Abstract
The Alzheimer's disease-related β-amyloid precursor protein (β-APP) is metabolized to a number of potentially amyloidogenic peptides that are believed to be pathogenic. Application of relatively low concentrations of the soluble forms of these peptides has previously been shown to block high-frequency stimulation-induced long-term potentiation (LTP) of glutamatergic transmission in the hippocampus. The present experiments examined how these peptides affect low-frequency stimulation-induced long-term depression (LTD) and the reversal of LTP (depotentiation). We discovered that β-amyloid peptide (Aβ1–42) and the Aβ-containing C -terminus of β-APP (CT) facilitate the induction of LTD in the CA1 area of the intact rat hippocampus. The LTD was frequency- and NMDA receptor-dependent. Thus, although low-frequency stimulation alone was ineffective, after intracerebroventricular injection of Aβ1–42, it induced an LTD that was blocked byd-(−)-2-amino-5-phosphonopentanoic acid. Furthermore, an NMDA receptor-dependent depotentiation was induced in a time-dependent manner, being evoked by injection of CT 10 min, but not 1 hr, after LTP induction. These use- and time-dependent effects of the amyloidogenic peptides on synaptic plasticity promote long-lasting reductions in synaptic strength and oppose activity-dependent strengthening of transmission in the hippocampus. This will result in a profound disruption of information processing dependent on hippocampal synaptic plasticity.
Keywords: Alzheimer's disease, long-term potentiation (LTP), long-term depression (LTD), depotentiation, amyloid β peptide (Aβ), C terminus fragment, β-amyloid precursor protein (β-APP)
Synaptic pathology is considered a major and early contributor to the cognitive deficits and reduced cerebral activity of Alzheimer's disease (Terry et al., 1991; Mesulam, 1999). The relationship between synaptic dysfunction and other hallmarks of the disease, including amyloid deposition, remains controversial (Arriagada et al., 1992; Cummings and Cotman, 1996;Mattson, 1997). Recent modifications of the amyloid hypothesis suggest that rather than β-amyloid (Aβ)-containing plaques being solely or primarily responsible, soluble, diffusable products (including monomeric and oligomeric forms) of the β-amyloid precursor protein (β-APP) may play a critical role (Lambert et al., 1998; Hartley et al., 1999; Lue et al., 1999; McLean et al., 1999). β-APP is believed to be metabolized to Aβ in two main stages (Selkoe, 1999; Sinha and Lieberburg, 1999; Selkoe and Wolfe, 2000). First, the N terminus is removed by β-secretase to yield a membrane spanning 99 amino acid-long C-terminal (CT) fragment. Subsequently, this is cleaved intramembrane by γ-secretase to yield Aβ. Both CT and Aβ1–42 are particularly amyloidogenic and highly neurotoxic (Suh, 1997; Selkoe, 1999; Lu et al., 2000). Transgenic mice overexpressing wild-type and mutant β-APP show evidence of disruption of synaptic morphology, altered excitatory synaptic transmission or plasticity, and impairment of learning, often before amyloid plaque deposition (Nalbantoglu et al., 1997; Baekelandt et al., 1999; Chapman et al., 1999; Hsia et al., 1999; Larson et al., 1999; Moechars et al., 1999;Janus et al., 2000; Kumar-Singh et al., 2000; Mucke et al., 2000; Van Leuven, 2000). This strongly supports the involvement of misprocessed β-APP in the early synaptic and behavioral changes of Alzheimer's disease.
The role of β-APP-related amyloidogenic peptides in mediating synaptic disruption has been examined by studying their direct effects on synaptic mechanisms, especially long-term potentiation (LTP). LTP is a neurophysiological model of activity-dependent changes in synaptic strength that are believed to underlie information storage (Elgersma and Silva, 1999; Roman et al., 1999; Luscher et al., 2000; Martin et al., 2000). Previously, application of relatively low concentrations of the soluble forms of potentially amyloidogenic β-APP fragments has been reported to affect LTP of glutamatergic transmission in the hippocampus both in vitro and in vivo (Wu et al., 1995b; Cullen et al., 1997; Lambert et al., 1998; Itoh et al., 1999;Chen et al., 2000). In particular, acute injection of Aβ1–42 and CT blocked LTP of AMPA receptor-mediated transmission in the intact hippocampus at a time when baseline transmission was not affected (Cullen et al., 1997). The question arises as to whether and how these peptides affect long-term depression (LTD), the other main form of synaptic plasticity in the brain.
The present experiments show that low doses of Aβ1–42 and CT can facilitate the induction of LTD in a frequency-dependent manner. The Aβ-facilitated LTD was blocked by the NMDA receptor antagonistd-(−)-2-amino-5-phosphonopentanoic acid (D-AP5). Furthermore, LTP was reversed when CT was applied after the conditioning high-frequency stimulation within a defined time window and in a manner sensitive to D-AP5. These results emphasize the potential importance of an NMDA receptor-dependent promotion of LTD mechanisms in the actions of amyloidogenic β-APP fragments on synaptic plasticity and transmission.
MATERIALS AND METHODS
Animals and surgery. Male Wistar rats (200–300 gm) were used in these experiments. During surgery, the rats were anesthetized with urethane (ethyl carbamate, 1.5 gm/kg, i.p.). The body temperature was maintained at 37.4–38°C for the duration of the experiments.
Cannula implantation. A stainless-steel cannula (22 gauge, 0.7 mm outer diameter, 13 mm length) was implanted above the right lateral ventricle (1 mm lateral to the midline and 4 mm below the surface of the dura). Injection was made via an internal cannula (28 gauge, 0.36 mm outer diameter). The solutions were injected in a 5 μl volume over a 3 min period. Verification of the placement of the cannula was performed postmortem by checking the spread of ink dye after intracerebroventricular injection.
