Abstract
This laboratory has demonstrated that a single methamphetamine (METH) injection rapidly and reversibly decreases the activity of the dopamine transporter (DAT), as assessed ex vivo in synaptosomes prepared from treated rats. This decrease does not occur because of residual drug introduced by the original injection or nor is it associated with a change in binding of the DAT ligand WIN35428. The purpose of this study was to elucidate the mechanism or mechanisms of this METH effect by determining whether direct application of this stimulant to synaptosomes causes changes in DAT similar to those observed ex vivo. Similar to the ex vivoeffect, incubation of striatal synaptosomes with METH decreased DAT activity, but not WIN35428 binding: the effect on activity was not eliminated by repeated washing of synaptosomes. Also, as observedex vivo, incubation with 3,4-methylenedioxymethamphetamine, but not cocaine or methylphenidate, caused a METH-like reduction in DAT function. The rapid and reversible METH-induced diminution in DAT activity did not occur because of a change in membrane potential, as assessed in vitro andex vivo by [3H]tetraphenylphosphonium accumulation. However, the METH-related decline in DAT function may be attributed to phosphorylation because NPC15437, a protein kinase C inhibitor, attenuated the METH-induced decline in DAT function. Similarities between previously reported effects ex vivo of a single METH injection on serotonin and norepinephrine transporter function and effects of direct METH application in vitro were also found. Together, these data demonstrate that the in vitro incubation model mimics the rapid and reversible effects observed after a single METH injection.
Keywords: in vitro, rapid and reversible, serotonin, norepinephrine, transport, phosphorylation
It has been established that the monoamine transporters [i.e., dopamine (DA), serotonin (5-HT), and norepinephrine (NE)] are the biological targets for stimulants such as amphetamine, a metabolite of methamphetamine (METH) (Azzaro et al., 1973). These transporters are affected acutely by promoting the release of neurotransmitters via a carrier-mediated efflux (Fischer and Cho, 1979; Liang and Rutledge, 1982), as well as through the reversal of the transporter (Sulzer et al., 1995). In addition to these acute changes in transporter activity, multiple high-dose administrations of METH cause a persistent loss of transporter protein (Wagner et al., 1980;Ricaurte et al., 1982), as well as depress tyrosine hydroxylase activity (Kogan et al., 1976; Hotchkiss and Gibb, 1980).
Investigations from this laboratory have demonstrated that the acute effects caused by a single and multiple administrations of METH are distinct from long-term transporter deficits presumably associated with nerve terminal degeneration (Fleckenstein et al., 1997; Kokoshka et al., 1998b). For instance, dopamine transporter (DAT) function rapidly and reversibly decreases after a single injection of METH (Fleckenstein et al., 1997), whereas multiple administrations of METH cause a rapid decrease in DAT activity that is partially reversed (Fleckenstein et al., 1997; Kokoshka et al., 1998b). Similar to DAT activity, serotonin transporter (SERT) function is also rapidly and reversibly affected by single and multiple METH administrations (Kokoshka et al., 1998a;Haughey et al., 2000b). These acute transporter changes reflect aVmax reduction and do not occur because of residual METH introduced by the original injection, as suggested by findings that the effects observed are still present after successive washing of METH-treated synaptosomes (Fleckenstein et al., 1997; Kokoshka et al., 1998a,b). Like the activities of DAT and SERT, norepinephrine transporter (NET) activity decreases after both single and multiple METH administrations (Fleckenstein et al., 1997; Kokoshka et al., 1998a; Haughey et al., 2000a). However, the changes in NET activity occur because of a direct competitive interaction of METH with the transporter; as suggested by findings that the effect is associated with an increase in Km and is eliminated by successive synaptosomal washing (Haughey et al., 2000a).
The mechanism or mechanisms responsible for these METH-induced acute effects on DAT and SERT was not apparent from reports of these rapid changes. However, recent in vitro studies by Kim et al. (2000) may have important relevance to these rapid in vivooutcomes of METH administration. This group reported that incubation of striatal synaptosomes in the presence of 10 μmMETH caused a reduction in DAT activity that was not likely to be because of METH acting as a competitive inhibitor of dopamine uptake. The characteristics of the in vitro synaptosomal effects of METH reported by these investigators resemble some of the features associated with the rapid and reversible responses of DAT to in vivo administration of METH, as described above. The present study delineates this association and suggests that the in vitroeffect of METH and the acute and reversible consequence of in vivo treatment with this stimulant on monoamine transporters are likely the same phenomenon. In addition, it was demonstrated in vitro as well as in vivo that the METH-induced reduction in DAT activity does not occur because of changes in synaptosomal membrane potential, as assessed by [3H]tetraphenylphosphonium ([3H]TPP+) accumulation. Instead, using the in vitro synaptosomal system, it was established that some protein kinase C (PKC) inhibitors, including the highly selective PKC inhibitor NPC15437, block the METH-induced decline in DA uptake. This suggests that phosphorylation may mediate this effect and that the synaptosomal in vitrosystem is an appropriate model to help elucidate the mechanism or mechanisms responsible for the decrease in DA uptake caused by METH treatment.
MATERIALS AND METHODS
Animals. Male Sprague Dawley rats (140–330 gm; Simonsen Laboratories, Gilroy, CA) were group-housed in wire hanging cages, maintained at 22 ± 1°C on a 14/10 hr light/dark cycle, and provided with food and water ad libitum. On the day of the experiment, six to eight rats were housed in nontransparent Plexiglas cages lined with screened sawdust shavings. Rats were killed by decapitation. All procedures were conducted in accordance with approved National Institutes of Health guidelines.
Drugs and chemicals. (±)-METH hydrochloride, 3,4-methylenedioxymethamphetamine hydrochloride (MDMA) (“ecstasy”), and cocaine hydrochloride were generously supplied by the National Institute on Drug Abuse (Rockville, MD). Methylphenidate hydrochloride (MPD) was obtained from Ciba Geigy (Summit, NJ). Drugs were administered as indicated in figure legends; doses were calculated as the respective free base. Pargyline hydrochloride, valinomycin, HEPES, EDTA, chelerythrine chloride, and NPC15437 were purchased from Sigma (St. Louis, MO). Desipramine hydrochloride was purchased from Research Biochemicals International (Natick, MA). Citalopram hydrochloride was obtained from H. Lundbeck A/S (Copenhagen, Denmark). [7,8-3H]Dopamine (49 Ci/mmol) and [3H]TPP+(32 Ci/mmol) were purchased from Amersham Life Sciences (Arlington Heights, IL). [ring-2,5,6-3H]levo-Norepinephrine (51.8 Ci/mmol) and [N-methyl-3H]WIN35428 (84.5 Ci/mmol) were purchased from New England Nuclear (Boston, MA).
