 We studied the effects of high mobility group box chromosomal protein 1 (HMGB1) and toll-like receptor (TLR4) in diisonoyl phthalate (DINP)-induced asthma.
We studied the effects of high mobility group box chromosomal protein 1 (HMGB1) and toll-like receptor (TLR4) in diisonoyl phthalate (DINP)-induced asthma.
Abstract
We studied the effects of high mobility group box chromosomal protein 1 (HMGB1) and toll-like receptor (TLR4) in diisonoyl phthalate (DINP)-induced asthma. Mice with DINP-induced asthma were treated with a TLR4-signaling inhibitor or anti-HMGB1 antibody, and various markers of asthma were measured 24 h later. DINP increased airway hyperresponsiveness, numbers of cells in BALF, numbers of inflammatory cells (leukocytes, lymphocytes, monocytes, eosinophils, neutrophils, basophils) in blood, mucus production, pulmonary fibrosis, Th2 type cytokine levels in BALF, and lung cell apoptosis. On the other hand, administrations of TLR4-signaling inhibitors (TAK-242) or anti-HMGB1 antibodies to a mouse model of DINP-induced asthma reduced biological markers of asthma. These results show TLR4 and HMGB1 both contribute to DINP-induced asthma, and that the inhibitions of TLR4 or HMGB1 offer potential means of treating asthma induced by phthalates like DINP.
1. Introduction
Asthma is one of the most common chronic diseases and is estimated to affect about 300 million people worldwide. The incidence of asthma appears to increase with the adoption of a western lifestyle, and as the urban population is expected to increase by 14% in 2025, the number of asthmatic patients is expected to increase significantly over the next 20 years.1 The prevalences of bronchial asthma and allergic respiratory diseases are increasing in recently industrialized countries in-line with changes in environmental factors such as air pollution. Over the past two decades, interest has grown in the relation between air pollution and human health.2 Asthma is characterized by intermittent manifestations of wheezing, coughing, and dyspnea caused by airway inflammation, mucinous hyperalgesia, and increased bronchial responses to various stimuli.3 Generally, asthma is caused by a Th1 to Th2 cell imbalance, and cytokines secreted by these cells are known to play important roles in the regulation and amplification of inflammatory response in asthma. IL-4, IL-5 and IL-13 are mainly secreted by Th2 cells whereas IFN-r and IL-2 are secreted by Th1 cells, and Th2 cell-associated cytokines play important roles in the pathophysiologies of allergic diseases such as asthma.4
Phthalates have been used for more than 50 years to increase the plasticity of rigid plastics such as polyvinyl chloride (PVC) and are approved for use in consumer products such as children's toys, medicines, nutritional supplements, cleaning materials, lubricants, insecticides, solvents, adhesives, paints, and building materials. Due to the widespread use of phthalates worldwide (annual production now exceeds 3 million metric tons per year) the impact of phthalate exposure on human health has become an issue of concern.5 Phthalates are found in food and water and in indoor dust. Contaminated food is the main cause of exposure, but its presence in indoor dust means infants have higher phthalate intakes than adults.6 No study has yet reported that DINP itself causes asthma in humans. However, studies have shown that DINP is associated with a variety of inflammatory diseases, including asthma. Long-term oral exposure to DINP aggravates allergic contact dermatitis (ACD) in mice model by activation of NF-kB.7 Exposure to diisonoyl phthalate (DINP) has been reported to activate allergic airway inflammation by stimulating the phosphoinositide 3-kinase (PI3K)/Akt pathway in rat pups,8 and recently, we showed DINP suppresses Th1 differentiation and promotes Th2 polarization from naive CD4+ T cells in vitro, and reported that Th2 type immune response in animals sensitized and challenged with DINP involved the up-regulations of Th2-mediated cytokines or immunoglobulin E in vivo.9 These studies show that DINP provides a risk for asthma induction in humans.
HMGB1 (high mobility group box chromosomal protein 1) is a protein that specifically binds to the linker region of nucleosomal DNA, enhances nucleosome stability, and enhances transcription factor interactions.10 The translocation of HMGB1 from the intracellular to the extracellular compartment is an important event in host defense and inflammation. HMGB1 is released from inflammatory cells stimulated by molecules released by exogenous pathogens or endogenous inflammatory mediators or secreted by necrotic cells or apoptotic bodies.11 HMGB1 triggers inflammatory response and tissue injury in various organ systems including heart, kidney, pancreas, and liver. Furthermore, lung diseases such as asthma, acute respiratory distress syndrome, cystic fibrosis, and lung cancer have all been associated with HMGB1 up-regulation,12 and HMGB1 secreted by affected cells has been shown to bind to receptors such as TLR2 (Toll-like receptor 2), TLR4, and RAGE (receptor for advanced glycation endproducts), the latter of which transduces intracellular signals that activate MAPK (mitogen-activated protein kinase) and NF-κB (nuclear factor kappaB), which when activated induce the productions of pro-inflammatory cytokines, which in turn, exacerbate airway inflammation.13
In previous studies, we demonstrated that exposing C57BL/6 to DINP induces asthma, but as yet, relationships between DINP exposure and HMGB1 and TLR4 proteins have not been studied. In the present study, we investigated the roles played by TLR4 and HMGB1 in mice with DINP-induced asthma using a TLR4-signaling inhibitor and anti-HMGB1 antibodies.
