Abstract
The CB1 cannabinoid receptor is a constitutively active receptor that can sequester Gi/o-proteins and prevent other Gi/o-coupled receptors from signaling (Bouaboula et al., 1997; Pan et al., 1998; Vasquez and Lewis, 1999). G-protein sequestration occurs because the population of CB1 cannabinoid receptors exists in both an inactive G-protein-precoupled RGGDP state and a constitutively active R*GGTPstate. We tested the hypothesis that the distal C-terminal tail acts to prevent G-protein activation. We found that truncation of the distal C-terminal tail of the CB1 receptor (CB1–417) enhanced both the constitutive activity and the ability of the receptor to sequester G-proteins. In addition, we tested the hypothesis that the conserved aspartate (D2.50) in the second transmembrane domain of the CB1 cannabinoid receptor is crucial for constitutive activity and G-protein sequestration. We found that the mutation of aspartate to asparagine (CB1-D164N) abolished G-protein sequestration and constitutive receptor activity without disrupting agonist-stimulated activity. We conclude that the CB1-D164N mutation and the C-terminal truncation shift the population of receptors in opposite directions. The CB1-D164N mutation shifts the receptor into an inactive R state upcoupled from G-proteins, whereas the C-terminal truncation (CB1–417) shifts the receptor into the active R*GGTP state. Thus the distal C-terminal tail acts to constrain the receptor from activating G-proteins, whereas the aspartate (D2.50) in the second transmembrane domain stabilizes the receptor in both the inactive RGGDP state and the active R*GGTP state.
Keywords: G-protein-coupled receptors, patch clamp, calcium channels, constitutive activity, receptor states, cannabinoid, tonic activity, C terminal
The discovery of the CB1 cannabinoid receptor (Howlett and Fleming, 1984; Howlett, 1985; Matsuda et al., 1990) and the demonstration that cannabinoid receptors are the most abundant G-protein-coupled receptor in the brain (Herkenham et al., 1990) stimulated questions about its physiological function. The active ingredient in marijuana, Δ9-tetrahydrocannabinol, binds to the CB1 receptor and is effective in alleviating pain and nausea, stimulating appetite, and affecting memory and mood. Endogenous cannabinoid ligands, anandamide and 2-arachidonylglycerol, are made in response to neuronal activity (Devane et al., 1992; Di Marzo et al., 1994, 1998; Stella et al., 1997) and act as fast retrograde messengers (Kreitzer and Regehr, 2001; Ohno-Shosaku et al., 2001; Wilson and Nicoll, 2001). CB1 receptors are found in high density in GABAergic neurons (Matsuda et al., 1993; Tsou et al., 1998), and CB1 receptors inhibit GABA release (Katona et al., 1999; Hajos et al., 2000; Hoffman and Lupica, 2001; Wilson and Nicoll, 2001) by inhibiting Ca2+ channels (Mackie and Hille, 1992;Mackie et al., 1995; Pan et al., 1996; Twitchell et al., 1997; Shen and Thayer, 1998; Sullivan, 1999).
The CB1 cannabinoid receptor is unusual because it is constitutively active and therefore is able to transduce a biological signal in the absence of ligand (Bouaboula et al., 1997; Landsman et al., 1997;MacLennan et al., 1998; Pan et al., 1998; Coutts et al., 2000). The CB1 receptor is also unusual in that it can sequester Gi/o-proteins and prevent other Gi/o-coupled receptors from signaling (Bouaboula et al., 1997; Vasquez and Lewis, 1999). However, the structural basis of constitutive activity and G-protein sequestration is unknown. A peptide fragment representing the juxtamembrane C-terminal tail of the CB1 cannabinoid receptor can activate G-proteins (Howlett et al., 1998). We hypothesized that the proximal C-terminal tail of the CB1 cannabinoid receptor is responsible for constitutive activity and that the distal C-terminal tail acts to prevent G-protein activation. We predicted that removal of the distal C-terminal tail (CB1–417) would enhance both the constitutive activity and the ability of the CB1 cannabinoid receptor to sequester G-proteins.
Mutation of aspartate to asparagine in the second transmembrane domain of the CB1 receptor (CB1-D164N) selectively blocked coupling to inwardly rectifying K+ channels while leaving coupling to the Ca2+ channels intact (Roche et al., 1999). Thus the CB1-D164N mutation may destabilize G-protein coupling. We therefore hypothesized that the aspartate in the second transmembrane domain of the CB1 receptor plays a crucial role in stabilizing the G-protein-coupled conformation of the receptor. We predicted that the CB1-D164N mutation would destabilize G-protein coupling and block the ability of the CB1 receptor to sequester G-proteins and to adopt a constitutively active conformation. We found that the CB1-D164N mutant receptor was not constitutively active and could not sequester G-proteins. We also found that truncation of the distal C terminal resulted in a receptor with enhanced constitutive activity and a greater ability to sequester G-proteins.
MATERIALS AND METHODS
Molecular biological procedures. The human brain cannabinoid receptor CB1 cDNA (from Dr. Tom I. Bonner, Laboratory of Cell biology, National Institute of Mental Health, Bethesda, MD) was subcloned into the mammalian expression vector pCI (Promega, Madison, WI) as described previously (Pan et al., 1996). The C-terminal-truncated CB1–417 receptor was constructed by using PCR techniques to delete amino acids 418–472. To truncate the CB1 receptor, we amplified a DNA segment from a restriction site (StuI) in the middle of the coding sequence of CB1 to the truncation site by PCR via Taq polymerase (Promega). The upstream PCR primer included the StuI site, GTG CGT CAT CCT CCA CTC. A stop codon was included in the downstream primer, CGA GAT CTC GTC AGC CTT CAC AAG AGG GAA AC. The PCR conditions were 30 cycles of 95°C for 1 min, 50°C for 1 min, and 72°C for 1.5 min. The PCR product was subcloned into pGEM-T (Promega). Both pCI-CB1 and the PCR product in pGEM-T were digested with StuI andNotI. The PCR fragment excised from pGEM-T was ligated back into pCI-CB1 to replace the original excised segment and was transformed into JM109 cells (Promega). Colonies were screened for ligation of the PCR fragment by size restriction analysis by usingStuI and NotI and were confirmed by sequencing (sequencing facility of the Medical College of Georgia). The mutant CB1 receptor in which the aspartate in the second transmembrane domain was mutated to asparagine, CB1-D164N in pcDNA3, was a gift from Dr. Kenneth Mackie (University of Washington, Seattle, WA). Preparation of plasmid DNA was accomplished with a plasmid prep kit (Qiagen, Santa Clarita, CA).