Electrode implantation. Electrodes were made and implanted as described previously (Cullen et al., 1997; Xu et al., 1998). Briefly, twisted-wire bipolar electrodes were constructed from Teflon-coated tungsten wires (625 μm inner core diameter, 750 μm external diameter). Recordings of field EPSPs were made from the stratum radiatum in the CA1 area of the right hippocampal hemisphere in response to stimulation of the ipsilateral Schaffer collateral–commissural pathway. The electrode implantation sites were identified using stereotaxic coordinates, with the recording site located 3.4 mm posterior to bregma and 2.5 mm lateral to the midline, and stimulating electrodes 4.2 mm posterior to bregma and 3.8 mm lateral to midline. The correct placement of electrodes in the CA1 region was confirmed via electrophysiological criteria and postmortem analysis.
Electrophysiology. Test EPSPs were evoked at a frequency of 0.033 Hz and an intensity evoking a response that was 50% of maximum. LTP was induced using high-frequency stimulation (HFS) consisting of square pulses (0.2 msec duration) of 10 trains of 20 stimuli with an interstimulus interval of 5 msec (200 Hz) and an intertrain interval of 2 sec. The stimulation intensity was raised to give an EPSP of 75% maximum during HFS. Low-frequency stimulation consisted of 1.3 Hz (1200 pulses), 3 Hz (900 pulses), or 10 Hz (270 pulses) using the test pulse intensity.
Compounds. Aβ1–42 was purchased from Bachem (Essex, UK) and stored as a stock solution (0.1 mm) in pyrogen-free distilled water in the presence of NH4 (final concentration, 0.00025%) at −20°C. Recombinant CT was produced as described previously (Chong et al., 1994). Briefly, a single 105-residue C-terminal fragment of β-APP was synthesized by expression of cDNA in Escherichia coli and purified on an ion-exchange column (Q-Sepharose; ∼90% purity confirmed by Coomaasie staining of SDS-PAGE gels). It was stored in pyrogen-free distilled water as a stock solution (0.1 mm) at −20°C until the time of the experiment. D-AP5 (Tocris Cookson, Bristol, UK) was stored as a stock solution (20 mm) at 4°C. The solutions were filtered through a Millipore millex GV4 0.22 μm filter (Millipore, Bedford, MA) before being used for intracerebroventricular injections. Assuming a rat brain volume of ∼2 ml, the initial concentration of peptide that reaches the CA1 synapses after the injection of 1–2 pmol should be in the low nanomolar range, which would rapidly transfer into different compartments, as found in Alzheimer's disease brain (Lue et al., 1999; McLean et al., 1999).
Data analysis. All data points were normalized to the final baseline response (30 min). Values given in the text are the mean ± SEM for 10 min epochs at the times indicated. Statistical comparisons were performed using two-tailed Wilcoxon signed-rank and rank sum tests. The probability level interpreted as significant wasp < 0.05.
RESULTS
Enhancement of the ability of low-frequency stimulation to induce LTD
We examined the effect of Aβ1–42 and CT on the induction of LTD using doses (1–2 pmol, i.c.v.) that were 25–100 times lower than those that affected baseline synaptic transmission (Cullen et al., 1996, 1997). In addition, we used conditioning stimulation frequencies (1–10 Hz) that failed to induce LTD in the urethane anesthetized ratin vivo (Doyle et al., 1997; Xu et al., 1997).
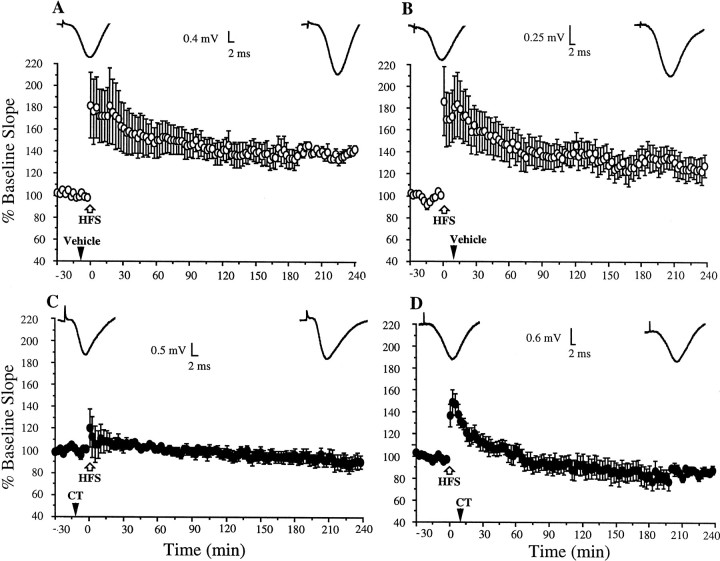
Both Aβ1–42 and CT facilitated the induction of LTD in a frequency-dependent manner (Fig. 1). In the case of Aβ1–42, the enablement was observed at 3 Hz but not 10 Hz. The application of 3 Hz conditioning stimulation 10 min after the injection of 1 pmol Aβ1–42 induced a persistent reduction in synaptic responses. Thus, the EPSP slope measured 70.6 ± 6.3% baseline EPSP slope ± SEM at 2 hr (n = 6; p< 0.05; compared with 107.9 ± 6.9% in vehicle-injected animals;n = 7) (Fig.1A,C). In contrast, 10 Hz conditioning stimulation applied 10 min after the injection of this dose of Aβ1–42 failed to affect synaptic strength, the EPSP slope measuring 103 ± 4.9% at 2 hr (n = 5) (Fig.1D), a value similar to controls (95.9 ± 4.5%;p > 0.05; n = 6) (Fig.1B).
Fig. 1.