2. Materials and methods
2.1. Reagents
Diisononyl phthalate was purchased from Sigma-Aldrich (St Louis, USA) and TAK-242 (Toll-like receptor 4-signaling inhibitor; a TLR4-signaling inhibitor) was purchased from MedChem Express. Anti-high mobility group box 1 (HMGB1) antibody was purchased from R&D Systems. Purified rat anti-mouse IL-4, purified rat anti-mouse IL-5, purified rat anti-mouse IL-13, purified rat anti-mouse IFN-γ, biotin rat anti-mouse IL-4, biotin rat anti-mouse IL-5, biotin rat anti-mouse IL-13, and biotin rat anti-mouse IFN-γ were obtained from BD Biosciences (San Diego, USA) and used in the ELISA experiments.
2.2. Animals
Eight-week-old female C57BL/6 mice were bred and maintained under specific pathogen-free conditions at Orient Bio (Seongnam, Korea). Animals were individually housed in standard polycarbonate cages under a 12 h light/dark cycle at 22 ± 2 °C and 50%–60% RH and provide a standard animal diet and water. All animal experiments were carried out according to the procedures approved by the Sunchon National University Institutional Animal Care and Use Committee (SCNU IACUC) for the care and use of laboratory animals. All procedures were approved by the SCNU IACUC (permit number: SCNU IACUC-2018-08). Mice were acclimatized for five days before experiments. Animal experiments were performed under Zoletil/Rumpun anesthesia.
2.3. Induction of airway inflammation using DINP
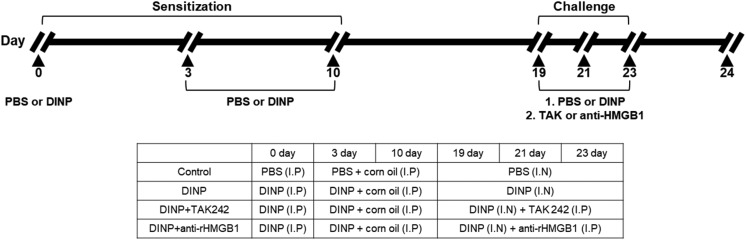
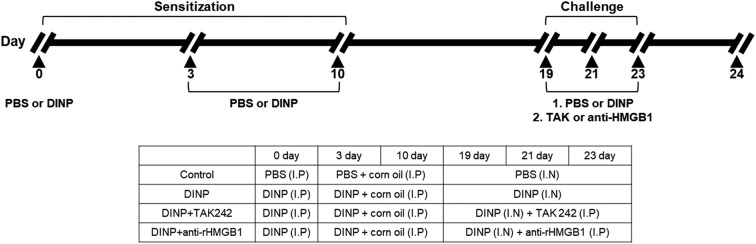
Airway inflammation was induced as previously described.9 Mice were randomly divided into four groups (n = 5): control group, DINP group, DINP + TAK242 group, and DINP + anti-HMGB1 antibody group. C57BL/6 mice were primarily sensitized with an intraperitoneal (i.p.) injection of DINP in 0.2 mL of PBS on day 0. On days 3 and 10, mice were secondarily sensitized with an i.p. injection of DINP (50 mg kg–1) dissolved in corn oil. On days 19, 21, and 23, the mice were challenged intranasally with phosphate-buffered saline (PBS) or 50 mg kg–1 of DINP dissolved in 50 μL. During challenge, mice in the DINP + TAK242 and DINP + anti-HMGB1 antibody groups received an intravenous (i.v.) injection of 3 mg kg–1 TAK-242 or 10 mg kg–1 anti-HMGB1 antibody, respectively, on each day of challenge via a tail vein (Fig. 1). 24 h after the last airway challenge, blood was collected from the retro orbital plexus and inflammatory cell counts in blood (white blood cells, neutrophils, lymphocytes, monocytes, eosinophils, and basophils) were determined using a HEMAVET 950 (Drew Scientific Inc). After sacrifice by cervical dislocation, bronchoalveolar lavage (BAL) was performed four times with 0.5 ml of saline to collect BALF. After centrifuging samples, BALF supernatants were combined and stored at –20 °C until required for ELISA of IL-4, IL5, and IFN-γ. Red blood cells in BALF were removed using tris-buffered ammonium chloride. Total cells in BLAF were counted using a hemocytometer.
