BIT may be involved in mucus hypersecretion associated with airway inflammatory diseases.
BIT may be involved in mucus hypersecretion associated with airway inflammatory diseases.
Abstract
Mucus plays an important role in protecting the respiratory tract from irritants. However, mucus hypersecretion is a major indicator of airway diseases. 1,2-Benzisothiazolin-3-one (BIT), as a microbicide, induces asthmatic inflammation. Therefore, we focused on the effects of BIT-related mucin secretion in airway epithelial cells. Our in vivo study showed increased mucus and MUC5AC expressions in the bronchioles of mice that inhaled BIT. For investigating the signaling pathways, we performed experiments in human airway epithelial cells. BIT induced the MUC5AC expression and significantly increased the phosphorylation of extracellular signal-regulated kinase 1/2 (ERK1/2), p38, and nuclear factor kappa-light-chain-enhancer of activated B cells (NF-κB). The specific inhibitors of ERK1/2, p38, and NF-κB blocked the BIT-induced MUC5AC expression. Therefore, these results suggest that BIT induces the MUC5AC expression via the ERK1/2, p38, and NF-κB pathways in human airway epithelial cells, which may be involved in mucus hypersecretion associated with airway inflammatory diseases.
1. Introduction
Biocides are widely used in agriculture, forestry, and industry for the prevention of infection from the short-lived biocides in industrial water treatment to disinfectants in swimming pools. Among the biocides, benzisothiazolinone (BIT) is commonly used as a fungicide and preservative in cosmetics and house cleaners. However, when the use of BIT increases, it cannot be considered safe. BIT has a low molecular weight and can be easily penetrated. Hence, it is classified as an irritant of the skin, eyes, and lungs. According to previous studies, exposure to BIT at excess doses and long duration causes skin sensitization due to its ability to react with skin proteins. BIT at the concentration of 20 ppm causes the sensitization of allergic contact dermatitis.1,2 Moreover, higher concentrations of BIT have been shown to disrupt balanced metabolism by causing the inhibition of enzymes involved in the central metabolic pathway of the Kreb's cycle. Besides, it also inhibits cell activity due to the progressive loss of protein thiols from multiple pathways in the cells.3 The inhalation of BIT causes occupational asthma and rhinitis.4 Many research findings indicate that BIT causes skin diseases, but studies on respiratory diseases are still lacking. BIT is also used in the spray form such as in fabric conditioners and lotions, which can cause respiratory problems. It is known to cause asthma and rhinitis in people who have been exposed to BIT at a sufficient dose and duration, but the precise mechanisms are unknown.
Mucociliary clearance (MCC) in the respiratory tract acts as the first physical barrier of the innate immune defense mechanism, by which inhaled environmental stimuli, including microbes and irritants.5 MCC depends on two important constituents: mucus production and mucus transport. Mucus in the airway is mainly composed of water, ions, lipids, and various macromolecules. Mucus overproduction leads to abnormal ciliary beat frequency, dehydration of the airway surface liquid, and disrupted MCC.6 The disruption of MCC increases the risk of developing bacterial infections in the airway, leading to many respiratory diseases including chronic rhinosinusitis, asthma, chronic obstructive pulmonary disease (COPD), and cystic fibrosis.7,8 Impaired mucus clearance exacerbates airway hyper-reactivity, goblet cell metaplasia, and airway inflammation.9 Mucin is the principal determinant of the viscoelastic properties of mucus, which is present as a gel layer overlying the surface of the airways to preserve the respiratory tract. A total 21 types of human mucin genes have been identified: 14 out of 21 are found in the airways. Among these airway-related mucin genes, MUC5AC encodes structurally related gel-forming glycoproteins. Nowadays, strategies for blocking mucin secretion by targeting specific signals have gained attention as potential therapeutic treatments.10 Therefore, the aim of this study was to determine the effects of BIT on mucus production and the MUC5AC expression in mice airway and the underlying signaling pathways in human airway epithelial NCI-H292 cells.
2. Materials and methods
2.1. Materials
BIT was purchased from Sigma Aldrich (Saint Louis, MO, USA). Human airway epithelial NCI-H292 cells (ATCC, Manassas, VA, USA) were used. Specific inhibitors were purchased as follows: U0126 from Calbiochem (San Diego, CA, USA), SB203580 from BIOMOL (Plymouth Meeting, PA, USA) and BAY 11-7085 from Sigma-Aldrich (Saint Louis, MO, USA). ERK1/2, p38, and NF-kB antibodies were purchased from Cell Signaling Technology (Danvers, MA, USA). The secondary antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA).