[3H]Neurotransmitter uptake and binding assays. Uptake of [3H]neurotransmitter was determined as described previously by Kokoshka et al. (1998b). Briefly, fresh striatal tissue was homogenized in ice-cold 0.32m sucrose and centrifuged (800 ×g for 12 min; 4°C), the supernatant (S1) fractions were carefully removed and centrifuged (22,000 × g for 15 min; 4°C) to obtain the synaptosomal-containing pellet (P2). The resulting pellet (P2) was resuspended in an ice-cold modified Krebs' buffer (in mm): 126 NaCl, 4.8 KCl, 1.3 CaCl2, 16 sodium phosphate, 1.4 MgSO4, 11 dextrose, 1 ascorbic acid, pH 7.4. In METH preincubation experiments, samples were preincubated with 10 μm METH for 30 min at 37°C, unless otherwise specified in the figure legends (i.e., in the legend to Fig. 6). After 30 min, resuspended P2 fractions were “washed” by centrifugation (22,000 × g for 15 min; 4°C). The resulting pellet (P3) was then resuspended in ice-cold Krebs' buffer and once again centrifuged (22,000 × g for 15 min; 4°C) to obtain a P4 pellet that was subsequently resuspended and assayed. Assays were conducted in Krebs' buffer. Each assay tube contained synaptosomal tissue (i.e., resuspended P4 obtained from 1.5, 7.5, and 10 mg original wet weight from striatal and hippocampal tissue, for dopamine, serotonin and norepinephrine, respectively) and 1 μm pargyline. Nonspecific values for DA, 5-HT, and NE transport were determined in the presence of 1 mm cocaine, 10 μmcitalopram, and 1 μm desipramine, respectively. After preincubation of assay tubes for 10 min at 37°C, assays were initiated by addition of [3H]neurotransmitter [final concentrations were (in nm): 0.5 for DA and 5 for 5-HT and NE]. Samples were incubated at 37°C for 3 (DA and 5-HT) or 5 (NE) min. [3H]WIN35428 binding (0.5 nm final concentration) was conducted in phosphate-buffered 0.32 m sucrose, pH 7.4, with synaptosomes obtained from 2 mg (original wet weight) of striatal tissue per reaction tube, and samples were incubated on ice for 2 hr. Samples were then filtered through Whatman GF/B filters (Brandel, Gaithersburg, MD) soaked previously in 0.05% polyethylenimine. Filters were washed rapidly three times with 3 ml of ice-cold 0.32 m sucrose using a filtering manifold (Brandel). Radioactivity trapped in filters was counted using a liquid scintillation counter. The remaining synaptosomal tissue (i.e., resuspended P4 not used for the uptake assay) was retained, and protein was determined according to the method of Lowry et al. (1951).
Fig. 6.
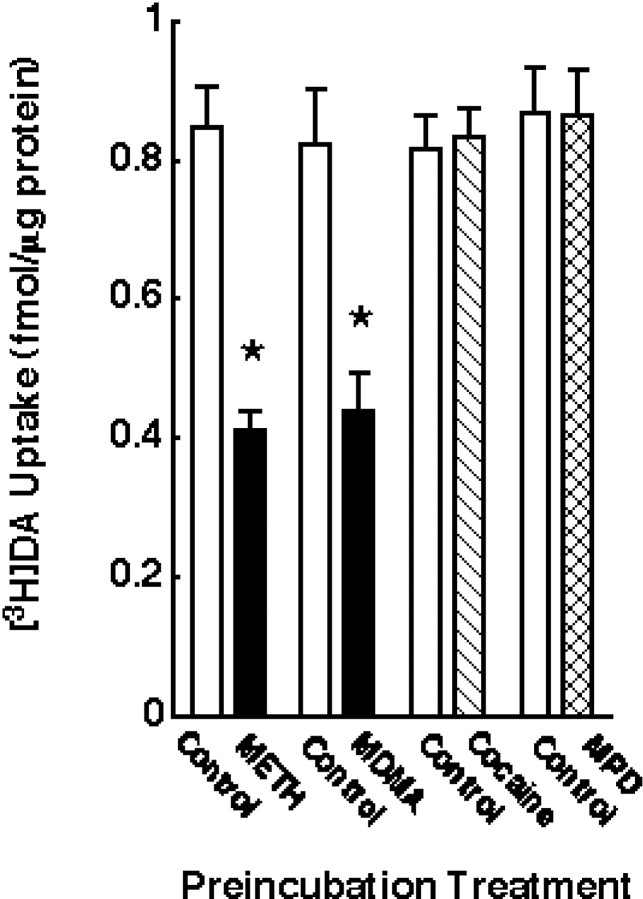
Effect of psychostimulant preincubation on [3H]DA uptake. Striatal synaptosomes were preincubated with (in μm): 10 METH, 10 MDMA, 10 cocaine, and 10 MPD or assay buffer for 30 min at 37°C. Before assaying [3H]DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods.Columns represent means, and vertical linesare 1 SEM of determinations from three independent experiments.Asterisks indicate values for drug-treated synaptosomes that differ significantly from their respective controls (p ≤ 0.05).
[3H]TPP+accumulation assay.[3H]TPP+accumulation was measured using a modification of the method of Pauwels and Laduron (1986). Briefly, fresh tissue was homogenized in ice-cold 0.32 m sucrose containing 3.0 mm HEPES and 1.0 mm EDTA, pH 7.4. The homogenate was centrifuged (800 × g for 12 min; 4°C), and the supernatant (S1) fractions were carefully removed and centrifuged (22,000 × g for 15 min; 4°C) to obtain the synaptosomal-containing pellet (P2). The resulting pellet (P2) was resuspended in ice-cold sucrose–HEPES. In METH incubation experiments, samples were incubated with 10 μmMETH for 30 min at 37°C. After 30 min preincubation periods, resuspended P2 fractions were washed by centrifugation (22,000 ×g for 15 min; 4°C). The resulting pellet (P3) was then resuspended in ice-cold sucrose–HEPES and once again centrifuged (22,000 × g for 15 min; 4°C) to obtain a P4 pellet that was subsequently resuspended and assayed. Assays were conducted in 5 and 75 mm KCl incubation buffers containing the following (in mm): 135 NaCl, 50 HEPES, 1.8 CaCl2, 0.8 MgSO4, and 5.5 glucose, pH 7.4. Each assay tube contained synaptosomal tissue (i.e., resuspended P4 obtained from 16 mg of original wet weight from striatal tissue). Nonspecific values for [3H]TPP+accumulation were determined in the presence of 75 mm KCl incubation buffer. Valinomycin (final concentration: 0.5 μm and 5% ethanol) was added to all assay tubes to eliminate the accumulation of [3H]TPP+ by intrasynaptosomal mitochondria (Scott and Nicholls, 1980). After preincubation of assay tubes for 10 min at 30°C, assays were initiated by addition of [3H]TPP+(final concentration, 5 nm). Samples were incubated at 30°C for 10 min. Samples were then filtered through Whatman GF/B filters soaked previously in 5 mm KCl buffer. Filters were washed rapidly three times with 3 ml of ice-cold 0.32 m sucrose using a Brandel filtering manifold. Radioactivity trapped in filters was counted using a liquid scintillation counter. The remaining synaptosomal tissue (i.e., resuspended P4 not used for the uptake assay) was retained, and protein was determined according to the method of Lowry et al. (1951). Membrane potential calculations were made by applying the Nernst equation and using 3.6 μl/mg as the intrasynaptosomal volume (Ramos et al., 1979).
