Abstract
Dengue is one of the most serious mosquito-borne infectious diseases in the world. Aedes albopictus is the most invasive mosquito and one of the primary vectors of dengue. Vector control using insecticides is the only viable strategy to prevent dengue virus transmission. In Guangzhou, after the 2014 pandemic, massive insecticides have been implemented. Massive insecticide use may lead to the development of resistance, but few reports are available on the status of insecticide resistance in Guangzhou after 2014. In this study, Ae. albopictus were collected from four districts with varied dengue virus transmission intensity in Guangzhou from 2015 to 2017. Adult Ae. albopictus insecticide susceptibility to deltamethrin (0.03%), permethrin(0.25%), DDT(4%), malathion (0.8%) and bendiocarb (0.1%) was determined by the standard WHO tube test, and larval resistance bioassays were conducted using temephos, Bacillus thuringiensis israelensis (Bti), pyriproxyfen (PPF) and hexaflumuron. Mutations at the voltage-gated sodium channel (VGSC) gene and acetylcholinesterase (AChE) gene were analyzed. The effect of cytochrome P450s on the resistance of Ae. albopictus to deltamethrin was tested using the synergistic agent piperonyl butoxide (PBO). The results showed that Ae. albopictus populations have rapidly developed very high resistances to multiple commonly used insecticides at all study areas except malathion, Bti and hexaflumuron. We found 1534 codon mutations in the VGSC gene that were significantly correlated with the resistance to pyrethroids and DDT, and 11 synonymous mutations were also found in the gene. The resistance to deltamethrin can be significantly reduced by PBO but may generated cross-resistance to PPF. Fast emerging resistance in Ae. albopictus may affect mosquito management and threaten the prevention and control of dengue, similar to the resistance in Anopheles mosquitoes has prevented the elimination of malaria and call for timely and guided insecticide management.
Author summary
Guangzhou is the most epidemic area of dengue in China. Massive insecticides have been used to control the vector mosquito Ae. albopictus, as no specific vaccines are available for dengue. Regular monitoring of insecticide susceptibility is essential for insecticide resistance management. In this study, the insecticide resistances of Ae. albopictus in Guangzhou were comparatively analyzed from 2015 to 2017. The results displayed that Ae. albopictus had rapidly generated high resistance to the most commonly used adult insecticide pyrethroid (deltamethrin and permethrin) and larvicide organophosphate (temephos). The combination of malathion for adult mosquitoes and Bti or hexaflumuron for larvae might be a better choice for vector control. Resistance to deltamethrin can be significantly reduced by PBO but may generated cross-resistance to PPF. F1534S and F1534L mutations in the VGSC gene were significantly correlated with resistance to pyrethroids. This study indicated that the insecticide resistances had been generated in Ae. albopictus in Guangzhou which was correlated with the dengue epidemic responses.
Introduction
Dengue is one of the most rapidly spreading mosquito-borne diseases in the world. Currently, 3.9 billion people in 128 countries or regions are at risk of dengue fever [1–3]. Guangzhou, the largest city in southern China and the capital of Guangdong Province, has become the epicenter of dengue outbreaks in China. In Guangzhou, the number of dengue cases accounted for 50% of the national incidence between 1978–2011 and an epidemic had occurred once every 3–4 years since the 1990s [4, 5]. In particular in 2014, a pandemic of dengue broke out in Guangzhou with more than 37,000 cases reported [6].
Aedes albopictus is the most invasive mosquito and is widely distributed in China, from Hainan in the south to Dalian in the north, while Aedes aegypti is only distributed in Hainan, Yunnan and a small area of the southernmost part of Guangdong Province [7]. Ae. albopictus is the main vector for dengue virus in China and in Guangzhou Ae. albopictus is the sole vector of dengue virus [8, 9]. Currently, due to the lack of effective drugs and vaccines against dengue, vector management is the main strategy to prevent and control mosquito-borne diseases, including dengue [10–13]. In China, chemical control through the use of insecticides is one of the major tools for the control of vector mosquitoes [14, 15]. During the outbreak of dengue in Guangzhou in 2014, more than 27,000 kg of pyrethroids were used for ultralow-volume (ULV) spraying to control adult Ae. albopictus, and a large amount of temephos, an organophosphate larvicide, was used for larval control. Chemical insecticides were also frequently used for focal hot-spot control of sporadic dengue transmission in Guangzhou [16, 17]. At the same time, agricultural insecticide usage in rural areas and residential insecticide usage in the city affected the resistance of Ae. albopictus in Guangzhou, although insecticide use is greater in the public health field. The government also regularly organized the patriotic health campaign to clean up aquatic mosquito habitats in Guangzhou.
With the extensive use of insecticides, insecticide resistance has become a threat. Since 2014, resistance to some insecticides has been reported in Ae. albopictus in limited regions of Guangzhou. Li et al. reported that the Ae. albopictus adult population in Yuexiu had developed resistance to dichlorodiphenyltrichloroethane (DDT), and deltamethrin [18]. The reports of insecticide resistance in Guangzhou raise serious concerns about the efficacy of chemical insecticides against Ae. albopictus and the dengue transmission control policy in China. Current research on the resistance mechanism of Ae. albopictus mainly focuses on target-site insensitivity and increased metabolic detoxification of insecticides[19]. Non-synonymous mutations in the voltage-gated sodium channel (VGSC) gene that cause resistance to pyrethroids and DDT insecticides are known as knockdown resistance (kdr) [18, 20, 21] and mutations (ace-1) in the acetylcholinesterase (AChE) gene cause a resistance to carbamates and organophosphates [22, 23]. However Grigoraki et al. reported that the resistance of Ae. albopictus to temephos is associated with elevated carboxylesterases (CCEs) which is caused by up-regulation of CCEae3a gene [24], and no difference was detected between resistant and susceptible CCEae3a_aeg variants [25]. Detoxification pathways are very complex and can be divided into three major gene families, monooxygenases (P450s), carboxylesterases (COEs), and glutathione S-transferases (GSTs) [26]. P450s are related to pyrethroid resistance in Ae. albopictus [27].
Determine the insecticide resistance status and mechanisms of Ae. albopictus in Guangzhou is very important for local vector control. In this study, Ae. albopictus was collected from four districts in Guangzhou during 2015–2017 and the resistances to the currently used insecticides was comparatively analyzed through a series of experiments. The aim was to characterize the spatial distribution, temporal changes, and mechanism of insecticide resistance in Guangzhou, and provide guidance for monitoring and controlling vector mosquitoes and mosquito-borne diseases.
