Abstract
Neurons in the inferior colliculus (IC) that are excited by one ear and inhibited by the other [excitatory—inhibitory (EI) neurons] can code interaural intensity disparities (IIDs), the cues animals use to localize high frequencies. Although EI properties are first formed in a lower nucleus and imposed on some IC cells via an excitatory projection, many other EI neurons are formed de novo in the IC. By reversibly inactivating the dorsal nucleus of the lateral lemniscus (DNLL) in Mexican free-tailed bats with kynurenic acid, we show that the EI properties of many IC cells are formed de novo via an inhibitory projection from the DNLL on the opposite side. We also show that signals excitatory to the IC evoke an inhibition in the opposite DNLL that persists for tens of milliseconds after the signal has ended. During that period, strongly suppressed EI cells in the IC are deprived of inhibition from the DNLL and respond to binaural signals as weakly inhibited or monaural cells. By relieving inhibition at the IC, we show that an initial binaural signal essentially reconfigures the circuit and thereby allows IC cells to respond to trailing binaural signals that were inhibitory when presented alone. Thus, DNLL innervation creates a property in the IC that is not possessed by lower neurons or by collicular EI neurons that are not innervated by the DNLL. That property is a change in responsiveness to binaural signals, a change dependent on the reception of an earlier sound. These features suggest that the circuitry linking the DNLL with the opposite central nucleus of the IC is important for the processing of IIDs that change over time, such as the IIDs generated by moving stimuli or by multiple sound sources that emanate from different regions of space.
Keywords: GABA, persistent inhibition, precedence effect, inferior colliculus, sound localization, dorsal nucleus of lateral lemniscus
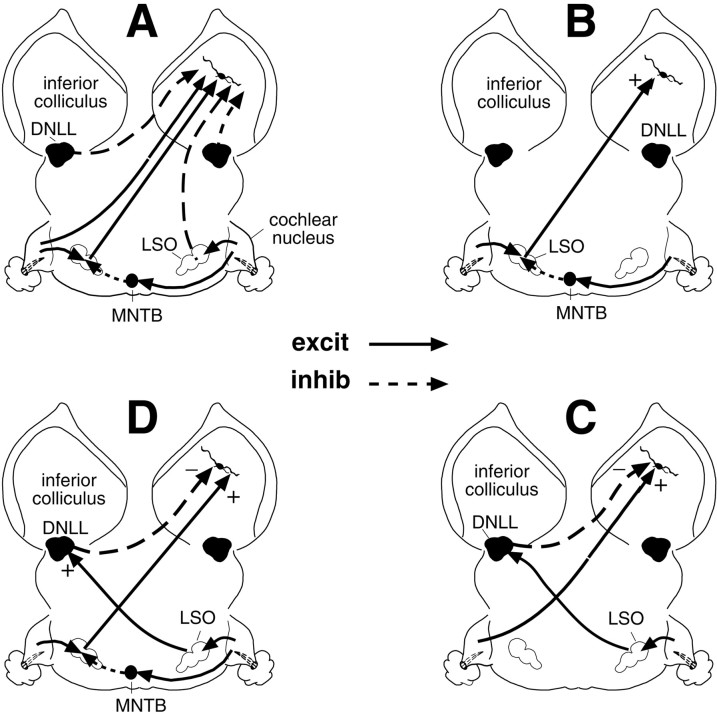
The projections from the vast majority of lower auditory nuclei converge at a common destination in the central nucleus of the inferior colliculus (ICc) (for review, see Aitkin, 1986; Oliver and Huerta, 1992). This large convergence of inputs suggests that substantial transformations occur in the ICc; yet the response properties of ICc neurons appear to be similar to those of the lower nuclei from which they receive their innervation. An example is excitatory–inhibitory (EI) neurons in the ICc, neurons that are excited by one ear and inhibited by the other ear. These neurons are sensitive to interaural intensity disparities (IIDs), the cues animals use to localize high-frequency sounds (Erulkar, 1972; Mills, 1972). EI neurons are initially formed in the lateral superior olive (LSO) (Boudreau and Tsuchitani, 1968; Finlayson and Caspary, 1991). They are also the dominant type in the dorsal nucleus of the lateral lemniscus (DNLL), a nucleus the neurons of which are almost exclusively GABAergic (Brugge et al., 1970; Adams and Mugniani, 1984; Covey, 1993; Yang and Pollak, 1994a,b,c; Winer et al., 1995). The DNLL, like the ICc, is one of the principal targets of both the ipsilateral and contralateral LSOs (Glendenning et al., 1981; Ross et al., 1988; Shneiderman et al., 1988, 1999; Yang et al., 1996). The DNLL, in turn, sends strong GABAergic projections bilaterally to the ICc (Ross et al., 1988; Shneiderman et al., 1988, 1999;Gonzalez-Hernandez et al., 1996; Kelly et al., 1998). Thus, EI cells of the ICc are strongly innervated by both LSOs and DNLLs, the lower nuclei the neuronal populations of which are EI (Fig.1A).
