Abstract
BACKGROUND
Osimertinib is an epidermal growth factor receptor tyrosine kinase inhibitor (EGFR-TKI) that is selective for both EGFR-TKI sensitizing and T790M resistance mutations in patients with non–small-cell lung cancer. The efficacy of osimertinib as compared with platinum-based therapy plus pemetrexed in such patients is unknown.
METHODS
In this randomized, international, open-label, phase 3 trial, we assigned 419 patients with T790M-positive advanced non–small-cell lung cancer, who had disease progression after first-line EGFR-TKI therapy, in a 2:1 ratio to receive either oral osimertinib (at a dose of 80 mg once daily) or intravenous pemetrexed (500 mg per square meter of body-surface area) plus either carboplatin (target area under the curve, 5 [AUC5]) or cisplatin (75 mg per square meter) every 3 weeks for up to six cycles; maintenance pemetrexed was allowed. In all the patients, disease had progressed during receipt of first-line EGFR-TKI therapy. The primary end point was investigator-assessed progression-free survival.
RESULTS
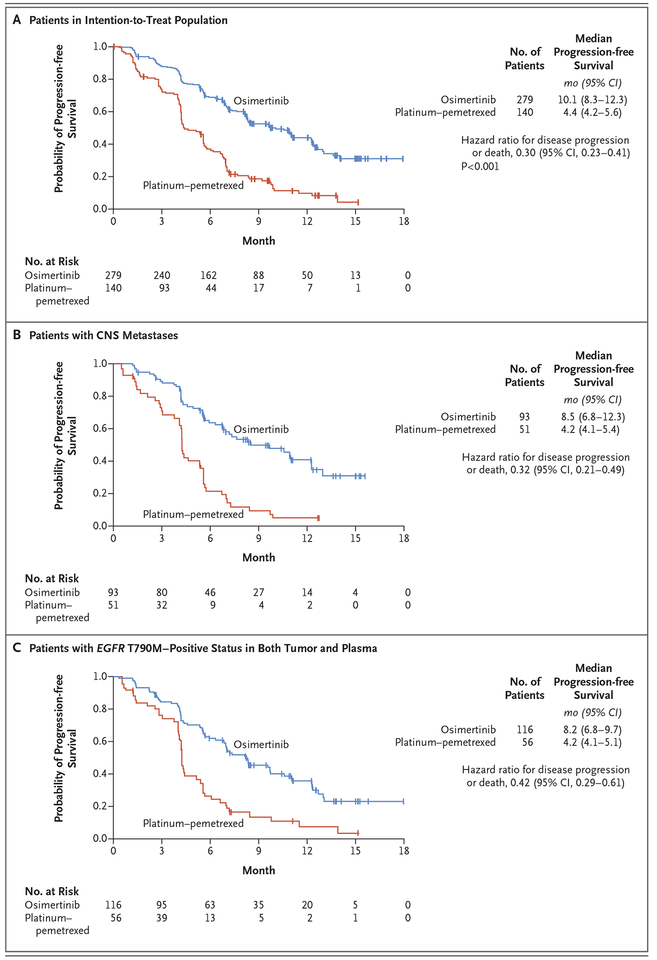
The median duration of progression-free survival was significantly longer with osimertinib than with platinum therapy plus pemetrexed (10.1 months vs. 4.4 months; hazard ratio; 0.30; 95% confidence interval [CI], 0.23 to 0.41; P<0.001). The objective response rate was significantly better with osimertinib (71%; 95% CI, 65 to 76) than with platinum therapy plus pemetrexed (31%; 95% CI, 24 to 40) (odds ratio for objective response, 5.39; 95% CI, 3.47 to 8.48; P<0.001). Among 144 patients with metastases to the central nervous system (CNS), the median duration of progression-free survival was longer among patients receiving osimertinib than among those receiving platinum therapy plus pemetrexed (8.5 months vs. 4.2 months; hazard ratio, 0.32; 95% CI, 0.21 to 0.49). The proportion of patients with adverse events of grade 3 or higher was lower with osimertinib (23%) than with platinum therapy plus pemetrexed (47%).
CONCLUSIONS
Osimertinib had significantly greater efficacy than platinum therapy plus pemetrexed in patients with T790M-positive advanced non–small-cell lung cancer (including those with CNS metastases) in whom disease had progressed during first-line EGFR-TKI therapy. (Funded by AstraZeneca; AURA3 ClinicalTrials.gov number, .)
Among patients with advanced non–small-cell lung cancer with a mutant epidermal growth factor receptor (EGFR), EGFR tyrosine kinase inhibitors (TKIs) are the standard first-line therapy.1–4 Despite high tumor response rates with first-line EGFR-TKIs, disease progresses in a majority of patients after 9 to 13 months of treatment.5–12 At the time of progression, about 60% of patients (regardless of race or ethnic background) are found to have a p.Thr790Met point mutation (T790M) in the gene encoding EGFR.13–16 The presence of the T790M variant reduces binding of first-generation or second-generation EGFR-TKIs to the ATP-binding pocket of EGFR, thereby reducing EGFR-TKI–mediated inhibition of downstream signaling and potentially leading to disease progression.17–19
Osimertinib is an oral, irreversible EGFR-TKI that is selective for both EGFR and T790M resistance mutations with activity in the central nervous system (CNS).19–21 In the phase 1 component of AURA, a phase 1/2 trial (ClinicalTrials.gov number, ), the objective response rate for osimertinib in patients with T790M-positive non–small-cell lung cancer was 61%; the median duration of progression-free survival was 9.6 months.22 These findings were confirmed in a pooled analysis of two subsequent phase 2 studies of osimertinib (at a dose of 80 mg once daily) in 411 patients with T790M-positive non–small-cell lung cancer, in which the response rate was 66% on blinded independent central review and the median duration of progression-free survival was 11.0 months.23 On the basis of these results, the Food and Drug Administration approved osimertinib under the Breakthrough Therapy Designation Program.24 A confirmatory, randomized, open-label, international, phase 3 trial (AURA3) was conducted to show the superiority of osimertinib over platinum therapy plus pemetrexed (followed by optional pemetrexed maintenance) as standard of care for patients with centrally confirmed T790M-positive advanced non–small-cell lung cancer after first-line EGFR-TKI therapy. Here, we report the results from AURA3.