Methods
Study sites
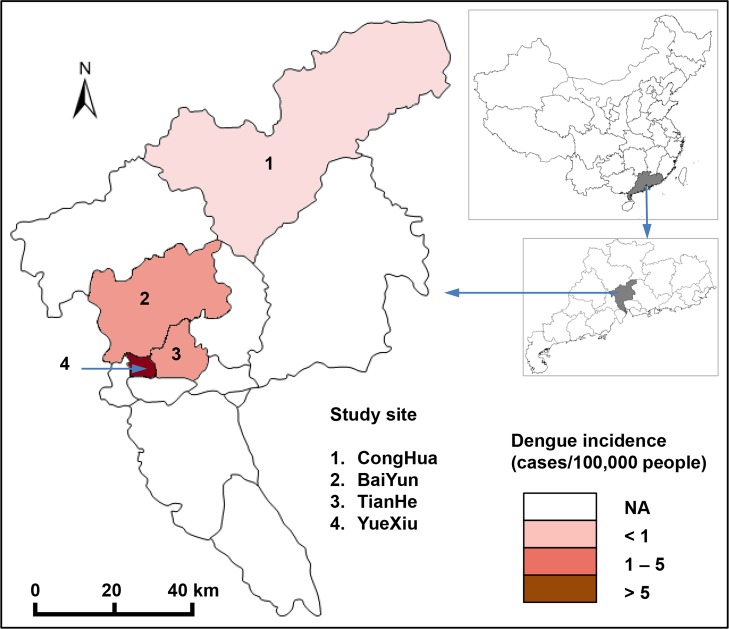
The study was conducted in four districts in Guangzhou, Guangdong Province, China, from 2015 to 2017: 1) Yuexiu district is located in the old downtown area, 2) Tianhe district is located in the new downtown area, 3) Baiyun district is located in the suburban area, and 4) Conghua district is located in the rural area. The reported dengue incidence varied among the four districts. The study sites and dengue incidence rates was marked in Fig 1, which was created by ArcGIS 10.2. The research site is a subtropical area with a monsoon climate. The annual average annual temperature is 20–22°C, the average relative humidity is 77%, and the annual rainfall is approximately 1720 mm.
Fig 1. The study sites and corresponding dengue incidence rates in Guangzhou, China.
The map was created using ArcGIS.
Mosquito strains and collection
The Foshan strain of Ae. albopictus was used as a control in this study, which was collected from Foshan City in 1983, and kept in the laboratory without insecticide exposure since then.
Ae. albopictus larvae were collected from three localities in each of the four districts, with representatives samples collected from parks, schools and residential areas, and all collection was done on public land (S1 Table). The larvae were housed in a steel tank with a size of 23 cm*29 cm*6.5 cm, and 1.5–2 L of dechlorinated tap water and small turtle food for feeding were added to the tank. Adult mosquitoes were housed in 20 cm*45 cm*30 cm yarn cages and fed with 10% glucose water. The female mosquitoes were bloodfed from an anesthetized mouse for spawning. The larvae were reared in the laboratory until adulthood. In the laboratory, the temperature was maintained at 26 ± 2°C, the relative humidity was 70 ± 10%, and the light: dark cycle was 14 h: 10 h. Non-blood-fed F1-generation female mosquitoes aged 3–5 days were used for the resistance test.
R24 is a laboratory resistant strain selected with deltamethrin for 24 generations from susceptible Ae. albopictus populations. Selection was performed by exposing each generation of fourth-stage larvae for 24 h to a 50% lethal concentration (LC50) of deltamethrin. The LC50 was determined by a larval bioassay. After 24 generations, the LC50 of deltamethrin increased from 0.001 mg/L to 0.033 mg/L.
Adult resistance bioassays
The adult resistance bioassays were performed using five insecticides, including the four major classes of insecticides currently used which were recommended by WHO Pesticides Evaluation Scheme (WHOPES) [28], i.e., type II pyrethroid: deltamethrin; type I pyrethroid: permethrin; organophosphates: malathion; organochlorine: DDT; and carbamate: bendiocarb, following the standard WHO tube test protocol [29,30]. We used deltamethrin (0.03%), permethrin (0.25%), malathion (0.8%) and bendiocarb (0.1%) test for 1h and DDT (4%) test for 0.5h by the standard WHO tube test. Testing kits and insecticide-impregnated papers with standard diagnostic doses were provided by the Universiti Sains Malaysia, Penang, Malaysia. In each holding tube, 25 adult female mosquitoes were tested with five replicates of field mosquitoes and two replicates of controls. The number of adult mosquitoes knocked down was recoded every ten minutes and used to calculate the value of 50% knockdown times (KDT50). After 1 h of exposure, the mosquitoes were transferred to holding tubes and fed on a 10% sucrose solution for 24 h. Mortality was scored after 24 h of recovery to determine the susceptibility status. After the bioassay, the dead and live mosquitoes were separated and stored individually in 95% alcohol for subsequent DNA analysis.
Adult synergistic test
The effect of cytochrome P450s on the resistance of Ae. albopictus to deltamethrin was tested using the synergistic agent piperonyl butoxide (PBO) following WHO guidelines [29,30]. Test paper with 4% PBO was prepared from 95% PBO (Yien Co. Ltd, Shanghai, China). Experiments on sets of 25 field-collected female mosquitoes were performed separately for 1) exposure to PBO alone for 1 h; 2) exposure to PBO for 1 h followed by exposure to deltamethrin for 1 h; and 3) exposure to deltamethrin alone for 1 h; and 4) control: no exposure to any agent. After the 1 h experiments, experimental mosquitoes were transferred to holding tubes, and mortality at 24 h was documented. This process was repeated five times.