Amyloidogenic fragments of β-APP facilitate the induction of LTD in the rat hippocampus in vivo.A, B, Conditioning stimulation at either 3 or 10 Hz had no significant persistent effect on field EPSP slope in the CA1 area of vehicle-injected control animals. C, D, Injection of Aβ1–42 (1 pmol in 5 μl, i.c.v.) 10 min before the conditioning stimulation enabled the induction of LTD in a frequency-dependent manner, LTD being induced at 3 Hz (C) but not 10 Hz (D). E, Pretreatment with the NMDA receptor antagonist D-AP5 (100 nmol) 5 min before the Aβ1–42 prevented the induction of LTD. F, Injection of the C-terminal fragment of β-APP (CT, 2 pmol) 10 min before 10 Hz conditioning stimulation enabled the induction of LTD.Insets show typical recordings at the beginning and end of the experiments. Values are the mean ± SEM EPSP slope;n = 5–10 per group. The time of the intracerebroventricular injection is indicated by a black arrowhead, and 3 or 10 Hz stimulation is indicated by ablack bar.
The requirement for NMDA receptor activation in the induction of the LTD was assessed because this is necessary for the induction of LTD by low-frequency stimulation in the Schaffer collateral–commissural pathway (Bear and Abraham, 1996; Manahan-Vaughan, 1997) and because Aβ can selectively enhance NMDA receptor-mediated synaptic transmission (Wu et al., 1995a). In animals injected with the antagonist D-AP5 (100 nmol, i.c.v.) 5 min before Aβ1–42, subsequent 3 Hz conditioning stimulation did not induce LTD, the EPSP slope measuring 113 ± 4.3% at 2 hr (n = 5; p < 0.05 compared with Aβ1–42-treated animals; p > 0.05 compared with baseline) (Fig. 1E).
In the presence of CT, LTD was induced by stimulation at 10 Hz but not 1.3 Hz. Thus, the application of 10 Hz conditioning stimulation 10 min after injection of a dose of 2 pmol CT induced a depression of the EPSP slope that persisted for the duration of the experiment (77.9 ± 5.2% at 2 hr; n = 10; p < 0.05 compared with vehicle-injected animals) (Fig. 1F). In contrast, 1.3 Hz conditioning stimulation after the administration of this dose of CT did not induce LTD (100.5 ± 3.0%;n = 7; p > 0.05; data not illustrated).
Time-dependent reversal of high-frequency stimulation-induced LTP
Because the induction of LTD and depotentiation share common properties, we examined the possibility that facilitation of depotentiation may have contributed to the previously observed block of LTP by amyloidogenic β-APP fragments (Cullen et al., 1997).
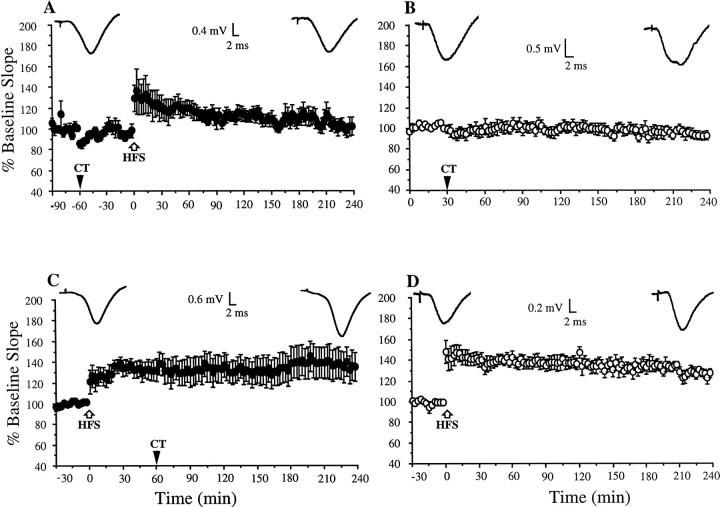
We therefore compared the ability of CT to block LTP when injected before and after the high-frequency conditioning stimulation. Consistent with our previous study (Cullen et al., 1997), 1 pmol CT, when injected 10 min before the HFS, blocked LTP completely (97.7 ± 4.8% at 2 hr post-HFS; n = 7;p > 0.05 compared with pre-HFS baseline;p < 0.05 compared with water-injected animals; 140.6 ± 10.4%; n = 7) (Fig.2A,C). Although the level of short-term potentiation appeared to be diminished, this was not statistically significant (p > 0.05 compared with water-injected controls, measured 10 min post-HFS).
Fig. 2.
The C-terminal fragment of β-amyloid precursor protein (CT) promotes the reversal of LTP. A, B, Stable LTP was induced with high-frequency stimulation (HFS, white arrow) in animals administered the vehicle either 10 min before (A) or 10 min after (B) the HFS. C, D, LTP was blocked when 1 pmol (5 μl, i.c.v.) was injected either 10 min before (C) or after (D) the HFS. Insets show typical recordings at the beginning and end of the experiments. Values are the mean ± SEM EPSP slope;n = 6–7 per group. The time of intracerebroventricular injection is indicated by a black arrowhead.
Remarkably, the injection of CT (1 pmol) 10 min after the HFS resulted in a block of LTP comparable with that caused by injection of the peptide 10 min pre-HFS. Thus, after the injection of CT, the synaptic responses gradually returned to baseline. The EPSP slope at 2 hr post-HFS measured 91 ± 10.9% (n = 6;p > 0.05 compared with pre-HFS baseline;p < 0.05 compared with vehicle-injected animals; 140.2 ± 11.4%; n = 7) (Fig.2B,D).