METHODS
TRIAL PATIENTS
Eligible patients who were screened at 126 trial centers from August 2014 through September 2015 had histologic or cytologic evidence of locally advanced or metastatic non–small-cell lung cancer and of disease progression after first-line EGFR-TKI therapy. The documented presence of an EGFR mutation and central confirmation of the T790M variant on the cobas EGFR Mutation Test (Roche Molecular Systems) after first-line EGFR-TKI treatment was required. All patients were required to provide a blood sample at screening to test for T790M in plasma circulating tumor DNA (ctDNA) on the cobas EGFR Mutation Test, version 2. Patients with stable, asymptomatic CNS metastases that had not been treated with glucocorticoids for at least 4 weeks before the first dose of a trial drug were eligible for inclusion. Complete eligibility criteria are provided in the trial protocol, available with the full text of this article at NEJM.org.
TRIAL DESIGN AND TREATMENT
Patients were stratified according to Asian or non-Asian race and were randomly assigned in a 2:1 ratio to receive oral osimertinib (at a dose of 80 mg once daily) or intravenous pemetrexed (500 mg per square meter of body-surface area) plus either carboplatin (target area under the curve 5 [AUC5]) or cisplatin (75 mg per square meter) every 3 weeks for up to six cycles. Patients without disease progression after four cycles of platinum therapy plus pemetrexed (platinum–pemetrexed group) could continue maintenance pemetrexed according to the approved label.
Treatment continued until disease progression, the development of unacceptable side effects, or a request by either the patient or the physician to discontinue treatment. Patients could receive the trial treatment beyond the point of disease progression (as defined according to the Response Evaluation Criteria in Solid Tumors [RECIST], version 1.1) as long as they were receiving clinical benefit, as judged by the investigator.
According to an amendment to the protocol on December 22, 2014, patients who had been assigned to receive platinum–pemetrexed could cross over to the osimertinib group after objective disease progression, according to investigator assessment and as confirmed by blinded independent central review. All the patients provided written informed consent before screening.
TRIAL END POINTS
The primary efficacy end point was the duration of progression-free survival as determined by investigator assessments, according to RECIST, version 1.1. A sensitivity analysis of progression-free survival by blinded independent central review was conducted. Secondary objectives included the response rate according to investigator assessment, response duration, disease control rate, tumor shrinkage, overall survival, patient-reported outcomes, and safety and side-effect profiles. Predefined subgroup analyses included the duration of progression-free survival and response rate among patients for whom EGFR T790M status was determined by means of a plasma ctDNA test and among those with CNS metastases.
ASSESSMENTS
We performed baseline tumor assessments within 28 days after the initiation of the randomized treatment, with subsequent assessments performed every 6 weeks until objective disease progression. Brain imaging was required only in patients with known or suspected CNS metastases. Assessments for survival were performed every 6 weeks after objective disease progression or withdrawal from treatment. The duration of progression-free survival was defined as the time from randomization until the date of objective disease progression or death in the absence of progression, regardless of whether the patient had withdrawn from randomized therapy or received another anticancer therapy before progression. (Details regarding secondary efficacy end points are provided in the Supplementary Appendix, available at NEJM.org.)
We assessed adverse events using the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI CTCAE), version 4.0 (see the Supplementary Appendix for further details). Adverse events that were deemed by the investigators to be possibly related to a trial regimen are described in Table S6 in the Supplementary Appendix. We assessed patient-reported outcomes using the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire–Core 30 items (EORTC QLQ-C30) and EORTC QLQ–Lung Cancer 13 items. (Additional details are provided in the Supplementary Methods section in the Supplementary Appendix.)
TRIAL OVERSIGHT
The trial was conducted in accordance with the provisions of the Declaration of Helsinki, Good Clinical Practice guidelines (as defined by the International Conference on Harmonisation), applicable regulatory requirements, and the policy on bioethics and human biologic samples of the trial sponsor, AstraZeneca. The trial was designed by the principal investigators and the sponsor. The sponsor was responsible for the collection and analysis of the data and had a role in data interpretation. The authors vouch for the completeness and accuracy of the data and the data analyses and adherence to the protocol. This report was written by the first author, with medical-writing support funded by the sponsor, and was reviewed and approved for submission for publication by all the coauthors and the sponsor. The first author had full access to the data and had final responsibility for the decision to submit the manuscript for publication. The statistical analysis plan is available at NEJM.org.
STATISTICAL ANALYSIS
All the patients who underwent randomization were included in the intention-to-treat population, which was used for all efficacy analyses. The safety analysis included all the patients in the intention-to-treat population who had received at least one dose of a trial drug and for whom data were available after the administration of the drug.
