Abstract
GABAB receptors are G-protein-coupled receptors that mediate slow synaptic inhibition in the brain and spinal cord. These receptors are heterodimers assembled from GABAB1 and GABAB2 subunits, neither of which is capable of producing functional GABAB receptors on homomeric expression. GABAB1, although able to bind GABA, is retained within the endoplasmic reticulum (ER) when expressed alone. In contrast, GABAB2 is able to access the cell surface when expressed alone but does not couple efficiently to the appropriate effector systems or produce any detectable GABA-binding sites. In the present study, we have constructed chimeric and truncated GABAB1and GABAB2 subunits to explore further GABABreceptor signaling and assembly. Removal of the entire C-terminal intracellular domain of GABAB1 results in plasma membrane expression without the production of a functional GABABreceptor. However, coexpression of this truncated GABAB1subunit with either GABAB2 or a truncated GABAB2 subunit in which the C terminal has also been removed is capable of functional signaling via G-proteins. In contrast, transferring the entire C-terminal tail of GABAB1 to GABAB2 leads to the ER retention of the GABAB2subunit when expressed alone. These results indicate that the C terminal of GABAB1 mediates the ER retention of this protein and that neither of the C-terminal tails of GABAB1or GABAB2 is an absolute requirement for functional coupling of heteromeric receptors. Furthermore although GABAB1 is capable of producing GABA-binding sites, GABAB2 is of central importance in the functional coupling of heteromeric GABAB receptors to G-proteins and the subsequent activation of effector systems.
Keywords: GABAB, GPCR, trafficking, signaling, intracellular retention, G-protein coupling, chimeras, receptor subunits
GABA is the most widely expressed inhibitory neurotransmitter in the mammalian CNS and mediates its actions via both ionotropic (GABAA/C) and metabotropic (GABAB) receptors (Bowery, 1993;Mott and Lewis, 1994; Rabow et al., 1995). GABABreceptors are members of the group 3 (C) family of G-protein-coupled receptors (GPCRs) (for review, see Couve et al., 2000), and the modulation of GABAB receptors is thought to be involved in a number of physiological and disease processes, including nociception, cognitive impairment, epilepsy, and spasticity, and also in the etiology of drug addiction (Bettler et al., 1998). GABAB receptors are unique among the group 3 GPCRs in that they are believed to be heterodimers of GABAB1 and GABAB2 subunits, each of which is unable to form a functional GABAB receptor in its own right (for review, seeMarshall et al., 1999). The heterodimerization of GABAB1b and GABAB2 has been shown to be mediated, at least in part, by interactions between two homologous α-helical coiled-coil domains present in the intracellular C terminals of both GABAB1b and GABAB2 (Kammerer et al., 1999; Kuner et al., 1999). Although the initial papers describing the cloning and expression of GABAB1 reported some functional activity for GABAB1 when expressed alone in mammalian cells (Kaupmann et al., 1997, 1998a), a number of subsequent studies have reported that, when expressed alone, GABAB1 is not able to inhibit adenylate cyclase activity effectively (White et al., 1998; Kuner et al., 1999; Ng et al., 1999), nor can it efficiently couple to K+ channels in either Xenopusoocytes (Jones et al., 1998; Kaupmann et al., 1998b; Ng et al., 1999) or human embryonic kidney (HEK)-293 cells (Jones et al., 1998; Kuner et al., 1999). Furthermore, GABAB1 is unable to inhibit calcium channel activity when injected alone into sympathetic neurons (Couve et al., 1998; Filippov et al., 2000). These findings are at least partly explained by the fact that, when expressed alone in heterologous systems, GABAB1 is not expressed on the cell surface but is retained within intracellular membranes (Couve et al., 1998), and although it is able to bind GABAB ligands, its pharmacological profile with respect to agonist binding is different from that of endogenous receptors (Kaupmann et al., 1997). However, coexpression of GABAB1 with GABAB2 results in the correct trafficking of both subunits to the cell surface as heterodimers and the formation of functional receptors with pharmacology similar to that of GABAB receptorsin vivo (Jones et al., 1998; Kaupmann et al., 1998b; White et al., 1998; Kuner et al., 1999; Ng et al., 1999). Thus the dimerization of GABAB2 with GABAB1 results in an increase in the affinity of the receptor for GABAB agonists, despite the fact that agonists are thought to bind specifically to GABAB1, showing that there is some form of cooperativity in ligand binding between GABAB1and GABAB2 (for review, see Bowery and Enna, 2000).
In this study we have generated a number of C-terminal truncations of GABAB1 and chimeric subunits between GABAB1 and GABAB2 and used these molecules to investigate the roles of the two subunits in the intracellular trafficking and downstream signaling of GABAB receptors.
MATERIALS AND METHODS
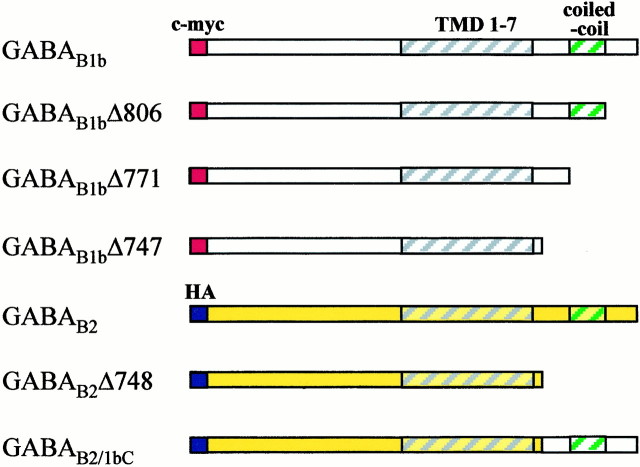
Construction of truncated and chimeric GABAB receptor subunits. A full-length human GABAB1b cDNA was tagged by site-directed mutagenesis (Transformer SDM kit; Clontech, Cambridge, UK) with thec-myc epitope (EQKLISEEDL) recognized by the 9E10 mouse monoclonal antibody (Roche Diagnostics). The tag was introduced after amino acid 35 of the nascent protein. GABAB2 was hemagglutinin (HA)-tagged (AAAYPYDVPDYA; recognized by 3F10 rat monoclonal antibody; Roche Diagnostics) after amino acid 42 of the nascent protein. Both full-length cDNAs were cloned into pcDNA3.1 (Invitrogen, San Diego, CA). GABAB1b and GABAB2deletion mutants and chimeras were generated by PCR from these full-length, tagged subunit cDNAs. All the PCR primers used hadHindIII restriction enzyme sites engineered into them to facilitate cloning and are described in Table1. GABAB1bΔ806 and GABAB1bΔ771 were amplified in single PCR reactions and cloned into pcDNA3.1/V5-His. GABAB1bΔ747 and GABAB2Δ748 were generated by PCR and cloned into pCMV-5 (Stratagene, La Jolla, CA). GABAB2/1bC was generated by ligation of GABAB2Δ748 and GABAB1b(750–844) and cloned into pcDNA3.1 (Invitrogen). GABAB1b/2C was generated by ligation of GABAB1Δ747 and GABAB2(751–941) and cloned into pcDNA3.1. The truncated and chimeric receptor subunits are all shown schematically in Figure 1. All experiments were performed using epitope-tagged wild-type, deletion, and chimeric receptor subunits unless otherwise stated.
Table 1.
