 The present study aims to investigate the protective effects of zinc oxide nanoparticles (ZnO NPs) on doxorubicin-induced testicular injury.
The present study aims to investigate the protective effects of zinc oxide nanoparticles (ZnO NPs) on doxorubicin-induced testicular injury.
Abstract
The present study aims to investigate the protective effects of zinc oxide nanoparticles (ZnO NPs) on doxorubicin-induced testicular injury. Forty mature male rats were randomly allocated into four equal groups: G1 (control), G2 (3 mg per kg BW of zinc oxide nanoparticles was administered), G3 (6 mg per kg BW of doxorubicin was intraperitoneally injected), and G4 (doxorubicin + ZnO NPs). Some fertility parameters, antioxidant status, genotoxicity assay, and a histopathological examination were used for this investigation. The doxorubicin-treated group showed a significant decrease in the index weight of reproductive organs, epididymal sperm count, motility%, and live sperm% and a significant increase in sperm abnormalities. Moreover, GSH and CAT activities were significantly decreased, and MDA content was significantly increased in the doxorubicin-treated group. Interestingly, co-administration of ZnO NPs significantly reduced the doxorubicin-induced changes in the investigated parameters. In addition, ZnO NPs alone did not show any undesirable effects on the sperm parameters, testis or DNA. However, its administration improves the reproductive parameters and significantly increases the testosterone level. We concluded that the administration of ZnO NPs at 3 mg per kg BW ameliorated the testicular toxicity and genotoxicity caused by doxorubicin through its antioxidant and androgenic activity.
1. Introduction
Doxorubicin (sold as Adriamycin), or hydroxydaunorubicin, is an anthracycline antibiotic drug isolated from Streptomyces peucetius var. caesius.1 The metabolic conversion of doxorubicin to doxorubicinol is mediated by cytoplasmic NADPH-dependent monomeric carbonyl reductases.2 Doxorubicinol toxicity is associated with reactive oxygen species (ROS) formation which its chelating with iron affecting bio-macromolecules and production of free radicals.3,4 Moreover, ROS causes damage to membranes and macromolecules through lipid peroxidation, oxidative stress, DNA fragmentation and protein oxidation.5–7
Doxorubicin-based chemotherapy for treatment of a wide spectrum of both hematological and solid tumors. It is also associated with toxicities to different organs like heart, kidney, liver, and testis.8–10 Its genotoxicity has also been reported, through increased cellular apoptosis and its high affinity towards chromosomal DNA and nucleosome eviction and replacement.3,11,12 The occurrence of male infertility after doxorubicin treatment is a serious problem. Several studies have reported that doxorubicin has a harmful effect on the testicular tissue, induces testicular damage and apoptosis, and decreases DNA synthesis.13–16
Nanomaterials are important products used in various fields due to their prominent characteristics. Zinc oxide nanoparticles (ZnO NPs) are nano-sized particles of less than 100 nm, which have been considered as an important factor in the area of biomedicine.17,18 Nanoparticles can rapidly enter the bloodstream and reach different organs through blood circulation.19 ZnO NPs are characterized by a high catalytic activity and a large surface area.20 Furthermore, ZnO NPs receive more attention in commercial and biomedical applications as antibacterial, anti-inflammation, anticancer, anti-diabetic agents and also for their ability to release zinc ions.21,22 Zn plays structural, catalytic, and regulatory roles in more than 300 mammalian enzymes. It also has an important role in maintaining critical cellular processes like DNA replication, DNA repair, cell division and protection against oxidative stress.23 Moreover, Dani and Dhawan24 and Malekirad et al.25 reported that Zn is a protecting antioxidant due to its ability to bring the malondialdehyde (MDA) levels to near normal levels. Additionally, Raajshreer and Durairaj26 revealed that their synthesized ZnO NPs possess antioxidant activity with respect to mechanisms of both reducing activities and free radical scavenging.
Pei et al.27 showed the beneficial effects of nano-ZnOs at low doses in weanling pigs on intestinal morphology and microflora, growth performance, and immunity. Also, Zhao et al.28 used nanoparticles at three concentrations, 20, 60, and 100 mg kg–1, as a feed additive in broilers and revealed that low dose (20 mg kg–1) of ZnO nanoparticles improve growth performance and antioxidative ability. Moreover, in mice, ZnO-NPs at low doses after long-term feeding are relatively biocompatible as a nutritional additive compared to ZnO microparticles (ZnO-MPs) and Zn ions.29
On the other hand, many studies reported that ZnO NPs act as a testicular toxicant in high doses such as 50, 150, 300 and 350 mg kg–1 in mice.30,31 But, low doses (5 mg kg–1) of ZnO NPs have some beneficial effects on male rat reproduction.32 Ibraheem and Ibrahim33 also showed that using nanoparticles in low doses (20 μg per kg BW) may have a protective effect through antioxidant mechanisms, but at high doses (150 μg per kg BW) they may have many levels of harmful effects.
The study of ZnO NP protective effects against the undesirable side effects of drugs is very meaningful and necessary. Therefore, the aim of this study was to focus on investigating the protective effects of zinc oxide nanoparticles in low doses (3 mg per kg BW) versus potential adverse effects of doxorubicin on some fertility parameters, antioxidant status, genotoxicity and testicular histopathological changes in male rats.