Neuron preparation and microinjection. Superior cervical ganglion (SCG) neurons were isolated from adult male Wistar rats (350–375 gm) in accordance with National Institutes of HealthGuidelines for the Care and Use of Laboratory Animals in Research and approved by the Committee on Animal Use for Research and Education at the Medical College of Georgia. All efforts were made to minimize animal suffering and to use only the number of animals necessary to produce reliable scientific data. Isolated superior cervical ganglia were treated with 0.3 mg/ml trypsin, 0.45 mg/ml collagenase D (Boehringer Mannheim, Indianapolis, IN), and 0.1 mg/ml DNase in Earle's balanced salt solution for 1 hr at 35°C in a shaking water bath. Then the flask was shaken vigorously by hand for 10 sec to dissociate the neurons. Dissociated neurons were plated onto poly-l-lysine-coated 35 mm culture dishes in MEM (Life Technologies, Gaithersburg, MD) with 10% fetal calf serum, 1% glutamine, and 1% penicillin–streptomycin. Neurons were incubated in a humidified incubator at 37°C in 5% CO2. After 4–5 hr to allow neurons to attach to the culture dishes, CB1, CB1–417, or CB1-D164N plasmid cDNA was microinjected directly into the nucleus of single SCG neurons in concentrations of 50 or 100 ng/μl in water. The pEGFP-N1 plasmid (10 ng/μl) containing the coding sequence of the enhanced green fluorescent protein (Clontech, Palo Alto, CA) was used as a coinjection marker. The plasmid solution was centrifuged (16,000 ×g) in nonheparinized hematocrit tubes for 20 min to remove suspended debris. Injection pipettes were pulled from fiber-filled capillary glass (1B120F-4; World Precision Instruments, Sarasota, FL) on a P-97 Flaming-Brown micropipette puller (Sutter Instrument, Novato, CA). SCG neurons were microinjected with an Eppendorf 5246 transjector and 5171 micromanipulator (Madison, WI), using an injection pressure of 75–100 hPa and an injection time of 0.3–0.4 sec.
Electrophysiological recording of Ca2+currents. Ca2+ currents from rat SCG neurons were recorded at room temperature (22–26°C) 16–20 hr after injection by the whole-cell variant of the patch-clamp technique (Hamill et al., 1981) with an Axopatch 200A patch-clamp amplifier (Axon Instruments, Foster City, CA). The pipettes for patch recording were pulled from borosilicate glass capillaries (Corning 7052; Garner Glass, Claremont, CA). Patch electrodes were coated with Sylgard 184 (Dow Corning, Midland, MI) and fire-polished on a microforge (Narishige, Tokyo, Japan). Pipette resistances ranged from 2.8 to 3.5 MΩ when filled with the internal solution described below. The cell membrane capacitance and series resistance were compensated electronically to >80%. Whole-cell currents were low-pass filtered at 5 kHz, using the Bessel filter of the clamp amplifier.
Voltage-clamp protocols were generated with a Power Macintosh 8600/200 computer (Apple Computer, Cupertino, CA) equipped with a PCI-16 Host Interface card connected to an ITC-16 Data Acquisition Interface (Instrutech, Port Washington, NY) using Pulse Control 5.0 extended operations (Richard J. Bookman, Jack D. Herrington, and Kenneth R. Newton, University of Miami, Miami, FL) with IGOR software (WaveMetrics, Lake Oswego, OR). Ca2+currents were elicited by voltage steps from a holding potential of −80 mV and digitized at 180 μsec per point. A double-pulse protocol consisting of two 25 msec steps to +5 mV was used to elicit Ca2+ currents. The first step to +5 mV elicited the control Ca2+ current. The second step to +5 mV was preceded by a 50 msec step to +80 mV. The current elicited by the second voltage step to +5 mV was facilitated when compared with the control current elicited by the first voltage step. Current amplitudes were measured isochronally 10 msec after the first voltage step to +5 mV.
Solutions. To isolate Ca2+currents for whole-cell recording, we bathed the cells in an external solution that contained (in mm): 140 tetraethylammonium methanesulfonate, 10 HEPES, 15 glucose, 10 CaCl2, and 0.0001 tetrodotoxin, pH 7.4 (adjusted with methanesulfonic acid). The intracellular solution consisted of (in mm): 120N-methyl-d-glucamine, 20 tetraethylammonium chloride, 10 HEPES, 11 EGTA, 1 CaCl2, 4 Mg-ATP, 0.1 Na2-GTP, and 14 phosphocreatine, pH 7.2 (adjusted with methanesulfonic acid).
The SF-77B Perfusion Fast-Step device (Warner Instrument, Hamden, CT) was used to apply the cannabinoid receptor agonist WIN 55, 212-2 mesylate (RBI/Sigma, St. Louis, MO), the cannabinoid receptor inverse agonist SR 141716A (a gift from Sanofi-Synthélabo, Paris, France), and the α2-adrenergic agonist UK 14304 (RBI/Sigma). Stock solutions of 10 mm WIN 55,212-2, SR 141716A, and UK 14304 were prepared in dimethylsulfoxide. On the day of the experiment the stock solution of WIN 55,212-2, SR 141716A, and UK 14304 was diluted to 1 μm in external solution and briefly sonicated (20 sec) to facilitate dispersion. This concentration of DMSO in external solution had no effect on the Ca2+ current.
Results are presented as means ± SEM where appropriate. Statistical significance was determined by Student's ttest. The differences were considered significant at p< 0.05.
RESULTS
Deletion of the distal C-terminal tail enhances the constitutive activity and the ability of the CB1 cannabinoid receptor to sequester G-proteins
The C-terminal-truncated CB1–417 receptor in which amino acids 418–472 were deleted was tested for both constitutive activity and G-protein sequestration. Constitutive activity of the CB1 cannabinoid receptor resulted in a tonic inhibition of the voltage-dependent Ca2+ current that was reversed by the CB1 inverse agonist SR 141716A. Both the wild-type CB1 and the truncated CB1–417 receptors were constitutively active. SR 141716A increased the Ca2+ current in neurons expressing CB1 and CB1–417 cannabinoid receptors (Fig. 1). To compare the constitutive activity between the wild-type CB1 and truncated CB1–417 cannabinoid receptors, we injected two different concentrations of receptor cDNA into the nuclei of SCG neurons. In SCG neurons that were injected with 100 ng/μl wild-type CB1 receptor cDNA, SR 141716A increased the Ca2+current 60.2 ± 13.7% (n = 10). In neurons that were injected with 100 ng/μl CB1–417 cDNA, SR 141716A increased Ca2+ current 110.0 ± 2.3% (n = 10) (Fig. 1). The difference between these groups was not significant (p = 0.08). However, in neurons that were injected with 50 ng/μl CB1–417 cDNA, the increase in the Ca2+ current by SR 141716A was significantly (p < 0.05) greater compared with the wild-type CB1 receptor. SR 141716A increased the Ca2+ current 101.1 ± 18.9% (n = 5) in neurons that were injected with 50 ng/μl CB1–417 cDNA compared with 43.1 ± 7.5% (n = 4) in neurons that were injected with 50 ng/μl CB1 cDNA. These results indicate that at a reduced receptor population the number of C-terminal-truncated CB1–417 receptors that are in a constitutively active state is greater than the number of constitutively active wild-type CB1 receptors. To confirm these results, we tested the effect of the cannabinoid agonist WIN 55,212-2. Previous work has shown that the C-terminal-truncated CB1 receptor has a similar affinity for WIN 55,212-2 (Jin et al., 1999). If the truncated CB1–417 cannabinoid receptor has a greater constitutive activity, then a larger number of the cannabinoid receptor population should be in the active R*GGTP state and a cannabinoid agonist would be predicted to have little additional effect. Inhibition of the Ca2+ current by WIN 55,212-2 was significantly (p < 0.05) smaller in neurons expressing the truncated CB1–417 receptor (Fig. 1). WIN 55,212-2 (1 μm) inhibited the Ca2+ current 43.7 ± 6.5% (n = 7) in neurons expressing wild-type CB1 receptors compared with 22.6 ± 3.0 (n = 5) in neurons expressing the truncated CB1–417 receptors (Nie and Lewis, 2001).