Fig. 1. Experimental protocol for induction of airway inflammation along with treatment scheme.
2.4. Assessment of airway hyperresponsiveness to TAK-242 or anti-HMGB1 antibody in OVA-induced asthmatic mice
Airway resistance (Rn) was measured as an indicator of airway hyperresponsiveness (AHR) in DINP-induced asthmatic mice, as previously described.9 AHR measurements were obtained using methacholine after last challenge. Briefly, after last challenge, mice were anesthetized using a mixture of Zoletil and Rumpun and tracheostomized using an 18G metal cannula. Animals were then placed in a flow-type body plethysmograph and connected to a small-animal ventilator (FlexiVent, SCIREQ Inc., Montreal, Canada) by an endotracheal cannula. Methacholine (MCh) doses were administered using a nebulizer (Aeroneb) and concentrations were progressively doubled in the range 0 to 50 mg ml–1.
2.5. Measurement of inflammatory cytokine levels in the BALF samples of mice treated with DINP plus TAK-242 or anti-HMGB1 antibody
The levels of IL-4, IL-5, and IFN-γ in BALF samples were determined by ELISA (enzyme-linked immunosorbent assay). The lower detection limits of these assays were 1.11 pg ml–1 (IL-4, IL-5, and IL-13) and 10 pg ml–1 (IFN-γ). Briefly, 2 μg ml–1 of anti-IL-4, anti-IL-5, or anti-IL13 antibody or IFN-r were coated on a 96-well plate. Standards and samples were processed and treated with biotin-anti-IL-4, biotin-anti-IL-5, biotin-anti-IL-13, or biotin-anti-IFN-r and then with avidin-peroxidase. Substrates were treated and absorbances were measured at 405 nm. All samples were tested in triplicate for standard curves.
2.6. Histological analysis of lung tissues in DINP plus TAK-242 or anti-HMGB1 antibody treated mice
After sacrifice by cervical dislocation, left lungs were collected, fixed in 4% formalin at room temperature for 4 hours, transferred to PBS, dehydrated using an ethanol series and xylene, paraffin embedded, and sectioned at 4 μm. Sections were stained with hematoxylin and eosin (H&E), periodic acid-Schiff (PAS), or Pico-sirius red. Images were acquired using a microscope equipped with a ×20 or ×40 objective.
For immunohistochemistry, paraffin embedded sections were deparaffinized, rinsed at room temperature, hydrated, and treated with 3% hydrogen peroxidase to quench endogenous peroxidase activity. Sections were then blocked with endogenous avidin and biotin, as per the manufacturer's instructions. Inflammatory cells, PAS positive cells, lung fibrosis, and the expression of caspase-3 were analyzed using the Image J program.
2.7. Statistical analysis
The Student's t-test was used to determine the significances of intergroup differences. Results are presented as mean ± SDs and the analysis was performed using SPSS program (SPSS, Chicago, IL). P values of p < 0.001, p < 0.01, or p < 0.05 were considered to be statistically significant.
3. Results
3.1. Effects of TAK-242 or anti-HMGB1 antibody on airway hyperresponsiveness (AHR)
To determine the effects of TAK-242 and HMGB1 (anti-high mobility group box 1) antibodies on airway hyperresponsiveness (AHR) in our DINP-induced asthma model, we measured airway resistance (Rn) using a Flexibent. Rn values in DINP-induced asthmatic mice increased with methacholine concentration whereas those of non-asthmatic controls did not. At a methacholine concentration of 50 mg ml–1, the administrations of TAK-242 or anti-HMGB1 antibody to DINP-induced asthmatic mice significantly reduced Rn values (Fig. 2). These results show that TLR4 and HMGB1 influence the asthma-inducing effects DINP.
Fig. 2. Assessment of allergen-induced airway hyperresponsiveness by the forced oscillation technique. Newtonian resistance (Rn) was determined by DINP-induced asthma micel model. All data were expressed as means ± SD (n = 2). a, b, and c: The means not sharing a common letter are significantly different between groups at p < 0.05 as measured by one-way ANOVA with Duncan's multiple-range test.
