Abstract
Japanese Encephalitis virus (JEV) is a zoonotic flavivirus that represents the most significant etiology of childhood viral neurological infections throughout the Asia. During the last 20 years, JEV genotype dominance has shifted from genotype III (GIII) to genotype I (GI). To date, the exact mechanism of this displacement is still not known. Culex (Cx.) mosquitoes are the most common species in China and play an essential role in maintaining JEV enzootic transmission cycle. In this study, we used Cx. pipiens mosquitoes from China as an in vivo mosquito model to explore if mosquitoes played a potential role in JEV genotype shift. We exposed female Cx. pipiens mosquitoes orally to either GI or GIII JEV strains. Midgut, whole mosquitoes, secondary organs, and salivary glands of JEV-infected mosquitoes were collected at 7 and 14 days of post infection (dpi) and subjected to measure the infection rate, replication kinetics, dissemination rate and transmission potential of the infected JEV strains in Cx. pipiens mosquitoes by 50% tissue culture infective dose assay. We found that Cx. pipiens mosquito was competent vector for both GI and GIII JEV infection, with similar infection rates and growth kinetics. After the establishment of infection, Cx. pipiens mosquitoes disseminated both JEV genotypes to secondary organs at similar rates of dissemination. A few GI-infected mosquito salivary glands (16.2%) were positive for GI virus, whereas GIII virus was undetectable in GIII-infected mosquito salivary glands at 7 dpi. However, 29.4% (5/17) and 36.3% (8/22) were positive for GI- and GIII-infected mosquito salivary glands at 14 dpi, respectively, showing an increase in JEV positive rate. No statistical difference in the transmission rate between GI- and GIII-infected mosquitoes was detected. Our experiment data demonstrated that GI and GIII viruses have similar infectivity in Cx. pipiens mosquitoes, suggesting that Cx. pipiens mosquitoes from China may not play a critical role in JEV genotype shift. Although the current data were obtained solely from Cx. pipiens mosquitoes, it is likely that the conclusion drawn could be extrapolated to the role of mosquitoes in JEV genotype shift.
Author summary
Japanese encephalitis virus (JEV) causes encephalitis and reproductive disorder in humans and pigs respectively, causing a serious impact on public health and pig industry. In nature JEV life cycle include both vertebrates (birds and pigs) as well as invertebrates (mosquitoes). Phylogenetic studies confirmed that JEV has five geographically and epidemiologically distinct genotypes (GI-V). Genotype III (GIII) was an endemic strain in Asia, but recently genotype I (GI) has displaced GIII as the most frequently isolated virus genotype. It is unclear if mosquitoes play a role in this genotype shift or not. Both genotypes are endemic in China and primarily transmitted by different species of Culex (Cx.) mosquitoes. Cx. Mosquitoes are the most common species in China and their role in this genotype shift was not studied previously. In the present study, we used Cx. pipiens mosquitoes from China as an in vivo mosquito model and challenged with GI and GIII JEV strains to explore if mosquitoes played a potential role in JEV genotype shift. Our investigation showed that GI and GIII viruses had similar infectivity in Cx. pipiens mosquitoes which highlighted that Cx. pipiens mosquitoes from China may not play a critical role in the genotype shift. Although the current data were obtained solely from Cx. pipiens mosquitoes, it is likely that the conclusion drawn could be extrapolated to the role of mosquitoes in JEV genotype shift.
Introduction
JEV is one of the leading encephalitis causing virus in the world [1]. According to World Health organization (WHO) more than 24 countries from South Asia and Western Pacific regions have exposed to JEV [1, 2]. JEV transmission cycle include both vertebrates (birds and pigs) as well as invertebrates (mosquitoes). Like other arboviruses, JEV is also transmitted by several Culex (Cx.), Aedes (Ae.), Anopheles (An.) and Armigeres (Ar.) mosquito species [3, 4]. However, Cx. mosquitoes have received much attention because they play a major role in transmission of JEV [4, 5]. Pigs and water birds act as an amplifying/reservoir host and later have an important role in its dispersion [6]. Usually, JEV is transmitted from infected birds/pigs to a susceptible host by mosquitoes [7]. Humans are considered dead end host of JEV infection, because humans infected with JEV seldom develop high viremia therefore, mosquitoes cannot get infection from JEV-infected persons [8].
JEV has a positive sense RNA genome belonging to flavivirus genus within flaviviridae family with three structural and seven non-structural proteins. Phylogenetic analysis indicated that it has five geographically and epidemiologically distinct genotypes (genotype I-V). Genotype III (GIII) had been the most dominant strain and source of outbreaks throughout the years. It was constantly circulating until 1990 throughout Asia, but recent studies have shown the emergence of genotype I (GI) which have displaced GIII [9–13]. According to previous data, GI diverged from Vietnam and spread towards North China, followed by Japan and Korea [14]. However, the mechanism responsible for the JEV genotype shift is unknown. Analysis of GI isolate multiplication shows that the infectivity titers after 24–48 hours post infection are significantly higher in avian and mosquito cells compared to GIII isolates. This indeed implies that high multiplicative ability of GI virus in mosquito infection may have resulted in a decreased incubation period that leads to higher GI enzootic transmission cycles and displaced GIII [15]. Another comparative study of JEV genetics reveals that this genotype shift might be due to differences in the amino acid sequences of NS5 RNA-dependent RNA polymerase between GI and GIII strains, that may help GI to achieve more efficient replication [16]. Although these previously provided data helps us to develop our understanding about JEV genetics and epidemiology, but exact mechanisms involved in GI emergence need to be elucidated.
JEV employs multiple species of mosquitoes to maintain its transmission cycle in nature [17]. In addition to the well-characterized Cx. tritaeniorhynchus, a number of other mosquito species are also competent for JEV infection. JEV has been isolated worldwidely from 17 species of Cx. mosquitoes, such as Cx. pipiens, Cx. theileri, Cx. modestus, Cx. quinquefasciatus, Cx. fuscocephalus, Cx. annulirostris, Cx. gelidus, Cx. whitmorei, Cx. epidesmus, Cx. vishnui, and Cx. pseudovishnui, and from 20 other mosquito species, such as Ar. subalbatus, Ae. vexans, Ae. lineatopennis and An. sinensis [18]. Several species of mosquitoes distributed in China, including Cx. tritaeniorhynchus, Cx. pipiens, Cx. theileri, Cx. modestus, Cx. fuscocephalus, Ar. subalbatus, Ae. vexans and An. sinensis, are competent vectors for JEV infection [19].