Fig. 1.
Schematic diagrams showing some principal connections from lower nuclei to EI neurons in the ICc (A) and the various ways that EI properties can be formed by subsets of those projections (B–D).A, The DNLL, shown in black, is a purely GABAergic nucleus that provides strong inhibitory projections to both the ipsilateral and contralateral ICc. Excitatory projections are shown as solid lines, and inhibitory projections aredashed lines. B, Excitatory projections from LSO to the contralateral ICc are shown. It is via this pathway that the excitatory–inhibitory properties first formed in the LSO can be imposed on their targets in the ICc. C, This circuit shows how EI properties can be formed de novo in the ICc. Stimulation of the ear contralateral to the ICc drives a lower monaural nucleus, shown generically here as the cochlear nucleus, which provides the excitation to the ICc. Stimulation of the ear ipsilateral to the ICc excites the DNLL, which then provides the inhibition that suppresses the contralaterally evoked excitation in the ICc.D, This circuit shows how EI properties, first formed in the LSO, can be modified in the ICc via the convergence of LSO and DNLL projections. The net effect of this convergence is to create EI cells in the ICc that are suppressed by lower intensities at the ipsilateral ear than they would be if they received only the LSO projection.excit, Excitatory; inhib, inhibitory;MNTB, medial nucleus of the trapezoid body.
The projections from the contralateral LSO to both the DNLL and ICc are excitatory, and it is via these crossed projections that the EI properties of the LSO are imposed on the DNLL and ICc (Fig.1B,C) (Saint Marie et al., 1989; Saint Marie and Baker, 1990; Glendenning et al., 1992; Park and Pollak, 1993, 1994). However, studies over the past several years have shown that although the EI properties of some ICc neurons are derived from innervation by the LSO, the EI properties of many ICc cells are either modified substantially or even formed de novo in the ICc (Faingold et al., 1991; Li and Kelly, 1992; Vater et al., 1992; Faingold et al., 1993; Park and Pollak, 1993, 1994). In this regard, the GABAergic projections from the DNLL to the opposite ICc play critical roles (Fig.1C,D).
What is unclear is what functional dividend derives from modifying or forming EI properties de novo in the ICc, because EI cells are already formed in the LSO. One hypothesis proposes that the reception of a first signal reconfigures the circuit by functionally inactivating the DNLL for a period of time. During that period, EI cells in the IC are deprived of their inhibitory inputs from the DNLL and are temporarily transformed from strongly inhibited into weakly inhibited EI or even monaural cells (Yang and Pollak, 1994c; Pollak, 1997). In short, there would be a change in the responsiveness of ICc cells to binaural signals, a change dependent on the reception of an earlier sound that inactivates the DNLL. Thus, at least one consequence of DNLL projections to the ICc is to influence the processing of multiple sound sources that originate from different regions of space.
Here we provide evidence supporting this hypothesis with recordings from the auditory system of Mexican free-tailed bats, where we block GABAergic inhibition at the DNLL and ICc as well as reversibly inactivate the DNLL while recording from ICc neurons. We then discuss the functional relevance of these features for sound localization and suggest that DNLL inhibition of the ICc may be one of the neural mechanisms that underlie the precedence effect.
