009.12.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arbuckle JL (1996). Full information estimation in the presence of incomplete data In Marcoulides GA & Schumacker RE (Eds.), Advanced structural equation modeling: Issues and techniques (pp. 243–277). Mahwah, NJ: Erlbaum. [Google Scholar]
- Atherton OE, Tackett JL, Ferrer E, & Robins RW (2017). Bidirectional pathways between relational aggression and temperament from late childhood to adolescence. Journal of Research in Personality, 67, 75–84. doi: 10.1016/j.jrp.2016.04.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai S, & Repetti RL (2015). Short‐term resilience processes in the family. Family Relations, 64(1), 108–119. doi: 10.1111/fare.12101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú JJ, Perzow SD, & Wadsworth ME (2016). What constitutes effective coping and efficient physiologic regulation following psychosocial stress depends on involuntary stress responses. Psychoneuroendocrinology, 73, 42–50. doi: 10.1016/j.psyneuen.2016.07.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bendezú JJ, & Wadsworth ME (2017). If the coping fits, use it: Preadolescent recent stress exposure differentially predicts post-TSST salivary cortisol recovery. Developmental Psychobiology, 59(7), 848–862. doi: 10.1002/dev.21542 [DOI] [PubMed] [Google Scholar]
- Bendezú JJ, & Wadsworth ME (2018). Person-centered examination of salivary cortisol and alpha-amylase responses to psychosocial stress: Links to preadolescent behavioral functioning and coping. Biological Psychology, 132,143–153. doi: 10.1016/j.biopsycho.2017.11.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger M, & Sarnyai Z (2015). ‘More than skin deep’: Stress neurobiology and mental health consequences of racial discrimination. Stress: The International Journal on the Biology of Stress, 18(1), 1–10. doi: 10.3109/10253890.2014.989204 [DOI] [PubMed] [Google Scholar]
- Blair C, Granger DA, Willoughby M, Mills-Koonce R, Cox M, Greenberg MT,…Fortunato CK (2011). Salivary cortisol mediates effects of poverty and parenting on executive functions in early childhood. Child Development, 82(6), 1970–1984. doi: 10.1111/j.1467-8624.2011.01643.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Call KT, Riedel AA, Hein K, McLoyd V, Petersen A, & Kipke M (2002). Adolescent health and well-being in the twenty-first century: A global perspective. Journal of Research on Adolescence, 12(1), 69–98. doi: 10.1111/1532-7795.00025 [DOI] [Google Scholar]
- Capaldi DM, & Rothbart MK (1992). Development and validation of an early adolescent temperament measure. Journal of Early Adolescence, 12(2), 153–173. doi: 10.1177/0272431692012002002 [DOI] [Google Scholar]
- Cauce AM, Cruz R, Corona M, & Conger R (2011). The face of the future: Risk and resilience in minority youth In Carlo G, Crockett LJ, & Carranza MA (Eds.), Health disparities in youth and families: Research and applications. (Vol. 57, pp. 13–32). New York: Springer Science + Business Media. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conger RD (2013). Rural children at risk. Monographs of the Society for Research in Child Development, 78(5), 127–138. doi: 10.1111/mono.12055 [DOI] [PubMed] [Google Scholar]
- Céspedes YM, & Huey SJ Jr. (2008). Depression in Latino adolescents: A cultural discrepancy perspective. Cultural Diversity and Ethnic Minority Psychology, 14(2), 168–172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chida Y, & Steptoe A (2008). Positive psychological well-being and mortality: A quantitative review of prospective observational studies. Psychosomatic Medicine, 70(7), 741–756. doi: 10.1097/PSY.0b013e31818105ba [DOI] [PubMed] [Google Scholar]