Cx. pipiens is one of the most widely distributed Cx. species in the world, especially in temperate regions, and lives in close contact with humans as well as animals [20]. Cx. pipiens is considered an important secondary or regional vector in certain areas such as temperate regions [18]. Both GI and GIII viruses have been isolated from Cx. pipiens [19], suggesting that Cx. pipiens is a potential vector for both genotypes transmission. Given that GI isolate shows higher infectivity than GIII isolates in mosquito cells [15], we therefore used Cx. pipiens mosquitoes from China as an in vivo mosquito model to examine its vector potential for GI and GIII JEV infection as well as the difference in infectivity between GI and GIII viruses to get an insight into its role in JEV genotype shift. We found that Cx. pipiens mosquitoes from China were competent vector for both GI and GIII JEV infection, with similar infection rates, growth kinetics, dissemination rates and transmission rates, showing that GI and GIII viruses have similar infectivity in Cx. pipiens mosquitoes.
Materials and methods
Cells and viruses
Aedes albopictus C6/36 cells were maintained at 28°C in SILAC™ RPMI 1640 (Life Technologies Ltd, Grand Island, USA) and Baby Hamster Kidney BHK-21 cells were maintained at 37°C in Dulbecco Modified Eagle media DMEM (Life Technologies) supplemented with 10% heat-inactivated fetal bovine serum (FBS), 100 IU of penicillin and streptomycin per ml for JEV production [5, 21–23]. JEV GI SH7 strain (GeneBank accession no MH753129) was isolated from Cx. tritaeniorhynchus in 2016 and GIII SH15 strain (GeneBank accession no. MH753130) was isolated from An. sinensis in 2016. Both strains were passaged fewer than seven times in cultured cells, including three passages for plaque purification and one passage on C6/36 cells for mosquito infection. The 50% tissue culture infective dose (TCID50) were determined on BHK-21 cells [23]. Fresh virus suspensions were used in whole experiment.
Mosquitoes rearing and infections
Cx. pipiens mosquitoes used for this experiment were provided by Dr. Zhu Huiman from Second Military Medical University Shanghai, PR China. Mosquitoes were maintained on 10% sucrose ad libitum solution and kept at 28°C with 70% to 80% relative humidity and 12-h-light–12-h-dark photoperiod in cages according to standard conditions [24, 25]. For per os infection, 5–7 days-old female mosquitoes were deprived of sugar and water for 48 and 24 hours, respectively. Viremic blood meals were prepared by mixing virus stocks with defebrinated mice blood and delivered through Hemotek membrane feeding apparatus (Discovery Workshop) and cotton pledget for one hour. Mosquitoes were cold anesthetized on ice prior to sorting fully engorged mosquitoes. 5–6 engorged mosquitoes were immediately collected to determine the quantities of viruses ingested through the blood meals. Titers of viremic blood meals and engorged mosquitoes are summarized in Table 1.
Table 1. JEV titers of viremic blood meals and engorged mosquitoes.
| JEV strains | GI (SH7) | GIII (SH15) | p value* |
|---|---|---|---|
| Viremic blood meals (log TCID50/ml) | 8.30±0.30 | 8.65±0.15 | 0.4063 |
| Engorged mosquitoes (log TCID50/ml) | 4.90±0.80 | 4.78±0.46 | 0.6255 |
*, tested by Student’s t-test.
Sample collection and JEV titration
After oral feeding, engorged mosquitoes were randomly divided into different groups (n>13) and held for extrinsic incubation period. At 7 and 14 dpi samples were collected in DMEM and JEV titration was performed by TCID50 assay [4, 26, 27] to determine the infection rate, growth kinetics, dissemination rate, and transmission rate [28, 29]. Infection rate was defined as the number of mosquitoes with infectious JEV in the midgut divided by the total number of engorged mosquitoes tested. Dissemination rate was defined by the detection of infectious JEV from homogenized secondary organs (legs, wings and heads) among mosquitoes with positive midguts. Transmission rate was defined as the number of mosquitoes with infectious JEV in the salivary glands divided by the total number of mosquitoes with positive midguts. Mosquitoes were caught by mechanical aspiration from cages and anesthetized using ice. Dissection of individual mosquito was conducted under stereomicroscope using dissecting needles to collect midgut for infection rate [30], secondary organs for dissemination rate, and salivary glands for transmission rates, as described previously by Coleman et al [30]. Whole mosquitoes were used to assess JEV growth kinetics. To avoid cross-contamination of virus across the midgut, secondary organs and salivary glands, these organs were dissected carefully using different dissecting needles and dipped in 75% ethanol followed by distal water [27]. Quantification of each sample were performed by TCID50 assay on BHK-21 cells according to previously described method [4, 23].
Statistical analysis
Student t-test or Fisher’s Exact test was performed for statistical analysis. A p value of <0.05 was considered significant. Graph Pad Prism software (version 7) was used for all statistical analysis.