2.2. Cell viability assay
Cell viability and proliferation were detected by Water-soluble tetrazolium salt-1 (WST-1). EZ-Cytox cell viability assay kit (Daeil Lab, Seoul, Korea) was used after incubation with BIT for 48 h. The absorbance of samples was measured at 450 nm (reference wavelength 600–650 nm) using a microplate reader (Tecan Austria GmbH, Austria).
2.3. Animal husbandry and mice inhalation
Wild-type BALB/c mice (male, 5–6 weeks old, weighing 15–18 g, n = 8) were purchased from Koatech (Pyeongtaek, Gyunggi-do, Korea). The mice were housed in polycarbonate cages under controlled temperatures (20–25 °C) and humidity (40–45%) under a regulated 12 h light/dark cycle. The mice were nebulized with PBS and exposed to BIT (0.4 or 0.8 mg kg–1) for 1 h every 5 days using the Whole Body Plethysmograph system (Buxco, DSI, MN, USA). All animals were treated in accordance with the Guidelines for the Care and Use of Laboratory Animals issued by the Yeungnam University, and all of the experimental protocols were approved by the Ethical Committee of Yeungnam University (YUMC-AEC2018-012). Animal lungs were harvested under anesthesia using Zoletil/Rompun.
2.4. RT-PCR and real-time PCR
RT-PCR and real-time PCR were performed using the GeneAmp RNA core Kit and iQ SYBR Green Supermix (Bio-Rad, Hercules, CA, USA), respectively, according to previously published protocols.11–13 The PCR products were quantified and normalized to the expression of a housekeeping gene, glyceraldehyde 3-phosphate dehydrogenase.
2.5. Enzyme-linked immunosorbent assay (ELISA)
Cell supernatants were transferred to an F96 Cert. Maxisorp Nunc-Immuno plate (Fisher Scientific, Lenexa, KS, USA). Subsequently, the samples were incubated with the primary antibody rabbit anti-MUC5AC (Santa Cruz Biotechnology) for 24 h and then incubated with the horseradish peroxidase-conjugated secondary antibody for 1 h. Optical density was measured at 450 nm using an ELISA reader (BIO-TEK Instruments, Winooski, VT, USA). Results were expressed as fold increase from the baseline control.
2.6. Western blot analysis
The cell lysate samples were separated using 10% SDS-PAGE and transferred onto a PVDF membrane. The membrane was incubated overnight with the primary antibody at 4 °C. After washing, the blots were incubated with a secondary antibody for 1 h. Bands were detected after the exposure of the membrane to an imaging system (Chemiluminescence image system, Eberhardzell, Germany).
2.7. Cell transfections with siRNAs
Small interfering RNAs were transfected into cells using Lipofectamine® 2000 (Invitrogen, Carlsbad, CA, USA) according to the manufacturer's protocol.13
2.8. Immunohistochemistry (IHC) and periodic-acid-Schiff (PAS) staining
Immunohistochemistry analysis was performed on 4 μm-thick sections of paraffin-embedded mice lung tissues treated with or without BIT. For the negative control, non-immune serum immunoglobulin G was used instead of the primary antibody. The slides were counterstained with hematoxylin and mounted.14 PAS staining was performed using the PAS staining kit (Merck Millipore, Darmstadt, Germany) according to the manufacturer's protocol. Immunohistochemistry and PAS staining of MUC5AC was analyzed on three different sections in five tissues per group and classified by two independent blinded observers using a semi-quantitative method.
2.9. Statistical analysis
Statistical analyses were performed using SPSS version 12.0 software (SPSS, Chicago, IL, USA). Data were expressed as means ± S.D. Comparisons were done using the unpaired t-test or Kruskal–Wallis test followed by the Mann–Whitney test. For all the tests, p-value < 0.05 was considered statistically significant.
3. Results
3.1. BIT induced MUC5AC expression in the bronchioles of mice
BIT belongs to the group of isothiazolinones (Fig. 1A). The results of real-time PCR showed significantly increased mRNA expression levels of MUC5AC in the lung tissues of BIT-inhaled mice (Fig. 1B).