- Davis EP, Bruce J, & Gunnar MR (2002). The anterior attention network: Associations with temperament and neuroendocrine activity in 6-year-old children. Developmental Psychobiology, 40(1), 43–56. doi: 10.1002/dev.10012 [DOI] [PubMed] [Google Scholar]
- DeSantis AS, Adam EK, Doane LD, Mineka S, Zinbarg RE, & Craske MG (2007). Racial/ethnic differences in cortisol diurnal rhythms in a community sample of adolescents. Journal of Adolescent Health, 41(1), 3–13. doi: 10.1016/j.jadohealth.2007.03.006 [DOI] [PubMed] [Google Scholar]
- Dishion TJ, & Connell A (2006). Adolescents’ resilience as a self-regulatory process: promising themes for linking intervention with developmental science. Annals of the New York Academy of Sciences, 1094, 125–138. [DOI] [PubMed] [Google Scholar]
- Doane LD, Mineka S, Zinbarg RE, Craske M, Griffith JW, & Adam EK (2013). Are flatter diurnal cortisol rhythms associated with major depression and anxiety disorders in late adolescence? The role of life stress and daily negative emotion. Development and Psychopathology, 25(3), 629–642. doi: 10.1017/S0954579413000060 [DOI] [PubMed] [Google Scholar]
- Doom JR, & Gunnar MR (2013). Stress physiology and developmental psychopathology: Past, present, and future. Development and Psychopathology, 25(4, Pt 2), 1359–1373. doi: 10.1017/S0954579413000667 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dressendörfer RA, Kirschbaum C, Rohde W, Stahl F, & Strasburger CJ (1992). Synthesis of a cortisol-biotin conjugate and evaluation as a tracer in an immunoassay for salivary cortisol measurement. Journal of Steroid Biochemistry and Molecular Biology, 43(7), 683–692. [DOI] [PubMed] [Google Scholar]
- Dyson R, Robertson GC, & Wong MM (2015). Brief report: Peer group influences and adolescent internalizing problems as mediated by effortful control. Journal of Adolescence, 41, 131–135. doi: 10.1016/j.adolescence.2015.03.004 [DOI] [PubMed] [Google Scholar]
- Eisenberg N (2015). Self-regulation: Conceptual issues and relations to developmental outcomes in childhood and adolescence In Oettingen G & Gollwitzer PM (Eds.), Self-regulation in adolescence. (pp. 57–77). New York: Cambridge University Press. [Google Scholar]
- Eisenberg N, & Valiente C (2004). Elaborations on a theme: Beyond main effects in relations of parenting to children’s coping and regulation. Parenting: Science and Practice, 4(4), 319–323. doi: 10.1207/s15327922par0404_2 [DOI] [Google Scholar]
- Ellis BJ, Essex MJ, & Boyce WT (2005). Biological sensitivity to context: II. Empirical explorations of an evolutionary–developmental theory. Development and Psychopathology, 17(2), 303–328. doi: 10.1017/S0954579405050157 [DOI] [PubMed] [Google Scholar]
- Ellis LK, & Rothbart MK (2001). Revision of the Early Adolescent Temperament Questionnaire. Poster presented at the 2001 Biennial Meeting of the Society for Research in Child Development, Minneapolis, Minnesota. [Google Scholar]
- Fisher SL, Bucholz KK, Reich W, Fox L, Kuperman S, Kramer J, & …Bierut LJ (2006). Teenagers are right--parents do not know much: An analysis of adolescent-parent agreement on reports of adolescent substance use, abuse, and dependence. Alcoholism: Clinical and Experimental Research, 30(10), 1699–1710. doi: 10.1111/j.1530-0277.2006.00205.x [DOI] [PubMed] [Google Scholar]
- Fries E, Dettenborn L, & Kirschbaum C (2009). The cortisol awakening response (CAR): Facts and future directions. International Journal of Psychophysiology, 72(1), 67–73. doi: 10.1016/j.ijpsycho.2008.03.014 [DOI] [PubMed] [Google Scholar]
- Gartstein MA, Bridgett DJ, Dishion TJ, & Kaufman NK (2009). Depressed mood and maternal report of child behavior problems: Another look at the depression-distortion hypothesis. Journal of Applied Developmental Psychology, 30(2), 149–160. doi: 10.1016/j.appdev.2008.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger DA, Fortunato CK, Beltzer EK, Virag M, Bright MA, & Out D (2012). Focus on methodology: Salivary bioscience and research on adolescence: An integrated perspective. Journal of Adolescence, 35(4), 1081–1095. doi: 10.1016/j.adolescence.2012.01.005 [DOI] [PubMed] [Google Scholar]
