Abstract
The extent of synchronization within and between the nuclei of the basal ganglia is unknown in Parkinson's disease. The question is an important one because synchronization will increase postsynaptic efficacy at subsequent projection targets. We simultaneously recorded local potentials (LPs) from the globus pallidus interna (GPi) and subthalamic nucleus (STN) in four awake patients after neurosurgery for Parkinson's disease. Nuclei from both sides were recorded in two patients so that a total of six ipsilateral GPi–STN LP recordings were made. Without medication, the power within and the coherence between the GPi and STN was dominated by activity with a frequency <30 Hz. Treatment with the dopamine precursor levodopa reduced the low-frequency activity and resulted in a new peak at ∼70 Hz. This was evident in the power spectrum from STN and GPi and in the coherence between these nuclei. The phase relationship between the nuclei varied in a complex manner according to frequency band and the presence of exogenous dopaminergic stimulation. Synchronization of activity does occur between pallidum and STN, and its pattern is critically dependent on the level of dopaminergic activity.
Keywords: globus pallidus interna, subthalamic nucleus, coherence, synchronization, Parkinson's disease, dopamine
Studies in 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP)-treated primates and in patients with Parkinson's disease have found an increase in firing rate and a tendency toward bursting in the neurons of the globus pallidus interna (GPi) and subthalamic nucleus (STN) (Filion and Tremblay, 1991; Bergman et al., 1994; Sterio et al., 1994; Hutchison et al., 1997a, 1998; Merello et al., 1999). These changes are likely to influence the projection targets of the basal ganglia in the thalamus and brainstem, although not as much as if the postsynaptic efficacy of neuronal activity was increased through the synchronization of discharges emanating from these nuclei. There is some evidence for the synchronization of neuronal discharges within the GPi of MPTP-treated primates (Nini et al., 1995), but, to date, there is no evidence for significant synchronization in patients with Parkinson's disease.
Here we look for synchronization within and between the human GPi and ipsilateral STN in the presence and relative absence of dopaminergic stimulation, by recording from the basal ganglia in patients undergoing functional neurosurgery for severe Parkinson's disease. Patients were recorded after withdrawal and reinstitution of treatment with the dopamine precursor levodopa, which elevates levels of dopamine and its metabolites in the parkinsonian brain, without significant change in noradrenaline or serotonin (Scatton et al., 1983). To avoid surgery-related time constraints, we recorded local potentials (LPs) postoperatively from the different contacts of macroelectrodes rather than the action potentials of individual neurons using intraoperative microelectrodes. The use of bipolar contacts increased the likelihood that only local potentials were recorded because activity generated near one contact in a contact pair is more likely to give a deflection than activity generated at a distance and picked up by both contacts. We further confirmed the local generation of recorded potentials by demonstrating reversal in the polarity of the cumulant density estimates between consecutive bipolar pairs of contacts.
The LP is the product of synchronous activity in a population of neurons. In the cerebral cortex, the timing of neuronal discharge is closely related to fluctuations in the local field potential (LFP) (Creutzfeldt et al., 1966; Frost, 1968), and, by analogy, we considered fluctuations in the LP within GPi and STN to be a surrogate marker of the synchronization of neuronal discharge in these nuclei. The validity of this marker was checked by looking for coherence between GPi and STN, because the presence of the latter would suggest that LFP oscillations in GPi were locked to postsynaptic effects in STN and visa versa.
MATERIALS AND METHODS
Patients and surgery. All four patients (mean age of 59 years; range of 49–64 years; two females; mean duration of disease, 17 years; range of 9–27 years) participated with informed consent. Their mean United Parkinson's Disease Rating Scale (UPDRS) motor scores were 66 (range of 48–80) and 20 (range of 11–38) off and on medication, respectively. Patients took a mean daily dosage of 770 mg of levodopa (range of 400–1500 mg) and a single dose of 200 mg during the recording session. UPDRS scores on and off treatment and the efficacy of stimulation at different macroelectrode contacts were assessed independently of the experiment and blind to any of the results.
The operative procedure and beneficial clinical effects of stimulation have been described previously (Siegfried and Lippitz, 1994; Limousin et al., 1995; Starr et al., 1998). Macroelectrodes were inserted after GPi and STN had been identified by nontelemetric ventriculography and localized using microelectrode recording and microstimulation while the subject was awake. The coordinates at the tip of contact 0 were 19–24 mm from the midline of the patient, 2 mm in front of the midcommissural point, and 6 mm below the anterior commissure (AC)–posterior commissure (PC) line for Gpi, and 12 mm from the midline, 0 mm from the midcommissural point, and 4–5 mm below the AC–PC line for STN. Macroelectrode position was confirmed postoperatively using magnetic resonance imaging (MRI). The macroelectrodes in the pallidum and STN were models 3387 and 3389 (Medtronic Neurological Division, Minneapolis, MN) with four platinum–iridium cylindrical surfaces (1.27 mm diameter and 1.5 mm length) and center-to-center separations of 3 and 2 mm, respectively. Contact 0 was the most caudal, and contact 3 was the most rostral. It was estimated that contacts 0–2 of the pallidal electrode were potentially in GPi, whereas only contacts 0 and/or 1 of the STN electrode were in the STN. However, the exact position of the macroelectrode contacts cannot be determined antemortem. Postoperative stimulation of the macroelectrodes at clinically effective frequencies and intensities led to neither visual or somaesthetic responses nor muscle activation.