Results
Efficiency in the establishment of JEV infection in midgut of Cx. pipiens mosquitoes from China
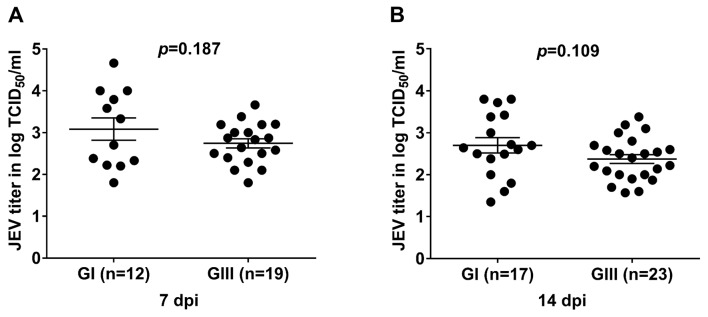
Cx. pipiens mosquitoes from China were used as a in vivo model to examine its vector potential for GI and GIII JEV infection as well as the difference in infectivity between GI and GIII viruses. Midgut is one of mosquito tissues used for analysis of the vector permissive to flavivirus infection [31–34]. Therefore, we determined the replication titers in midguts of Cx. pipiens to explore its vector competence for different JEV genotype infection. The mosquitoes were orally infected with GI and GIII viruses and six of mosquitoes for each group were randomly collected from the engorged mosquitoes immediately after blood feeding for detection of JEV titers. No significant difference in JEV titers was observed between GI- and GIII-infected groups (Table 1). JEV replication in the midgut of JEV-infected mosquitoes was determined by TCID50 assay at 7 and 14 dpi. Infection rates in GI-infected group were 42.8% (12/28) at 7 dpi and 37.7% (17/45) at 14 dpi, which were similar to those 57.5% (19/33) at 7 dpi and 44.2% (23/52) at 14 dpi in GIII-infected group (Table 2). At 7 dpi, similar replication titers (p = 0.187) between GI-infected group (3.083logTCID50 /ml) and GIII-infected group (2.748logTCID50 /ml) were observed (Fig 1A). However, the replication titers were slightly declined at 14 dpi in both JEV-infected groups, but no significant difference (p = 0.109) in replication titers were detected between GI-infected group (2.70logTCID50 /ml) and GIII-infected group (2.37logTCID50 /ml) (Fig 1B). Cumulatively, these data indicated that Cx. pipiens mosquito was competent vector for both GI and GIII JEV infection, with similar infection rate and replication titers.
Table 2. Summary of the infection, dissemination and transmission rates of JEV infected mosquitoes.
| JEV strains | GI (SH7) | GIII (SH15) | p value* | |
|---|---|---|---|---|
| Infection rate | 7 dpi | 42.8% (12/28) | 57.5% (19/33) | 0.3087 |
| 14 dpi | 37.7% (17/45) | 44.2% (23/52) | 0.5422 | |
| Dissemination rate | 7 dpi | 27.2% (3/11) | 31.5% (6/19) | >0.9999 |
| 14 dpi | 23.5% (4/17) | 34.7% (8/23) | 0.6989 | |
| Transmission rate | 7 dpi | 16.6% (2/12) | 0% (0/13) | 0.2200 |
| 14 dpi | 29.4% (5/17) | 36.3% (8/22) | 0.7401 |
*, tested by Fisher’s Exact test.
Fig 1. Viral titers in midgut of JEV-infected mosquitoes.
Cx. pipiens mosquitoes were orally infected with GI or GIII viruses and midguts of the infected mosquitoes were collected at 7 dpi (A) and 14 dpi (B) for measurement of JEV titers by TCID50 assay. n, the numbers of mosquitoes tested positive for JEV. The p values were generated by Student t-test between GI- and GIII-infected groups. A p value of <0.05 was considered significant.
Replication kinetics of JEV in Cx. pipiens mosquitoes
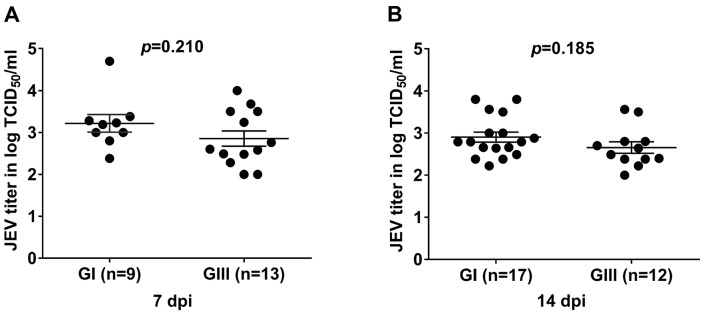
JEV replicates in various tissues of mosquitoes [33, 35, 36], we therefore determined replication kinetics in whole mosquitoes infected with GI or GIII JEV. Following oral infection, the Cx. pipiens mosquitoes were collected at 7 and 14 dpi and JEV titers were measured by TCID50 assay. Among 16 and 27 GI-infected mosquitoes collected at 7 and 14 dpi, 9 and 17 were tested positive for JEV, respectively. While out of 20 and 25 GIII-infected mosquitoes collected at 7 and 14 dpi, 13 and 12 were tested positive for JEV, respectively. The replication titers in the JEV-positive mosquitoes were further compared between GI- and GIII-infected groups. No significant difference in replication titers between GI- and GIII-infected mosquitoes were detected at both 7 and 14 dpi. As shown in Fig 2, the replication titers in GI-infected group were 3.21logTCID50 /ml at 7 dpi (n = 9), which was statistically similar (p = 0.2107) with that (2.85logTCID50 /ml) in GIII-infected group (n = 13) (Fig 2A). At 14 dpi, the replication titer (2.90logTCID50 /ml) in GI-infected group (n = 17) was also relatively higher than that (2.65logTCID50 /ml) in GIII-infected group (n = 12), but no significant difference was detected (p = 0.1859) (Fig 2B). Overall, these results suggested that the GI and GIII JEV replicated with similar kinetics in Cx. pipiens mosquitoes.
Fig 2. Viral titers in whole mosquitoes of JEV-infected mosquitoes.
Cx. pipiens mosquitoes were orally infected with GI or GIII viruses and whole mosquitoes were collected at 7 dpi (A) and 14 dpi (B) for measurement of JEV titers by TCID50 assay on BHK-21 cells. n, the numbers of mosquitoes tested positive for JEV. The p values were generated by Student t-test between GI- and GIII-infected groups. A p value of <0.05 was considered significant.