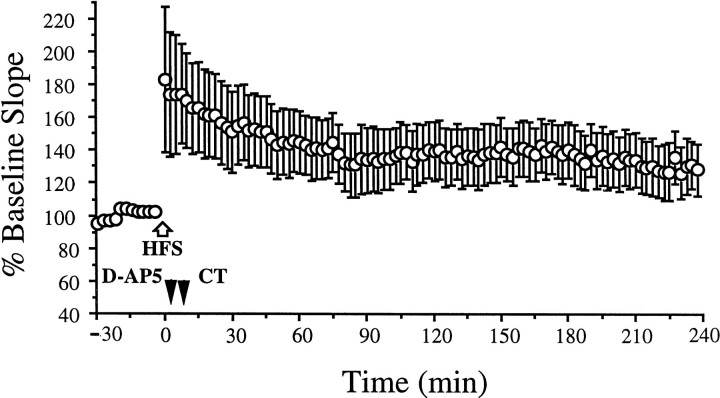
We reasoned that if the mechanism for the CT-mediated reversal of HFS-induced LTP was similar to the facilitation of low-frequency stimulation-induced LTD, it should depend on the activation of NMDA receptors. D-AP5 (100 nmol) was injected intracerebroventricularly 5 min post-HFS, and CT (1 pmol) was administered 5 min later. In these animals, stable LTP was induced (Fig. 3) (138.8 ± 17.8% at 2 hr post-HFS; n = 7;p < 0.05 compared with pre-HFS baseline;p > 0.05 compared with water-injected animals;p < 0.05 compared with CT-injected animals). Thus, the reversal of LTP by CT was blocked by D-AP5, indicating that it is NMDA receptor-dependent.
Fig. 3.
CT-facilitated reversal of LTP is NMDA receptor-dependent. Stable LTP was induced with HFS (white arrow) in animals injected with the NMDA receptor antagonist D-AP5 (100 nmol; black arrowhead) 5 min before the administration of CT 105 (1 pmol) and 5 min after the HFS (n = 7).
We then determined whether CT had similar effects when administered 1 hr before or after the HFS. In animals injected with CT 1 hr before the tetanus, LTP was blocked (Fig.4A) (113.7 ± 5.7% at 2 hr; n = 6; p > 0.05 compared with pre-HFS baseline; p < 0.05 compared with noninjected animals). A control set of experiments confirmed that this dose of CT (1 pmol) did not affect baseline transmission evoked at the test stimulation frequency of 0.033 Hz over a 3 hr recording period (Fig. 4B) (96.7 ± 4.1% at 3 hr;p > 0.05 compared with pre-injection baseline;n = 6).
Fig. 4.
Time window for the block of LTP by CT.A, When CT (1 pmol) was injected 1 hr before the application of HFS (white arrow), LTP was blocked.B, CT had no effect on baseline synaptic transmission over this time period in a nontetanized pathway. C, In contrast, LTP was not affected when CT was injected 1 hr after the HFS.D, This HFS protocol induced stable LTP that lasted at least 3 hr in noninjected animals. Insets show typical recordings at the beginning and end of the experiments. Values are the mean ± SEM EPSP slope; n = 6 per group. The time of intracerebroventricular injection is indicated by black arrowheads.
In contrast, when CT was administered 1 hr after the HFS, LTP remained stable (133.5 ± 9.9 and 137.2 ± 14.2% at 1 and 3 hr, respectively; n = 6; p < 0.05) (Fig.4C). In control experiments, the HFS protocol used in these experiments was found to induce a robust LTP that persisted for at least 3 hr after tetanus in noninjected animals (Fig.4D) (136.8 ± 5.8% pre-HFS baseline EPSP slope at 3 hr; n = 6).
DISCUSSION
Aβ1–42 and the C -terminus of β-APP were found to exert powerful activity- and time-dependent effects on synaptic plasticity. The activity-dependence of the action of these peptides was demonstrated especially when their effect on LTD induction was examined. Although low-frequency conditioning stimulation alone had no effect on transmission, it induced a gradually developing LTD in the presence of the peptides at certain stimulation frequencies but not others. The ability to induce LTD with previously ineffective conditioning stimulation indicates that activity patterns which normally fail to induce synaptic plasticity can trigger LTD in the presence of small amounts of these β-APP fragments.
The time-dependence of the action of the peptides was clearly evident in the experiments on the effect of CT on LTP, the block LTP being found to operate within a defined time window. Thus the potentiated synaptic responses returned to the original pre-HFS baseline level within 1 hr when CT was applied 10 min before or after, but not 1 hr after, the high-frequency conditioning stimulation. Administration of CT 1 hr before the HFS also blocked LTP, but synaptic responses returned to baseline levels more gradually. A similar depotentiation was caused when the Aβ-containing solution was injected 10 min, but not 1 hr, after high-frequency stimulation (I. Klyubin, R. Anwyl, and M. J. Rowan, unpublished observations). The present findings indicate that persistent increases in synaptic strength have a greater vulnerability to interference if the peptide is active within a critical time period and places special focus on the importance of the interval shortly after induction.
The conditions governing the ability to induce LTD and reverse previously established LTP in the intact adult rat hippocampus have proved to be elusive (Stäubli and Lynch, 1990; Errington et al., 1995; Doyère et al., 1996; Doyle et al., 1996, 1997; Heynen et al., 1996; Manahan-Vaughan, 1997; Xu et al., 1997). Similar low-frequency stimulation protocols that can initiate LTD in vitro have been found to trigger depotentiation and, under some conditions, are effective in vivo. The selectivity for certain frequencies of conditioning to preferentially initiate LTD and depotentiation is thought to be related to a requirement for a critical NMDA receptor-mediated rise in intracellular Ca2+ over sufficient time to activate key protein phosphatases (Bear and Abraham, 1996). Once LTP has been induced, it has been reported to become gradually resistant to reversal (Xu et al., 1998; Stäubli and Scafidi, 1999). Intriguingly, agents that affect cell–cell adhesion were reported to lead to depotentiation within a defined time window that is similar to that found in the present studies (Stäubli et al., 1998). Similarly, protein synthesis inhibitors can prevent the development of a later phase of LTP if administered soon after the tetanus (Otani et al., 1989; Huang et al., 1996). However, unlike the β-APP fragments in the present study, neither of these interventions has been reported to promote LTD.
