Abstract
We characterized a subpopulation of dorsal root ganglion (DRG) sensory neurons that were previously identified as preferential targets of enkephalins. This group, termed P-neurons after their “pear” shape, sequentially required nerve growth factor (NGF) and basic fibroblast growth factor (bFGF) for survival in vitroduring different developmental stages. Embryonic P-neurons required NGF, but not bFGF. NGF continued to promote their survival, although less potently, up to postnatal day 2 (P2). Conversely, at P5, they needed bFGF but not NGF, with either factor having similar effects at P2. This trophic switch was unique to that DRG neuronal group. In addition, neither neurotrophin-3 (NT-3) nor brain-derived neurotrophic factor influenced their survival during embryonic and postnatal stages, respectively. The expression of NGF (Trk-A) and bFGF (flg) receptors paralleled the switch in trophic requirement. No single P-neuron appeared to coexpress bothTrk-A and flg. In contrast, all of them coexpressed flg and substance P, providing a specific marker of these cells. Immunosuppression of bFGF in newborn animals greatly reduced their number, suggesting that the factor was requiredin vivo. bFGF was present in the DRG and spinal cord, as well as in skeletal muscle, the peripheral projection site of P-neurons, as revealed by tracer DiIC183. The lack of requirement of NT-3 for survival and immunoreactivity for the neurofilament of 200 kDa distinguished them from muscle proprioceptors, suggesting that they are likely to be unmyelinated muscle fibers. Collectively, their properties indicate that P-neurons constitute a distinct subpopulation of sensory neurons for which the function may be modulated by enkephalins.
Keywords: dorsal root ganglion, NGF, bFGF, switch, survival, trophic dependence, rat, sensory neurons, subpopulation, muscle innervation
Several sensory neuron subpopulations have been identified in the dorsal root ganglia (DRGs) of mammals on the basis of anatomical, electrophysiological, functional, cytochemical, and trophic criteria (Perl, 1992; McMahon et al., 1994; Gilabert and McNaughton, 1997; Baudet et al., 2000). These studies have greatly furthered our understanding of how sensory neurons transmit specific sensory modalities, providing rich insights on central issues such as the transmission of pain (Wood and Docherty, 1997; Caterina and Julius, 1999; Wood and Perl, 1999; Caterina et al., 2000).
We have shown recently that activation of δ-opioid receptors (DORs) inhibited high-voltage-activated Ca2+currents (HVACCs) in primary rat sensory neurons (Acosta and López, 1999), validating the cellular mechanism that is suspected to mediate the action of enkephalins and exogenous δ-opioid compounds on the transmission of normal and pain sensation (Dickenson et al., 1987; Standifer et al., 1994). Acosta and López (1999) identified a neuronal subpopulation, referred to as P-neurons after their distinctive “pear-shaped” soma in vitro, in which the frequency of DOR-mediated Ca2+ current inhibition was remarkably high (75%) when compared with the other cell types (18–35%). Those findings suggested that their function could be preferentially modulated by enkephalins and prompted a characterization of the properties of that subpopulation to clearly determine whether they represent a distinct group. P-neurons are of medium size and express substance P (SP), properties that have been useful for classifying primary sensory neurons (Harper and Lawson, 1985; Cardenas et al., 1995; Gilabert and McNaughton, 1997). However, further refinement is needed because several subpopulations share such properties. We hypothesize that P-neurons are likely to constitute a previously uncharacterized group because both their unmistakable shapein vitro and preferential coupling of DOR to their HVACCs have been noted only recently (Acosta and López, 1999).
Taking advantage of their distinct morphology to unequivocally identify P-neurons in culture, this study determined unique characteristics of that cell group concerning trophic requirements during development, anatomical projections, and cytochemical features. Most distinctively, P-neurons sequentially required nerve growth factor (NGF) and basic fibroblast growth factor (bFGF) for survival along embryonic and postnatal stages. The need of specific trophic factors has provided a powerful criterion to define distinct subpopulations of sensory neurons and infer their possible physiological role (Levi-Montalcini and Angeletti, 1968; Kucera et al., 1995; Lewin and Barde, 1996; Davies, 1997). In particular, the switch of neurotrophic requirements has been recognized recently, underscoring a developmental complexity that goes beyond the classical view of target-derived trophic support (Birren et al., 1993; Molliver and Snider, 1997; Enokido et al., 1999; Baudet et al., 2000; Enomoto et al., 2000). P-neurons also displayed a recognizable cytochemical pattern and supplied sensory innervation to skeletal muscle. These data strongly indicate that they constitute a subpopulation of sensory neurons with distinctive developmental and cellular characteristics, in addition to the previously reported high sensitivity to enkephalins (Acosta and López, 1999).
MATERIALS AND METHODS
Cell culture. All procedures were in accordance with the Guide for the Care and Use of Laboratory Animals of the Society for Neuroscience. Sensory neurons from DRGs of rat embryos or newborn rats (up to 7 d old) were isolated as described previously (Acosta and López, 1999). Briefly, embryonic or postnatal DRGs were enzymatically dissociated by incubating the tissue for 15–30 min at 37°C with 0.125% trypsin and 0.625% collagenase, or 0.25% trypsin and 1.25% collagenase, respectively. The enzymatic activity was halted by adding 1 ml of Eagle minimal essential medium supplemented with 10% fetal bovine serum (MEM10). After centrifugation at 2000 rpm for 5 min, the pellet was resuspended in MEM10 containing different trophic factors or the compound K252a at the concentrations specified in Results. A final step of cell dissociation was performed mechanically by passing the material through Pasteur pipettes of increasingly smaller tip diameters. Approximately 70 μl of the cell suspension were plated on coverslips coated with 0.25% collagen and 0.05% poly-d-lysine. Embryonic day 18 (E18) cultures were grown on poly-d-lysine 1 mg/ml alone (∼300 ng/mm2) because the neurons showed some tendency to detach from the mixed substrate. No differences were found in the survival of postnatal neurons grown in any of those substrates. Plating cell density was standardized, using a Neubauer chamber, to ∼104 cells per milliliter. The coverslips were placed in an incubator (36°C, 5% CO2) for 1–2 hr to allow for cell adhesion. Then, MEM10 alone or supplemented with trophic factors or K252a was added to the culture dishes containing the coverslips until reaching a volume of ∼2 ml. The cultures were kept in those conditions for 24 hr to permit the stabilization of neuronal number and morphological phenotypes. Then, we performed the first neuronal counting, which was defined as the initial condition in all survival assays. Immediately after the first counting, the MEM10 was completely replaced by defined media N2 alone (control groups) or supplemented with trophic factors or K252a. Half of the media was replaced every 48 hr thereafter, but a fresh aliquot of trophic factors or K252a was added daily to the media.
The cultures consisted of a mixed population of neuronal and non-neuronal cells. Two consecutive applications of 5–10 μm β-arabinocytofuranoside (at days 2 and 3) were used to eliminate dividing fibroblasts. In some cultures the dissociated ganglia were passed throughout a 20% Percoll gradient by centrifugation at 2500 rpm for 6–8 min to reduce the fibroblast population. Penicillin–streptomycin (150 U/150 μg per milliliter, respectively) was always included in the media. The following definitions were used in this study: E0 was defined as the day of mating, embryonic age was defined relative to E0, postnatal day 0 (P0) was the day of birth, and postnatal age was defined with respect to P0.
Evaluation of neuronal survival. Neuronal survival was assessed in cultures grown on etched grid coverslips from Bellco Glass (Vineland, NJ); alphanumeric coordinates on the coverslips allowed counting the cells within an identified region of the culture. A minimum of 500 neurons were counted in each replication of the experiments. Taking advantage of their relatively small fractional contribution to the culture population, the survival of each individual P-neuron was followed and recorded. Survival was estimated as the average percentage (±SEM) of cells remaining alive relative to their number at the initial condition. The neurons were counted daily until no P-neurons remained in the culture. The culture density at the time of the first neuronal counting, estimated using the coverslip etched grid, already reflected the survival-promoting effect of the different factors at embryonic or postnatal stages. The survival of P-neurons showed no correlation with the observed density. For example, similar survival rates after 90 hr in vitro were obtained with bFGF, despite a threefold difference in density after the first 24 hr in culture. Dying cells were recognized on the basis of signs such as pyknosis, shrinkage and fragmentation, membrane disruption, loss of adhesion to the substratum or complete lysis. Because, as noted above, the morphological integrity of each individual P-neuron was recorded, the chances of mistakenly counting a live cell as dead, or vice versa, were negligible. P-neurons never became round over the entire duration of the experiment. Very few neurons identified as round at the time of the first counting became pear-shaped by the time of the second counting (48 hr after plating), keeping that phenotype thereafter, a change that was unrelated to the presence of any of the trophic factors. Those cells were counted as P-neurons and might have caused, at the most, an overestimation of survival of 10%. The number of replications of the experiments is stated in Results or Figure legends.
The results were analyzed statistically using a two-way ANOVA test [treatment × days in vitro (DIV)]. The percentage of P-neuron survival was the dependent variable, and the repeated measures were provided by the survival percentage at each consecutive DIV. The interaction between factors was evaluated with multiple comparison tests (post hoc analysis with the Tukey test;p = 0.05). For E18, P2, and P5, the interaction values were F(6,18) = 12.63,p < 0.0001; F(6,18) = 12.97, p < 0.0001; andF(8,24) = 5.69, p < 0.0001, respectively.
Immunocytochemistry. The cells were withdrawn from the incubator 24 hr after plating and washed for 5 min with PBS, then fixed with 4% paraformaldehyde–sucrose for 20 min at 37°C and washed again for 5 min with PBS. In experiments detecting endogenous proteins (bFGF, substance P, neurofilament 200 kDa, β-tubulin isoform III), the cells were permeabilized with 0.2% Triton X-100 for 5 min, and nonspecific binding sites were blocked with 5% bovine serum albumin (BSA) for 1 hr. Surface antigen detection (Trk-A andflg) did not use permeabilization. The coverslips were covered with primary antibodies, usually overnight at 4°C, in 1% BSA at the dilutions indicated in Results. The primary antibodies were subsequently washed three times with PBS, and the cells were incubated at room temperature for 1 hr with the corresponding secondary antibodies, labeled with fluorescein isothyocianate (FiTC) or rhodamine. Finally, the cells were washed three times, and the coverslips were mounted on glasses with FluorSave (Calbiochem, La Jolla, CA). In double-staining experiments, the described procedure was repeated after the incubation with the second primary antibody. Images were acquired using an epifluorescence microscope (Axiovert TM-31; Zeiss, Oberkochen, Germany) and recorded on optical disk.
A series of preliminary experiments established the appropriate dilutions of primary antibodies as those allowing a clear distinction from the background and causing no labeling of cell types (neurons or other) that were known to be negative for the tested antigen. The antibodies against Trk-A (α-Trk-A),flg (α-flg), and bFGF (α-bFGF) were tested with Western immunoblots following standard protocols (Cáceres et al., 1992) and found to specifically label the bands corresponding to the molecular weight of their target proteins (see Figs. 5, bottom panel,6B, 9A). The proteins for Western blotting were obtained from tissues of E20, P1, or P5 rats (as indicated in the corresponding figure legends), homogenized at 4°C in Ripa 1×. Samples were centrifuged at 14,000 rpm for 15 min; the supernatant was recovered, centrifuged again, and kept at −20°C. The proteins were quantified using colorimetric methods. In all cases, 20 μg of proteins were seeded in each lane. Although a 1:200 dilution of α-Trk-A labeled a single band corresponding toTrk-A (∼135 kDa), it only moderately labeled cultured embryonic neurons at a 1:20 dilution, a result attributed to disruption of the trypsin-sensitive extracellular loop of Trk-Arecognized by the antibody after the enzymatic treatment required for the DRG dissociation. We used α-Trk-A at a 1:50 dilution because it yielded the same number of labeled neurons as lower dilutions, with a minor loss of fluorescence intensity. Theα-flg produced satisfactory labeling in the range 1:400–1:1000.