Larval resistance bioassays
Mosquito larval resistance bioassays were conducted using four insecticides which were recommended by WHOPES [31]: 1) organophosphate, temephos; 2) microbial bacterial toxin, Bti; 3) hormonal insect growth regulators, pyriproxyfen (PPF); 4) the chitin biosynthesis inhibitor, hexaflumuron; following WHO guidelines [32]. Industrial grade temephos (87.4%) and PPF (98.3%) were provided by the Chinese Centers for Disease Control and Prevention. Bti (7000 ITU/mg) was provided by Wuhan Nature’s Favour Bioengineering. Hexaflumuron (99.0%) was provided by Shanghai Yien. Twenty-five 3-4-instar Ae. albopictus larvae were added to 99 mL of dechlorinated tap water and 1 mL of different concentrations of insecticide solution. Nine concentration gradients for each insecticide were tested during the experiment, with concentrations ranging between 10% and 90% mortality, three replicates per concentration. For temephos and Bti, the number of dead larvae was counted 24 h after the experiment, and the LC50 was calculated. For PPF and hexaflumuron, emergence inhibition was measured daily until complete mortality or adult emergence, and IE50 was calculated.
DNA extraction and kdr, ace-1 mutation detection
Genomic DNA was extracted from individual mosquitoes using the Extract-N-Amp Tissue PCR Kit (Sigma Aldrich) following the manufacturer’s protocol. Extracted DNA was stored at 4°C or used immediately for PCR. For each insecticide, 48 surviving individuals and 20 dead individuals were used to extract genomic DNA for mutation detection of target genes for insecticide resistance. DNA was extracted from individual mosquito and this is only done for adults which tested by the standard WHO tube test. Samples exposed to deltamethrin, permethrin, and DDT were genotyped at the voltage-gated sodium channel (VGSC) gene to detect mutations within domains II, III and IV, following the protocol by Kasai et al., 2011 [33]. Samples exposed to bendiocarb were genotyped at the ace-1 gene to detect mutations within G119 following the protocol by M. Weill et al., 2004 [34]. The details of the primers and PCR conditions are given in S2 Table. A total of 204 samples were sequenced for the kdr gene, and 68 samples were sequenced for the ace-1 gene. The sequences were aligned and analyzed using BioEdit (http://www.mbio.ncsu.edu/BioEdit/bioedit.html).
Survey of insecticide usage
A survey on the use of pesticides in public regions was carried out via questionnaires from March to September of 2017 in the Yuexiu, Tianhe, Baiyun and Conghua districts of Guangzhou. The survey sites were selected near the collection sites of the Ae. albopictus samples. The surveys targeted employees of Pest Control Organizations (PCOs) and the local street administrators responsible for mosquito control. The contents of the survey included the species targeted and the frequency of insecticides used to control adult and larval mosquitoes. Each site completed 30 questionnaires.
Statistical analysis
LC50 and KDT50 were estimated using the log-probit models. For larvae bioassays, the resistant status was measured by the resistant ratio (RR50), i.e., the ratio of LC50 (or IE50) for the field population over LC50 (or IE50) for the laboratory-susceptible strain. Larval resistance status was defined as susceptible if RR50 < 5, moderately resistant if 5 < RR50 < 10, and highly resistant if RR50 > 10 [28]. Post hoc Tukey’s HSD test of analysis of variance (ANOVA) was used to compare differences in RR50 among different study sites. For adult bioassays, resistant status was defined by mortality rate: Resistant if mortality < 90%, probably resistant if mortality was between 90 and 98%, and susceptible if mortality > 98% [27, 28]. The relationship between nonsynonymous mutations and resistance was verified by Fisher's exact test or the χ2-test (when all n >5), and the odds ratio (OR) was calculated for each mutation. The χ2-test was used to compare differences in adult mortalities between deltamethrin and deltamethrin + PBO groups at different study sites.
Results
Ae. albopictus developed high resistance to currently used insecticides
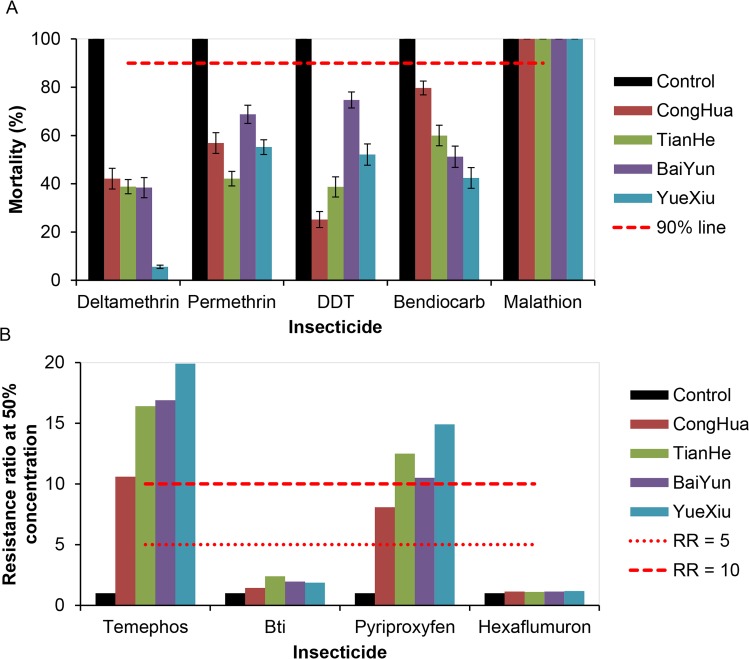
Ae. albopictus adult populations in the four districts were all resistant to four insecticides (deltamethrin, permethrin, DDT and bendiocarb) (mortality<90%) except malathion (mortality >98%) (Fig 2A, S3 Table). The lowest mortality rate against deltamethrin and bendiocarb was 5.6% and 42.4% in the populations from Yuexiu, respectively, 42.1% to permethrin in Tianhe, and 25.2% to DDT in Conghua (Fig 2A, S3 Table). Ae. albopictus larvae from all four districts were still sensitive to Bti and hexaflumuron (RR50<5), but displayed high resistance to temephos and pyriproxyfen (RR50 > 10), and moderate resistance to pyriproxyfen (5 ≤ RR50 ≤ 10) in the Conghua population (Fig 2B, S4 Table). Comparatively, resistance to temephos and PPF were significantly higher in the urban areas, i.e., Yuexiu, Tianhe and Baiyun, than in the rural areas, Conghua.
Fig 2. The resistance of Aedes albopictus to currently used insecticides.