Forward and reverse primers used to amplify truncated and chimeric GABAB receptor subunits
| PCR product | Forward primer (5′–3′) | Reverse primer (5′–3′) |
|---|---|---|
| GABAB1bΔ806 | CCCGAATTCATGGGGC | TTAGAGCTGCTGCCGAGA |
| CCGGGGCC | CTGGAGTTG | |
| GABAB1bΔ771 | CCCGAATTCATGGGGC | TTACTTCTCCTCCTCGTTG |
| CCGGGGCC | TTGTTGGTC | |
| GABAB1bΔ747 | CCCGAATTCATGGGGC | CCCAAGCTTCCTCGGGTG |
| CCGGGGCC | ATCAGCCTG | |
| GABAB1b(750–844) | CCCAAGCTTGCAGTCG | CCCCTCGAGTCACTTATA |
| GAGGCGCAGG | AAGCAAATGCACTCGAC | |
| GABAB2Δ748 | CCCGAATTCATGGCTTC | CCCAAGCTTCTCAGGGTG |
| CCCGCGGAGC | ATGAGCTTCGGC | |
| GABAB2(751–941) | CCCAAGCTTCCCAGAT | CCCCTCGAGTTACAGGCC |
| GCAGCAACGCAGAAC | CGAGACCATGACTC |
Fig. 1.
GABAB receptor subunit truncations and chimeras used in transfection experiments. c-myc and HA are epitope tags for immunocytochemistry and Western blotting. TMD1–7 are the seven transmembrane domains; coiled-coil is the C-terminal domain implicated in the interaction between GABAB1b and GABAB2.
Culture and transfection of HEK-293 cells. All cell culture reagents were obtained from Life Technologies, Paisley, UK. HEK-293 cells were maintained in DMEM supplemented with 10% fetal calf serum and 1% nonessential amino acids. Exponentially growing cells were transfected using Lipofectamine Plus (Life Technologies) according to the manufacturer's instructions and then incubated for 24 hr to allow for protein expression before analysis.
Antibodies and immunofluorescence. A rabbit antiserum specific for GABAB1b was raised against the peptide CHSPHLPRPHPRVPPHPS (amino acids 31–47) and affinity purified as described previously (Calver et al., 2000). The rabbit antiserum specific for GABAB2 was raised against a GST fusion protein, which contained the entire intracellular C terminal of the rat GABAB2 protein (amino acids 745–941), and has been described previously (Calver et al., 2000).
For immunocytochemistry, cells on glass coverslips were fixed with 4% paraformaldehyde for 5 min and then either permeabilized with 0.1% Triton X-100 for 10 min or washed with PBS. Cells were incubated in primary antibody (anti c-myc or anti-HA; 1:5000 in PBS; 60 min), washed in PBS, and then incubated in goat anti-mouse IgG-FITC (for antic-myc) or goat anti-rat IgG-FITC (for anti-HA; both obtained from Sigma, Poole, UK, and used at 1:100 in PBS; 45 min). Cells were then washed in PBS, mounted in Citifluor (Citifluor, London, UK), and viewed using a Leica laser-scanning confocal microscope.
Immunoprecipitation and Western blotting. Crude membranes from rat whole brain were prepared as described previously (Benke et al., 1999). Transiently transfected HEK-293 cells were lysed with ice-cold 1% (v/v) Triton X-100 including protease inhibitors (protease inhibitor cocktail tablets; Roche Diagnostics). After centrifugation the lysate supernatants were precleared with normal rabbit serum and then incubated overnight with anti-GABAB1bantibody (5 μg). Antigen–antibody complexes were immunoprecipitated with Protein A–Sepharose, washed extensively in PBS containing Triton X-100, and then resuspended in sample buffer containing 2% (v/v) 2-mercaptoethanol and boiled for 5 min. Eluted proteins were resolved by discontinuous SDS-PAGE and transferred to a polyvinylidene difluoride membrane using a semidry transfer system (Bio-Rad, Hercules, CA). The membranes were blocked with 5% nonfat milk in PBS and 0.05% Tween 20 and then incubated overnight with anti-GABAB2 antibody at 0.1 μg/ml in blocking solution. Immunoreactive bands were detected with a goat anti-rabbit antibody conjugated to horseradish peroxidase followed by chemiluminescence detection (ECL; Amersham).
Radioligand binding. Transfected cells were homogenized in ice-cold 50 mm Tris-HCl and 2.5 mmMgCl2 buffer, pH 7.4, using a Kinematic Ultra-Turrax homogenizer. The homogenates were then centrifuged at 35,000 × g for 15 min at 4°C. Membrane pellets were resuspended in the buffer and homogenized and centrifuged as before. The final membrane pellet was resuspended in buffer and stored at −80°C until required. Binding assays consisted of 50 μl of displacing compound or buffer, 400 μl of membrane suspension (corresponding to ∼30 μg of protein/well), and 50 μl of [3H]CGP-54626 (specific activity, 40 Ci/mmol). In competition binding experiments, 10 concentrations of the competing ligands were tested, at a final [3H]CGP-54626 concentration of 2 nm. Nonspecific binding was defined using 1 mm GABA or 10 μmCGP-62349. The experiments were terminated by rapid filtration over Whatman GF/B glass fiber filters, presoaked with 0.3% (v/v) polyethyleneimine, and washed with 6 ml of ice-cold 50 mm Tris-HCl buffer. Radioactivity was determined by liquid scintillation spectrometry using a Packard 2700 liquid scintillation counter. The concentration of GABA inhibiting specific [3H]CGP-54626 binding by 50% (IC50) was determined by iterative curve fitting using a four-parameter logistic fit (Grafit, Erithacus Software). pKi values (−log of the inhibition constant) were then calculated from the IC50 values by the method described by Cheng and Prusoff (1973); theKD had been determined previously in the present system and was 4.2 ± 0.8 nm(data not shown).
Calcium mobilization assay. Transfected cells were seeded into black-walled 96-well plates (Corning Costar Ltd.) at a density of 30,000 cells/well and incubated at 37°C in 5% CO2 for 24 hr before use. Cells were loaded with media containing 4 μm Fluo-3 (Molecular Probes, Eugene, OR), a Ca2+-sensitive dye, in the presence of 2.5 mm probenecid and incubated for 60 min at 37°C in 5% CO2. Cells were then washed four times with 125 μl of modified Tyrode's buffer (145 mm NaCl, 2.5 mm KCl, 10 mm HEPES, 10 mm glucose, 1.2 mm MgCl2, 2.5 mmprobenecid, and 0.15 mm CaCl2) and then incubated in 150 μl of the same buffer for 20 min at 37°C in 5% CO2. Agonist was added, and the resulting intracellular calcium mobilization was recorded using a fluorimetric-imaging plate reader (FLIPR; Molecular Devices, Palo Alto, CA). Peak fluorescence was determined for each agonist addition, and the data were iteratively curve-fitted using a four-parameter logistic model (Bowen and Jerman, 1995). In addition to HEK-293 cells, all of the functional data presented here have been repeated and confirmed in another cell line, Chinese hamster ovary (CHO)-K1 cells (data not shown).