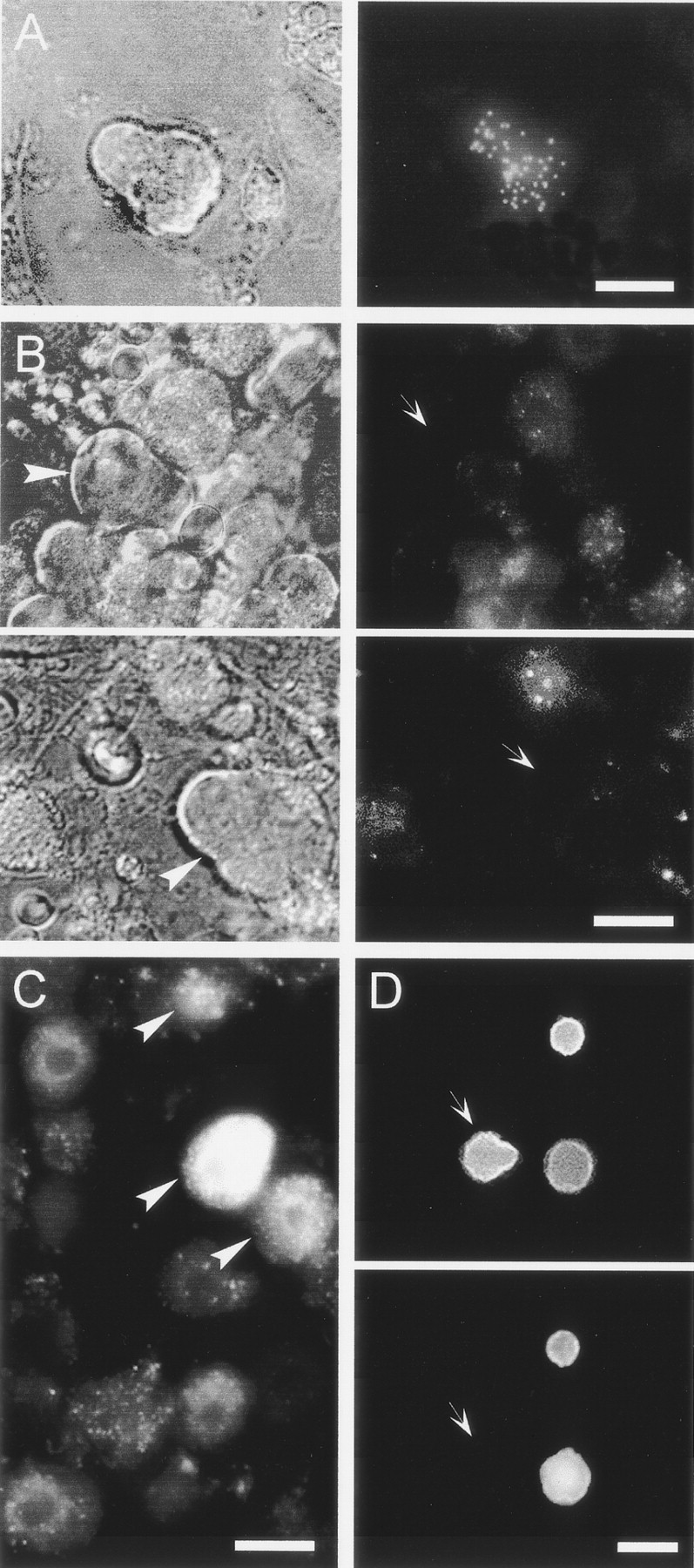
Fig. 5.
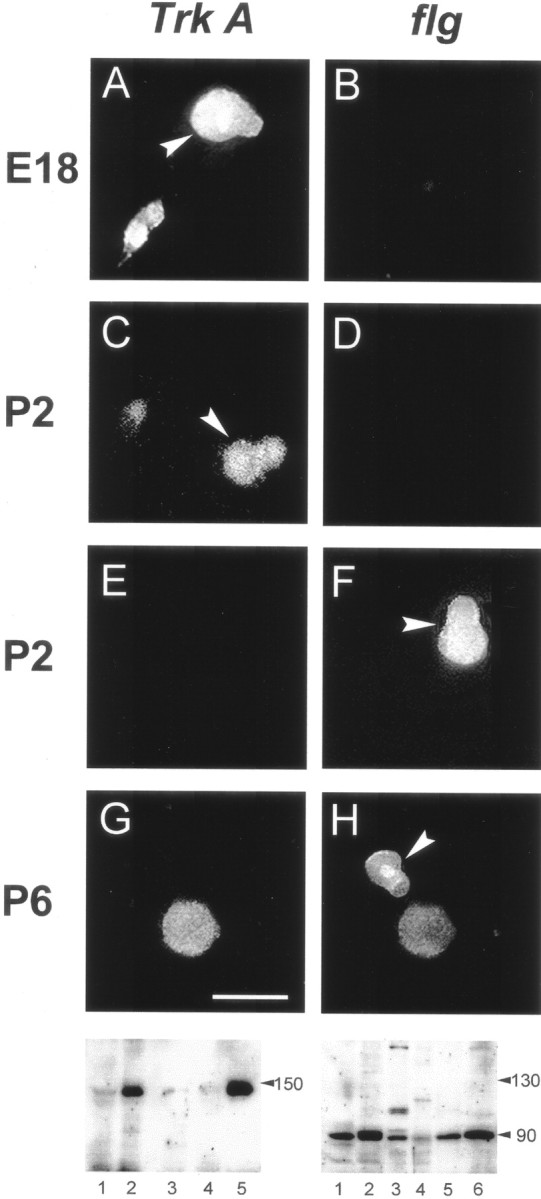
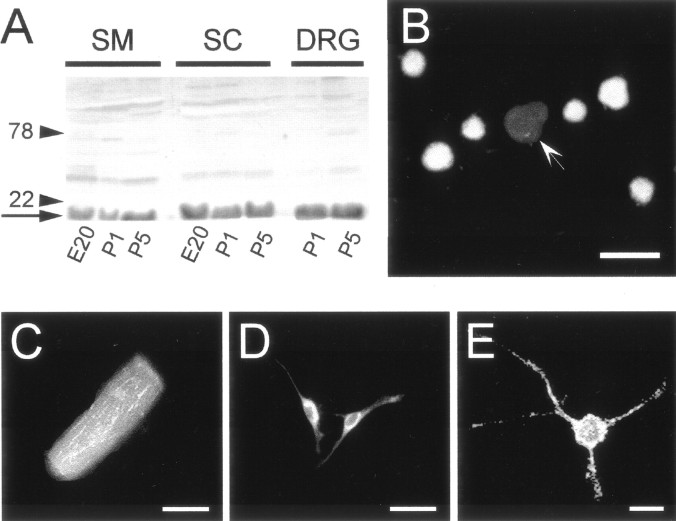
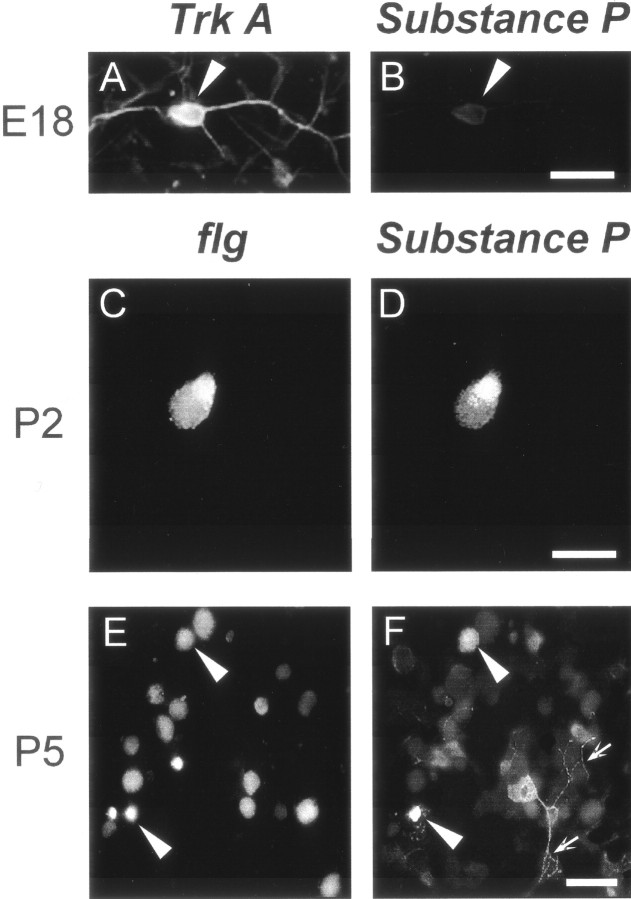
Expression of Trk-A andflg in P-neurons at different ages. Detection ofTrk-A-like (left column) andflg-like (right column) immunoreactivity as revealed with mouse α-Trk-A (1:50) and rabbit α-flg (1:500) antibodies, respectively, in cultures established from animals of ages shown on the left. Eachrow corresponds to a same view field but with the filter selected for FiTC (left) or rhodamine (right), which labeled the secondary antibodies (arrowheads indicate P-neurons). All experiments were performed 24 hr after plating the neurons. E18 P-neurons were immunoreactive for Trk-A (A) but not flg (B). At the age corresponding to the switchover of trophic requirement (P2), a set of P-neurons displayed Trk-Aimmunoreactivity (C) and a different set displayed flg immunoreactivity (F). No P-neurons were immunoreactive for both or for none of the proteins (D, E). P6 P-neurons were immunoreactive for flg (H) but notTrk-A (G). Scale bar (shown inG): A, B, 18 μm;C, D, 20 μm; E,F, 15 μm; and G, H, 30 μm. Bottom, Western blots assaying the expression ofTrk-A (left) and flg(right) in different tissues with the antibodies used for neuronal immunolabeling. The lanes correspond to spinal cord (1), DRG (2), skeletal muscle (3), kidney (4), and brain (5) inleft panel, and to DRG (1), lung (2), skeletal muscle (3), spinal cord (4), heart (5), and brain (6) in right panel (20 μg of tissue proteins seeded in each lane). The monoclonal α-Trk-A labeled a single band that corresponded to the molecular weight (MW) of Trk-A (∼140 kDa). The rabbit α-flg labeled a single band corresponding to the estimated MW of FGFR-1 (∼90 kDa) in all tissues, except in skeletal muscle. The extra bands in skeletal muscle presumably reflected the antibody reaction with the C-terminal epitope of truncated forms of FGFR-1 expressed in that tissue (Templeton and Hauschka, 1992).
Fig. 6.
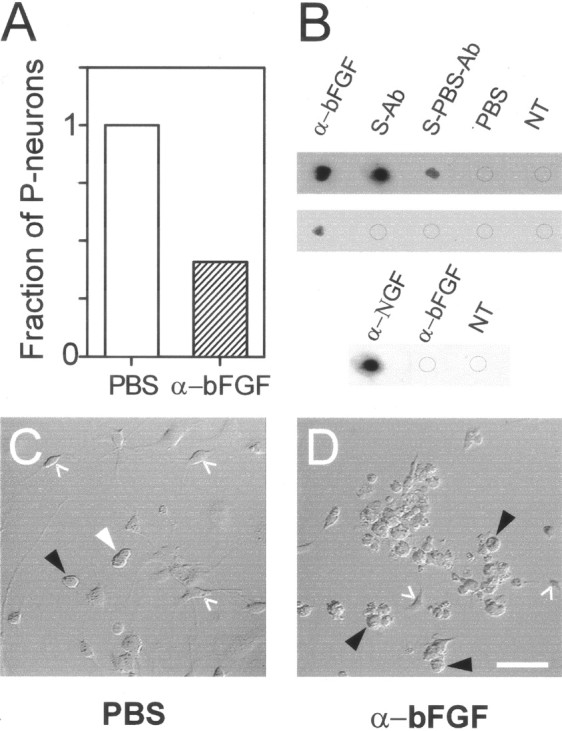
Effect in vivo of an antibody against α-bFGF on the number of P-neurons during the early postnatal period. A, The proportion of P-neurons in culture was 2.4-fold smaller in α-bFGF-injected animals (1.3%) as compared with controls (3.17%). Number of P-neurons and round neurons counted were 45 of 3458 and 78 of 2463, respectively.B, Immunodot blots showing that theα-bFGF can be found in rat serum 1 hr (first row), but not 12 hr (second row), after its intraperitoneal administration. Eachdot illustrates the result of treating a substrate of pure human bFGF (250 ng in 10 μl) with α-bFGF (1:50) as positive control, or serum (without dilution) from animals treated with α-bFGF for 3 d plus one extra dosis 1 hr before bleeding (S-Ab), PBS for 3 d plus one dosis of α-bFGF 1 hr before bleeding (S-PBS-Ab), PBS for 3 d (PBS), or from untreated control animals (NT). The immunodot blot reaction was revealed with a secondary antibody. The reaction reliably detected circulating α-bFGF 1 hr after the last antibody injection but not after 12 hr. The antibody α-bFGF did not cross-react with mNGF 7S (25 ng/μl) (third row). C, D, Representative Nomarski-interference photographs of cultures obtained from rats that received one injection per day of PBS (100 μl) (C) or α-bFGF (100 μl, 1:10) (D) during postnatal days 2–4. The number of non-neuronal cells that depend on the supply of bFGF (glia and fibroblasts) was greatly reduced after the antibody treatment.White arrowhead, P-neuron; black arrowheads, round neuron; white angles, non-neuronal cells (fibroblasts or glia). At a 1:50 dilution, the antibody injection produced a minor reduction in the number of P-neurons. Scale bar, 50 μm. Cultures were plated in video microscopy chambers and kept in MEM10 for 24 hr, and the media was replaced by PBS for image acquisition 24 hr later. Throughout the experiment, the cultures were maintained at 37°C and supplemented with 10 ng/ml bFGF to support the survival of P-neurons.