We used the log-rank test stratified according to Asian or non-Asian race to compare the duration of progression-free survival between the two treatment groups. We used the Breslow approach for handling tied events and the Kaplan–Meier method to summarize the results. Data for patients who had not had a progression event or had not died at the time of the analysis were censored at the time of the last RECIST assessment.
We determined that 221 events of progression or death would provide a power of 80% to reject the null hypothesis of no significant difference in the duration of progression-free survival between the two treatment groups, assuming a treatment effect hazard ratio of 0.67 with a P value of 0.05 indicating two-sided statistical significance. (Additional details are provided in the Supplementary Methods section in the Supplementary Appendix.) The data cutoff date was April 15, 2016.
RESULTS
PATIENTS
Of the 1036 patients who were screened, a total of 419 patients underwent randomization (279 to the osimertinib group and 140 to the platinum–pemetrexed group) (Fig. S1 in the Supplementary Appendix). The demographic and clinical characteristics of the patients at baseline were balanced in the two groups (Table 1). At the time of data cutoff, the mean duration of treatment was 8.6 months (median, 8.1; range, 0.2 to 18.5) in the osimertinib group and 4.8 months (median, 4.2; range, 0.4 to 14.5) in the platinum–pemetrexed group. Of the 140 patients in the platinum–pemetrexed group, 136 (97%) received treatment; of these patients, 100 (74%) completed at least four cycles of platinum–pemetrexed, with 73 (54%) receiving maintenance pemetrexed monotherapy. At the time of the data cutoff, 166 patients (59%) in the osimertinib group and 16 (12%) in the platinum–pemetrexed group were still receiving the assigned treatment. Patients who were included in the plasma ctDNA analysis are described in Figure S2 in the Supplementary Appendix. A total of 172 patients with positive results for T790M on both tumor and plasma testing were included in the analysis.
Table 1.
Demographic and Clinical Characteristics of the Patients at Baseline.*
| Characteristic | Osimertinib (N = 279) |
Platinum-Pemetrexed (N = 140) |
|---|---|---|
| Median age (range) — yr | 62 (25–85) | 63 (20–90) |
| Female sex — no. (%) | 172 (62) | 97 (69) |
| Race — no. (%)† | ||
| White | 89 (32) | 45 (32) |
| Asian | 182 (65) | 92 (66) |
| Other | 8 (3) | 3 (2) |
| No history of smoking — no. (%) | 189 (68) | 94 (67) |
| Disease classification — no. (%) | ||
| Adenocarcinoma histology not otherwise specified | 232 (83) | 122 (87) |
| Metastatic disease | 266 (95) | 138 (99) |
| CNS metastases‡ | 93 (33) | 51 (36) |
| Extrathoracic visceral metastases§ | 145 (52) | 80 (57) |
| Type of EGFR mutation — no. (%)¶ | ||
| T790M‖ | 275 (99) | 138 (99) |
| Exon 19 deletion | 191 (68) | 87 (62) |
| Exon 21 L858R | 83 (30) | 45 (32) |
| G719X | 4 (1) | 2 (1) |
| S768I | 1 (<1) | 1 (1) |
| Exon 20 insertion | 1 (<1) | 2 (1) |
| No. of previous anticancer regimens for advanced disease — no. (%)** | ||
| 1 | 269 (96) | 134 (96) |
| 2 | 9 (3) | 6 (4) |
| 3 | 1 (<1)†† | 0 |
| Previous EGFR-TKI therapy — no. (%) | 279 (100) | 139 (99) |
| Gefitinib | 166 (59) | 87 (62) |
| Erlotinib | 96 (34) | 49 (35) |
| Afatinib | 20 (7) | 4 (3) |
CNS denotes central nervous system, EGFR epidermal growth factor receptor, and TKI tyrosine kinase inhibitor.
Race was self-reported. The category of “other” includes black, American Indian, and Alaska Native.
CNS metastases were determined from baseline data for the CNS lesion site, medical history, surgery, or radiotherapy. One patient was identified as having locally advanced disease in the brain.
Extrathoracic visceral metastases were determined on the basis of baseline data for which the disease site was described as adrenal, ascites, brain or CNS, gastrointestinal, genitourinary, hepatic (including gallbladder), liver, other CNS, pancreas, peritoneum, or spleen. Also included were other metastatic sites, such as those occurring in the eye and thyroid, as identified as extrathoracic visceral sites by AstraZeneca physicians.
EGFR mutations were identified by means of the cobas EGFR Mutation Test from a biopsy sample obtained after confirmation of disease progression while the patient was receiving the most recent treatment regimen.
Six patients (four in the osimertinib group and two in the platinum–pemetrexed group) did not have centrally confirmed T790M mutation–positive status that was documented in the trial database. Three patients (two in the osimertinib group and one in the platinum–pemetrexed group) were subsequently found to have positive results on testing for the T790M mutation. Therefore, three patients (two in the osimertinib group and one in the platinum–pemetrexed group) were T790M-negative in the tumor sample and underwent randomization in error. One of the three patients who had T790M-negative results in the tumor sample had T790M-positive results in plasma.