What mechanisms mediate the facilitation of LTD and LTP reversal by these peptides? The block of LTP persistence was clearly not caused by a change in the response during the high-frequency conditioning stimulation because injection of the peptide 10 min before and after the HFS had similar effects. Moreover, the LTP reversal did not occur simply because the synaptic responses were larger after LTP induction, because the peptide was ineffective when administered 1 hr after the HFS, a time when there was still marked potentiation. The discovery that the blocking of NMDA receptors by D-AP5 completely prevented the peptide-facilitated LTD and LTP reversal points to the importance of NMDA receptor-dependent processes. Because low-frequency stimulation-induced LTD (Manahan-Vaughan, 1997) and depotentiation (Doyle et al., 1996) in this pathway have been reported to be NMDA receptor-dependent, there are two main probable mechanisms for the requirement for NMDA receptor activation. First, the peptides may have directly facilitated NMDA receptor-mediated transmission sufficiently to trigger LTD or LTP reversal. Indeed the amyloidogenic β-APP fragment Aβ1–40 can selectively enhance NMDA receptor-mediated synaptic currents measured in the hippocampus with voltage clamp (Wu et al., 1995a). In contrast, another amyloidogenic peptide, Aβ25–35, was reported not to affect NMDA receptor-mediated transmission, but this was based on measurement of epileptic activity in low Mg2+ (Ye and Qiao, 1999). Second, intracellular signaling mechanisms promoting or required for LTD induction may have been affected. For example, a critical rise in postsynaptic Ca2+ is required for LTD induction (Bear and Abraham, 1996). All of the amyloidogenic β-APP fragments have been reported to enhance Ca2+ entry or destabilize intracellular Ca2+ storage (Fraser et al., 1997;Mattson, 1997; Suh, 1997; Kim et al., 2000). Such an action would be expected to facilitate LTD induction. The difference in the frequency at which Aβ1–42 and CT facilitated LTD induction may be the result of a differential ability to affect intracellular Ca2+ at the doses tested.
High concentrations of Aβ1–42 and CT can directly cause a long-lasting depression of baseline synaptic transmission in the hippocampus and cerebellum, although the use-dependence of this depression has not been investigated (Cullen et al., 1997; Hartell and Suh, 2000). In the case of CT, but not Aβ1–42, the depression was blocked by a nitric oxide synthase inhibitor and was associated with an increase in intracellular Ca2+ levels in cerebellar Purkinje cells (Hartell and Suh, 2000). This was proposed to be caused by the formation or opening of relatively nonselective cation channels in the plasma membrane.
Although Aβ1–42 is found extracellularly in Alzheimer's disease brain, there is growing evidence for the importance of intracellular accumulation (Gouras et al., 2000). We have previously reported (Wu et al., 1995a) that intracellular application of Aβ1–40 had the same ability to enhance NMDA receptor-mediated synaptic transmission as when applied exogenously. Similarly, CT can elicit strong inward currents when injected inside as well as outside cells (Fraser et al., 1997;Suh, 1997). It seems reasonable therefore to expect that actions similar to those reported here may apply to the effects of raised levels of intracellular β-APP metabolites on synaptic plasticity. It will be important to examine the effects on synaptic plasticity of potential new therapeutic agents for Alzheimer's disease that inhibit γ-secretase activity because a significant elevation of intracellular C-terminal fragments should accompany the reduction in Aβ production.
The observed frequency- and time-dependent ability of low concentrations of potentially amyloidogenic peptide fragments of β-APP to promote persistent reductions and prevent persistent enhancements of synaptic transmission should result in a gradual, activity-dependent, long-lasting decline in baseline transmission. We have previously reported (Cullen et al., 1996) a delayed (>5 hr) decline in synaptic transmission lasting at least 48 hr after a single intracerebroventricular injection of a low dose of Aβ1–40. Consistent with the present study, the delayed depression was also NMDA receptor-dependent. Such activity-dependent reductions in synaptic transmission are putative substrates for the reductions in cerebral activity of patients with preclinical Alzheimer's disease (Rapoport, 2000).
In conclusion, if, as is generally believed (Elgersma and Silva, 1999;Roman et al., 1999; Martin et al., 2000), synaptic plasticity is engaged during learning and memory, the profound alterations seen here with very low levels of β-APP fragments will have a significant impact on disease symptoms. Moreover, if activity-dependent changes in synaptic strength underlie synaptic remodeling in neurodegeneration (Neill, 1995; McEachern and Shaw, 1996; Mesulam, 1999), they may play an important role in disease progression.
Footnotes
This research was supported by grants from the Health Research Board of Ireland, Enterprise Ireland, the Irish Higher Education Authority, the Wellcome Trust, and the National Creative Research Initiative Grant (2000–2003) from the Ministry of Science and Technology. We thank Dr. William Cullen for assistance in the preparation of the illustrations.
Correspondence should be addressed to Dr. Michael J. Rowan, Department of Pharmacology and Therapeutics, Zoology Building, Trinity College, Dublin 2, Ireland. E-mail: mrowan@tcd.ie.
Dr. Kim's present address: Center for Neurobiology and Behavior, Columbia University, 1051 Riverside Drive, New York, NY 10032.