A: Resistance of adule Aedes albopictus to currently used insecticides, 2017. If the mortality is less than 90%, then the population is considered resistant. Error bars indicate 95% CIs. B: Resistance of larval Aedes albopictus to currently used insecticides, 2017. If the resistance ratio at 50% concentration (RR50) is <5, then the population is considered susceptible, when the RR50 is between 5 and 10, the population is considered to have moderate resistance, and when the RR50 is >10, the population is considered highly resistant.
Ae. albopictus insecticide resistance may develop very fast
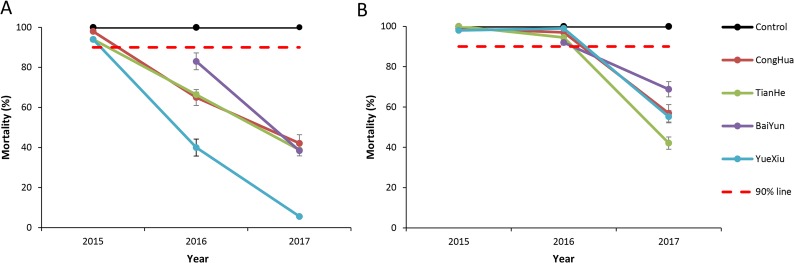
Pyrethroids are the most commonly used insecticides to control the adult Ae. albopictus in Guangzhou. In 2015, Ae. albopictus populations was susceptible to deltamethrin in Conghua and possible resistant in Tianhe and Yuexiu (Fig 3A). In 2016, Ae. albopictus populations in all four districts became resistant to deltamethrin (Fig 3A). In 2017, Ae. albopictus mortality against deltamethrin was significantly decreased at all study sites compared to 2016, and the mortality in Yuexiu was only 5.6% (Fig 3A). The average mortality against deltamethrin was 95.3% in 2015. It decreased to 63.6% in 2016 and dropped again to 31.2% in 2017 (ANOVA, all p < 0.05), with a 30% decrease every year (Fig 3A). The decrease in Ae. albopictus mortality against permethrin was also very fast from 2016 (average 95.6%) to 2017 (55.8%), with a 40% decrease in one year (Fig 3B).
Fig 3. Resistance of Aedes albopictus to pyrethroid insecticides, 2015–2017.
A: Resistance of Aedes albopictus to deltamethrin, 2015–2017. If mortality less than 90%, the population is considered resistant. Error bars indicate 95% CIs .B: Resistance of Aedes albopictus to permethrin, 2015–2017. If mortality less than 90%, the population is considered resistant. Error bars indicate 95% CIs.
Resistance to pyrethroids and DDT associated with 1534 codon mutations
Sequences of domains II (480 bp), III (346 bp) and IV (280 bp) of the VGSC gene were obtained from resistant and susceptible mosquitoes after deltamethrin, permethrin and DDT adult bioassays. Three synonymous mutations in domain II, 5 synonymous mutations in domain III, and 3 synonymous mutations in domain IV were detected (S5 Table). In domain III, non-synonymous mutations were detected at codon 1534, where wild-type TTC (Phe) was changed to either TCC (Ser) or CTC (Leu). Data analysis showed that the F1534S and F1534L mutations were significantly associated with the resistance to deltamethrin, permethrin and DDT (p<0.05) (Table 1).
Table 1. Kdr mutations at position F1534 of the VGSC gene in Aedes albopictus.
| Insecticide | Phenotype | N | Genotype | Odds Ratio (95% CI) | |||||
|---|---|---|---|---|---|---|---|---|---|
| FF | FS | SS | FL | LL | F1534S | F1534L | |||
| Deltamethrin | S | 20 | 17 | 3 | 0 | 0 | 0 | 1 | 1 |
| R | 41 | 2 | 1 | 17 | 3 | 18 | 4.44 (1.12, 17.52)* | NA ** | |
| Permethrin | S | 19 | 11 | 5 | 0 | 1 | 1 | 1 | 1 |
| R | 42 | 1 | 4 | 22 | 5 | 11 | 4.55 (1.38, 15.05) * | 27.20 (4.60, 160.69) ** | |
| DDT | S | 18 | 15 | 2 | 1 | 0 | 0 | 1 | 1 |
| R | 44 | 5 | 7 | 15 | 11 | 6 | 5.00 (1.27, 19.74)* | NA ** | |
NA: Not Available. Significance level
*p<0.05
**p<0.01
The 194-bp fragment in exon 5 of the AChE gene was obtained for ace-1 mutation detection, but no amino acid mutation was found in the G119 site in the 33 successfully sequenced samples.
PBO significantly reduced resistance to deltamethrin
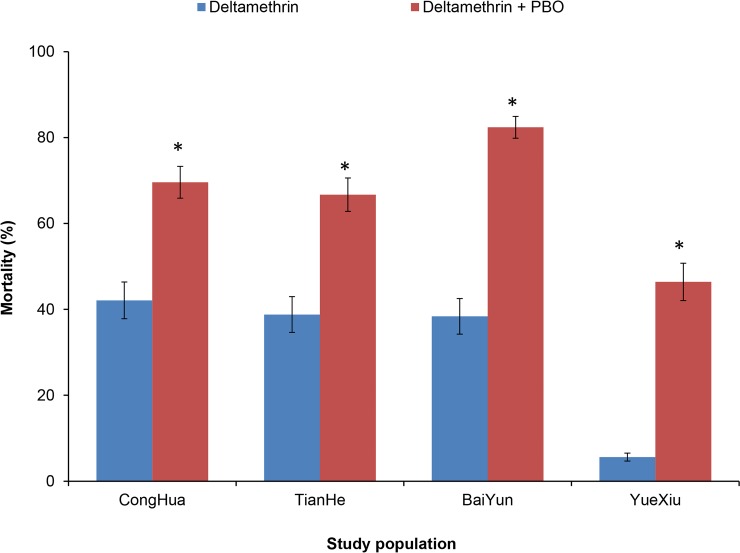
Exposing mosquitoes to PBO before exposing them to deltamethrin significantly increased Ae. albopictus mortality compared to directly exposing them to deltamethrin (χ2-test, all p < 0.01) (Fig 4), indicating that PBO can reduce the resistance of Ae. albopictus to deltamethrin through anti-P450s activity.
Fig 4. The effect of PBO on the resistance of Aedes albopictus to deltamethrin.
Exposing mosquitoes to PBO before exposing them to deltamethrin significantly increased Aedes albopictus mortality compared to directly exposing them to deltamethrin. Error bars indicate 95% CIs. *: p < 0.01.
