Abstract
The drug rapamycin has beneficial effects in a number of animal models of neurodegeneration and aging including mouse models of Alzheimer’s disease. Despite its compelling preclinical record, no clinical trials have tested rapamycin or other mTOR inhibitors in patients with Alzheimer’s disease. We argue that such clinical trials should be undertaken.
Alzheimer’s disease (AD) and other dementias represent an increasing burden on society worldwide. It is estimated that 5.4 million Americans currently suffer from AD, with one to four family members generally serving as a caregiver for each of these individuals. Unless effective therapies are developed and implemented, this burden will grow as the demographic shift toward older ages continues and is expected to reach 13.8 million affected individuals in the United States by mid-century. Age is the greatest risk factor for AD, with the risk of developing AD estimated to double every 5 years over the age of 65, and the risk of death from AD increasing approximately 700-fold between the ages of 55 and 85 (1). The growing elderly population combined with the increasing risk of AD with age has led some to predict that AD will “break Medicare” in the United States and the health care economies of many other countries.
Currently, there are no effective treatments available to delay or prevent the onset and progression of AD, despite significant investments in research dollars aimed at developing such therapies. Indeed, more than half of the annual research budget of the National Institute on Aging has been earmarked for research on AD for several years now (2). Many factors likely contribute to the limited progress toward effective AD therapies, including the still poorly understood molecular mechanisms of disease pathogenesis and the limited ability to predict disease onset at early stages where intervention is likely to be most effective. We speculate that an additional major barrier to progress, possibly the most important, is the lack of attention paid to the role of the aging process itself as a critical factor in AD.
Over the past two decades, research on the biology of aging, referred to as geroscience, has made substantial progress in elucidating the genetic, molecular, and biochemical mechanisms of aging (2). A small number of “hallmarks of aging,” driven by the activity of genes that regulate aging, have been identified that play important and evolutionarily conserved roles in the decline in function and increase in disease associated with old age (3). By targeting genes that regulate aging and the molecular processes that they represent, researchers have been able to increase life span and delay age-associated decline in every laboratory animal where this has been attempted. In principle, targeting these same processes should also be effective at delaying the onset of specific age-related diseases, including but not limited to AD, and, in some cases, perhaps even reversing specific disease-related pathologies (4).
We argue here that increased attention should be placed toward understanding which physiological changes of aging contribute to an increased risk of AD and toward clinical testing of interventions that act at the interface of AD and normative aging. Specifically, interventions that are effective at both attenuating normative aging and attenuating disease progression in preclinical models of AD should become a high priority for preclinical discovery and testing. Failure to appreciate the mechanisms that underlie age-associated changes in brain and organismal physiology is likely to limit the efficacy of any strategy aimed at delaying or preventing AD.
IS RAPAMYCIN A PRECLINICAL CANDIDATE FOR TREATING AD?
The drug rapamycin is currently the most effective and reproducible pharmacological approach for directly targeting the aging process to increase life span and health span in laboratory animals (5). Rapamycin positively impacts most hallmarks of aging, and it has been shown to increase life span in each of the major invertebrate model organisms and in rodents (4). Rapamycin increases life span by 10 to 30% in multiple strains of mice when started either early or late in life, and when administered continuously (6, 7), intermittently (8), or transiently (9). Notably, a single 3-month treatment regimen was recently shown to increase remaining life expectancy of mice by up to 60% (9). Not only does rapamycin treatment increase life span but it also delays, or even reverses, nearly every age-related disease or decline in function in which it has been tested in mice, rats, and companion dogs, including cancers, cardiac dysfunction, kidney disease, obesity, cognitive decline, periodontal disease, macular degeneration, muscle loss, stem cell function, and immune senescence (10–12).