3.2. Effects of TAK-242 or anti-HMGB1 antibody on inflammatory cell populations in BALF and blood
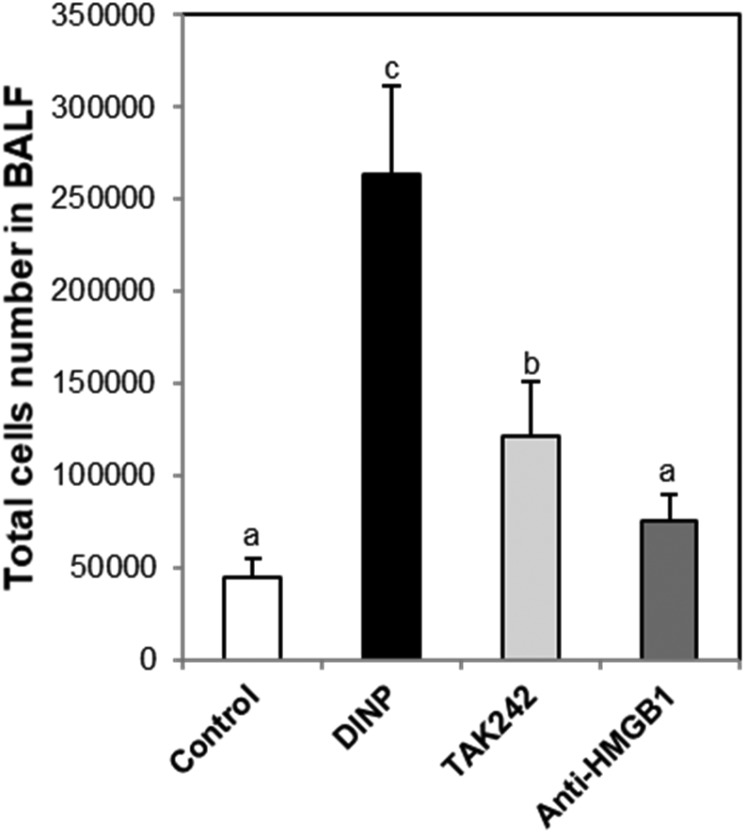
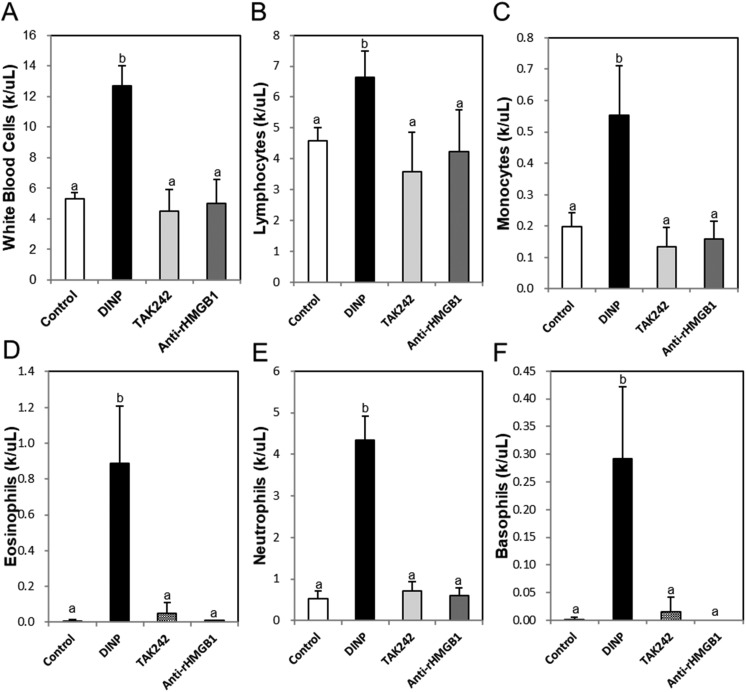
Exposure of DINP significantly increased the total number of cells in BALF, and these increases were significantly reduced by TAK-242 or anti-HMGB1 antibody (Fig. 3). Inflammatory cell populations (e.g., white blood cells (Fig. 4A), lymphocytes (Fig. 4B), monocytes (Fig. 4C), eosinophils (Fig. 4D), neutrophils (Fig. 4E), and basophils (Fig. 4F)) were significantly higher in the DINP group than in the control group, these increases were reduced in the DINP + TAK-242 or anti-HMGB1 antibody groups.
Fig. 3. Effect of TAK242 and anti-HMGB1 antibody on total cell number in BALF. Mice were sacrificed 24 h after the last challenge, and the lungs were lavaged four times with 0.5 mL ice-cold saline via a tracheal cannula. The BALF was then centrifuged and the pellets were re-suspended with 1000 μL and used for cell counting. a, b, and c: The means not sharing a common letter are significantly different between groups at p < 0.05 as measured by one-way ANOVA with Duncan's multiple-range test.
Fig. 4. Effects of TAK242 and anti-HMGB1 antibody on the population of inflammatory cells in blood. (A) White blood cell, (B) lymphocyte (C) monocyte, (D) eosinophil (E) neutrophil, and (F) basophil were analyzed by HEMAVET 950. a and b: The means not sharing a common letter are significantly different between groups at p < 0.05 as measured by one-way ANOVA with Duncan's multiple-range test.