RESULTS
Intracellular retention of GABAB1 is mediated by the coiled-coil motif within the C terminal of the protein
Using PCR amplification from a full-length, myc-tagged GABAB1 cDNA, we constructed a number of truncated coding sequences for this subunit and subcloned them into mammalian expression vectors, as described in Materials and Methods and Table 1. The first of these, GABAB1bΔ806, was a truncation removing the most distal part of the C terminal, up to the end of the coiled-coil domain shown previously to be involved in the interaction with GABAB2 (Kammerer et al., 1999;Kuner et al., 1999). The second truncation (GABAB1bΔ771) was similar but removed an additional 35 amino acids covering the GABAB2-interacting stretch of the coiled-coil domain. The final truncation of GABAB1b(GABAB1bΔ747) removed the entire intracellular C terminal except the four amino acids downstream of the putative seventh transmembrane domain (Fig. 1). As expected, when transfected into HEK-293 cells in isolation, GABAB1b was not detected by immunocytochemistry on the cell surface but could only be visualized after permeabilization of the cells with detergent (Fig.2A,B) (Couve et al., 1998). Similarly, GABAB1bΔ806 was not expressed on the cell surface but could be detected after membrane disruption with Triton X-100 (Fig. 2C,D). In contrast however, GABAB1bΔ771, which lacks the coiled-coil domain shown previously to interact with GABAB2, was readily detectable by immunofluorescence on the cell surface of intact transfected cells, as well as after permeabilization (Fig.2E,F). In addition, the truncated GABAB1b subunit lacking the entire C terminal, GABAB1bΔ747, was also expressed on the cell surface at levels similar to those obtained in cotransfection experiments with GABAB2 (Fig.2G,H). Thus the normal intracellular retention of GABAB1b in the absence of GABAB2 is mediated via the putative α-helical coiled-coil motif in the same region in which the GABAB1b–GABAB2 interaction occurs.
Fig. 2.
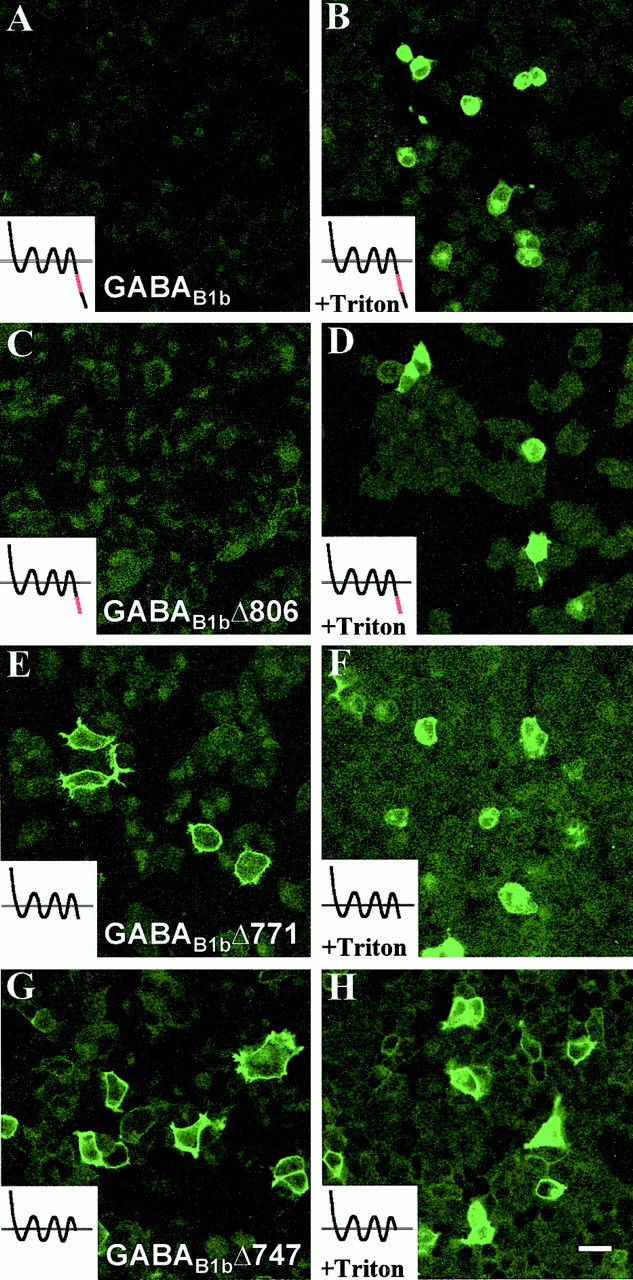
Intracellular retention of GABAB1b is mediated by the coiled-coil motif within the C terminal of the protein.A–H, HEK-293 cells were transiently transfected with constructs encoding either the full-length GABAB1b(A, B) or progressive deletions of GABAB1b(C–H). All of the full-length and truncated subunits were tagged with a c-myc epitope recognized by the 9E10 antibody. Transfected cells were examined after 24 hr by immunofluorescence using the 9E10 antibody either with (B, D, F, H) or without (A, C, E, G) permeabilization. GABAB1b and GABAB1bΔ806 are both retained intracellularly (A–D), whereas GABAB1bΔ771 and GABAB1bΔ747 are both expressed on the cell surface (E–H).Insets, Diagrams of the truncations are shown, with the coiled-coil region necessary for receptor dimerization indicated inred. All of the transfections were performed and analyzed at least three times, and the results with each construct were consistent. Scale bar, 10 μm.
The C-terminal domain of GABAB1 is sufficient to sequester GABAB2 within the cell
We were therefore interested in whether the intracellular C-terminal tail of GABAB1b was able to redirect the normally cell surface-expressed GABAB2subunit to intracellular membranes. To investigate this, we replaced the entire intracellular tail of GABAB2 (amino acids 751–941) with the equivalent domain of GABAB1b (amino acids 750–844) to generate the chimeric subunit GABAB2/1bC (Fig. 1). As a control, we also generated a truncated GABAB2subunit that lacked the entire intracellular tail (GABAB2Δ748; Fig. 1). When expressed alone in transient transfections, full-length GABAB2is transported to and detectable by immunocytochemistry both on the cell surface (Fig. 3A) and intracellularly (Fig. 3B), as has been described previously (Martin et al., 1999). Similarly, our deletion mutant GABAB2Δ748 could be detected readily in transfected cells both with and without detergent permeabilization and with the same cellular distribution as its full-length counterpart (Fig. 3C,D). However, when the chimeric receptor subunit GABAB2/1bC was expressed transiently in HEK-293 cells, although intracellular expression was seen after permeabilization, there was no evidence of transport of the subunit to the plasma membrane (Fig. 3E,F). When this chimera was coexpressed transiently with wild-type GABAB2, however, this intracellular retention was overcome, and the chimeric GABAB2/1bC was transported to the cell surface (Fig. 3G,H). The full-length GABAB2 used for this cotransfection was not tagged with the HA epitope, so the immunofluorescence seen using the anti-HA antibody 3F10 on unpermeabilized cells was specific to the HA-tagged GABAB2/1bC.
Fig. 3.