Patients were classified as having received more than one previous line of therapy if they received any of the following: adjuvant or neoadjuvant chemotherapy administered less than 6 months before the start of EGFR-TKI therapy; more than one EGFR-TKI (switching from a first-generation EGFR-TKI to a second-generation EGFR-TKI, or restarting EGFR-TKI after >12 months off treatment) administered sequentially; or the addition of anticancer agents such as cytotoxic chemotherapy or a c-Met monoclonal antibody toward the end of a previous monotherapy EGFR-TKI regimen.
One patient in the osimertinib group was treated with fulvestrant followed by letrozole before starting EGFR-TKI.
POST-TRIAL TREATMENT
After the discontinuation of randomized treatment, 67 of 279 patients (24%) in the osimertinib group and 96 of 136 patients (71%) in the platinum–pemetrexed group received subsequent anticancer treatment, including osimertinib, radio-therapy, platinum and nonplatinum chemotherapy, and other EGFR-TKIs (Fig. S1 in the Supplementary Appendix). In the platinum–pemetrexed group, 82 of 136 patients (60%) crossed over to receive osimertinib, with 63 of 82 patients (77%) receiving ongoing treatment at the time of data cutoff. The subsequent duration of exposure to osimertinib ranged from 0.1 months to 12.5 months (median, 4.2).
EFFICACY
Progression-free Survival
At the time of data cutoff, the median follow-up for all patients was 8.3 months. Progression events occurred in 140 patients (50%) in the osimertinib group and in 110 (79%) in the platinum–pemetrexed group. The duration of progression-free survival was significantly longer in the osimertinib group than in the platinum–pemetrexed group (median, 10.1 months vs. 4.4 months; hazard ratio after adjustment for Asian or non-Asian race, 0.30; 95% confidence interval [CI], 0.23 to 0.41; P<0.001) (Fig. 1A). The estimated proportion of patients who were alive and progression-free at 6 months was 69% (95% CI, 63 to 74) in the osimertinib group and 37% (95% CI, 29 to 45) in the platinum–pemetrexed group; at 12 months, the proportions were 44% (95% CI, 37 to 51) and 10% (95% CI, 5 to 17), respectively. The duration of progression-free survival according to blinded independent central review was consistent with the investigator-assessed durations, with a median of 11.0 months versus 4.2 months (adjusted hazard ratio, 0.28; 95% CI, 0.20 to 0.38; P<0.001). (Additional details are provided in the Supplementary Results section in the Supplementary Appendix.)
Figure 1 (facing page). Duration of Progression-free Survival, According to Subgroup.
Shown are Kaplan–Meier estimates of the duration of progression-free survival as assessed by investigators in the intention-to-treat population (Panel A), in patients with central-nervous-system (CNS) metastases (Panel B), and in patients with EGFR T790M–positive status in both tumor and plasma (Panel C). The tick marks indicate censored data. Progression events that occurred after two or more missed visits (i.e., 14 weeks) after the last assessment were censored at the last assessment, according to Response Evaluation Criteria in Solid Tumors. CI denotes confidence interval.
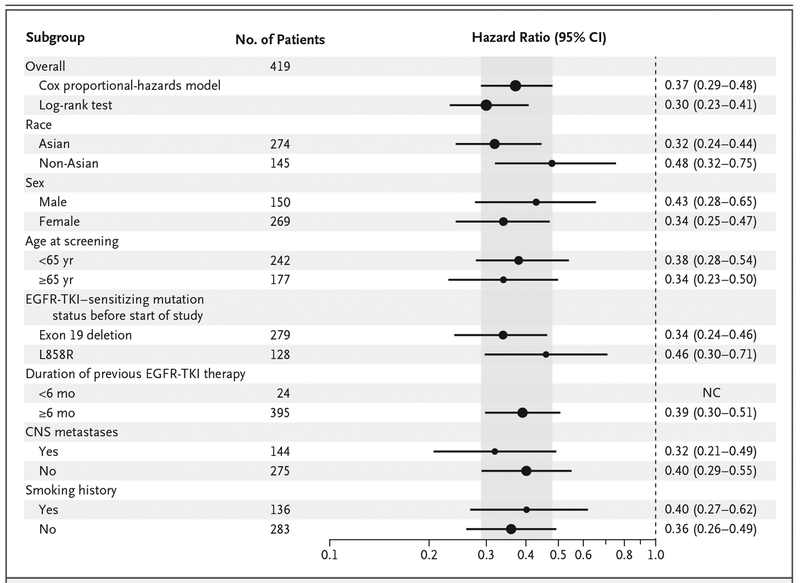
The hazard ratio for progression-free survival favored osimertinib across all predefined subgroups that were analyzed (hazard ratio, <0.50 for each subgroup) (Fig. 2), including patients with CNS metastases (median duration of progression-free survival, 8.5 months vs. 4.2 months; hazard ratio, 0.32; 95% CI, 0.21 to 0.49) (Fig. 1B). (Details regarding the duration of progression-free survival among patients without CNS metastases are provided in Fig. S3 in the Supplementary Appendix.)
Figure 2. Subgroup Analyses of Progression-free Survival.
A hazard ratio of less than 1 indicates a lower risk of progression in the osimertinib group. The Cox proportional-hazards model includes randomized treatment, the subgroup covariate of interest, and the treatment according to subgroup interaction. The size of the circles is proportional to the number of events. Overall population analyses are presented from both a Cox proportional-hazards model and the primary analysis (U and V statistics from a log-rank test stratified according to race). If there were fewer than 20 events in any subgroup, then the analysis was not performed. The shaded area indicates the 95% CI for the overall hazard ratio (all patients). NC denotes could not be calculated.