Data analysis. The data presented represent means ± SEM. Statistical analyses between two groups were conducted using the two-tailed, unpaired Student's t test. Analyses among multigroup data were conducted using ANOVA, followed by a Fisher least significant difference test. Differences among groups were considered significant if the probability of error was <5%.
RESULTS
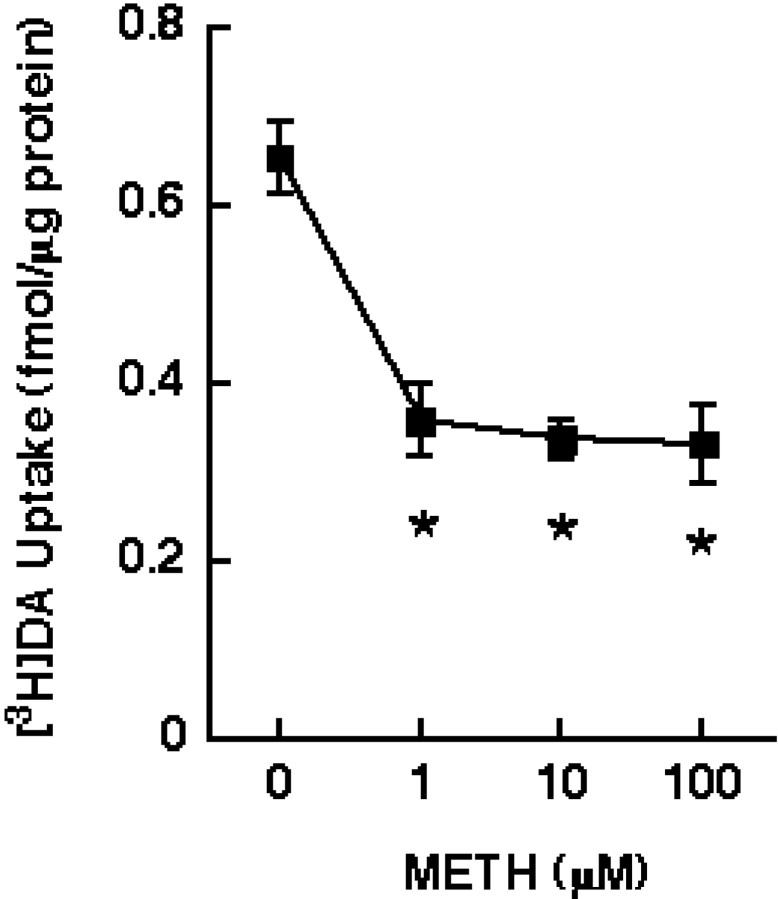
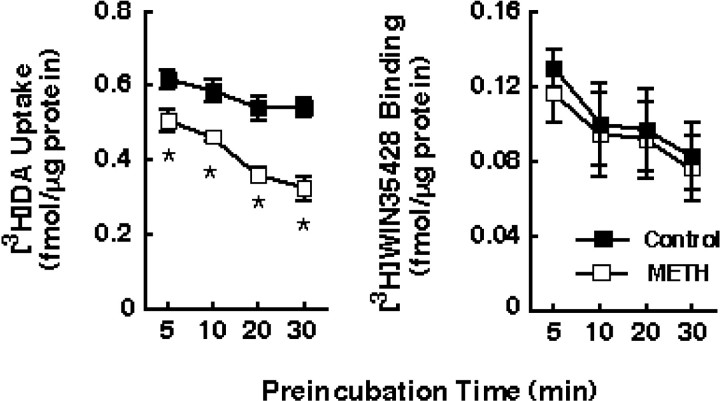
Results presented in Figure 1indicate that preincubation of striatal synaptosomes at 37°C with various METH concentrations (i.e., 1–100 μm) reduced [3H]DA uptake by ∼50%. Based on the amphetamine brain levels (i.e., 8–15 μm) reported byClausing et al. (1995) after multiple administrations of 5 mg/kg drug and based on the data presented in Figure 1, a 10 μm METH concentration was used in subsequent experiments. This METH-induced reduction in [3H]DA uptake occurred rapidly (i.e., within 5 min) and increased in magnitude with increasing time of preincubation (Fig.2A). The largest decrease was achieved at 30 min; consequently, this time point was used in the subsequent experiments because viability of synaptosomes was compromised with longer periods of preincubation. These reductions in DAT function caused by METH preincubation were not associated with a significant change in binding of the DAT ligand, [3H]WIN35428 (Fig.2B). The decline in DAT activity after the 30 min preincubation in the presence of 10 μm METH was associated with a diminution in transporterVmax (in femtomoles per milligram of protein per 3 min: 2162 and 1324 for control and METH-treated synaptosomes, respectively) with no change inKm (in nm: 98 and 96 for control and METH-treated synaptosomes, respectively). In contrast, no drug effect was observed after preincubation of striatal synaptosomes at 4°C, suggesting that the reduction in [3H]DA uptake did not occur because of residual drug (i.e., the same METH concentration was used at 37°C and 4°C, and yet no effect was observed at 4°C).
Fig. 1.
METH preincubation at various concentrations decreased [3H]DA uptake. Striatal synaptosomes were preincubated with various concentrations of METH (in μm: 1–100) or assay buffer for 30 min at 37°C and later assayed at 37°C for the influx of [3H]DA. Before assaying DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods. Values represent means, and vertical lines are 1 SEM of determinations from three independent experiments. Asterisks indicate values for METH-treated synaptosomes that differ significantly from controls (p ≤ 0.05).
Fig. 2.
Preincubation with METH decreased [3H]DA uptake without changing [3H]WIN35428 binding in striatal synaptosomes. Synaptosomes were preincubated with 10 μm METH or assay buffer for various time points (5–30 min) at 37°C. Before assaying [3H]DA uptake and [3H]WIN35428 binding, synaptosomal preparations were washed two extra times, as described in Materials and Methods. Values represent means, and vertical lines are 1 SEM of determinations from three independent experiments.Asterisks indicate values for METH-treated synaptosomes that differ significantly from controls (p≤ 0.05).