REFERENCES
- 1.Arriagada PV, Growdon JH, Hedleywhyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer's disease. Neurology. 1992;42:631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 2.Baekelandt V, Moechars D, Laenen I, Lorent K, Van Leuven F. Disturbance of the glutamatergic system in mice transgenic for the amyloid precursor protein. Alzheimers Rep. 1999;2:359–368. [Google Scholar]
- 3.Bear M, Abraham W. Long-term depression in the hippocampus. Annu Rev Neurosci. 1996;19:437–462. doi: 10.1146/annurev.ne.19.030196.002253. [DOI] [PubMed] [Google Scholar]
- 4.Chapman PF, White GL, Jones MW, Cooper-Blacketer D, Marshall VJ, Irizarry M, Younkin L, Good MA, Bliss TV, Hyman BT, Younkin SG, Hsiao KK. Impaired synaptic plasticity and learning in aged amyloid precursor protein transgenic mice. Nat Neurosci. 1999;2:271–276. doi: 10.1038/6374. [DOI] [PubMed] [Google Scholar]
- 5.Chen QS, Kagan BL, Hirakura Y, Xie CW. Impairment of hippocampal long-term potentiation by Alzheimer amyloid beta-peptides. J Neurosci Res. 2000;60:65–72. doi: 10.1002/(SICI)1097-4547(20000401)60:1<65::AID-JNR7>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 6.Chong YH, Jung JM, Choi W, Park CW, Choi KS, Suh YH. Bacterial expression, purification of full-length and carboxyl-terminal fragment of Alzheimer amyloid precursor protein and their proteolytic processing by thrombin. Life Sci. 1994;54:1259–1268. doi: 10.1016/0024-3205(94)00853-1. [DOI] [PubMed] [Google Scholar]
- 7.Cullen WK, Wu J, Anwyl R, Rowan MJ. β-Amyloid produces a delayed NMDA receptor-dependent reduction in synaptic transmission in rat hippocampus. NeuroReport. 1996;8:87–92. doi: 10.1097/00001756-199612200-00018. [DOI] [PubMed] [Google Scholar]
- 8.Cullen WK, Suh YH, Anwyl R, Rowan MJ. Block of LTP in rat hippocampus in vivo by β-amyloid precursor protein fragments. NeuroReport. 1997;8:3213–3217. doi: 10.1097/00001756-199710200-00006. [DOI] [PubMed] [Google Scholar]
- 9.Cummings B, Cotman C. Image analysis of β-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet. 1996;346:1524–1528. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- 10.Doyère V, Errington ML, Laroche S, Bliss TVP. Low-frequency trains of paired stimuli induce long-term depression in area CA1 but not in dentate gyrus of the intact rat. Hippocampus. 1996;6:52–57. doi: 10.1002/(SICI)1098-1063(1996)6:1<52::AID-HIPO9>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 11.Doyle C, Holscher C, Rowan MJ, Anwyl R. The selective neuronal NO synthase inhibitor 7-nitro-indazole blocks both long-term potentiation and depotentiation of field EPSPs in rat hippocampal CA1 in vivo. J Neurosci. 1996;16:418–424. doi: 10.1523/JNEUROSCI.16-01-00418.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Doyle CA, Cullen WK, Rowan MJ, Anwyl R. Low-frequency stimulation induces homosynaptic depotentiation but not long-term depression of synaptic transmission in the adult anaesthetized and awake rat hippocampus in vivo. Neuroscience. 1997;77:75–85. doi: 10.1016/s0306-4522(96)00427-7. [DOI] [PubMed] [Google Scholar]
- 13.Elgersma Y, Silva A. Molecular mechanisms of synaptic plasticity and memory. Curr Opin Neurobiol. 1999;9:209–213. doi: 10.1016/s0959-4388(99)80029-4. [DOI] [PubMed] [Google Scholar]
- 14.Errington ML, Bliss TVP, Richter-Levin G, Yenk K, Doyère V, Laroche S. Stimulation at 1–5 Hz does not produce long-term depression or depotentiation in the hippocampus of the adult rat in vivo. J Neurophysiol. 1995;74:1793–1799. doi: 10.1152/jn.1995.74.4.1793. [DOI] [PubMed] [Google Scholar]
- 15.Fraser S, Suh Y-H, Djamgoz M. Ionic effects of the Alzheimer's disease β-amyloid precursor protein and its metabolic fragments. Trends Neurosci. 1997;20:67–72. doi: 10.1016/s0166-2236(96)10079-5. [DOI] [PubMed] [Google Scholar]
- 16.Gouras G, Tsai J, Näslund J, Vincent B, Edgar M, Checler F, Greenfield J, Haroutunian V, Buxbaum J, Xu H, Greengard P, Relkin N. Intraneuronal Aβ42 accumulation in human brain. Am J Pathol. 2000;156:15–20. doi: 10.1016/s0002-9440(10)64700-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hartell NA, Suh YH. Peptide fragments of β-amyloid precursor protein: effects on parallel fiber-Purkinje cell synaptic transmission in rat cerebellum. J Neurochem. 2000;74:1112–1121. doi: 10.1046/j.1471-4159.2000.741112.x. [DOI] [PubMed] [Google Scholar]
- 18.Hartley DM, Walsh DM, Ye CPP, Diehl T, Vasquez S, Vassilev PM, Teplow DB, Selkoe DJ. Protofibrillar intermediates of amyloid β-protein induce acute electrophysiological changes and progressive neurotoxicity in cortical neurons. J Neurosci. 1999;19:8876–8884. doi: 10.1523/JNEUROSCI.19-20-08876.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Heynen AJ, Abraham WC, Bear MF. Bidirectional modification of CA1 synapses in the adult hippocampus in vivo. Nature. 1996;381:163–166. doi: 10.1038/381163a0. [DOI] [PubMed] [Google Scholar]