MATERIALS AND METHODS
Surgical procedure. Mexican free-tailed bats,Tadarida brasilensis mexicana, were used in this study. We used these animals because their brainstem auditory nuclei are fundamentally similar to those of less specialized mammals, but they are greatly hypertrophied (Grothe et al., 1994; Park et al., 1996;Grothe and Park, 1998). The relatively large size makes nuclei, such as the IC, readily accessible, whereas the small absolute size allows them to be reversibly inactivated by iontophoresing drugs.
Each animal was anesthetized with methoxyflurane inhalation (Metofane; Pitman-Moore, Inc.) and the neuroleptic Innovar-Vet (Fentanyl and Droperidol; 0.02 mg/gm of body weight; Pitman-Moore, Inc.), injected intraperitoneally. The hair on the head was removed with a depilatory, and the head was secured in a head holder with a bite bar. The muscles and skin overlying the skull were reflected, and lidocaine (Elkins-Sinn, Cherry Hill, NJ) was applied topically to all open wounds. The surface of the skull was cleared of tissue, and a foundation layer of cyanoacrylate and small glass beads was placed on the surface. The IC is greatly hypertrophied and is so large that it protrudes between the cortex and the cerebellum. Both ICs are clearly visible through the thin braincase as two prominent structures framed by suture lines. By the use of visible landmarks formed by the ICs and the suture lines, a small opening in the skull was made over the left IC with a scalpel blade. In experiments in which we recorded from the DNLL, a small opening was made over the right IC, and in experiments in which we inactivated the DNLL while recording from the ICc, small openings were made over each IC.
The bat was then transferred to a heated recording chamber, where it was placed in a restraining cushion constructed of foam molded to the animal's body. The restraining cushion was attached to a platform mounted on a custom-made stereotaxic instrument (Schuller et al., 1986). A small metal rod was cemented to the foundation layer on the skull and then attached to a bar mounted on the stereotaxic instrument to ensure a uniform positioning of the head. A ground electrode was placed between the reflected muscle and the skin. Recordings were begun after the bats recovered from the anesthetic. The bats typically lie quietly in the restraining cushion and show no signs of pain or discomfort. Supplementary doses of the neuroleptic were given if the bat struggled or otherwise appeared in discomfort.
After the animal was fixed in the stereotaxic instrument, the electrode was positioned over the right IC while being viewed with an operating microscope. The electrode was advanced to a depth of 300 μm to ensure that recordings were obtained from neurons in the central nucleus of the inferior colliculus. The electrode was subsequently advanced from outside of the experimental chamber with a piezoelectric microdrive (Burleigh 7121W). For recordings from the DNLL, the instrument in which the bat was held was first rotated and adjusted to maximize the extent of the DNLL that would be encountered by the electrode penetration. By the use of visual landmarks, the electrode was then positioned on the surface of the IC so that it would enter the DNLL at a depth of ∼1600 μm. As with ICc recordings, the electrode was subsequently advanced from outside of the experimental chamber with a piezoelectric microdrive.
Several criteria were used to determine when the electrode had left the ICc and entered the DNLL. As the electrode was advanced through the ICc, there was an abrupt change in the best frequency (BF, the frequency to which the cluster or single unit was most sensitive) of the background activity at a depth of ∼1600 μm. This change signaled that the electrode had left the ICc and entered the DNLL. For the next 200–300 μm, both the multiunit activity and single units encountered displayed prominent sustained activity in response to contralateral tone bursts, and this activity was strongly suppressed when tone bursts were presented simultaneously to the ipsilateral (inhibitory) ear. A second abrupt change in BF and a change from binaural activity to monaural activity that was influenced only by sound to the contralateral ear signaled when the electrode left the DNLL and entered the intermediate nucleus of the lateral lemniscus. To ensure that these changes in BF, as well as discharge patterns and binaural properties, indicate DNLL location, in 16 experiments electrode location was verified with small iontophoretic injections (2–5 nA for 20–60 sec) of rhodamine-conjugated dextran (Molecular Probes, Eugene, OR). These response features have proven to be reliable indicators in previous studies of the mustache bat's DNLL (Markovitz and Pollak, 1993, 1994; Yang and Pollak, 1994a,b,c).