2. Materials and methods
2.1. Chemicals
Doxorubicin was purchased from Eimc United Pharmaceuticals, Badr City, Cairo, Egypt. ZnO NPs were purchased from Sigma-Aldrich (Germany) in the form of dispersion with an average nanoparticle size of ∼35 nm and a concentration of 50 wt% water. The particle size distribution (hydrodynamic diameter) was found to be <100 nm using the dynamic light scattering (DLS) technique, pH was 7 ± 0.1, and density was 1.7 g ml–1 at 25 °C. The diameters of the nanoparticles were found to be less than 35 nm using a transmission electron microscope (TEM). ZnO NPs were suspended in saline (0.9%) before use according to Torabi et al.34 and dispersed by ultrasonication to avoid particle aggregation.
2.2. Animals and experimental design
Mature adult male Wistar rats (5 months old, weighing 180–200 g) were housed in plastic cages under the same hygienic and environmental conditions with temperature and light controlled on a 12 h light /12 h dark period and were fed a standard laboratory diet (Damanhour Feed Co., Behera, Egypt) and water. The rats were acclimatized two weeks before the experiment. The duration of the experiment was 8 weeks to cover the complete spermatogenic cycle in rats which is 56 days.35 All animal procedures were performed in accordance with the Guidelines for Care and Use of Laboratory Animals of ICLAS-2015. All procedures and experiments were accepted by the local ethics committee of animal use from the Faculty of Veterinary Medicine, Alexandria University-Institutional Animal Care and Use Committee (AU-IACUC).
Forty rats were randomly allocated into 4 groups (n = 10 per each): Group 1 (control) rats were administered 2 ml per kg BW saline (a vehicle for doxorubicin) by gavage five times weekly for 8 weeks together with intraperitoneal injection of 2 ml per kg BW saline (a vehicle for the ZnO NPs) three times weekly for 8 weeks. Group 2 (ZnO NPs) rats were administered ZnO NPs (3 mg per kg BW by gavage) five times weekly for 8 weeks. Group 3 (doxorubicin) rats were intraperitoneally injected with 6 mg per kg BW doxorubicin, three times weekly for 8 weeks.36 Group 4 (doxorubicin + ZnO NPs) rats received a combination of both doxorubicin and ZnO NPs with the same dose as in G2 and G3.
2.3. Sample collection
At the end of 8 weeks, the rats were individually weighed, euthanized by isoflurane inhalation and then sacrificed. Blood samples were collected and centrifuged at 3000 rpm for 15 min for serum separation. Serum was kept at –20 °C until the determination of the testosterone level. Testis, epididymis, and accessory sex organs (seminal vesicles and prostate glands) were dissected out immediately from each rat, grossly examined and weighed. The index weight (I.W.) of each organ was calculated according to Matousek.37 After that, epididymis of each rat from the same group was used for semen analysis and the other one kept at –80 °C for quantifying DNA damage by comet assay. One testis, from each rat, was rapidly kept in 10% neutral buffered formalin for histopathological processing. The other testis was kept at –20 °C for assaying of GSH and CAT activities and malondialdehyde (MDA) content.
2.4. Sperm examination and testosterone assay
Epididymal sperm counting was conducted immediately as previously described.38 Cauda epididymis was minced in 5 ml physiological saline. An aliquot of the epididymis sperm suspension was diluted in an alkaline aqueous solution and the spermatozoa counting microscopically using a hemacytometer (Neubauer, Marienfeld, Germany). Live spermatozoa and sperm progressive motility were evaluated as described by Sönnmez et al.39 Epididymis sperm abnormality% was evaluated according to Bearden and Faquay.40 The contents of each epididymis were mixed with a drop of eosin–nigrosin stain, and a thin film was spread on a clean slide. A total of 300 spermatozoa per slide were randomly examined microscopically. The preserved sera were used for the estimation of testosterone level by an enzyme-linked immunosorbent assay (ELISA) kit (Immunometrics Ltd, London, UK) according to Demetrious.41
2.5. Assessment of glutathione and catalase activities, and malondialdehyde content
Testis was homogenized in chilled Tris-HCl buffer (pH 7.4) and centrifuged (12 000g, 30 min at 4 °C). The clear supernatant was used for spectrophotometric assessment of the following: GSH using Ellman's reagent,42 CAT enzyme activity based on the reaction of the CAT enzyme with a known quantity of H2O243 and lipid peroxidation (LPO) as malondialdehyde (MDA), after the reaction with thiobarbituric acid.44
2.6. DNA damage by comet assay
Sperm DNA damage was quantified using single-cell gel electrophoresis (comet assay) according to Simon and Carrell.45 Briefly, frozen sperm was thawed on ice and suspended in PBS at a concentration of 6 × 106 cells per ml, and then 10 μl was mixed with 75 ml of 0.5% low-melting-point agarose (Sigma-Aldrich, Germany) at 37 °C. This cell suspension was pipetted on the pre-treated slides (0.6% normal melting agarose). The slides were covered with a coverslip, and they were left to solidify at ambient temperature. The slides were submerged in a fresh lysis solution (Sigma-Aldrich, Germany) for 1 h at 4 °C. Then, 1 M dichloro-diphenyl-trichloroethane (DDT) (0.25 ml) (Sigma-Aldrich, Germany) was added for a further 30 min at 4 °C. Subsequently, 1 ml of 100 mM lithium diiodosalicylate (LIS) (Sigma-Aldrich, Germany) was added and incubated at ambient temperature for 90 min. The slides were placed in a fresh alkaline electrophoresis buffer (0.3 M NaOH, 1 mM Na2EDTA, pH 13) for 10 min to allow for unwinding of the DNA.