On the basis of mutation status before the initiation of the trial, the hazard ratio for progression-free survival was 0.34 (95% CI, 0.24 to 0.46) among patients with an EGFR exon 19 deletion and 0.46 (95% CI, 0.30 to 0.71) among those with an EGFR L858R mutation. Among Asian patients, the hazard ratio for progression-free survival was 0.32 (95% CI, 0.24 to 0.44), as compared with 0.48 (95% CI, 0.32 to 0.75) among non-Asian patients. The median duration of progression-free survival among patients with tumor and plasma T790M-positive status was 8.2 months in the osimertinib group versus 4.2 months in the platinum–pemetrexed group (hazard ratio, 0.42; 95% CI, 0.29 to 0.61) (Fig. 1C). Among the patients receiving osimertinib, there was no significant difference in benefit between patients with T790M-positive status on both tumor and plasma analyses and those in the intention-to-treat population.
Objective Response and Duration of Response
The response rate was significantly better in the osimertinib group (71%; 95% CI, 65 to 76) than in the platinum–pemetrexed group (31%; 95% CI, 24 to 40) (odds ratio, 5.39; 95% CI, 3.47 to 8.48; P<0.001) (Table 2). A similar finding was observed in the subgroup of patients with T790M-positive status on both tumor and plasma analyses (89 of 116 patients [77%] vs. 22 of 56 patients [39%]; odds ratio, 4.96; 95% CI, 2.49 to 10.15; P<0.001). Among the patients who had a response to treatment at the time of data cutoff, disease progression or death was reported in 88 of 197 patients (45%) in the osimertinib group and in 36 of 44 patients (82%) in the platinum–pemetrexed group. On the basis of investigator assessment, the median response duration was 9.7 months (95% CI, 8.3 to 11.6) in the osimertinib group and 4.1 months (95% CI, 3.0 to 5.6) in the platinum–pemetrexed group. Data regarding the best percentage change from baseline in target lesions are provided in Figure S4 in the Supplementary Appendix. At the time of data cutoff, 61 patients (15%) had died: 35 (13%) in the osimertinib group and 26 (19%) in the platinum–pemetrexed group. Data for the overall survival analysis were not complete at the time of this report.
Table 2.
Response to Treatment (Intention-to-Treat Population).*
| Response | Osimertinib (N = 279) |
Platinum-Pemetrexed (N = 140) |
Odds Ratio (95% CI)† |
P Value‡ |
|---|---|---|---|---|
| Type of response — no. (%) | ||||
| Complete | 4 (1) | 2 (1) | ||
| Partial | 193 (69) | 42 (30) | ||
| Stable disease for ≥6 wk | 63 (23) | 60 (43) | ||
| Progression | 18 (6) | 26 (19) | ||
| RECIST progression | 15 (5) | 22 (16) | ||
| Death | 3 (1) | 4 (3) | ||
| Could not be evaluated | 1 (<1) | 10 (7) | ||
| Objective response rate | 5.39 (3.47–8.48) | <0.001 | ||
| Percentage of patients | 71 | 31 | ||
| 95% CI | 65–76 | 24–40 | ||
| Disease control rate§ | 4.76 (2.64–8.84) | <0.001 | ||
| Percentage of patients | 93 | 74 | ||
| 95% CI | 90–96 | 66–81 | ||
| Time to response¶ | ||||
| Median no. of wk | 6.1 | 6.4 | ||
| 95% CI | NC-NC | 6.3–7.0 | ||
| ≤6 wk after randomization — no./total no. (%)‖ | 161/197 (82) | 29/44 (66) | ||
| Duration of response** | ||||
| Median no. of months | 9.7 | 4.1 | ||
| 95% CI | 8.3–11.6 | 3.0–5.6 | ||
| >6 mo — no./total no. (%) | 96/197 (49) | 12/44 (27) | ||
| >9 mo — no./total no. (%) | 56/197 (28) | 4/44 (9) | ||
| >12 mo — no./total no. (%) | 21/197 (11) | 1/24 (2) | ||
Tumor responses were assessed by the investigators according to Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1. Complete response and partial response did not require confirmation, according to RECIST, version 1.1., guidance on randomized studies, since the control group served as an appropriate means of interpretation of data. CI denotes confidence interval, and NC could not be calculated.
Odds ratios were calculated with the use of logistic regression adjusted for Asian or non-Asian race. An odds ratio of more than 1 favors osimertinib.
P values were calculated by means of the likelihood ratio test, which compared two models (one model with race as the only covariate and the other model with both treatment factor and race as covariates).
The disease control rate is the proportion of patients who had a complete response, a partial response, or stable disease lasting at least 6 weeks before any disease-progression event.
The time to tumor response was calculated from the date of randomization to the date of the first documentation of a partial or complete response.
A 1-week window was allowed at approximately 6 weeks.
The duration of response was calculated with the use of the Kaplan–Meier method from the time of the first documented response until the date of progression or the last RECIST assessment for patients who did not have disease progression.
Patient-Reported Outcomes
In a mixed model for repeated-measures analysis, patient-reported outcomes were better in the osimertinib group than in the platinum–pemetrexed group across five prespecified symptoms during the overall period from randomization until 6 months (Table S4 in the Supplementary Appendix).