Dissemination of JEV in Cx. pipiens mosquitoes
To determine whether JEV-infected mosquitoes could disseminate virus to secondary organs, Cx. pipiens mosquitoes were infected orally with GI or GIII JEV and the secondary organs including legs, wings and heads from JEV-infected mosquitoes that were tested positive for JEV in midguts were collected at 7 and 14 dpi for detection of JEV presence. No statistical difference in the dissemination rates between GI- and GIII-infected mosquitoes was tested at both 7 dpi (Fisher’s Exact test, p>0.9999) and 14 dpi (Fisher’s Exact test, p = 0.6989). As shown in Table 2, the dissemination rate of GI-infected mosquitoes was 27.2%, which was not significantly different to that (31.5%) of GIII-infected mosquitoes at 7 dpi. Similar results were observed at 14 dpi between GI- (23.5%) and GIII- (34.7%) infected mosquitoes. These results suggested that Cx. pipiens mosquitoes disseminated JEV to secondary organs after the establishment of infection, with similar dissemination rate between GI and GIII viruses.
Viral load in salivary glands of JEV infected Cx. pipiens mosquitoes
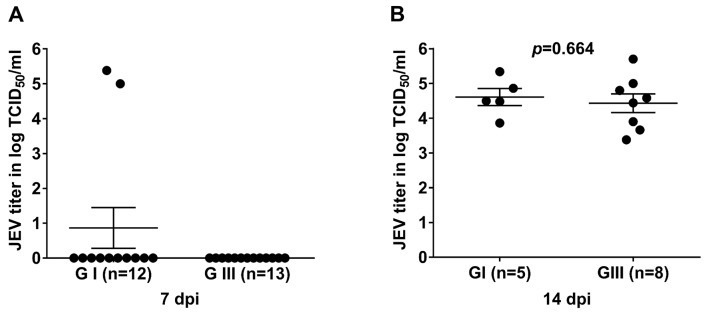
Saliva plays crucial role in transmission of flaviviruses [27, 37, 38], we therefore determined the viral load in salivary glands of JEV-infected mosquitoes to compare the potentials of Cx. pipiens mosquitoes in JEV transmission. The salivary glands of JEV-infected mosquitoes that were tested positive for JEV in midguts were subjected to analysis of viral load by TCID50 assay. Out of 12 GI-infected mosquitoes tested, two showed positive for JEV in the salivary glands at 7 dpi, whereas no mosquito was detected positive in the salivary gland among 13 GIII-infected mosquitoes tested (Fig 3A, Table 2). However, 29.4% (5/17) and 36.3% (8/22) were positive for GI and GIII viruses in the salivary glands at 14 dpi, respectively; showing an increase in JEV positive rate, but no significant difference was detected between GI- and GIII-infected groups (Table 2). At 14 dpi, JEV titer in the salivary glands of GI-infected mosquitoes was 4.60logTCID50/ml that was not significantly different (p = 0.6648) from that (4.33logTCID50/ml) in the salivary glands of GIII-infected mosquitoes (Fig 3B). It has previously been shown that virus must go through the midgut barrier and then spread to salivary glands. Therefore, the time of virus detected in salivary glands was later than the time of detection in midgut [39, 40]. Altogether these results indicated that the Cx. pipiens mosquitoes had potential for transmission of both genotype viruses, with similar transmission rates.
Fig 3. Viral titers in salivary glands of JEV-infected mosquitoes.
Cx. pipiens mosquitoes were orally infected with GI or GIII viruses and salivary glands of the infected mosquitoes were collected at 7 dpi (A) and 14 dpi (B) for measurement of JEV titers by TCID50 assay on BHK-21 cells. n, the numbers of mosquitoes tested positive for JEV in midguts. The p values were generated by Student t-test between GI- and GIII-infected groups. A p value of <0.05 was considered significant.
Discussion
In recent years, multiple reports indicated that GI JEV has taken over GIII, as the most frequently isolated strain in a number of Asian countries [41–43]. The mechanism of this shifting is still unclear. A previous observation describes that GI strain has superior multiplication kinetics in C6/36 cells as compared to GIII strain [15], implying that mosquitoes may play a potential role in JEV genotype shift. Cx. pipiens mosquito is one of the most common and high density Cx. species [44–46] and lives in close contact with humans as well as animals with blood-feeding behavior [20, 47]. In addition, Cx. pipiens mosquito is easy to be reared and experimentally infected with flavivirus in laboratorial condition [48–50]. In response to JEV infection, Cx. pipiens shows susceptibility similar to Cx. tritaeniorhyncus and has been considered as an effective laboratory vector for JEV infection [51]. Therefore, we used Cx. pipiens mosquito as an in vivo mosquito model to explore whether mosquito could play a role in JEV genotype shift. Although Cx. tritaeniorhyncus is the primary vector for JEV transmission, other species of Cx. mosquitoes, such as Cx. pipiens, are considered important secondary or regional vectors in certain areas such as temperate regions [18]. It has been reported that the increasing JE cases are observed in the area with very less density of Cx. tritaeniorhyncus (1%) and high population of Cx. pipiens (>60%) [45], suggesting that Cx. pipiens other than Cx. tritaeniorhyncus may play a major role in JEV transmission in certain areas. In addition, Cx. pipiens feeds mostly on birds (83%) [49, 52] that act as the reservoir and amplifying hosts for maintaining JEV transmission cycle and have been speculated to be involved in the increased JE cases and genotype shift [45, 53].
We have previously compared the replication efficiency of 3 GI isolates with 4 GIII isolates in mosquito cells and found no significant difference in replication efficiency between GI and GIII isolates tested, suggesting similar replication efficiency between GI and GIII isolates in mosquito cells [53]. Based on these findings, we selected one GI isolate (SH7 strain) and one GIII isolate (SH15 strain) to compare the replication efficiency in Cx. pipiens mosquitoes. Both strains were isolated from mosquitoes in 2016 and have replication kinetics similar to the average replication kinetics of their respective genotype isolates in mosquito, swine and avian cells [53]. Therefore, we considered that these two strains could be representative of their respective genotypes.
We demonstrated experimentally that Cx. pipiens mosquito was competent vector for both GI and GIII infection. The infection rates and growth kinetics in the orally infected Cx. pipiens mosquitoes showed no significant difference between GI- and GIII-infected groups. After development of infection, Cx. pipiens mosquitoes disseminated both JEV genotypes to secondary organs at similar dissemination rate. GI and GIII were detectable in the salivary glands with 29.4% to 36.3% positive rate at 14 dpi, suggesting a potential of Cx. pipiens mosquitoes for transmission of both genotype viruses. However, no significant difference in the transmission rates between GI- and GIII-infected mosquitoes was detected. As whole, our experiment data demonstrated that GI and GIII viruses have similar infectivity in Cx. pipiens mosquitoes, suggesting that Cx. pipiens mosquitoes from China may not play a critical role in JEV genotype shift. However, this conclusion was generated by the use of a single representative JEV strain from each genotype, further studies with more different GI and GIII JEV strains should be conducted to confirm this conclusion.