Recordings. Subjects were supine and were recorded at rest and while they performed isometric contraction of the wrist extensors, so as to maintain the wrist dorsiflexed by ∼30° from the primary position. The forearm was supported in pronation, and wrist extension was unconstrained. During each task, LPs from the GPi and STN macroelectrodes were recorded simultaneously with contralateral wrist extensor EMG. EMG was picked up with bipolar 9-mm-diameter Ag–AgCl electrodes. The degree of EMG activity was monitored on-line with verbal feedback being given so that activity was matched on and off medication. Deep brain activity was recorded from the adjacent four contacts of each macroelectrode (0–1, 1–2, and 2–3). EMG was band-pass filtered at 10–300 Hz and amplified (1000×). LPs and EEG were filtered at 1–300 Hz and amplified (100–500,000×). Signals were sampled at 1 kHz and recorded and monitored on-line using a custom-written program.
Analysis. A fast Fourier transform was performed on nonoverlapping sections of equal length (Halliday et al., 1995). Results were averaged across sections, and the autospectra, cross-spectra, and from this coherence were determined over 0–200 Hz. A total of 1027 segments (mean of 178 per pair of nuclei from each subject) were averaged for each of the four conditions (rest and tonic voluntary contraction on and off levodopa). The segment length used was 1024 points, giving a frequency resolution of 0.98 Hz. The variances of autospectra were stabilized using a logarithmic (log10) transform. The variance of the coherence was normalized by transforming the square root of the coherence (a complex valued function termed coherency) at each frequency using the Fisher transform. This results in values of constant variance for each record given by 1/2L, where L is the number of segment lengths used to calculate the coherence. A χ2 test was then used to test the hypothesis of equal coherence values in the original records at each frequency (Amjad et al., 1997). As multiple comparisons were performed, the confidence limits (CL) for autospectra, coherence, and χ2 spectra were calculated after a Bonferroni correction. Phase was only analyzed over those frequencies showing significant coherence between STN and GPi. The constant time lag between the two signals was calculated from the slope of the phase estimate after a line had been fitted by linear regression. The time lag was only calculated from the gradient if the number of contiguous data points included in the segment was equal to or more than eight, and a linear relationship accounted for >80% of the variance. There often appeared to be more than one component of differing slope. The limits of individual components were defined by the turning points of the best-fit second- or third-order polynomial fitted to all contiguous plotted points. The polynomials accounted for >80% of the variance and had the same or more than eight data points per model order. The results of phase analysis are useful in suggesting which nucleus is active first over a particular frequency band. However, 95% CL were generally large so that inferences about the precise temporal differences between structures could not be made, especially because bipolar electrodes may degrade phase information (Mima and Hallett, 1999). It should also not be assumed that the lags and leads reported here only reflect conduction delays.
The cumulant density, equivalent to the cross-correlation between signals, was calculated from the inverse Fourier transform of the cross-spectrum. Reversals in the polarity of the cumulant density estimate between consecutive bipolar pairs of contacts were used to determine the source of oscillatory activity. Only peaks in the cumulant density that were above the Bonferroni corrected 95% CL were assessed.
RESULTS
GPi and STN LPs
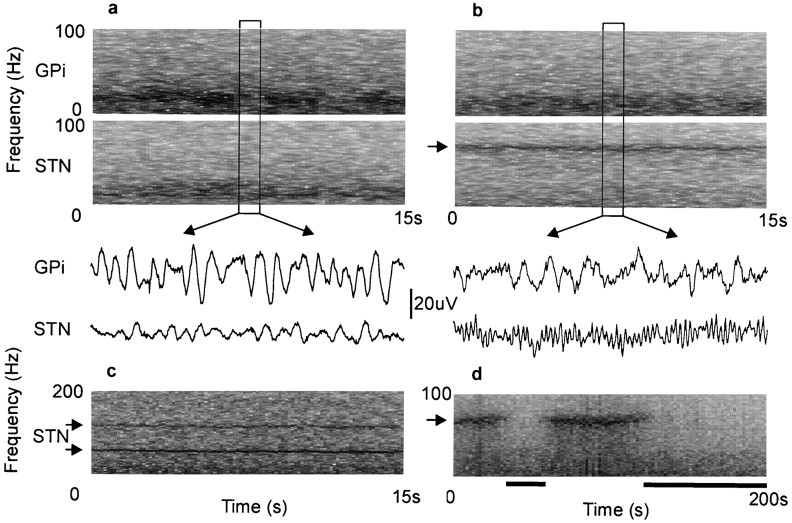
Four patients with idiopathic Parkinson's disease were studied 3–8 d after the simultaneous surgical implantation of macroelectrodes in the basal ganglia, in the period between implantation and subsequent subcutaneous rerouting to an internal stimulator (Fig.1). Two patients were recorded twice as both sides were implanted over the course of the study. This resulted in LPs being sampled from a total of six GPi and six STN. Each patient was recorded at rest and during tonic voluntary contraction of the extensor muscles of the contralateral forearm after the overnight withdrawal of their antiparkinsonian medication and again 1.5 hr after ingestion of levodopa. Figure 2 shows representative examples of the raw LP signals picked up in GPi and STN and variations in their frequency content over time. Off treatment, the records from both nuclei are dominated by oscillatory activity with a frequency below 30 Hz (Fig. 2a). These oscillations are reduced after the ingestion of levodopa, when a sharply tuned band of activity appears in STN at ∼70 Hz (Fig. 2b). Similar features were seen in all subjects. After treatment, two of the patients also showed a smaller peak in STN at ∼140 Hz, a harmonic of the major activity at 70 Hz (Fig. 2c). One patient became drowsy during recordings made after levodopa, whereupon the 70 Hz oscillations became greatly diminished (Fig. 2d).