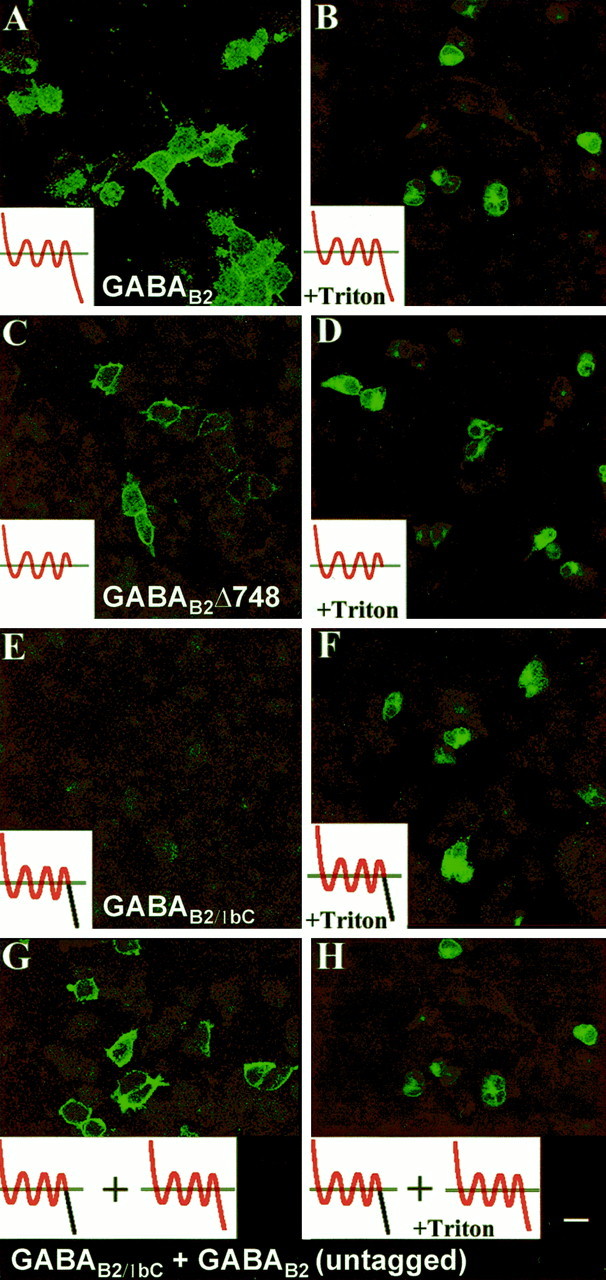
The C-terminal domain of GABAB1bis sufficient to sequester GABAB2 within the cell.A–H, HEK-293 cells were transfected with constructs encoding either the full-length GABAB2 (A, B), GABAB2Δ748 (C, D), GABAB2/1bC (E, F), or GABAB2/1bC + GABAB2 (G, H). All of the full-length and truncated subunits were tagged with an HA epitope recognized by the 3F10 antibody, except for the full-length GABAB2 used in the cotransfection (G, H) that was not epitope-tagged. Transfected cells were examined after 24 hr by immunofluorescence using the 3F10 antibody either with (B, D, F, H) or without (A, C, E, G) permeabilization. Both full-length GABAB2 and GABAB2Δ748 when transfected alone are expressed on the cell surface (A–D), whereas GABAB2/1bC is retained within the cell (E, F). The HA-tagged GABAB2/1bC is able to reach the cell surface and be detected by anti-HA, however, when coexpressed with the untagged GABAB2 (G, H). Insets, Diagrams of the truncations and chimeras are shown; GABAB2 sequences are shown inred, whereas GABAB1b sequences are shown inblack. All of the transfections were performed and analyzed at least three times, and the results with each construct were consistent. Scale bar, 10 μm.
C-terminally truncated GABAB1b subunits are able to bind GABA
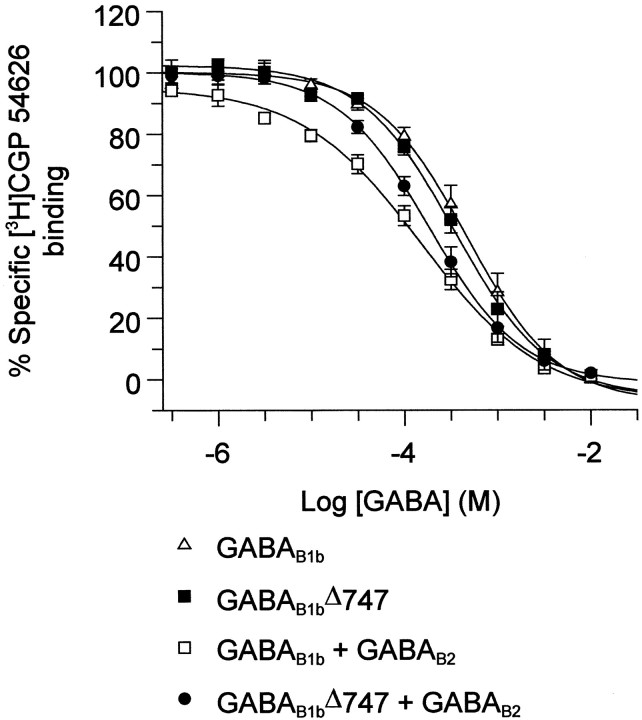
We investigated the effect of C-terminal truncation and subsequent cell surface localization of the GABAB1bΔ747 subunit on the ability of the subunit to bind GABA. Removal of the intracellular C terminal of GABAB1b had no effect on the ability of GABA to displace the antagonist CGP-54626 in competition binding assays. Furthermore, the potency of GABA at this truncated receptor subunit was not significantly different from its potency at the full-length GABAB1b subunit (Fig.4) (p < 0.001, one-way ANOVA with post hoc t test). As has been reported previously (for review, see Bowery and Enna, 2000), coexpression of GABAB1b with GABAB2resulted in a significant increase in the potency of GABA binding compared with GABA binding at GABAB1b alone, and such a shift in potency was also observed when the truncated GABAB1b subunit was coexpressed with GABAB2 (Fig. 4) (p < 0.001, one-way ANOVA with post hoc t test).
Fig. 4.
Removal of the C terminal of GABAB1bdoes not affect ligand binding. Cells transiently transfected with either GABAB1b alone, GABAB1b + GABAB2, GABAB1bΔ747 alone, or GABAB1bΔ747 + GABAB2 specifically bound [3H]CGP-54626, and this could be completely displaced by GABA (10 mm), with pKi values of 3.60 ± 0.13, 4.14 ± 0.05, 3.70 ± 0.10, and 4.00 ± 0.09, respectively. Data are expressed as means ± SEM (n = 4–6). All receptor subunits were epitope-tagged as described in Materials and Methods.
C-terminally truncated GABAB1b subunits are nonfunctional when expressed alone but couple to G-proteins when coexpressed with GABAB2
To determine whether cell surface expression of the GABAB1b subunit was sufficient to form a functional GABAB receptor, we tested the ability of C-terminally truncated GABAB1b to activate G-proteins in response to GABA. Although GABABreceptors are known to inhibit adenylate cyclase via their interaction with Gi, we cotransfected the chimeric G-protein Gqi5 (Conklin et al., 1993) in transient transfections so that stimulation of a GABABreceptor would activate the phospholipase C pathway, resulting in mobilization of intracellular calcium, which could then be measured on a FLIPR. Coexpression of GABAB1b and GABAB2 together with Gqi5 in HEK-293 cells resulted in robust calcium mobilization in response to agonist, whereas coexpression of GABAB1b and Gqi5 gave no response in this functional assay, even at a GABA concentration of 0.1 mm (Fig. 5A). When we tested the cell surface-expressed C-terminally truncated GABAB1bΔ747 with Gqi5 in this system, it also exhibited no functional coupling to the G-protein when expressed on its own (Fig. 5A).
Fig. 5.