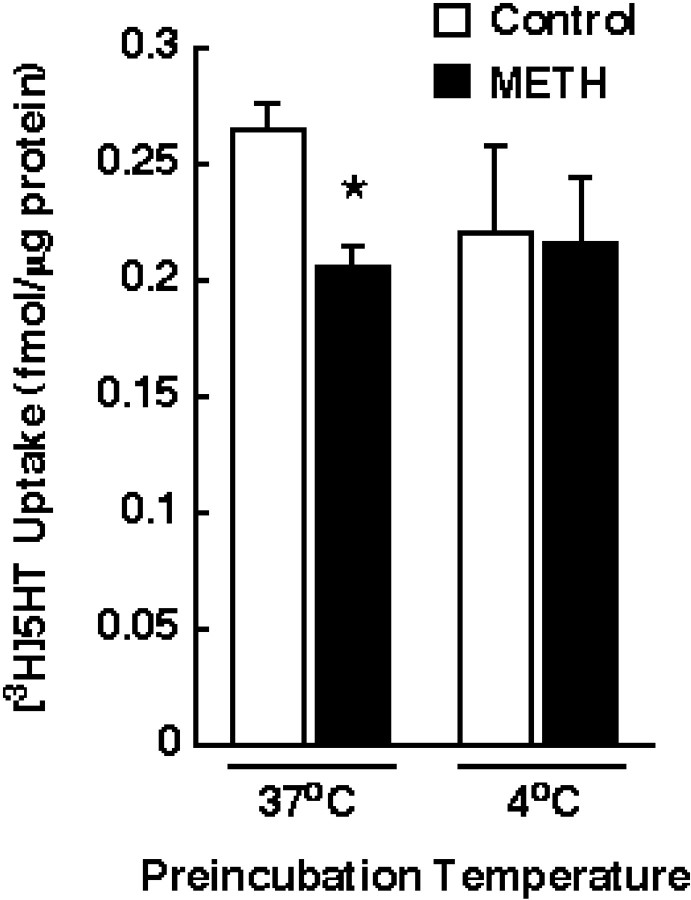
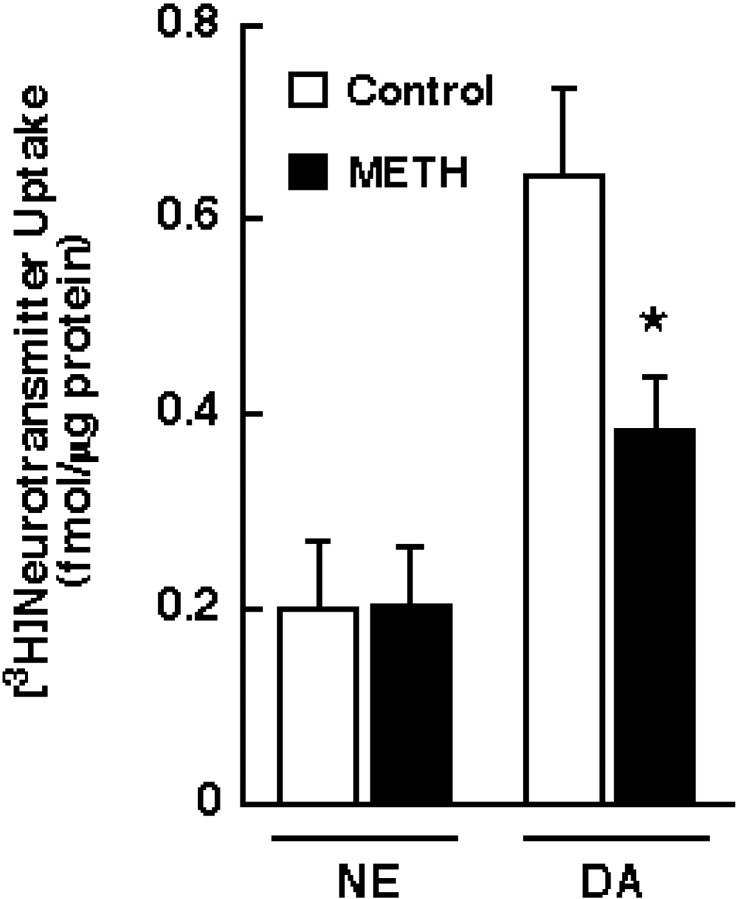
The results presented in Figure 3indicate that the METH-induced decrease was not restricted to DAT, because a reduction in [3H]5-HT uptake was observed when synaptosomes were preincubated with 10 μm METH at 37°C. Similar to DAT activity, SERT function was not altered by METH when synaptosomes were preincubated at 4°C. The data in Figure 4 demonstrate that [3H]NE uptake (as assessed in washed hippocampal synaptosomes because the striatum contains few NE terminals), unlike [3H]DA uptake, was not decreased by METH preincubation.
Fig. 3.
Effect of METH preincubation on [3H]5-HT uptake. Striatal synaptosomes were preincubated with 10 μm METH or assay buffer for 30 min at 37 or 4°C. Before assaying [3H]5-HT influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods. Columns represent means, andvertical lines are 1 SEM of determinations from three independent experiments. The asterisk indicates a value for METH-treated synaptosomes that differ significantly from the respective control group (p ≤ 0.05).
Fig. 4.
Effect of METH preincubation on [3H]NE uptake. Hippocampal and striatal synaptosomes were preincubated with 10 μm METH or assay buffer for 30 min at 37°C and assayed at 37°C for the influx of [3H]NE and [3H]DA, respectively. Before assaying [3H]NE and [3H]DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods.Columns represent means, and vertical linesare 1 SEM of determinations from four independent experiments. Theasterisk indicates a value for METH-treated synaptosomes that differs significantly from the control (p ≤ 0.05).
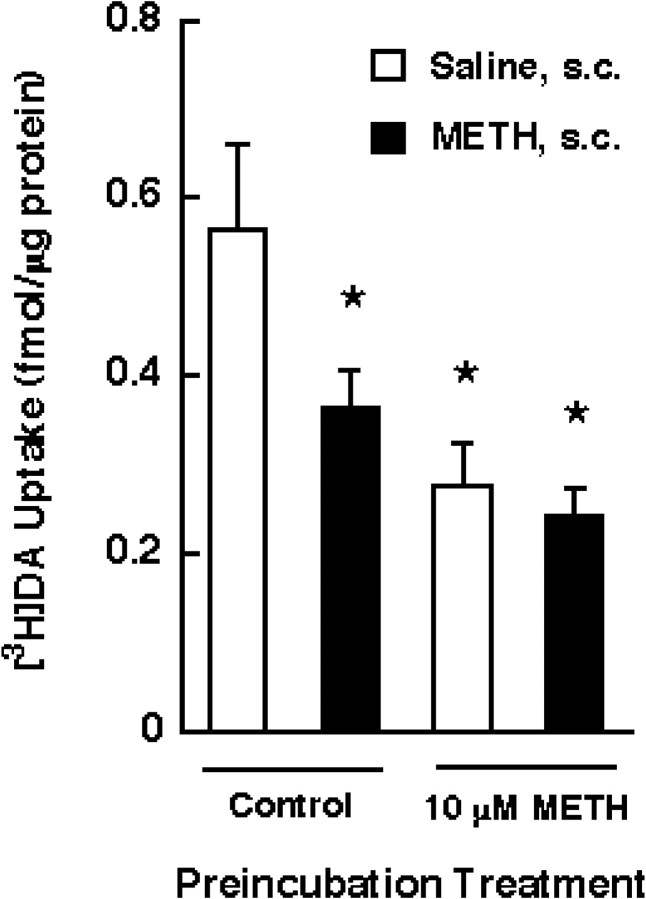
To determine whether the in vitro phenomenon was related to that of an in vivo single or multiple administration of METH, rats were treated in vivo with a single injection (15 mg/kg, s.c.) of METH or saline. The synaptosomes prepared from drug- or saline-treated rats (in vivo) were then preincubated in the presence of 10 μm METH (in vitro), as described above, and DAT activity was assessed by [3H]DA uptake. As shown in Figure 5, METH preincubation in vitro did not further diminish DAT activity in synaptosomal preparations from drug-treated rats compared with the two 10 μm METH groups, suggesting that the in vitro and ex vivo models represent the same phenomenon.