Fig. 9.
Expression of bFGF immunoreactivity at sites with which P-neurons relate anatomically, revealing potential sources of bFGF at the time it was required for P-neuron survival. All tissues were isolated from animals of age P3–P5. A, Western blot showing the expression pattern of bFGF in skeletal muscle (SM), spinal cord (SC), andDRG at ages indicated under the correspondinglanes. Labeled bands of ∼18 kDa (arrow), the MW of bFGF, indicate expression of that protein in all of those tissues. The locations of 22 and 78 kDa MW markers in a gel run in parallel are indicated byarrowheads and corresponding MW numbers.B, DRG cultured cells. Some round neurons expressed bFGF immunoreactivity. In contrast, P-neurons themselves were always negative (arrow). C, At P3, 90% of skeletal muscle fibers, the presumptive innervation target of P-neurons according to our data (Fig. 7), were clearly positive for bFGF. A representative example is shown here. D, Glial cells (presumptive type-2 astrocytes, identified with double staining with an antibody against α-GFAP) (data not shown) from the same ganglia used in A were also immunolabeled for bFGF. E, Photograph of a bFGF-positive neuron from the spinal cord. Experiments were performed using a mouse monoclonal antibody (α-bFGF) at 1:200 dilution. Scale bars:B, 40 μm; C, 20 μm;D, 100 μm; E, 15 μm.
Identification of targets innervated by P-neurons. To determine the peripheral tissue innervated by P-neurons, we injected several possible targets with the lipophilic fluorescent neuronal tracer DiIC18(3) (DiI). A single peripheral site was injected per animal. After being selectively taken up by neuronal termini present in the region of application, this compound diffuses along the cell membrane without crossing to other neurons (Honig and Hume, 1989). Its detection in cultured P-neurons was used to qualitatively specify their peripheral target. To this end, the DRGs were dissected and cultured on poly-d-lysine-covered glass in low-volume videomicroscopy chambers 3 d after the injections, a time at which the dye presumably reached the somata of sensory neurons according to its diffusion rate on lipid membranes in vivo (6–7 mm/d). A very small volume of DiI at low concentration (2–5 μl of 1% DiI inN,N-dimethylformamide) was injected into skin, subcutaneous tissue, skeletal muscle, or joints with a Gilmont microsyringe. This protocol minimized the possibility of dye diffusion to neighboring tissues, so that the chances of assigning labeled P-neurons to a wrong target were negligible. Because this injection protocol might miss a sparse cutaneous innervation by P-neurons, we also evaluated this possibility by injecting several subcutaneous sites of a rat with larger volumes of DiI at high concentration (six injections of 10 μl each, 3% DiI).
Treatment with antibody against bFGF in vivo. For 3 d, rats of age P2 received daily injections of 100 μl ofα-bFGF diluted 1:10 in PBS. The control group was injected similarly with PBS alone. At P6, DRG sensory neurons from test and control groups were isolated, and all neurons present in the culture were counted 24 hr after plating to evaluate the number of P-neurons in treated animals and controls. The serum of PBS- or antibody-injected animals was examined with the technique of dot immunoblots to assess whether α-bFGF reached the bloodstream after its intraperitoneal injection. The serum was obtained 1 or 12 hr after an injection of PBS or antibody. To separate the serum, the blood from injected animals was collected in heparinized Eppendorf tubes (50 U/ml), incubated for 1 hr at 37°C, and kept overnight at 4°C. Then, samples were centrifuged at 10,000 rpm for 10 min at 4°C, and the supernatant was collected and centrifuged again. The new supernatant (serum, ∼100 μl) was kept at −20°C until used. To detect theα-bFGF, 10 μl dots containing 250 ng of pure human bFGF were added to a nitrocellulose membrane, dried, and allowed to bind to the paper for 1 hr. This resulted in a final concentration of bFGF of ∼25 μg/ml, considered optimal for this technique. Then, the nitrocellulose membranes were washed three times with TBS for 5 min and blocked overnight at 4°C with a 5% milk suspension in TBS plus 0.05% Tween 20 (TBST). On the following day, the membranes were incubated for 18 hr at 4°C with the sera or with α-bFGFdiluted in TBST. Finally, the membranes were washed several times with TBST and incubated with a biotinylated mouse anti-rabbit IgG (1:400) for 1 hr at room temperature, which would then conjugate the rabbitα-bFGF present in the serum of injected animals. After three washouts with TBST, the membranes were exposed to a peroxidase–extravidin complex (1:2000) for 30 min at room temperature. The Enhanced Chemiluminescence reaction kit was used to detect the antibody. Exposure time was 20–30 sec.
Electrophysiology. Na+, K+, and Ba2+currents were recorded using the whole-cell configuration of the patch-clamp technique (Hamill et al., 1981). Appropriate external and pipette solutions for each current type were used as described elsewhere (Acosta and López, 1999; Everill and Kocsis, 1999).
Antibodies and reagents. The neuronal tracer DiIC18(3) was obtained from Molecular Probes (Eugene, OR). Rat tail collagen type I was from Biomedical Technologies (Stoughton, MA), and the enzymes used for tissue dissociation were from Worthington (Lakewood, NJ). All trophic factors and K252a were from Alomone Labs (Jerusalem, Israel). The rabbit (Rb) polyclonal anti-human bFGF (affinity purified, raised in rabbit against a peptide corresponding to amino acids 3–17 mapping within the N-terminal region of human bFGF precursor), Rb polyclonal anti-rat FGF receptor 1 (affinity purified, raised in rabbit against a peptide corresponding to amino acids 808–822 mapping within the C-terminal region of human FGFR-1), and mouse monoclonal anti-TrkA (clone 6G10) were from Research Diagnostics (Flanders, NJ). The monoclonal anti-bovine bFGF antibody was from Chemicon (Temecula, CA). The antibody against substance P was from Sera-Lab (commercialized by Accurate Chemical & Scientific Corp.,Westbury, NY). The monoclonal mouse anti-β tubulin isotype III (clone SDL.3D10), polyclonal Rb anti-neurofilament 200, and all other reagents were obtained from Sigma (St. Louis, MO). Stock solutions of trophic factors were prepared in sterile water; aliquots were maintained at −70°C for no more than 3 months. Stock solution of K252a was prepared in cell culture-tested DMSO to yield a 1:2000 dilution of the organic solvent in the culture medium. No adverse effects on neurons have been reported for this concentration of DMSO. The photosensitive K252a was stored in a light-proof container at −20°C until used, and the dishes treated with this compound were protected from light.
RESULTS
Subpopulation of P-neurons in culture
The distinct pear-shaped soma of the sensory neurons, which for that reason we have designated P-neurons, allowed different independent observers to readily and consistently identify them in DRG primary cultures (Fig. 1A). The other subpopulations were classified according to the diameter of their round somata into small (<15 μm), medium (15–26 μm), and large (>26 μm) neurons (Perl, 1992; Gilabert and McNaughton, 1997). By size, P-neurons corresponded to the medium size group. Inspection of a large number of cultures established from rats of increasing ages (E18–P9), at 1 d intervals, showed that they contributed 4–7% of the total population in vitro throughout that period, with the larger percentages at postnatal stages. They might represent a subset of a larger subpopulation, of which only a fraction assumes a pear-shaped soma. It was this feature, however, that allowed their unequivocal identification as a separate group. They exhibited characteristics of healthy neurons such as the development of axons after several days in vitro, as shown by labeling the axonal protein class-III β-tubulin (Fig. 1B) (Ferreira and Caceres, 1992), and a normal expression of currents through several voltage-dependent ion channels (Na+, K+, and Ca2+) (Fig. 1C). P-neurons were present already from the moment of cell plating, as well as in cultures grown on different substrates such as laminin (10 μg/ml), fibronectin (15 μg/ml), and collagen (1 μg/mm2) (data not shown). Thus, their peculiar shape, which exhibited little change in long-term cultures (up to 14 d), did not appear to reflect either cell injury or abnormal cell function.
Fig. 1.
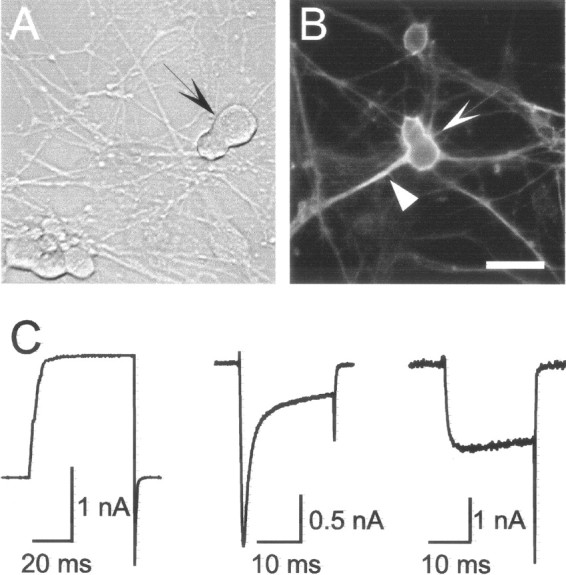
Morphological phenotype and ion currents of “pear”-shaped sensory neurons (P-neurons) in culture.A, Typical P-neuron (arrow) in a DRG culture from a rat embryo (E18). Phase contrast photograph after 8 DIV. For comparison, round sensory neurons were included in the view field (bottom left). B, Labeling of the soma (sharp arrowhead) and axon (triangle arrowhead) of a P-neuron with an antibody against the class III isoform of β-tubulin. Culture from a rat of age P5 after 2 DIV, supplemented with bFGF (10 ng/ml). Scale bar indicates 20 μm inA and 30 μm in B. C, Expression of voltage-activated ion currents in three different postnatal P-neurons. The left, middle, and right panels show whole cell currents through K+, Ca2+ plus Na+, and Ca2+ channels, respectively, recorded under voltage clamp. The permeant ions were K+, Ca2+ plus Na+, and Ba2+, and the current was activated with voltage pulses to 0–10 mV from holding potentials of −70 or −80 mV.
Requirement of NGF and bFGF for the survival of P-neurons during development
Distinct subpopulations of sensory neurons require for survival during development one or more specific trophic factors (Levi-Montalcini and Angeletti, 1968; Ruit et al., 1992; Kucera et al., 1995; Lewin and Barde, 1996; Davies, 1997). To characterize the properties of P-neurons, we examined whether they depended on specific trophic factors during embryonic and early postnatal development by evaluating their survival in cultures established from animals of increasing ages (E18–P5), supplemented with NGF, bFGF, neurotrophin-3 (NT-3), or brain-derived neurotrophic factor (BDNF). The results were compared with the survival rates of round neurons in the same cultures.