Resistance to PPF may associated with cross resistance to deltamethrin
PPF is an insect growth regulator and has never been used previously in mosquito control in Guangzhou. However, resistance to PPF was detected in Ae. albopictus collected in all four study sites (Fig 2B). Comparative analysis on Ae. albopictus larval resistance to deltamethrin from four field populations and one laboratory selected resistant strain, R24, showed that all of them were resistant to deltamethrin as well as PPF (Table 2). Because R24 resistance to deltamethrin was artificially selected by exposing fully susceptible laboratory Ae. albopictus larvae to deltamethrin after 24 generations, this strain has never been exposed to any other insecticide; therefore, Ae. albopictus resistance to PPF could be explained by cross resistance to pyrethroids.
Table 2. Resistance to PPF and deltamethrin in Aedes albopictus from different populations, 2017.
| Population name |
Deltamethrin | pyriproxyfen | ||
|---|---|---|---|---|
| LC50 (95%CI) (mg/L) | RR50b | IE50 (95%CI) (μg/L) | RR50 | |
| Controla | 0.001 (0.001, 0.001) | 1.00 | 0.073 (0.064, 0.083) | 1.00 |
| Yuexiu | 0.067 (0.061, 0.094) | 67.0 | 1.091 (1.053, 1.142) | 14.9 |
| Tianhe | 0.036 (0.035, 0.038) | 36.0 | 0.913 (0.839, 1.062) | 12.5 |
| Baiyun | 0.049 (0.046, 0.053) | 49.0 | 0.767 (0.625, 0.806) | 10.5 |
| Conghua | 0.037 (0.032, 0.040) | 37.0 | 0.590 (0.555, 0.627) | 8.08 |
| R24c | 0.033 (0.030, 0.036) | 33.0 | 0.774 (0.663, 0.862) | 10.6 |
a Control: Laboratory-susceptible strain
b RR50: resistant ratio, LC50 (or IE50) test population/LC50 (or IE50) laboratory-susceptible strain
c R24: Laboratory-susceptible strains selected for 24 generations by deltamethrin
Resistance and insecticide application
Our survey found that four major classes of insecticides (pyrethroids, organophosphates, organochlorine and carbamate) were currently used in Guangzhou (Table 3). Pyrethroids (mainly type I permethrin S-biomethrin, and type II beta-cypermethrin) were the most commonly used adulticides, while organophosphates (mainly temephos and fenthion) were the most commonly used larvicides (Table 3). The frequency of insecticide usage in urban areas was more frequent than that in the rural district of Conghua (Table 3). Conghua in the rural area did not use the insecticide routinely. The survey also found that Bti and nicotine (imidacloprid) have gradually become the new choices for larvae control (Table 3). Adulticides were more frequently used than larvicides.
Table 3. Survey of insecticide usage in four districts of Guangzhou, China, 2017.
| District | N | Adulticide | Frequency | Larvicide | Frequency |
|---|---|---|---|---|---|
| Conghua | 30 | Pyrethrin: Beta-cypermethrin, Cypermethrin, S-bioallethrin |
None or 1time/year | Organophosphate: Fenthion | When necessary a |
| Tianhe | 30 | Pyrethrin: Permethrin, Meperfluthrin, Alphacypermethrin, S-bioallethrin |
1–2 times/month | Organophosphate:Temephos; Microbial bacterial toxins: Bti; Nicotine: Imidacloprid |
When necessary |
| Baiyun | 30 | Pyrethrin: Permethrin, Meperfluthrin, Alphacypermethrin, Beta-cypermethrin; Organophosphate: DDVP; Carbamate: Propoxur |
2–3 times/month | Organophosphate: Fenthion Nicotine: Imidacloprid |
When necessary |
| Yuexiu | 30 | Pyrethrin: Permethrin, Meperfluthrin, Alphacypermethrin, Beta-cypermethrin, Tefluthrin, S-bioallethrin; Organophosphate: DDVP; Carbamate: Propoxur |
1–2 times/month, 2–3 times/week when an outbreak occurs |
Organophosphate: Temephos, Fenthion; Microbial bacterial toxin: Bti; Carbamate: Fenobucarb; Nicotine: Imidacloprid |
When necessary |
a When necessary: used during outbreaks
Discussion
In this study, we characterized the current insecticide resistance in Ae. albopictus in Guangzhou, China in the following ways: (1) Increasing. In 2017, Ae. albopictus populations in four districts were all resistant to the four tested insecticides with sensitivity only to malathion (Fig 2), whereas in 2014, all were susceptibility but had only low or moderate resistance to some insecticide in very limited areas of Guangzhou [18, 35]. In 2017, all four tested districts were resistant (Fig 2). (2) Rapid. The resistance of Ae. albopictus to pyrethroids has changed from susceptibility or moderately resistant to resistant within three years, 2015–2017 (Fig 3). (3) Multiresistance. In 2014, Ae. albopictus was resistant only to limited pyrethroids and DDT, whereas in 2017, it was resistant to all four types of insecticides except malathion[11,19]. In 2016–2017, the Mosquito and Oviposition Positive Index (MOI) in Guangzhou is greater than 5 (MOI>5 is the risk of dengue virus transmission) [36]. At the same time, in 2015, dengue fever affected 31 towns in 8 districts in Guangzhou and 108 cases were reported; in 2016, dengue fever expanded to 61 towns in 10 districts, and 253 cases were reported; in 2017, dengue fever expanded to 122 towns in 11 districts and cases increased to 950, only less than 2013 and 2014 in the past 10 years [6].The quick generation, wide distribution, and increasing insecticide resistance to multiple agents in Ae. albopictus is bound to affect mosquito management and threaten the prevention and control of dengue, similar to the resistance in Anopheles mosquitoes has prevented the elimination of malaria [37,38].