Fig. 1. MUC5AC gene expression increased in the bronchioles of BIT-inhaled mice. (A) Chemical structure of 1,2-benzisothiazolin-3-one (BIT). (B) Results of real-time PCR show significantly increased MUC5AC gene expression in the lung tissue of BIT-inhaled mice. Bars indicate mean ± S.D. of three independent experiments performed in triplicates. *p < 0.05 compared with zero value.
3.2. BIT induced mucus secretion and MUC5AC expression in the bronchioles of mice
To confirm the effect of BIT on mucus production and MUC5AC protein production, PAS staining and IHC analysis were performed. The results of PAS staining showed abundant mucus production in the bronchioles of BIT-inhaled mice compared to that of PBS-inhaled mice (Fig. 2A). IHC analysis revealed increased MUC5AC protein production in BIT-inhaled mice compared to that in PBS-inhaled mice, which was confirmed by the increased immunoreactivity mainly in the epithelium (Fig. 2C). Semi-quantitative histological analysis showed significantly higher immunoreactivity scores for MUC5AC in the bronchioles of BIT-inhaled mice compared to that for the bronchioles of PBS-inhaled mice (Fig. 2B and D).
Fig. 2. Mucus secretion and MUC5AC expression increased in the bronchioles of BIT-inhaled mice. (A) Periodic-acid-Schiff (PAS) staining of the bronchioles showed that mucus production increased in BIT-inhaled mice (arrow). PAS positive substances are stained pink and the nuclei are blue. The upper panel shows the lung tissue at ×100 magnification, and the lower panel shows the lung tissue at ×400 magnification. (C) Immunohistochemical staining showed that the MUC5AC expression was significantly increased in the bronchioles of BIT-inhaled mice than that in PBS-inhaled mice (×400 magnification; immunopositive cells appear brown). (B and D) Semi-quantitative histological analysis showed that the immunoreactivity scores of MUC5AC in BIT-inhaled mice were significantly higher than that for PBS-inhaled mice. Bars indicate mean ± S.D. of three independent experiments performed in triplicates. Scale bar, 100 μm. *p < 0.05 compared with zero value.
3.3. BIT induced MUC5AC expression in human airway epithelial NCI-H292 cells
BIT did not inhibit cell viability and proliferation at concentrations up to 200 μM (Fig. 3A). In vitro experiments were performed to evaluate the specific mechanism of the effect of BIT on the MUC5AC expression in human airway epithelial NCI-H292 cells. To determine the effect of BIT, we performed RT-PCR and ELISA (Fig. 3B). The results showed that the MUC5AC gene expression and protein production were significantly increased by BIT at 50 μM (Fig. 3C). Additionally, this effect was evident from 4 h after incubation and peaked at 8 h after exposure to BIT (Fig. 3D).
Fig. 3. The effects of BIT on cell viability were measured using a WST-1 assay and BIT-induced MUC5AC expression in human airway epithelial NCI-H292 cells. (A) BIT did not affect cell viability up to 200 μM. (B and C) RT-PCR and ELISA results showed BIT concentration-dependent increase in MUC5AC mRNA expression and protein production. (D) Results of real-time PCR showed that the MUC5AC mRNA expression peaked at 8 h after exposure to BIT. Images are representative of three separate experiments performed in triplicates. Bars indicate mean ± S.D. of three independent experiments performed in triplicates. ELISA, enzyme-linked immunosorbent assay; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; RT-PCR, reverse transcription polymerase chain reaction. *p < 0.05 compared with zero value.
3.4. BIT activated the phosphorylation of ERK1/2, p38, and NF-κB in human airway epithelial NCI-H292 cells
To identify the signaling pathway involved in BIT (50 μM)-induced MUC5AC expression, the ERK1/2, p38, and NF-κB signaling pathways were investigated. The results suggested that BIT significantly increased the phosphorylation of ERK1/2, p38, and NF-κB (Fig. 4A). Furthermore, to determine the involvement of ERK1/2, p38, and NF-κB signaling pathways in the BIT-induced MUC5AC production, we treated the human airway epithelial NCI-H292 cells with the ERK1/2 pathway inhibitor U0126, p38 inhibitor SB203580, and NF-κB inhibitor BAY 11-7085. The results showed that the inhibitors significantly attenuated BIT-induced MUC5AC mRNA expressions and protein levels (Fig. 4B and C). Additionally, the knockdown of ERK1, ERK2, p38, and NF-κB by siRNA transfection confirmed that the BIT-induced MUC5AC mRNA expression was significantly blocked (Fig. 4D).