Electrophoresis was performed for 10 min at 25 V and 300 A. The slides were submerged with three changes of a neutralization buffer (0.4 M Tris, pH 7.5; Sigma-Aldrich, Inc. Germany) for 5 min, stained with 50 μl of 20 mg mL–1 ethidium bromide (Sigma-Aldrich, Inc. Germany) and then analyzed immediately. 50–100 random spermatozoa/slide were analyzed using a fluorescence microscope (with excitation filter 420–490 nm [issue 510 nm]) attached to a CCD video camera (Polytec GmbH, Waldbronn, Germany). Tail length, percent tail DNA, and tail moment were determined using the KOMET 5.0 image analysis system (Kinetic Imaging Ltd, Liverpool, United Kingdom).
2.7. Histopathologic examination
The collected testicular specimens were fixed in a 10% neutral buffered formalin solution, dehydrated in ascending grades of ethanol (≥99.9%, International Company for sup. Med. Industries, Egypt), cleared in xylene (99.5%, Chemajet Chem. Co. Egypt) and then embedded in paraffin wax. Tissue sections of 5–6 μm thickness were made and stained with hematoxylin and eosin (H and E) according to Bancroft and Stevens.46
2.8. Statistical analysis
Data were statistically analyzed by one-way analysis of variance (ANOVA) followed by Duncan's multiple range tests. The data were presented as means ± standard error, and P-values ≤0.05 were considered statistically significant. The analysis was performed using Instant Statistical Analysis Package (Prism 6.0 GraphPad Software, San Diego, CA, USA).
3. Results
There was no mortality in the animals treated with doxorubicin and/or ZnO NPs during the study period. The index weight of testes, epididymis, and accessory sex organs were significantly reduced in the doxorubicin-treated and co-treated with ZnO NPs groups versus the control (P ≤ 0.05). However, the index weight of the reproductive organs showed a significant (P ≤ 0.05) increase in the group co-treated with doxorubicin and ZnO NPs compared with the doxorubicin-treated group as shown in Table 1.
Table 1. Effect of doxorubicin and ZnO NPs on the index weight of the reproductive organs in male rats.
| Groups | Index weight of testes (g per 100 g BW) | Index weight of epididymis (g per 100 g BW) | Index weight of accessory sex organs (g per 100 g BW) |
| G1 (Control) | 1.15 ± 0.03a | 0.70 ± 0.01a | 0.80 ± 0.03a |
| G2 (ZnO NPs) | 1.17 ± 0.04a | 0.67 ± 0.02a | 0.88 ± 0.03a |
| G3 (Doxorubicin) | 0.62 ± 0.09c | 0.47 ± 0.06c | 0.31 ± 0.08c |
| G4 (Doxorubicin + ZnO NPs) | 0.86 ± 0.06b | 0.58 ± 0.02b | 0.46 ± 0.05b |
In addition, the obtained results of the epididymal sperm analysis are presented in Table 2. The epididymal sperm count, motility%, live sperm, and serum testosterone level were significantly (P ≤ 0.05) decreased, and the sperm abnormality% was significantly (P ≤ 0.05) increased in the doxorubicin-treated group as compared with the control group. While the co-treatment with ZnO NPs showed a significant increase in these reproductive performance parameters and a decrease in the sperm abnormality% compared to the doxorubicin-treated group, these parameters (sperm count, motility%, live sperm, and serum testosterone level) showed significant differences versus the control group (Table 2). G2 administered with ZnO NPs alone revealed a significant (P ≤ 0.05) increase in the sperm count, motility%, live sperm, and serum testosterone level as compared with the control group (Table 2).
Table 2. Effect of doxorubicin and ZnO NPs on the reproductive performance parameters.
| Groups | Sperm cell count (×106 ml–1) | Sperm motility (%) | Sperm viability (%) | Sperm abnormalities (%) | Testosterone (ng ml–1) |
| G1 (Control) | 301.55 ± 1.32b | 88.50 ± 1.06b | 90.56 ± 1.32b | 12.00 ± 0.04b | 3.28 ± 0.43b |
| G2 (ZnO NPs) | 425.15 ± 2.24a | 95.75 ± 1.86a | 96.75 ± 1.44a | 10.15 ± 0.60b | 5.27 ± 0.34a |
| G3 (Doxorubicin) | 106.86 ± 1.94d | 50.00 ± 2.16d | 55.20 ± 2.42d | 24.60 ± 0.86a | 1.87 ± 0.26d |
| G4 (Doxorubicin + ZnO NPs) | 225.00 ± 1.81c | 78.33 ± 1.96c | 83.11 ± 1.96c | 15.95 ± 0.74ab | 2.84 ± 0.64c |
As shown in Table 3, the GSH and CAT activities, an oxidative stress marker, were significantly decreased, and the MDA content, an indicator of lipid peroxidation, was significantly (P ≤ 0.05) increased in the doxorubicin-treated group compared with the control group. While the ZnO NP co-administration with doxorubicin in G4 successfully reduced the oxidative stress in the testicular tissue, the GSH and CAT activities were significantly increased and the MDA content was significantly (P ≤ 0.05) decreased in G4 compared with G3 which was treated with doxorubicin (Table 3).