Mutations in the VGSC gene have been correlated with the resistance of vector mosquitoes including Ae. albopictus [33,39,40]. The present study also found that F1534S and F1534L mutations were significantly correlated with the resistance of Ae. albopictus to deltamethrin, permethrin and DDT (p<0.05) (Table 1). At present, pyrethroids are the most commonly used insecticides in China; therefore, sensitive and specific techniques based on the detection of 1534 mutations must be developed to monitor the resistance of Ae. albopictus to pyrethroids. However, the mechanism for insecticide resistance in mosquitoes is very complicated. In addition to the correlation of kdr mutations with resistance, the increased expression and enhanced activity of P450s have also been proven to be associated with mosquito resistance to pyrethroids in the present study and in other studies [41–43]. Only P450s changes have been reported in the resistant mosquitoes, and no kdr mutations were reported [44]. Therefore, clarifying the mechanism of insecticide resistance in vector mosquitoes and developing suitable monitoring systems, especially those easily used in the field, remain challenging.
In the era of rapidly emerging and widely distributed insecticide resistance, it is important to make suitable and updated guidelines for insecticide usage. PBO is an inhibitor of monooxygenase, such as P450s [26,45]. The present study proved that PBO can significantly reduce the resistance of Ae. albopictus to deltamethrin by anti-P450s (Fig 4), which could be used as a synergistic agent to enhance the effect of pyrethroids. PPF is an insect growth regulator and has been used as an automatically disseminated insecticide to control habitats, especially for Aedes mosquitoes [46,47]. Recently, PPF has also been used to sterilize adult Anopheles mosquitoes by reducing their fecundity and longevity [48]. Unexpectedly, in the present study, the higher resistance to PPF was widely detected in Ae. albopictus populations in Guangzhou probably because of the cross resistance to pyrethroids (Fig 2B). Yunta C et al. also proved that PPF is metabolized by P450s and associated with pyrethroid resistance in Anopheles gambiae [41]. Considering the higher and widely distributed resistance to pyrethroids, they should be cautious when using PPF as the larvicide or as the synergistic agent for adulticide. In mosquito incense and aerosol insecticides used daily among residents, the active ingredient is primiarly pyrethroid (such as transfluthrin and s- bioallethrin). More and more residents are using mosquito nets, electric mosquito swatters or mosquito killer lamps to prevent mosquito bites. In Conghua, vegetable farmers use DDVP and beta-cypermethrin to prevent Plutella xylostella. According to our research, the Ae. albopictus populations were still sensitive to malathion, hexaflumuron and Bti. Similarly, in Brazil where pyrethroid and temephos resistance has developed, local health authorities recommend the use of malathion against adult mosquitoes and chitin synthesis inhibitors against larvae (Controle de vetores. http://www.saude.gov.br/vigilancia-em-saude/controle-de-vetores). Therefore, we suggest useing malathion against adult mosquitoes and hexaflumuron or Bti against larvae for dengue vector control in Guangzhou. Timely monitoring of resistance is critical for the proper management of insecticides. Additionally, every year at the end of February or early in March, the Guangzhou government launches a patriotic health campaign to focus on cleaning up aquatic mosquito habitats for one month, which is important for cleaning the over winter eggs of Ae. albopictus and reducing the population density of mosquitoes.
In conclusion, increased and more widespread insecticide resistance to multiple agents has been rapidly developing in Ae. albopictus, the primary dengue virus vector in China. Extensive applications and inapposite applications of insecticides were likely one reason for development of resistance generation. The 1534 codon mutations in the VGSC gene were significantly correlated with resistance to pyrethroids and possibly used as a biomarker to monitor insecticide resistance. PBO can significantly reduce the resistance of Ae. albopictus to deltamethrin an act as a synergistic agent of pyrethroids. Gravid Ae. albopictus does not oviposit all eggs into one place, although they used to lay eggs in different breeding sites. By treatment of a breeding site with an insecticide such pyriproxyfen (PPF), gravid mosquitoes could be contacted and contaminated with PPF when they oviposit the eggs. Then, when they fly to neighborhood breeding sites to lay the remaining eggs, the contaminated PPF would be transferred automatically to cryptic habitats [49, 50]. PPF may display cross resistance to deltamethrin, and the concentration should be cautiously considered when used as an automatically disseminated insecticide. Fast emerging resistance in Ae. albopictus raises the alarm for dengue vector control and calls for timely and guided insecticide management.
Supporting information
(DOCX)
(DOCX)
aControl: Laboratory-susceptible strain
(DOCX)
aControl: Laboratory-susceptible strain.
(DOCX)
a SNPs: Single nucleotide polymorphisms.
(DOCX)
Acknowledgments
The authors would like to thank American Journal Experts for the English language review.
Data Availability
All relevant data are within the manuscript and its Supporting Information files.
Funding Statement
This research was supported by a combination of funding from the: National Nature Science Foundation of China (81829004, 81420108024 and 1830087), URL: http://www.nsfc.gov.cn; National Institutes of Health, USA (AI136850) URL:https://www.nih.gov/grants-funding; Science Foundation of Guangdong Province (2014A030312016), URL:http://www.gdstc.gov.cn; the Guangzhou Synergy Innovation Key Program for Health (201807010005 and 201803040006), URL:http://www.gzsi.gov.cn; the funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Bhatt S, Gething PW, Brady OJ, Messina JP, Farlow AW, Moyes CL, et al. The global distribution and burden of dengue. Nature. 2013;496(7446):504–7. 10.1038/nature12060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady OJ, Gething PW, Bhatt S, Messina JP, Brownstein JS, Hoen AG, et al. Refining the global spatial limits of dengue virus transmission by evidence-based consensus. PLoS neglected tropical diseases. 2012;6(8):e1760 10.1371/journal.pntd.0001760 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Organization WH. World malaria report 2015: World Health Organization; 2016. [Google Scholar]