SAFETY AND ADVERSE EVENTS
Adverse events occurred in 273 of 279 patients (98%) in the osimertinib group and in 135 of 136 (99%) in the platinum–pemetrexed group. Adverse events with a maximum grade of 1 were reported in 93 patients (33%) in the osimertinib group and in 15 (11%) in the platinum–pemetrexed group; adverse events with a maximum grade of 2 were reported in 117 (42%) and in 56 (41%), respectively. Fewer patients reported adverse events of grade 3 or more in the osimertinib group than in the platinum–pemetrexed group (63 [23%] vs. 64 [47%]). A summary of adverse events of grade 3 or more is provided in Table S5 in the Supplementary Appendix. (Details regarding the safety analysis for patients who crossed over from platinum–pemetrexed to osimertinib are provided in the Supplementary Results section in the Supplementary Appendix.)
In the osimertinib group, the most commonly reported adverse events were diarrhea (in 113 patients [41%]), rash (in 94 [34%]), dry skin (in 65 [23%]), and paronychia (in 61 [22%]) (Table 3). The most commonly reported adverse events in the platinum–pemetrexed group were nausea (in 67 patients [49%]), decreased appetite (in 49 [36%]), constipation (in 47 [35%]), and anemia (in 41 [30%]). Adverse events that were deemed by the investigators to be possibly related to a trial regimen are described in Table S6 in the Supplementary Appendix.
Table 3.
Adverse Events.*
| Adverse Event | Osimertinib (N = 279) |
Platinum-Pemetrexed (N = 136) |
||
|---|---|---|---|---|
| Any Grade | Grade ≥3 | Any Grade | Grade ≥3 | |
| number (percent) | ||||
| Diarrhea | 113 (41) | 3 (1) | 15 (11) | 2 (1) |
| Rash† | 94 (34) | 2 (1) | 8 (6) | 0 |
| Dry skin† | 65 (23) | 0 | 6 (4) | 0 |
| Paronychia† | 61 (22) | 0 | 2 (1) | 0 |
| Decreased appetite | 50 (18) | 3 (1) | 49 (36) | 4 (3) |
| Cough | 46 (16) | 0 | 19 (14) | 0 |
| Nausea | 45 (16) | 2 (1) | 67 (49) | 5 (4) |
| Fatigue | 44 (16) | 3 (1) | 38 (28) | 1 (1) |
| Stomatitis | 41 (15) | 0 | 21 (15) | 2 (1) |
| Constipation | 39 (14) | 0 | 47 (35) | 0 |
| Pruritus | 35 (13) | 0 | 6 (4) | 0 |
| Vomiting | 31 (11) | 1 (<1) | 27 (20) | 3 (2) |
| Back pain | 29 (10) | 1 (<1) | 12 (9) | 1 (1) |
| Thrombocytopenia† | 28 (10) | 1 (<1) | 27 (20) | 10 (7) |
| Nasopharyngitis | 28 (10) | 0 | 7 (5) | 0 |
| Headache | 28 (10) | 0 | 15 (11) | 0 |
| Dyspnea | 24 (9) | 3 (1) | 18 (13) | 0 |
| Neutropenia† | 22 (8) | 4 (1) | 31 (23) | 16 (12) |
| Leukopenia† | 22 (8) | 0 | 20 (15) | 5 (4) |
| Anemia† | 21 (8) | 2 (1) | 41 (30) | 16 (12) |
| Asthenia | 20 (7) | 3 (1) | 20 (15) | 6 (4) |
| Pyrexia | 18 (6) | 0 | 14 (10) | 0 |
| Alanine aminotransferase elevation | 18 (6) | 3 (1) | 15 (11) | 1 (1) |
| Aspartate aminotransferase elevation | 14 (5) | 3 (1) | 15 (11) | 1 (1) |
| Malaise | 11 (4) | 0 | 14 (10) | 0 |
Listed are adverse events that were reported in at least 10% of the patients in any group. Safety analyses included all the patients who received at least one dose of a trial drug (safety analysis set). Included are adverse events with an on-set date on or after the date of first dose and up to and including 28 days after the discontinuation of the trial drug or the day before the first administration of crossover treatment. Some patients had more than one adverse event.
This category represents a grouped term for the event. If a patient had multiple preferred-term level events within a specific grouped term adverse event, then the maximum grade (according to the Common Terminology Criteria for Adverse Events) across those events was counted.
Interstitial lung disease–like adverse events were reported in 10 patients (4%) in the osimertinib group (nine events of grade 1 or 2 in severity and one death) and in 1 patient (1%) in the platinum–pemetrexed group (one grade 3 event). A prolongation in the QT interval was recorded in 10 patients (4%) in the osimertinib group and 1 patient (1%) in the platinum–pemetrexed group, with all events of grade 1 or 2 in severity except for one grade 3 event in the osimertinib group. (Additional details are provided in the Supplementary Results section in the Supplementary Appendix.)
Osimertinib was associated with a lower rate of adverse events leading to permanent discontinuation than was platinum–pemetrexed (in 19 patients [7%] and 14 patients [10%], respectively). Fatal adverse events were reported in 4 patients in the osimertinib group (respiratory failure in 2, pneumonitis in 1, and ischemic stroke in 1). One fatal adverse event of hypovolemic shock was reported in the platinum–pemetrexed group.
DISCUSSION
In this trial, we found that patients with T790M-positive advanced non–small-cell lung cancer who received osimertinib had better response rates and a longer duration of progression-free survival than did those receiving platinum therapy plus pemetrexed after first-line EGFR-TKI therapy. The progression-free survival benefit with osimertinib was observed across all predefined subgroups, with hazard ratios of less than 0.50, including in patients with asymptomatic CNS metastases. In five prespecified measures of patient-reported symptoms, osimertinib had better results than platinum–pemetrexed.