- 20.Hsia AY, Masliah E, McConlogue L, Yu GQ, Tatsuno G, Hu K, Kholodenko D, Malenka RC, Nicoll RA, Mucke L. Plaque-independent disruption of neural circuits in Alzheimer's disease mouse models. Proc Natl Acad Sci USA. 1999;96:3228–3233. doi: 10.1073/pnas.96.6.3228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Y-Y, Nguyen P, Abel T, Kandel E. Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- 22.Itoh A, Akaike T, Sokabe M, Nitta A, Iida R, Olariu A, Yamada K, Nabeshima T. Impairments of long-term potentiation in hippocampal slices of β-amyloid-infused rats. Eur J Pharmacol. 1999;382:167–175. doi: 10.1016/s0014-2999(99)00601-9. [DOI] [PubMed] [Google Scholar]
- 23.Janus C, Chishti M, Westaway D. Transgenic mouse models of Alzheimer's disease. Biochim Biophys Acta. 2000;1502:63–75. doi: 10.1016/s0925-4439(00)00033-8. [DOI] [PubMed] [Google Scholar]
- 24.Kim HS, Park CH, Cha SH, Lee JH, Lee S, Kim Y, Rah JC, Jeong SJ, Suh YH. Carboxyl-terminal fragment of Alzheimer's APP destabilizes calcium homeostasis and renders neuronal cells vulnerable to excitotoxicity. FASEB J. 2000;14:1508–1517. doi: 10.1096/fj.14.11.1508. [DOI] [PubMed] [Google Scholar]
- 25.Kumar-Singh S, Dewachter I, Moechars D, Lubke U, De Jonghe C, Ceuterick C, Checler F, Naidu A, Cordell B, Cras P, Van Broeckhoven C, Van Leuven F. Behavioral disturbances without amyloid deposits in mice overexpressing human amyloid precursor protein with Flemish (A692G) or Dutch (E693Q) mutation. Neurobiol Dis. 2000;7:9–22. doi: 10.1006/nbdi.1999.0272. [DOI] [PubMed] [Google Scholar]
- 26.Lambert MP, Barlow AK, Chromy BA, Edwards C, Freed R, Liosatos M, Morgan TE, Rozovsky I, Trommer B, Viola KL, Wals P, Zhang C, Finch CE, Krafft GA, Klein WL. Diffusible, nonfibrillar ligands derived from Aβ1–42 are potent central nervous system neurotoxins. Proc Natl Acad Sci USA. 1998;95:6448–6453. doi: 10.1073/pnas.95.11.6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Larson J, Lynch G, Games D, Seubert P. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- 28.Lu D, Rabizadeh S, Chandra S, Shayya R, Ellerby L, Ye X, Salvesen G, Koo E, Bredesen D. A second cytotoxic proteolytic peptide derived from amyloid beta-protein precursor. Nat Med. 2000;6:397–404. doi: 10.1038/74656. [DOI] [PubMed] [Google Scholar]
- 29.Lue LF, Kuo YM, Roher AE, Brachova L, Shen Y, Sue L, Beach T, Kurth JH, Rydel RE, Rogers J. Soluble amyloid beta peptide concentration as a predictor of synaptic change in Alzheimer's disease. Am J Pathol. 1999;155:853–862. doi: 10.1016/s0002-9440(10)65184-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Luscher C, Nicoll R, Malenka R, Muller D. Synaptic plasticity and dynamic modulation of the postsynaptic membrane. Nat Neurosci. 2000;3:545–550. doi: 10.1038/75714. [DOI] [PubMed] [Google Scholar]
- 31.Manahan-Vaughan D. Group 1 and 2 metabotropic glutamate receptors play differential roles in hippocampal long-term depression and long-term potentiation in freely moving rats. J Neurosci. 1997;17:3303–3311. doi: 10.1523/JNEUROSCI.17-09-03303.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Martin S, Grimwood P, Morris RGM. Synaptic plasticity and memory: an evaluation of the hypothesis. Annu Rev Neurosci. 2000;23:649–711. doi: 10.1146/annurev.neuro.23.1.649. [DOI] [PubMed] [Google Scholar]
- 33.Mattson MP. Cellular actions of β-amyloid precursor protein and its soluble and fibrillogenic derivatives. Physiol Rev. 1997;77:1081–1132. doi: 10.1152/physrev.1997.77.4.1081. [DOI] [PubMed] [Google Scholar]
- 34.McEachern JC, Shaw CA. An alternative to the LTP orthodoxy: a plasticity-pathology continuum model. Brain Res Rev. 1996;22:51–92. [PubMed] [Google Scholar]
- 35.McLean CA, Cherny RA, Fraser FW, Fuller SJ, Smith MJ, Beyreuther K, Bush AI, Masters CL. Soluble pool of A beta amyloid as a determinant of severity of neurodegeneration in Alzheimer's disease. Ann Neurol. 1999;46:860–866. doi: 10.1002/1531-8249(199912)46:6<860::aid-ana8>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 36.Mesulam MM. Neuroplasticity failure in Alzheimer's disease: bridging the gap between plaques and tangles. Neuron. 1999;24:521–529. doi: 10.1016/s0896-6273(00)81109-5. [DOI] [PubMed] [Google Scholar]
- 37.Moechars D, Dewachter I, Lorent K, Reverse D, Baekelandt V, Naidu A, Tesseur I, Spittaels K, Van Den Haute C, Checler F, Godaux E, Cordell B, Van Leuven F. Early phenotypic changes in transgenic mice that overexpress different mutants of amyloid precursor protein in brain. J Biol Chem. 1999;274:6483–6492. doi: 10.1074/jbc.274.10.6483. [DOI] [PubMed] [Google Scholar]