- 4.Wu JY, Lun ZR, James AA, Chen XG. Dengue Fever in mainland China. The American journal of tropical medicine and hygiene. 2010;83(3):664–71. 10.4269/ajtmh.2010.09-0755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lai S, Huang Z, Zhou H, Anders KL, Perkins TA, Yin W, et al. The changing epidemiology of dengue in China, 1990–2014: a descriptive analysis of 25 years of nationwide surveillance data. BMC medicine. 2015;13(1):100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lu Jian⁃yun, Chen Zong⁃qiu, Ma Meng⁃meng, Cai Rui⁃bin, Su Wen-zhe, Li Yi⁃lan, et al. Epidemic trend of dengue fever in Guangzhou city from 2008 to 2017. Journal of Tropical Medicine. 2018; 18(07):973–9. [Google Scholar]
- 7.Gong D, Zhou H. Progress in Dengue fever important vector Aedes albopctus in China. Chin J Vector Biol & Control. 2009;20(6):607–10. 10.1088/0031-9155/50/15/N02 [DOI] [Google Scholar]
- 8.Peng HJ, Lai HB, Zhang QL, Xu BY, Zhang H, Liu WH, et al. A local outbreak of dengue caused by an imported case in Dongguan China. BMC public health. 2012;12:83 10.1186/1471-2458-12-83 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Li Y, Kamara F, Zhou G, Puthiyakunnon S, Li C, Liu Y, et al. Urbanization increases Aedes albopictus larval habitats and accelerates mosquito development and survivorship. PLoS neglected tropical diseases. 2014;8(11):e3301 10.1371/journal.pntd.0003301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abramides GC, Roiz D, Guitart R, Quintana S, Gimenez N. Control of the Asian tiger mosquito (Aedes albopictus) in a firmly established area in Spain: risk factors and people's involvement. Transactions of The Royal Society of Tropical Medicine and Hygiene. 2013;107(11):706–14. 10.1093/trstmh/trt093 [DOI] [PubMed] [Google Scholar]
- 11.Abramides GC, Roiz D, Guitart R, Quintana S, Guerrero I, Giménez N. Effectiveness of a multiple intervention strategy for the control of the tiger mosquito (Aedes albopictus) in Spain. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2011;105(5):281–8. 10.1016/j.trstmh.2011.01.003 [DOI] [PubMed] [Google Scholar]
- 12.Banks S, Murray N, WILDER‐SMITH A, Logan J. Insecticide‐treated clothes for the control of vector‐borne diseases: a review on effectiveness and safety. Medical and veterinary entomology. 2014;28(S1):14–25. [DOI] [PubMed] [Google Scholar]
- 13.Fonseca DM, Unlu I, Crepeau T, Farajollahi A, Healy SP, Bartlett‐Healy K, et al. Area‐wide management of Aedes albopictus. Part 2: Gauging the efficacy of traditional integrated pest control measures against urban container mosquitoes. Pest management science. 2013;69(12):1351–61. 10.1002/ps.3511 [DOI] [PubMed] [Google Scholar]
- 14.Liu S, Cui F, Yan S, Qiao C. Investigation of organophosphate and pyrethroid resistance in vector mosquitoes in China. Chin J Vector Biol Control. 2011;22:184–9. [Google Scholar]
- 15.Liu S, Cui F, Yan S. Investigation on the resistance of vector mosquitoes to organochlorines and carbamates in China. Chin J Vector Biol Control. 2011;22:82-–5. [Google Scholar]
- 16.Li C, Yan Z, Jian G, Wu H, Hu Z. Resistance of Aedes albopictus to commonly used insecticides in urban area of Guangzhou. Chin J Hygienic Insecticides Equipments. 2013;6:487–9. [Google Scholar]
- 17.Meng FX, Wang YG, Feng L, Liu QY. Review on dengue prevention and control and integrated mosquito management in China. Chinese Journal of Vector Biology & Control. 2015;29(1):113–22. [Google Scholar]
- 18.Li Y, Xu J, Zhong D, Zhang H, Yang W, Zhou G, et al. Evidence for multiple-insecticide resistance in urban Aedes albopictus populations in southern China. Parasites & vectors. 2018;11(1):4 10.1186/s13071-017-2581-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Moyes CL, Vontas J, Martins AJ, Ng LC, Koou SY, Dusfour I, et al. Contemporary status of insecticide resistance in the major Aedes vectors of arboviruses infecting humans. PLoS neglected tropical diseases. 2017;11(7):e0005625 10.1371/journal.pntd.0005625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Marcombe S, Farajollahi A, Healy SP, Clark GG, Fonseca DM. Insecticide resistance status of United States populations of Aedes albopictus and mechanisms involved. PloS one. 2014;9(7):e101992 10.1371/journal.pone.0101992 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chatterjee M, Ballav S, Maji AK, Basu N, Sarkar BC, Saha P. Polymorphisms in voltage-gated sodium channel gene and susceptibility of Aedes albopictus to insecticides in three districts of northern West Bengal, India. 2018;12(1):e0006192 10.1371/journal.pntd.0006192 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chen CD, Nazni WA, Lee HL, Norma-Rashid Y, Lardizabal ML, Sofian-Azirun M. Temephos resistance in field Aedes (Stegomyia) albopictus (Skuse) from Selangor, Malaysia. Tropical biomedicine. 2013;30(2):220–30. Epub 2013/08/21. . [PubMed] [Google Scholar]
- 23.Lee RM, Choong CT, Goh BP, Ng LC, Lam-Phua SG. Bioassay and biochemical studies of the status of pirimiphos-methyl and cypermethrin resistance in Aedes (Stegomyia) aegypti and Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Singapore. Tropical biomedicine. 2014;31(4):670–9. Epub 2015/03/18. . [PubMed] [Google Scholar]
- 24.Grigoraki L, Lagnel J, Kioulos I, Kampouraki A, Morou E, Labbe P, et al. Transcriptome profiling and genetic study reveal amplified carboxylesterase genes implicated in temephos resistance, in the Asian tiger mosquito Aedes albopictus. PLoS neglected tropical diseases. 2015;9(5):e0003771 10.1371/journal.pntd.0003771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Grigoraki L, Balabanidou V, Meristoudis C, Miridakis A, Ranson H, Swevers L, et al. Functional and immunohistochemical characterization of CCEae3a, a carboxylesterase associated with temephos resistance in the major arbovirus vectors Aedes aegypti and Ae. albopictus. Insect biochemistry and molecular biology. 2016;74:61–7. 10.1016/j.ibmb.2016.05.007 [DOI] [PubMed] [Google Scholar]
- 26.Liu N. Insecticide resistance in mosquitoes: impact, mechanisms, and research directions. Annual review of entomology. 2015;60:537–59. 10.1146/annurev-ento-010814-020828 . [DOI] [PubMed] [Google Scholar]
- 27.Xu J, Su X, Bonizzoni M, Zhong D, Li Y, Zhou G, et al. Comparative transcriptome analysis and RNA interference reveal CYP6A8 and SNPs related to pyrethroid resistance in Aedes albopictus. 2018;12(11):e0006828 10.1371/journal.pntd.0006828 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.WHO recommended insecticides for space spraying against mosquitoes. Available from:https://www.who.int/neglected_diseases/vector_ecology/vector-control/Space_Spray_products_February_2016.pdf?ua = 1.