Electrodes. Both single-barrel glass pipettes filled with 1m NaCl and 2% fast green, pH 7.4, and “piggy back” multibarrel micropipettes (Havey and Caspary, 1980) were used. Fast green was used to enhance the visibility of the electrode. The recording electrodes, used either singly or when mounted on multibarrel electrodes, were constructed from capillary glass that was pulled and blunted to a tip diameter of 1–2 μm under microscopic observation. Multibarrel electrodes were pulled from a five-barrel blank (A-M Systems) and blunted to 15–20 μm. A single-barrel pipette was then attached to the five-barrel pipette and glued with cyanoacrylate so that the tip of the single-barrel pipette protruded 10–15 μm from the blunted tip of the five-barrel pipette. The single-barrel micropipette was used for recording single-unit activity and was filled with buffered 1 m NaCl and 2% fast green, pH 7.4. One barrel of the five-barrel pipette was the balancing barrel and was filled with buffered 1 m NaCl and 2% fast green. The other barrels were filled with various substances that depended on the experiment. With ICc recordings, two to four barrels were filled with solutions of bicuculline methiodide (10 mm in 0.165m NaCl, pH 3.0; Sigma, St. Louis, MO), an antagonist of GABAA receptors (Borman, 1988), and in some experiments one barrel was also filled with glutamic and aspartic acid (500 mm each in dH2O, pH 9–10). When recording from the DNLL, two barrels were filled with a cocktail of bicuculline methiodide and the glycine receptor antagonist strychnine HCl (Cooper et al., 1982) (both were 10 mm in 0.165 m NaCl, pH 3.0; Sigma). The two other barrels were filled with glutamic and aspartic acid (500 mm each in dH2O, pH 9–10). As explained in Results, because neither ICc nor DNLL cells were spontaneously active, glutamic and aspartic acid were used to generate background activity against which inhibition evoked by stimulation of the ipsilateral ear could be observed. For DNLL inactivation experiments, all drug barrels were filled with a solution of kynurenic acid (75 mm in 0.165m NaCl, pH 9–10; Sigma), a broad-spectrum antagonist of glutamatergic receptors (Collingridge and Lester, 1989). Drugs were retained in the electrode with a 15–20 nA current of opposite polarity. The drug and balancing barrels were connected via silver–silver chloride wires to a six-channel microiontophoresis constant-current generator (Medical Systems Neurophore BH-2) that was used to generate and monitor ejection and retention currents. The sum channel was used to balance current in the drug barrels and reduce current effects. The recording barrel was connected by a silver–silver chloride wire to a Dagan AC amplifier (model 2400).
Acoustic stimuli and data acquisition. Sine waves from a Wavetek function generator (model 136) were shaped into tone bursts with a custom-made analog switch. Tone burst frequency was monitored by a frequency counter. Tone bursts, as well as frequency-modulated (FM) sweeps, were also generated digitally by a Power Macintosh 7300. FM sweeps were always digitally generated and could have any desired duration as well as starting and terminal frequency. All stimuli, whether generated digitally or by the function generator, had 0.2 msec rise and fall times and were presented four times per second. The computer could generate either one or two signals (tone bursts or FM sweeps), with interpulse intervals selected by the investigator. The interpulse interval will be referred to simply as the “interval” and indicates the time from the end of the first signal to the beginning of the second or trailing signal. In addition, each signal could be presented either monaurally or binaurally. A Power Macintosh 7300 computer was used to control stimulus parameters via connections to a real time clock and a pair of digital attenuators (Wilsonics, model PATT) through a 24-bit digital interface NuBus card (National Instruments DIO-24) and a custom-made digital distributor. The output of each independently controlled channel of the attenuator was sent to two 1/4 inch Brüel and Kjaer (B&K) microphones biased with 200 V of DC and driven as speakers. The frequency response of each speaker was measured with another B&K microphone, the output of which was fed to a sound-level meter (B&K, model 2608) that measured sound pressure level in decibels (0.0002 dynes/cm2). At the start of each experiment, speakers, with the protective grid attached, were inserted into the funnels formed by the bat's pinnas and were positioned adjacent to the external auditory meatus. The pinnas were folded onto the housing of the microphones and wrapped with Scotch tape. The binaural cross-talk with this arrangement was attenuated by 35–40 dB.