Table 3. Effect of doxorubicin and ZnO NPs on testicular oxidative stress biomarkers.
| Groups | GSH (μmol g–1 wet tissue) | Catalase (unit g–1 wet tissue) | MDA (nmol g–1 wet tissue) |
| G1 (Control) | 10.49 ± 0.30b | 7.21 ± 0.18a | 4.88 ± 0.2b |
| G2 (ZnO NPs) | 16.89 ± 0.41a | 7.17 ± 0.17a | 4.79 ± 0.16b |
| G3 (Doxorubicin) | 6.59 ± 0.18c | 3.23 ± 0.26c | 12.20 ± 0.38a |
| G4 (Doxorubicin + ZnO NPs) | 9.83 ± 0.36b | 5.41 ± 0.30b | 4.62 ± 0.55b |
In the present study, doxorubicin induced a significant increase in DNA damage as indicated by a significant (P ≤ 0.05) increase in the tail length, tail DNA percentage and tail moment in the sperm cell of G3 (Table 4). In contrast, the rats co-treated with doxorubicin and ZnO NPs in G4 revealed a significant (P ≤ 0.05) decrease in all parameters of DNA damage in the testis (tail length, DNA tail percentage, and tail moment) when compared to the doxorubicin-treated group (G3).
Table 4. The DNA damage level in the sperm cells of control and different treated groups.
| Groups | Tail length (μm) | Tail DNA % | Tail moment (Unit) |
| G1 (Control) | 2.21 ± 0.16c | 2.34 ± 0.07c | 4.74 ± 0.94c |
| G2 (ZnO NPs) | 2.06 ± 0.19c | 2.28 ± 0.21c | 4.48 ± 0.84c |
| G3 (Doxorubicin) | 3.17 ± 0.17a | 3.52 ± 0.18a | 11.18 ± 1.19a |
| G4 (Doxorubicin + ZnO NPs) | 2.89 ± 0.06b | 3.21 ± 0.06b | 9.26 ± 0.36b |
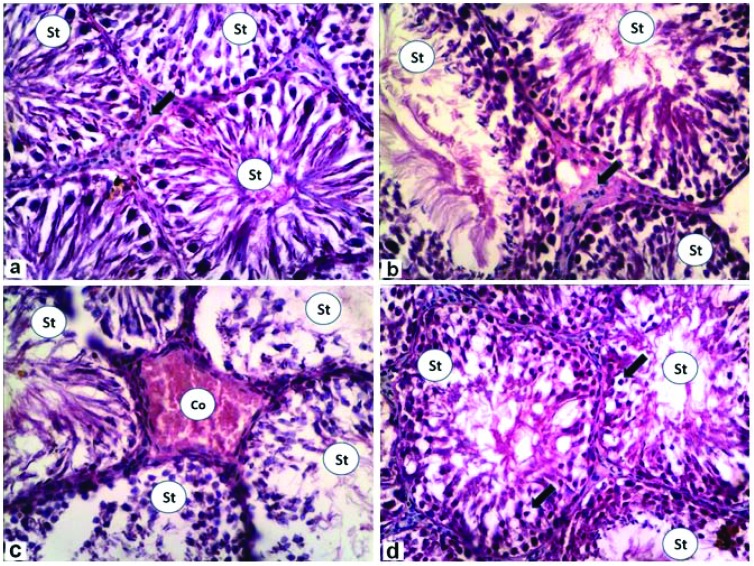
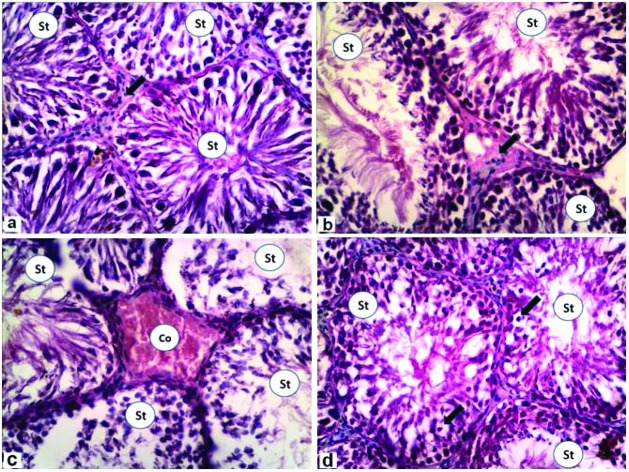
Testicular histological sections were analyzed to evaluate structural changes and damage caused by in vivo exposure to the various treatments. The results of the testicular histopathological finding are shown in Fig. 1a–d; the control rats showed normal seminiferous tubules with normal spermatogenesis and interstitial Leydig cells (Fig. 1a). In the rats treated with ZnO NPs, the testis showed normal seminiferous tubules with normal spermatogenesis (Fig. 1b). G3 treated with doxorubicin was affected by less spermatogenesis and severely congested interstitial capillaries (Fig. 1c). Seminiferous tubules in the rats co-treated with doxorubicin and ZnO NPs appeared nearly normal, except for the presence numerous intratubular vacuolations (Fig. 1d).