A previous in vitro observation indicates that the infectivity titers of GI isolate after 24–48 hours post infection are significantly higher in mosquito cells compared to GIII isolates [15]. This observation is partially in contrast with our findings that no significant difference in infectivity titers between GI and GIII strains was observed in Cx. pipiens mosquitoes. However, our findings are consistent with a previous in vivo observation [5], in which similar infection rate, dissemination rate and transmission rate are observed between GI- and GIII-infected North American Cx. quinquefasciatus mosquitoes. Our data together with the previous in vivo observations, suggested that Cx. species are competent vectors for both GI and GIII JEV infection with similar infectivity and that Cx. pipiens mosquitoes may not play a critical role in JEV genotype shift. This conclusion is further supported by Wispelaere et al’s observation [4], in which they analyze the vector competence of European Cx. pipiens mosquitoes for GIII and genotype V (GV) JEV infection and noted similar infectivity between GIII- and GV- infected Cx. pipiens mosquitoes.
An interesting observation from our experiments was the earlier viral load detected in salivary glands of GI-infected Cx. pipiens mosquitoes. Out of 12 GI-infected mosquitoes tested, two showed positive for JEV in the salivary glands at 7 dpi, whereas no mosquito was detected positive in the salivary gland among 13 GIII-infected mosquitoes tested. Saliva plays crucial role in JEV transmission. The earlier viral load in salivary glands of GI-infected mosquitoes could be taken into account as a potential factor when dissecting the mechanisms responsible for JEV genotype shift.
Genetic drift during systemic arbovirus infection of mosquitoes randomly generates tissue and saliva specific progeny arbovirus [40, 54–56]. For example, mutant progeny of West Nile virus (WNV) is transiently detected in the saliva of infected individual mosquito between feeding episodes. The mutant WNV has advantage in competitive fitness relative to the reference WNV in Cx. quinquefasciatus mosquitoes, but becomes extinct in some individual mosquito during competitive fitness assays [57]. We did not know whether the earlier viral load detected in the salivary glands of GI-infected mosquitoes was the progeny virus specific to salivary glands generated by genetic drift or attributable to the enhanced vector fitness generated by convergent evolution. Future studies with virus isolation and genome sequencing under different time intervals should be conducted to determine the reasons responsible for the earlier viral load detected in the salivary glands of GI-infected Cx. pipiens mosquitoes, which could be useful for elucidating the mechanisms of JEV genotype shift.
JEV enzootic transmission cycle is maintained by both vertebrates (wild birds and pigs) and invertebrates (mosquitoes) [3, 4, 17, 58]. In addition to mosquitoes, pig serves as a major amplifying host of JEV in Asia, especially in China pork industry has grown exponentially (87%) within the last twenty years [47] and also close proximity of pig breeding farms to suburban areas increases the risk of JE cases [59]. Birds serve as an amplifying/reservoir host of JEV. Previous studies reported that avian species can develop viremia either with natural exposure or by challenging in laboratory [17, 60–62]. In 2009, Saito et al. suggested that wild ducks can play a role in JEV reservoir in Hakkaido, Japan [63]. These findings further supported by Yang et al., where he had reported 84%-88.5% sero-prevalence of JEV in different wild birds including ducks [64]. A most recent study in Korea demonstrated that distribution of wading birds and the incidence of JE cases are correlated [45]. Recently, our lab also reported that GI replicates more efficiently than GIII in avian and porcine cells, particularly in avian cells with titers reaching 22.9−225.3 fold higher than GIII. In addition, GI-inoculated ducklings developed higher viremia titers and showed a relatively longer viremic duration than GIII-inoculated ducklings [53]. These reports suggest that pigs/birds may have some important role in JEV genotype shift that need to be explored in future.
In addition to JEV hosts, phylogenetic studies suggest that the mechanism of JEV genotype shift might be due to amino acid variations between GI and GIII viral proteins, especially the variation in JEV envelope protein that plays major roles in mediating virus entry and pathogenicity [9, 15, 65], and the variation in JEV nonstructural protein 5 that plays essential roles in methylation of the 5’ RNA cap structure, viral replication and antagonization of the interferon response [66]. These amino acid variations alone or in combination with variations in other proteins or genomes may lead to GI viruses with increased host fitness and enhanced multiplicative ability in hosts such as birds and pigs and eventually displacement of GIII as a dominant genotype. This hypothesis is currently under investigation in our laboratory.
In conclusion, we compared the infectivity of GI and GIII viruses in Cx. pipiens mosquitoes and found that Cx. pipiens mosquito is competent vector for both GI and GIII JEV infection, with similar infection rate and growth kinetics. Cx. pipiens mosquitoes were able to disseminate both JEV genotype to secondary organs at similar level of dissemination. Both JEV genotypes were detectable in the salivary glands of infected mosquitoes at similar transmission rate, suggesting the potential of Cx. pipiens mosquitoes for transmission of both genotype viruses. Our experiment data demonstrated that GI and GIII viruses had similar infectivity in Cx. pipiens mosquitoes, suggesting that Cx. pipiens mosquitoes from China may not play a critical role in JEV genotype shift. However, this conclusion was generated by the use of a single representative JEV strain from each genotype, further studies with more different GI and GIII JEV strains should be conducted to confirm this conclusion. Although the current data were obtained solely from Cx. pipiens mosquitoes, it is likely that the conclusion drawn could be extrapolated to the role of mosquitoes in JEV genotype shift.