Fig. 1.
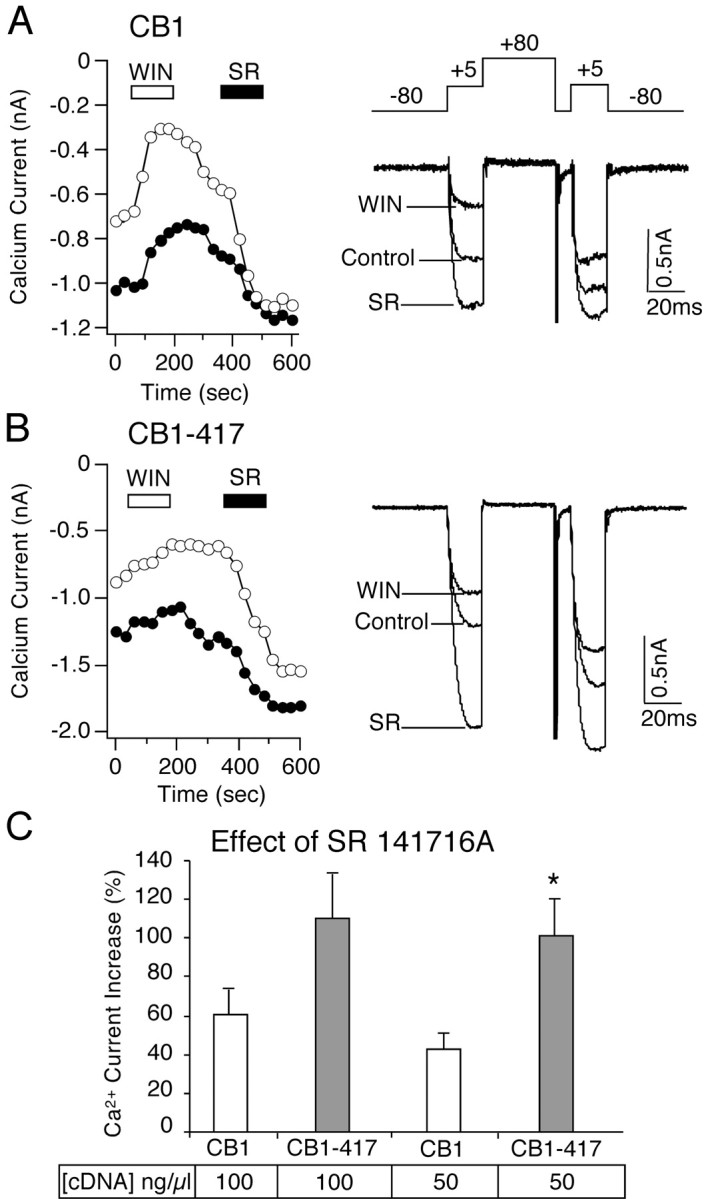
Truncation of the C-terminal tail of the CB1 cannabinoid receptor enhances constitutive activity. A, Left, Ca2+ current amplitude is plotted over the time course of the experiment (open circles, amplitude elicited by the first voltage step to +5 mV; filled circles, amplitude from the second step to +5 mV of a double-pulse protocol shown above the Ca2+ current traces on the right). In an SCG neuron expressing wild-type CB1 receptors (from 100 ng/μl cDNA injection) the application of the cannabinoid agonist WIN 55,212-2 (WIN) decreased the Ca2+current. Application of the CB1 inverse agonist SR 141716A (SR) increased the Ca2+ current.A, Right, Superimposed current traces in the absence (Control) and presence of 1 μm WIN 55,212-2 (WIN) or 1 μm SR 141716A (SR). B, Left, In an SCG neuron expressing the C-terminal-truncated CB1–417 receptor (from 100 ng/μl cDNA injection), WIN 55,212-2 produced a small inhibition of the Ca2+ current. A subsequent application of SR 141716A enhanced the Ca2+ current. B, Right, Superimposed current traces in the absence (Control) and presence of WIN 55,212-2 (WIN) or SR 141716A (SR).C, Bar graph of the enhancement of the Ca2+ current in the presence of SR 141716A in SCG neurons that were injected with 100 or 50 ng/μl wild-type CB1 cannabinoid receptor cDNA or truncated CB1–417 receptor cDNA. *p < 0.05 relative to 50 ng/μl CB1 cDNA injection.
As an additional test of the enhanced constitutive activity of the CB1–417 receptor, we tested whether its ability to sequester G-proteins would be enhanced. We have shown previously that the wild-type CB1 cannabinoid receptor can sequester G-proteins and prevent α2-adrenergic and somatostatin receptors from signaling (Vasquez and Lewis, 1999). G-protein sequestration occurs because the receptor resides in both an inactive G-protein-precoupled RGGDP state and a constitutively active R*GGTP state. If the truncated CB1–417 receptors primarily populate the constitutively active R*GGTP state by depopulating the inactive R state that is uncoupled from G-proteins, then as a population they should be better able to sequester G-proteins and prevent other receptors from signaling. To test this hypothesis, we injected SCG neurons with either 100 or 50 ng/μl CB1 receptor cDNA and tested for native α2-adrenergic receptor signaling. In SCG neurons the α2-adrenergic receptor agonist UK 14304 inhibited the Ca2+ current 44.5 ± 5.7% (n = 12) (Fig.2C). In neurons that were injected with either wild-type CB1 or CB1–417 cDNA (100 ng/μl), the effect of UK 14304 was abolished (p < 0.01). UK 14304 inhibited the Ca2+ current only 1.5 ± 4.2% (n = 4) in neurons expressing CB1 receptors and 0.2 ± 2.0% (n = 5) in neurons expressing CB1–417 receptors (Fig. 2C). However, when the wild-type CB1 cDNA concentration was reduced to 50 ng/μl, the effect of the α2-adrenergic agonist UK 14304 was partially restored (Fig. 2A,C). UK 14304 inhibited the Ca2+ current 20.0 ± 3.6% (n = 4) in neurons that were injected with 50 ng/μl CB1 cDNA. In contrast, in SCG neurons that were injected with 50 ng/μl CB1–417 cDNA the UK 14304 still had no effect (Fig.2B,C). UK 14304 inhibited the Ca2+ current 0.6 ± 1.2% (n = 5) in neurons that were injected with 50 ng/μl CB1–417 cDNA. These results suggest that the truncated CB1–417 receptor has an enhanced ability to sequester G-proteins.