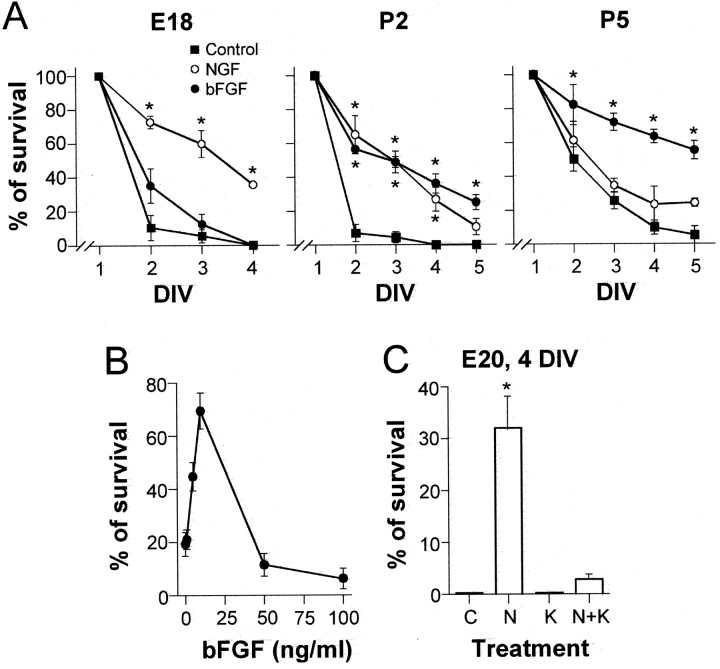
The survival of P-neurons depended on NGF and bFGF, but not on NT-3 or BDNF. The plots of Figure 2A illustrate the survival of P-neurons over 4–5 DIV in defined media alone (control), or supplemented with NGF or bFGF. The requirement of NGF and bFGF occurred in a sequential fashion during development. At E18, NGF (50 ng/ml) promoted the survival of 35.6 ± 0.5% of P-neurons at 4 DIV (Fig.2A, left), whereas none survived in defined media alone or with bFGF (10 ng/ml). The difference between the survival observed with NGF and any of the other treatments (control or bFGF) was statistically significant over the entire period assayed (2–4 DIV). Conversely, at P5 63.5 ± 3.9 and 55.3 ± 5.3% of P-neurons survived with bFGF at 4 and 5 DIV, respectively, whereas the effect of NGF was barely above control (Fig. 2A, right). The effect of bFGF was statistically significant with respect to both NGF and control. NGF and bFGF had similar effects at P2 (Fig. 2A,middle), with ∼50% of P-neurons surviving after 3 DIV in the presence of either factor alone, an effect that differed statistically from the virtual lack of survival observed in control conditions. At P3, bFGF promoted considerably more survival than NGF (44.2 ± 8.2 and 15.8 ± 2.2%, respectively, at 3 DIV). The modest survival that was observed with nonsupplemented media after 3 DIV at P5 (25.5 ± 5.1%) most likely reflected the progressive drop in trophic factor requirements of sensory neurons after birth (Levi-Montalcini and Angeletti, 1968). The unmistakable morphology of P-neurons, together with the alphanumeric-coded grid of the coverslips onto which the cells were plated, allowed us to individually follow each P-neuron over several DIV, assuring that the observations corresponded to that single subpopulation of sensory neurons.
Fig. 2.
Time course of the survival of P-neurons in cultures supplemented with NGF or bFGF from animals of different ages. A, Average percentage (±SEM) of surviving P-neurons as a function of DIV, relative to their number at 1 DIV (taken as 100%), in cultures established from rats of ages E18, P2, and P5. The cultures were maintained in defined media alone (squares) or defined media supplemented with 50 ng/ml NGF (white circles) or 10 ng/ml bFGF (black circles). Each plot contains data from three experiments. NGF but not bFGF promoted the survival of E18 neurons (left panel). The reversed result was obtained with P5 neurons (right panel). At P2 (middle panel), the factors had similar effects. Analysis of the data at 2–5 DIV (ANOVA, and Tukey post hoc test;p = 0.05) indicated highly significant statistical differences (asterisks) between the NGF treatment and bFGF treatment or control (E18), between NGF or bFGF treatment and control (P2), and between bFGF treatment and NGF or control (P5). B, Dose–response curve of the average survival (±SD) of P-neurons from P5 animals (estimated at 3 DIV) as a function of bFGF concentration (n = 2). C, The survival-promoting effect of NGF (50 ng/ml) on E20 P-neurons was completely blocked byTrk-A kinase activity inhibitor K252a (50 nm) (n = 2). The barsindicate the percentage of survival (±SD) after 4 DIV in defined media alone (C) or supplemented with NGF (N), K252a (K), or both (N + K) at the concentrations specified above. K252a prevents the effect of NGF in a statistically significant way (see asterisk).
The concentrations of NGF and bFGF used in these experiments were those that optimally promote survival as shown elsewhere for NGF (Ruit et al., 1992) and here for bFGF (Fig. 2B). In agreement with other reports in neurons, 5–10 ng/ml bFGF was the most effective concentration, with smaller and larger concentrations being ineffective and detrimental, respectively (Schmidt and Kater, 1993; Abe and Saito, 2000). The alkaloid K252a (50 nm), an inhibitor of the tyrosine kinase pathway that is activated by the high-affinity receptor of NGF (Trk-A) (Koizumi et al., 1988), prevented the effect of that neurotrophin on the survival of E20 P-neurons (Fig.2C). Dose–response data showed that 50 nm K252a inhibits 80% of Trk-A kinase activity, fully blocks the Trk-A-mediated differentiation of PC12 cells, and has negligible effects on other tyrosine kinase receptors (Berg et al., 1992). Our results are thus consistent with NGF acting through the signaling pathway involving Trk-A and tyrosine kinase activity, and not through its low-affinity receptor p75 (Lewin and Barde, 1996) or other tyrosine kinase pathways. The effect of NGF most likely reflected its well known ability to prevent embryonic sensory neurons from entering programmed cell death (Vogel, 1993; Yao and Cooper, 1995). Postnatal P-neurons deprived of bFGF underwent nuclear changes typically associated with apoptosis (data not shown).
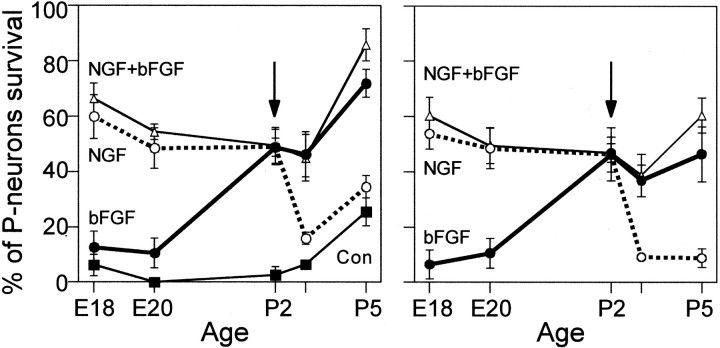
Figure 3 (left) summarizes all of our data on the extent and time course of the dependence of P-neurons on NGF and bFGF as a function of the age of the animal at the time of the isolation of sensory neurons (E18–P5). The data points indicate the average percentage (±SEM) of surviving neurons after 3 DIV in control media alone or supplemented with NGF alone, bFGF alone, or both factors. Both factors together promoted a survival rate similar to that achieved individually by the most effective factor at any given age. The switchover of survival dependence, defined as the day at which the survival-promoting effects of NGF and bFGF were similar, occurred at P2. The gradual increase in survival rate over P3–P5 with any factor alone or combined was similar to that observed in control cultures (Fig. 3, left) and was assumed to reflect the decrease in trophic factor requirements after birth. To show more clearly the switch in trophic factor requirement, the background survival that was obtained in the absence of factors was subtracted as follows. In each experiment, the control values (no treatment) were subtracted from the treatment values. Then, the data were averaged (Fig. 3, right).
Fig. 3.
Switch in the requirement of P-neurons from NGF to bFGF at different developmental stages.Left, average percentage (±SEM) of survival of P-neurons after 3 DIV, relative to their initial number, in cultures established from animals of different embryonic and postnatal ages, maintained in defined media alone (control, squares), supplemented with NGF (50 ng/ml; white circles), bFGF (10 ng/ml; black circles), or both NGF + bFGF (50 and 10 ng/ml, respectively; triangles). Right, same data as in left, but subtracting in each experiment the percentage of survival in control conditions (defined media alone) to make the trophic switch clearer. The switch of trophic dependence occurred at P2, defined as the day at which NGF and bFGF had similar effects (arrow). The survival obtained with both factors was not larger than that observed with the most effective factor at a given age, although it appeared to approach the sum of NGF and bFGF effects at E18 and P5. The survival-promoting effects of the trophic factors were statistically significant, as indicated in Figure2. For better visualization of the trophic switch, statistically significant differences were not labeled with asterisks in these summary plots.
The sequential requirement of NGF and bFGF was unique to P-neurons
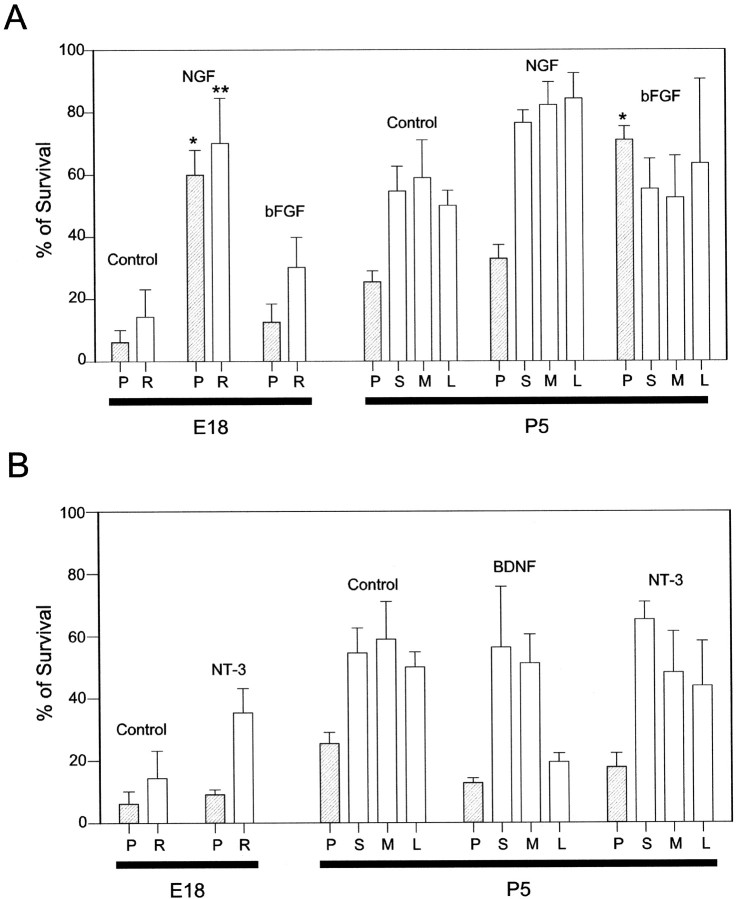
Only P-neurons required NGF prenatally and bFGF postnatally. None of the other sensory subpopulations (round small, medium, and large) depended on those factors in a sequential fashion. However, NGF or bFGF did affect the survival of embryonic or postnatal round sensory neurons in the way illustrated in Figure4A. We focused on the survival of round neurons in cultures that were obtained from E18 and P5 animals because the switch in trophic dependence of P-neurons was most obvious at those ages. Classifying E18 round neurons into small, medium, and large groups was uncertain because their size in culture changed considerably with trophic factors at that early stage, and the size differences in the first two groups were subtle. Therefore, E18 round neurons were lumped together into a single class (“round”). P5 round neurons were readily sorted into small, medium, and large categories. As expected from the literature (Silos-Santiago et al., 1995), NGF greatly enhanced, in a statistically significant manner, the survival of round E18 neurons (70.1 ± 14.5% at 3 DIV) when compared with control cultures (14.3 ± 8.3% at 3 DIV) (Fig. 4A, left). In contrast to its lack of effect on postnatal P-neurons, NGF also enhanced the survival of P5 round neurons, with equal effectiveness among categories (between 76 and 84% at 3 DIV), although the increase in survival over the control was smaller because of the expected drop in the requirement of trophic factors of postnatal sensory neurons (Fig. 4A,right) (see Ehrhard and Otten, 1994; Wewetzer et al., 1999). bFGF promoted the survival of E18 round neurons but had no effect on the survival of small, medium, or large P5 round neurons, as illustrated in Figure 4A. In the presence of bFGF, 30.2 ± 9.7% of embryonic round neurons survived after 3 DIV, compared with 14.3 ± 8.3% in control cultures, and ∼55% of postnatal round neurons survived after 3 DIV regardless of the presence of the factor in the culture. Thus, the pattern of bFGF effect on round neurons clearly differed from that observed with P-neurons. NT-3 caused some increase in the survival of E18 round neurons and small P5 round neurons. The former was an expected result because NT-3 is essential for the survival of round neurons that will supply proprioceptive afferents to skeletal muscle spindles at ∼E17–E18 (Ernfors et al., 1994). BDNF appeared toxic to large P5 round neurons, in agreement with other reports (Koh et al., 1995) and references therein (Fig.4B, right). Neither NT-3 (10 ng/ml) nor BDNF (50 ng/ml) had any observable effect on the survival of embryonic or postnatal P-neurons (Fig. 4B).