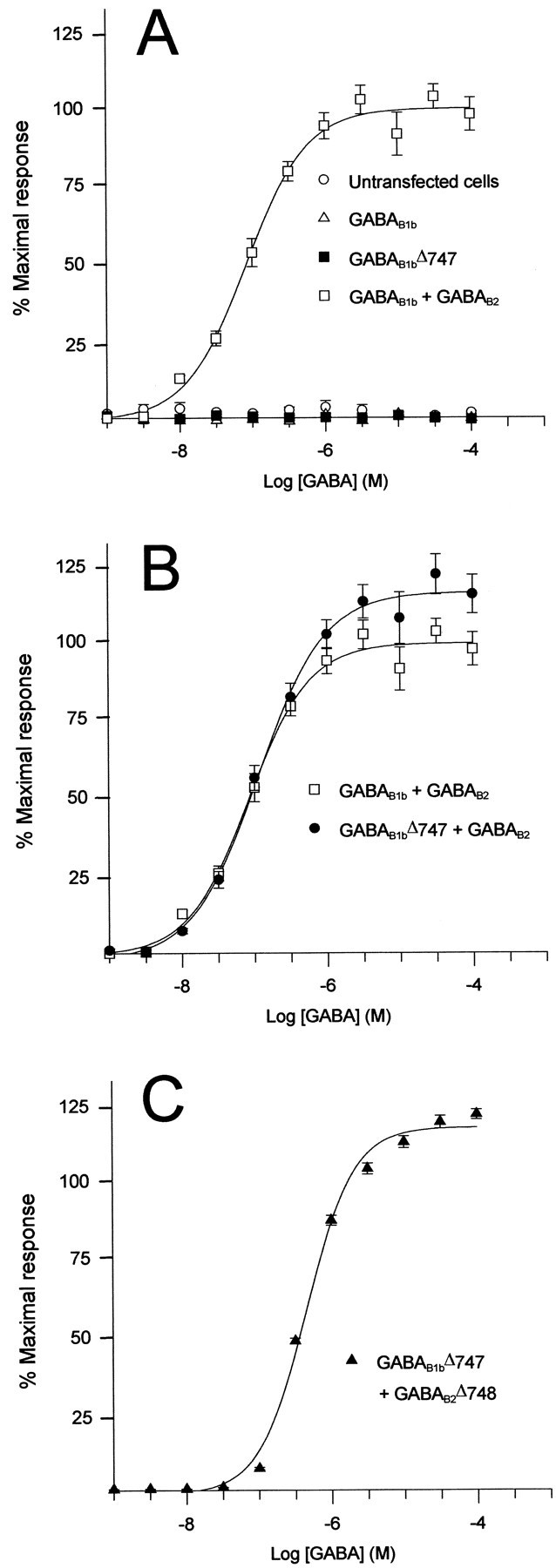
GABAB1b subunits lacking the intracellular C terminal are nonfunctional, whereas heterodimers formed between the C-terminally truncated GABAB1 and either full-length or C-terminally truncated GABAB2 signal via G-proteins. HEK-293 cells were cotransfected with the expression constructs shown together with the chimeric G-protein Gqi5 and assayed for intracellular Ca2+ mobilization in response to GABA stimulation in a FLIPR. A, No response was observed from mock-transfected cells or from cells transfected with GABAB1b or GABAB1bΔ747 on their own, whereas a robust functional response was seen when GABAB1b was cotransfected with GABAB2(pEC50 = 7.08 ± 0.02). B, A similar response was seen when GABAB2 was cotransfected with GABAB1bΔ747 or when GABAB2 was cotransfected with GABAB1b [pEC50(GABAB1b + GABAB2) = 7.08 ± 0.02; pEC50 (GABAB1bΔ747 + GABAB2) = 6.93 ± 0.05].C, A functional GABAB receptor was also detected in FLIPR when GABAB1bΔ747 was cotransfected with GABAB2Δ748 and Gqi5 (pEC50= 6.34 ± 0.01). The data in A–C are taken from a single representative experiment. All receptor subunits were epitope-tagged as described in Materials and Methods.
We next tested the effect of coexpression of the GABAB2 subunit with the C-terminally truncated GABAB1b on the coupling of the receptor to the chimeric G-protein Gqi5. Both the full-length GABAB1b subunit and the C-terminally truncated GABAB1bΔ747, when coexpressed with GABAB2, were capable of activating Gqi5 and activating the downstream phospholipase C pathway (Fig. 5B). The functional response of the truncated GABAB1bwith GABAB2, as measured by the EC50 in response to GABA, was not significantly different from that of the wild-type GABAB1bsubunit expressed with GABAB2[pEC50 (GABAB1b + GABAB2) = 7.08 ± 0.02; pEC50 (GABAB1bΔ747 + GABAB2) = 6.93 ± 0.05;n = 4–6]. In addition to HEK-293 cells, all of the functional experiments presented here have also been performed in another cell line, CHO-K1 cells, with qualitatively identical results.
Functional coupling of the GABAB receptor to G-proteins requires neither the C-terminal of GABAB1b nor the C-terminal of GABAB2
Because we had shown that the C-terminal domain of GABAB1b was not necessary for GABAB receptor heterodimers to couple functionally to Gqi5, we investigated the importance of the C-terminal of GABAB2 with respect to the correct functionality of the receptor. We again performed the calcium mobilization assay experiments on the FLIPR, using cells transiently transfected with the C-terminally truncated GABAB1bΔ747, a C-terminally truncated GABAB2 subunit (GABAB2Δ748), and Gqi5. This also resulted in the expression of a receptor complex capable of coupling to Gqi5 (Fig.5C). The EC50 of this response was significantly lower than that for the wild-type receptor (p < 0.001, F test), although it is unclear whether this reflects genuine differences in the ability of the mutant receptor subunits to couple to G-proteins in a nontransient system [pEC50(GABAB1bΔ747 + GABAB2Δ748) = 6.34 ± 0.01].