Spikes were fed to a window discriminator and then to a Power Macintosh 7300 computer controlled by a custom-made real time clock. Raster displays, peristimulus time histograms, and rate-level functions were generated and graphically displayed. Unless otherwise noted, each raster display was generated from the discharges evoked by 20 presentations of a signal at a fixed intensity.
After a unit was isolated, its BF, threshold at BF, and binaural type were determined audiovisually. Binaural properties were determined by presenting a BF tone burst 10–30 dB above threshold, first to the contralateral (excitatory) ear, then to the ipsilateral (inhibitory) ear, and then to both ears simultaneously. When presented simultaneously, ipsilateral intensities, ranging from 20–30 dB below to 20–30 dB above the intensity of the contralateral ear, were presented to determine the ipsilateral intensities that suppressed contralaterally evoked discharges.
Spike counts evoked by increasing ipsilateral intensities of binaural signals were normalized in terms of the percentage change from the maximum response. Maximum response was the spike count evoked by the contralateral signal alone or the spike count evoked by a binaural signal with the lowest ipsilateral intensity, an ipsilateral intensity that was always below threshold for evoking inhibition.
In many ICc recordings we presented an initial and a trailing signal to evaluate the effects of an initial signal on the binaural properties evoked by trailing signals. In all of these experiments, the first signal was always a 5–10 msec FM signal that swept downward by 10–30 kHz. The initial and terminal frequencies were adjusted so that the frequencies swept through the BF of the neuron. The binaural trailing signals were either FM sweeps, identical to the first FM signal, or BF tone bursts. We did not use tone bursts as the initial signals because in the vast majority of ICc neurons, BF tone bursts at the contralateral (excitatory) ear evoke an excitation followed by a long-lasting inhibition (Klug et al., 1999; Bauer et al., 2000). The inhibition prevents ICc neurons from responding to trailing signals and creates long recovery times. In contrast, FM sweeps produce such inhibition much less frequently, and most ICc neurons recover within a few milliseconds (G. Pollak et al., 1977; G. D. Pollak et al., 1977).
After recording the responses of a cell to these signals, pharmacological agents were iontophoretically applied, and the responses to the same signals were recorded again. During application of bicuculline or the cocktail of bicuculline and strychnine, rate-level functions were monitored while ejection currents of from 10 to 60 nA were applied. For each ejection current, rate-level functions were obtained until the rate-level function stabilized. After the responses were stable, the complement of tone bursts and FM signals was presented again, and the same response features were obtained for comparison with those obtained before the application of drugs. The ejection current was then switched off, and the cell was allowed to recover. Recovery was complete when both the shape of the rate-level function returned to its predrug form and the maximum spike count returned to the predrug value. In many cases, however, contact with the unit was lost before recovery was complete. Because recovery times were usually 10–20 min, when neurons were lost before recovery was attained, we allowed at least 30 min before searching for another neuron.
Procedures for reversibly inactivating the DNLL. We opted to inactivate the DNLL with iontophoretic application of kynurenic acid rather than with pressure. The main reason was that we wanted to minimize any potential tissue damage caused by the mechanical deformations that result from the relatively large volumes of liquid injected with pressure. Thus, we wanted to inactivate repeatedly the DNLL and be confident that the previous inactivation had not caused damage to the DNLL.
We first determined the dimensions of the DNLL. The DNLL stains intensely with cytochrome oxidase and stands out clearly from other structures (Markovitz and Pollak, 1994). We processed four brains for cytochrome oxidase and determined the largest rostrocaudal, mediolateral, and dorsoventral extent of the DNLL. On average, the dimensions were as follows: rostrocaudal 554 μm, mediolateral 524 μm, and dorsoventral 282 μm. The histological and other procedures used to determine these dimensions are described below. Assuming that kynurenic acid spreads spherically from the injection site, we calculated that the required radius of spread had to be ∼275 μm to inactivate most of the DNLL.