- Gunnar MR, Sebanc AM, Tout K, Donzella B, & van Dulmen MH (2003). Peer rejection, temperament, and cortisol activity in preschoolers. Developmental Psychobiology, 43(4), 346–358. doi: 10.1002/dev.10144 [DOI] [PubMed] [Google Scholar]
- Hajat A, Diez-Roux A, Franklin TG, Seeman T, Shrager S, Ranjit N,…Kirschbaum C (2010). Socioeconomic and race/ethnic differences in daily salivary cortisol profiles: The multi-ethnic study of atherosclerosis. Psychoneuroendocrinology, 35(6), 932–943. doi: 10.1016/j.psyneuen.2009.12.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilt LM, Armstrong JM, & Essex MJ (2012). Early family context and development of adolescent ruminative style: Moderation by temperament. Cognition and Emotion, 26(5), 916–926. doi: 10.1080/02699931.2011.621932 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hostinar CE, McQuillan MT, Mirous HJ, Grant KE, & Adam EK (2014). Cortisol responses to a group public speaking task for adolescents: Variations by age, gender, and race. Psychoneuroendocrinology, 50, 155–166. doi: 10.1016/j.psyneuen.2014.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juster R-P, McEwen BS, & Lupien SJ (2010). Allostatic load biomarkers of chronic stress and impact on health and cognition. Neuroscience and Biobehavioral Reviews, 35(1), 2–16. doi: 10.1016/j.neubiorev.2009.10.002 [DOI] [PubMed] [Google Scholar]
- King KM, Lengua LJ, & Monahan KC (2013). Individual differences in the development of self-regulation during pre-adolescence: Connections to context and adjustment. Journal of Abnormal Child Psychology, 41(1), 57–69. doi: 10.1007/s10802-012-9665-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kishton JM, & Widaman KF (1994). Unidimensional versus domain representative parceling of questionnaire items: An empirical example. Educational and Psychological Measurement, 54(3), 757–765. [Google Scholar]
- Klimes-Dougan B, Thai M, Westlund Schreiner M, Bortnova A, Cullen K, & Gunlicks-Stoessel M (2018). Elevations in cortisol awakening response in depressed adolescents with a history of non-suicidal self injury. Biological Psychiatry, 83,(9), S184. [Google Scholar]
- Kwak Y, Taylor ZE, Anaya LY, Feng Y, Evich CD, & Jones BL (2017). Cumulative family stress and diurnal cortisol responses in Midwest Latino families. Hispanic Journal of Behavioral Sciences, 39(1), 82–97. doi: 10.1177/0739986316684130 [DOI] [Google Scholar]
- Laceulle OM, Nederhof E, Karreman A, Ormel J, & Aken MAG (2012). Stressful events and temperament change during early and middle adolescence: The TRAILS Study. European Journal of Personality, 26(3), 276–284. doi: 10.1002/per.832 [DOI] [Google Scholar]
- Lengua LJ (2006). Growth in temperament and parenting as predictors of adjustment during children’s transition to adolescence. Developmental Psychology, 42(5), 819–832. doi: 10.1037/0012-1649.42.5.819 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lengua LJ, Zalewski M, Fisher P, & Moran L (2013). Does HPA-axis dysregulation account for the effects of income on effortful control and adjustment in preschool children? Infant and Child Development, 22(5), 439–458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisonbee JA, Pendry P, Mize J, & Gwynn EP (2010). Hypothalamic-pituitary-adrenal and sympathetic nervous system activity and children’s behavioral regulation. Mind, Brain, and Education, 4(4), 171–181. doi: 10.1111/j.1751-228X.2010.01096.x [DOI] [Google Scholar]