The C-terminal interaction between GABAB1 and GABAB2 is not necessary for the formation of heterodimers
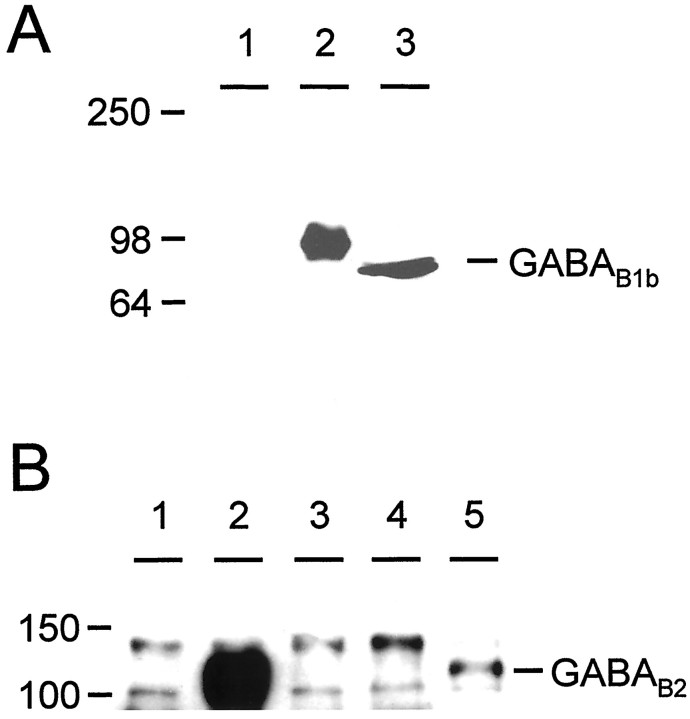
It has been well documented that GABAB1b and GABAB2 form heterodimers, both in transfected cells and in native tissues, and that the only reported region of dimerization is between the α-helical coiled-coil motifs present in the C terminals of the two subunits (Kammerer et al., 1999; Kuner et al., 1999). However we have demonstrated here that this interaction is not necessary for a functional response of GABABreceptor heterodimers. We performed immunoprecipitation experiments to investigate further whether this coiled-coil interaction was indeed necessary for heterodimerization of GABAB1b and GABAB2 subunits. We raised antibodies in rabbits against an N-terminal peptide of GABAB1b (see Materials and Methods) that recognized single bands on Western blots both with cells transfected with GABAB1b and with brain membranes (Fig.6A). We did consistently observe a small difference in size between the human tagged recombinant GABAB1b and the rat brain-derived GABAB1b, but we would suggest that this may reflect differences in expression and post-translational modification between the recombinant human and endogenous rat receptor subunits. Both these bands could be specifically blocked by preincubation of the antiserum with the immunizing peptide (data not shown). After immunoprecipitation with anti-GABAB1b and immunodetection on Western blots with anti-GABAB2 (Calver et al., 2000), heterodimers could be readily detected in membrane preparations from cells transfected with both full-length subunits together but not in cells transfected with either GABAB1bΔ748 or GABAB2 alone (Fig. 6B). However, when GABAB2 was transiently coexpressed with GABAB1b lacking a C terminal, a band corresponding to GABAB2 was detected after immunoprecipitation with anti-GABAB1b and immunoblotting with anti-GABAB2 antibodies (Fig.6B). Although this band is less intense than that observed after coexpression of the two full-length subunits, it nevertheless indicates that GABAB1b and GABAB2 are capable of forming heterodimers in the absence of the C-terminal coiled-coil interaction. Other bands were also observed in all lanes above and below those corresponding to GABAB2, but these were also observed in untransfected cells (Fig. 6B, lane 1) and thus represent nonspecific bands unrelated to the transfected GABAB cDNAs.
Fig. 6.
Heterodimers between GABAB1b and GABAB2 form in the absence of the C-terminal coiled-coil interaction. A, Western blot to show specificity of anti-GABAB1b antibody. Single bands are observed in lanes containing either rat brain membranes (lane 3) or cells transfected with GABAB1b(lane 2); no bands are observed in untransfected cells (lane 1). B, Immunoprecipitation from transfected cell membranes with anti-GABAB1b antibody followed by Western blotting with anti-GABAB2. HEK-293 cell membranes were prepared from mock-transfected cells (lane 1) and from cells transiently transfected with GABAB1b + GABAB2 (lane 2), GABAB1bΔ748 (lane 3), GABAB2(lane 4), or GABAB1bΔ748 + GABAB2 (lane 5). This experiment clearly demonstrates an interaction between GABAB1bΔ748 and GABAB2 (lane 5), in addition to the strong interaction between GABAB1b and GABAB2(lane 2). Numbers on the leftindicate the position of molecular weight markers, expressed in kilodaltons.
DISCUSSION
We have shown in this study that the C-terminal intracellular domain of the GABAB1b receptor subunit is responsible for the retention of the subunit within the cell when expressed in the absence of GABAB2. When this domain is removed from the GABAB1b subunit, the truncated GABAB1b is trafficked to the cell surface independently of GABAB2. We have also shown that the C-terminal stretch of 35 amino acids responsible for intracellular retention is the same domain that mediates the interaction etween GABAB1b and GABAB2 and is situated within the putative “coiled-coil” domain between amino acids 771 and 806 of GABAB1b. In addition, the C-terminal domain of GABAB1b is sufficient to cause the normally cell surface-expressed GABAB2 to be retained within the cell, when it is exchanged for the equivalent region of the native GABAB2 (chimera GABAB2/1bC). There are a number of other examples in which the C terminal of a receptor or ion channel is responsible for its intracellular retention in the absence of accessory molecules (McIlhinney et al., 1998; Zerangue et al., 1999). For example, the ionotropic NMDA receptor subunit NR1 is retained within cells in the absence of the NR2 subunit (McIlhinney et al., 1998). This intracellular retention appears to be mediated by the C terminal of the NR1 protein, because splice variants in this region are able to reach the cell surface in the absence of NR2 (Okabe et al., 1999). In addition, GPCRs for which the C terminal appears to be important for targeting to the plasma membrane include the metabotropic glutamate receptors (Stowell and Craig, 1999; Chan et al., 2000) and the serotonin receptor 5-HT1B (Jolimay et al., 2000).
The intracellular retention of the chimeric GABAB2/1bC can be overcome by the coexpression of GABAB2/1bC with the full-length GABAB2, presumably as a result of the interaction between the two C terminals. These data suggest that the coiled-coil interaction between GABAB2 and GABAB1b competes with a similar coiled-coil interaction between GABAB1b and another unknown, presumably ER-associated protein, which in the absence of GABAB2 mediates the retention of the GABAB1b subunit within the ER. This would not be surprising because coiled-coil motifs are secondary structures present in a large number of proteins and have been implicated in the formation of several multimeric protein complexes (Lupas, 1996).
It is known that expression of GABAB1b alone results in the formation of nonfunctional GABABreceptors, at least in part because of the fact that when expressed alone it is not present on the cell surface (Couve et al., 1998). However, cell surface expression of GABAB1 alone is not sufficient to form a functional GABABreceptor, as demonstrated by a recent study in which coexpression of metabotropic glutamate receptor 4 (mGluR4) in Xenopusoocytes resulted in cell surface expression of GABAB1, although not as a GABAB1–mGluR4 heterodimer (Sullivan et al., 2000). In this system the GABAB1 subunit was unable to couple to members of the Kir3.0 family of potassium channels or to couple negatively to adenylate cyclase. The results we present here support these findings, because the C-terminally truncated cell surface-expressed GABAB1b subunit, when expressed alone, binds GABA but is unable to couple functionally to the chimeric G-protein Gqi5. It is only by coexpressing the truncated GABAB1b with GABAB2 that we were able to reconstitute a functional G-protein-coupled receptor. Interestingly we also detected a functional receptor when we coexpressed the C-terminally truncated GABAB1bwith C-terminally truncated GABAB2. This suggests that neither of the intracellular C terminals of GABAB1b or GABAB2 is necessary for the coupling of the GABAB receptor to its second messenger system, although we cannot exclude the possibility that the functionality of the receptor is altered in a more subtle manner by such deletions. This is consistent with the findings of Gomeza et al. (1996), who demonstrated that although all of the intracellular domains of mGluR1 play a role in G-protein coupling, none of them apart from intracellular loop two is absolutely required for the activation and downstream signaling of this receptor.
The data presented here also suggest that it may be the GABAB2 subunit that binds to G-proteins and subsequently initiates the downstream signaling cascades. Alternatively, it may be that the intracellular loops of GABAB1b are in fact the important regions for G-protein coupling and that the presence of GABAB2 is required for GABAB1b to assume the correct tertiary structure to mediate this interaction. However, when one compares the sequences of the intracellular loops of GABAB2 with those of GABAB1 and the other seven transmembrane receptors that are known to bind and couple to G-proteins, it is not unreasonable to suggest that the amino acids present in the GABAB2subunit intracellular loops render it a more attractive candidate for G-protein coupling than do the corresponding residues present in GABAB1.