Fig. 1.
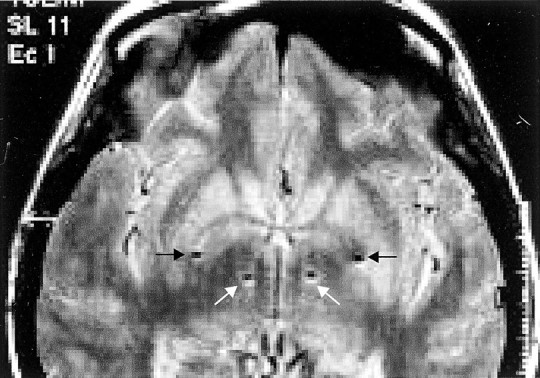
T1-weighted magnetic resonance axial view in a patient with Parkinson's disease after implantation of the left then right pallidum and STN. The sites of the deepest contacts (0) are shown and lie in STN (white arrows) and GPi (black arrows), bilaterally. The scale to the right is in centimeters.
Fig. 2.
Raw LP signals picked up in GPi12 and STN12/01 at rest and variations in their frequency content over time.a, Off treatment, the records from both nuclei are dominated by activity with a frequency below 30 Hz. b, Same patient after the ingestion of levodopa. Low-frequency activity is reduced, and a sharply tuned band of activity appears in STN at ∼70 Hz (arrow). In both a andb, a 1 sec segment of corresponding LP is shown under the frequency sonograms. c, Another patient recorded on treatment, showing activity in STN at ∼70 and 140 Hz (arrows). d, A patient who fell drowsy during treatment with levodopa. The peak in activity at ∼70 Hz (arrow) is present when alert but not drowsy (denoted byblack bars and defined as eyes closed and low-voltage slow activity and α dropout in EEG).
STN-GPi coherence off levodopa
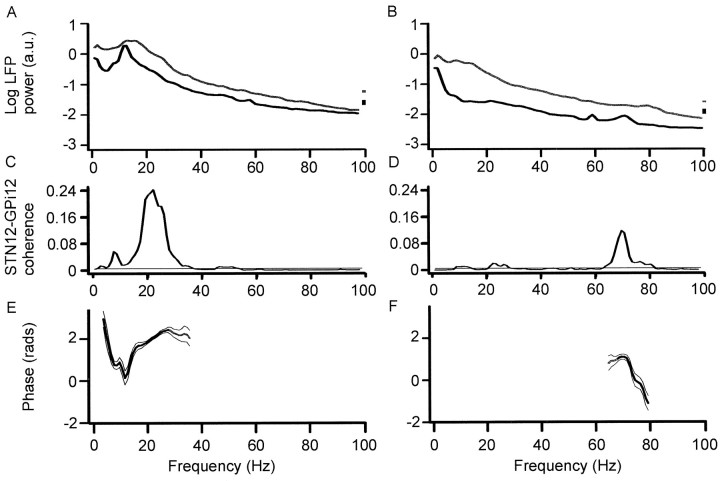
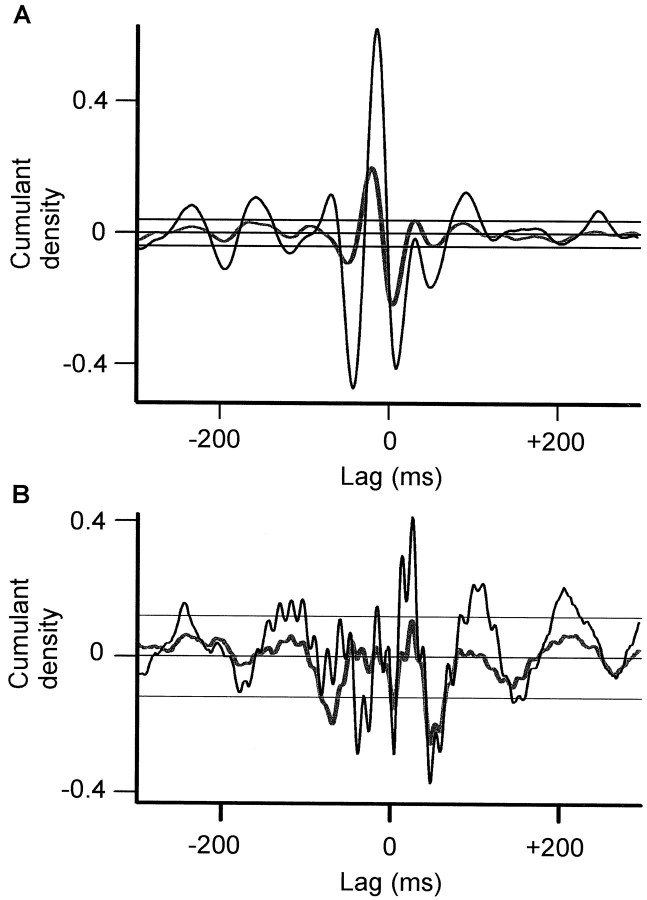
Figures 3 and 5 illustrate the power, coherence, and phase spectra at rest and during tonic extension of the contralateral wrist in the different treatment states for activities recorded from macroelectrode contacts STN12 and GPi12, pooled across all subjects. These contacts were chosen because they included at least one contact from the pair yielding the best clinical improvement upon high-frequency stimulation in each patient and also demonstrated the highest coherence with one another. At rest, off antiparkinsonian medication, the coherence between LPs recorded at STN12 and GPi12 demonstrated two peaks (Fig. 3B). The first was relatively small and centered at ∼6 Hz. STN led GPi at this frequency as shown by the net negative phase slope in Figure3E. The second peak was much larger and centered at ∼20 Hz. Up to 25% of the activity at this frequency was synchronized between GPi and STN. GPi led STN over this β band, as shown by the net positive slope in Figure 3E. Cumulant density estimates showed polarity reversal at contact 1 in GPi and STN (Fig.4A), suggesting that oscillations arose at this contact and were not volume conducted from elsewhere. In the case of GPi, which is big enough to accommodate several macroelectrode contacts, this result implies some functional somatotopy within the nucleus and is in accord with other evidence suggesting somatotopy in the human GPi (Bejjani et al., 1997; Krack et al., 1998).