3.3. Effects of TAK-242 and anti-HMGB1 antibody on the productions of Th1/Th2 mediated cytokines in the BALF samples of DINP-induced asthmatic mice
To confirm the effects of TAK-242 or anti-HMGB1 antibody administration on the productions of Th1 and Th2-associated cytokines in DINP-induced asthmatic mice, we measured IL-4, IL-5, IL-13, and IFN-γ concentrations in BALF samples. Levels of IL-4, IL-5, and IL-13 (all secreted by Th2 cells) were higher in the DINP group than in the control group, whereas IFN-γ levels were lower in the DINP group than in the control group. On the other hand, TAK-242 or anti-HMGB1 antibody co-treatments reduced IL-4, IL-5, and IL-13 levels and increased IFN-γ levels as compared with the DINP group (Table 1).
Table 1. Effects of TAK242 or anti-HMGB1 on TH1- and TH2 cytokine levels of in BALF.
| Control | DINP | TAK242 | Anti-HMGB1 | |
| IL-4 (pg ml–1) | 6.026 ± 2.846b | 39.03 ± 5.96c | N.Da | N.Da |
| IL-5 (pg ml–1) | N.Da | 22.464 ± 1.879d | 0.921 ± 1.393b | 7.929 ± 0.758c |
| IL-13 (pg ml–1) | 0.408 ± 0.007a | 1.116 ± 0.11c | 0.465 ± 0.024ab | 0.532 ± 0.046 b |
| IFN-y (pg ml–1) | 777.4 ± 282.8 b | N.Da | 507.4 ± 344.676b | 613.6 ± 565.267 b |
3.4. Effects of TAK-242 and of anti-HMGB1 antibody on histological changes in asthmatic mice
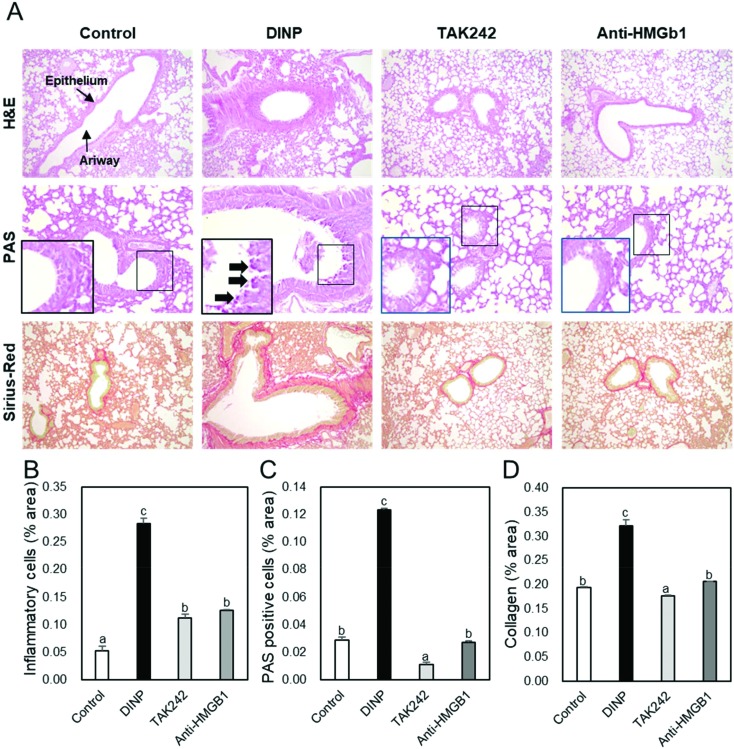
H&E, PAS, and Pico sirius red staining were used to examine the effects of TAK-242 and anti-HMGB1 antibody on lung histology in DINP-induced asthmatic mice, (Fig. 5A). Airways of mice in the DINP group were narrower than those of controls and many inflammatory cells were observed around airways. However, inflammatory cell infiltration was lower in the DINP + TAK-242 or anti-HMGB1 antibody groups than in the DINP group (Fig. 5B). Also, mucin and collagen productions were greater in the DINP group than in controls, but lower in the DINP + TAK-242 or anti-HMGB1 antibody groups than in the DINP group (Fig. 5C and D). In addition, caspase-3 immunohistochemistry showed its expression was higher in the DINP group than in controls, but lower in the DINP + TAK-242 or anti-HMGB1 antibody groups than in the DINP group (Fig. 6B).