Fig. 1. Photomicrographs of rat testes stained with H&E (×400). (a) Control group showing normal seminiferous tubules with nearly normal spermatogenesis (St) and interstitial Leydig cells (arrow). (b) Zinc oxide nanoparticles group showing increased size of seminiferous tubules with normal spermatogenesis (St). (c) Doxorubicin group showing that most of seminiferous tubule with less spermatogenesis (St), while the interstitial capillaries appeared severely congested (Co). (d) Doxorubicin + zinc oxide nanoparticles group showing numerous intratubular vacuolations (arrows) with some normal spermatogenesis in seminiferous tubules (St).
4. Discussion
The adverse effects of cancer chemotherapy have been recognized in multiple organ systems including the testis. It was suggested that the combination of a chemotherapy drug together with cell protective agents may be an appropriate way to reduce its hazardous effects. In the present study, the testicular toxicity observed in the doxorubicin-treated group (G3) included a significant reduction of the index weight of the testes, epididymis, and accessory sex organs. Also, a significant (P ≤ 0.05) decrease in the sperm count, motility% and percentage of live spermatozoa and a significant increase in the sperm abnormality% were observed compared to the control (G1). These findings coincided with the results of several previous studies.8,9,13,14,47 So, all these changes in the sperm profiles may lead to an increase in the incidence or confirmation of the infertility induced by the doxorubicin treatment. However, concomitant treatment with ZnO NPs at 3 mg kg–1 diminished the reduction of rats’ reproductive organ weights and the semen parameters induced by the doxorubicin treatment. A previous study demonstrated that ZnO NPs induced testicular damage in a dose-dependent manner in mice, where mice treated with 5 mg kg–1 ZnO NPs daily for 35 days showed no significant alteration in testicular parameters (sperm count, sperm motility and sperm abnormality) compared with the control group, while a dose of 50 and 300 mg kg–1 induced testicular damage.30 In addition, Afifi et al.48 reported that treatment with ZnO NPs alone or in combination with insulin has the ability to increase the sperm count, motility and serum testosterone level in diabetic rats. Also, Mohamed and Abdelrahman49 found that ZnO NPs (10 mg per kg day–1) have an obvious improvement in testicular and epididymal structural changes and sperm morphological abnormalities detected in nicotine (1 mg per kg day–1)-treated rats for 30 days.
Testosterone is critically involved in normal spermatogenesis and normal function of the reproductive tract.50,51 Moreover, previous studies reported that testosterone is associated with semen quality (sperm concentration and motility) and fertility.52,53 The doxorubicin-treated group showed a significant decrease of the testosterone level compared to the control group, and this indicated a decline in the testicular function as stated by Ateşşahin et al.54 As such, the drop and deficiency in the serum testosterone level can impact sperm production and semen quality.55,56 So, the decreased serum testosterone level in G3 may lead to the deteriorating effects on the sperm parameters.
Release of luteinizing hormone (LH) and follicle stimulating hormone (FSH) from the pituitary gland was increased by Zn, and they stimulate the testosterone production. Additionally, Zn also prevents the aromatase enzyme from the conversion of testosterone to excess estrogen.57 In the present study, the rats treated with ZnO NPs alone had increased testosterone levels compared to the other groups (Table 2). Moreover, there was a significant increase in the sperm count in the rats administered with ZnO NPs compared to the control rats due to their androgenic activity. Furthermore, co-administration of ZnO NPs with doxorubicin (G4) showed a significant increase of the serum testosterone level compared to G3 and showed a good protective effect in the testis against the adverse, inflammatory and damaging effect of doxorubicin.
Glutathione (GSH) and catalase (CAT) are used as markers of oxidative stress. GSH provides reducing equivalents in the form of free thiol groups, and CAT is an antioxidant enzyme protects the structure of the membranes from destruction and removes peroxide from the body.58,59 Decreased CAT activities are associated with increased oxidative stress.60 In the present study, the doxorubicin-treated group (G3) showed high levels of oxidative stress due to the effect of doxorubicin on oxidative stress markers, where there was a significant decrease in GSH and CAT activities and a significant increase in the testicular MDA concentration, the indicator of increased lipid peroxidation and oxidative damage caused by ROS. So, the oxidative stress produced by doxorubicin cytotoxicity may explain the testicular deterioration. Similarly, other studies showed a decrease in the activities of enzymic and non-enzymic antioxidants with an elevation of LPO in the testes of doxorubicin-treated rats.6,9,13
In the rat group administered with ZnO NPs alone (G2), GSH had a higher increase than in the control rats, which may be due to the increasing Zn concentration in the testicular tissue due to the ZnO nanoparticle dissociation. Additionally, Singh et al.61 reported that ZnO nanoparticles can be used as promising antioxidants in the biological system. So co-administration of ZnO NPs (3 mg per kg BW) induced a significant decrease in the testicular MDA concentration and significantly increased GSH and CAT activities compared with G3, which was treated with doxorubicin only. These results showed that co-administration of ZnO NPs could give protection against testicular LPO and oxidative stress induced by doxorubicin. Moreover, Dawei et al.18 reported that ZnO NPs have the ability to protect cell membrane integrity against oxidative stress damage, reduce the MDA level and increase antioxidant enzyme activities. Moreover, ZnO NPs at low doses have a hepatoprotective effect through the scavenging of free radicals or promotion of antioxidant activities, which detoxify the free radicals.62
Doxorubicin produces its antitumor effects and toxicity by the formation of intracellular ROS and free radicals which intercalated with DNA and led to subsequent inhibition of topoisomerase.63 Regarding doxorubicin-induced genotoxicity, the results from the G3 group showed a significant (P ≤ 0.05) increase in DNA-damaging parameters (tail length, DNA tail percentage and tail moment) in sperm cells compared with the control (G1). As mammalian spermatozoa are rich in polyunsaturated fatty acids and because of the inherent deficiency of the superoxide dismutase (SOD) family in testis, this may play a role in the susceptibility of spermatozoa to damage by free radicals.64 Normal sperm functioning needs a low physiological level of reactive oxygen species (ROS),65 and if ROS levels became above the physiological level it leads to deterioration in the sperm functioning.66 ROS and their metabolites can attack DNA, proteins, and lipids and alter enzymatic systems, cause cell death, and finally lead to a decline in the semen parameters concomitant with male infertility.67 Additionally, Minotti et al.4 and Carvalho et al.68 reported that doxorubicin induced cell damage through the production of ROS, lipid peroxidation, and DNA fragmentation. However, in the present study, co-treatment with a low dose of ZnO NPs attenuated the sperm cell DNA damage induced by doxorubicin. In addition, El-Masry et al.36 revealed that rats injected doxorubicin and ZnO NPs (5 mg per kg BW) showed less DNA fragmentation in liver tissues.