Among patients receiving osimertinib, the AURA3 outcomes (median progression-free survival duration of 10.1 months and response rate of 71%) were in line with results of the phase 1/2 AURA and AURA2 studies.22,23 Similarly, the treatment outcomes with platinum–pemetrexed (a median progression-free survival duration of 4.4 months and response rate of 31%) were broadly in line with cisplatin–pemetrexed treatment in a T790M-positive population (as defined according to the results on a plasma ctDNA test) in the IMPRESS trial.25 Chemotherapy was the standard control at the time of trial initiation. As evidence emerges on immunotherapy, future studies are needed to address the role of such therapy among patients with EGFR mutation–positive non–small-cell lung cancer.
The findings of AURA3 support the feasibility of detecting EGFR T790M from plasma ctDNA samples, in line with previous reports.26,27 Improvement in outcomes with osimertinib over platinum–pemetrexed in the tumor and plasma ctDNA T790M-positive subgroup was similar to that in the intention-to-treat population. However, because of the high false negative rates with plasma ctDNA T790M testing, the analysis of a biopsy sample is recommended for patients with a plasma T790M-negative result who have disease progression after receiving first-line EGFR-TKI.26 We cannot address clinical outcomes of patients with potential false positive results (i.e., T790M-positive results on plasma ctDNA testing and negative results on tumor testing) in this trial because of the requirement for a positive tumor sample for enrollment.
In the BLOOM study (), in which 20 patients with leptomeningeal metastases from EGFR mutation–positive non–small-cell lung cancer were treated with osimertinib (at a dose of 160 mg once daily), preliminary results showed radiologic improvement in 7 patients.21 In our trial, the benefit of osimertinib in the subgroup of patients with CNS metastases was shown by a longer duration of progression-free survival than among those treated with platinum–pemetrexed. Independent radiologic assessment of all intracranial metastases is ongoing.
In AURA3, the safety profile for osimertinib was consistent with that reported previously and differed from that in the platinum–pemetrexed group.23 The safety profile in the platinum–pemetrexed group was consistent with that observed in the cisplatin–pemetrexed group in the IMPRESS trial.28 Overall, adverse events tended to be more severe in the platinum–pemetrexed group, despite the longer treatment duration with osimertinib.
In conclusion, osimertinib was more effective than combination platinum-based chemotherapy in patients with T790M-positive non–small-cell lung cancer (including those with CNS metastases) after disease progression with first-line EGFR-TKI therapy.
Supplementary Material
Supported by AstraZeneca.
Dr. Mok reports receiving fees for serving on advisory boards from AstraZeneca, Roche/Genentech, Eli Lilly and Company, Merck Serono, ACEA Biosciences, Bristol-Myers Squibb, AVEO-Biodesix, Pfizer, Boehringer Ingelheim, Novartis Pharmaceuticals, GlaxoSmithKline, Clovis Oncology, Amgen, Janssen, Bio-Marin Pharmaceuticals, SFJ Pharmaceuticals, Merck Sharp & Dohme, Vertex Pharmaceuticals, Oncogenex, and Celgene, consulting fees from geneDecode, lecture fees from AstraZeneca, Roche/Genentech, Eli Lilly and Company, Merck Serono, Bristol-Myers Squibb, Pfizer, Boehringer Ingelheim, Novartis Pharmaceuticals, GlaxoSmithKline, Clovis Oncology, Amgen, Merck Sharp & Dohme, and Prime Oncology, and holding stock in Sanomics; Dr. Wu, receiving lecture fees from AstraZeneca, Roche, Eli Lilly and Company, Pfizer, and Sanofi; Dr. Garassino, receiving consulting and lecture fees from AstraZeneca; Dr. Ramalingam, receiving fees for serving on advisory boards from Astra-Zeneca, AbbVie, Bristol-Myers Squibb, Boehringer Ingelheim, Celgene, Novartis Pharmaceuticals, Genentech, and Eli Lilly and Company; Dr. Shepherd, receiving fees for serving on advisory boards from AstraZeneca and Eli Lilly and Company, and holding stock in AstraZeneca; Dr. Akamatsu, receiving honoraria and lecture fees from AstraZeneca, Eli Lilly and Company, Chugai Pharmaceutical Co., Pfizer, Taiho Pharmaceutical Co., Nippon Boehringer Ingelheim, and Novartis Pharmaceuticals; Dr. Lee, receiving travel support from AstraZeneca; Dr. Sebastian, receiving personal fees from AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Merck Sharp & Dohme, Eli Lilly and Company, Pfizer, Roche, and Novartis Pharmaceuticals; Dr. Templeton, Dr. Mann, Dr. Marotti, and Dr. Ghiorghiu being employees of AstraZeneca, and Dr. Templeton holding stock in AstraZeneca; and Dr. Papadimitrakopoulou, receiving consulting fees from AstraZeneca. No other potential conflict of interest relevant to this article was reported.
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
We thank the patients and their families, as well as the staff members at all trial sites; and Thomas Hudson, Ph.D., of iMed Comms, for medical-writing support.
Footnotes
A complete list of the AURA3 Investigators is provided in the Supplementary Appendix, available at NEJM.org.