Fig. 5.
Effects of a single administration and preincubation with METH on [3H]DA uptake. Rats received METH (15 mg/kg, s.c.) or saline (1 ml/kg, s.c.) 1 hr before decapitation. Striatal synaptosomes were preincubated with 10 μm METH or assay buffer for 30 min at 37°C, as described in Materials and Methods, and assayed for the influx of [3H]DA. Before assaying DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods. Columns represent means, and vertical lines are 1 SEM of determinations from four independent experiments. Asterisks indicate values for METH-treated synaptosomes that differ significantly from the saline/control group (p ≤ 0.05).
To determine the selectivity of the transient METH-related reduction in DAT function observed in vitro, synaptosomes were preincubated with (in μm): 10 MDMA, 10 cocaine, or 10 MPD. MDMA, like METH, decreased [3H]DA uptake (i.e., to 47 and 51% of control for MDMA and METH, respectively) (Fig.6). In contrast, preincubation with cocaine and MPD was without effect in [3H]DA uptake (Fig. 6).
The in vitro model was used next to determine whether synaptosomal membrane potential changes contributed to the METH-induced decline in DAT activity. To achieve this objective, [3H]TPP+accumulation assays were conducted in vitro. As presented in Table 1, in vitro incubation of striatal synaptosomes with 10 μm METH did not alter synaptosomal membrane potential. Furthermore, [3H]TPP+accumulation assays were also conducted ex vivo after a single injection of METH; these assays, like those conducted in vitro, showed no alterations in synaptosomal membrane potential.
Table 1.
Effect of 10 μm METH preincubation on synaptosomal membrane potential in rat striatal synaptosomes
| Treatment | Synaptosomal membrane potential ± SEM | [3H]DA uptake ± SEM | |
|---|---|---|---|
| In vitro | |||
| Control | 62.01 ± 1.98 | 0.381 ± 0.051 | |
| 10 μm METH | 57.89 ± 1.29 | 0.176 ± 0.026* | |
| In vivo | |||
| Saline | 59.43 ± 2.54 | 0.506 ± 0.023 | |
| METH | 59.94 ± 7.19 | 0.358 ± 0.014* |
For in vitro experiments, synaptosomes were preincubated with 10 μm METH or assay buffer for 30 min at 37°C as described in Materials and Methods, and assayed for the accumulation of [3H]TPP+. Values represent means and 1 SEM of determinations from three independent experiments. For in vivo experiments, rats received a single administration of METH (15 mg/kg, s.c.) or saline (1 ml/kg, s.c.) 1 hr before decapitation (n = 6 for each group). Values for synaptosomal membrane potentials represent millivolts; values for [3H]DA uptake represent femtomoles per microgram of protein.
Values different from respective control groups (p ≤ 0.05).
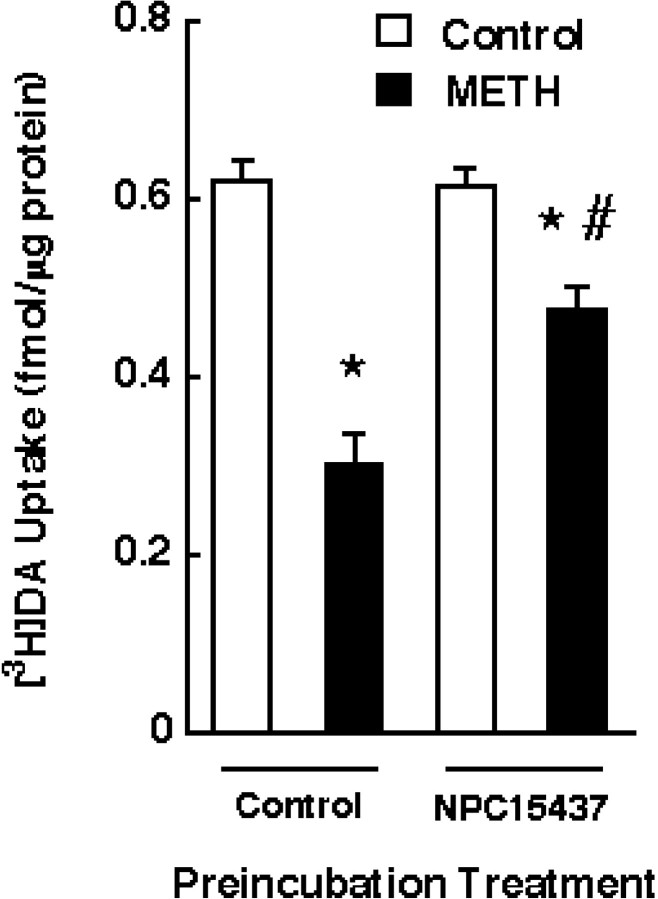
Finally, the in vitro model was used to examine the role of phosphorylation in the METH-induced DAT changes. Synaptosomes were pretreated with 15 μm NPC15437 for 5 min before METH exposure. The results presented in Figure7 demonstrate that pretreatment with NPC15437 significantly attenuated the in vitro METH-induced decrease in synaptosomal [3H]DA uptake. In contrast, pretreatment with a second PKC inhibitor, chelerythrine, did not attenuate the METH-induced reduction in synaptosomal [3H]DA uptake (Table2).
Fig. 7.
Effect of NPC15437 pretreatment on the decrease in [3H]DA uptake in striatal synaptosomes induced by METH preincubation. Striatal synaptosomes were pretreated with 15 μm NPC15437 for 5 min and subsequently preincubated with 10 μm METH or assay buffer for 30 min at 37°C. Before assaying [3H]DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods.Columns represent means, and vertical linesare 1 SEM of determinations from three independent experiments.Asterisks indicate values for METH-treated synaptosomes that differ significantly from controls; #, a value for NPC15437-treated synaptosomes that differs significantly from METH-treated synaptosomes (p ≤ 0.05).
Table 2.