Rapamycin is an inhibitor of the mechanistic target of rapamycin (mTOR), a nutrient and growth factor–responsive kinase. Within cells, rapamycin binds to the FK506 binding protein 12 (FKBP12), and the FKBP12-rapamycin complex inhibits the activity of mTOR complex 1 (mTORC1). There are no confirmed off-target effects of rapamycin, but due to the central role of mTORC1 in regulating growth and metabolism, rapamycin has complex, context-dependent cellular effects including potential inhibition of mRNA translation, induction of autophagy, and altered mitochondrial metabolism (5). In addition, chronic treatment with rapamycin can indirectly inhibit mTOR complex 2 (mTORC2). These complicated cellular interactions likely contribute to pleiotropic phenotypes associated with rapamycin treatment.
In addition to its robust effects at attenuating normative aging, rapamycin has also been shown to have beneficial effects in several different mouse models of AD that exhibit amyloidosis alone, or amyloidosis plus tauopathy, or primary tauopathy. Indeed, the breadth and depth of positive preclinical data for rapamycin are perhaps greater than for any other potential AD therapy at this time. Such beneficial effects of rapamycin include reducing amyloid-β(Aβ) deposition, reducing pathogenic tau phosphorylation and abundance of misfolded tau species including neurofibrillary tangles, restoring cerebral blood flow and cerebromicrovascular density, preserving blood-brain barrier integrity, preventing human tau-induced neuronal loss, and improving cognitive function (13–20). Beneficial outcomes have been seen in several different mouse models of AD including 3× transgenic mice, P301S mice, hAPP(J20) mice, transgenic 2576 mice, APP/PS1 mice, ApoE4 transgenic mice, and a viral vector-based mouse model of AD in which tau P301L is expressed in layer II of the lateral entorhinal cortex of the mouse brain (13–20). Improvements after rapamycin treatment have been observed in these animal models when initiated either before the onset of disease symptoms or after symptoms and pathology are already present (17, 19, 21). In addition to studies with rapamycin, genetic inhibition of mTOR rescued memory deficits, improved cognitive function, and decreased tau and Aβ deposits (22, 23). The rapamycin derivative temsirolimus also improved spatial learning and memory and prevented apoptosis in the hippocampus of AD mouse models (24).
Given the large number of studies showing that rapamycin can attenuate both normative aging and AD-like disease in preclinical models, along with the fact that rapamycin is a U.S. Food and Drug Administration–approved drug with known dosing and side effect profiles, it seems reasonable that rapamycin should be tested in clinical trials for efficacy in AD patients or in patients with mild cognitive impairment (MCI). Yet, to the best of our knowledge, such a clinical trial has not been proposed or initiated nor has there even been an analysis of whether organ transplant patients taking rapamycin are at a reduced risk of AD. A recent search of the National Institutes of Health (NIH) clinical trials database at clinicaltrials.gov shows that the search terms “Alzheimer Disease” (condition or disease) and “rapamycin” (other term) yielded no results.
IS FEAR OF FAILURE THE BARRIER?
Despite compelling preclinical evidence that rapamycin might delay or perhaps even reverse AD progression, and clinical data indicating that rapamycin could be safely used to treat AD, a clinical trial to determine whether rapamycin can slow or reverse AD has not yet been initiated. In an attempt to understand why not, we contacted several colleagues in both academia and industry and posed this question to them. The array of potential reasons we received was both surprising and enlightening. They can be considered in two groups: those that are true for any AD clinical trial and those that are specific for rapamycin.
The former category can be summarized essentially as “fear of failure.” Clinical trials are expensive and time consuming; clinical trials for AD may be particularly difficult to interpret, and there is a perception that many AD trials have already failed, resulting in a waste of resources. The unstated implication here seems to be that we should not undertake any new clinical trial for AD unless we know for sure that it is going to be successful. Of course, it is impossible to know whether a properly designed clinical trial is going to be successful at the outset of the trial. Thus, subscribing to this line of reasoning means accepting that we should not perform any clinical trials for AD, ensuring no development of new therapies, which is simply not acceptable. NIH funding, congressional mandates for AD research, and foundation funding for AD research all come with the expectation that potential therapies for AD will be developed and that these therapies will be tested when there is a reasonable probability that they could improve the health and well-being of patients. We are by no means arguing that additional resources should not also be put toward basic and preclinical research on AD. Indeed, we feel that the development of predictive biomarkers that are suitable for shorter proof-of-concept clinical trials, in particular, would greatly accelerate testing of potential therapies and should be pursued with vigor. However, the idea that we should forego all clinical trials for AD because past trials have failed is simply not a reasonable proposition and must be rejected.