- Lopez-Duran NL, Kovacs M, & George CJ (2009). Hypothalamic-pituitary-adrenal axis dysregulation in depressed children and adolescents: A meta-analysis. Psychoneuroendocrinology, 34(9), 1272–1283. doi: 10.1016/j.psyneuen.2009.03.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loukas A, & Roalson LA (2006). Family environment, effortful control, and adjustment among European American and Latino early adolescents. Journal of Early Adolescence, 26(4), 432–455. doi: 10.1177/0272431606291939 [DOI] [Google Scholar]
- Mayer SE, Abelson JL, & Lopez-Duran NL (2014). Effortful control and context interact in shaping neuroendocrine stress responses during childhood. Hormones and Behavior, 66(2), 457–465. doi: 10.1016/j.yhbeh.2014.06.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marceau K, Ruttle PL, Shirtcliff EA, Essex MJ, & Susman EJ (2015). Developmental and contextual considerations for adrenal and gonadal hormone functioning during adolescence: Implications for adolescent mental health. Developmental Psychobiology, 57(6), 742–768. doi: 10.1002/dev.21214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin CG, Bruce J, & Fisher PA (2012). Racial and ethnic differences in diurnal cortisol rhythms in preadolescents: The role of parental psychosocial risk and monitoring. Hormones and Behavior, 61(5), 661–668. doi: 10.1016/j.yhbeh.2012.02.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure H, Snodgrass JC Jr., Eddy J, McDade T, Hyers M, & Johnstone-Díaz A (2013). Integrating biomarkers into research with Latino immigrants in the United States. Advances in Anthropology, 3(2), 112–120. doi: 10.4236/aa.2013.32015 [DOI] [Google Scholar]
- McEwen BS, Gray J, & Nasca C (2015). Recognizing resilience: Learning from the effects of stress on the brain. Neurobiology of Stress, 1, 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller GE, Chen E, & Zhou ES (2007). If it goes up, must it come down? Chronic stress and the Hypothalamic-Pituitary-Adrenocortical axis in humans. Psychological Bulletin, 133(1), 25–45. doi: 10.1037/0033-2909.133.1.25 [DOI] [PubMed] [Google Scholar]
- Muris P, Meesters C, & Blijlevens P (2007). Self-reported reactive and regulative temperament in early adolescence: Relations to internalizing and externalizing problem behavior and ‘Big Three’ personality factors. Journal of Adolescence, 30(6), 1035–1049. doi: 10.1016/j.adolescence.2007.03.003 [DOI] [PubMed] [Google Scholar]
- Nelemans SA, Hale WW III, Branje SJT, van Lier PAC, Jansen LMC, Platje E,…Meeus WHJ (2014). Persistent heightened cortisol awakening response and adolescent internalizing symptoms: A 3-year longitudinal community study. Journal of Abnormal Child Psychology, 42(5), 767–777. doi: 10.1007/s10802-013-9820-2 [DOI] [PubMed] [Google Scholar]
- Nigg JT (2017). Annual Research Review: On the relations among self‐regulation, self‐control, executive functioning, effortful control, cognitive control, impulsivity, risk‐taking, and inhibition for developmental psychopathology. Journal of Child Psychology and Psychiatry, 58(4), 361–383. doi: 10.1111/jcpp.12675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Gilbert K, & Hilt LM (2015). Rumination and self-regulation in adolescence In Oettingen G & Gollwitzer PM (Eds.), Self-regulation in adolescence. (pp. 311–331). New York: Cambridge University Press. [Google Scholar]
- Obradović J (2012). How can the study of physiological reactivity contribute to our understanding of adversity and resilience processes in development? Development and Psychopathology, 24(2), 371–387. doi: 10.1017/S0954579412000053 [DOI] [PubMed] [Google Scholar]
- Oldehinkel AJ, Hartman CA, Nederhof E, Riese H, & Ormel J (2011). Effortful control as predictor of adolescents’ psychological and physiological responses to a social stress test: The Tracking Adolescents’ Individual Lives Survey. Development and Psychopathology, 23(2), 679–688. doi: 10.1017/S0954579411000095 [DOI] [PubMed] [Google Scholar]