Effects of chelerythrine on the METH-induced decrease in [3H]DA uptake
| Treatment | [3H]DA uptake ± SEM |
|---|---|
| Control | 0.64 ± 0.06 |
| 10 μm METH | 0.31 ± 0.03* |
| 0.1 μm chelerythrine | 0.61 ± 0.09 |
| 0.1 μm chelerythrine/10 μmMETH | 0.29 ± 0.02* |
| 1 μmchelerythrine | 0.57 ± 0.08 |
| 1 μmchelerythrine/10 μm METH | 0.27 ± 0.04* |
Striatal synaptosomes were pretreated with 1 or 0.1 μm chelerythrine for 5 min and then preincubated with 10 μm METH or assay buffer for 30 min at 37°C. Before assaying [3H]DA influx, synaptosomal preparations were washed two extra times, as described in Materials and Methods. Values represent means and 1 SEM of determinations from three independent experiments. Values for [3H]DA uptake represent femtomoles per microgram of protein.
Values different from respective control groups (p ≤ 0.05).
DISCUSSION
The intent of this study was to elucidate the mechanism whereby METH administration rapidly and reversibly reduces DAT activity in striatal synaptosomes. Recently, the findings by Kim et al. (2000)suggested that in vitro application of METH to the synaptosomes may cause an effect similar to that observed rapidly afterin vivo administrations of METH. For instance, this group reported that the in vitro METH-induced reduction in [3H]DA uptake was not associated with a change in [3H]WIN35428 binding, as shown previously in vivo by Fleckenstein et al. (1997) andKokoshka et al. (1998b). Like the in vivo findings ofFleckenstein et al. (1997) and Kokoshka et al. (1998b), this group also reported that the in vitro changes in transporter activity occurred because of a decline in Vmax. It was also determined that, as observed ex vivo in synaptosomes prepared from METH-treated rats (Kokoshka et al., 1998a), glutamate uptake was unaltered after in vitro preincubation with 10 μm METH. Moreover, this in vitro system also parallels previous in vivo reports that showed no alteration in [3H]DA uptake in the presence of cocaine and MPD (Fleckenstein et al., 1999). Thus, the observations made by this group resemble those effects of METH previously reported in vivo, which further suggested that the in vitro synaptosomal system is an appropriate model for the rapid and reversible changes caused by in vivoadministrations of METH.
To confirm the possibility that this in vitro model resembles those effects observed in vivo, we further characterized the in vitro effect and found substantial parallels. For example, the proposed in vitro model not only resembles the METH-induced ex vivo changes in DAT function, but is also similar to the METH-induced effects in other monoamine transporter activities, such as SERT and NET. Kokoshka et al. (1998a)and Fleckenstein et al. (1999) showed that SERT function, like DAT activity, is reduced after a single administration of METH. This decrease was not a result of residual METH introduced by the original injection of the drug (Kokoshka et al., 1998a). Similarly, results in Figure 3 are consistent with these in vivo findings ofKokoshka et al. (1998a) and Fleckenstein et al. (1999), in that SERT function decreased by 22% after synaptosomes were exposed in vitro to 10 μm METH at 37°C. These data further establish that this reduction in [3H]5-HT uptake did not occur because of a direct interaction of residual METH with the transporter protein, because synaptosomal exposure to 10 μm METH did not alter [3H]5-HT uptake at 4°C, whereas transporter activity was reduced at 37°C after successive washing of the synaptosomal preparation (Fig. 3). Unlike DAT and SERT, NET activity was not reduced after hippocampal synaptosomes were exposed to 10 μm METH (Fig. 4). These results are concordant with the recent observations of Haughey et al. (2000a)that demonstrated that unlike DAT and SERT, [3H]NE uptake returned to control levels after successive washing of drug-treated synaptosomes (Fig. 4).
Interestingly, the results presented in Figure 5 further support the possibility that the in vitro model represents acute changes in DAT function after in vivo METH treatment. These data demonstrate that after in vitro preincubation with 10 μm METH, the DAT activity is reduced comparably in striatal synaptosomes prepared from saline- and METH-treated animals. The fact that the in vivo treatment with METH did not further add to the decline in DAT activity caused by in vitro preincubation with this drug suggests that these two treatments cause the same phenomenon.
These comparisons are significant for the following reasons: first, the reversible changes after in vivo treatment with METH are likely the same as those resulting from in vitropreincubation with this stimulant. The similarities between thein vitro and the rapid in vivo models are confirmed by findings indicating that, similar to the in vivo model (Fleckenstein et al., 1999), cocaine and MPD were without effect on DAT activity after the in vitropreincubation (Fig. 6). Second, the rapid transporter phenomenon does not necessarily reflect a neurotoxic process because it (1) occurs quickly and is reversible and (2) is comparably caused by MDMA, a drug not particularly toxic to DA systems (Stone et al., 1987; O'Hearn et al., 1988; Ricaurte et al., 1993) except at high doses in rats (Commins et al., 1987) and mice (Logan et al., 1988). Still, the decrease in DAT function may, under certain conditions, promote the development of neurotoxicity; this possibility remains to be explored.
If these acute reversible changes in DAT function do not occur because of damage to the monoamine terminals, the mechanism or mechanisms by which METH causes the transient reduction in DAT activity is unclear. Using the in vitro model to address this issue, and knowing that monoamine transporters belong to the Na+/Cl−-dependent family (Uhl and Hartig, 1992; Borowsky and Hoffman, 1995), changes in ion fluxes were investigated as a possible mechanism causing the acute decline in DAT activity. The results suggest that ion fluxes did not contribute to the transient diminution of DAT action, as suggested by the findings that the synaptosomal membrane potential was not alteredin vitro in the presence of 10 μmMETH, nor was it altered in vivo after a single injection of METH, as assessed by [3H]TPP+accumulation (Table 1).
Another mechanism whereby METH might cause rapid and reversible reductions in DAT activity is phosphorylation. It has been determined that DAT is extensively glycosylated and that its sequence contains phosphorylation consensus sites for protein kinase A, PKC, and Ca2+-calmodulin kinase (Giros et al., 1991; Kilty et al., 1991; Shimada et al., 1991; Vandenbergh et al., 1992), suggesting that these transporters may be subject to regulation by phosphorylation. This is supported by studies using DAT-expressing COS cells that have shown that activation of PKC by phorbol esters leads to a decrease in the transport of DA into the transfected cell line (Kitamaya et al., 1994). This observation is also in accordance with other reports that state that PKC-induced regulation decreases DAT activity in cell culture systems (Huff et al., 1997;Zhang et al., 1997) as well as in mouse and rat synaptosomes (Copeland et al., 1996; Vaughan et al., 1997). The role of phosphorylation in the METH-related changes in DAT function was examined using the PKC inhibitors NPC15437 and chelerythrine. As demonstrated by Kim et al. (2000), chelerythrine application did not prevent the METH-induced decrease in DAT function. However, NPC15437 treatment attenuated the METH-induced decrease in DA uptake. Interestingly, Kim et al. (2000) reported that another PKC inhibitor, bisindolylmaleimide (BIS), attenuated the METH-induced decrease in DA uptake; however, BIS per se decreased DA uptake and, hence, the authors concluded that a role for PKC in an in vitro model remained to be established. Our data accomplish this by demonstrating that a PKC inhibitor can substantially attenuate the METH-induced decrease in DAT function, without eliciting an effect per se.