Data Availability
All relevant data are within the manuscript
Funding Statement
The study was supported by the National Key Research and Development Program of China (No. 2017YFD0501805, No. 2016YFD0500404) awarded to KL and YQ, Project of Shanghai Science and Technology Commission (No. 17391901600) awarded to ZM, Natural Science Foundation of Shanghai (No. 19ZR1469000) award to JW, Project of International Science and Technology Cooperation (No. 2014DFE30140) award to ZM, Shanghai Agricultural Applied Technology Development Program (No. G20130506) award to ZM. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Campbell GL, Hills SL, Fischer M, Jacobson JA, Hoke CH, Hombach JM, et al. 2011. Estimated global incidence of Japanese encephalitis: a systematic review. Bulletin of the World Health Organization.89:766–74. 10.2471/BLT.10.085233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gould E, Solomon T. 2008. Pathogenic flaviviruses. The Lancet.371(9611):500–9. [DOI] [PubMed] [Google Scholar]
- 3.Van Den Hurk A, Nisbet D, Hall R, Kay B, Mackenzie J, Ritchie S. 2003. Vector competence of Australian mosquitoes (Diptera: Culicidae) for Japanese encephalitis virus. Journal of medical entomology.40(1):82–90. 10.1603/0022-2585-40.1.82 [DOI] [PubMed] [Google Scholar]
- 4.De Wispelaere M, Desprès P, Choumet V. 2017. European Aedes albopictus and Culex pipiens are competent vectors for Japanese encephalitis virus. PLoS neglected tropical diseases.11(1):e0005294 10.1371/journal.pntd.0005294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Huang Y-JS, Hettenbach SM, Park SL, Higgs S, Barrett AD, Hsu W-W, et al. 2016. Differential infectivities among different Japanese encephalitis virus genotypes in Culex quinquefasciatus mosquitoes. PLoS neglected tropical diseases.10(10):e0005038 10.1371/journal.pntd.0005038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Endy T, Nisalak A. Japanese encephalitis virus: ecology and epidemiology Japanese encephalitis and West Nile viruses: Springer; 2002. p. 11–48. [DOI] [PubMed] [Google Scholar]
- 7.Ricklin ME, García-Nicolás O, Brechbühl D, Python S, Zumkehr B, Nougairede A, et al. 2016. Vector-free transmission and persistence of Japanese encephalitis virus in pigs. Nature communications.7:10832 10.1038/ncomms10832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Weaver SC, Barrett AD. 2004. Transmission cycles, host range, evolution and emergence of arboviral disease. Nature Reviews Microbiology.2(10):789 10.1038/nrmicro1006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schuh AJ, Ward MJ, Brown AJL, Barrett AD. 2013. Phylogeography of Japanese encephalitis virus: genotype is associated with climate. PLoS neglected tropical diseases.7(8):e2411 10.1371/journal.pntd.0002411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schuh AJ, Guzman H, Tesh RB, Barrett AD. 2013. Genetic diversity of Japanese encephalitis virus isolates obtained from the Indonesian archipelago between 1974 and 1987. Vector-Borne and Zoonotic Diseases.13(7):479–88. 10.1089/vbz.2011.0870 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Su C-L, Yang C-F, Teng H-J, Lu L-C, Lin C, Tsai K-H, et al. 2014. Molecular epidemiology of Japanese encephalitis virus in mosquitoes in Taiwan during 2005–2012. PLoS neglected tropical diseases.8(10):e3122 10.1371/journal.pntd.0003122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gao X, Liu H, Wang H, Fu S, Guo Z, Liang G. 2013. Southernmost Asia is the source of Japanese encephalitis virus (genotype 1) diversity from which the viruses disperse and evolve throughout Asia. PLoS neglected tropical diseases.7(9):e2459 10.1371/journal.pntd.0002459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Do LP, Bui TM, Hasebe F, Morita K, Phan NT. 2015. Molecular epidemiology of Japanese encephalitis in northern Vietnam, 1964–2011: genotype replacement. Virology journal.12(1):51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller RH, Masuoka P, Klein TA, Kim H-C, Somer T, Grieco J. 2012. Ecological niche modeling to estimate the distribution of Japanese encephalitis virus in Asia. PLoS neglected tropical diseases.6(6):e1678 10.1371/journal.pntd.0001678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schuh AJ, Ward MJ, Brown AJL, Barrett AD. 2014. Dynamics of the emergence and establishment of a newly dominant genotype of Japanese encephalitis virus throughout Asia. Journal of virology.JVI. 02686–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Li J, Guo D, Huang L, Yin M, Liu Q, Wang Y, et al. 2014. The interaction between host Annexin A2 and viral Nsp9 is beneficial for replication of porcine reproductive and respiratory syndrome virus. Virus research.189:106–13. 10.1016/j.virusres.2014.05.015 [DOI] [PubMed] [Google Scholar]
- 17.Van den Hurk AF, Ritchie SA, Mackenzie JS. 2009. Ecology and geographical expansion of Japanese encephalitis virus. Annual review of entomology.54:17–35. 10.1146/annurev.ento.54.110807.090510 [DOI] [PubMed] [Google Scholar]
- 18.Pearce JC, Learoyd TP, Langendorf BJ, Logan JG. 2018. Japanese encephalitis: the vectors, ecology and potential for expansion. Journal of travel medicine.25(Suppl_1):S16–S26. 10.1093/jtm/tay009 [DOI] [PubMed] [Google Scholar]
- 19.Zheng Y, Li M, Wang H, Liang G. 2012. Japanese encephalitis and Japanese encephalitis virus in mainland China. Reviews in medical virology.22(5):301–22. 10.1002/rmv.1710 [DOI] [PubMed] [Google Scholar]
- 20.Zhao T, Lu B. 1995. Biosystematics of Culex pipiens complex in China. Insect Science.2(1):1–8. [Google Scholar]
- 21.Krishnan MN, Ng A, Sukumaran B, Gilfoy FD, Uchil PD, Sultana H, et al. 2008. RNA interference screen for human genes associated with West Nile virus infection. Nature.455(7210):242 10.1038/nature07207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lin C-C, Yang C-F, Tu C-H, Huang C-G, Shih Y-T, Chuang C-K, et al. 2007. A novel tetraspanin C189 upregulated in C6/36 mosquito cells following dengue 2 virus infection. Virus research.124(1–2):176–83. 10.1016/j.virusres.2006.11.002 [DOI] [PubMed] [Google Scholar]