The present study confirmed that doxorubicin induced testicular and spermatozoa toxicity associated with oxidative damage and it was found that such toxicity could be improved by using ZnO NPs (3 mg per kg BW) as antioxidants. Moreover, Zhao et al.28 confirmed that an appropriate concentration of nano-ZnO stimulates Cu–Zn-SOD activity and this enhancement will suppress ROS generation. Sharma et al.69 also reported that nZnO enhances and improves the activities of antioxidants and decreases the levels of free radicals. Moreover, ZnO NPs (10 mg per kg BW) have the ability to protect the testicular tissue against the oxidative stress induced by diabetes in rats.48 Furthermore, diabetic rats treated with ZnO NPs revealed an increase in serum antioxidant enzyme (PON-1) levels, and nitric oxide also significantly decreased malondialdehyde (MDA), IL-1α and CRP (inflammatory markers) and fasting blood sugar.70
5. Conclusion
We conclude that co-treatment of doxorubicin with ZnO NPs at low doses significantly ameliorates doxorubicin-induced testicular toxicity and genotoxicity in rats due to its pharmacological role as a potent androgenic and antioxidant agent. Also, we confirm that doxorubicin as chemotherapy carries the risk of testicular damage and susceptibility of infertility.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
We would like to thank Prof. Dr Mostafa Mansour, Pathology Department, Faculty of Veterinary Medicine, Alexandria University, Egypt, for his help in the histopathological examination of this work.
References
- Arcamone F., Cassinelli G., Fantini G., Biotechnol. Bioeng., 1969, 116 , 1101 –1110 , , Pubmed ID: 5365804 . [DOI] [PubMed] [Google Scholar]
- Kassner N., Huse K., Martin H. J. U. Drug Metab. Dispos. 2008;36(10):2113–2120. doi: 10.1124/dmd.108.022251. [DOI] [PubMed] [Google Scholar]
- Quiles J. L., Huertas J. R., Battino M., Toxicology, 2002, 180 , 79 –95 , , Pubmed ID: 12324201 . [DOI] [PubMed] [Google Scholar]
- Minotti G., Menna P., Salvatorelli E., Pharm. Rev., 2004, 56 , 185 –229 , , Pubmed ID: 15169927 . [DOI] [PubMed] [Google Scholar]
- Othman A. I., El-Missiry M. A., Amer M. A., Life Sci., 2008, 83 , 563 –568 , , Pubmed ID: 18793653 . [DOI] [PubMed] [Google Scholar]
- Saalu L. C., Osinub A. A., Olaguniu J. I. Int. J. Cancer Res. 2010;6(1):1–9. doi: 10.3923/ijcr.2010.1.9. [DOI] [Google Scholar]
- Sah S. K., Khatiwada S., Chaudhary D. Nepal J. Biotechnol. 2015;3(1):10–14. doi: 10.3126/njb.v3i1.14223. [DOI] [Google Scholar]
- Kato M., Makino S., Kimura H., J. Toxicol. Sci., 2001, 26 , 51 –59 , , Pubmed ID: 11255793 . [DOI] [PubMed] [Google Scholar]
- Prahalathan C., Selvakumar E., Varalakshmi P., Clin. Chim. Acta, 2005, 360 , 160 –166 , , Pubmed ID: 15964560 . [DOI] [PubMed] [Google Scholar]
- Shivakumar P., Rani M. U., Reddy A. G., Toxicol. Int., 2012, 193 , 241 –244 , , Pubmed ID: 23293460 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hida H., Coudray C., Calop J., Biol. Trace Elem. Res., 1995, 47 , 111 –116 , , Pubmed ID: 7779536 . [DOI] [PubMed] [Google Scholar]
- Yang F., Kemp C. J., Henikoff S., Curr. Biol., 2013, 239 , 782 –787 , . http://www.ncbi.nlm.nih.gov/pmc/articles/PMC3678987/ . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K. M., Lee I. C., Kim S. H., Andrologia, 2012, 44 , 796 –803 , , Pubmed ID: 22212014 . [DOI] [PubMed] [Google Scholar]
- Vijay K. M., Mahesh V., Srinivas P. Int. J. Pharma Bio Sci. 2013;4(1):473–484. [Google Scholar]
- Rizk S. M., Zaki H. F., Mina M. A. M., Food Chem. Toxicol., 2014, 67 , 176 –186 , , Pubmed ID: 24593989 . [DOI] [PubMed] [Google Scholar]
- Yang C. C., Chen Y. T., Chen C. H., Am. J. Transl. Res., 2017, 912 , 5275 –5288 , http://www.ajtr.org . [PMC free article] [PubMed] [Google Scholar]