A possible consequence of phosphorylation is internal trafficking of the monoamine transporters. METH-induced internalization is suggested by the findings of Saunders et al. (2000), who demonstrated that treatment with amphetamine internalized the human DAT expressed on human embryonic kidney cells, thereby decreasing its function. These observations are consistent with reports by Pristupa et al. (1998) andMelikian and Buckley (1999) that showed that activation of PKC results in decreased transporter activity that is attributable to intracellular transporter sequestration.
In summary, these observations demonstrate that METH in vitro alters DAT activity in a manner that parallels what occursin vivo after a single administration of this stimulant. Furthermore, we speculate that this METH-induced decrease in DA uptake may be mediated by internalization of DAT via a PKC-mediated mechanism. Other related drugs such as MDMA, but not cocaine or MPD, appear to cause a similar decrease in DA uptake. This effect appears to be reversible and perhaps relates to an important means of physiologically regulating the activity of DA neurons.
Footnotes
This research was supported by United States Public Health Service Grants DA00869, DA04222, DA11389, and DA00378.
Correspondence should be addressed to Dr. Annette E. Fleckenstein, Department of Pharmacology and Toxicology, 30 South 2000 East, Room 201, University of Utah, Salt Lake City, UT 84112. E-mail:fleckenstein@hsc.utah.edu.
REFERENCES
- 1.Azzaro AJ, Rutledge CO. Selectivity of release of norepinephrine, dopamine, and 5-hydroxytryptamine by amphetamine in various regions of rat brain. Biochem Pharmacol. 1973;22:2801–2813. doi: 10.1016/0006-2952(73)90147-0. [DOI] [PubMed] [Google Scholar]
- 2.Borowsky B, Hoffman BJ. Neurotransmitter transporters: molecular biology function and regulation [review]. Int Rev Neurobiol. 1995;38:139–199. doi: 10.1016/s0074-7742(08)60526-7. [DOI] [PubMed] [Google Scholar]
- 3.Clausing P, Gough B, Holson RR, Slikker W, Jr, Bowyer JF. Amphetamine levels in brain microdialysate, caudate/putamen, substantia nigra, and plasma after dosage that produces either behavioral or neurotoxic effects. J Pharmacol Exp Ther. 1995;274:614–621. [PubMed] [Google Scholar]
- 4.Commins DL, Vosmer G, Virus RM, Woolverton WL, Schuster CR, Seiden LS. Biochemical and histological evidence that methylenedioxymethylamphetamine (MDMA) is toxic to neurons in the rat brain. J Pharmacol Exp Ther. 1987;241:338–345. [PubMed] [Google Scholar]
- 5.Copeland BJ, Vogelsberg V, Neff NH, Hadjiconstantinou M. Protein kinase C activators decrease dopamine uptake into striatal synaptosomes. J Pharmacol Exp Ther. 1996;277:1527–1532. [PubMed] [Google Scholar]
- 6.Fischer JF, Cho AK. Chemical release of dopamine from striatal homogenates: evidence for an exchange diffusion model. J Pharmacol Exp Ther. 1979;208:203–209. [PubMed] [Google Scholar]
- 7.Fleckenstein AE, Metzger RR, Wilkins DG, Gibb JW, Hanson GR. Rapid and reversible effects of methamphetamine on dopamine transporters. J Pharmacol Exp Ther. 1997;282:834–838. [PubMed] [Google Scholar]
- 8.Fleckenstein AE, Haughey HM, Metzger RR, Kokoshka JM, Riddle EL, Hanson JE, Gibb JW, Hanson GR. Differential effects of psychostimulants and related agents on dopaminergic and serotonergic transporter function. Eur J Pharmacol. 1999;382:45–49. doi: 10.1016/s0014-2999(99)00588-9. [DOI] [PubMed] [Google Scholar]
- 9.Giros B, el Mestikawy S, Bertrand L, Caron MG. Cloning and functional characterization of a cocaine-sensitive dopamine transporter. FEBS Lett. 1991;295:149–154. doi: 10.1016/0014-5793(91)81406-x. [DOI] [PubMed] [Google Scholar]
- 10.Haughey HM, Brown JM, Wilkins DG, Hanson GR, Fleckenstein AE. Differential effects of methamphetamine on Na+/Cl−-dependent transporters. Brain Res. 2000a;863:59–65. doi: 10.1016/s0006-8993(00)02094-1. [DOI] [PubMed] [Google Scholar]
- 11.Haughey HM, Fleckenstein AE, Metzger RR, Hanson GR. Effects of methamphetamine on serotonin transporter activity: role of dopamine and hyperthermia. J Neurochem. 2000b;75:1608–1617. doi: 10.1046/j.1471-4159.2000.0751608.x. [DOI] [PubMed] [Google Scholar]
- 12.Hotchkiss AJ, Gibb JW. Long-term effects of multiple doses of methamphetamine on tryptophan hydroxylase and tyrosine hydroxylase activity in rat brain. J Pharmacol Exp Ther. 1980;214:257–262. [PubMed] [Google Scholar]
- 13.Huff RA, Vaughan RA, Kuhar MJ, Uhl GR. Phorbol esters increase dopamine transporter phosphorylation and decrease transport Vmax. J Neurochem. 1997;68:225–232. doi: 10.1046/j.1471-4159.1997.68010225.x. [DOI] [PubMed] [Google Scholar]
- 14.Kilty JE, Lorang D, Amara SG. Cloning and expression of a cocaine-sensitive rat dopamine transporter. Science. 1991;254:578–579. doi: 10.1126/science.1948035. [DOI] [PubMed] [Google Scholar]
- 15.Kim S, Westphalen R, Callahan B, Hatzidimitriou G, Yuan J, Ricaurte GA. Toward development of an in vitro model of methamphetamine-induced dopamine nerve terminal toxicity. J Pharmacol Exp Ther. 2000;293:625–633. [PubMed] [Google Scholar]
- 16.Kitamaya S, Dohi T, Uhl GR. Phorbol esters alter functions of the expressed dopamine transporter. Eur J Pharmacol. 1994;268:115–119. doi: 10.1016/0922-4106(94)90180-5. [DOI] [PubMed] [Google Scholar]
- 17.Kogan FJ, Nichols WK, Gibb JW. Influence of methamphetamine on nigral and striatal tyrosine hydroxylase activity and/or striatal dopamine levels. Eur J Pharmacol. 1976;36:363–371. doi: 10.1016/0014-2999(76)90090-x. [DOI] [PubMed] [Google Scholar]