Fig. 4.
Differential effects of NGF, bFGF, NT-3, and BDNF on the survival of round and P-neurons of the DRG in culture. The experiments were performed at developmental stages at which the differences in the requirements for NGF or bFGF by P-neurons are most salient (E18 and P5) and show that the switch of survival requirements from NGF to bFGF was restricted to P-neurons. A, Average percentage (±SEM) survival of E18 and P5 round neurons after 3 DIV (open bars), relative to their number at 1 DIV (taken as 100%), in the presence of 50 ng/ml NGF or 10 ng/ml bFGF. Round neurons were lumped into a single group (R) at E18 and subclassified into small (S; <15 μm), medium (M; 15–26 μm), and large (L; >26 μm) neurons at P5. P-neuron data were included for comparison (hatched bars). The control group received no trophic factors. NGF dramatically enhanced the survival of E18 round and P-neurons (3 replications). The effect of NGF on P5 round neurons of any size group was mild, but larger than that observed on P5 P-neurons. bFGF promoted the survival of E18 round neurons, but not of P5 round neurons. B, Percentage survival of E18 and P5 P-neurons and round neurons after 3 DIV with NT-3 (10 ng/ml) or BDNF (50 ng/ml). NT-3 had no effect on the survival of E18 or P5 P-neurons, and slightly enhanced that of round neurons (E18 and small P5). BDNF was rather detrimental to P-neurons and large P5 round neurons (2 replications). Statistically significant differences between a given treatment and the control are labeled with * (P-neurons data) or ** (round neurons data) (ANOVA; Tukey post hoc test; p = 0.05).
Expression of NGF and bFGF receptors in embryonic and postnatal P-neurons
We studied by immunofluorescence microscopy the expression in P-neurons of Trk-A and the high-affinity bFGF receptor (flg or FGF-receptor 1) before, during, and after the switch in trophic requirements. These experiments addressed the important point that if Trk-A and flg are to mediate the survival-promoting effect of NGF and bFGF, respectively, then they must be present in the membrane of P-neurons at the appropriate times during development. Figure5 illustrates representative examples of the expression of Trk-A and flg immunoreactivity in P-neurons, as observed at the ages E18, P2, and P6. In all cases, the immunolabeling experiments were performed after 1 DIV. The expression of Trk-A or flg qualitatively mirrored the change in the requirements for their corresponding ligands (three experimental replications). Thus, during the embryonic period in which only NGF enhanced survival (E18–E20), all P-neurons (n= 21 cells) expressed Trk-A, but none expressedflg (Fig. 5A,B). At P2, the age at which NGF and bFGF had similar survival promoting effects, a group of P-neurons expressed Trk-A (n = 12 cells), whereas a different set expressed flg(n = 14 cells) (Fig.5C–F). We never observed coexpression ofTrk-A and flg in any given neuron or lack of expression of either receptor. However, we cannot rule out the possibility that lower levels of expression of those receptors, although functional, could be below the detection sensitivity of our immunolabeling technique. In fact, some degree of undetected coexpression might explain, at least partially, why the survival that was obtained with both NGF and bFGF during P2 did not approached the sum of the survivals obtained with those factors separately. At P5–P6, a stage at which only bFGF promoted survival, we detected expression offlg but not Trk-A (n = 23 cells, from four experiments) (Fig. 5G,H). The mouse α-Trk-A and rabbit α-flg were used at 1:50 and 1:500 dilutions, respectively.
Expression of Trk-A or flg was not restricted to P-neurons. A number of studies have shown expression of theTrk-A mRNA and the protein in several types of rat sensory neurons (Mu et al., 1993; McMahon et al., 1994; White et al., 1996). Similarly, these neurons have been reported to express flg(Grothe and Wewetzer, 1996). In accord with those reports, we foundTrk-A immunoreactivity in round embryonic neurons and small and medium round postnatal neurons, and we found flgimmunoreactivity in medium and large cells (Fig.5B,G). Coexpression of both receptors appeared very infrequently in round neurons (an example is shown in Fig. 5G,H). No switch in the expression from Trk-A to flg was observed in round neurons, although these experiments could not completely rule out that possibility.
Western blot data indicated that the antibodies specifically reacted with their targets. Thus, the monoclonal α-Trk-Alabeled a single band of ∼140 kDa in spinal cord, DRG, and whole brain and failed to react with proteins from muscle and kidney (Fig. 5,bottom left), in agreement with the expression of Trk-A in those tissues (Lomen-Hoerth and Shooter, 1995; Wheeler et al., 1998). The polyclonal α-flg labeled a single band of ∼90 kDa in DRG, lung, spinal cord, heart, and brain (Fig. 5, bottom right), as expected from the literature (Perderiset et al., 1992; Hughes and Hall, 1993; Sugi et al., 1995). The presence of more than one band in skeletal muscle most probably reflected the antibody reaction with the C-terminal epitope of truncated forms of FGFR-1 that was present in that tissue (Templeton and Hauschka, 1992).
Requirement of bFGF by postnatal P-neuronsin vivo
To investigate whether the survival promoting effect of bFGF on P-neurons that was observed in vitro also occurred in vivo, postnatal rats received daily intraperitoneal injections of an antibody against bFGF (α-bFGF) during the days P2–P5 aimed at sequestering the factor that P-neurons needed from P2 onward. This strategy has been successfully used for studying the dependence of sensory neurons on NGF and other trophic factors (Carroll et al., 1992; Ruit et al., 1992) or NT-3 (Oakley et al., 1995; Zhou and Rush, 1995a; Lefcort et al., 1996). We compared the number of P-neurons in DRG cultures obtained from injected animals with their number in control cultures obtained from PBS-injected animals. If P-neurons did require bFGF in vivo, then there should be a deficit of those cells in cultures of antibody-injected animals. In agreement with this expectation, there was a substantial reduction in the number of P-neurons found in cultures obtained from antibody-injected animals, with a 2.4-fold reduction of their total number (Fig.6A). The detection by immunoblot of circulating antibody shortly after its intraperitoneal injection gave support to our assumption that it reached the peripheral nervous system through the bloodstream (Fig. 6B). In addition, in cultures of antibody-injected animals, there was a marked reduction in the number of fibroblasts and glial cells, which were ubiquitous in cultures from control or PBS-injected animals. This further indicated that the circulating antibody effectively neutralized the endogenous bFGF, because those cell types require that factor for survival (Vescovi et al., 1993). Representative photographs of cultures from control and Ab-injected animals are shown in Figure 6,C and D. In addition to the reduced number of P-neurons and non-neuronal cells, the culture of Ab-treated animals differed from the typical cultures in several respects. The neurons had a grainy profile and tended to form clusters. In agreement with the lack of effect of bFGF on postnatal round neurons (Fig.4A), their number did not seem to be significantly reduced in antibody-injected animals. However, they had thinner nerve fibers at the time of the DRG isolation, and therefore we cannot exclude more subtle effects perhaps attributable to the loss of non-neuronal cells (Jessen and Mirsky, 1999) and references therein.
Innervation of peripheral tissues by P-neurons
To further characterize the P-neuron sensory subpopulation, we studied to which peripheral tissues they provide afferent innervation. Several potential targets, such as skin, subcutaneous tissue, skeletal muscle, and joints were injected in vivo with the intensely fluorescent lipophilic neuroanatomical tracer DiI, which is selectively taken up by nerve cell terminals and diffuses centrally along the neuronal membranes (Schroeder and McCleskey, 1993). If P-neurons peripheral terminals selectively innervate any of the above targets, then the tracer should eventually reach the somata of P-neurons only after the injection of the specific target(s).
Inspection of DRG cultures prepared 2–3 d after DiI administration into a single peripheral site revealed labeled P-neurons only after skeletal muscle dye injections. Of 20 labeled neurons found in that case, seven were P-neurons (of an estimated population of 100 P-neurons), a reasonable yield of our minimum dye injection protocol (see Materials and Methods) (Fig.7A). Single injections into skin (n = 6), subcutaneous tissue (n = 13), or joints (n = 10) only labeled round neurons. Injections of a higher concentration of DiI into a large fraction of the subcutaneous tissue (∼30–40%) confirmed the lack of innervation of that tissue by P-neurons (Fig. 7B). As expected, that dye application yielded a larger number of labeled round neurons (Fig.7C). In these experiments, the phenotype of P-neurons was accurately identified under phase contrast before obtaining the images, as illustrated in Figure 7.
Fig. 7.
Peripheral target innervated by P-neurons as revealed by the retrograde transport of the lipophilic fluorescent neuronal tracer DiI (1%) injected into several tissues of newborn rats (P2). Cultures of DRG were examined for fluorescently labeled neurons (arrowheads) 3–4 d after a single injection of DiI.A, Phase contrast Nomarski photograph (left) and patchy pattern of DiI labeling (right) of the same P-neuron after the injection of a single 2 μl of DiI in the main muscle mass of both hindlegs.B, Dye injections into skin or subcutaneous targets never labeled P-neurons. Phase contrast photographs of two P-neurons (left, arrowheads) lacking DiI labeling (right, arrows) after large DiI injections at high concentration into several cutaneous sites of a rat.C, Round sensory neurons from the same animal in B labeled with DiI. Round labeled neurons (arrowheads) were also observed after injecting a very small volume (3 μl) of a low concentration of DiI into a single cutaneous site (data not shown).D, Photographs of the same view field after the treatment with antibodies against the β-tubulin isotype III (top) and the 200 kDa neurofilament protein (bottom). P-neurons showed no immunoreactivity for the 200 kDa neurofilament (arrows), whereas some round neurons were labeled. All neurons were positive for the β-tubulin isotype III, which clearly delineated the soma shape. Scale bars:A, D, 20 μm; B, 18 μm;C, 15 μm. The data were replicated three to four times.
Sensory innervation of skeletal muscle includes large myelinated fast-conducting and unmyelinated slow-conducting fibers (Zhou and Rush, 1995b). Our data suggest that P-neurons correspond to the second group. First, P-neurons showed no immunoreactivity for 200 kDa protein subunit of neurofilaments, an exclusive marker of large myelinated fast-conducting sensory neurons (Lawson et al., 1984) (Fig.7D). As expected, the antibody clearly labeled the large round neurons present in the same cultures. Second, the survival of embryonic fast-conducting myelinated muscle afferents (proprioceptors innervating muscle spindles) both in vivo and in vitro require NT-3 (Hory-Lee et al., 1993; Oakley et al., 1995,1997; Wright et al., 1997), a neurotrophin that had no effect on the survival of P-neurons (Fig. 4B). Lastly, the size of proprioceptors is among the largest of sensory neurons, whereas P-neurons fall into the medium size group.
Coexpression of substance P and other markers in P-neurons
We have previously shown that the somata of postnatal neurite-free P-neurons in culture display immunoreactivity for SP (Acosta and López, 1999), a peptide that has been involved in the transmission of nociceptive stimuli (Holland and Goldstein, 1990). Here, we have confirmed and extended those results. Every single postnatal P-neuron present in the culture was immunoreactive for SP (n = 50, replicated at least in eight assays). As is well known, SP immunoreactivity can be found in other sensory neuron subpopulations, particularly in small round neurons (O'Brien et al., 1989; Nothias et al., 1993). Unlike P-neurons, however, only a fraction of those subpopulations expressed the peptide (data not shown). Interestingly, two replications of double-labeling experiments showed that whenever embryonic P-neurons were immunoreactive forTrk-A, they lacked SP immunoreactivity (n = 12) (Fig.8A,B). In contrast, SP and flg strictly coexpressed in postnatal P-neurons (n = 8) (Fig. 8C,D). Thus, in addition to their unique requirement of NGF and bFGF, this one-to-one correlation between flg and SP expression was a unique marker further typifying these cells. Of the round neurons that expressed SP, only a minor fraction (∼7%; 2 of 29 neurons) showed immunoreactivity for flg (Fig.8E,F). Conversely, round neurons expressing flg lacked immunoreactivity for SP (data not shown).
Fig. 8.