Fig. 4. BIT-induced MUC5AC expression in human airway epithelial NCI-H292 cells was associated with ERK1/2, p38 and NF-κB activation. (A) Results of western blot analysis showed that BIT induced the phosphorylation of ERK1/2, p38 and NF-κB. (B and C) Results of RT-PCR and ELISA showed that U0126 (a specific ERK1/2 inhibitor), SB203580 (a specific p38 inhibitor), and BAY 11-7085 (a specific NF-κB inhibitor) significantly attenuated the BIT-induced MUC5AC mRNA expression and protein levels. (D) Results of RT-PCR showed that the knockdown of ERK1, ERK2, p38 and NF-κB by siRNA significantly blocked BIT-induced MUC5AC mRNA expression. Images are representative of three separate experiments performed in triplicates. Bars indicate mean ± S.D. of three independent experiments performed in triplicates. Con, control; ELISA, enzyme-linked immunosorbent assay; ERK, extracellular signal-regulated kinase; GAPDH, glyceraldehyde 3-phosphate dehydrogenase; NF-κB, nuclear factor kappa-light-chain-enhancer of activated B cells; p-ERK, phosphorylated ERK; p-NF-κB, phosphorylated NF-κB; p-p38, phosphorylated p38. *p < 0.05 compared with the zero value, †p < 0.05 compared with BIT (50 μM) alone.
4. Discussion
Mucin is the major component of mucus, which is synthesized by the goblet cells and submucosal glands to protect and lubricate the luminal surfaces of respiratory tracts.15 Goblet cell hyperplasia and metaplasia are caused by various stimuli, including bacteria, particles, and chemical irritants.16 Healthy individuals have a few mucin-producing cells in the airway epithelium, whereas asthma and COPD patients have a higher number of goblet cells due to mucin hypersecretion.17 Mucins have two major subfamilies: secreted and membrane-tethered mucins. Among the secreted mucins, MUC5AC is a major gel-forming mucin, which is a marker of goblet cells in the human airway epithelium. Previous studies have reported increased levels of MUC5AC in patients with asthma.18 Excessive mucin secretion contributes to airway obstruction and impairment of MCC, which lead to bacterial colonization in the airway and the associated exacerbations. Patients with airway diseases, such as rhinosinusitis, COPD, asthma, and cystic fibrosis, exhibit mucin hypersecretion and adhesive mucus in the airway.19–22 Consequently, mucin hypersecretion contributes to morbidity and mortality in airway diseases.
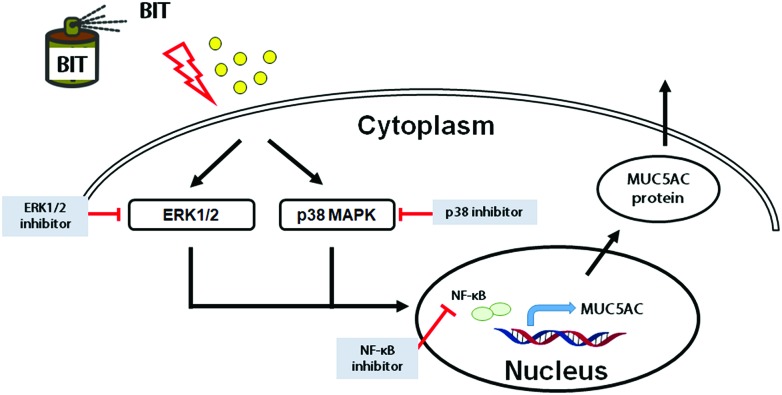
Isothiazolones are heterocyclic chemical compounds, which are widely used as antimicrobial agents. The derivatives of isothiazolones include methylisothiazolinone (MIT), chloromethylisothiazolinone (CMIT), and BIT.1 These biocides have been used for industrial applications requiring long-term preservation for microbe control. The CMIT/MIT chemical mixture has been shown to increase the levels of inflammatory cytokines and induce the infiltration of immune cells in human liver epithelial cells.23 In addition, MIT induces cell death by the activation of ERK1/2 via a 12-lipoxygenase-mediated pathway in neuron-enriched cultured cells.24,25 However, the effect of BIT on airway mucin production has not been determined, and its associated signaling pathway has not been explored. In line with the increasing evidences, we hypothesized that the exposure to ambient biocides can be associated with respiratory