Fig. 2.
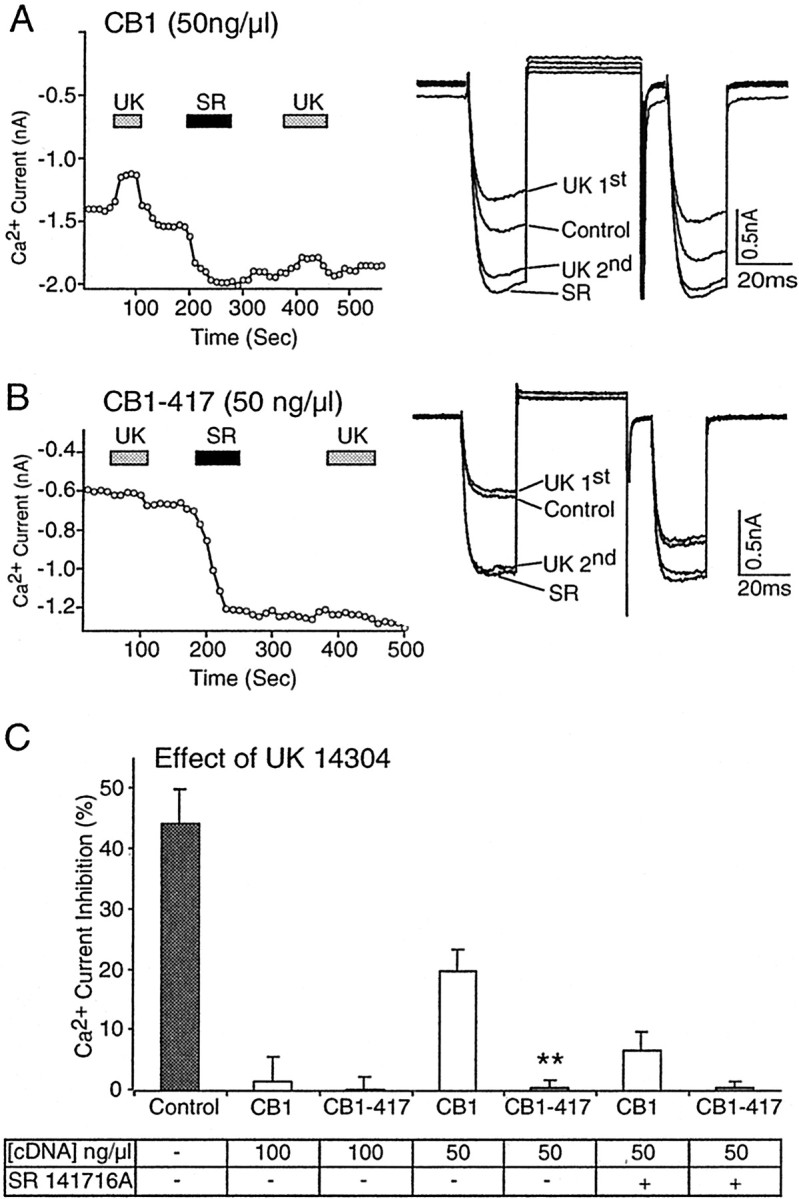
G-protein sequestration is enhanced by truncation of the distal C-terminal tail of the CB1 cannabinoid receptor.A, Left, In an SCG neuron that was injected with 50 ng/μl CB1, the cDNA application of the α2-adrenergic agonist UK 14304 (UK) produced a small inhibition of the Ca2+ current. Application of the CB1 cannabinoid receptor inverse agonist SR 141716A (SR) increased the Ca2+ current and blocked the effect of a subsequent application of UK 14304. A, Right, Superimposed current traces in the absence (Control) and presence of the first and second application of UK 14304 (UK) and SR 141716A (SR). B, Left, In an SCG neuron that was injected with 50 ng/μl CB1–417, cDNA application of UK 14304 had no effect on the Ca2+ current. Application of SR 141716A produced a large increase in the Ca2+current, and a subsequent application of UK 14304 also had no effect.B, Right, Superimposed current traces in the absence (Control) and presence of the first and second application of UK 14304 (UK) and SR 141716A (SR). C, Bar graph of Ca2+ current inhibition by UK 14304 in neurons expressing wild-type CB1 or truncated CB1–417 receptors from cDNA injections at the concentrations indicated in the table. The effect of UK 14304 in control neurons was abolished in neurons that were injected with 100 ng/μl CB1 or CB1–417 cDNA. The effect of UK 14304 was partially restored in neurons that were injected with 50 ng/μl CB1, but not with CB1–417 cDNA. The effect of UK 14304 was abolished after the application of SR 141716A for CB1-injected neurons (50 ng/μl). **p < 0.01 relative to wild-type CB1.
The partial restoration of α2-adrenergic receptor signaling in neurons that were injected with 50 ng/μl wild-type CB1 cDNA was abolished after the application of the inverse agonist SR 141716A (1 μm) (Fig. 2A). SR 141716A traps the CB1 cannabinoid receptor in its inactive G-protein-precoupled RGGDP state and prevents the G-proteins from coupling to α2-adrenergic receptors. In neurons that were injected with 50 ng/μl CB1 cDNA, the first application of UK 14304 inhibited the Ca2+ current 20.0 ± 3.6% (n = 4). After application of SR 141716A the UK 14304 inhibited the Ca2+ current only 6.7 ± 3.0% (n = 4) (Fig. 2C) in agreement with our previous work (Vasquez and Lewis, 1999). In contrast, in neurons that were injected with 50 ng/μl CB1–417 cDNA, the effect of UK 14304 was no different either before or after SR 141716A. In neurons that were injected with 50 ng/μl CB1–417 cDNA, UK 14304 inhibited the Ca2+ current 0.6 ± 1.2% (n = 5) before the application of SR 141716A and 0.6 ± 1.0% (n = 5) after SR 141716A (Fig.2C).