Related to this is an argument that preclinical studies of AD should not be used to guide clinical development of therapies, in large part because none of the mouse models of AD accurately capture the full spectrum of AD pathologies in patients. By extension, demonstrating efficacy of a drug in a mouse model of AD is, therefore, not strong enough evidence to move forward into clinical testing. Whereas the logic of this reasoning is debatable, it is important to consider the larger body of data for rapamycin. As already discussed, rapamycin and other methods of inhibiting mTORC1 are effective not only in one mouse model of AD but also in each of four different well-established mouse models of AD amyloidopathy, two mouse models of primary tauopathy, and a model of combined AD amyloidopathy and tauopathy (13–20, 22–24). Not only that, but rapamycin blocks or ameliorates the majority of AD-relevant pathologies in mouse models and also restores cognitive function. Further, there is substantial evidence that the mTOR signaling pathway, the target of rapamycin, is perturbed in brain tissue from AD patients as well as in animal models of AD (25–28), providing evidence for common biological pathways underpinning rapamycin’s efficacy. Perhaps most importantly, mTOR is a central regulator of the greatest risk factor for AD, which is aging itself (Fig. 1). Inhibition of mTOR by rapamycin effectively delays aging and reverses age-associated functional decline in mice (5). It appears to ameliorate functional deficits of the aged heart in dogs and improves immune responses in older people (30).
Fig. 1. mTOR links aging and AD.
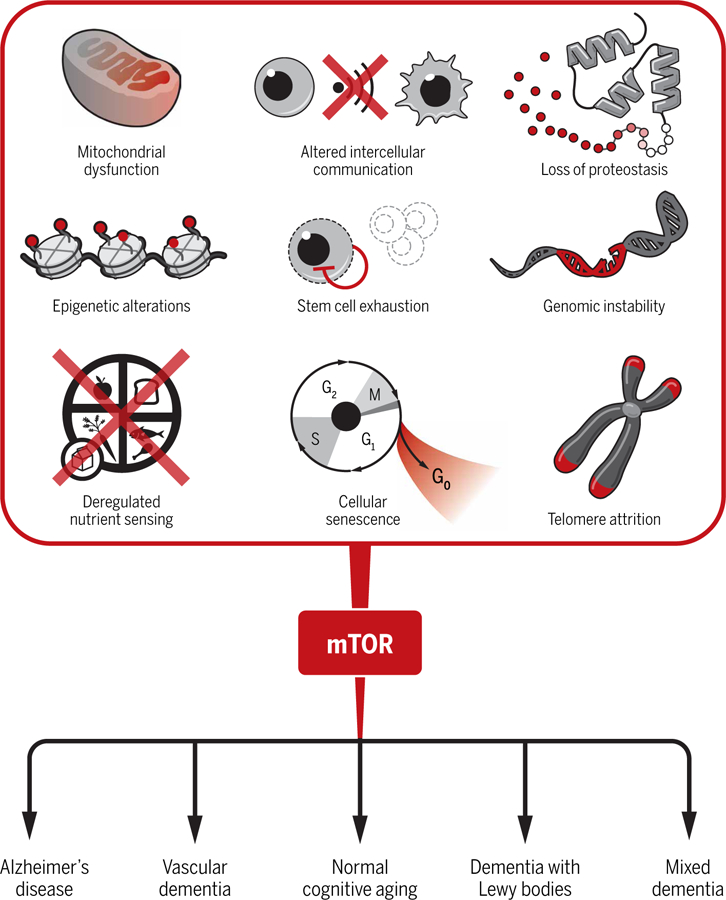
The top part of the figure depicts the involvement of mTOR in key processes common to aging. These hallmarks of aging contribute, in different degrees, to an increased risk for AD and other age-associated neurological diseases, as well as cognitive decline during normative aging. Rapamycin and other pharmacological interventions that attenuate mTOR activity may be beneficial for delaying the progression of AD and other age-associated dementias, as well as for preserving brain function in the healthy elderly.