Fig. 5. The effect of TAK242 and anti-HMGB1 antibody on the histology of lung tissue in the DINP-induced murine model of asthma. The C57BL/6 mice were sensitized and challenged with DINP for asthma induction. At the end of the experiment, the mice lungs were removed. (A) The lungs were stained by H&E (×200), PAS (×400), and Picro Sirius Red (×200). The percentages of (B) inflammatory cells, (C) PAS-positive cells, and (D) collagen in the lung sections were measured via Image J program. a, b, and c: The means not sharing a common letter are significantly different between groups at p < 0.05 as measured by one-way ANOVA with Duncan's multiple-range test.
Fig. 6. The effect of TAK242 and anti-HMGB1 antibody on the histology of lung tissue in the DINP-induced murine model of asthma. The C57BL/6 mice were sensitized and challenged with DINP for asthma induction. At the end of the experiment, the mice lungs were removed. (A) The lungs were stained by caspase-3 (×200) immunohistochemistry. The percentages of (B) caspase-3 positive cells in the lung sections were measured via Image J program. a, b, and c: The means not sharing a common letter are significantly different between groups at p < 0.05 as measured by one-way ANOVA with Duncan's multiple-range test.
4. Discussion
Plastics, one of the most preferred materials in today's industry, pose a serious threat to the environment and human beings, both directly and indirectly. Hazardous chemicals exposed in the course of handling plastics present risks such as cancer, immune dysfunction, and endocrine disruption.14 The relationship between toxic chemicals derived from plastics and respiratory diseases of the municipal waste workers (MWWs) are unknown, but exposure to a number of pathogens, toxic substances, and chemicals is associated with a higher prevalence of respiratory symptoms and a greater decrease in lung function of MWWs.15 These data indicate that many people, including MWWs, are exposed to plastic-related risks in a variety of ways.
Phthalates such as di(2-ethylhexyl) phthalate (DEHP), butyl benzyl phthalate (BBP), diisobutyl phthalate (DIBP), and diethylphthalate (DEP) are widely used to produce household articles and consumables, though DEHP is more commonly used. However, industrial consumers have recently shown a shift away from DEHP toward DIDP, DINP, and DPHP.16 Especially DINP is mainly used for children's toys. In carrying out this study, we found no minimum recommended dose not affecting humans. However, based on spongiosis hepatis in rats, the recommended acceptable daily intake (ADI) level is 120 μg kg–1 d–1. The relatively low estimated exposure to DINP from soft plastic toys is lower than 120 μg kg–1 d–1. Therefore, exposure of DINP from children's toys will not pose a health risk.17 Nonetheless, studies have shown that DINP acts as a sensitizer and that sustained exposure to this substance can cause airway inflammation. DINP increases the production of IL-4 by activating CD4+ cells and increases in IL-4 induce allergic response.18 As has been reported previously, DINP induces asthma by altering the Th1/Th2 equilibrium.9 In the present study, we found TLR4 and HMGB1 protein were involved in the asthma-inducing effects of DINP as these effects were diminished by TAK-242 or anti-HMGB1 antibody in our murine DINP-induced model of asthma. The reason for using anti-HMGB1 antibody as a method to inhibit the activity of HMGB1 in this study is due to its low toxicity and high specificity.
The innate immune system senses invasions by pathogenic microorganisms using toll-like receptors (TLRs), which recognize specific molecular patterns on the surfaces of microorganisms. This sensing ability results in the activation of defense systems and the development of antigen-specific acquired immunity.19 The TLR-related pathway has a critical effect on the development of asthma by the interaction between the genes associated with TLRs.20 Interestingly, the activation of TLR4 a feature of cardiovascular disease, diabetes, obesity, and neuroinflammatory diseases as well as asthma and results in the productions of ROS and RNS (reactive oxygen and nitrogen species), which may result in chronic inflammation and oxidative stress.21 Hammad et al. reported that house dust mite induces asthma through TLR4 on airway structural cells.22 Furthermore, when mice were exposed to cigarette smoke, up-regulations of TLR4 and TLR4-mediated signal transduction were found to increase airway inflammatory responses significantly.23 These studies show TLR4 plays an important role in the development of asthma, and thus, we considered exposure to DINP would influence TLR4 signaling, and investigated the effect of TAK-242, a TLR4-signaling inhibitor, on our murine model of asthma. IL-4 is an important cause of allergic inflammation and is associated with the ε isotype switch and IgE secretion by B lymphocytes.24 IL-5 is responsible for the maturation and release of eosinophils in bone marrow25 and IL-13 induces mucus secretion, airway hyperresponsiveness, and fibrosis.26 On the other hand, IFN-γ produced by Th1 cells inhibits the differentiation of naïve CD4+ T cell into Th2 cells.27 In mice with DINP-induced asthma, TAK242 reduced the productions of IL-4, IL-5, and IL-13 in lung tissues as compared with the DINP group and increased the production of IFN-r. Furthermore, TAK242 inhibited the DINP-induced productions of Th2 type cytokines, numbers of leukocytes, lymphocytes, monocytes, eosinophils, basophils, and neutrophils in serum, mucin production, and pulmonary fibrosis. These results show TAK242 alleviated these general features of asthma in our mouse model, and show the TLR4 signaling pathway was involved in the induction of asthma in our DINP model.