- Ormel J, Oldehinkel AJ, Ferdinand RF, Hartman CA, De Winter AF, Veenstra R, & .. Verhulst FC (2005). Internalizing and externalizing problems in adolescence: General and dimension-specific effects of familial loadings and preadolescent temperament traits. Psychological Medicine, 35(12), 1825–1835. doi: 10.1017/S0033291705005829 [DOI] [PubMed] [Google Scholar]
- Poon JA, Turpyn CC, Hansen A, Jacangelo J, & Chaplin TM (2016). Adolescent substance use & psychopathology: Interactive effects of cortisol reactivity and emotion regulation. Cognitive Therapy and Research, 40(3), 368–380. doi: 10.1007/s10608-015-9729-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potochnick SR, & Perreira KM (2010). Depression and anxiety among first-generation immigrant Latino youth. Journal of Nervous and Mental Disease, 198(7), 470–477. doi: 10.1097/NMD.0b013e3181e4ce24 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pruessner JC, Kirschbaum C, Meinlschmid G, & Hellhammer DH (2003). Two formulas for computation of the area under the curve represent measures of total hormone concentration versus time-dependent change. Psychoneuroendocrinology, 28(7), 916–931. doi: 10.1016/S0306-4530(02)00108-7 [DOI] [PubMed] [Google Scholar]
- Pruessner M, Pruessner JC, Hellhammer DH, Bruce Pike G, & Lupien SJ (2007). The associations among hippocampal volume, cortisol reactivity, and memory performance in healthy young men. Psychiatry Research, 155(1), 1–10. [DOI] [PubMed] [Google Scholar]
- Pérez-Edgar K (2015). Effortful control in adolescence: Individual differences within a unique developmental window In Oettingen G & Gollwitzer PM (Eds.), Self-regulation in adolescence. (pp. 78–99). New York: Cambridge University Press. [Google Scholar]
- Rickard NS, Chin T-C, & Vella-Brodrick DA (2016). Cortisol awakening response as an index of mental health and well-being in adolescents. Journal of Happiness Studies, 17(6), 2555–2568. doi: 10.1007/s10902-015-9706-9 [DOI] [Google Scholar]
- Rothbart MK, & Bates JE (2006). Temperament In Damon W, Lerner R (Eds.) & Eisenberg N (Volume Ed.), Handbook of child psychology (6th ed.): Vol. 3: Social, emotional, and personality development (pp. 99–176). New York: Wiley. [Google Scholar]
- Shiner RL, Allen TA, & Masten AS (2017). Adversity in adolescence predicts personality trait change from childhood to adulthood. Journal of Research in Personality, 67, 171–182. doi: 10.1016/j.jrp.2016.10.002 [DOI] [Google Scholar]
- Sladek MR, Doane LD, & Stroud CB (2016). Individual and day-to-day differences in active coping predict diurnal cortisol patterns among early adolescent girls. Journal of Youth and Adolescence, 1–15. doi: 10.1007/s10964-016-0591-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stalder T, Kirschbaum C, Kudielka BM, Adam EK, Pruessner JC, Wüst S,…Clow A (2016). Assessment of the cortisol awakening response: Expert consensus guidelines. Psychoneuroendocrinology, 63, 414–432. doi: 10.1016/j.psyneuen.2015.10.010 [DOI] [PubMed] [Google Scholar]
- Stein GL, Gonzales RG, Coll CG, & Prandoni JI (2016). Latinos in rural, new immigrant destinations: A modification of the integrative model of child development (Crockett LJ & Carlo G Eds. Vol. Rural ethnic minority youth and families in the United States: Theory, research, and applications). Cham, Switzerland: Springer International Publishing. [Google Scholar]
- Steinberg L (2015). How to improve the health of American adolescents. Perspectives on Psychological Science: A Journal of the Association for Psychological Science, 10, 711–715. doi: 10.1177/1745691615598510 [DOI] [PubMed] [Google Scholar]
- Sturge-Apple ML, Davies PT, Cicchetti D, Hentges RF, & Coe JL (2016). Family instability and children’s effortful control in the context of poverty: Sometimes a bird in the hand is worth two in the bush. Development and Psychopathology, 29(3), 685–696. [DOI] [PubMed] [Google Scholar]