Substance P expression strictly paralleled the expression of flg in cultured P-neurons, as illustrated by double-labeling immunofluorescence experiments. Left column shows examples of Trk-A (top row) or flg (other rows) immunoreactive neurons, and right column shows examples of substance P (SP) immunoreactive neurons. The rightand left panels in each row show the same view field. At E18 (top row), all P-neurons (arrowheads) expressed Trk-A(A) and none expressed SP immunoreactivity (B) (n = 12). A large number of round neurons were also immunoreactive for Trk-A(data not shown). At this stage there was no detectable immunolabeling for flg in P-neurons. At P2 (middle row), the P-neurons that were immunoreactive for flg(C) were also immunoreactive for SP (D) (n = 8). In contrast, those negative for flg were also negative for SP (data not shown). Only flg-positive P-neurons contained substance P. In non-P-neurons, coexpression of flg and SP was infrequent (∼7%). An example from P5 animals is shown inE and F. Arrowheadsindicate round neuron somata that coexpressed flg and SP, out of 29 round neurons present in the field. Arrowsindicate axons that showed SP, but not flg, immunoreactivity. In all cases, we used a rat monoclonal antibody against SP at a dilution of 1:20. Scale bars: A,B, 15 μm; C, D, 20 μm;E, F, 50 μm.
Potential sources of bFGF
Like other primary sensory neurons, the cell bodies of P-neurons are located within the DRG itself; their pseudounipolar axons project centrally and peripherally to the spinal cord and, as shown before, to skeletal muscle, respectively. Assessing whether the sites with which P-neuron somas or fibers anatomically relate (DRG, spinal cord, skeletal muscle) are potential sources of bFGF is important to hypothesize on how the switch in trophic dependence might correlate with developmental events, such as the establishment of innervation. The presence of bFGF was determined in the relevant peripheral tissues using Western blots at different developmental stages (E20, P1, and P5) (Fig.9A). bFGF was present in skeletal muscle as well as in the spinal cord and the DRG at all ages tested. Interestingly, the highest expression of the factor in muscle coincided with the time at which all P-neurons became bFGF-dependent. A more indirect evidence of the bFGF presence in the tissues examined above was obtained from a set of separate immunocytochemical experiments that were performed during the postnatal stage at which that factor promoted survival (P3–P6). The representative data of Figure 9 show that neurons and glial cells of the DRG (Fig.9B,D) and spinal cord (Fig.9E), as well as skeletal muscle fibers (Fig. 9C), were immunoreactive for bFGF. In sharp contrast, P-neuron themselves or fibroblasts completely lacked immunolabeling (Fig.9B,D). These data indicate that P-neurons may potentially obtain bFGF from several types of cells. Our data show that the factor is available in sites reachable by P-neurons at the time they become bFGF-dependent.
DISCUSSION
Distinguishing a group of sensory neurons as a distinct, homogenous subpopulation usually requires determining several characteristics of the cells, with their specific requirements of trophic factors during development being a major defining criterion (Cameron et al., 1992; Perl, 1992; Snider, 1994; Lewin and Barde, 1996). We report a set of properties of the cell group that we have referred to as P-neurons, which, together with our previous study (Acosta and López, 1999), indicate that they constitute a distinct subpopulation of the rat DRG. A defining feature of P-neurons was their requirement of NGF prenatally and bFGF postnatally for survival during development, because no other type of sensory neurons studied here or elsewhere depended on that specific sequence of trophic factors (Vescovi et al., 1993; Grothe and Wewetzer, 1996; Ogilvie et al., 2000). Originally reported by Buchman and Davies (1993), this pattern of trophic dependence has been increasingly recognized in sensory (Molliver et al., 1997) and other neuronal types (Davies, 1994). For instance, mouse trigeminal neurons prenatally switch from BDNF–NT-3 to NGF (Buchman and Davies, 1993), and IB4-positive mouse sensory neurons switch from NGF to GDNF early after birth (Molliver et al., 1997). The need of more than a trophic factor, either sequentially or simultaneously, has also been strongly implied by work done in null mutants for neurotrophins or their receptors (White et al., 1996; Liebl et al., 1997). P-neurons specifically express Trk-A (E18) orflg (P5) at the ages at which their respective ligands act as sole survival factors, a correlation suggesting that the membrane exchange of the relevant receptors may be linked to the regulation of the trophic switch in vivo, as hypothesized elsewhere (Hashino et al., 1999; Baudet et al., 2000). It is at present unclear to what extent NGF and bFGF act on overlapping groups of P-neurons.
Like most developing DRG neurons, embryonic P-neurons depend on NGF (Levi-Montalcini and Angeletti, 1968; Kucera et al., 1995; Lewin and Barde, 1996) and become independent of it shortly after birth. However, they have not been previously identified as a specific group depleted after prenatal NGF deprivation. This treatment causes the loss of most small diameter neurons, presumably peptidergic, and mediating nociceptive functions in the adult (Davies et al., 1987; Lewin and Mendell, 1993; Snider, 1994). From our data, P-neurons do not appear to be contemplated in that group. The former represent ∼70% of the total population, are claimed to express SP or CGRP, and mostly innervate skin (for review, see Snider, 1994), whereas the medium size P-neurons contribute a relatively small fraction, have a different morphological phenotype in culture, do not express SP whenever they express Trk-A, and innervate skeletal muscle but not cutaneous tissue (see below). This last finding is especially interesting in view of the fact that embryonic NGF-dependent neurons are assumed to include most nociceptors, despite being presently unclear whether muscle nociceptors require that neurotrophin (Lewin and Barde, 1996; Snider and McMahon, 1998). With regard to SP expression in P-neurons, it was detected at times that closely agree with previous findings in the rat DRG (Hall et al., 1997).
This is the first report, as far as we are aware, showing a clear survival-promoting effect of bFGF on peripheral sensory neurons. Previous studies reported a minor effect, if any, on the survival of chick DRG neurons (Eckenstein et al., 1990; Oppenheim et al., 1992), whereas it clearly acts as survival factor on central neurons (Beck et al., 1993; Grothe and Wewetzer, 1996). Most commonly, bFGF has been found to be a powerful mitogen and differentiating factor (Birren and Anderson, 1990; Birren et al., 1993; Vescovi et al., 1993; Vaccarino et al., 1999). A strong indication that bFGF is required in vivo for the survival of P-neurons is the large reduction in the number of those cells in newborn animals injected with the antibody against that factor. In particular, our result rules out that the dependence on bFGF observed in vitro results from the axotomy caused by tissue dissociation (Ji et al., 1995).
Our data fit only partially into the classical view that the survival of primary sensory neurons during development depends on the obtention of specific target-derived trophic factors by their growing axons (Levi-Montalcini and Angeletti, 1968; Lewin and Barde, 1996). P-neurons depend on NGF mostly before the development of target innervation by growing sensory axons (Reynolds et al., 1991; Coggeshall et al., 1994) (but see Mirnics and Koerber, 1995). Their requirement of NGF better correlates with the period of naturally occurring death, which peaks between E15 and E19, and is over just after birth. This conforms to the more recent notion that NGF is required by embryonic sensory neurons during the period of massive neuronal death, supplied by sources other than the peripheral targets, before their innervation (Coggeshall et al., 1994; Davies, 1997; Wetts and Vaughn, 1998). Accordingly, Trk genes (receptors for neurotrophins) are expressed early in development (Mu et al., 1993). Although we do not know whether P-neurons require NGF before E18, the factor appears to regulate their survival over a relatively extended time (E18–P2). One possible early source of trophic factors is the spinal cord (Snider et al., 1992; Fitzgerald et al., 1993; Coggeshall et al., 1994). Unlike NGF, the onset of bFGF effect occurs at an age in which sensory axons have reached skeletal muscle fibers, a target of P-neurons innervation (see below) (Coggeshall et al., 1994). Consistent with the idea of target-derived factor, bFGF is present in skeletal muscle at P3–P4, although it is also found in neuronal and glial cells of the spinal cord and the DRG, as shown by others (Moore et al., 1991; Ji et al., 1995; Grothe et al., 1997).
The notion of trophic switch implies that exactly the same cells initially requiring some trophic factor subsequently need another. No conclusive proof of this has been possible for two main reasons (Birren et al., 1993; Molliver et al., 1997; Enokido et al., 1999; Enomoto et al., 2000): (1) the neurons requiring each trophic factor might be generated in different waves of neurogenesis when the switch occurs very early in development, and (2) the lack of a property, or of a marker uniquely identifying a single subpopulation of living neurons. Although this study does not show a trophic switch in a single neuron, it leaves little room for an alternative explanation. Both NGF and bFGF requirements occurred after the termination of neurogenesis, and the phenotype of P-neurons allowed their unequivocal identification in culture. Moreover, the stable proportion of P-neurons over ages E18–P5, together with the specific effects of NGF and bFGF at E18 and P5, respectively, would imply that if the switch occurred in different groups, then NGF-dependent P-neurons must differentiate into round cells postnatally, whereas a matching number of separate, round neurons must differentiate into bFGF-dependent P-neurons. This speculation and the additional requirement that those two unrelated subpopulations should display a coordinated change in their sensitivity to the survival factors (Fig. 3) seem highly unreasonable.
Additional features distinguish the group of P-neurons. They project to skeletal muscle but not skin or joints. They are, however, different from the proprioceptors innervating muscle spindles. These are large, express the 200 kDa neurofilament but not peptides, and require NT-3 for survival during embryonic life (Hory-Lee et al., 1993; Kucera et al., 1995; Zhou and Rush, 1995b). P-neurons are smaller, contain SP but not the 200 kDa neurofilament, and do not require NT-3. Thus, they are likely to fall into the group of unmyelinated muscle afferents (Abrahams, 1986; Mense, 1996), although we cannot exclude projections to visceral targets. Interestingly, and consistent with our data, chick embryonic muscle sensory neurons require NGF (Hory-Lee et al., 1993), and adult muscle afferents only rarely express Trk-A(McMahon et al., 1994). The expression of the pain-related peptide SP in P-neurons merits some comments. First, the coexpression offlg and SP is a specific marker of postnatal P-neurons. Second, it suggests that they might have a nociceptive function in view of the fact that that all SP-containing sensory neurons have been reported to respond to noxious stimuli, and mostly project to deep, noncutaneous peripheral targets (Levine et al., 1993; Zheng and Lawson, 1994; Lawson et al., 1997). P-neurons might represent a subset of a larger subpopulation, of which only a fraction adopts a pear shape, and perhaps included in some group of sensory afferents previously studied. Nonetheless, this is the first study that characterizes them as a specific group. On the basis of this report and our previous work, we hypothesize that P-neurons represent nociceptors of skeletal muscle for which function could be strongly regulated by enkephalins (Acosta and López, 1999). Alternatively, they might correspond to fine afferents involved in SP-mediated, circulatory reflexes (Kniffeki et al., 1981; Wilson and Hand, 1997).
Footnotes
This work was supported by grants from the International Foundation for Science (F/2632-F2), Fondo Nacional para las Ciencias y la Tecnología (PICT 5-01202), Beca Carillo-Oñativia, and Secretaria de Ciencia y Técnica–Universidad Nacional de Córdoba (UNC) (163/99). H.S.L is a career member of Consejo Nacional de Investigaciones Científicas y Técnicas (CONICET) and professor of the Facultad de Psicología, UNC. C.G.A. is the recipient of a fellowship from CONICET. A.R.F. is a recipient of a fellowship from CONICOR and Agencia Cordoba Ciencia. We thank P. Marchetti for help with the statistical analysis, Dr. A. Lorenzo for helpful comments, Dr. Salvarezza for help with some experiments, and P. Panzetta for the gift of neurofilament antibody.
C.G.A. and A.R.F. contributed equally to this work.
Correspondence should be addressed to Héctor S. López, Instituto de Investigación Médica Mercedes y Martin Ferreyra, Casilla de Correo 389, (5000) Córdoba, Argentina. E-mail: hlopez@immf.uncor.edu.