Fig. 3.
Autospectra of LP power (A,B), coherence spectra between STN12 and GPi12 (C, D), and respective phase spectra (E, F) after withdrawal (A, C, E) or reinstitution (B, D, F) of levodopa. Data pooled from all records at rest in four patients with Parkinson's disease (over 6 experimental sessions). Off medication, there is coherence between STN12 and GPi12 at ∼6 and 20 Hz. Regression analysis of phase suggested that STN led GPi by 50 ± 19 msec from 3.9 to 11.7 Hz (r2= 0.853; p = 0.0004), whereas GPi led STN by 20 ± 5 msec from 11.7 to 27.3 Hz (r2 = 0.851;p < 0.0001). It should be noted that the 95% confidence limits for these and ensuing temporal differences were broad, although they never encompassed zero. The low-frequency activity is reduced on treatment when a peak appears at ∼70 Hz in the autospectrum of STN12 (B) and coherence spectrum (D). STN led GPi by 31 ± 6 msec (r2 = 0.928;p < 0.0001). In this and Figure 5, phase is shown in black (rather than gray) when it met criteria for measurement as defined in Materials and Methods, bin size is 0.98 Hz, and vertical bars and thin lines are 95% CL in power spectra and in coherence and phase spectra, respectively.
Fig. 5.
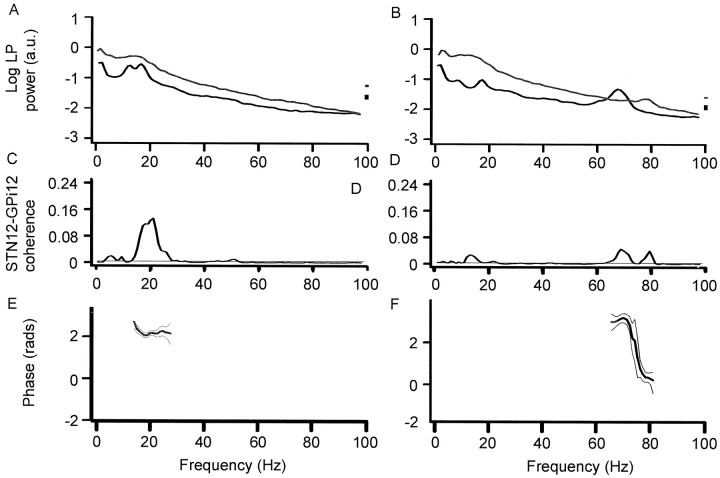
Autospectra of LP power (A,B), coherence spectra between STN12 and GPi12 (C, D), and respective phase spectra (E, F) after withdrawal (A, C, E) or reinstitution (B, D, F) of levodopa. Data pooled from all records in which the contralateral wrist was tonically extended in four patients with Parkinson's disease (over 6 experimental sessions). Off medication, there is coherence between STN12 and GPi12 at ∼20 Hz. The temporal difference between STN and GPi was indeterminate, with the best-fit line accounting for only 17% of the variance. The low-frequency activity is reduced on treatment when a peak appears at ∼70 Hz in the autospectrum of STN12 (B) and coherence spectrum (D). Regression analysis of phase suggested that STN led GPi by 47 ± 9 msec from 69.3 to 82.0 Hz (r2 = 0.910;p < 0.0001).
Fig. 4.
Cumulant density estimates after withdrawal (A) and reinstitution of levodopa (B) of levodopa recorded at rest.Black and thick gray lines are calculated from STN12–GPi12 and STN01–GPi12, respectively. STN01–GPi12 has been inverted. The close superimposition of waves indicates polarity reversal around contact 1 in STN for both the slow activity inA and fast (70 Hz) activity evident in B. The horizontal lines are the 95% CL.
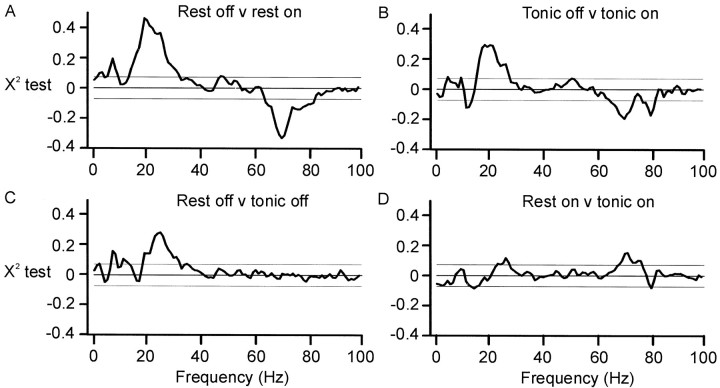
The picture was similar during tonic contractions made off levodopa (Fig. 5C), except that coherence between STN12 and GPi12 was not as high (Fig.6C, confirmed by χ2 test) and phase was indeterminate (Fig. 5E). Cumulant density estimates again showed polarity reversal at contact 1 in GPi and STN (data not shown).
Fig. 6.