Mutation of aspartate in the second transmembrane domain of the CB1 receptor abolishes constitutive activity and G-protein sequestration
Mutation of aspartate in the second transmembrane domain of the rat CB1 cannabinoid receptor, CB1-D164N, disrupts activation of inwardly rectifying K+ channels while leaving Ca2+ channel modulation intact (Roche et al., 1999). Because modulation of both ion channels is mediated by receptor activation of G-proteins, we hypothesized that the D164N mutation impairs G-protein coupling. Wild-type CB1 cannabinoid receptors exist in an inactive G-protein-precoupled RGGDP state and a constitutively active R*GGTP state. The wild-type CB1 receptor also is predicted to populate an inactive G-protein-uncoupled R state. We hypothesized that the D164N mutation destabilizes the receptor such that more of the receptors are in the inactive R state uncoupled from Gi/o-proteins. Thus we predicted that the mutant CB1-D164N receptor would not be constitutively active and would not sequester G-proteins. If the CB1-D164N receptor is not constitutively active, then the CB1 inverse agonist SR 141716A should have little effect. If the CB1-D164N receptor does not sequester G-proteins, then signaling by the α2-adrenergic receptor should not be affected. In SCG neurons that were injected with CB1-D164N cDNA (100 ng/μl), the cannabinoid agonist WIN 55,212-2 (1 μm) inhibited the Ca2+current (Fig. 3A) in agreement with the results of others (Roche et al., 1999). Ca2+ current inhibition was similar for both CB1-D164N and wild-type CB1 receptors. WIN 55,212-2 inhibited the Ca2+ current 34.2 ± 4.8% (n = 6) in neurons expressing CB1-D164N receptors compared with 43.7 ± 6.5% (n = 7) in neurons expressing CB1 receptors (Fig.4A). However, unlike for the wild-type CB1 receptors the effect of SR 141716A was abolished (p < 0.05) in neurons expressing CB1-D164N receptors. In an SCG neuron expressing CB1-D164N receptors, SR 141716A (1 μm) produced a very small increase in the Ca2+ current (Fig. 3B). SR 141716A increased the Ca2+ current 60.2 ± 13.7% (n = 10) in neurons expressing wild-type CB1 receptors but only by 11.6 ± 6.9% (n = 5) in neurons expressing CB1-D164N receptors (Fig.4B). Thus the CB1-D164N receptor does not appear to be constitutively active.
Fig. 3.
Expression of the mutant CB1-D164N receptor does not interfere with signaling by the α2-adrenergic receptor. A, Left, In an SCG neuron that was injected with 100 ng/μl CB1-D164N cDNA, the cannabinoid agonist WIN 55,212-2 (1 μm) inhibited the Ca2+ current. In this neuron the effect of WIN 55,212-2 partially desensitized. A subsequent application of the α2-adrenergic agonist UK 14304 (1 μm) inhibited the Ca2+current. A, Right, Superimposed current traces in the absence (Control) and presence of WIN 55,212-2 (WIN) and UK 14304 (UK).B, Left, In an SCG neuron that was injected with 100 ng/μl CB1-D164N cDNA, the CB1 inverse agonist SR 141716A (1 μm) slightly increased the Ca2+current. A subsequent application of UK 14304 (1 μm) inhibited the Ca2+ current. B, Right, Superimposed current traces in the absence (Control) and presence of SR 141716A (SR) and UK 14304 (UK).
Fig. 4.
The D164N mutant CB1 receptor is not constitutively active but can be activated by a cannabinoid agonist.A, Bar graph of the Ca2+ current inhibition in the presence of the cannabinoid agonist WIN 55,212-2 (1 μm) in neurons that were injected with CB1 or CB1-D164N cDNA (100 ng/μl). B, Bar graph of the increase in the Ca2+ current by the CB1 inverse agonist SR 141716A in neurons that were injected with wild-type CB1 or CB1-D164N cDNA (100 ng/μl). *p < 0.05 relative to the CB1 receptor.
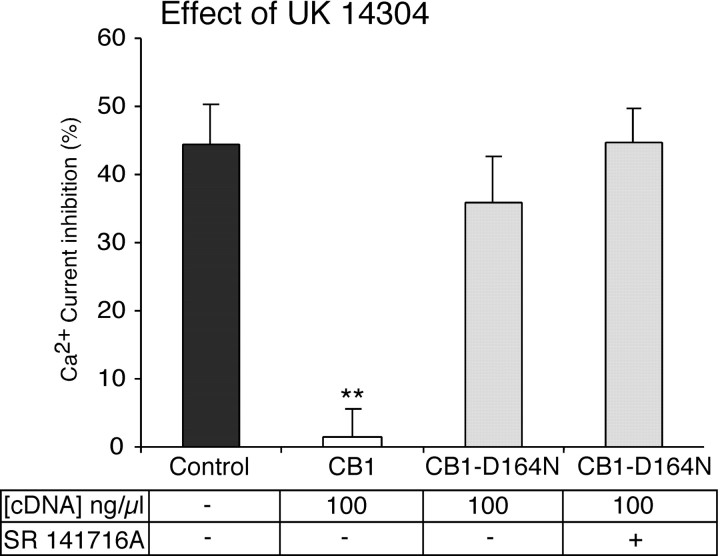
To test whether CB1-D164N receptors can sequester G-proteins, we tested the α2-adrenergic agonist UK 14304 in neurons that were injected with 100 ng/μl CB1-D164N cDNA. In SCG neurons expressing CB1-D164N receptors, UK 14304 (1 μm) produced a robust inhibition of the Ca2+current (Fig. 3). The effect of UK 14304 was no different between uninjected control neurons and neurons that were injected with CB1-D164N cDNA. UK 14304 inhibited the Ca2+ current 44.5 ± 5.7% (n = 12) in control neurons and 35.8 ± 6.8% (n = 5) in neurons expressing CB1-D164N receptors (100 ng/μl cDNA-injected) (Fig. 5). In contrast, UK 14304 had no effect (1.5 ± 4.2%; n= 4) in neurons expressing wild-type CB1 receptors (100 ng/μl cDNA-injected) (Fig. 5). Thus mutation of aspartate in the second transmembrane domain of the CB1 receptor abolished the ability of the CB1 receptor to sequester G-proteins and interfere with signaling by the G-protein-coupled α2-adrenergic receptor.
Fig. 5.
The CB1-D164N cannabinoid receptor does not sequester G-proteins. Bar graph of Ca2+ current inhibition by the α2-adrenergic agonist UK 14304 in uninjected control SCG neurons and in neurons expressing wild-type CB1 or CB1-D164N receptors. The effect of UK 14304 was abolished in neurons that were injected with wild-type CB1 receptor cDNA, but not in neurons that were injected with CB1-D164N cDNA. The effect of UK 14304 was no different in neurons expressing CB1-D164N receptors after the application of SR 141716A (1 μm). The concentration of receptor cDNA that was injected and the application of SR 141716A are indicated in the table. **p < 0.01 relative to control neurons or CB1-D164N-injected neurons.
G-protein sequestration by the wild-type CB1 receptor can occur in both inactive RGGDP and active R*GGTP receptor conformations (Vasquez and Lewis, 1999). Because the mutant CB1-D164N receptor failed to sequester G-proteins, we predicted that this receptor fails to adopt the inactive G-protein-precoupled RGGDP conformation. To determine whether the mutant CB1-D164N receptor can adopt the inactive G-protein-precoupled RGGDP state, we tested the ability of SR 141716A to stabilize the RGGDPstate. We have shown previously that SR 141716A acting on the wild-type CB1 receptor can sequester G-proteins by trapping the CB1 receptor in the RGGDP conformation and preventing signaling by the α2-adrenergic receptor (Vasquez and Lewis, 1999). SR 141716A failed to abolish the inhibition of the Ca2+ current by the α2-adrenergic agonist UK 14304 in neurons expressing CB1-D164N receptors (100 ng/μl). Ca2+ current inhibition by UK 14304 (1 μm) was 35.8 ± 6.8% (n = 5) before and 44.7 ± 5.0% (n = 4) after the application of SR 141716A (Fig. 5). Thus the CB1-D164N receptor, unlike the wild-type CB1 receptor, does not precouple to G-proteins in their inactive RGGDP state. These results suggest that the CB1-D164N receptor occupies an inactive state uncoupled from G-proteins but can couple to G-proteins in the presence of the cannabinoid agonist WIN 55,212-2 (Figs. 3A, 4A).
DISCUSSION
CB1 cannabinoid receptors are constitutively active, which results in a tonic inhibition of Ca2+ channels when expressed in SCG neurons. The CB1 cannabinoid receptors reside in two G-protein-coupled states, a constitutively active R*GGTP and an inactive RGGDP state (Bouaboula et al., 1997; Vasquez and Lewis, 1999). By stabilizing the inactive RGGDPstate, the CB1 inverse agonist SR 141716A reverses the constitutive activity by depopulating the active R*GGTP state. SR 141716A thus enhances the Ca2+ current in neurons expressing the CB1 receptors. By stabilizing the inactive RGGDP state, SR 141716A also prevents these G-proteins from interacting with other G-protein-coupled receptors.
In the present study SR 141716A produced a greater enhancement of the Ca2+ current when the distal C-terminal tail of the CB1 receptor was truncated. When 50 ng/μl cDNA was injected into SCG neurons, the enhancement of the Ca2+ current in the presence of SR 141716A was significantly (p < 0.05) greater for the C-terminal-truncated CB1–417 cannabinoid receptor compared with the wild-type CB1 receptor. However, when the cDNA injection concentration was 100 ng/μl, the enhancement of the Ca2+ current in the presence of SR 141716A approached but was not significantly (p = 0.08) greater for the CB1–417 cannabinoid receptor compared with the wild-type CB1 receptor. At the 100 ng/μl injection concentration both the wild-type CB1 and the C-terminal-truncated CB1–417 receptor populations are greater; therefore, the number of receptors that are constitutively active is greater. When the receptor density was reduced by injecting 50 ng/μl receptor cDNA, SR 141716A produced a larger increase in the Ca2+ current in neurons expressing CB1–417 receptors compared with wild-type CB1 receptors. Thus truncation of the distal C-terminal tail of the cannabinoid receptor promotes the constitutively active R*GGTP conformational state of the receptor. If a receptor is already in the active R*GGTPconformation, then the effect of the cannabinoid agonist WIN 55,212-2, which stabilizes the active R*GGTP state, would be predicted to have little additional effect. Consistent with this prediction, the effect of WIN 55,212-2 on both the Ca2+ channels and the inwardly rectifying K+ channels was reduced by truncation of the distal C terminal (Jin et al., 1999; Nie and Lewis, 2001). The distal C-terminal tail of the cannabinoid receptor thus acts to constrain the receptor to its inactive RGGDPconformation and slows its transition to the active R*GGTP conformation.
Several other studies on G-protein-coupled receptors also have shown a role for the C terminal in constitutive activity. Deletion of the C terminal of the β-adrenergic receptor enhances its constitutive activity (Parker and Ross, 1991), and the constitutive activity of the D5 dopamine receptor is critically dependent on the C terminal (Demchyshyn et al., 2000). Additionally, shorter C-terminal variants of 5-HT4 receptors have been shown to have greater constitutive activity (Claeysen et al., 1999). Thus, for a subset of G-protein-coupled receptors that show constitutive activity, the C-terminal tail appears to play a critical role in limiting G-protein activation.
The wild-type CB1 cannabinoid receptor is unusual because it is both constitutively active and it sequesters Gi/o-proteins, preventing other Gi/o-coupled receptors from signaling (Vasquez and Lewis, 1999). The α2-adrenergic receptor agonist UK 14304 inhibits the Ca2+ current in SCG neurons by activating native Gi/o-coupled α2-adrenergic receptors (Schofield, 1990,1991). When CB1 cannabinoid receptors are expressed in SCG neurons, the inhibitory effect of the α2-adrenergic agonist UK 14304 on the Ca2+ current is abolished. The effect of UK 14304 is abolished because the CB1 cannabinoid receptors sequester a common pool of Gi/o-proteins (Vasquez and Lewis, 1999). Sequestration of Gi/o-proteins by the CB1 cannabinoid receptor occurs in both the inactive RGGDP as well as the active R*GGTP conformations (Vasquez and Lewis, 1999).
The results of the present study show that truncation of the distal C-terminal tail of the CB1 cannabinoid receptor enhanced the ability of the receptor to sequester G-proteins. Injection of 100 ng/μl of either CB1 or CB1–417 cDNA abolished signaling by the α2-adrenergic receptor. Reducing the cDNA concentration to 50 ng/μl partially restored α2-adrenergic receptor signaling in neurons expressing wild-type CB1 receptors, but not in CB1–417 receptors. Thus deletion of the distal C terminal of the CB1 receptor enhanced the ability of the receptor to sequester G-proteins. The opposite effect was obtained by the mutation of aspartate in the second transmembrane domain. The mutant CB1-D164N receptor failed to sequester G-proteins.
Unlike the wild-type CB1 receptor, the mutant CB1-D164N receptor showed little ability to adopt the constitutively active R*GGTP state. The active R*GGTP state causes a tonic inhibition of the Ca2+ current in SCG neurons that is reversed by the CB1 inverse agonist SR 141716A. SR 141716A failed to increase the Ca2+ current in neurons expressing the mutant CB1-D164N receptors, indicating that very few receptors are in the active R*GGTP state. The mutant CB1-D164N receptor also does not precouple to G-proteins in the inactive RGGDP state. SR 141716A traps the wild-type CB1 receptor in the inactive RGGDPstate, which results in G-protein sequestration and the complete block of α2-adrenergic receptor signaling. Signaling by UK 14304 was unaffected by SR 141716A in neurons expressing mutant CB1-D164N receptors. Given that the affinity of SR 141716A is unchanged by the mutation of aspartate to asparagine in the second transmembrane domain (Tao and Abood, 1998), our results suggest that this amino acid plays a critical role in stabilizing both the inactive G-protein-precoupled RGGDP and the active R*GGTP receptor conformations in the absence of an agonist. However, in the presence of the cannabinoid agonist WIN 55,212-2 the mutant CB1-D164N receptor shows robust G-protein coupling, suggesting that the mutant receptor can undergo the conformational changes to the active R*GGTP conformation.
Previous work has shown that mutation of the aspartate residue in the second transmembrane domain of the CB1 receptor causes it to lose its ability to activate inwardly rectifying K+channels and to inhibit forskolin-stimulated cAMP accumulation, but not its ability to inhibit Ca2+ channels (Tao and Abood, 1998; Roche et al., 1999). Similar results were found for the α2-adrenergic receptor. Mutation of the aspartate residue in the second transmembrane domain of the α2-adrenergic receptor blocked G-protein coupling to inwardly rectifying K+channels, but not to Ca2+ channels (Surprenant et al., 1990). Our study found that mutation of the aspartate residue in the second transmembrane domain (D2.50) blocked both constitutive activity and the ability of the CB1 receptor to sequester G-proteins. These results suggest that the D2.50 aspartate residue is critical for maintaining a receptor conformation with a high affinity for Gi/o-proteins in the absence of an agonist. However, because the cannabinoid agonist WIN 55,212-2 is able to activate the mutant CB1-D164N receptor and cause Ca2+ channel modulation, this indicates that the mutant receptor can adopt an agonist-occupied conformational state with high affinity for Gi/o-proteins. The agonist-occupied G-protein-coupled mutant receptor may select for specific Gi/o-proteins that affect Ca2+ channels, but not inwardly rectifying K+ channels or adenylyl cyclase.
Mutagenesis studies of several G-protein-coupled receptors indicated an interaction between aspartate (D2.50) and asparagine (N7.49) residues in the second and seventh transmembrane domains that regulate receptor activation (Zhou et al., 1994; Xu et al., 1999; Wilson et al., 2001). However, in the crystal structure of the inactive state of rhodopsin the aspartate (D2.50) and asparagine (N7.49) residues are not close enough to interact directly, but they have an indirect interaction via a bridging water molecule (Palczewski et al., 2000). Modeling studies on the CB1 receptor suggest that aspartate (D2.50) interacts with asparagine (N7.49) only in the active receptor conformation (P. Reggio, personal communication). Our results with the CB1 receptor suggest that the aspartate residue D2.50 plays a critical role in G-protein binding. The aspartate residue in the second transmembrane domain of the CB1 receptor allows the receptor the intrinsic flexibility to switch from an inactive state uncoupled to G-proteins into two G-protein-coupled states, an inactive RGGDP state responsible for Gi/o-protein sequestration and an active R*GGTP state responsible for constitutive activity.
In summary, the aspartate-to-asparagine mutation in the second transmembrane domain disrupts G-protein coupling, causing the CB1 cannabinoid receptor to exist primarily in the G-protein-uncoupled R state. Thus the aspartate in the second transmembrane domain of the CB1 cannabinoid receptor plays a critical role in stabilizing both the inactive RGGDP and the active R*GGTP G-protein-coupled receptor conformations in the absence of agonist. The distal C-terminal tail of the CB1 cannabinoid receptor acts to constrain the receptor from activating G-proteins. Deletion of the distal C-terminal tail promotes the active R*GGTP conformation of the receptor. Thus the aspartate-to-asparagine mutation in the second transmembrane domain shifts the CB1 cannabinoid receptor into the G-protein-uncoupled R state, whereas truncation of the distal C terminal promotes the constitutively active R*GGTP receptor conformation.
Footnotes
This work was supported by Grant DA10350 from the National Institute on Drug Abuse. We thank Dr. Kenneth Mackie for CB1-D164N, Dr. Tom I. Bonner for CB1, and Sanofi-Synthélabo (Paris, France) for SR141716A.
Correspondence should be addressed to Dr. Deborah L. Lewis, Department of Pharmacology and Toxicology, Medical College of Georgia, 1120 15th Street, Augusta, GA 30912. E-mail: dlewis@mail.mcg.edu.
REFERENCES
- 1.Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand JP, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 2.Claeysen S, Sebben M, Becamel C, Bockaert J, Dumuis A. Novel brain-specific 5-HT4 receptor splice variants show marked constitutive activity: role of the C-terminal intracellular domain. Mol Pharmacol. 1999;55:910–920. [PubMed] [Google Scholar]
- 3.Coutts AA, Brewster N, Ingram T, Razdan RK, Pertwee RG. Comparison of novel cannabinoid partial agonists and SR141716A in the guinea-pig small intestine. Br J Pharmacol. 2000;129:645–652. doi: 10.1038/sj.bjp.0703094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Demchyshyn LL, McConkey F, Niznik HB. Dopamine D5 receptor agonist high affinity and constitutive activity profile conferred by carboxyl-terminal tail sequence. J Biol Chem. 2000;275:23446–23455. doi: 10.1074/jbc.M000157200. [DOI] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Di Marzo V, Fontana A, Cadas H, Schinelli S, Cimino G, Schwartz J, Piomelli D. Formation and inactivation of endogenous cannabinoid anandamide in central neurons. Nature. 1994;372:686–691. doi: 10.1038/372686a0. [DOI] [PubMed] [Google Scholar]
- 7.Di Marzo V, Melck D, Bisogno T, De Petrocellis L. Endocannabinoids: endogenous cannabinoid receptor ligands with neuromodulatory action. Trends Neurosci. 1998;21:521–528. doi: 10.1016/s0166-2236(98)01283-1. [DOI] [PubMed] [Google Scholar]
- 8.Hajos N, Katona I, Naiem SS, MacKie K, Ledent C, Mody I, Freund TF. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 9.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 10.Herkenham M, Lynn AB, Little MD, Johnson MR, Melvin LS, de Costa BR, Rice KC. Cannabinoid receptor localization in brain. Proc Natl Acad Sci USA. 1990;87:1932–1936. doi: 10.1073/pnas.87.5.1932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hoffman AF, Lupica CR. Direct actions of cannabinoids on synaptic transmission in the nucleus accumbens: a comparison with opioids. J Neurophysiol. 2001;85:72–83. doi: 10.1152/jn.2001.85.1.72. [DOI] [PubMed] [Google Scholar]
- 12.Howlett AC. Cannabinoid inhibition of adenylate cyclase. Biochemistry of the response in neuroblastoma cell membranes. Mol Pharmacol. 1985;27:429–436. [PubMed] [Google Scholar]
- 13.Howlett AC, Fleming RM. Cannabinoid inhibition of adenylate cyclase. Pharmacology of the response in neuroblastoma cell membranes. Mol Pharmacol. 1984;26:532–538. [PubMed] [Google Scholar]
- 14.Howlett AC, Song C, Berglund BA, Wilken GH, Pigg JJ. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol Pharmacol. 1998;53:504–510. doi: 10.1124/mol.53.3.504. [DOI] [PubMed] [Google Scholar]
- 15.Jin W, Brown S, Roche JP, Hsieh C, Celver JP, Kovoor A, Chavkin C, Mackie K. Distinct domains of the CB1 cannabinoid receptor mediate desensitization and internalization. J Neurosci. 1999;19:3773–3780. doi: 10.1523/JNEUROSCI.19-10-03773.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Katona I, Sperlagh B, Sik A, Kafalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kreitzer AC, Regehr WG. Retrograde inhibition of presynaptic calcium influx by endogenous cannabinoids at excitatory synapses onto Purkinje cells. Neuron. 2001;29:717–727. doi: 10.1016/s0896-6273(01)00246-x. [DOI] [PubMed] [Google Scholar]
- 18.Landsman RS, Burkey TH, Consroe P, Roeske WR, Yamamura HI. SR141716A is an inverse agonist at the human cannabinoid CB1 receptor. Eur J Pharmacol. 1997;334:R1–R2. doi: 10.1016/s0014-2999(97)01160-6. [DOI] [PubMed] [Google Scholar]
- 19.Mackie K, Hille B. Cannabinoids inhibit N-type calcium channels in neuroblastoma–glioma cells. Proc Natl Acad Sci USA. 1992;89:3825–3829. doi: 10.1073/pnas.89.9.3825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mackie K, Lai Y, Westenbroek R, Mitchell R. Cannabinoids activate an inwardly rectifying potassium conductance and inhibit Q-type calcium currents in AtT20 cells transfected with rat brain cannabinoid receptor. J Neurosci. 1995;15:6552–6561. doi: 10.1523/JNEUROSCI.15-10-06552.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.MacLennan SJ, Reynen PH, Kwan J, Bonhaus DW. Evidence for inverse agonism of SR141716A at human recombinant cannabinoid CB1 and CB2 receptors. Br J Pharmacol. 1998;124:619–622. doi: 10.1038/sj.bjp.0701915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Matsuda LA, Lolait SJ, Brownstein MJ, Young AC, Bonner TI. Structure of a cannabinoid receptor and functional expression of the cloned cDNA. Nature. 1990;346:561–564. doi: 10.1038/346561a0. [DOI] [PubMed] [Google Scholar]
- 23.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor mRNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 24.Nie J, Lewis DL (2001) The proximal and distal C-terminal tail domains of the CB1 cannabinoid receptor mediate G-protein coupling. Neuroscience, in press. [DOI] [PubMed]
- 25.Ohno-Shosaku T, Maejima T, Kano M. Endogenous cannabinoids mediate retrograde signals from depolarized postsynaptic neurons to presynaptic terminals. Neuron. 2001;29:729–738. doi: 10.1016/s0896-6273(01)00247-1. [DOI] [PubMed] [Google Scholar]
- 26.Palczewski K, Kumasaka T, Hori T, Behnke CA, Motoshima H, Fox BA, Le Trong I, Teller DC, Okada T, Stenkamp RE, Yamamoto M, Miyano M. Crystal structure of rhodopsin: a G-protein-coupled receptor. Science. 2000;289:739–745. doi: 10.1126/science.289.5480.739. [DOI] [PubMed] [Google Scholar]
- 27.Pan X, Ikeda SR, Lewis DL. Rat brain cannabinoid receptor modulates N-type Ca2+ channels in a neuronal expression system. Mol Pharmacol. 1996;49:707–714. [PubMed] [Google Scholar]
- 28.Pan X, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- 29.Parker EM, Ross EM. Truncation of the extended carboxyl-terminal domain increases the expression and regulatory activity of the avian β-adrenergic receptor. J Biol Chem. 1991;266:9987–9996. [PubMed] [Google Scholar]
- 30.Roche JP, Bounds S, Brown S, Mackie K. A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G-protein signaling and prevents receptor internalization. Mol Pharmacol. 1999;56:611–618. doi: 10.1124/mol.56.3.611. [DOI] [PubMed] [Google Scholar]
- 31.Schofield GG. Norepinephrine blocks a calcium current of adult rat sympathetic neurons via an α2-adrenoceptor. Eur J Pharmacol. 1990;180:37–47. doi: 10.1016/0014-2999(90)90590-3. [DOI] [PubMed] [Google Scholar]
- 32.Schofield GG. Norepinephrine inhibits a Ca2+ current in rat sympathetic neurons via a G-protein. Eur J Pharmacol. 1991;207:195–207. doi: 10.1016/0922-4106(91)90031-c. [DOI] [PubMed] [Google Scholar]
- 33.Shen M, Thayer SA. The cannabinoid agonist Win55,212-2 inhibits calcium channels by receptor-mediated and direct pathways in cultured rat hippocampal neurons. Brain Res. 1998;783:77–84. doi: 10.1016/s0006-8993(97)01195-5. [DOI] [PubMed] [Google Scholar]
- 34.Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- 35.Sullivan JM. Mechanisms of cannabinoid-receptor-mediated inhibition of synaptic transmission in cultured hippocampal pyramidal neurons. J Neurophysiol. 1999;82:1286–1294. doi: 10.1152/jn.1999.82.3.1286. [DOI] [PubMed] [Google Scholar]
- 36.Surprenant A, Shen KZ, North RA, Tatsumi H. Inhibition of calcium currents by noradrenaline, somatostatin, and opioids in guinea-pig submucosal neurones. J Physiol (Lond) 1990;431:585–608. doi: 10.1113/jphysiol.1990.sp018349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tao Q, Abood ME. Mutation of a highly conserved aspartate residue in the second transmembrane domain of the cannabinoid receptors, CB1 and CB2, disrupts G-protein coupling. J Pharmacol Exp Ther. 1998;285:651–658. [PubMed] [Google Scholar]
- 38.Tsou K, Brown S, Sanudo-Pena MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 39.Twitchell W, Brown S, Mackie K. Cannabinoids inhibit N- and P/Q-type calcium channels in cultured rat hippocampal neurons. J Neurophysiol. 1997;78:43–50. doi: 10.1152/jn.1997.78.1.43. [DOI] [PubMed] [Google Scholar]
- 40.Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;19:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wilson MH, Highfield HA, Limbird LE. The role of a conserved inter-transmembrane domain interface in regulating α2a-adrenergic receptor conformational stability and cell-surface turnover. Mol Pharmacol. 2001;59:929–938. doi: 10.1124/mol.59.4.929. [DOI] [PubMed] [Google Scholar]
- 42.Wilson RI, Nicoll RA. Endogenous cannabinoids mediate retrograde signaling at hippocampal synapses. Nature. 2001;410:588–592. doi: 10.1038/35069076. [DOI] [PubMed] [Google Scholar]
- 43.Xu W, Ozdener F, Li JG, Chen C, de Riel JK, Weinstein H, Liu-Chen LY. Functional role of the spatial proximity of Asp114(2.50) in TMH 2 and Asn332(7.49) in TMH 7 of the mu-opioid receptor. FEBS Lett. 1999;447:318–324. doi: 10.1016/s0014-5793(99)00316-6. [DOI] [PubMed] [Google Scholar]
- 44.Zhou W, Flanagan C, Ballesteros JA, Konvicka K, Davidson JS, Weinstein H, Millar RP, Sealfon SC. A reciprocal mutation supports helix 2 and helix 7 proximity in the gonadotropin-releasing hormone receptor. Mol Pharmacol. 1994;45:165–170. [PubMed] [Google Scholar]