- Medina C., Santos-Martinez M. J., Radomski A., Br. J. Pharmacol., 2007, 150 , 552 –558 , , Pubmed ID: 17245366 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawei A., Zhisheng W., Anguo Z. J. Anim. Vet. Adv. 2009;9(10):1964–1967. [Google Scholar]
- Babadi V. Y., Najafi L., Najafi A., J. Pharm. Biomed. Sci., 2012, 23 , 1 –4 , , Pubmed ID: 16616095 . [Google Scholar]
- Shoeb M., Braj R. S., Javed A. K., Wasi K., Brahma N. S. Adv. Nat. Sci.: Nanosci. Nanotechnol. 2013;4:035015. [Google Scholar]
- Osmond M. J., Mccall M. J. Nanotoxicology. 2010;4(1):15–41. doi: 10.3109/17435390903502028. [DOI] [PubMed] [Google Scholar]
- Jiang J., Pi J., Cai J., Bioinorg. Chem. Appl., 2018. , 1062562 10.1155/2018/1062562 , , 18 pages . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy B., Baghel R. P. S., Mohanty T. K. Indian J. Anim. Nutr. 2013;30(4):339–350. doi: 10.1093/humrep/deu075. [DOI] [Google Scholar]
- Dani V., Dhawan D. K., Indian J. Med. Res., 2005, 122 , 338 –342 , , Pubmed ID: 16394327 . [PubMed] [Google Scholar]
- Malekirad A. A., Oryan S. H., Babapor V., Toxicol. Ind. Health, 2010, 26 , 331 –337 , , Pubmed ID: 20371635 . [DOI] [PubMed] [Google Scholar]
- Raajshreer K., Durairaj B. Int. J. Appl. Pharm. 2017;9(5):116–120. doi: 10.22159/ijap.2017v9i5.20847. [DOI] [Google Scholar]
- Pei X., Xiao Z., Liu L. J. Sci. Food Agric. 2019;99(3):1366–1374. doi: 10.1002/jsfa.9312. [DOI] [PubMed] [Google Scholar]
- Zhao C. Y., Tan S. X., Xiao X. Y. Biol. Trace Elem. Res. 2014;160(3):361–367. doi: 10.1007/s12011-014-0052-2. [DOI] [PubMed] [Google Scholar]
- Liu J., Ma X., Xu Y. Toxicol. Res. 2017;6:134. doi: 10.1039/c6tx00370b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Talebi A. R., Khorsandi L., Moridian M., J. Assist. Reprod. Genet., 2013, 309 , 1203 –1209 , , Pubmed ID: 23949131 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moridian M., Khorsandi L., Talebi A. R., Bratisl. Lek. Listy, 2015, 1165 , 321 –325 , , Pubmed ID: 25924642 . [DOI] [PubMed] [Google Scholar]
- Morsi M. H., Physiological effects of Nano-zinc on Reproductive performance and Immunity of Male Rat, Thesis (PhD), Faculty of Veterinary Medicine, Alexandria University, Egypt, 2015. [Google Scholar]
- Ibraheem S. R., Ibrahim M. R. MJS. 2016;27(5):1–10. doi: 10.23851/mjs.v27i5.160. [DOI] [Google Scholar]
- Torabi M., Kesmati M., Harooni H. E., Varzi H. N. Indian J. Pharmacol. 2013;45(5):508–512. doi: 10.4103/0253-7613.117784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russell L. D., Ettlin R. A. and Hikim A. P., et al., The classification of timing of spermatogenesis, in Histological and Histopathological evaluation of the testis, Cache River Press, 1990, pp. 41–58. [Google Scholar]
- El-Masry S. A., Sobhy H. M., Mansour M. k. Egypt. J. Chem. Environ. 2015;1(1):866–882. [Google Scholar]
- Matousek J., J. Reprod. Fertil., 1969, 19 , 63 –72 , , Pubmed ID: 5793915 . [DOI] [PubMed] [Google Scholar]
- Yokoi K., Uthus E. O., Nielsen F. H., Biol. Trace Elem. Res., 2003, 93 , 141 , , Pubmed ID: 12835498 . [DOI] [PubMed] [Google Scholar]
- Sönnmez M., Türk G., Yüce A., Theriogenology, 2005, 63 , 2063 –2072 , , Pubmed ID: 15823361 . [DOI] [PubMed] [Google Scholar]
- Bearden H. and Faquay J., Applied animal reproduction, Reston Publishing Co Inc, Reston Virginia, 1980, pp. 158–160. [Google Scholar]
- Demetrious J. A., Testosterone in methods, in Clinical chemistry, ed. A. G. Tech and L. A. Klapalan, CVMOS Co., 2nd edn, 1987, p. 268. [Google Scholar]
- Sedlak J., Lindsay R. H., Anal. Biochem., 1968, 25 , 192 –205 , , pii/0003269768900924 . [DOI] [PubMed] [Google Scholar]
- Aebi H., Catalase, in Methods of Enzymatic Analysis, ed. H. U. Bergmeryer and H. Ulrich, Verlag Chemic, Weinhein, New York, 2nd edn, 1974, p. 67. [Google Scholar]