A, Comparison of pooled coherence at rest off and on levodopa. B, Comparison of pooled coherence during tonic wrist extension off and on levodopa. In bothA and B, coherence was greater at ∼20 and 70 Hz off and on levodopa, respectively. C, Comparison of pooled coherence at rest and during tonic wrist extension when off levodopa. Coherence was greater at rest at ∼6 and 20 Hz.D, Comparison of pooled coherence at rest and during tonic wrist extension after treatment with levodopa. Coherence was greater at rest at ∼70 Hz.
STN–GPi coherence after levodopa
The picture was very different after levodopa. The latter was effective in improving parkinsonism (see Materials and Methods). Both at rest and during tonic activity power in the STN12 and GPi12, LPs dropped at frequencies under 30 Hz and a new peak appeared in the LPs at ∼70 Hz (Figs. 3B, 5B). This was paralleled by a striking reduction in coherence between STN12 and GPi12 LPs at ∼20 Hz and by an increase in coherence at ∼70 Hz (Figs.3B, 5B). There was no significant coherence between STN12 and GPi12 above 90 Hz in the pooled or individual data. The differences between coherence spectra on and off levodopa were confirmed by a χ2 test on the pooled data (Fig. 6A,B). Thus, the synchronization of activities in GPi and STN shifted to much higher frequencies after treatment with levodopa. STN tended to phase lead GPi over these higher frequencies, as shown by the net negative gradients in Figures 3F and 5F. After treatment, cumulant density estimates showed polarity reversal of the 70 Hz activity at contact 1 in GPi and STN, suggesting that the fast oscillations arose within these nuclei (Fig. 4B), although during tonic wrist extension, there was an additional slow wave that did not phase reverse and probably represented volume conduction from a more distant source (data not shown). The coherence between STN12 and GPi12 LPs at ∼70 Hz after levodopa tended to be greater at rest than during tonic extension of the wrist (Fig.6D, see χ2 test). Note that the small peak in coherence at ∼6 Hz at rest was greater off medication than after levodopa (Fig. 6A) and greater off medication but at rest than during tonic wrist extension (Fig.6C), in keeping with a tremor-related phenomenon.
DISCUSSION
Our results show a marked change in the nature of oscillatory activity within and between GPi and STN in untreated and treated patients with Parkinson's disease. Off levodopa LPs in both nuclei were dominated by low-frequency activity with coherence between the two signals at ∼6 and 20 Hz. Conversely, after treatment with levodopa doses sufficient to improve parkinsonism, this low-frequency synchronization was diminished and replaced by synchronization at ∼70 Hz. Power changes were in the same direction as changes in coherence so that the latter were not attributable to alterations in the nonlinear components of the signals. These dramatic effects of levodopa on pallidal–STN oscillations may have been exerted through the projection of the substantia nigra pars compacta to the putamen and thence to STN or directly via a dopaminergic substantia nigra pars compacta–STN projection (Parent and Hazrati, 1995). In line with the latter, the overall electrophysiological activity of the STN is known to increase after the direct iontophoretic application of dopamine in the rat (Cambell et al., 1985).
Origin of low- and high-frequency activities
Macroelectrode sites were deemed to be within GPi and STN on the basis of their stereotactic coordinates, postoperative MRI, and the antiparkinsonian effects of high-frequency stimulation at each site. In addition, the phase reversal of the cumulant density estimates indicated that the activities recorded by contacts GPi12 and STN12 were generated locally rather than picked up through volume conduction from more distant sources.
The networks subserving synchronized oscillations at above 15 Hz, which represent the bulk of the activity recorded here, are obscure. To date, there have been no reports of such oscillatory activity in cross-correlations of single units within or between GPi and STN. Nevertheless, recent investigations of organotypic cocultures suggest that the STN–pallidal network can support rhythmic activity, albeit at frequencies under 4 Hz (Plenz and Kital, 1999). Cortical (Magill et al., 2000; Nambu et al., 2000) and subcortical influences, such as those from the striatum (Ryan and Clark, 1992), thalamus (Bevan et al., 1995; Smith et al., 1998), and pedunculopontine nucleus (Inglis and Winn, 1995), may further modify this innate rhythmicity.
Phase relationships between GPi and STN
Phase relationships between GPi and STN are open to several interpretations. However, we would choose to view them in the light of current models of the basal ganglia (Albin et al., 1989; Alexander and Crutcher, 1990; Parent and Hazrati, 1995; Chesselet and Delfs, 1996;Smith et al., 1998). Off levodopa, activity in GPi led that in STN at ∼20 Hz. This would be consistent with the common driving of STN and GPi by globus pallidus externa (GPe) (with conduction and neuronal delays to STN being longer than to GPi). Also off levodopa, activity in STN at ∼6 Hz led that in GPi and might contribute to the postulated synchronization of output from GPi at low frequency in untreated Parkinson's disease (Brown and Marsden, 1998).
Dopaminergic stimulation dramatically changed the pattern of functional connectivity. Oscillatory interactions between STN and GPi were dominated by activity at ∼70 Hz in which STN led GPi, whereas those below 30 Hz were greatly diminished. This would be consistent with the driving of GPi by STN at high frequency when on treatment, either through direct STN–GPi projections or via STN–GPe–GPi pathways.
The above may well not be the only interactions between GPi and STN; some may involve asynchronous and nonoscillatory activity and escape detection through frequency analysis of LPs. Nevertheless, the connections identified above are likely to be quantitatively important because the postsynaptic effects of synchronized inputs are greater than those of asynchronous ones.