REFERENCES
- 1.Masters GA, Temin S, Azzoli CG, et al. Systemic therapy for Stage IV non-small-cell lung cancer: American Society of Clinical Oncology Clinical Practice Guideline update. J Clin Oncol 2015; 33: 3488–515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Novello S, Barlesi F, Califano R, et al. Metastatic non-small-cell lung cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: Suppl 5: v1–v27. [DOI] [PubMed] [Google Scholar]
- 3.Tan DS, Yom SS, Tsao MS, et al. The International Association for the Study of Lung Cancer consensus statement on optimizing management of EGFR mutation-positive non-small cell lung cancer: status in 2016. J Thorac Oncol 2016; 11: 946–63. [DOI] [PubMed] [Google Scholar]
- 4.National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: NSCLC (version 3.2017). 2016. (http://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf). [DOI] [PubMed]
- 5.Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non–small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–8. [DOI] [PubMed] [Google Scholar]
- 6.Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol 2010; 11: 121–8. [DOI] [PubMed] [Google Scholar]
- 7.Zhou C, Wu YL, Chen G, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol 2011; 12: 735–42. [DOI] [PubMed] [Google Scholar]
- 8.Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol 2012; 13: 239–46. [DOI] [PubMed] [Google Scholar]
- 9.Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of afatinib or cisplatin plus pemetrexed in patients with meta-static lung adenocarcinoma with EGFR mutations. J Clin Oncol 2013; 31: 3327–34. [DOI] [PubMed] [Google Scholar]
- 10.Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the phase III, randomized, open-label, ENSURE study. Ann Oncol 2015; 26: 1883–9. [DOI] [PubMed] [Google Scholar]
- 11.Wu Y-L, Zhou C, Hu C-P, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol 2014; 15: 213–22. [DOI] [PubMed] [Google Scholar]
- 12.Mok TS, Wu Y-L, Thongprasert S, et al. Gefitinib or carboplatin–paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–57. [DOI] [PubMed] [Google Scholar]
- 13.Kobayashi S, Boggon TJ, Dayaram T, et al. EGFR mutation and resistance of non–small-cell lung cancer to gefitinib. N Engl J Med 2005; 352: 786–92. [DOI] [PubMed] [Google Scholar]
- 14.Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res 2013; 19: 2240–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sequist LV, Waltman BA, Dias-Santa-gata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med 2011; 3: 75ra26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oxnard GR, Arcila ME, Sima CS, et al. Acquired resistance to EGFR tyrosine kinase inhibitors in EGFR-mutant lung cancer: distinct natural history of patients with tumors harboring the T790M mutation. Clin Cancer Res 2011; 17: 1616–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yun CH, Mengwasser KE, Toms AV, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A 2008; 105: 2070–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sos ML, Rode HB, Heynck S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res 2010; 70: 868–74. [DOI] [PubMed] [Google Scholar]
- 19.Cross DA, Ashton SE, Ghiorghiu S, et al. AZD9291, an irreversible EGFR TKI, overcomes T790M-mediated resistance to EGFR inhibitors in lung cancer. Cancer Discov 2014; 4: 1046–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ballard P, Yates JW, Yang Z, et al. Pre-clinical comparison of osimertinib with other EGFR-TKIs in EGFR-mutant NSCLC brain metastases models, and early evidence of clinical brain metastases activity. Clin Cancer Res 2016; 22: 5130–40. [DOI] [PubMed] [Google Scholar]
- 21.Yang JC-H, Kim D-W, Kim S-W, et al. Osimertinib activity in patients (pts) with leptomeningeal (LM) disease from non-small cell lung cancer (NSCLC): updated results from BLOOM, a phase I study. J Clin Oncol 2016; 34: Suppl: 9002 abstract. [Google Scholar]
- 22.Jänne PA, Yang JC-H, Kim D-W, et al. AZD9291 in EGFR inhibitor–resistant non–small-cell lung cancer. N Engl J Med 2015; 372: 1689–99. [DOI] [PubMed] [Google Scholar]
- 23.Yang J, Ramalingam SS, Jänne PA, Cantarini M, Mitsudomi T. LBA2_PR: Osimertinib (AZD9291) in pre-treated pts with T790M-positive advanced NSCLC: updated Phase 1 (P1) and pooled Phase 2 (P2) results. J Thorac Oncol 2016; 11: Suppl: S152–S153. [Google Scholar]
- 24.FDA approves new pill to treat certain patients with non-small cell lung cancer. Press release of the Food and Drug Administration, November 13, 2015 (http://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm472525.htm).
- 25.Soria J, Kim S, Wu Y, et al. Gefitinib/chemotherapy vs chemotherapy in EGFR mutation-positive NSCLC resistant to first-line gefitinib: IMPRESS T790M subgroup analysis. J Thorac Oncol 2015; 10: Suppl: S207–S208. [Google Scholar]
- 26.Oxnard GR, Thress KS, Alden RS, et al. Association between plasma genotyping and outcomes of treatment with osimertinib (AZD9291) in advanced non-small-cell lung cancer. J Clin Oncol 2016; 34: 3375–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Jenkins S, Yang J, Ramalingam S, et al. 134O_PR: Plasma ctDNA analysis for detection of EGFR T790M mutation in patients (pts) with EGFR mutation-positive advanced non-small cell lung cancer (aNSCLC). J Thorac Oncol 2016; 11: 4 Suppl: S153–S154. [Google Scholar]
- 28.Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol 2015; 16: 990–8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.