As well as trafficking and G-protein coupling, our data have novel implications for the heterodimerization of GABABreceptors, which until now has been thought to be solely mediated by the C-terminal coiled-coil interaction between GABAB1b and GABAB2. In this study, we have shown that when the intracellular tail of GABAB1b is removed, the resulting truncated subunit is still capable of forming functional heterodimers with GABAB2, although the efficiency of the heterodimerization is reduced. This demonstrates that other sequences exist within the two GABAB subunits that must be capable of interacting with each other. It is unclear from the data presented here whether this interaction occurs within the extracellular N terminals of the subunits or alternatively within the transmembrane domains, but further truncation and chimera experiments are in progress to identify such interactions. Other GPCRs have been shown to heterodimerize, such as the κ and δ opioid receptors (Jordan and Devi, 1999) and the somatostatin (SST5) and dopamine (D2) receptors (Rocheville et al., 2000), in the absence of C-terminal coiled-coil domains. Such receptors must therefore use other protein–protein interactions to form dimers. Interestingly, the interaction between SST5 and D2 was demonstrated recently by the functional rescue of an inactive C-terminally truncated SST5 receptor by a full-length D2 receptor, demonstrating that a C-terminal interaction between these two receptors is not necessary for the formation of heterodimers (Rocheville et al., 2000).
In summary, our data support a model in which the GABAB1b subunit, when expressed alone, is retained within the cell by protein–protein interactions between its C-terminal coiled-coil domain and, presumably, components of the ER. In the presence of GABAB2, the equivalent coiled-coil domain in the C terminal of GABAB2competes for these interactions, and thus the intracellular retention of GABAB1b is overcome, and the subunit is able to reach the cell surface. In addition, the elements of the GABAB receptor responsible for G-protein coupling are probably not present within the C terminal of either the GABAB1b or the GABAB2subunit and may indeed lie within the intracellular domains of GABAB2.
Notes added in proof. Since the submission of this manuscript, two papers have appeared in press that confirm a number of our observations. First, Margeta-Mitrovic et al. (2000) have reported the presence of an intracellular retention motif in the C terminal of GABAB1, although, in contrast to the data shown in our paper, they also report that the C-terminal coiled-coil interaction between GABAB1 and GABAB2 is necessary to form a functional GABAB receptor. Second, Schwartz et al. (2000)have identified a splice variant of GABAB1,termed GABAB1e, consisting of just the extracellular N terminal of GABAB1. This subunit is capable of forming (nonfunctional) heterodimers with GABAB2, confirming that the coiled-coil interaction is not necessary for the heterodimerization of GABAB receptors. The data presented here both confirm and extend these observations.
Footnotes
A.C. and S.J.M. are supported by the Wellcome Trust and the Medical Research Council.
A.R.C. and M.J.R. contributed equally to this work.
Correspondence should be addressed to Dr. Andrew Calver, Department of Neuroscience Research, SmithKline Beecham Pharmaceuticals, New Frontiers Science Park, Third Avenue, Harlow, Essex CM19 5AW, United Kingdom. E-mail: Andrew_R_Calver@sbphrd.com.
REFERENCES
- 1.Benke D, Honer M, Michel C, Bettler B, Mohler H. Gamma-aminobutyric acid type B receptor splice variant proteins GBR1a and GBR1b are both associated with GBR2 in situ and display differential regional and subcellular distribution. J Biol Chem. 1999;274:27323–27330. doi: 10.1074/jbc.274.38.27323. [DOI] [PubMed] [Google Scholar]
- 2.Bettler B, Kaupmann K, Bowery N. GABAB receptors: drugs meet clones. Curr Opin Neurobiol. 1998;8:345–350. doi: 10.1016/s0959-4388(98)80059-7. [DOI] [PubMed] [Google Scholar]
- 3.Bowen WP, Jerman JC. Nonlinear regression using spreadsheets. Trends Pharmacol Sci. 1995;16:413–417. doi: 10.1016/s0165-6147(00)89091-4. [DOI] [PubMed] [Google Scholar]
- 4.Bowery NG. GABAB receptor pharmacology. Annu Rev Pharmacol Toxicol. 1993;33:109–147. doi: 10.1146/annurev.pa.33.040193.000545. [DOI] [PubMed] [Google Scholar]
- 5.Bowery NG, Enna SJ. Gamma-aminobutyric acid (B) receptors: first of the functional metabotropic heterodimers. J Pharmacol Exp Ther. 2000;292:2–7. [PubMed] [Google Scholar]
- 6.Calver AR, Medhurst AD, Robbins MJ, Charles KJ, Evans ML, Harrison DC, Stammers M, Hughes SA, Hervieu G, Couve A, Moss SJ, Middlemiss DM, Pangalos MN. mRNA and protein distribution of GABAB receptor splice variants in human and rodent central nervous system and peripheral tissues. Neuroscience. 2000;100:155–170. doi: 10.1016/s0306-4522(00)00262-1. [DOI] [PubMed] [Google Scholar]
- 7.Chan WY, Ciruela F, Soloviev MM, McIlhinney RA. The C-terminal domain of the mGluR receptors mGluR1A and mGluR1B regulate their intracellular targetting. Eur J Neurosci. 2000;12:462. [Google Scholar]
- 8.Cheng Y, Prusoff WH. Relationship between the inhibition constant (Ki) and the concentration of inhibitor which causes 50 percent inhibition (IC50) of an enzymatic reaction. Biochem Pharmacol. 1973;22:3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- 9.Conklin BR, Farfel Z, Lustig KD, Julius D, Bourne HR. Substitution of three amino acids switches receptor specificity of Gq alpha to that of Gi alpha. Nature. 1993;363:274–276. doi: 10.1038/363274a0. [DOI] [PubMed] [Google Scholar]
- 10.Couve A, Filippov AK, Connolly CN, Bettler B, Brown DA, Moss SJ. Intracellular retention of recombinant GABAB receptors. J Biol Chem. 1998;273:26361–26367. doi: 10.1074/jbc.273.41.26361. [DOI] [PubMed] [Google Scholar]
- 11.Couve A, Moss SJ, Pangalos MN. GABAB receptors: a new paradigm in G protein signalling. Mol Cell Neurosci. 2000;16:296–312. doi: 10.1006/mcne.2000.0908. [DOI] [PubMed] [Google Scholar]