Effects of pathological synchronization below 30 Hz
In the presence of deficient dopaminergic input, as in untreated Parkinson's disease, subthalamo-pallidal networks seem to be favored that oscillate at frequencies below 30 Hz. There appear to be two main effects. First, there are oscillations at 4–10 Hz in which STN leads GPi. These oscillations may be related to tremor as reported in microelectrode single-unit studies in monkeys (Bergman et al., 1994,1998) and parkinsonian patients (Hutchison et al., 1997b; Taha et al., 1997; Magnin et al., 2000). Certainly, in one of our cases with a florid rest tremor off medication, there was coherence between the STN LP and EMG at tremor frequency. The second oscillatory activity occurred in the β range, with a peak centered at ∼20 Hz. Here GPi phase led STN. Both activities may disrupt normal motor function (Wichmann and DeLong, 1996). Artificial driving of the pallidum in the cat (Hassler and Dieckmann, 1967; Dieckmann, 1968) or of STN in humans (Demeret et al., 1999) at low frequency brings on or exacerbates parkinsonism, suggesting that the spontaneous synchronization at low frequency may contribute to the abnormal pattern of movement in Parkinson's disease (Brown and Marsden, 1998). This hypothesis deserves further investigation, because it may help explain some of the paradoxical effects of functional neurosurgery (see below).
Effects of synchronization at ∼70 Hz
Our results imply that elements within the pallidum and STN form a functional network that ordinarily, in the presence of a normal dopaminergic drive, resonates at ∼70 Hz. That such a rhythm is important for the optimal organization of voluntary movement is strongly suggested by the antiparkinsonian effects of stimulation of the same nuclei at frequencies likely to cause resonance within this network, at or near the base frequency of 70 Hz or its second harmonic (Siegfried and Lippitz, 1994; Limousin et al., 1995; Starr et al., 1998). However, the oscillations occurring after the reinstitution of dopaminergic stimulation are unlikely to be directly related to the execution of voluntary movement because they occur at rest as well as during motor activity. As such, they could be related to attentional processes operating in the executive domain, acting through the thalamus to favor cortico-cortical interactions in the gamma band (Hassler, 1980; Brown and Marsden, 1998). In support of this is the disappearance of the 70 Hz activity with drowsiness in one patient, although one might have expected the same activity to be increased rather than reduced during tonic voluntary contraction. Alternatively, the 70 Hz activity could act as a carrier rhythm for motor commands. The narrow band nature of the high-frequency activity evident in individual power and coherence spectra would be particularly suited to a carrier function.
Implications for functional neurosurgery
The present findings could help explain the paradoxical results of functional neurosurgery for Parkinson's disease. Hitherto, the efficacy of this treatment has been difficult to explain in terms of the known physiology of the basal ganglia. There are two surgical techniques, lesioning of GPi or STN and stimulation of the same sites at high frequency through implanted macroelectrodes (Siegfried and Lippitz, 1994; Limousin et al., 1995; Gill and Heywood, 1997; Starr et al., 1998). Focal lesions of GPi should destroy the major output of the basal ganglia to the motor cortex and abolish their contributions to normal voluntary movement. Lesions would therefore be expected to impair motor performance, but the reverse is seen in Parkinson's disease. On the other hand, the similarity between the effects of stimulation at frequencies in excess of 50 Hz and focal lesioning might imply that the former works through the induction of a virtual lesion by depolarization block (Limousin et al., 1995). However, human GPi neurons discharge at frequencies of ∼85–140 Hz in Parkinson's disease, suggesting that neural elements are more likely to be driven than blocked by high-frequency stimulation (Hutchison et al., 1997a; Merello et al., 1999; Magnin et al., 2000).
These paradoxical observations could be reconciled by the present findings if we are correct in hypothesizing that the low- and high-frequency modes of the subthalamic-pallidal circuit impair and promote motor function, respectively. In this case, the low-frequency activity could be blocked with beneficial effect by either exogenous dopaminergic stimulation or the focal destruction of GPi or STN. At the same time, therapeutic stimulation of either nucleus at high frequency might artificially drive a circuit that normally requires dopaminergic stimulation to resonate in its optimal mode.
Footnotes
This work was supported by the Medical Research Council. We thank J. C. Rothwell for his assistance in recording one of the patients and D. H. Halliday and D. Buckwell for computer programs.
Correspondence should be addressed to Dr. P. Brown, Medical Research Council Human Movement and Balance Unit, Institute of Neurology, Queen Square, London WCIN 3BG, UK. E-mail: p.brown@ion.ucl.ac.uk.