- 23.Rodriguez-Andres J, Rani S, Varjak M, Chase-Topping ME, Beck MH, Ferguson MC, et al. 2012. Phenoloxidase activity acts as a mosquito innate immune response against infection with Semliki Forest virus. PLoS pathogens.8(11):e1002977 10.1371/journal.ppat.1002977 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Xi Z, Ramirez JL, Dimopoulos G. 2008. The Aedes aegypti toll pathway controls dengue virus infection. PLoS pathogens.4(7):e1000098 10.1371/journal.ppat.1000098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Clemons A, Mori A, Haugen M, Severson DW, Duman-Scheel M. 2010. Culturing and egg collection of Aedes aegypti. Cold Spring Harbor Protocols.2010(10):pdb. prot5507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kenney JL, Romo H, Duggal NK, Tzeng W-P, Burkhalter KL, Brault AC, et al. 2017. Transmission incompetence of Culex quinquefasciatus and Culex pipiens pipiens from North America for Zika virus. The American journal of tropical medicine and hygiene.96(5):1235–40. 10.4269/ajtmh.16-0865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liu Z, Zhou T, Lai Z, Zhang Z, Jia Z, Zhou G, et al. 2017. Competence of Aedes aegypti, Ae. albopictus, and Culex quinquefasciatus mosquitoes as Zika virus vectors, China. Emerging infectious diseases.23(7):1085 10.3201/eid2307.161528 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weger-Lucarelli J, Rückert C, Chotiwan N, Nguyen C, Luna SMG, Fauver JR, et al. 2016. Vector competence of American mosquitoes for three strains of Zika virus. PLoS neglected tropical diseases.10(10):e0005101 10.1371/journal.pntd.0005101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Turell MJ, Wilson WC, Bennett KE. 2010. Potential for North American mosquitoes (Diptera: Culicidae) to transmit rift valley fever virus. Journal of medical entomology.47(5):884–9. 10.1603/me10007 [DOI] [PubMed] [Google Scholar]
- 30.Coleman J, Juhn J, James AA. 2007. Dissection of midgut and salivary glands from Ae. aegypti mosquitoes. Journal of visualized experiments: JoVE.(5). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Girard YA, Klingler KA, Higgs S. 2004. West Nile virus dissemination and tissue tropisms in orally infected Culex pipiens quinquefasciatus. Vector-borne and zoonotic diseases.4(2):109–22. 10.1089/1530366041210729 [DOI] [PubMed] [Google Scholar]
- 32.Romoser WS, Wasieloski LP Jr, Pushko P, Kondig JP, Lerdthusnee K, Neira M, et al. 2004. Evidence for arbovirus dissemination conduits from the mosquito (Diptera: Culicidae) midgut. Journal of medical entomology.41(3):467–75. 10.1603/0022-2585-41.3.467 [DOI] [PubMed] [Google Scholar]
- 33.Salazar MI, Richardson JH, Sánchez-Vargas I, Olson KE, Beaty BJ. 2007. Dengue virus type 2: replication and tropisms in orally infected Aedes aegypti mosquitoes. BMC microbiology.7(1):9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hanley KA, Nelson JT, Schirtzinger EE, Whitehead SS, Hanson CT. 2008. Superior infectivity for mosquito vectors contributes to competitive displacement among strains of dengue virus. BMC ecology.8(1):1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kramer LD, Ciota AT. 2015. Dissecting vectorial capacity for mosquito-borne viruses. Current opinion in virology.15:112–8. 10.1016/j.coviro.2015.10.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cheng G, Liu Y, Wang P, Xiao X. 2016. Mosquito defense strategies against viral infection. Trends in parasitology.32(3):177–86. 10.1016/j.pt.2015.09.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Jin L, Guo X, Shen C, Hao X, Sun P, Li P, et al. 2018. Salivary factor LTRIN from Aedes aegypti facilitates the transmission of Zika virus by interfering with the lymphotoxin-β receptor. Nature immunology.19(4):342 10.1038/s41590-018-0063-9 [DOI] [PubMed] [Google Scholar]
- 38.McKimmie C, Pingen M, Bryden S, Lefteri D. 2018. Host inflammatory response to mosquito bites enhances the severity of arbovirus infection. Isbt Science Series.13(1):76–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Franz A, Kantor A, Passarelli A, Clem R. 2015. Tissue barriers to arbovirus infection in mosquitoes. Viruses.7(7):3741–67. 10.3390/v7072795 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grubaugh ND, Weger-Lucarelli J, Murrieta RA, Fauver JR, Garcia-Luna SM, Prasad AN, et al. 2016. Genetic drift during systemic arbovirus infection of mosquito vectors leads to decreased relative fitness during host switching. Cell host & microbe.19(4):481–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pan J-r, Yan J-y, Zhou J-y, Tang X-w, He H-q, Xie R-h, et al. 2016. Sero-molecular epidemiology of Japanese encephalitis in Zhejiang, an eastern province of China. PLoS neglected tropical diseases.10(8):e0004936 10.1371/journal.pntd.0004936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chai C, Wang Q, Cao S, Zhao Q, Wen Y, Huang X, et al. 2018. Serological and molecular epidemiology of Japanese encephalitis virus infections in swine herds in China, 2006–2012. Journal of veterinary science.19(1):151–5. 10.4142/jvs.2018.19.1.151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fulmali PV, Sapkal GN, Athawale S, Gore MM, Mishra AC, Bondre VP. 2011. Introduction of Japanese encephalitis virus genotype I, India. Emerging infectious diseases.17(2):319 10.3201/eid1702.100815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhao T, Lu B. 1996. Hybridization of Culex pipiens complex of China. Acta Zootaxonomica Sinica.21(2):218–23. [Google Scholar]