RAPAMYCIN: AN UNDESERVED BAD REPUTATION?
In addition to the fear of failure described above, there are also several misperceptions that may have limited consideration of rapamycin as a potential clinical intervention for AD. There is no question that side effects have been associated with the use of rapamycin and other mTOR inhibitors in patients, the most common of which include mouth sores (similar to canker sores), an increase in blood lipids, impaired wound healing, gastrointestinal discomfort, and the potential for an increased risk of infection. It is important to keep in mind, however, that these side effects have largely been observed in patients who have received an organ transplant or who are being treated for cancer and who are often simultaneously taking other medications. Moreover, the side effects associated with rapamycin are dose-dependent and reversible; therefore, it should be fairly straightforward to establish safe dosing guidelines for an AD clinical trial, which may include intermittent administration.
There are few data on adverse events associated with rapamycin monotherapy in older individuals. A recent clinical trial reported relatively mild side effects, and no negative impact on the immune system nor changes in blood glucose, insulin secretion, or insulin sensitivity, in healthy 70- to 95-year-old individuals given rapamycin for at least 8 weeks (31). Another study has also reported mild side effects associated with 6 weeks of treatment of healthy elderly people with the rapamycin-derivative RAD001; this study documented improved, not impaired, immune function (32). In this study, the most common side effects at the highest dose tested (20 mg/week) were mouth ulcers (17%), headache (17%), fatigue (7.5%), and neutropenia (6%). All side effects were reduced at a lower dose (5 mg/week) that was actually more effective at boosting an immune response to flu vaccine. Granted, the people taking the mTOR inhibitors in these studies were only on the drug for 6 to 16 weeks. However, it is notable that there were no serious adverse events attributed to the treatment in either study, providing evidence that rapamycin is well tolerated as a monotherapy in elderly people.
Even if we take the unlikely worst-case scenario—that the side effects from rapamycin treatment in AD patients would be comparable to those experienced by organ transplant and cancer patients—a reasoned argument can be made that such side effects would be acceptable if AD disease progression could be attenuated. Whereas some patients are unable or unwilling to endure the side effects, many people tolerate high-dose rapamycin therapy for years with little, if any, discomfort. Indeed, a recent study indicates that less than 5% of patients with lymphangioleiomyomatosis taking rapamycin reported side effects after 1 year of continuous treatment; of those who did report side effects, they were relatively mild, consisting primarily of mouth sores, nausea, and diarrhea (33). It seems likely that most patients with AD, their caregivers, and family members would tolerate this level of risk and inconvenience for a chance at delaying AD progression.
What about the argument that we do not know the right dose of rapamycin to test in an AD clinical trial. Whereas it is true that we are lacking clinical data on the dose of rapamycin that would be most effective at combating AD (if any dose is), there are abundant clinical data on biological efficacy and side effects of rapamycin and rapamycin derivatives for other indications. Given the long history of rapamycin use to prevent organ transplant rejection, along with the studies in healthy elderly people discussed above (31, 32), it seems reasonable to consider testing doses of rapamycin used in these different studies.