REFERENCES
- 1.Albin RL, Young AB, Penney JB. The functional anatomy of basal ganglia disorders. Trends Neurosci. 1989;12:366–376. doi: 10.1016/0166-2236(89)90074-x. [DOI] [PubMed] [Google Scholar]
- 2.Alexander GE, Crutcher ME. Functional architecture of the basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- 3.Amjad AM, Halliday DM, Rosenberg JR, Conway BA. An extended difference of coherence test for comparing and combining several independent coherence estimates: theory and application to the study of motor units and physiological tremor. J Neurosci Methods. 1997;73:69–79. doi: 10.1016/s0165-0270(96)02214-5. [DOI] [PubMed] [Google Scholar]
- 4.Bejjani B, Damier P, Arnulf I, Bonnet AM, Vidailhet M, Dormont D, Pidoux B, Cornu P, Marsault C, Agid Y. Pallidal stimulation for Parkinson's disease: two targets? Neurology. 1997;49:1564–1569. doi: 10.1212/wnl.49.6.1564. [DOI] [PubMed] [Google Scholar]
- 5.Bergman H, Wichmann T, Karmon B, DeLong MR. The primate subthalamic nucleus. II. Neuronal activity in the MPTP model of parkinsonism. J Neurophysiol. 1994;72:507–520. doi: 10.1152/jn.1994.72.2.507. [DOI] [PubMed] [Google Scholar]
- 6.Bergman H, Feingold A, Nini A, Raz A, Slovin H, Abeles M, Vaadia E. Physiological aspects of information processing in the basal ganglia of normal and parkinsonian primates. Trends Neurosci. 1998;21:32–38. doi: 10.1016/s0166-2236(97)01151-x. [DOI] [PubMed] [Google Scholar]
- 7.Bevan MD, Francis CF, Bolam JP. The glutamate-enriched cortical and thalamic input to neurons in the subthalamic nucleus of the rat. J Comp Neurol. 1995;361:491–511. doi: 10.1002/cne.903610312. [DOI] [PubMed] [Google Scholar]
- 8.Brown P, Marsden CD. What do the basal ganglia do? Lancet. 1998;351:1801–1804. doi: 10.1016/s0140-6736(97)11225-9. [DOI] [PubMed] [Google Scholar]
- 9.Cambell GA, Eckardt MJ, Weight FF. Dopaminergic mechanisms in subthalamic nucleus of rat: analysis using horseradish peroxidase and microiontophoresis. Brain Res. 1985;333:261–270. doi: 10.1016/0006-8993(85)91580-x. [DOI] [PubMed] [Google Scholar]
- 10.Chesselet MF, Delfs JM. Basal ganglia and movement disorders: an update. Trends Neurosci. 1996;18:417–422. doi: 10.1016/0166-2236(96)10052-7. [DOI] [PubMed] [Google Scholar]
- 11.Creutzfeldt OD, Watanabe S, Lux HD. Relations between EEG phenomena and potentials of single cortical cells. I. Evoked responses after thalamic and epicortical stimulation. Electroencephalogr Clin Neurophysiol. 1966;20:1–18. doi: 10.1016/0013-4694(66)90136-2. [DOI] [PubMed] [Google Scholar]
- 12.Demeret S, Bejjani B-P, Arnulf I, Damier P, Gervais D, Houeto JL, Pridoux B, Agid Y. Low frequency subthalamic stimulation worsens parkinsonian symptoms. Neurology. 1999;[Suppl 2] 52:A406. [Google Scholar]
- 13.Dieckmann G. Cortical synchronised and desynchronised responses evoked by stimulation of the putamen in cats. J Neurol Sci. 1968;7:385–391. doi: 10.1016/0022-510x(68)90157-3. [DOI] [PubMed] [Google Scholar]
- 14.Filion M, Tremblay L. Abnormal spontaneous activity of globus pallidus neurons in monkeys with MPTP-induced parkinsonism. Brain Res. 1991;547:142–151. [PubMed] [Google Scholar]
- 15.Frost JD. EEG-intracellular potential relationships in isolated cerebral cortex. Electroencephalogr Clin Neurophysiol. 1968;24:434–443. doi: 10.1016/0013-4694(68)90103-x. [DOI] [PubMed] [Google Scholar]
- 16.Gill SS, Heywood P. Bilateral dorsolateral subthalamotomy for advanced Parkinson's disease. Lancet. 1997;350:1224. doi: 10.1016/s0140-6736(05)63455-1. [DOI] [PubMed] [Google Scholar]
- 17.Halliday DM, Rosenberg JR, Amjad AM, Breeze P, Conway BA, Farmer SF. A framework for the analysis of mixed time series/point process data: theory and application to the study of physiological tremor, single motor unit discharges and electromyograms. Prog Biophys Mol Biol. 1995;64:237–278. doi: 10.1016/s0079-6107(96)00009-0. [DOI] [PubMed] [Google Scholar]
- 18.Hassler R. Brain mechanisms of intention and attention with introductory remarks on other volitional processes. Prog Brain Res. 1980;54:585–614. doi: 10.1016/S0079-6123(08)61680-5. [DOI] [PubMed] [Google Scholar]
- 19.Hassler R, Dieckmann G. Arrest reaction, delayed inhibition and unusual gaze behaviour resulting from stimulation of the putamen in awake unrestrained cats. Brain Res. 1967;5:504–508. doi: 10.1016/0006-8993(67)90021-2. [DOI] [PubMed] [Google Scholar]
- 20.Hutchison WD, Levy R, Dostrovsky JO, Lozano AM, Lang AE. Effects of apomorphine on globus pallidus neurons in parkinsonian patients. Ann Neurol. 1997a;42:767–775. doi: 10.1002/ana.410420513. [DOI] [PubMed] [Google Scholar]
- 21.Hutchison WD, Lozano AM, Tasker AE, Dostrovsky JO. Identification and characterization of neurons with tremor-frequency activity in human globus pallidus. Exp Brain Res. 1997b;113:557–563. doi: 10.1007/pl00005606. [DOI] [PubMed] [Google Scholar]