diseases. Therefore, we investigated the in vivo effects of BIT on mucus secretion and MUC5AC expression in mice lungs. Our results showed that the amount of mucus production increased in the BIT-nebulized mice group compared to that of the control group. An increase in the MUC5AC expression was also recorded in the bronchioles of the BIT-inhaled mice. These findings suggested that BIT is associated with mucin secretion in the mice respiratory tract. Previous studies have reported that the mucus production in the human airway can be increased by various stimuli involving MAPKs or NF-κB activation.26,27 To further confirm the signaling pathway involved in BIT-induced MUC5AC expression in human airway NCI-H292 epithelial cells, we performed in vitro studies. We aimed to determine that the BIT-induced MUC5AC expression involved MAPK and NF-κB. Our results showed that BIT activated ERK1/2, p38, and NF-κB. MAPK inhibitors and NF-κB inhibitor significantly blocked BIT-induced MUC5AC expression. In addition, these results were confirmed by cell transfection with siRNAs. The knockdown of ERK1, ERK2, p38, and NF-κB inhibited the BIT-induced MUC5AC expression. These results suggest a possible association of BIT with the expression of MUC5AC via the ERK1/2, p38 and NF-κB pathways in human airway epithelial cells (Fig. 5). However, there are some limitations in this study: we only determined whether BIT induced mucus and MUC5AC expression in mice but not the signaling pathway. Further studies are necessary to elucidate the signaling pathway for mucus production in mice.
Fig. 5. The schematic of the signaling pathway of BIT-induced MUC5AC expression. BIT induced MUC5AC expression via the activation of ERK1/2, p38, and NF-κB in human airway epithelial cells.
5. Conclusion
This study demonstrates that BIT induced the MUC5AC expression via the ERK1/2, p38, and NF-κB pathways in airway epithelial cells. Further studies are needed to explore the expressions of other types of airway mucin genes and the specific signaling pathway in relation to the effect of BIT on human airway epithelial cells. Our findings provide basic information about the aggravation of airway inflammation and the related mucus hypersecretion associated with BIT.
Author contributions
SYK and HGK: experimentation; YSC: study design; HGN: data collection, writing; CHB: manuscript revision; SYS: data analysis, data interpretation; YDK: study concept, supervision.
Conflicts of interest
The authors have declared that they have no conflicts of interest.
Acknowledgments
This work was supported by the 2016 Yeungnam University Research Grant.
References
- Aalto-Korte K., Ackermann L., Henriks-Eckerman M. L., Valimaa J., Reinikka-Railo H., Leppanen E., Jolanki R. Contact Dermatitis. 2007;57:365–370. doi: 10.1111/j.1600-0536.2007.01278.x. [DOI] [PubMed] [Google Scholar]
- Basketter D. A., Rodford R., Kimber I., Smith I., Wahlberg J. E. Contact Dermatitis. 1999;40:150–154. doi: 10.1111/j.1600-0536.1999.tb06013.x. [DOI] [PubMed] [Google Scholar]
- Jacobson A., Williams T. M. Chim. Oggi. 2000;18:105–108. [Google Scholar]
- Moscato G., Omodeo P., Dellabianca A., Colli M. C., Pugliese F., Locatelli C., Scibilia J. Occup. Med. 1997;47:249–251. doi: 10.1093/occmed/47.4.249. [DOI] [PubMed] [Google Scholar]