Although rapamycin concentrations that were efficacious have been demonstrated in the brains of AD mice (17), there are limited data regarding the efficiency with which rapamycin is able to cross the blood-brain barrier in humans. At least one study has shown that oral delivery of rapamycin led to pharmacologically relevant concentrations of the drug that were detectable in brain tumors in 14 of 14 patients (34). Thus, rapamycin is clearly able to cross the blood-brain barrier in people to some extent, and some rapamycin derivatives may be even more effective in this regard. Delivery of rapamycin to the brain may be further facilitated by blood-brain barrier breakdown associated with aging (35). In addition, numerous studies in mice have shown that rapamycin effectively inhibits mTOR signaling in the brain and has substantial effects on brain physiology (13, 17, 20, 36, 37).
It is possible that rapamycin might only be effective at delaying AD if treatment is started before the disease has progressed to the point of clinical diagnosis. However, at least a subset of preclinical studies reported positive effects of rapamycin in mouse models even after substantial AD-like cognitive deficits and histopathology were present (17, 20). These observations, combined with the ability of rapamycin to improve function in other tissues, most notably the cardiac and immune systems (29, 32, 38–41), raise the possibility that cognitive function could be improved in patients with early- or moderate-stage AD even after substantial cognitive decline. Optimally, we would recommend clinical trials for rapamycin efficacy both in patients with MCI who are likely to progress to a diagnosis of AD and in patients recently diagnosed with AD.
Unfortunately, the fact that rapamycin is off-patent and available as a generic medication may have played a major role in the lack of clinical testing for efficacy against AD. Certainly, there is little incentive for large pharmaceutical companies to invest in its development and testing in this context. The lack of a strong profit motive should not preclude testing by the NIH or through federally or privately funded investigators, and it remains unclear why this has not yet happened.
CONCLUSION
Despite robust preclinical evidence that rapamycin may be effective at slowing AD progression, there has not yet been a single clinical trial to test this potentially transforming hypothesis. A number of studies have shown benefits from rapamycin in the context of normative aging and robust protection in a subset of animal models of AD. Rapamycin has been used extensively in the clinic, with well-understood dosing and safety information. Rapamycin and other mTOR inhibitors appear to be well tolerated in elderly subjects, with limited side effects that are reversible, are dose-dependent, and would be acceptable for an AD therapy. We therefore argue strongly for the initiation of clinical trials to test rapamycin as a drug to delay disease progression in AD patients as soon as possible.
Acknowledgments:
M.K. and V.G. are supported by the University of Washington and UT Health San Antonio Nathan Shock Centers of Excellence in the Basic Biology of Aging, as well as NIH grants P30AG013280 and P30AG013319.
Footnotes
Competing interests: M.K. serves on the Scientific Advisory Board of resTORbio Inc. V.G. is an inventor on U.S. patent applications 13/128,800, 14/435,306, and 61/790,485 regarding the use of encapsulated rapamycin for treating a variety of conditions; V.G. also consults for Rapamycin Holdings Inc.
REFERENCES AND NOTES
- 1.Qiu C, Kivipelto M, von Strauss E, Epidemiology of Alzheimer’s disease: Occurrence, determinants, and strategies toward intervention. Dialogues Clin. Neurosci 11, 111–128 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kaeberlein M, Rabinovitch PS, Martin GM, Healthy aging: The ultimate preventative medicine. Science 350, 1191–1193 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.López-Otín C, Blasco MA, Partridge L, Serrano M, Kroemer G, The hallmarks of aging. Cell 153, 1194–1217 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaeberlein M, Translational geroscience: A new paradigm for 21st century medicine. Transl. Med. Aging 1, 1–4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Johnson SC, Sangesland M, Kaeberlein M, Rabinovitch PS, Modulating mTOR in aging and health. Interdiscip. Top. Gerontol 40, 107–127 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Harrison DE, Strong R, Sharp ZD, Nelson JF, Astle CM, Flurkey K, Nadon NL, Wilkinson JE, Frenkel K, Carter CS, Pahor M, Javors MA, Fernandez E, Miller RA, Rapamycin fed late in life extends lifespan in genetically heterogeneous mice. Nature 460, 392–395 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Miller RA, Harrison DE, Astle CM, Baur JA, Boyd R, de Cabo R, Fernandez E, Flurkey K, Javors MA, Nelson JF, Orihuela CJ, Pletcher S, Sharp ZD, Sinclair D, Starnes JW, Wilkinson JE, Nadon NL, Strong R, Rapamycin, but not resveratrol or simvastatin, extends life span of genetically heterogeneous mice. J. Gerontol. A Biol. Sci. Med. Sci 66, 191–201 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Leontieva OV, Paszkiewicz GM, Blagosklonny MV, Weekly administration of rapamycin improves survival and biomarkers in obese male mice on high-fat diet. Aging Cell 13, 616–622 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bitto A, Ito TK, Pineda VV, LeTexier NJ, Huang HZ, Sutlief E, Tung H, Vizzini N, Chen B, Smith K, Meza D, Yajima M, Beyer RP, Kerr KF, Davis DJ, Gillespie CH, Snyder JM, Treuting PM, Kaeberlein M, Transient rapamycin treatment can increase lifespan and healthspan in middle-aged mice. eLife 5, e16351 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson SC, Martin GM, Rabinovitch PS, Kaeberlein M, Preserving youth: Does rapamycin deliver? Sci. Transl. Med 5, 211fs240 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.An JY, Darveau R, Kaeberlein M, Oral health in geroscience: Animal models and the aging oral cavity. Geroscience 40, 1–10 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kennedy BK, Lamming DW, The mechanistic target of rapamycin: The grand ConducTOR of metabolism and aging. Cell Metab 23, 990–1003 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Caccamo A, Majumder S, Richardson A, Strong R, Oddo S, Molecular interplay between mammalian target of rapamycin (mTOR), amyloid-β, and tau: Effects on cognitive impairments. J. Biol. Chem 285, 13107–13120 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Majumder S, Richardson A, Strong R, Oddo S, Inducing autophagy by rapamycin before, but not after, the formation of plaques and tangles ameliorates cognitive deficits. PLOS ONE 6, e25416 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Siman R, Cocca R, Dong Y, The mTOR inhibitor rapamycin mitigates perforant pathway neurodegeneration and synapse loss in a mouse model of early-stage Alzheimer-type tauopathy. PLOS ONE 10, e0142340 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lin A-L, Jahrling JB, Zhang W, DeRosa N, Bakshi V, Romero P, Galvan V, Richardson A, Rapamycin rescues vascular, metabolic and learning deficits in apolipoprotein E4 transgenic mice with presymptomatic Alzheimer’s disease. J. Cereb. Blood Flow Metab 37, 217–226 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin A-L, Zheng W, Halloran JJ, Burbank RR, Hussong SA, Hart MJ, Javors M, Shih Y-Y, Muir E, Solano Fonseca R., Strong R, Richardson AG,Lechleiter JD, Fox PT, Galvan V, Chronic rapamycin restores brain vascular integrity and function through NO synthase activation and improves memory in symptomatic mice modeling Alzheimer’s disease. J. Cereb. Blood Flow Metab 33, 1412–1421 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ozcelik S, Fraser G, Castets P, Schaeffer V, Skachokova Z, Breu K, Clavaguera F, Sinnreich M, Kappos L, Goedert M, Tolnay M, Winkler DT, Rapamycin attenuates the progression of tau pathology in P301S tau transgenic mice. PLOS ONE 8, e62459 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Spilman P, Podlutskaya N, Hart MJ, Debnath J, Gorostiza O, Bredesen D, Richardson A, Strong R, Galvan V, Inhibition of mTOR by rapamycin abolishes cognitive deficits and reduces amyloid-β levels in a mouse model of Alzheimer’s disease. PLOS ONE 5, e9979 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Van Skike CE, Jahrling JB, Olson AB, Sayre NL, Hussong SA, Ungvari Z, Lechleiter JD, Galvan V, Inhibition of mTOR protects the blood-brain barrier in models of Alzheimer’s disease and vascular cognitive impairment. Am. J. Physiol. Heart Circ. Physiol 314, H693–H703 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pierce A, Podlutskaya N, Halloran JJ, Hussong SA, Lin P-Y, Burbank R, Hart MJ, Galvan V, Over-expression of heat shock factor 1 phenocopies the effect of chronic inhibition of TOR by rapamycin and is sufficient to ameliorate Alzheimer’s-like deficits in mice modeling the disease. J. Neurochem 124, 880–893 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Caccamo A, De Pinto V, Messina A, Branca C, Oddo S, Genetic reduction of mammalian target of rapamycin ameliorates Alzheimer’s disease-like cognitive and pathological deficits by restoring hippocampal gene expression signature. J. Neurosci 34, 7988–7998 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Caccamo A, Belfiore R, Oddo S, Genetically reducing mTOR signaling rescues central insulin dysregulation in a mouse model of Alzheimer’s disease. Neurobiol. Aging 68, 59–67 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jiang T, Yu J-T, Zhu X-C, Tan M-S, Wang H-F, Cao L, Zhang Q-Q, Shi J-Q, Gao L, Qin H, Zhang Y-D, Tan L, Temsirolimus promotes autophagic clearance of amyloid-beta and provides protective effects in cellular and animal models of Alzheimer’s disease. Pharmacol. Res 81, 54–63 (2014). [DOI] [PubMed] [Google Scholar]
- 25.Tramutola A, Triplett JC, Di Domenico F, Niedowicz DM, Murphy MP, Coccia R, Perluigi M, Butterfield DA, Alteration of mTOR signaling occurs early in the progression of Alzheimer disease (AD): Analysis of brain from subjects with pre-clinical AD, amnestic mild cognitive impairment and late-stage AD. J. Neurochem 133, 739–749 (2015). [DOI] [PubMed] [Google Scholar]
- 26.Di Domenico F, Barone E, Perluigi M, Butterfield DA, The triangle of death in Alzheimer’s disease brain: The aberrant cross-talk among energy metabolism, mammalian target of rapamycin signaling, and protein homeostasis revealed by redox proteomics. Antioxid. Redox Signal 26, 364–387 (2017). [DOI] [PubMed] [Google Scholar]
- 27.Sun Y-X, Ji X, Mao X, Xie L, Jia J, Galvan V, Greenberg DA, Jin K, Differential activation of mTOR complex 1 signaling in human brain with mild to severe Alzheimer’s disease. J. Alzheimers Dis 38, 437–444 (2014). [DOI] [PubMed] [Google Scholar]
- 28.Chiang ACA, Fowler SW, Savjani RR, Hilsenbeck SG, Wallace CE, Cirrito JR, Das P, Jankowsky L, Combination anti-Aβ treatment maximizes cognitive recovery and rebalances mTOR signaling in APP mice. J. Exp. Med 215, 1349–1364 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Urfer SR, Kaeberlein TL, Mailheau S, Bergman PJ, Creevy E, Promislow DEL, Kaeberlein M, A randomized controlled trial to establish effects of short-term rapamycin treatment in 24 middle-aged companion dogs. Geroscience 39, 117–127 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mannick JB, Morris M, Hockey H-P, Roma G, Beibel M, Kulmatycki K, Watkins M, Shavlakadze T, Zhou W, Quinn D, Glass DJ, Klickstein LB, TORC1 inhibition enhances immune function and reduces infections in the elderly. Sci. Transl. Med 10, eaaq1564 (2018). [DOI] [PubMed] [Google Scholar]