- 29.Organization WH. Test procedures for insecticide resistance monitoring in malaria vector mosquitoes. 2016.
- 30.Organization WH. Monitoring and managing insecticide resistance in Aedes mosquito populations: Interim guidance for entomologists. 2016.
- 31.Organization WH. WHOPES-recommended compounds and formulations for control of mosquito larvae Geneva: WHO Pesticides Evaluation Scheme (WHOPES); 2011. [Google Scholar]
- 32.Organization WH. Guidelines for laboratory and field testing of mosquito larvicides. Geneva: World Health Organization, 2005. [Google Scholar]
- 33.Kasai S, Ng LC, Lam-Phua SG, Tang CS, Itokawa K, Komagata O, et al. First detection of a putative knockdown resistance gene in major mosquito vector, Aedes albopictus. Japanese journal of infectious diseases. 2011;64(3):217–21. [PubMed] [Google Scholar]
- 34.Weill M, Malcolm C, Chandre F, Mogensen K, Berthomieu A, Marquine M, et al. The unique mutation in ace‐1 giving high insecticide resistance is easily detectable in mosquito vectors. Insect molecular biology. 2004;13(1):1–7. [DOI] [PubMed] [Google Scholar]
- 35.Yiguan W, Xin L, Chengling L, Su T, Jianchao J, Yuhong G, et al. A survey of insecticide resistance in Aedes albopictus (Diptera: Culicidae) during a 2014 dengue fever outbreak in Guangzhou, China. Journal of economic entomology. 2016;110(1):239–44. [DOI] [PubMed] [Google Scholar]
- 36.Xiao-bo Liu, Yu-hong Guo, Hai-xia Wu, Quan-cheng Li, Yu-juan Yue, Dong-sheng Ren, et al. Surveillance on the density of Aedes mosquito larvae in China, 2015–2017. Chinese Journal of Vector Biology and Control. 2018; 29(04):325–330. [Google Scholar]
- 37.Ranson H, Lissenden N. Insecticide resistance in African Anopheles mosquitoes: a worsening situation that needs urgent action to maintain malaria control. Trends in parasitology. 2016;32(3):187–96. 10.1016/j.pt.2015.11.010 [DOI] [PubMed] [Google Scholar]
- 38.Hemingway J, Ranson H, Magill A, Kolaczinski J, Fornadel C, Gimnig J, et al. Averting a malaria disaster: will insecticide resistance derail malaria control? The Lancet. 2016;387(10029):1785–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen H, Li K, Wang X, Yang X, Lin Y, Cai F, et al. First identification of kdr allele F1534S in VGSC gene and its association with resistance to pyrethroid insecticides in Aedes albopictus populations from Haikou City, Hainan Island, China. Infectious diseases of poverty. 2016;5:31 10.1186/s40249-016-0125-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Xu J, Bonizzoni M, Zhong D, Zhou G, Cai S, Li Y, et al. Multi-country Survey Revealed Prevalent and Novel F1534S Mutation in Voltage-Gated Sodium Channel (VGSC) Gene in Aedes albopictus. PLoS neglected tropical diseases. 2016;10(5):e0004696 10.1371/journal.pntd.0004696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yunta C, Grisales N, Nasz S, Hemmings K, Pignatelli P, Voice M, et al. Pyriproxyfen is metabolized by P450s associated with pyrethroid resistance in An. gambiae. Insect biochemistry and molecular biology. 2016;78:50–7. 10.1016/j.ibmb.2016.09.001 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stevenson BJ, Pignatelli P, Nikou D, Paine MJ. Pinpointing P450s associated with pyrethroid metabolism in the dengue vector, Aedes aegypti: developing new tools to combat insecticide resistance. PLoS neglected tropical diseases. 2012;6(3):e1595 10.1371/journal.pntd.0001595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Balabanidou V, Kampouraki A, MacLean M, Blomquist GJ, Tittiger C, Juárez MP, et al. Cytochrome P450 associated with insecticide resistance catalyzes cuticular hydrocarbon production in Anopheles gambiae. Proceedings of the National Academy of Sciences. 2016;113(33):9268–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang X, Zhong D, Fang Q, Hartsel J, Zhou G, Shi L, et al. Multiple resistances and complex mechanisms of Anopheles sinensis mosquito: a major obstacle to mosquito-borne diseases control and elimination in China. PLoS neglected tropical diseases. 2014;8(5):e2889 10.1371/journal.pntd.0002889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hemingway J, Ranson H. Insecticide resistance in insect vectors of human disease. Annual review of entomology. 2000;45(1):371–91. [DOI] [PubMed] [Google Scholar]
- 46.Diseases PFIT, Organization WH. Dengue: guidelines for diagnosis, treatment, prevention and control Geneva: World Health Organization; 2009;6(12):990. [PubMed] [Google Scholar]
- 47.Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, Morrison AC. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proceedings of the National Academy of Sciences. 2009;106(28):11530–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tiono AB, Ouédraogo A, Ouattara D, Bougouma EC, Coulibaly S, Diarra A, et al. Efficacy of Olyset Duo, a bednet containing pyriproxyfen and permethrin, versus a permethrin-only net against clinical malaria in an area with highly pyrethroid-resistant vectors in rural Burkina Faso: a cluster-randomised controlled trial. The Lancet. 2018;392(10147):569–80. [DOI] [PubMed] [Google Scholar]
- 49.Chandel K, Suman DS, Wang Y, Unlu I, Williges E, Williams GM, et al. Targeting a hidden enemy: pyriproxyfen autodissemination strategy for the control of the container mosquito Aedes albopictus in cryptic habitats. PLoS neglected tropical diseases. 2016;10(12):e0005235 10.1371/journal.pntd.0005235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Devine GJ, Perea EZ, Killeen GF, Stancil JD, Clark SJ, Morrison AC. Using adult mosquitoes to transfer insecticides to Aedes aegypti larval habitats. Proceedings of the National Academy of Sciences. 2009;106(28):11530–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOCX)
aControl: Laboratory-susceptible strain
(DOCX)
aControl: Laboratory-susceptible strain.
(DOCX)
a SNPs: Single nucleotide polymorphisms.
(DOCX)
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.