- 18.Kokoshka JM, Metzger RR, Wilkins DG, Gibb JW, Hanson GR, Fleckenstein AE. Methamphetamine treatment rapidly inhibits serotonin, but not glutamate, transporters in rat brain. Brain Res. 1998a;799:79–83. doi: 10.1016/s0006-8993(98)00472-7. [DOI] [PubMed] [Google Scholar]
- 19.Kokoshka JM, Vaughan RA, Hanson GR, Fleckenstein AE. Nature of methamphetamine-induced rapid and reversible changes in dopamine transporters. Eur J Pharmacol. 1998b;361:269–275. doi: 10.1016/s0014-2999(98)00741-9. [DOI] [PubMed] [Google Scholar]
- 20.Liang NY, Rutledge CO. Evidence for carrier-mediated efflux of dopamine from corpus striatum. Biochem Pharmacol. 1982;31:2479–2484. doi: 10.1016/0006-2952(82)90057-0. [DOI] [PubMed] [Google Scholar]
- 21.Logan BJ, Laverty R, Sanderson WD, Yee YB. Differences between rats and mice in MDMA (methylenedioxymethylamphetamine) neurotoxicity. Eur J Pharmacol. 1988;152:227–234. doi: 10.1016/0014-2999(88)90717-0. [DOI] [PubMed] [Google Scholar]
- 22.Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 23.Melikian HE, Buckley KM. Membrane trafficking regulates the activity of the human dopamine transporter. J Neurosci. 1999;19:7699–7710. doi: 10.1523/JNEUROSCI.19-18-07699.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.O'Hearn E, Battaglia G, de Souza EB, Kuhar MJ, Molliver ME. Methylenedioxyamphetamine (MDA) and methylenedioxymethamphetamine (MDMA) cause selective ablation of serotonergic axon terminals in forebrain: immunocytochemical evidence for neurotoxicity. J Neurosci. 1988;8:2788–2803. doi: 10.1523/JNEUROSCI.08-08-02788.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pauwels PJ, Laduron PM. TPP+ accumulation in rat brain synaptosomes as a probe for Na+ channels. Eur J Pharmacol. 1986;132:289–293. doi: 10.1016/0014-2999(86)90618-7. [DOI] [PubMed] [Google Scholar]
- 26.Pristupa ZB, McConkey F, Liu F, Man HY, Lee FJ, Wang YT, Niznik HB. Protein kinase-mediated bi-directional and functional regulation of the human dopamine transporter. Synapse. 1998;30:79–87. doi: 10.1002/(SICI)1098-2396(199809)30:1<79::AID-SYN10>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 27.Ricaurte GA, Guillery RW, Seiden LS, Schuster CT, Moore RY. Dopamine nerve terminal degeneration produced by high doses of methylamphetamine in the rat brain. Brain Res. 1982;235:93–103. doi: 10.1016/0006-8993(82)90198-6. [DOI] [PubMed] [Google Scholar]
- 28.Ricaurte GA, Markowska AL, Wenk GL, Hatzidimitriou G, Wlos J, Olton DS. 3,4-Methylenedioxymethamphetamine, serotonin, and memory. J Pharmacol Exp Ther. 1993;266:1097–1105. [PubMed] [Google Scholar]
- 29.Ramos S, Grollman EF, Lazo PS, Dyer SA, Habig WH, Hardegree MC, Kaback HR, Kohn LD. Effect of tetanus toxin on the accumulation of the permeant lipophilic cation tetraphenylphosphonium by guinea pig brain synaptosomes. Proc Natl Acad Sci USA. 1979;76:4783–4787. doi: 10.1073/pnas.76.10.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Saunders C, Ferrer JV, Shi L, Chen J, Merrill G, Lamb ME, Leeb-Lundberg LMF, Carvelli LC, Javitch JA, Galli A. Amphetamine-induced loss of human dopamine transporter activity: an internalization-dependent and cocaine-sensitive mechanism. Proc Natl Acad Sci USA. 2000;97:6850–6855. doi: 10.1073/pnas.110035297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Scott ID, Nicholls DG. Energy transduction in intact synaptosomes. Influence of plasma-membrane depolarization on the respiration and membrane potential of internal mitochondria determined in situ. Biochem J. 1980;186:21–33. doi: 10.1042/bj1860021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimada S, Kitamaya S, Lin CL, Patel A, Nanthakumar E, Gregor P, Kuhar M, Uhl GR. Cloning and expression of a cocaine-sensitive dopamine transporter complementary DNA. Science. 1991;254:576–578. doi: 10.1126/science.1948034. [DOI] [PubMed] [Google Scholar]
- 33.Stone DM, Merchant KM, Hanson GR, Gibb JW. Immediate and long-term effects of 3,4-methylenedioxymethamphetamine on serotonin pathways in brain of rat. Neuropharmacology. 1987;26:1677–1683. doi: 10.1016/0028-3908(87)90117-1. [DOI] [PubMed] [Google Scholar]
- 34.Sulzer D, Chen TK, Lau YY, Kristensen H, Rayport S, Ewing A. Amphetamine redistributes dopamine from synaptic vesicles to the cytosol and promotes reverse transport. J Neurosci. 1995;15:4102–4108. doi: 10.1523/JNEUROSCI.15-05-04102.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Uhl GR, Hartig PR. Transporter explosion: update on uptake [review]. Trends Pharmacol Sci. 1992;13:421–425. doi: 10.1016/0165-6147(92)90133-q. [DOI] [PubMed] [Google Scholar]
- 36.Vandenbergh DJ, Persico AM, Uhl GR. A human dopamine transporter cDNA predicts reduced glycosylation, displays a novel repetitive element, and provides racially-dimorphic TaqIRFLPs. Brain Res Mol Brain Res. 1992;15:161–166. doi: 10.1016/0169-328x(92)90165-8. [DOI] [PubMed] [Google Scholar]
- 37.Vaughan RA, Huff RA, Uhl GR, Kuhar MJ. Protein kinase C-mediated phosphorylation and functional regulation in striatal synaptosomes. J Biol Chem. 1997;272:15541–15546. doi: 10.1074/jbc.272.24.15541. [DOI] [PubMed] [Google Scholar]
- 38.Wagner GC, Ricaurte GA, Seiden LS, Schuster CR, Miller RJ, Westley J. Long-lasting depletions of striatal dopamine and loss of dopamine uptake sites following repeated administration of methamphetamine. Brain Res. 1980;181:151–160. doi: 10.1016/0006-8993(80)91265-2. [DOI] [PubMed] [Google Scholar]
- 39.Zhang L, Coffey LL, Reith ME. Regulation of the functional activity of the human dopamine transporter by protein kinase C. Biochem Pharmacol. 1997;53:677–688. doi: 10.1016/s0006-2952(96)00898-2. [DOI] [PubMed] [Google Scholar]