- 22.Hutchison WD, Allan RJ, Opitz H, Levy R, Dostrovsky JO, Lang AE, Lozano AM. Neurophysiological identification of the subthalamic nucleus in surgery for Parkinson's disease. Ann Neurol. 1998;44:622–628. doi: 10.1002/ana.410440407. [DOI] [PubMed] [Google Scholar]
- 23.Inglis WL, Winn P. The pedunculopotine tegmental nucleus. Where the striatum meets the reticular formation. Prog Neurobiol. 1995;47:1–29. doi: 10.1016/0301-0082(95)00013-l. [DOI] [PubMed] [Google Scholar]
- 24.Krack P, Pollak P, Limousin P, Hoffmann D, Benazzouz A, Le Bas JF, Koudsie A, Benabid AL. Opposite motor effects of pallidal stimulation in Parkinson's disease. Ann Neurol. 1998;43:180–192. doi: 10.1002/ana.410430208. [DOI] [PubMed] [Google Scholar]
- 25.Limousin P, Pollak P, Benazzouz A, Hoffmann D, Le Bas JF, Broussolle E, Perret JE, Benabid AL. Effect on parkinsonian signs and symptoms of bilateral subthalamic nucleus stimulation. Lancet. 1995;345:91–95. doi: 10.1016/s0140-6736(95)90062-4. [DOI] [PubMed] [Google Scholar]
- 26.Magill PJ, Bolam JP, Bevan MD. Relationship of activity in the subthalamic nucleus-globus pallidus network to cortical electroencephalogram. J Neurosci. 2000;20:820–833. doi: 10.1523/JNEUROSCI.20-02-00820.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Magnin M, Morel A, Jeanmond D. Single unit analysis of the pallidum, thalamus and subthalamic nucleus in parkinsonian patients. Neuroscience. 2000;96:549–564. doi: 10.1016/s0306-4522(99)00583-7. [DOI] [PubMed] [Google Scholar]
- 28.Merello M, Balej J, Delfino M, Cammarota A, Betti O, Leiguarda R. Apomorphine induces changes in GPi spontaneous outflow in patients with Parkinson's disease. Mov Disord. 1999;14:45–49. doi: 10.1002/1531-8257(199901)14:1<45::aid-mds1009>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 29.Mima T, Hallett M. Corticomuscular coherence: a review. J Clin Neurophysiol. 1999;16:501–511. doi: 10.1097/00004691-199911000-00002. [DOI] [PubMed] [Google Scholar]
- 30.Nambu A, Tokuno H, Hamada I, Kita H, Imanishi M, Akazawa T, Ikeuchi Y, Hasegawa N. Excitatory cortical inputs to pallidal neurons via the subthalamic nucleus in the monkey. J Neurophysiol. 2000;84:289–300. doi: 10.1152/jn.2000.84.1.289. [DOI] [PubMed] [Google Scholar]
- 31.Nini A, Feingold A, Slovin H, Bergman H. Neurons in the globus pallidus do not show correlated activity in the normal monkey, but phase-locked oscillations appear in the MPTP model of parkinsonism. J Neurophysiol. 1995;74:1800–1805. doi: 10.1152/jn.1995.74.4.1800. [DOI] [PubMed] [Google Scholar]
- 32.Parent A, Hazrati L-N. Functional anatomy of the basal ganglia. II. The place of the subthalamic nucleus and external pallidum in basal ganglia circuitry. Brain Res Rev. 1995;20:128–154. doi: 10.1016/0165-0173(94)00008-d. [DOI] [PubMed] [Google Scholar]
- 33.Plenz D, Kital ST. A basal ganglia pacemaker formed by the subthalamic nucleus and external globus pallidus. Nature. 1999;400:677–682. doi: 10.1038/23281. [DOI] [PubMed] [Google Scholar]
- 34.Ryan LJ, Clark KB. Alteration of neuronal responses in the subthalamic nucleus following globus pallidus and neostriatal lesions in rats. Brain Res Bull. 1992;29:319–327. doi: 10.1016/0361-9230(92)90063-4. [DOI] [PubMed] [Google Scholar]
- 35.Scatton B, Javoy-Agid F, Rouquier L, Dubois B, Agid Y. Reduction of cortical dopamine, noradrenaline, serotonin and their metabolites in Parkinson's disease. Brain Res. 1983;275:321–328. doi: 10.1016/0006-8993(83)90993-9. [DOI] [PubMed] [Google Scholar]
- 36.Siegfried J, Lippitz B. Bilateral chronic electrostimulation of ventroposterolateral pallidum: a new therapeutic approach for alleviating all parkinsonian symptoms. Neurosurgery. 1994;35:1126–1130. doi: 10.1227/00006123-199412000-00016. [DOI] [PubMed] [Google Scholar]
- 37.Smith Y, Bevan MD, Shink E, Bolam JP. Microcircuitry of the direct and indirect pathways of the basal ganglia. Neuroscience. 1998;86:353–387. doi: 10.1016/s0306-4522(98)00004-9. [DOI] [PubMed] [Google Scholar]
- 38.Starr PA, Vitek J, Bakay RAE. Ablative surgery and deep brain stimulation for Parkinson's disease. Neurosurgery. 1998;43:989–1015. doi: 10.1097/00006123-199811000-00001. [DOI] [PubMed] [Google Scholar]
- 39.Sterio D, Beric A, Dogali M, Fazzini E, Alfaro G, Devinsky O. Neurophysiological properties of pallidal neurons in Parkinson's disease. Ann Neurol. 1994;35:586–591. doi: 10.1002/ana.410350512. [DOI] [PubMed] [Google Scholar]
- 40.Taha JM, Favre J, Baumann TK, Burchiel KJ. Tremor control after pallidotomy in patients with Parkinson's disease: correlation with microrecording findings. J Neurosurg. 1997;86:642–647. doi: 10.3171/jns.1997.86.4.0642. [DOI] [PubMed] [Google Scholar]
- 41.Wichmann T, DeLong MR. Functional and pathophysiological models of the basal ganglia. Curr Opin Neurobiol. 1996;6:751–758. doi: 10.1016/s0959-4388(96)80024-9. [DOI] [PubMed] [Google Scholar]