- 12.Filippov AK, Couve A, Pangalos MN, Walsh FS, Brown DA, Moss SJ. Heteromeric assembly of GABAB1 and GABAB2 receptor subunits inhibits Ca2+ current in sympathetic neurons. J Neurosci. 2000;20:2867–2874. doi: 10.1523/JNEUROSCI.20-08-02867.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gomeza J, Joly C, Kuhn R, Knopfel T, Bockaert J, Pin J-P. The second intracellular loop of metabotropic glutamate receptor 1 cooperates with the other intracellular domains to control coupling to G-proteins. J Biol Chem. 1996;271:2199–2205. doi: 10.1074/jbc.271.4.2199. [DOI] [PubMed] [Google Scholar]
- 14.Jolimay N, Louis F, Langlois X, Hamon M, Darmon M. The cytosolic C-terminal domain of the rat 5-HT1B receptor possesses an axonal/apical targetting signal. Eur J Neurosci. 2000;12:409. doi: 10.1523/JNEUROSCI.20-24-09111.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones KA, Borowsky B, Tamm JA, Craig DA, Durkin MM, Dai M, Yao WJ, Johnson M, Gunwaldsen C, Huang LY, Tang C, Shen Q, Salon JA, Morse K, Laz T, Smith KE, Nagarathnam D, Noble SA, Branchek TA, Gerald C. GABAB receptors function as a heteromeric assembly of the subunits GABAB1 and GABAB2. Nature. 1998;396:674–679. doi: 10.1038/25348. [DOI] [PubMed] [Google Scholar]
- 16.Jordan BA, Devi LA. G-protein-coupled receptor heterodimerization modulates receptor function. Nature. 1999;399:697–700. doi: 10.1038/21441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kammerer RA, Frank S, Schulthess T, Landwehr R, Lustig A, Engel J. Heterodimerization of a functional GABAB receptor is mediated by parallel coiled-coil alpha-helices. Biochemistry. 1999;38:13263–13269. doi: 10.1021/bi991018t. [DOI] [PubMed] [Google Scholar]
- 18.Kaupmann K, Huggel K, Heid J, Flor PJ, Bischoff S, Mickel SJ, McMaster G, Angst C, Bittiger H, Froestl W, Bettler B. Expression cloning of GABAB receptors uncovers similarity to metabotropic glutamate receptors. Nature. 1997;386:239–246. doi: 10.1038/386239a0. [DOI] [PubMed] [Google Scholar]
- 19.Kaupmann K, Schuler V, Mosbacher J, Malitschek B, Bischoff S, Bittiger H, Heid J, Froestl W, Leonhard S, Pfaf T, Karschin A, Bettler B. Human γ-aminobutyric acid type B receptors are differentially expressed and regulate inwardly rectifying K+ channels. Proc Natl Acad Sci USA. 1998a;95:14991–14996. doi: 10.1073/pnas.95.25.14991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kaupmann K, Malitschek B, Schuler V, Heid J, Froestl W, Beck P, Mosbacher J, Bischoff S, Kulik A, Shigemoto R, Karschin A, Bettler B. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature. 1998b;396:683–687. doi: 10.1038/25360. [DOI] [PubMed] [Google Scholar]
- 21.Kuner R, Kohr G, Grunewald S, Eisenhardt G, Bach A, Kornau HC. Role of heteromer formation in GABAB receptor function. Science. 1999;283:74–77. doi: 10.1126/science.283.5398.74. [DOI] [PubMed] [Google Scholar]
- 22.Lupas A. Coiled coils: new structures and new functions. Trends Biochem Sci. 1996;21:375–382. [PubMed] [Google Scholar]
- 23.Margeta-Mitrovic M, Jan YN, Jan LY. A trafficking checkpoint controls GABAB receptor heterodimerization. Neuron. 2000;27:97–106. doi: 10.1016/s0896-6273(00)00012-x. [DOI] [PubMed] [Google Scholar]
- 24.Marshall FH, Jones KA, Kaupmann K, Bettler B. GABAB receptors—the first 7TM heterodimers. Trends Pharmacol Sci. 1999;20:396–399. doi: 10.1016/s0165-6147(99)01383-8. [DOI] [PubMed] [Google Scholar]
- 25.Martin SC, Russek SJ, Farb DH. Molecular identification of the human GABAB2: cell surface expression and coupling to adenylyl cyclase in the absence of GABAB1. Mol Cell Neurosci. 1999;13:180–191. doi: 10.1006/mcne.1999.0741. [DOI] [PubMed] [Google Scholar]
- 26.McIlhinney RA, Le Bourdelles B, Molnar E, Tricaud N, Streit P, Whiting PJ. Assembly intracellular targeting and cell surface expression of the human N-methyl-d-aspartate receptor subunits NR1a and NR2A in transfected cells. Neuropharmacology. 1998;37:1355–1367. doi: 10.1016/s0028-3908(98)00121-x. [DOI] [PubMed] [Google Scholar]
- 27.Mott DD, Lewis DV. The pharmacology and function of central GABAB receptors. Int Rev Neurobiol. 1994;36:97–223. doi: 10.1016/s0074-7742(08)60304-9. [DOI] [PubMed] [Google Scholar]
- 28.Ng GY, Clark J, Coulombe N, Ethier N, Hebert TE, Sullivan R, Kargman S, Chateauneuf A, Tsukamoto N, McDonald T, Whiting P, Mezey E, Johnson MP, Liu Q, Kolakowski LFJ, Evans JF, Bonner TI, O'Neill GP. Identification of a GABAB receptor subunit, gb2, required for functional GABAB receptor activity. J Biol Chem. 1999;274:7607–7610. doi: 10.1074/jbc.274.12.7607. [DOI] [PubMed] [Google Scholar]
- 29.Okabe S, Miwa A, Okado H. Alternative splicing of the C-terminal domain regulates cell surface expression of the NMDA receptor NR1 subunit. J Neurosci. 1999;19:7781–7792. doi: 10.1523/JNEUROSCI.19-18-07781.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rabow LE, Russek SJ, Farb DH. From ion currents to genomic analysis: recent advances in GABAA receptor research. Synapse. 1995;21:189–274. doi: 10.1002/syn.890210302. [DOI] [PubMed] [Google Scholar]
- 31.Rocheville M, Lange DC, Kumar U, Patel SC, Patel RC, Patel YC. Receptors for dopamine and somatostatin: formation of hetero-oligomers with enhanced functional activity. Science. 2000;288:154–157. doi: 10.1126/science.288.5463.154. [DOI] [PubMed] [Google Scholar]
- 32.Schwartz DA, Barry G, Eliasof SD, Petroski RE, Conlon PJ, Maki RA. Characterization of GABAB1e, a GABAB1 splice variant encoding a truncated receptor. J Biol Chem. 2000;275:32174–32181. doi: 10.1074/jbc.M005333200. [DOI] [PubMed] [Google Scholar]
- 33.Stowell JN, Craig AM. Axon/dendrite targeting of metabotropic glutamate receptors by their cytoplasmic carboxy-terminal domains. Neuron. 1999;22:525–536. doi: 10.1016/s0896-6273(00)80707-2. [DOI] [PubMed] [Google Scholar]
- 34.Sullivan R, Chateauneuf A, Coulombe N, Kolakowski LFJ, Johnson MP, Hebert TE, Ethier N, Belley M, Metters K, Abramovitz M, O'Neill GP, Ng GY. Coexpression of full-length gamma-aminobutyric acid(B) (GABAB) receptors with truncated receptors and metabotropic glutamate receptor 4 supports the GABAB heterodimer as the functional receptor. J Pharmacol Exp Ther. 2000;293:460–467. [PubMed] [Google Scholar]
- 35.White JH, Wise A, Main MJ, Green A, Fraser NJ, Disney GH, Barnes AA, Emson P, Foord SM, Marshall FH. Heterodimerization is required for the formation of a functional GABAB receptor. Nature. 1998;396:679–682. doi: 10.1038/25354. [DOI] [PubMed] [Google Scholar]
- 36.Zerangue N, Schwappach B, Jan Y, Jan L. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 1999;22:537–548. doi: 10.1016/s0896-6273(00)80708-4. [DOI] [PubMed] [Google Scholar]