- Munkholm M., Mortensen J. Clin. Physiol. Funct. Imaging. 2014;34:171–177. doi: 10.1111/cpf.12085. [DOI] [PubMed] [Google Scholar]
- Weiterer S., Schulte D., Muller S., Kohlen T., Uhle F., Weigand M. A., Henrich M. PLoS One. 2014;9:e91705. doi: 10.1371/journal.pone.0091705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daviskas E., Anderson S. D., Shaw J., Eberl S., Seale J. P., Yang I. A., Young I. H. Respirology. 2005;10:426–435. doi: 10.1111/j.1440-1843.2005.00738.x. [DOI] [PubMed] [Google Scholar]
- Ghosh A., Boucher R. C., Tarran R. Cell. Mol. Life Sci. 2015;72:3637–3652. doi: 10.1007/s00018-015-1946-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma J., Rubin B. K., Voynow J. A. Chest. 2018;154:169–176. doi: 10.1016/j.chest.2017.11.008. [DOI] [PubMed] [Google Scholar]
- Ha E. V. S., Rogers D. F. Pharmacology. 2016;97:84–100. doi: 10.1159/000442794. [DOI] [PubMed] [Google Scholar]
- Song S. Y., Woo H. J., Bae C. H., Kim Y. W., Kim Y. D. Laryngoscope. 2010;120:1046–1050. doi: 10.1002/lary.20844. [DOI] [PubMed] [Google Scholar]
- Woo H. J., Yoo W. J., Bae C. H., Song S. Y., Kim Y. W., Park S. Y., Kim Y. D. Exp. Lung Res. 2010;36:262–269. doi: 10.3109/01902140903427033. [DOI] [PubMed] [Google Scholar]
- Kwak S., Kim Y. D., Na H. G., Bae C. H., Song S. Y., Choi Y. S. Biochem. Biophys. Res. Commun. 2018;499:655–661. doi: 10.1016/j.bbrc.2018.03.206. [DOI] [PubMed] [Google Scholar]
- Bae C. H., Na H. G., Choi Y. S., Song S. Y., Kim Y. D. Clin. Exp. Otorhinolaryngol. 2018;11:124–132. doi: 10.21053/ceo.2017.00493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. F. Int. J. Biochem. Cell Biol. 2003;35:1–6. doi: 10.1016/s1357-2725(02)00083-3. [DOI] [PubMed] [Google Scholar]
- Shale D. J., Ionescu A. A. Eur. Respir. J. 2004;23:797–798. doi: 10.1183/09031936.0.00018404. [DOI] [PubMed] [Google Scholar]
- Rose M. C., Nickola T. J., Voynow J. A. Am. J. Respir. Cell Mol. Biol. 2001;25:533–537. doi: 10.1165/ajrcmb.25.5.f218. [DOI] [PubMed] [Google Scholar]
- Thornton D. J., Carlstedt I., Howard M., Devine P. L., Price M. R., Sheehan J. K. Biochem. J. 1996;316:967–975. doi: 10.1042/bj3160967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S. A., Ishinaga H., Takeuchi K. J. Inflammation. 2016;13:11. doi: 10.1186/s12950-016-0121-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers D. F. Novartis Found. Symp. 2001;234:65–83. doi: 10.1002/0470868678.ch5. [DOI] [PubMed] [Google Scholar]
- Rogers D. F. Curr. Opin. Pharmacol. 2004;4:241–250. doi: 10.1016/j.coph.2004.01.011. [DOI] [PubMed] [Google Scholar]
- Puchelle E., Bajolet O., Abely M. Paediatr. Respir. Rev. 2002;3:115–119. doi: 10.1016/s1526-0550(02)00005-7. [DOI] [PubMed] [Google Scholar]
- Park E. J., Kim S., Chang J. Environ. Toxicol. 2018;33:156–166. doi: 10.1002/tox.22503. [DOI] [PubMed] [Google Scholar]
- He K., Huang J., Lagenaur C. F., Aizenman E. J. Pharmacol. Exp. Ther. 2006;317:1320–1329. doi: 10.1124/jpet.106.103044. [DOI] [PubMed] [Google Scholar]
- Du S., McLaughlin B., Pal S., Aizenman E. J. Neurosci. 2002;22:7408–7416. doi: 10.1523/JNEUROSCI.22-17-07408.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bae C. H., Choi Y. S., Song S. Y., Kim Y. K., Kim Y. D. Int. Forum Allergy Rhinol. 2017;7:91–98. doi: 10.1002/alr.21844. [DOI] [PubMed] [Google Scholar]
- Kim Y. D., Choi Y., Na H., Bae C., Song S. Y. Am. J. Respir. Crit. Care Med. 2016;193:161–165. [Google Scholar]