- 38.Mucke L, Masliah E, Yu GQ, Mallory M, Rockenstein EM, Tatsuno G, Hu K, Kholodenko D, Johnson-Wood K, McConlogue L. High-level neuronal expression of Aβ1–42 in wild-type human amyloid protein precursor transgenic mice: synaptotoxicity without plaque formation. J Neurosci. 2000;20:4050–4058. doi: 10.1523/JNEUROSCI.20-11-04050.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nalbantoglu J, Tirado-Santiago G, Lahsaini A, Poirier J, Goncalves O, Verge G, Momoli F, Welner SA, Massicotte G, Julien JP, Shapiro ML. Impaired learning and LTP in mice expressing the carboxy terminus of the Alzheimer amyloid precursor protein. Nature. 1997;387:500–505. doi: 10.1038/387500a0. [DOI] [PubMed] [Google Scholar]
- 40.Neill D. Alzheimer's disease: maladaptive synaptoplasticity hypothesis. Neurodegeneration. 1995;4:217–232. doi: 10.1006/neur.1995.0027. [DOI] [PubMed] [Google Scholar]
- 41.Otani S, Marshall C, Tate W, Goddard G, Abraham W. Maintenance of long-term potentiation in rat dentate gyrus requires protein synthesis but not messenger RNA synthesis immediately post-tetanization. Neuroscience. 1989;28:519–526. doi: 10.1016/0306-4522(89)90001-8. [DOI] [PubMed] [Google Scholar]
- 42.Rapoport S. Functional brain imaging to identify affected subjects genetically at risk for Alzheimer's disease. Proc Natl Acad Sci USA. 2000;97:5696–5698. doi: 10.1073/pnas.120178897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Roman F, Truchet B, Marchetti E, Chaillan F, Soumireu-Mourat B. Correlations between electrophysiological observations of synaptic plasticity modifications and behavioral performance in animals. Prog Neurobiol. 1999;58:61–87. doi: 10.1016/s0301-0082(98)00076-8. [DOI] [PubMed] [Google Scholar]
- 44.Selkoe DJ. Translating cell biology into therapeutic advances in Alzheimer's disease. Nature. 1999;399:A23–A31. doi: 10.1038/399a023. [DOI] [PubMed] [Google Scholar]
- 45.Selkoe DJ, Wolfe MS. In search of gamma-secretase: presenilin at the cutting edge. Proc Natl Acad Sci USA. 2000;97:5690–5692. doi: 10.1073/pnas.97.11.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sinha S, Lieberburg I. Cellular mechanisms of β-amyloid production and secretion. Proc Natl Acad Sci USA. 1999;96:11049–11053. doi: 10.1073/pnas.96.20.11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Stäubli U, Lynch G. Stable depression of potentiated synaptic responses in the hippocampus with 1–5 Hz stimulation. Brain Res. 1990;513:113–118. doi: 10.1016/0006-8993(90)91096-y. [DOI] [PubMed] [Google Scholar]
- 48.Stäubli U, Scafidi J. Time-dependent reversal of long-term potentiation in area CA1 of the freely moving rat induced by theta pulse stimulation. J Neurosci. 1999;19:8712–8719. doi: 10.1523/JNEUROSCI.19-19-08712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Stäubli U, Chun D, Lynch G. Time-dependent reversal of long- term potentiation by an integrin antagonist. J Neurosci. 1998;18:3460–3469. doi: 10.1523/JNEUROSCI.18-09-03460.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Suh YH. An etiological role of amyloidogenic carboxyl-terminal fragments of the β-amyloid precursor protein in Alzheimer's disease. J Neurochem. 1997;68:1781–1791. doi: 10.1046/j.1471-4159.1997.68051781.x. [DOI] [PubMed] [Google Scholar]
- 51.Terry RD, Masliah E, Salmon DP, Butters N, Deteresa R, Hill R, Hansen LA, Katzman R. Physical basis of cognitive alterations in Alzheimer's disease: synapse loss is the major correlate of cognitive impairment. Ann Neurol. 1991;30:572–580. doi: 10.1002/ana.410300410. [DOI] [PubMed] [Google Scholar]
- 52.Van Leuven F. Single and multiple transgenic mice as models for Alzheimer's disease. Prog Neurobiol. 2000;61:305–312. doi: 10.1016/s0301-0082(99)00055-6. [DOI] [PubMed] [Google Scholar]
- 53.Wu J, Anwyl R, Rowan MJ. β-Amyloid selectively augments NMDA receptor-mediated synaptic transmission in rat hippocampus. NeuroReport. 1995a;6:2409–2413. doi: 10.1097/00001756-199511270-00031. [DOI] [PubMed] [Google Scholar]
- 54.Wu J, Anwyl R, Rowan MJ. β-Amyloid-(1–40) increases long-term potentiation in rat hippocampus in vitro. Eur J Pharmacol. 1995b;284:R1–R3. doi: 10.1016/0014-2999(95)00539-w. [DOI] [PubMed] [Google Scholar]
- 55.Xu L, Anwyl R, Rowan MJ. Behavioural stress facilitates the induction of long-term depression in the hippocampus. Nature. 1997;387:497–500. doi: 10.1038/387497a0. [DOI] [PubMed] [Google Scholar]
- 56.Xu L, Anwyl R, Rowan MJ. Spatial exploration induces a persistent reversal of long-term potentiation in rat hippocampus. Nature. 1998;394:891–894. doi: 10.1038/29783. [DOI] [PubMed] [Google Scholar]
- 57.Ye L, Qiao JT. Suppressive action produced by β-amyloid peptide fragment 31–35 on long-term potentiation in rat hippocampus is N-methyl-d-aspartate receptor-independent: it's offset by (-)huperzine A. Neurosci Lett. 1999;275:187–190. doi: 10.1016/s0304-3940(99)00795-8. [DOI] [PubMed] [Google Scholar]