REFERENCES
- 1.Abe K, Saito H. Neurotrophic effect of basic fibroblast growth factor is mediated by the p42/p44 mitogen-activated protein kinase cascade in cultured rat cortical neurons. Brain Res Dev Brain Res. 2000;122:81–85. doi: 10.1016/s0165-3806(00)00054-7. [DOI] [PubMed] [Google Scholar]
- 2.Abrahams V. Group III and IV receptors of skeletal muscle. Can J Physiol Pharmacol. 1986;64:509–514. doi: 10.1139/y86-083. [DOI] [PubMed] [Google Scholar]
- 3.Acosta C, López H. δ opioid receptor modulation of several voltage-dependent Ca2+ currents in rat sensory neurons. J Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baudet C, Mikaels A, Westphal H, Johansen J, Johansen T, Ernfors P. Positive and negative interactions of GDNF, NTN and ART in developing sensory neuron subpopulations, and their collaboration with neurotrophin. Development. 2000;127:4335–4344. doi: 10.1242/dev.127.20.4335. [DOI] [PubMed] [Google Scholar]
- 5.Beck KD, Knusel B, Hefti F. The nature of the trophic action of brain-derived neurotrophic factor, des(1–3)-insulin-like growth factor-1, and basic fibroblast growth factor on mesencephalic dopaminergic neurons developing in culture. Neuroscience. 1993;52:855–866. doi: 10.1016/0306-4522(93)90534-m. [DOI] [PubMed] [Google Scholar]
- 6.Berg MM, Sternberg DW, Parada LF, Chao MV. K-252a inhibits nerve growth factor-induced trk proto-oncogene tyrosine phosphorylation and kinase activity. J Biol Chem. 1992;267:13–16. [PubMed] [Google Scholar]
- 7.Birren SJ, Anderson DJ. A v-myc-immortalized sympathoadrenal progenitor cell line in which neuronal differentiation is initiated by FGF but not NGF. Neuron. 1990;4:189–201. doi: 10.1016/0896-6273(90)90094-v. [DOI] [PubMed] [Google Scholar]
- 8.Birren SJ, Lo L, Anderson DJ. Sympathetic neuroblasts undergo a developmental switch in trophic dependence. Development. 1993;119:597–610. doi: 10.1242/dev.119.3.597. [DOI] [PubMed] [Google Scholar]
- 9.Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118:989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 10.Cáceres A, Mautino J, Kosik K. Supression of MAP-2 in cultured cerebellar macroneurons inhibit minor neurite formation. Neuron. 1992;9:607–618. doi: 10.1016/0896-6273(92)90025-9. [DOI] [PubMed] [Google Scholar]
- 11.Cameron AA, Pover CM, Willis WD, Coggeshall RE. Evidence that fine primary afferent axons innervate a wider territory in the superficial dorsal horn following peripheral axotomy. Brain Res. 1992;575:151–154. doi: 10.1016/0006-8993(92)90436-d. [DOI] [PubMed] [Google Scholar]
- 12.Cardenas CG, Del Mar LP, Scroggs RS. Variation in serotonergic inhibition of calcium channel currents in four types of rat sensory neurons differentiated by membrane properties. J Neurophysiol. 1995;74:1870–1879. doi: 10.1152/jn.1995.74.5.1870. [DOI] [PubMed] [Google Scholar]
- 13.Carroll SL, Silos-Santiago I, Frese SE, Ruit KG, Milbrandt J, Snider WD. Dorsal root ganglion neurons expressing trk are selectively sensitive to NGF deprivation in utero. Neuron. 1992;9:779–788. doi: 10.1016/0896-6273(92)90040-k. [DOI] [PubMed] [Google Scholar]
- 14.Caterina M, Julius D. Sense and specificity: a molecular identity for nociceptors. Curr Opin Neurobiol. 1999;9:525–530. doi: 10.1016/S0959-4388(99)00009-4. [DOI] [PubMed] [Google Scholar]
- 15.Caterina M, Leffler A, Malmberg A, Martin W, Trafton J, Petersen-Zeitz K, Koltzenburg M, Basbaum A, Julius D. Impaired nociception and pain sensation in mice lacking the capsaicin receptor. Science. 2000;288:306–313. doi: 10.1126/science.288.5464.306. [DOI] [PubMed] [Google Scholar]
- 16.Coggeshall RE, Pover CM, Fitzgerald M. Dorsal root ganglion cell death and surviving cell numbers in relation to the development of sensory innervation in the rat hindlimb. Brain Res Dev Brain Res. 1994;82:193–212. doi: 10.1016/0165-3806(94)90163-5. [DOI] [PubMed] [Google Scholar]
- 17.Davies AM. Neurotrophic factors. Switching neurotrophin dependence. Curr Biol. 1994;4:273–276. doi: 10.1016/s0960-9822(00)00064-6. [DOI] [PubMed] [Google Scholar]
- 18.Davies AM. Neurotrophin switching: where does it stand? Curr Opin Neurobiol. 1997;7:110–118. doi: 10.1016/s0959-4388(97)80128-6. [DOI] [PubMed] [Google Scholar]
- 19.Davies AM, Bandtlow C, Heumann R, Korsching S, Rohrer H, Thoenen H. Timing and site of nerve growth factor synthesis in developing skin in relation to innervation and expression of the receptor. Nature. 1987;326:353–358. doi: 10.1038/326353a0. [DOI] [PubMed] [Google Scholar]
- 20.Dickenson AH, Sullivan AF, Knox R, Zajac JM, Roques BP. Opioid receptor subtypes in the rat spinal cord: electrophysiological studies with mu- and delta-opioid receptor agonists in the control of nociception. Brain Res. 1987;413:36–44. doi: 10.1016/0006-8993(87)90151-x. [DOI] [PubMed] [Google Scholar]
- 21.Eckenstein FP, Esch F, Holbert T, Blacher RW, Nishi R. Purification and characterization of a trophic factor for embryonic peripheral neurons: comparison with fibroblast growth factors. Neuron. 1990;4:623–631. doi: 10.1016/0896-6273(90)90120-5. [DOI] [PubMed] [Google Scholar]
- 22.Ehrhard PB, Otten U. Postnatal ontogeny of the neurotrophin receptors trk and trkB mRNA in rat sensory and sympathetic ganglia. Neurosci Lett. 1994;166:207–210. doi: 10.1016/0304-3940(94)90487-1. [DOI] [PubMed] [Google Scholar]
- 23.Enokido Y, Wyatt S, Davies AM. Developmental changes in the response of trigeminal neurons to neurotrophins: influence of birthdate and the ganglion environment. Development. 1999;126:4365–4373. doi: 10.1242/dev.126.19.4365. [DOI] [PubMed] [Google Scholar]
- 24.Enomoto H, Heuckeroth RO, Golden JP, Johnson EM, Milbrandt J. Development of cranial parasympathetic ganglia requires sequential actions of GDNF and neurturin. Development. 2000;127:4877–4889. doi: 10.1242/dev.127.22.4877. [DOI] [PubMed] [Google Scholar]
- 25.Ernfors P, Lee KF, Kucera J, Jaenisch R. Lack of neurotrophin-3 leads to deficiencies in the peripheral nervous system and loss of limb proprioceptive afferents. Cell. 1994;77:503–512. doi: 10.1016/0092-8674(94)90213-5. [DOI] [PubMed] [Google Scholar]
- 26.Everill B, Kocsis JD. Reduction in potassium currents in identified cutaneous afferent dorsal root ganglion neurons after axotomy. J Neurophysiol. 1999;82:700–708. doi: 10.1152/jn.1999.82.2.700. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira A, Caceres A. Expression of the class III beta-tubulin isotype in developing neurons in culture. J Neurosci Res. 1992;32:516–529. doi: 10.1002/jnr.490320407. [DOI] [PubMed] [Google Scholar]
- 28.Fitzgerald M, Kwiat GC, Middleton J, Pini A. Ventral spinal cord inhibition of neurite outgrowth from embryonic rat dorsal root ganglia. Development. 1993;117:1377–1384. doi: 10.1242/dev.117.4.1377. [DOI] [PubMed] [Google Scholar]
- 29.Gilabert R, McNaughton P. Enrichment of the fraction of nociceptive neurones in cultures of primary sensory neurons. J Neurosci Methods. 1997;71:191–198. doi: 10.1016/s0165-0270(96)00144-6. [DOI] [PubMed] [Google Scholar]
- 30.Grothe C, Wewetzer K. Fibroblast growth factor and its implications for developing and regenerating neurons. Int J Dev Biol. 1996;40:403–410. [PubMed] [Google Scholar]
- 31.Grothe C, Meisinger C, Hertenstein A, Kurz H, Wewetzer K. Expression of fibroblast growth factor-2 and fibroblast growth factor receptor 1 messenger RNAs in spinal ganglia and sciatic nerve: regulation after peripheral nerve lesion. Neuroscience. 1997;76:123–135. doi: 10.1016/s0306-4522(96)00355-7. [DOI] [PubMed] [Google Scholar]
- 32.Hall AK, Ai X, Hickman GE, MacPhedran SE, Nduaguba CO, Robertson CP. The generation of neuronal heterogeneity in a rat sensory ganglion. J Neurosci. 1997;17:2775–2784. doi: 10.1523/JNEUROSCI.17-08-02775.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hamill O, Marty A, Neher E, Sackmann B, Sigworth F. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;39:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 34.Harper AA, Lawson SN. Conduction velocity is related to morphological cell type in rat dorsal root ganglion neurones. J Physiol (Lond) 1985;359:31–46. doi: 10.1113/jphysiol.1985.sp015573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hashino E, Dolnick RY, Cohan CS. Developing vestibular ganglion neurons switch trophic sensitivity from BDNF to GDNF after target innervation. J Neurobiol. 1999;38:414–427. doi: 10.1002/(sici)1097-4695(19990215)38:3<414::aid-neu9>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 36.Holland LN, Goldstein BD. Changes of substance P-like immunoreactivity in the dorsal horn are associated with the “phasic” behavioral response to a formalin stimulus. Brain Res. 1990;537:287–292. doi: 10.1016/0006-8993(90)90370-q. [DOI] [PubMed] [Google Scholar]
- 37.Honig MG, Hume RI. Dil and diO: versatile fluorescent dyes for neuronal labelling and pathway tracing. Trends Neurosci. 1989;12:333–335. [PubMed] [Google Scholar]
- 38.Hory-Lee F, Russell M, Lindsay RM, Frank E. Neurotrophin 3 supports the survival of developing muscle sensory neurons in culture. Proc Natl Acad Sci USA. 1993;90:2613–2617. doi: 10.1073/pnas.90.7.2613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hughes SE, Hall PA. Immunolocalization of fibroblast growth factor receptor 1 and its ligands in human tissues. Lab Invest. 1993;69:173–182. [PubMed] [Google Scholar]
- 40.Jessen KR, Mirsky R. Schwann cells and their precursors emerge as major regulators of nerve development. Trends Neurosci. 1999;22:402–410. doi: 10.1016/s0166-2236(98)01391-5. [DOI] [PubMed] [Google Scholar]
- 41.Ji RR, Zhang Q, Zhang X, Piehl F, Reilly T, Pettersson RF, Hokfelt T. Prominent expression of bFGF in dorsal root ganglia after axotomy. Eur J Neurosci. 1995;7:2458–2468. doi: 10.1111/j.1460-9568.1995.tb01044.x. [DOI] [PubMed] [Google Scholar]
- 42.Kniffeki K, Mense S, Schmidt R. Muscle receptors with fine afferent fibers which may evoke circulatory reflexes. Circ Res. 1981;48:I25–31. [PubMed] [Google Scholar]
- 43.Koh JY, Gwag BJ, Lobner D, Choi DW. Potentiated necrosis of cultured cortical neurons by neurotrophins. Science. 1995;268:573–575. doi: 10.1126/science.7725105. [DOI] [PubMed] [Google Scholar]
- 44.Koizumi S, Contreras ML, Matsuda Y, Hama T, Lazarovici P, Guroff G. K-252a: a specific inhibitor of the action of nerve growth factor on PC 12 cells. J Neurosci. 1988;8:715–721. doi: 10.1523/JNEUROSCI.08-02-00715.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kucera J, Fan G, Jaenisch R, Linnarsson S, Ernfors P. Dependence of developing group Ia afferents on neurotrophin-3. J Comp Neurol. 1995;363:307–320. doi: 10.1002/cne.903630211. [DOI] [PubMed] [Google Scholar]
- 46.Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurons. J Comp Neurol. 1984;228:263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- 47.Lawson SN, Crepps BA, Perl ER. Relationship of substance P to afferent characteristics of dorsal root ganglion neurones in guinea-pig. J Physiol (Lond) 1997;505:177–191. doi: 10.1111/j.1469-7793.1997.00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Lefcort F, Clary DO, Rusoff AC, Reichardt LF. Inhibition of the NT-3 receptor TrkC, early in chick embryogenesis, results in severe reductions in multiple neuronal subpopulations in the dorsal root ganglia. J Neurosci. 1996;16:3704–3713. doi: 10.1523/JNEUROSCI.16-11-03704.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Levi-Montalcini R, Angeletti PU. Nerve growth factor. Physiol Rev. 1968;48:534–569. doi: 10.1152/physrev.1968.48.3.534. [DOI] [PubMed] [Google Scholar]