In addition to its intrinsic activity, HMGB1 induces the productions of cytokines, promotes chemotaxis, activates immune and endothelial cells and fibroblasts, and stimulates autoantibody production. HMGB1 is secreted during apoptosis, necrosis, pyroptosis, and NETosis and can interact with multiple immune sensors and receptors including RAGE, TLR2, TLR4, and TLR9.28 The interaction between HMGB1 and TLR4 plays a crucial role in the induction of inflammatory responses. For example, activation of TLR4 by HMGB1 induces the nuclear translocation of NF-κB (nuclear factor kappa B) and thus, the expressions of genes related to inflammation.29 NF-kB also plays vital roles by regulating the expressions of cytokines, chemokines, and cell adhesion molecules in airways under pathologic conditions, and these inflammatory mediators influence the types and numbers of inflammatory cells that infiltrate airway tissues.30 In the present study, HMGB1 inhibition with neutralizing antibodies suppressed DINP-induced inflammatory responses, including infiltration of inflammatory cells, airway inflammation, and Th2 type cytokine levels in BALF and reduced DINP-induced lung tissue damage. These results suggest HMGB1 played a critical role in out model of DINP-induced pulmonary inflammation.
The present study shows TLR4-signaling inhibition or HMGB1 neutralization in out DINP-induced model of asthma moderated airway inflammatory responses. Based on these results, we propose cells in lung tissue damaged by DINP release HMGB1 which binds to TLR4 and activates the NF-κB signaling pathway. Unfortunately, we did not examine the expressions of HMGB1 protein and NF-κB signaling-related genes in the lung tissues of DINP treated mice, which is a limitation of the present study. We suggest detailed understanding of the HMGB1–TLR4 interaction at a molecular level and of downstream signaling pathways in mice with DINP-induced asthma might have significant implications for the design of therapeutics targeting asthma induced by phthalate-based environmental pollutants like DINP. Several studies have shown that TLR4-signaling inhibition or HMGB1 neutralization reduces the pathologic features of asthma. For example, treatment of TAK242 in an HDM-induced asthma animal model suppresses airway remodeling in asthma by inhibiting the T-helper 2 response,31 ameliorates combined allergic rhinitis and asthma syndrome (CARAS) by reducing inflammatory monocytes infiltration in mice model.32 Moreover, HMGB1 neutralization attenuation of neutrophilic airway inflammation in asthma by suppressing Th17 polarization.33 Further studies on humans are needed to determine whether HMGB1 neutralization or TLR4-signaling inhibition is a potential treatment for allergic airway remodeling.
5. Conclusion
Administration of a TLR4-signaling inhibitor or anti-HGB1 antibody to DINP-induced asthmatic mice reduced airway hyperresponsiveness, numbers of inflammatory cells, DINP-induced Th2 cytokine productions, inflammatory cell accumulations, mucin production, and DINP-induced lung tissue damage. We suggest the inhibition of TLR4-signaling or the neutralization of HMGB1 might provide useful strategies for the treatment of DINP-induced asthma.
Author contributions
Yun-Ho Hwang and Sung-Tae Yee designed the study. Yun-Ho Hwang analyzed and interpreted the data. Yun-Ho Hwang and Youngjin Lee conducted the laboratory work. Yun-Ho Hwang wrote the manuscript, and Sung-Tae Yee and Man-Jeong Paik revised the manuscript. All authors read and approved this manuscript.
Conflicts of interest
There are no conflicts of interest to declare.
Acknowledgments
This research was supported by a National Research Foundation of Korea (NRF) grant funded by the Ministry of Science, ICT & Future Planning (MSIP) (2015R1A4A1041219).