- 31.Kraig E, Linehan LA, Liang H, Romo TQ, Liu Q, Wu Y, Benavides AD, Curiel TJ, Javors MA, Musi N, Chiodo L, Koek W, Gelfond JAL, Kellogg DL Jr., A randomized control trial to establish the feasibility and safety of rapamycin treatment in an older human cohort: Immunological, physical performance, and cognitive effects. Exp. Gerontol 105, 53–69 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mannick JB, Del Giudice G, Lattanzi M, Valiante NM, Praestgaard J, Huang B, Lonetto MA, Maecker HT, Kovarik J, Carson S, Glass DJ, Klickstein LB, mTOR inhibition improves immune function in the elderly. Sci. Transl. Med 6, 268ra179 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Bee J, Fuller S, Miller S, Johnson SR, Lung function response and side effects to rapamycin for lymphangioleiomyomatosis: A prospective national cohort study. Thorax 73, 369–375 (2017). [DOI] [PubMed] [Google Scholar]
- 34.Cloughesy TF, Yoshimoto K, Nghiemphu P, Brown, Dang J, Zhu S, Hsueh T, Chen Y, Wang W, Youngkin D, Liau L, Martin N, Becker D, Bergsneider M, Lai A, Green R, Oglesby T, Koleto M, Trent J, orvath S, Mischel PS, Mellinghoff IK, Sawyers CL, Antitumor activity of rapamycin in a phase I trial for patients with recurrent PTEN-deficient glioblastoma. PLOS Med 5, e8 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Montagne A, Barnes SR, Sweeney MD, Halliday MR, Sagare AP, Zhao Z, Toga AW, Jacobs RE, Liu CY, Amezcua L, Harrington MG, Chui HC, Law M, Zlokovic BV, Blood-brain barrier breakdown in the aging human hippocampus. Neuron 85, 296–302 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Skike CE, Galvan V, A perfect sTORm: The role of the mammalian target of rapamycin (mTOR) in cerebrovascular dysfunction of Alzheimer’s disease: A mini-review. Gerontology 64, 205–211 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Johnson SC, Yanos ME, Kayser E-B, Quintana A, Sangesland M, Castanza A, Uhde L, Hui J, Wall VZ, Gagnidze A, Oh K, Wasko BM, Ramos FJ, Palmiter RD, Rabinovitch PS, Morgan PG, Sedensky MM, Kaeberlein M, mTOR inhibition alleviates mitochondrial disease in a mouse model of Leigh syndrome. Science 342, 1524–1528 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dai D-F, Karunadharma PP, Chiao YA, Basisty N, Crispin D, Hsieh EJ, Chen T, Gu H, Djukovic D, Raftery D, Beyer RP, MacCoss MJ, Rabinovitch PS, Altered proteome turnover and remodeling by short-term caloric restriction or rapamycin rejuvenate the aging heart. Aging Cell 13, 529–539 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Neff F, Flores-Dominguez D, Ryan DP, Horsch M, Schröder S, Adler T, Afonso LC, Aguilar-Pimentel JA, Becker L, Garrett L, Hans W, Hettich MM, Holtmeier R, Hölter SM, Moreth K, Prehn C, Puk O, Racz, Rathkolb B, Rozman J, Naton B, Ordemann R, Adamski, Beckers J, Bekeredjian R, Busch DH, Ehninger G, Graw J, Höfler H, Klingenspor M, Klopstock T, Ollert M, Stypmann J, Wolf E, Wurst W, Zimmer A, Fuchs H, Gailus-Durner V, Hrabe de Angelis M., Ehninger D, Rapamycin extends murine lifespan but has limited effects on aging. J. Clin. Invest 123, 3272–3291 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Flynn JM, O’Leary MN, Zambataro CA, Academia EC, Presley MP, Garrett BJ, Zykovich A, Mooney SD, Strong R, Rosen CJ, Kapahi P, Nelson MD, Kennedy BK, Melov S, Late-life rapamycin treatment reverses age-related heart dysfunction. Aging Cell 12, 851–862 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen C, Liu Y, Zheng P, mTOR regulation and therapeutic rejuvenation of aging hematopoietic stem cells. Sci. Signal 2, ra75 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]