- 50.Levine JD, Fields HL, Basbaum AI. Peptides and the primary afferent nociceptor. J Neurosci. 1993;13:2273–2286. doi: 10.1523/JNEUROSCI.13-06-02273.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lewin GR, Barde YA. Physiology of the neurotrophins. Annu Rev Neurosci. 1996;19:289–317. doi: 10.1146/annurev.ne.19.030196.001445. [DOI] [PubMed] [Google Scholar]
- 52.Lewin GR, Mendell LM. Nerve growth factor and nociception. Trends Neurosci. 1993;16:353–359. doi: 10.1016/0166-2236(93)90092-z. [DOI] [PubMed] [Google Scholar]
- 53.Liebl DJ, Tessarollo L, Palko ME, Parada LF. Absence of sensory neurons before target innervation in brain-derived neurotrophic factor-, neurotrophin 3-, and TrkC-deficient embryonic mice. J Neurosci. 1997;17:9113–9121. doi: 10.1523/JNEUROSCI.17-23-09113.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lomen-Hoerth C, Shooter EM. Widespread neurotrophin receptor expression in the immune system and other nonneuronal rat tissues. J Neurochem. 1995;64:1780–1789. doi: 10.1046/j.1471-4159.1995.64041780.x. [DOI] [PubMed] [Google Scholar]
- 55.McMahon S, Armanini M, Ling L, Phillips H. Expression and coexpression of Trk receptors in subpopulations of adult primary sensory neurons projecting to identified peripheral targets. Neuron. 1994;12:1161–1171. doi: 10.1016/0896-6273(94)90323-9. [DOI] [PubMed] [Google Scholar]
- 56.Mense S. Group III and IV receptors in skeletal muscle: are they specific or polymodal? Prog Brain Res. 1996;113:83–100. doi: 10.1016/s0079-6123(08)61082-1. [DOI] [PubMed] [Google Scholar]
- 57.Mirnics K, Koerber HR. Prenatal development of rat primary afferent fibers. I. Peripheral projections. J Comp Neurol. 1995;355:589–600. doi: 10.1002/cne.903550408. [DOI] [PubMed] [Google Scholar]
- 58.Molliver D, Snider W. Nerve growth factor receptor TrkA is down-regulated during postnatal development by a subset of dorsal root ganglion neurons. J Comp Neurol. 1997;381:428–438. doi: 10.1002/(sici)1096-9861(19970519)381:4<428::aid-cne3>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 59.Molliver DC, Wright DE, Leitner ML, Parsadanian AS, Doster K, Wen D, Yan Q, Snider WD. IB4-binding DRG neurons switch from NGF to GDNF dependence in early postnatal life. Neuron. 1997;19:849–861. doi: 10.1016/s0896-6273(00)80966-6. [DOI] [PubMed] [Google Scholar]
- 60.Moore JW, Dionne C, Jaye M, Swain JL. The mRNAs encoding acidic FGF, basic FGF and FGF receptor are coordinately downregulated during myogenic differentiation. Development. 1991;111:741–748. doi: 10.1242/dev.111.3.741. [DOI] [PubMed] [Google Scholar]
- 61.Mu X, Silos-Santiago I, Carroll SL, Snider WD. Neurotrophin receptor genes are expressed in distinct patterns in developing dorsal root ganglia. J Neurosci. 1993;13:4029–4041. doi: 10.1523/JNEUROSCI.13-09-04029.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nothias F, Tessler A, Murray M. Restoration of substance P and calcitonin gene-related peptide in dorsal root ganglia and dorsal horn after neonatal sciatic nerve lesion. J Comp Neurol. 1993;334:370–384. doi: 10.1002/cne.903340304. [DOI] [PubMed] [Google Scholar]
- 63.Oakley RA, Garner AS, Large TH, Frank E. Muscle sensory neurons require neurotrophin-3 from peripheral tissues during the period of normal cell death. Development. 1995;121:1341–1350. doi: 10.1242/dev.121.5.1341. [DOI] [PubMed] [Google Scholar]
- 64.Oakley RA, Lefcort FB, Clary DO, Reichardt LF, Prevette D, Oppenheim RW, Frank E. Neurotrophin-3 promotes the differentiation of muscle spindle afferents in the absence of peripheral targets. J Neurosci. 1997;17:4262–4274. doi: 10.1523/JNEUROSCI.17-11-04262.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.O'Brien C, Woolf CJ, Fitzgerald M, Lindsay RM, Molander C. Differences in the chemical expression of rat primary afferent neurons which innervate skin, muscle or joint. Neuroscience. 1989;32:493–502. doi: 10.1016/0306-4522(89)90096-1. [DOI] [PubMed] [Google Scholar]
- 66.Ogilvie JM, Speck JD, Lett JM. Growth factors in combination, but not individually, rescue rd mouse photoreceptors in organ culture. Exp Neurol. 2000;161:676–685. doi: 10.1006/exnr.1999.7291. [DOI] [PubMed] [Google Scholar]
- 67.Oppenheim RW, Prevette D, Fuller F. The lack of effect of basic and acidic fibroblast growth factors on the naturally occurring death of neurons in the chick embryo. J Neurosci. 1992;12:2726–2734. doi: 10.1523/JNEUROSCI.12-07-02726.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perderiset M, Courty J, Mereau A, Chevet E, Barritault D. Purification of a heparin binding FGF receptor (HB-FGFR) from adult bovine brain membranes. Biochimie. 1992;74:1091–1096. doi: 10.1016/0300-9084(92)90007-2. [DOI] [PubMed] [Google Scholar]
- 69.Perl ER. Function of dorsal root ganglion neurons: an overview. In: Scott SA, editor. Sensory neurons: diversity, development, and plasticity. Oxford UP; New York: 1992. pp. 3–23. [Google Scholar]
- 70.Reynolds ML, Fitzgerald M, Benowitz LI. GAP-43 expression in developing cutaneous and muscle nerves in the rat hindlimb. Neuroscience. 1991;41:201–211. doi: 10.1016/0306-4522(91)90210-f. [DOI] [PubMed] [Google Scholar]
- 71.Ruit KG, Elliott JL, Osborne PA, Yan Q, Snider WD. Selective dependence of mammalian dorsal root ganglion neurons on nerve growth factor during embryonic development. Neuron. 1992;8:573–587. doi: 10.1016/0896-6273(92)90284-k. [DOI] [PubMed] [Google Scholar]
- 72.Schmidt MF, Kater SB. Fibroblast growth factors, depolarization, and substratum interact in a combinatorial way to promote neuronal survival. Dev Biol. 1993;158:228–237. doi: 10.1006/dbio.1993.1181. [DOI] [PubMed] [Google Scholar]
- 73.Schroeder JE, McCleskey EW. Inhibition of Ca2+ currents by a μ-opioid in a defined subset of rat sensory neurons. J Neurosci. 1993;13:867–873. doi: 10.1523/JNEUROSCI.13-02-00867.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Silos-Santiago I, Molliver DC, Ozaki S, Smeyne RJ, Fagan AM, Barbacid M, Snider WD. Non-TrkA-expressing small DRG neurons are lost in TrkA-deficient mice. J Neurosci. 1995;15:5929–5942. doi: 10.1523/JNEUROSCI.15-09-05929.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Snider WD. Functions of the neurotrophins during nervous system development: what the knockouts are teaching us. Cell. 1994;77:627–638. doi: 10.1016/0092-8674(94)90048-5. [DOI] [PubMed] [Google Scholar]
- 76.Snider WD, McMahon SB. Tackling pain at the source: new ideas about nociceptors. Neuron. 1998;20:629–632. doi: 10.1016/s0896-6273(00)81003-x. [DOI] [PubMed] [Google Scholar]
- 77.Snider WD, Zhang L, Yusoof S, Gorukanti N, Tsering C. Interactions between dorsal root axons and their target motor neurons in developing mammalian spinal cord. J Neurosci. 1992;12:3494–3508. doi: 10.1523/JNEUROSCI.12-09-03494.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Standifer KM, Chien CC, Wahlestedt C, Brown GP, Pasternak GW. Selective loss of delta opioid analgesia and binding by antisense oligodeoxynucleotides to a delta opioid receptor. Neuron. 1994;12:805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- 79.Sugi Y, Sasse J, Barron M, Lough J. Developmental expression of fibroblast growth factor receptor-1 (cek-1; flg) during heart development. Dev Dyn. 1995;202:115–125. doi: 10.1002/aja.1002020203. [DOI] [PubMed] [Google Scholar]
- 80.Templeton TJ, Hauschka SD. FGF-mediated aspects of skeletal muscle growth and differentiation are controlled by a high affinity receptor, FGFR1. Dev Biol. 1992;154:169–181. doi: 10.1016/0012-1606(92)90057-n. [DOI] [PubMed] [Google Scholar]
- 81. Vaccarino FM, Schwartz ML, Raballo R, Nilsen J, Rhee J, Zhou M, Doetschman T, Coffin JD, Wyland JJ, Hung YT. Changes in cerebral cortex size are governed by fibroblast growth factor during embryogenesis. Nat Neurosci 2 1999. 246 253 [Errata (1999) 2:485, 848] [DOI] [PubMed] [Google Scholar]
- 82.Vescovi AL, Reynolds BA, Fraser DD, Weiss S. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron. 1993;11:951–966. doi: 10.1016/0896-6273(93)90124-a. [DOI] [PubMed] [Google Scholar]
- 83.Vogel KS. Development of trophic interactions in the vertebrate peripheral nervous system. Mol Neurobiol. 1993;7:363–382. doi: 10.1007/BF02769183. [DOI] [PubMed] [Google Scholar]
- 84.Wetts R, Vaughn JE. Peripheral and central target requirements for survival of embryonic rat dorsal root ganglion neurons in slice cultures. J Neurosci. 1998;18:6905–6913. doi: 10.1523/JNEUROSCI.18-17-06905.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Wewetzer K, Lausch M, Christ B. Macrowell cultures identify a subpopulation of neonatal rat dorsal root ganglionic neurons displaying nerve growth factor independent survival. Neurosci Lett. 1999;276:9–12. doi: 10.1016/s0304-3940(99)00791-0. [DOI] [PubMed] [Google Scholar]
- 86.Wheeler EF, Gong H, Grimes R, Benoit D, Vazquez L. p75NTR and Trk receptors are expressed in reciprocal patterns in a wide variety of non-neural tissues during rat embryonic development, indicating independent receptor functions. J Comp Neurol. 1998;391:407–428. [PubMed] [Google Scholar]
- 87.White FA, Silos-Santiago I, Molliver DC, Nishimura M, Phillips H, Barbacid M, Snider WD. Synchronous onset of NGF and TrkA survival dependence in developing dorsal root ganglia. J Neurosci. 1996;16:4662–4672. doi: 10.1523/JNEUROSCI.16-15-04662.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wilson L, Hand G. The pressor reflex evoked by static contraction: neurochemistry at the site of the first synapse. Brain Res Brain Res Rev. 1997;23:169–209. doi: 10.1016/s0165-0173(96)00019-7. [DOI] [PubMed] [Google Scholar]
- 89.Wood J, Docherty R. Chemical activators of sensory neurons. Annu Rev Physiol. 1997;59:457–482. doi: 10.1146/annurev.physiol.59.1.457. [DOI] [PubMed] [Google Scholar]
- 90.Wood J, Perl E. Pain. Curr Opin Genet Dev. 1999;9:328–332. doi: 10.1016/s0959-437x(99)80049-5. [DOI] [PubMed] [Google Scholar]
- 91.Wright DE, Zhou L, Kucera J, Snider WD. Introduction of a neurotrophin-3 transgene into muscle selectively rescues proprioceptive neurons in mice lacking endogenous neurotrophin-3. Neuron. 1997;19:503–517. doi: 10.1016/s0896-6273(00)80367-0. [DOI] [PubMed] [Google Scholar]
- 92.Yao R, Cooper GM. Requirement for phosphatidylinositol-3 kinase in the prevention of apoptosis by nerve growth factor. Science. 1995;267:2003–2006. doi: 10.1126/science.7701324. [DOI] [PubMed] [Google Scholar]
- 93.Zheng F, Lawson SN. Immunocytochemical properties of rat renal afferent neurons in dorsal root ganglia: a quantitative study. Neuroscience. 1994;63:295–306. doi: 10.1016/0306-4522(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 94.Zhou XF, Rush R. Sympathetic neurons in neonatal rats require endogenous neurotrophin-3 for survival. J Neurosci. 1995a;15:6521–6530. doi: 10.1523/JNEUROSCI.15-10-06521.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Zhou XF, Rush RA. Peripheral projections of rat primary sensory neurons immunoreactive for neurotrophin 3. J Comp Neurol. 1995b;363:69–77. doi: 10.1002/cne.903630107. [DOI] [PubMed] [Google Scholar]