References
- Masoli M., Fabian D., Holt S. Allergy. 2004;59(5):469–478. doi: 10.1111/j.1398-9995.2004.00526.x. [DOI] [PubMed] [Google Scholar]
- D'Amato G., Liccardi G., D'Amato M. Clin. Exp. Allergy. 2005;35(9):1113–1124. doi: 10.1111/j.1365-2222.2005.02328.x. [DOI] [PubMed] [Google Scholar]
- Watson R. R., Zibadi S., Rafatpanah H. Nutr. Res. 2008;28(3):166–171. doi: 10.1016/j.nutres.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Barnes P. J. Respir. Res. 2001;2(2):64–65. doi: 10.1186/rr39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Zhao Y., Li L. Sci. Total Environ. 2012;427–428:60–69. doi: 10.1016/j.scitotenv.2012.03.090. [DOI] [PubMed] [Google Scholar]
- Bornehag C. G., Nanberg E. Int. J. Androl. 2010;33(2):333–345. doi: 10.1111/j.1365-2605.2009.01023.x. [DOI] [PubMed] [Google Scholar]
- Kang J., Song J., Shen S. Oncotarget. 2016;7(51):85472–85482. doi: 10.18632/oncotarget.13403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L., Chen J., Xie C. M. Biomed. Environ. Sci. 2015;28(3):190–198. doi: 10.3967/bes2015.025. [DOI] [PubMed] [Google Scholar]
- Hwang Y. H., Paik M. J., Yee Y. S. T. Toxicol. Lett. 2017;272:49–59. doi: 10.1016/j.toxlet.2017.03.014. [DOI] [PubMed] [Google Scholar]
- Lange S. S., Vasquez K. M. Mol. Carcinog. 2009;48(7):571–580. doi: 10.1002/mc.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schierbeck H., Lundbäck P., Palmblad K. Mol. Med. 2011;17(9–10):1039–1044. doi: 10.2119/molmed.2010.00264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang R., Chen R., Zhang Q. Mol. Aspects Med. 2014;40:1–116. doi: 10.1016/j.mam.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbalzano E., Quartuccio S., Di Salvo E. Clin. Mol. Allergy. 2017;15:12. doi: 10.1186/s12948-017-0068-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rustagi N., Pradhan S. K., Singh R. Indian J. Occup. Environ. Med. 2011;15(3):100–103. doi: 10.4103/0019-5278.93198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athanasiou M., Makrynos G., Dounias G. Occup. Med. 2010;60(8):618–623. doi: 10.1093/occmed/kqq127. [DOI] [PubMed] [Google Scholar]
- Kimber I., Dearman R. J. Toxicology. 2010;271(3):73–82. doi: 10.1016/j.tox.2010.03.020. [DOI] [PubMed] [Google Scholar]
- Babich M. A., Chen S. B., Greene M. A. Regul. Toxicol. Pharmacol. 2004;40(2):151–167. doi: 10.1016/j.yrtph.2004.06.005. [DOI] [PubMed] [Google Scholar]
- Lee M. H., Park J., Chung S. W. Int. Arch. Allergy Immunol. 2004;134(3):213–222. doi: 10.1159/000078768. [DOI] [PubMed] [Google Scholar]
- Akira S., Takeda K. Nat. Rev. Immunol. 2004;4(7):499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- Reijmerink N. E., Bottema R. W., Kerkhof M. Allergy. 2010;65(2):199–207. doi: 10.1111/j.1398-9995.2009.02111.x. [DOI] [PubMed] [Google Scholar]
- Lucas K., Maes M. Mol. Neurobiol. 2013;48(1):190–204. doi: 10.1007/s12035-013-8425-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammad H., Chieppa M., Perros F. Nat. Med. 2009;15(4):410–416. doi: 10.1038/nm.1946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y., Wang D., Wang B. Mol. Biol. Cell. 2017;28(1):201–209. doi: 10.1091/mbc.E16-02-0126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinke J. W., Borish L. Respir. Res. 2001;2(2):66–70. doi: 10.1186/rr40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenfeder S., Umland S. P., Cuss F. M., Respir. Res., 2001, 22 , 71 –79 , . Epub 2001 Mar 8 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rael E. L., Lockey R. F. World Allergy Organ. J. 2011;4(3):54–64. doi: 10.1097/WOX.0b013e31821188e0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung F. Mediators Inflammation. 2001;10(2):51–59. doi: 10.1080/09629350120054518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magna M., Pisetsky D. S. Mol. Med. 2014;20:138–146. doi: 10.2119/molmed.2013.00164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang J., Huang C., Yang J. Mol. Cell. Biochem. 2010;345(1–2):189–195. doi: 10.1007/s11010-010-0572-9. [DOI] [PubMed] [Google Scholar]
- Schuliga M. Biomolecules. 2015;5(3):1266–1283. doi: 10.3390/biom5031266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M., Wang Z. N., Yang L. F. Exp. Ther. Med. 2017;14(4):2911–2916. doi: 10.3892/etm.2017.4898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang H., Li T., Han X. Int. Immunopharmacol. 2019;73:254–260. doi: 10.1016/j.intimp.2019.05.021. [DOI] [PubMed] [Google Scholar]
- Zhang F., Huang G., Hu B. Mediators Inflamm. 2014;2014:257930. doi: 10.1155/2014/257930. [DOI] [PMC free article] [PubMed] [Google Scholar]