- Ohkawa H. P., Ohishi W., Yagi K., Anal. Biochem., 1979, 952 , 351 –358 , , pii/0003269779907383 . [DOI] [PubMed] [Google Scholar]
- Simon L., Carrell D. T. Methods Mol. Biol. 2013;927:137–146. doi: 10.1007/978-1-62703-038-0_13. [DOI] [PubMed] [Google Scholar]
- Bancroft J. D. and Stevens A., Theory and Practice of Histological Techniques, Churchill Livingston, New York, Edinburgh, London, 4th edn, 1996. [Google Scholar]
- Patil L. R., Balaraman R. Int. J. ChemTech. Res. 2009;1(3):879–884. doi: 10.1111/j.1439-0272.2011.01269.x. [DOI] [Google Scholar]
- Afifi M., Almaghrabi O. A., Kadasam N. M. BioMed. Res. Int. 2015:153573. doi: 10.1155/2015/153573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohamed D. A., Abdelrahman S. A. Cell Tissue Res. 2018:1–16. doi: 10.1007/s00441-018-2909-8. [DOI] [PubMed] [Google Scholar]
- Weinbauer G. F. and Nieschlag E., The role of testosterone in spermatogenesis, in Testosterone: action, deficiency, substitution, ed. E. Nieschlag and H. M. Behre, Springer, Heidelberg, 2nd edn, 1998, pp. 144–168. [Google Scholar]
- McLachlau R. I., Wreford N. G., O'Donnell L. J. Endocrinol. 1996;148(1):1–9. doi: 10.1677/joe.0.1480001. [DOI] [PubMed] [Google Scholar]
- Javed M. T., Khan A., Ali M. Vet. Arh. 2000;70:141–149. [Google Scholar]
- Meeker J. D., Godfrey-Bailey L., Hauser R. J. Androl. 2007;28(3):397–406. doi: 10.2164/jandrol.106.001545. [DOI] [PubMed] [Google Scholar]
- Ateşşahin A., Türk G., Karahan I., Fertil. Steril., 2006, 85 , 1216 –1222 , , Pubmed ID: 16616095 . [DOI] [PubMed] [Google Scholar]
- Gulia S., Sarkar M., Kumar V., Trop. Anim. Health Prod., 2010, 42 , 1143 –1148 , http://www.ncbi.nlm.nih.gov/pubmed/20213223 . [DOI] [PubMed] [Google Scholar]
- Semet M., Paci M., Saïas-Magnan J. Andrology. 2017;5:640–663. doi: 10.1111/andr.12366. [DOI] [PubMed] [Google Scholar]
- Al-Ani N. K., Al-Kawaz U., Saeed B. T. Am. J. Med. Sci. 2015;5(2):73–81. doi: 10.5923/j.ajmms.20150502.03. [DOI] [Google Scholar]
- Jozefczak M., Remans T., Vangronsveld J. Int. J. Mol. Sci. 2012;13(3):3145–3175. doi: 10.3390/ijms13033145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinzato Y. K., Lima P. H. O., de Campos K. E. Rev. Assoc. Med. Bras. 2009;55(4):384–388. doi: 10.1590/s0104-42302009000400010. [DOI] [PubMed] [Google Scholar]
- Duzgunera V., Sule Kaya S. Free Radicals Biol. Med. 2007;42(10):1481–1486. doi: 10.1016/j.freeradbiomed.2007.02.021. [DOI] [PubMed] [Google Scholar]
- Singh B. N., Rawat A., Khan K. S. PLoS One. 2014;9(9):e106937. doi: 10.1371/journal.pone.010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atef H. A., Mansour M. K., Ibrahim E. M. Int. J. Curr. Microbiol. Appl. Sci. 2016;5(12):795–818. [Google Scholar]
- Hrdina R., Gersl V. I., Klimtova T. Acta Med. 2000;43(3):75–82. [PubMed] [Google Scholar]
- Sikka S. C., J. Androl., 2004, 25 , 5 –18 , , Pubmed ID: 14662779 . [DOI] [PubMed] [Google Scholar]
- Agarwal A., Said T. M., Hum. Reprod. Update, 2003, 9 , 331 –345 , , Pubmed ID: 12926527 . [DOI] [PubMed] [Google Scholar]
- Aitken J. R., Baker M. A., Int. J. Androl., 2002, 25 , 191 –194 , , Pubmed ID: 12121567 . [DOI] [PubMed] [Google Scholar]
- Agarwal A., Virk G., Ong C. World J. Men's Health. 2014;32(1):1–17. doi: 10.5534/wjmh.2014.32.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho C., Santos R. X., Correia S., Curr. Med. Chem., 2009, 16 , 3267 –3285 , , Pubmed ID: 19548866 . [DOI] [PubMed] [Google Scholar]
- Sharma V., Singh P., Pandey A. K., Mutat. Res., 2012, 745 , 84 –91 , , Pubmed ID: 22198329 . [DOI] [PubMed] [Google Scholar]
- Hussein J., El-Banna M., Razik T. A. Int. J. Biol. Macromol. 2018;107:748–754. doi: 10.1016/j.ijbiomac.2017.09.056. [DOI] [PubMed] [Google Scholar]