- 45.Bae W, Kim JH, Kim J, Lee J, Hwang ES. 2018. Changes of epidemiological characteristics of Japanese encephalitis viral infection and birds as a potential viral transmitter in Korea. Journal of Korean medical science.33(9). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cui F, Qiao C-L, Shen B-C, Marquine M, Weill M, Raymond M. 2007. Genetic differentiation of Culex pipiens (Diptera: Culicidae) in China. Bulletin of entomological research.97(3):291–7. 10.1017/S0007485307004968 [DOI] [PubMed] [Google Scholar]
- 47.Erlanger TE, Weiss S, Keiser J, Utzinger J, Wiedenmayer K. 2009. Past, present, and future of Japanese encephalitis. Emerging infectious diseases.15(1):1 10.3201/eid1501.080311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Talavera S, Birnberg L, Nuñez AI, Muñoz-Muñoz F, Vázquez A, Busquets N. 2018. Culex flavivirus infection in a Culex pipiens mosquito colony and its effects on vector competence for Rift Valley fever phlebovirus. Parasites & vectors.11(1):310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Richards SL, Anderson SL, Lord CC, Smartt CT, Tabachnick WJ. 2012. Relationships between infection, dissemination, and transmission of West Nile virus RNA in Culex pipiens quinquefasciatus (Diptera: Culicidae). Journal of medical entomology.49(1):132–42. 10.1603/me10280 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vinogradova EB. Culex pipiens pipiens mosquitoes: taxonomy, distribution, ecology, physiology, genetics, applied importance and control: Pensoft Publishers; 2000. [Google Scholar]
- 51.Weng M-H, Lien J-C, Lin C-C, Yao C-W. 2000. Vector competence of Culex pipiens molestus (Diptera: Culicidae) from Taiwan for a sympatric strain of Japanese encephalitis virus. Journal of medical entomology.37(5):780–3. 10.1603/0022-2585-37.5.780 [DOI] [PubMed] [Google Scholar]
- 52.Hamer GL, Kitron UD, Goldberg TL, Brawn JD, Loss SR, Ruiz MO, et al. 2009. Host selection by Culex pipiens mosquitoes and West Nile virus amplification. The American journal of tropical medicine and hygiene.80(2):268–78. [PubMed] [Google Scholar]
- 53.Xiao C, Li C, Di D, Cappelle J, Liu L, Wang X, et al. 2018. Differential replication efficiencies between Japanese encephalitis virus genotype I and III in avian cultured cells and young domestic ducklings. PLoS neglected tropical diseases.12(12):e0007046 10.1371/journal.pntd.0007046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ciota AT, Ehrbar DJ, Van Slyke GA, Payne AF, Willsey GG, Viscio RE, et al. 2012. Quantification of intrahost bottlenecks of West Nile virus in Culex pipiens mosquitoes using an artificial mutant swarm. Infection, Genetics and Evolution.12(3):557–64. 10.1016/j.meegid.2012.01.022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Forrester NL, Guerbois M, Seymour RL, Spratt H, Weaver SC. 2012. Vector-borne transmission imposes a severe bottleneck on an RNA virus population. PLoS pathogens.8(9):e1002897 10.1371/journal.ppat.1002897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lequime S, Fontaine A, Gouilh MA, Moltini-Conclois I, Lambrechts L. 2016. Genetic drift, purifying selection and vector genotype shape dengue virus intra-host genetic diversity in mosquitoes. PLoS genetics.12(6):e1006111 10.1371/journal.pgen.1006111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Grubaugh ND, Fauver JR, Rückert C, Weger-Lucarelli J, Garcia-Luna S, Murrieta RA, et al. 2017. Mosquitoes transmit unique West Nile virus populations during each feeding episode. Cell reports.19(4):709–18. 10.1016/j.celrep.2017.03.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Nidaira M, Taira K, Okano S, Shinzato T, Morikawa T, Tokumine M, et al. 2009. Survey of Japanese encephalitis virus in pigs on Miyako, Ishigaki, Kume, and Yonaguni islands in Okinawa, Japan. Jpn J Infect Dis.62(3):220–4. [PubMed] [Google Scholar]
- 59.Lindahl J, Chirico J, Boqvist S, Thu HTV, Magnusson U. 2012. Occurrence of Japanese encephalitis virus mosquito vectors in relation to urban pig holdings. The American journal of tropical medicine and hygiene.87(6):1076–82. 10.4269/ajtmh.2012.12-0315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mackenzie JS, Gubler DJ, Petersen LR. 2004. Emerging flaviviruses: the spread and resurgence of Japanese encephalitis, West Nile and dengue viruses. Nature medicine.10(12s):S98. [DOI] [PubMed] [Google Scholar]
- 61.Nemeth N, Bosco-Lauth A, Oesterle P, Kohler D, Bowen R. 2012. North American birds as potential amplifying hosts of Japanese encephalitis virus. The American journal of tropical medicine and hygiene.87(4):760–7. 10.4269/ajtmh.2012.12-0141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Xiao C, Wang X, Cui G, Pang L, Xu J, Li C, et al. 2018. Possible pathogenicity of Japanese encephalitis virus in newly hatched domestic ducklings. Veterinary microbiology.227:8–11. 10.1016/j.vetmic.2018.10.016 [DOI] [PubMed] [Google Scholar]
- 63.Saito M, Osa Y, Asakawa M. 2009. Antibodies to flaviviruses in wild ducks captured in Hokkaido, Japan: risk assessment of invasive flaviviruses. Vector-Borne and zoonotic diseases.9(3):253–8. 10.1089/vbz.2008.0111 [DOI] [PubMed] [Google Scholar]
- 64.Yang D-K, Oh Y-I, Kim H-R, Lee Y-J, Moon O-K, Yoon H, et al. 2011. Serosurveillance for Japanese encephalitis virus in wild birds captured in Korea. Journal of veterinary science.12(4):373–7. 10.4142/jvs.2011.12.4.373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Han N, Adams J, Chen P, Guo Z-y, Zhong X-f, Fang W, et al. 2014. Comparison of genotypes I and III in Japanese encephalitis virus reveals distinct differences in their genetic and host diversity. Journal of virology.88(19):11469–79. 10.1128/JVI.02050-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Li X-D, Shan C, Deng C-L, Ye H-Q, Shi P-Y, Yuan Z-M, et al. 2014. The interface between methyltransferase and polymerase of NS5 is essential for flavivirus replication. PLoS neglected tropical diseases.8(5):e2891 10.1371/journal.pntd.0002891 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript