Abstract
cGMP has been implicated in the regulation of many essential functions in the brain, such as synaptic plasticity, phototransduction, olfaction, and behavioral state. Cyclic nucleotide phosphodiesterase (PDE) hydrolysis of cGMP is the major mechanism underlying the clearance of cGMP and is likely to be important in any process that depends on intracellular cGMP. PDE9A has the highest affinity for cGMP of any PDE, and here we studied the localization of this enzyme in the rat brain using in situ hybridization. PDE9A mRNA is widely distributed throughout the brain with varying regional expression. The pattern of PDE9A mRNA expression closely resembles that of soluble guanylyl cyclase (sGC) in the rat brain, suggesting a possible functional association or coupling of these two enzymes in the regulation of cGMP levels. Most of the brain areas expressing PDE9A mRNA also contain neuronal nitric oxide synthase (NOS), the enzymatic source of NO and the principal activator of sGC. PDE9A is the only cGMP-specific PDE with significant expression in the forebrain, and as such is likely to play an important role in NO–cGMP signaling.
Keywords: nitric oxide, guanylyl cyclase, in situhybridization, olfaction, memory, learning, sleep, basal forebrain, magnocellular, preoptic
The biological effects of cGMP are dependent on its intracellular concentration, determined by its rate of formation and its rate of hydrolysis. Cyclic nucleotide phosphodiesterases (PDEs) are a large group of enzymes that participate in a wide variety of functions in different organs, including the brain (Dousa, 1999). All known PDEs can be divided into three groups: (1) PDEs hydrolyzing both cAMP and cGMP (PDE1, PDE2, PDE3, PDE10, and PDE11), (2) PDEs hydrolyzing cAMP (PDE4, PDE7, and PDE8), and (3) cGMP-specific PDEs. Currently this last group of PDEs includes three families: PDE5, PDE6, and PDE9A, of which PDE5 has been found to be expressed mainly in the cerebellum (Kotera et al., 1997), and PDE6 is the phosphodiesterase involved in visual transduction in the retina (Stryer, 1986; Gillespie, 1990). PDE9A is the PDE with the highest affinity for cGMP (Soderling et al., 1998), and therefore is likely to be important in determining intracellular cGMP levels and therefore activation of cGMP-dependent signaling pathways. However, no previous studies have investigated the localization of PDE9A in the brain.
cGMP plays an important role in many processes in the CNS, including synaptic plasticity (Bernabeu et al., 1996; Barcellos et al., 2000;Halcak et al., 2000), phototransduction (Fesenko et al., 1985), and olfaction (Zufall and Leinders-Zufall, 1998). Nitric oxide (NO) is a powerful activator of the soluble form of the cGMP-synthesizing enzyme guanylyl cyclase (Katsuki et al., 1977; Miki et al., 1977). Investigations comparing distribution of the neuronal NO-generating enzyme (NOS) with that of nitric oxide-stimulated cGMP accumulation in the rat brain have shown a parallel distribution of NOS and NO-stimulated cGMP accumulation (Southam and Garthwaite, 1993; De Vente et al., 1998). cGMP is likely to be important in the regulation of behavioral state through the NO–cGMP signal transduction system (Burlet et al., 1999; Cudeiro et al., 2000). Studies using inhibitors of nitric oxide synthase have shown inhibition of sleep with blockade of enzyme activity in the rabbit (Kapas et al., 1994b) and the rat (Dzoljic and De Vries, 1994; Kapas et al., 1994a; Dzoljic et al., 1996;Burlet et al., 1999). Other studies have shown a facilitatory effect of NO on arousal and REM generation mechanisms in target areas of the laterodorsal tegmental nucleus (LDT) and pedunculopontine tegmental nucleus (PPT) (Williams et al., 1997): the thalamus (Pape and Mager, 1992) and medial pontine reticular formation (Leonard and Lydic, 1997). As a major mechanism underlying the clearance of cGMP, cyclic nucleotide PDE hydrolysis of cGMP may play an important role in behavioral state regulation and also in other processes in which the NO–cGMP signal transduction system is involved. Our initial Northern blot studies showed strong expression of PDE9A in several regions of the forebrain, and we pursued this observation further to determine the regional expression of PDE9A mRNA.
MATERIALS AND METHODS
Animals. All procedures used conform to the National Institutes of Health guidelines for the care and use of laboratory animals.
Isolation of total RNA from rat brain. Male Sprague Dawley rats at 4 weeks of age were anesthetized with sodium pentobarbital before different brain regions were excised and stored in RNAlater (Ambion, Austin, TX) at 4°C. Total RNAs were prepared using the RNeasy Midi kit (Qiagen, San Diego, CA) according to the manufacturer's instructions. The integrity and concentration of RNA samples were determined by agarose gel electrophoretic analysis and spectrophotometry.
Preparation of DNA and RNA probes. Using rat brain total RNA and primers I and IV, corresponding to the mouse cDNA sequence (GenBank accession number AF068247, nucleotides 529–546 and 1236–1216 accordingly) (Table 1), a rat PDE9A fragment was obtained by RT-PCR. Reverse transcription was performed using the RETROscript kit (Ambion). PCR was performed withTaq DNA polymerase under the conditions recommended by the manufacturer (Promega, Madison, WI). The PCR fragment (primers I, IV) was cloned in pGEM-T Easy vector and sequenced. This construct was used for synthesis of PCR-amplified products of rat PDE9A cDNA (primers I and II, probe A; III and IV, probe B) (Table 1). Probes A and B were labeled by the DECAprime II (Ambion) and used for Northern blot analysis. There are four mRNA transcripts for the PDE9A gene in humans, arising from alternative splicing of the first six exons at the N-terminal end (Guipponi et al., 1998). Probes A and B are targeted to regions of the rat PDE9A gene that are 3′ to the sequence corresponding to the first six exons of the human gene, and thus if multiple transcripts of the rat PDE9A gene exist, arising from alternative splicing similar to that in human, probes A and B should recognize all of these transcripts.
Table 1.
Oligonucleotides used for preparation DNA and RNA probes
| Oligo name | Sequence (5′-3′) | Nucleotide residues1-a |
|---|---|---|
| I | aagaagttgacacctcga | 529–546 |
| II | atatgttcttaaacagctcag | 668–649 |
| III | cgacatctcaccgctggagaa | 962–983 |
| IV | gacttcattggagatatcacagc | 1236–1216 |
Nucleotide residues correspond to the mouse PDE9A cDNA sequence reported in GenBank accession numberAF068247.
PCR products A and B were subcloned into pGEM-T Easy Vector. TheEcoRI–EcoRI fragments of these plasmids containing the PDE9A fragments were then subcloned into pGEM-9zf(-) vector. The orientation of each fragment and fidelity of the PCR were tested by sequence analysis. The templates for generation of the antisense and sense RNA probes were made by linearizing this new construction using XbaI and SalI. Riboprobes labeled with S35 were prepared using an RNA labeling kit (Amersham Pharmacia Biotech, Arlington Heights, IL) following the manufacturer's recommendations. The labeled probes were then stored at −20°C and used within 1 week. All figures shown were obtained from experiments using probe B (or its complement in Fig.2B), except Figure 5A, obtained using probe A. Probes A and B share 33–44% and 40–50% of homology, respectively, with other PDEs. The A and B probes gave identical results.
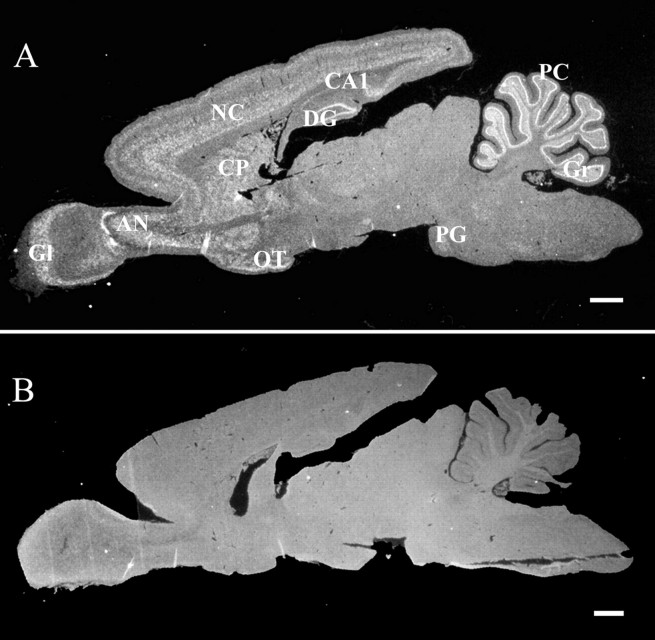
Fig. 2.
In situ hybridization assay of PDE9A expression in the whole rat brain. A, Antisense probe for PDE9A demonstrates specific labeling in discrete regions of the brain. Labeling, indicating PDE9A mRNA expression, was found in the glomerular cell layer (Gl) of the olfactory bulb, anterior olfactory nucleus (AN), neocortex (NC), olfactory tubercle (OT), caudoputamen (CP) of the striatum, dentate gyrus (DG), pontine gray nucleus (PG), Purkinje cells (PC), and granular layer (Gr) of the cerebellum. B, Corresponding sense probe shows background labeling. Scale bars, 1 mm.
Fig. 5.
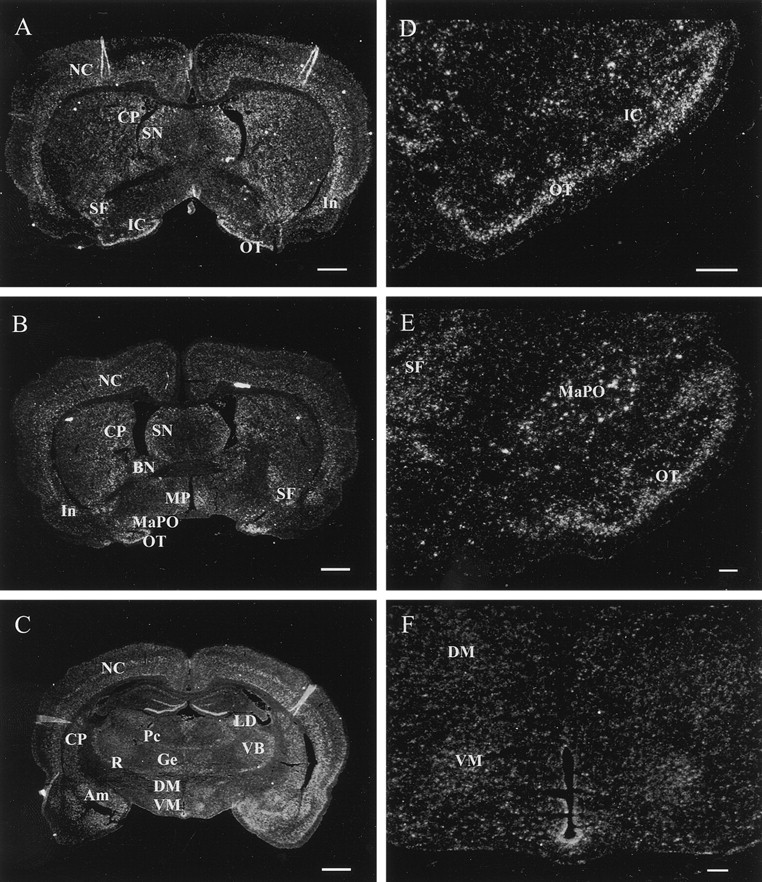
In situ hybridization assay of PDE9A expression in deep gray structures and the basal forebrain.A, A coronal section of the forebrain through the islands of Calleja. Labeling, indicating PDE9A mRNA expression, was found in the neocortex (NC), insular area of the allocortex (In), septal nucleus (SN), caudoputamen (CP), striatal fundus (SF), olfactory tubercle (OT), and islands of Calleja (IC).B, A coronal section of the forebrain through the magnocellular preoptic nucleus. Labeling was detected in the neocortex (NC), insular area of the allocortex (In), septal nucleus (SN), caudoputamen (CP), bed nucleus of stria terminalis (BN), striatal fundus (SF), olfactory tubercle (OT), magnocellular preoptic nucleus (MaPO), and medial preoptic nucleus (MP) of the hypothalamus. C, A coronal section of the forebrain through the thalamus and hippocampus. Labeling was observed in the paracentral nucleus (Pc), reticular nucleus (R), nucleus gelatinosus (Ge), lateral dorsal nucleus (LD), and ventrobasal complex of the thalamus (VB), and the ventromedial (VM) and dorsomedial (DM) nuclei of the hypothalamus. D, Higher magnification of the islands of Calleja. Strong labeling was seen in the olfactory tubercle (OT) and islands of Calleja (IC). E, Higher magnification of the preoptic magnocellular area of the basal forebrain. Strong labeling was found in the olfactory tubercle (OT), magnocellular preoptic nucleus (MaPO), and striatal fundus (SF).F, Higher magnification of the hypothalamus. Labeling was detected in the ventromedial (VM) and dorsomedial (DM) nuclei. Scale bars:A–C, 1 mm; D–F, 100 μm.
Northern blot analysis. Northern blot analysis was performed essentially as described (Selden, 1989). Ten micrograms of total RNA from the basal forebrain, cortex, cerebellum, medulla, midbrain, hippocampus, thalamus, pons, and olfactory bulb were separated on a denaturing agarose gel, transferred to a nylon membrane and hybridized with a 32P-labeled DNA probe at 42°C in ULTRAhyb (Ambion) overnight. The membrane was washed twice for 5 min in 2× SSC, 0.1% SDS at 42°C, twice for 15 min in 0.1× SSC, 0.1% SDS at 42°C and exposed to Kodak (Eastman Kodak, Rochester, NY) X-OMAT AR film.
Tissue preparation. Sprague Dawley rats, 26- to 30-d-old, were used. Rats were decapitated under sodium pentobarbital anesthesia (50 mg/kg, i.p.) and perfused through the ascending aorta with 50 ml of heparinized 0.1 m sodium phosphate buffer, pH 7.4 (PBS), containing 0.9% sodium chloride (NaCl), followed by 150 ml of freshly prepared 4% paraformaldehyde in PBS containing 0.25% glutaraldehyde (fixative solution). The brains were removed, post-fixed in the same fixative solution, then dehydrated in 50% ethanol for 3 hr, in 70% ethanol overnight, in 95% ethanol for 2 hr, twice in 100% ethanol for 2 hr each, cleared in xylene twice for 1 hr, then infiltrated with Paraplast Plus and embedded. Tissue was stored at 4°C until sectioning. For in situ hybridization 8 μm sections were cut on a rotary microtome and collected onto positively charged microscope slides (Superfrost Plus; Fisher Scientific, Pittsburgh, PA). The slides were dried at 37°C and stored at 4°C until use. To process the slides they were brought to room temperature, rinsed twice for 10 min in xylene to remove the paraffin, hydrated in a graded series of ethyl alcohol solutions (100, 95, 85, 70, 50, and 30%), then rinsed in 0.85% NaCl for 5 min and in PBS for 5 min, post-fixed in freshly prepared 4% paraformaldehyde in PBS for 30 min, rinsed twice in PBS for 5 min, treated with proteinase K (PK) solution (0.5 μg/ml PK, 50 mm EDTA, pH 8.0, 100 mm Tris, pH 8.0), rinsed in H2O, acetylated with 0.25% acetic anhydride in 0.1 m triethanolamine pH 8.0 for 10 min, rinsed in 2× SSC, dehydrated and delipidated through graded alcohol and chloroform, and dried for 30 min.
In situ hybridization. Hybridization was performed at 55°C for 16–20 hr in a solution of 50% deionized formamide, 10 mm Tris HCl, pH 7.5, 1 mmEDTA, pH 8.0, 1× Denhardt's solution, 10% dextran sulfate, 0.1% SDS, 0.1% sodium thiosulfate, 0.1% DTT, 0.02% sheared salmon sperm DNA, 0.02% yeast tRNA, and 0.1% total yeast RNA. The35S-labeled cRNA probe was added to the hybridization solution at a concentration of 107 cpm/ml. After hybridization the slides were rinsed in 2× SSC at room temperature, incubated in 20 μg/ml RNase A1 for 30 min at room temperature, washed once in 2× SSC at 50°C for 1 hr and twice in 0.2× SSC for 1 hr at 55 and 60°C. After these procedures, slides were dehydrated in an ethanol water series in the presence of 0.3 m ammonium acetate (50, 70, and 95%) and 100% ethanol, after which they were dried and exposed to Amersham Hyperfilm-βmax for 48 hr. Finally, the slides were dipped into undiluted Kodak NTB-2, exposed at 4°C for 3 to 4 weeks, and developed at 15°C with freshly prepared Kodak Developer D-19 and Fixer.
RESULTS
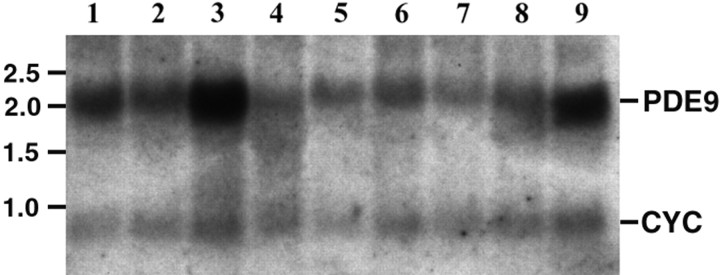
Northern blot analysis indicated that PDE9A mRNA was expressed in one transcript throughout the brain. The levels of this expression were different from region to region. The highest expression of PDE9A mRNA was detected in the basal forebrain, cerebellum, and olfactory bulb (Fig. 1, lanes 1, 3, 9).
Fig. 1.
Northern blot analysis of regional PDE9A expression. PDE9A mRNA expression was highest in the basal forebrain, cerebellum, and olfactory bulb. Northern blot contained 10 μg of rat total RNA per lane. The blot was hybridized with PDE9A and cyclophilin probes. Cyclophilin was measured to ensure equal loading of RNA on the blot. Relative size (in kilobases) is indicated on theleft based on mobility of an RNA ladder. Cyclophilin mRNA migrated at ∼0.7 kb. PDE9A was expressed in all nine brain tissues and migrated at ∼2.0 kb mRNA. 1, Basal forebrain; 2, cortex; 3, cerebellum;4, medulla; 5, midbrain;6, hippocampus; 7, thalamus;8, pons; 9, olfactory bulb.
To examine the localization of the PDE9A mRNA in the rat brain we performed in situ hybridization analysis. Sagittal section of the whole brain (Fig.2A) showed labeling in the: glomerular layer (Gl) of the olfactory bulb, anterior olfactory nucleus (AN), neocortex (NC), layers II, V, and VI, caudoputamen (CP) of the striatum, olfactory tubercle (OT), hippocampal area CA1, dentate gyrus (DG), pontine gray nuclei (PG), Purkinje cell (PC), and granular (Gr) layers of the cerebellum. No labeling was observed with the sense probe (Fig. 2B).
Olfactory system
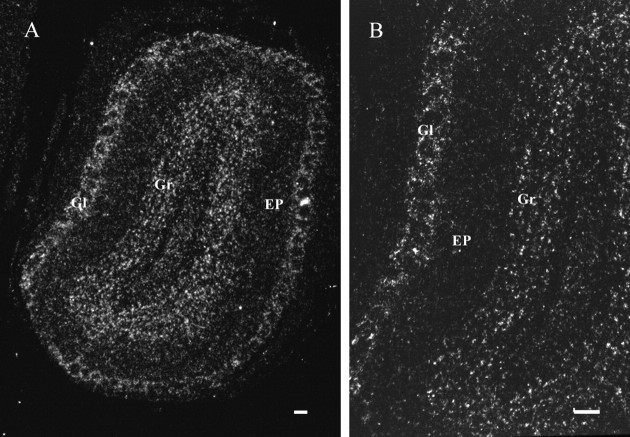
Within the main olfactory bulb, heavily labeled PDE9A mRNA-expressing cells were seen in the Gl and Gr layers (Fig.3). In the accessory olfactory bulb, strong expression was detected in the AN (Fig.2A).
Fig. 3.
In situ hybridization assay of PDE9A expression in the main olfactory bulb. A, A coronal section of the olfactory bulb. B, Higher magnification of a portion of the olfactory bulb. Labeling, indicating PDE9A mRNA expression, was found in the granular cell layer (Gr) and in the glomerular cell layer (Gl). Labeling of only scattered cells was found in the external plexiform layer (EP). Scale bars, 100 μm.
Cerebral cortex
The neocortex was rich in PDE9A mRNA-containing cells (Fig.4A). Intense hybridization signal was observed in layer V and less so in layers II and VI. In layers III and IV weak expression of PDE9A mRNA in scattered cells was observed. In addition, strong expression of PDE9A was found in the insular area of allocortex (In) (Fig.5A,B).
Fig. 4.
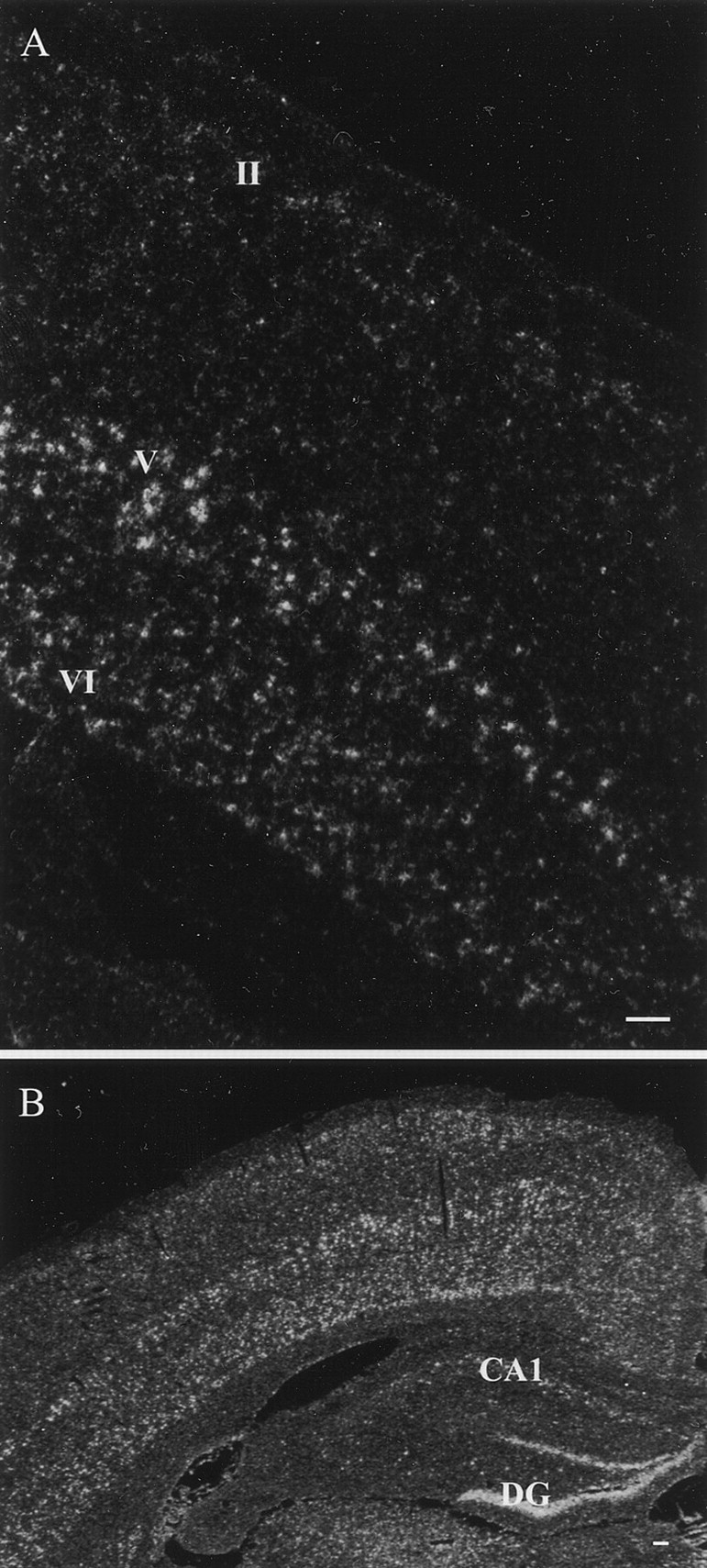
In situ hybridization assay of PDE9A expression in the neocortex. A, A coronal section of the neocortex. B, A coronal rat brain section at the hippocampal level. Labeling, indicating PDE9A mRNA expression, was found in the layers II, V, and VI of the neocortex, in the dentate nucleus (DG), and in the CA1 area of the hippocampus. Scale bars, 100 μm.
Hippocampus
PDE9A mRNA was strongly expressed in the DG and moderately in the pyramidal cell layer of the CA1 region of the hippocampus (Fig.4B). Interestingly, no detectable labeling of CA2–4 was observed, demonstrating a high degree of variation in expression within a given anatomical structure. Such variation within anatomically defined structures was seen in other locations as well.
Basal ganglia
In the medial CP and the bed nucleus (BN) of the stria terminalis the expression of PDE9A mRNA was moderate (Fig.5A,B). Strong labeling was detected in the striatal fundus (SF), part of the nucleus accumbens (Fig. 5A,B). There was also a strong signal in the septal nuclei (SN) (lateral/dorsal, lateral/intermediate, lateral/ventral) (Fig. 5A,B).
Basal forebrain
Extensive and intense labeling was observed also in the OT, the islands of Calleja (IC) (Fig. 5A,D), and the magnocellular preoptic nucleus (MaPO) (Fig. 5B,E). The amygdaloid nuclei (medial, basolateral, basomedial) also showed strong expression of PDE9A mRNA (Fig. 5C).
Thalamus and hypothalamus
The expression of PDE9A mRNA was moderate in the reticular thalamic nucleus (R) and weak in the paracentral thalamic nucleus (Pc), nucleus gelatinosus (Ge) of the thalamus, laterodorsal nucleus (LD) of the thalamus, and ventrobasal (VB) complex of the thalamus (Fig.5C). The medial preoptic (MP) area of hypothalamus showed moderate expression of PDE9A mRNA (Fig. 5B). In the ventrobasal nuclear complex of the hypothalamus [dorsomedial (DM) and ventromedial (VM) nuclei] weak PDE9A mRNA expression was detected (Fig. 5F).
Midbrain
In most of the midbrain areas expression of PDE9A mRNA was hardly distinguishable compared with the background. Only in the trochlear nucleus was weak expression of PDE9A mRNA detectable (data not shown).
Pons
PDE9A mRNA was expressed strongly in the PG (Fig. 2), the trigeminal nucleus (TN), and the inferior olive nucleus (ON) (Fig.6A,B). The facial (FN), raphe (RN), and dorsal tegmental (DT) nuclei showed moderate expression of PDE9A mRNA (Fig. 6A).
Fig. 6.
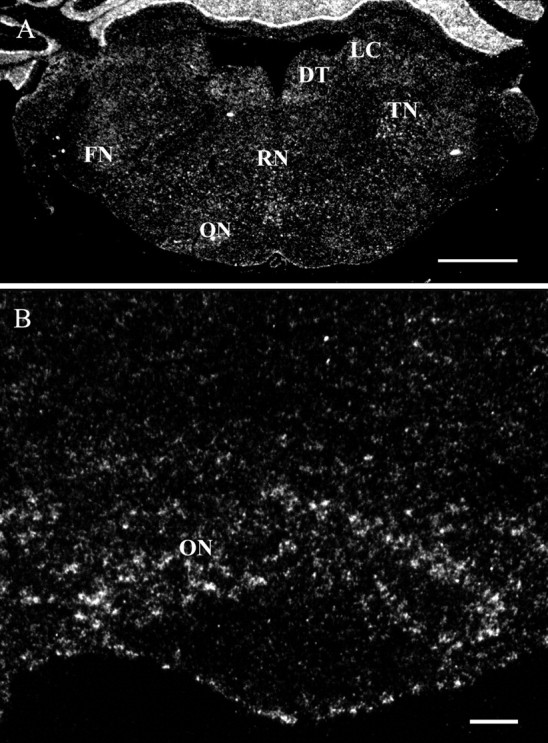
In situ hybridization assay of PDE9A expression in the pons. A, A coronal section of the pons. Labeling, indicating PDE9A mRNA expression, was found in the dorsal tegmental nucleus (DT), facial nucleus (FN), raphe nucleus (RN), trigeminal nucleus (TN), olive nucleus (ON), and locus coeruleus (LC).B, High magnification of the region containing olive neurons. Scale bars: A, 1 mm; B, 100 μm.
Cerebellum
The heaviest PDE9A mRNA was found in the PC layer, which contains the large cell bodies of Purkinje neurons that are arranged side by side in a single layer (Fig.7A,B). The Gr had weak expression of PDE9A mRNA (Fig. 7B). The molecular layer of cerebellum did not display any specific labeling for PDE9A mRNA (Fig.7B).
Fig. 7.
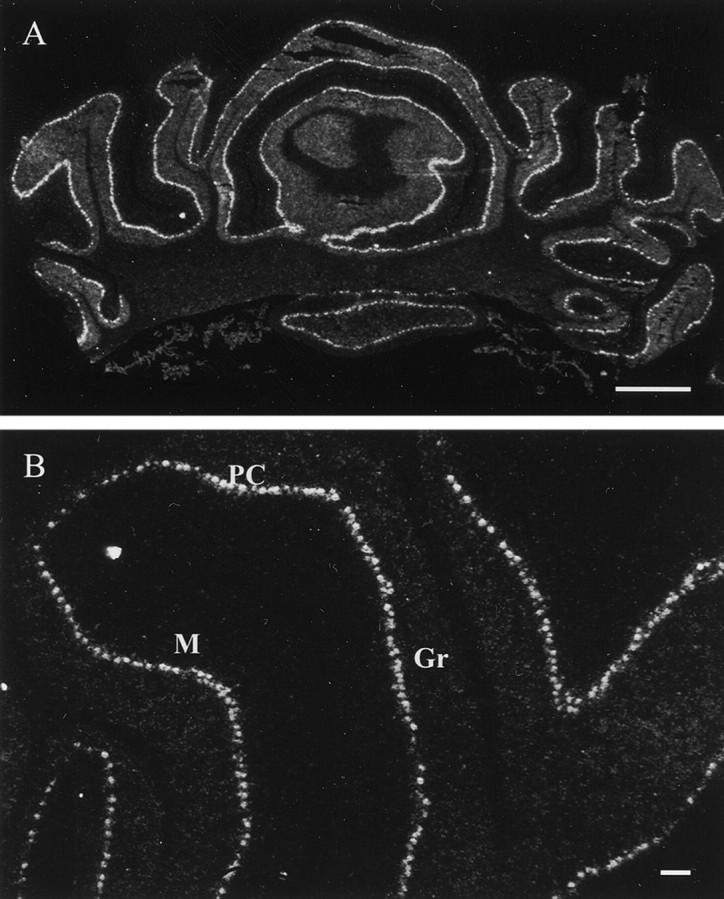
In situ hybridization assay of PDE9A expression in the cerebellum. A, A coronal section of the cerebellum. Labeling, indicating PDE9A mRNA expression, was found in the Purkinje cell layer (PC) and granular layer (Gr). No labeling was found in the molecular layer.B, High magnification of the cerebellum. Scale bars:A, 1 mm; B, 100 μm.
Table 2 summarizes the intensities of expression in various brain regions. The specificity of the riboprobes used for in situ hybridization was confirmed by four lines of evidence. First, there was no specific hybridization with the sense probe (Fig. 2B). Second, RNase-treated sections did not hybridize to the probe. Third, the results obtained using two different riboprobes were identical. Fourth, Northern blot analysis showed that the antisense riboprobes used in our experiment hybridized to unique rat PDE9A transcripts of 2 kb, demonstrating that no cross-hybridization to other transcripts occurred. No other PDE has a transcript of size similar to PDE9A (Soderling et al., 1998).
Table 2.
Summary of PDE9A mRNA expression in the rat by in situ hybridization
| Brain region | PDE9A mRNA |
|---|---|
| Olfactory bulb | |
| Glomerular layer | ++++ |
| Internal granular layer | ++++ |
| Anterior olfactory nucleus | ++++ |
| Allocortex | |
| Insular area | ++++ |
| Hippocampus | |
| Pyramidal cells in the CA1 | ++ |
| Dentate gyrus | ++++ |
| Neocortex | |
| Layer II | +++ |
| Layer III | + |
| Layer IV | + |
| Layer V | ++++ |
| Layer VI | +++ |
| Basal forebrain | |
| Olfactory tubercle | ++++ |
| Islands of Calleja | ++++ |
| Magnocellular preoptic nucleus | ++++ |
| Amygdala | +++ |
| Basal ganglia | |
| Septal nuclei | +++ |
| Caudate putamen | ++ |
| Bed nucleus of stria terminalis | ++ |
| Striatal fundus | ++ |
| Thalamus | |
| Reticular thalamic nucleus | ++ |
| Paracentral nucleus | + |
| Nucleus gelatinosus | + |
| Lateral dorsal nucleus | + |
| Ventrobasal complex | + |
| Hypothalamus | |
| Ventrobasal nuclear complex | + |
| Medial preoptic area | ++ |
| Midbrain | |
| Trochlear nucleus | + |
| Pons | |
| Facial nucleus | ++ |
| Pontine gray nucleus | +++ |
| Trigeminal nucleus | +++ |
| Raphe nucleus | ++ |
| Dorsal tegmental nucleus | ++ |
| Inferior olive nucleus | +++ |
| Cerebellum | |
| Purkinje cells | ++++ |
| Granular layer | + |
++++, Very strong; +++, strong; ++, moderate; +, weak.
DISCUSSION
In the present study the distribution pattern of PDE9A mRNA expression was investigated in the rat brain by in situhybridization using antisense RNA probes. The expression pattern generally agrees with that obtained by using Northern blot analysis. The strongest expression of PDE9A mRNA was detected in the following regions of the forebrain: the olfactory bulb and olfactory tubercle, the allocortex, the neocortex, the dentate gyrus and CA1 region of the hippocampus, specific thalamic nuclei, the islands of Calleja and magnocellular preoptic nucleus of the basal forebrain, the amygdala, and the striatal fundus. In the hindbrain, strong signal was observed in the pontine gray nucleus, the trigeminal and inferior olive nuclei of the pons, and the Purkinje cells of the cerebellum.
It is useful to compare the distribution of PDE9A with other PDEs that hydrolyze cGMP (Table 3). The distribution of PDE9A mRNA in the rat brain overlaps significantly with the distribution of PDE1B mRNA (Furuyama et al., 1994; Polli and Kincaid, 1994; Yan et al., 1994). PDE1B mRNA expression was found in the caudate putamen, nucleus accumbens, olfactory tubercle, olfactory bulb, dentate gyrus, pyramidal cells of the hippocampus (CA1–CA4), and cerebral cortex. All of these regions except CA2–CA4 of the hippocampus express PDE9A mRNA as well. In contrast to PDE9A mRNA expression, PDE1B mRNA expression was not detectable in the islands of Calleja and was expressed to a much lesser extent in Purkinje cells of the cerebellum. PDE2 mRNA is highly expressed in the limbic system of the rat brain (the medial habenula, CA1–CA3 areas and dentate gyrus of the hippocampus, subiculum, olfactory and entorhinal cortices, amygdala, and nucleus accumbens) (Repaske et al., 1993). Some regions of the limbic system, such as the CA1 region of the hippocampus, dentate gyrus, amygdala, and basal ganglia express PDE9A mRNA as well. Strong expression of PDE5 was found only in Purkinje cells of the cerebellum, where a very strong signal for PDE9A mRNA was also detected (Kotera et al., 1997). Expression of mRNA for PDE10, as well as PDE9A, PDE1B, and PDE1C, was observed in the olfactory tubercle (Fujishige et al., 1999). Such expression of different PDE families in the same brain region may indicate redundancy of function, different pathways for regulating different PDEs within the same cell, or expression in different cell types within the same region.
Table 3.
Comparison of PDE9A mRNA localization with other PDEs
| Brain regions | PDE9A3-a | PDE1A3-b | PDE1B3-b,3-c,3-d | PDE1C3-e | PDE23-f | PDE53-g | PDE103-h |
|---|---|---|---|---|---|---|---|
| Olfactory bulb | − | ||||||
| Glomerular layer | + | + | + | ||||
| External plexiform layer | − | − | + | ||||
| Internal granular layer | + | + | − | ||||
| Anterior olfactory nucleus | + | + | |||||
| Hippocampus | |||||||
| Pyramidal cells in the CA1 | + | + | + | − | + | ||
| Pyramidal cells in the CA2 | |||||||
| -CA4 | − | + | + | − | + | ||
| Dentate gyrus | + | − | + | − | + | ||
| Neocortex | − | ||||||
| Layer I | − | − | − | − | |||
| Layer II | + | ± | + | + | |||
| Layer III | ± | ± | + | + | |||
| Layer IV | ± | ± | + | + | |||
| Layer V | + | + | + | + | |||
| Layer VI | + | + | + | + | |||
| Basal forebrain | |||||||
| Olfactory tubercle | + | − | + | + | + | ||
| Islands of Calleja | + | + | − | ||||
| Magnocellular preoptic nucleus | + | ||||||
| Amygdala | + | + | + | + | + | ||
| Basal ganglia | |||||||
| Septal nuclei | + | ||||||
| Caudate putamen | + | ± | + | + | |||
| Striatum | − | − | − | − | + | ||
| Bed nucleus of stria terminalis | + | + | + | ||||
| Nucleus accumbens | + | + | + | + | |||
| Thalamus | |||||||
| Reticular thalamic nucleus | + | + | |||||
| Medial habenular nucleus | − | + | − | + | |||
| Hypothalamus | |||||||
| Medial preoptic area | + | ± | + | ||||
| Midbrain | ± | ± | + | ||||
| Pons | |||||||
| Facial nucleus | + | + | |||||
| Pontine gray nucleus | + | + | |||||
| Trigeminal nucleus | + | + | |||||
| Raphe nucleus | + | + | |||||
| Dorsal tegmental nucleus | + | + | |||||
| Inferior olive nucleus | + | + | |||||
| Cerebellum | |||||||
| Purkinje cells | + | − | + | + | + | ||
| Granular layer | ± | + | ± | − |
+, Significant signal present; ±, weak signal above background; −, no detectable signal.
This study (rat;35S-labeled riboprobes).
Yan et al. (1994) (mouse;35S-labeled riboprobes).
Furuyama et al. (1994) (rat;35S-labeled oligonucleotides).
Polli et al. (1994) (mouse;35S-labeled riboprobe).
Yan et al. (1996) (mouse;35S-labeled riborobe).
Repaske et al. (1993) (rat;35S-labeled riboprobe).
Kotera et al. (1997) (rat; nonradioactive riboprobe).
Fujishige et al. (1999) (rat; nonradioactive riboprobe).
It is likely that the expression of PDEs in a particular region correlates with their functional importance in that region. For example, it has been shown that the high level of cGMP-specific PDE6 in the retina underlies a crucial role for this enzyme family in the visual transduction cascade (Stryer, 1986; Gillespie, 1990), and the high level of cGMP-stimulated PDE (PDE2) in the adrenal cortex mediates most of the effects of atrial natriuretic peptide on aldosterone production (MacFarland et al., 1991).
Interestingly, the pattern of PDE9A mRNA expression closely resembles that of soluble guanylyl cyclase (sGC) in the rat brain (Matsuoka et al., 1992; Furuyama et al., 1993; Burgunder and Cheung, 1994; Giuili et al., 1994), suggesting a coordinated action of these two enzymes in the regulation of cGMP levels in the CNS. The localization of PDE9A mRNA also largely overlaps with NO synthase distribution (Bredt et al., 1990; Rodrigo et al., 1994), although in some cases they are located in adjacent cells and cell layers. In the cerebellum, for example, NO synthase is absent from the Purkinje cells, but its mRNA is heavily expressed in the nearby granule and basket cells. Similarly, a difference between PDE9A and NO synthase mRNA localization may occur in the striatum, where we detected expression of PDE9A mRNA throughout this area, whereas only some isolated cells were found to contain a strong signal corresponding to NOS mRNA (Giuili et al., 1994). These data are consistent with direct evidence obtained in the cerebellum that NO is an intercellular signaling agent (Garthwaite, 1991).
The strong PDE9A mRNA expression found in the magnocellular preoptic nucleus (MCP) of the basal forebrain, a region implicated in behavioral state control (McGinty and Sterman, 1968; Lucas and Sterman, 1975;Szymusiak and Satinoff, 1984; Szymusiak and McGinty, 1986, 1989a,b,1990; Detari and Vanderwolf, 1987), suggests a role for this enzyme in sleep–wake regulation. The MCP contains a population of large cholinergic neurons as well as noncholinergic neurons (Gritti et al., 1993). Arousal-related functions are mediated by magnocellular cholinergic neurons (Buzasaki and Gage, 1989), whereas GABAergic neurons located within magnocellular regions of the basal forebrain are hypothesized to mediate sleep-promoting functions (Szymusiak, 1995;Wenk, 1997). The interaction between GABAergic and cholinergic neurons in this region has been suggested to regulate behavioral state (Szymusiak, 1995). PDE9A may participate in this regulation as an important determinant of intracellular cGMP concentration. Important evidence supporting this hypothesis is the demonstration that the magnocellular preoptic nucleus contains nitric oxide synthase-expressing neurons (Bredt et al., 1990; Rodrigo et al., 1994) as well as projecting axons from the nitric oxide synthase containing cholinergic neurons of the LDT (Woolf and Butcher, 1986; Semba and Fibiger, 1989). The localization of PDE9A mRNA to preoptic magnocellular neurons has provided the first indication that PDE9A may play a role in the regulation of behavioral state.
PDE9A mRNA is highly expressed in the glomerular and granular cell layers of the olfactory bulb, where the expression of sGC (Matsuoka et al., 1992; Burgunder and Cheung, 1994) and NOS mRNA are also found (Bredt et al., 1990; Vincent and Kimura, 1992). It has already been suggested that the NO–cGMP signaling system is implicated in the formation of olfactory memory and also in olfactory adaptation (Bicker et al., 1996; Hopkins et al., 1996). PDE9A and other phosphodiesterases that hydrolyze cGMP are expected to be important for these processes as regulators of intracellular cGMP concentration.
NO and cGMP also appear to act as synaptic signaling agents in the hippocampus and cerebellum. They are involved in long-term depression (LTD) (Hartell, 1996) as well as long-term potentiation (LTP) (Schuman and Madison, 1991; Chetkovich et al., 1993; Selig et al., 1996; Son et al., 1998) and thus may play an important role in the biochemical mechanisms of learning and memory. Mechanisms controlling the formation and degradation of cGMP may have a key role in the modulation of LTD recorded from Purkinje neurons (Hartell, 1996). These cells express a high level of sGC (Matsuoka et al., 1992; Furuyama et al., 1993; Burgunder and Cheung, 1994; Giuili et al., 1994). We found that they also highly express PDE9A mRNA. It is known that simultaneous, repetitive activation of parallel fibers (PF), the axons of cerebellar granule cells that highly express NOS, and climbing fibers, the axons of inferior olivary neurons, leads to LTD of transmission at the PF–Purkinje cell synapse (Ito et al., 1982). It is possible that PDE9A participates in this process as an enzyme controlling cGMP levels. It was noticed that application of zaprinast, an inhibitor of PDE9A and other cGMP-specific PDEs, led to LTD of PF responses (Hartell, 1996). On the other hand, the application of the nonspecific phosphodiesterase inhibitor 1-methyl-3-isobutylxanthine (IBMX), to which PDE9A is not sensitive, led to a dramatic potentiation of the evoked PF excitatory response. This may indicate that PDE9A, which is IBMX-insensitive but zaprinast-sensitive, may be involved in this synaptic response and that cGMP accumulation is associated with synaptic depression at this synapse.
cGMP-regulated processes in the hippocampus play an important role in the early stages of memory consolidation (Bernabeu et al., 1996). Using a passive avoidance task, it was observed that the level of cGMP in the hippocampus increased immediately after training and that administration of an analog of cGMP into the hippocampus immediately after training enhanced memory performance. In addition, infusion of an sGC inhibitor immediately after training caused full elimination of the training effect (Bernabeu et al., 1997). In another study, the effects of 7-nitroindazole, a selective inhibitor of nNOS, and zaprinist were evaluated in an object recognition task in rats based on the differential exploration of new and familiar objects (Prickaerts et al., 1997). 7-Nitroindazole impaired the discrimination between objects, whereas zaprinist facilitated object recognition and restored the recognition deficit caused by 7-nitroindazole. These data suggest that the NO–cGMP signal transduction pathway is involved in memory formation in this task and that PDEs hydrolyzing cGMP, in particular PDE9A, which is expressed in the CA1 pyramidal neurons of the hippocampus, may participate as important determinants of intracellular cGMP concentration.
Thus, in the basal forebrain, olfactory bulb, cerebellum, and hippocampus, regions known to be associated with behavioral state regulation, olfaction, motor control, and learning, the NO–cGMP signaling pathway appears to play an important role. In these regions we have found strong expression of PDE9A. We therefore propose that in these regions and in the functions subserved by these regions, PDE9A is important because its high affinity for cGMP makes it a major regulator of intracellular cGMP concentration. Determining the precise cellular localization of PDE9A and the mechanisms underlying the regulation of its expression and activity will be crucial in understanding the exact physiological role of this enzyme.
Footnotes
This work was supported by National Heart Lung and Blood Institute Grant HL59595 and National Institute of Child Health and Human Development Grant HD18655.
Correspondence should be addressed to Dr. Paul A. Rosenberg, Department Neurology, Enders 349, Children's Hospital, 300 Longwood Avenue, Boston, MA 02115. E-mail: paul.rosenberg@tch.harvard.edu.
REFERENCES
- 1.Barcellos CK, Bradley PM, Burns BD, Webb AC. Effects of nitric oxide release in an area of the chick forebrain which is essential for early learning. Brain Res Dev Brain Res. 2000;121:79–87. doi: 10.1016/s0165-3806(00)00027-4. [DOI] [PubMed] [Google Scholar]
- 2.Bernabeu R, Schmitz P, Faillace MP, Izquierdo I, Medina JH. Hippocampal cGMP and cAMP are differentially involved in memory processing of inhibitory avoidance learning. NeuroReport. 1996;7:585–588. doi: 10.1097/00001756-199601310-00050. [DOI] [PubMed] [Google Scholar]
- 3.Bernabeu R, Schroder N, Quevedo J, Cammarota M, Izquierdo I, Medina JH. Further evidence for the involvement of a hippocampal cGMP/cGMP-dependent protein kinase cascade in memory consolidation. NeuroReport. 1997;8:2221–2224. doi: 10.1097/00001756-199707070-00026. [DOI] [PubMed] [Google Scholar]
- 4.Bicker G, Schmachtenberg O, De Vente J. The nitric oxide/cyclic GMP messenger system in olfactory pathways of the locust brain. Eur J Neurosci. 1996;8:2635–2643. doi: 10.1111/j.1460-9568.1996.tb01558.x. [DOI] [PubMed] [Google Scholar]
- 5.Bredt DS, Hwang PM, Snyder SH. Localization of nitric oxide synthase indicating a neural role for nitric oxide. Nature. 1990;347:768–770. doi: 10.1038/347768a0. [DOI] [PubMed] [Google Scholar]
- 6.Burgunder JM, Cheung PT. Expression of soluble guanylyl cyclase gene in adult rat brain. Eur J Neurosci. 1994;6:211–217. doi: 10.1111/j.1460-9568.1994.tb00263.x. [DOI] [PubMed] [Google Scholar]
- 7.Burlet S, Leger L, Cespuglio R. Nitric oxide and sleep in the rat: a puzzling relationship. Neuroscience. 1999;92:627–639. doi: 10.1016/s0306-4522(99)00031-7. [DOI] [PubMed] [Google Scholar]
- 8.Buzasaki G, Gage FH. The cholinergic nucleus basalis: a key structure in neocortical arousal. Experientia [Suppl] 1989;57:159–171. doi: 10.1007/978-3-0348-9138-7_16. [DOI] [PubMed] [Google Scholar]
- 9.Chetkovich DM, Klann E, Sweatt JD. Nitric oxide synthase-independent long-term potentiation in area CA1 of hippocampus. NeuroReport. 1993;4:919–922. doi: 10.1097/00001756-199307000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Cudeiro J, Rivadulla C, Grieve KL. A possible role for nitric oxide at the sleep/wake interface. Sleep. 2000;23:829–835. [PubMed] [Google Scholar]
- 11.De Vente J, Hopkins DA, Markerink-Van Ittersum M, Emson PC, Schmidt HHHW, Steinbusch HWM. Distribution of nitric oxide synthase and nitric oxide-receptive, cyclic GMP-producing structures in the rat brain. Neuroscience. 1998;87:207–241. doi: 10.1016/s0306-4522(98)00171-7. [DOI] [PubMed] [Google Scholar]
- 12.Detari L, Vanderwolf CH. Activity of identified cortically projecting and other basal forebrain neurones during large slow waves and cortical activation in anaesthetized rats. Brain Res. 1987;437:1–8. doi: 10.1016/0006-8993(87)91521-6. [DOI] [PubMed] [Google Scholar]
- 13.Dousa TP. Cyclic-3′ 5′-nucleotide phosphodiesterase isozymes in cell biology and pathophysiology of the kidney. Kidney Int. 1999;55:29–62. doi: 10.1046/j.1523-1755.1999.00233.x. [DOI] [PubMed] [Google Scholar]
- 14.Dzoljic MR, De Vries R. Nitric oxide synthase inhibition reduces wakefulness. Neuropharmacology. 1994;33:1505–1509. doi: 10.1016/0028-3908(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 15.Dzoljic MR, De Vries R, Van Leeuwen R. Sleep and nitric oxide: effects of 7-nitro indazole, inhibitor of brain nitric oxide synthase. Brain Res. 1996;718:145–150. doi: 10.1016/0006-8993(96)00102-3. [DOI] [PubMed] [Google Scholar]
- 16.Fesenko EE, Kolesnikov SS, Lyubarsky AL. Induction by cyclic GMP of cationic conductance in plasma membrane of retinal rod outer segment. Nature. 1985;313:310–313. doi: 10.1038/313310a0. [DOI] [PubMed] [Google Scholar]
- 17.Fujishige K, Kotera J, Omori K. Striatum- and testis-specific phosphodiesterase PDE10A isolation and characterization of a rat PDE10A. Eur J Biochem. 1999;266:1118–1127. doi: 10.1046/j.1432-1327.1999.00963.x. [DOI] [PubMed] [Google Scholar]
- 18.Furuyama T, Inagaki S, Takagi H. Localizations of alpha 1 and beta 1 subunits of soluble guanylate cyclase in the rat brain. Mol Brain Res. 1993;20:335–344. doi: 10.1016/0169-328x(93)90060-3. [DOI] [PubMed] [Google Scholar]
- 19.Furuyama T, Iwahashi Y, Tano Y, Takagi H, Inagaki S. Localization of 63-kDa calmodulin-stimulated phosphodiesterase mRNA in the rat brain by in situ hybridization histochemistry. Mol Brain Res. 1994;26:331–336. doi: 10.1016/0169-328x(94)90107-4. [DOI] [PubMed] [Google Scholar]
- 20.Garthwaite J. Glutamate, nitric oxide and cell-cell signalling in the nervous system. Trends Neurosci. 1991;14:60–67. doi: 10.1016/0166-2236(91)90022-m. [DOI] [PubMed] [Google Scholar]
- 21.Gillespie PG. Phosphodiesterases in visual transduction by rods and cones. In: Beavo JA, Houslay MD, editors. Structure, regulation, and drug action. Wiley; New York: 1990. pp. 163–184. [Google Scholar]
- 22.Giuili G, Luzi A, Poyard M, Guellaën G. Expression of mouse brain soluble guanylyl cyclase and NO synthase during ontogeny. Dev Brain Res. 1994;81:269–283. doi: 10.1016/0165-3806(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 23.Gritti I, Mainville L, Jones BE. Codistribution of GABA-with acetylcholine-synthesizing neurons in the basal forebrain of the rat. J Comp Neurol. 1993;329:438–457. doi: 10.1002/cne.903290403. [DOI] [PubMed] [Google Scholar]
- 24.Guipponi M, Scott HS, Kudoh J, Kawasaki K, Shibuya K, Shintani A, Asakawa S, Chen HM, Lalioti MD, Rossier C, Minoshima S, Shimizu N, Antonarakis SE. Identification and characterization of a novel cyclic nucleotide phosphodiesterase gene (PDE9A) that maps to 21q22.3: alternative splicing of mRNA transcripts, genomic structure and sequence. Hum Genet. 1998;103:386–392. doi: 10.1007/s004390050838. [DOI] [PubMed] [Google Scholar]
- 25.Halcak L, Pechanova O, Zigova Z, Klemova L, Novacky M, Bernatova I. Inhibition of NO synthase activity in nervous tissue leads to decreased motor activity in the rat. Physiol Res. 2000;49:143–149. [PubMed] [Google Scholar]
- 26.Hartell NA. Inhibition of cGMP breakdown promotes the induction of cerebellar long-term depression. J Neurosci. 1996;16:2881–2890. doi: 10.1523/JNEUROSCI.16-09-02881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hopkins DA, Steinbusch HW, Markerink-van Ittersum M, De Vente J. Nitric oxide synthase, cGMP, and NO-mediated cGMP production in the olfactory bulb of the rat. J Comp Neurol. 1996;375:641–658. doi: 10.1002/(SICI)1096-9861(19961125)375:4<641::AID-CNE6>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 28.Ito M, Sakurai M, Tongroach P. Climbing fibre induced depression of both mossy fibre responsiveness and glutamate sensitivity of cerebellar Purkinje cells. J Physiol (Lond) 1982;324:113–134. doi: 10.1113/jphysiol.1982.sp014103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kapas L, Fang J, Krueger JM. Inhibition of nitric oxide synthesis inhibits rat sleep. Brain Res. 1994a;664:189–196. doi: 10.1016/0006-8993(94)91969-0. [DOI] [PubMed] [Google Scholar]
- 30.Kapas L, Shibata M, Kimura M, Krueger JM. Inhibition of nitric oxide synthesis suppresses sleep in rabbits. Am J Physiol. 1994b;266:R151–R157. doi: 10.1152/ajpregu.1994.266.1.R151. [DOI] [PubMed] [Google Scholar]
- 31.Katsuki S, Arnold W, Mittal C, Murad F. Stimulation of guanylate cyclase by sodium nitroprusside, nitroglycerin and nitric oxide in various tissue preparations and comparison to the effects of sodium azide and hydroxylamine. J Cyclic Nucleotide Res. 1977;3:23–35. [PubMed] [Google Scholar]
- 32.Kotera J, Yanaka N, Fujishige K, Imai Y, Akatsuka H, Ishizuka T, Kawashima K, Omori K. Expression of rat cGMP-binding cGMP-specific phosphodiesterase mRNA in Purkinje cell layers during postnatal neuronal development. Eur J Biochem. 1997;249:434–442. doi: 10.1111/j.1432-1033.1997.t01-1-00434.x. [DOI] [PubMed] [Google Scholar]
- 33.Leonard TO, Lydic R. Pontine nitric oxide modulates acetylcholine release, rapid eye movement sleep generation, and respiratory rate. J Neurosci. 1997;17:774–785. doi: 10.1523/JNEUROSCI.17-02-00774.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lucas EA, Sterman MB. Effect of a forebrain lesion on the polycyclic sleep-wake cycle and sleep-wake patterns in the cat. Exp Neurol. 1975;46:368–388. doi: 10.1016/0014-4886(75)90142-9. [DOI] [PubMed] [Google Scholar]
- 35.MacFarland RT, Zelus BD, Beavo JA. High concentrations of a cGMP-stimulated phosphodiesterase mediate ANP-induced decreases in cAMP and steroidogenesis in adrenal glomerulosa cells. J Biol Chem. 1991;266:136–142. [PubMed] [Google Scholar]
- 36.Matsuoka I, Giuili G, Poyard M, Stengel D, Parma J, Guellaen G, Hanoune J. Localization of adenylyl and guanylyl cyclase in rat brain by in situ hybridization: comparison with calmodulin mRNA distribution. J Neurosci. 1992;12:3350–3360. doi: 10.1523/JNEUROSCI.12-09-03350.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McGinty DJ, Sterman MB. Sleep suppression after basal forebrain lesions in the cat. Science. 1968;160:1253–1255. doi: 10.1126/science.160.3833.1253. [DOI] [PubMed] [Google Scholar]
- 38.Miki N, Kawabe Y, Kuriyama K. Activation of cerebral guanylate cyclase by nitric oxide. Biochem Biophys Res Commun. 1977;75:851–856. doi: 10.1016/0006-291x(77)91460-7. [DOI] [PubMed] [Google Scholar]
- 39.Pape H-C, Mager R. Nitric oxide controls oscillatory activity in thalamocortical neurons. Neuron. 1992;9:441–448. doi: 10.1016/0896-6273(92)90182-d. [DOI] [PubMed] [Google Scholar]
- 40.Polli JW, Kincaid RL. Expression of a calmodulin-dependent phosphodiesterase isoform (PDE1B1) correlates with brain regions having extensive dopaminergic innervation. J Neurosci. 1994;14:1251–1261. doi: 10.1523/JNEUROSCI.14-03-01251.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Prickaerts J, Steinbusch HWM, Smits JFM, De Vente J. Possible role of nitric oxide cyclic GMP pathway in object recognition memory: effects of 7-nitroindazole and zaprinast. Eur J Pharmacol. 1997;337:125–136. doi: 10.1016/s0014-2999(97)01301-0. [DOI] [PubMed] [Google Scholar]
- 42.Repaske DR, Corbin JG, Conti M, Goy MF. A cyclic GMP-stimulated cyclic nucleotide phosphodiesterase gene is highly expressed in the limbic system of the rat brain. Neuroscience. 1993;56:673–686. doi: 10.1016/0306-4522(93)90364-l. [DOI] [PubMed] [Google Scholar]
- 43.Rodrigo J, Springall DR, Uttenthal O, Bentura ML, Abadia-Molina F, Riveros-Moreno V, Martinez-Murillo R, Polak JM, Moncada S. Localization of nitric oxide synthase in the adult rat brain. Philos Trans R Soc Lond B Biol Sci. 1994;345:175–221. doi: 10.1098/rstb.1994.0096. [DOI] [PubMed] [Google Scholar]
- 44.Schuman EM, Madison DV. A requirement for the intercellular messenger nitric oxide in long-term potentiation. Science. 1991;254:1503–1506. doi: 10.1126/science.1720572. [DOI] [PubMed] [Google Scholar]
- 45.Selden RF. Analysis of RNA by northern hybridization. In: Ausubel FM, Brent R, Kingston RE, Moore DD, Spidman JG, Smith JA, Struhl K, editors. Current protocols in molecular biology. Wiley; New York: 1989. pp. 4.9.1–4.9.5. [Google Scholar]
- 46.Selig DK, Segal MR, Liao D, Malenka RC, Malinow R, Nicoll RA, Lisman JE. Examination of the role of cGMP in long-term potentiation in the CA1 region of the hippocampus. Learn Mem. 1996;3:42–48. doi: 10.1101/lm.3.1.42. [DOI] [PubMed] [Google Scholar]
- 47.Semba K, Fibiger HC. Organizaation of central cholinergic systems. Prog Brain Res. 1989;79:37–63. doi: 10.1016/s0079-6123(08)62464-4. [DOI] [PubMed] [Google Scholar]
- 48.Soderling SH, Bayuga SJ, Beavo JA. Identification and characterization of a novel family of cyclic nucleotide phosphodiesterases. J Biol Chem. 1998;273:15553–15558. doi: 10.1074/jbc.273.25.15553. [DOI] [PubMed] [Google Scholar]
- 49.Son H, Lu YF, Zhuo M, Arancio O, Kandel ER, Hawkins RD. The specific role of cGMP in hippocampal LTP. Learn Mem. 1998;5:231–245. [PMC free article] [PubMed] [Google Scholar]
- 50.Southam E, Garthwaite J. The nitric oxide-cyclic GMP signalling pathway in rat brain. Neuropharmacology. 1993;32:1267–1277. doi: 10.1016/0028-3908(93)90021-t. [DOI] [PubMed] [Google Scholar]
- 51.Stryer L. Cyclic GMP cascade of vision. Annu Rev Neurosci. 1986;9:87–119. doi: 10.1146/annurev.ne.09.030186.000511. [DOI] [PubMed] [Google Scholar]
- 52.Szymusiak R. Magnocellular nuclei of the basal forebrain: substrates of sleep and arousal regulation. Sleep. 1995;18:478–500. doi: 10.1093/sleep/18.6.478. [DOI] [PubMed] [Google Scholar]
- 53.Szymusiak R, McGinty D. Sleep-related neuronal discharge in the basal forebrain of cats. Brain Res. 1986;370:82–92. doi: 10.1016/0006-8993(86)91107-8. [DOI] [PubMed] [Google Scholar]
- 54.Szymusiak R, McGinty D. Sleep-waking discharge of basal forebrain projection neurons in cats. Brain Res Bull. 1989a;22:423–430. doi: 10.1016/0361-9230(89)90069-5. [DOI] [PubMed] [Google Scholar]
- 55.Szymusiak R, McGinty D. Effects of basal forebrain stimulation on the waking discharge of neurons in the midbrain reticular formation of cats. Brain Res. 1989b;498:355–359. doi: 10.1016/0006-8993(89)91116-5. [DOI] [PubMed] [Google Scholar]
- 56.Szymusiak R, McGinty D. State-dependent neurophysiology of the basal forebrain: relationship to sleep, arousal, and thermoregulatory function. In: Mancia M, Marini G, editors. The diencephalon and sleep. Raven; New York: 1990. pp. 111–123. [Google Scholar]
- 57.Szymusiak R, Satinoff E. Ambient temperature-dependence of sleep disturbances produced by basal forebrain damage in rats. Brain Res Bull. 1984;12:295–305. doi: 10.1016/0361-9230(84)90057-1. [DOI] [PubMed] [Google Scholar]
- 58.Vincent SR, Kimura H. Histochemical mapping of nitric oxide synthase in the rat brain. Neuroscience. 1992;46:755–784. doi: 10.1016/0306-4522(92)90184-4. [DOI] [PubMed] [Google Scholar]
- 59.Wenk GL. The nucleus basalis magnocellularis cholinergic system: one hundred years of progress. Neurobiol Learn Mem. 1997;67:85–95. doi: 10.1006/nlme.1996.3757. [DOI] [PubMed] [Google Scholar]
- 60.Williams JA, Vincent SR, Reiner PB. Nitric oxide production in rat thalamus changes with behavioral state, local depolarization, and brainstem stimulation. J Neurosci. 1997;17:420–427. doi: 10.1523/JNEUROSCI.17-01-00420.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Woolf NJ, Butcher LL. Cholinergic systems in the rat brain: III. Projections from the pontomesencephalic tegmentum to the thalamus, tectum, basal ganglia, and basal forebrain. Brain Res Bull. 1986;16:603–637. doi: 10.1016/0361-9230(86)90134-6. [DOI] [PubMed] [Google Scholar]
- 62.Yan C, Bentley JK, Sonnenburg WK, Beavo JA. Differential expression of the 61 kDa and 63 kDa calmodulin-dependent phosphodiesterases in the mouse brain. J Neurosci. 1994;14:973–984. doi: 10.1523/JNEUROSCI.14-03-00973.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zufall F, Leinders-Zufall T. Role of cyclic GMP in olfactory transduction and adaptation. Ann NY Acad Sci. 1998;855:199–204. doi: 10.1111/j.1749-6632.1998.tb10566.x. [DOI] [PubMed] [Google Scholar]