- Susman EJ (2007). Toward a psychobiologic understanding of youth health disparities. Journal of Adolescent Health, 41(1), 1–2. doi: 10.1016/j.jadohealth.2007.04.002 [DOI] [PubMed] [Google Scholar]
- Taylor ZE, Jones BL, Anaya LY, & Evich CD (2017). Effortful control as a mediator between contextual stressors and adjustment in Midwestern Latino youth. Journal of Latina/o Psychology. doi: 10.1037/lat0000091 [DOI] [Google Scholar]
- Taylor ZE, Widaman KF, & Robins RW (2018). Longitudinal relations of economic hardship and effortful control to active coping in Mexican-origin youth. Journal of Research on Adolescence, 28(2), 396–411. doi: 10.1111/jora.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uchoa ET, Aguilera G, Herman JP, Fiedler JL, Deak T, & de Sousa MBC (2014). Novel aspects of glucocorticoid actions. Journal of Neuroendocrinology, 26(9), 557–572. doi: 10.1111/jne.12157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valiente C, Lemery-Chalfant K, & Swanson J (2009). Children’s responses to daily social stressors: Relations with parenting, children’s effortful control, and adjustment. Journal of Child Psychology and Psychiatry, 50(6), 707–717. doi: 10.1111/j.1469-7610.2008.02019.x [DOI] [PubMed] [Google Scholar]
- van Oort FA, Greaves‐Lord K, Ormel J, Verhulst FC, & Huizink AC (2011). Risk indicators of anxiety throughout adolescence: The TRAILS study. Depression and Anxiety, 28(6), 485–494. doi: 10.1002/da.20818 [DOI] [PubMed] [Google Scholar]
- Wadsworth ME, Bendezú JJ, Loughlin-Presnal J, Ahlkvist JA, Tilghman-Osborne E, Bianco H, & …Hurwich-Reiss E (2016). Unlocking the black box: A multilevel analysis of preadolescent children’s coping. Journal of Clinical Child and Adolescent Psychology, 53, 1–15. doi: 10.1080/15374416.2016.1141356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang FL, Eisenberg N, Valiente C, & Spinrad TL (2016). Role of temperament in early adolescent pure and co-occurring internalizing and externalizing problems using a bifactor model: Moderation by parenting and gender. Development and Psychopathology, 28(4pt2), 1487–1504. doi: 10.1017/S0954579415001224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittle S, YÜCel M, Fornito A, Barrett A, Wood SJ, Lubman DI,…Allen NB (2008). Neuroanatomical correlates of temperament in early adolescents. Journal of the American Academy of Child and Adolescent Psychiatry, 47(6), 682–693. doi: 10.1097/CHI.0b013e31816bffca [DOI] [PubMed] [Google Scholar]
- Yap MBH, Allen NB, O’Shea M, Di Parsia P, Simons JG, & Sheeber L (2011). Early adolescents’ temperament, emotion regulation during mother–child interactions, and depressive symptomatology. Development and Psychopathology, 23(1), 267–282. doi: 10.1017/S0954579410000787 [DOI] [PubMed] [Google Scholar]
- Yu R, Nieuwenhuis J, Meeus W, Hooimeijer P, Koot HM, & Branje S (2016). Biological sensitivity to context: Cortisol awakening response moderates the effects of neighbourhood density on the development of adolescent externalizing problem behaviours. Biological Psychology, 120, 96–107. doi: 10.1016/j.biopsycho.2016.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalewski M, Lengua LJ, Fisher PA, Trancik A, Bush NR, & Meltzoff AN (2012). Poverty and single parenting: Relations with preschoolers’ cortisol and effortful control. Infant and Child Development, 21(5), 537–554. doi: 10.1002/icd.1759 [DOI] [Google Scholar]
- Zeiders KH, Doane LD, & Roosa MW (2012). Perceived discrimination and diurnal cortisol: Examining relations among Mexican American adolescents. Hormones and Behavior, 61(4), 541–548. doi: 10.1016/j.yhbeh.2012.01.018 [DOI] [PMC free article] [PubMed] [Google Scholar]