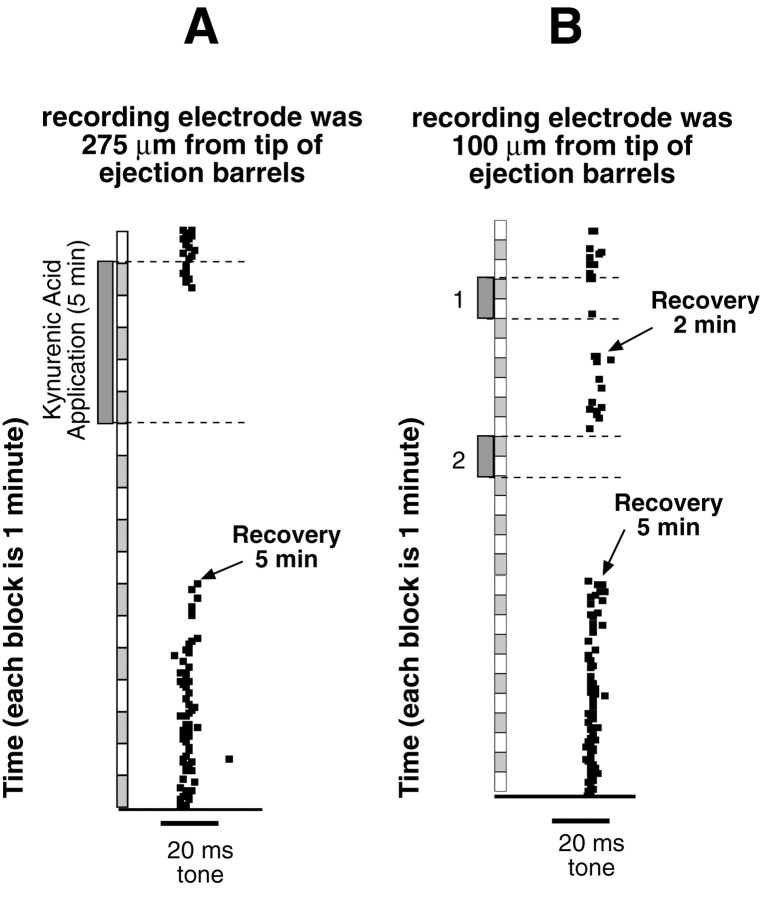
We next obtained an estimate of how far kynurenic acid spreads from the ejection barrel. This was accomplished by making multibarrel electrodes in which the tips of the recording electrodes were 100–275 μm beyond the tips of the multibarrel electrodes from which the kynurenic acid was ejected. We then recorded from 11 single units in the ICc of four bats. The ICc was used because it was easily accessible. With appropriate adjustments of ejection currents, discharges could be completely suppressed within 1–3 min, even when the recording electrode was 275 μm distant from the ejection electrodes, and discharges recovered within 3–5 min (e.g., Fig.2A). Reapplication of kynurenic acid again completely suppressed discharges, and responsiveness again recovered within 3–5 min (Fig.2B). The degree of suppression, the time it took to achieve suppression, and the time for recovery were all dosage dependent (e.g., Fig. 2B). These results provide confidence that iontophoretic application of kynurenic acid inactivated much of the DNLL and may even have spread slightly beyond the dorsoventral limits of the DNLL. Furthermore, it shows that inactivated neurons recover and suggests that repeated applications of kynurenic acid produce no tissue damage.
Fig. 2.
Inactivation of single-unit activity by iontophoresis of kynurenic acid. Tone bursts were presented once every 5 sec. Twelve tone bursts were presented each minute; responses to each presentation are shown as horizontally aligned squares. Tone bursts were presented for 1–2 min before kynurenic acid was applied, and the responses are shown as thecolumns of squares at thetop of each record. The times at which the iontophoresis of kynurenic acid was initiated and terminated are indicated bydashed horizontal lines. A, Iontophoretic application of kynurenic acid for 5 min (hatched vertical bar) blocked excitation at sites distant from the recording electrode. The tip of the recording electrode was 275 μm from the ejection barrels. Within 1 min, kynurenic acid completely suppressed discharges at the recording electrode. The neuron recovered in 5 min and responded to the BF tones bursts as it did before drug application. Ejection current was 200 nA. B, In this example, the tip of the recording electrode was 100 μm from the ejection barrels, and the neuron was inactivated twice. For the first inactivation (1), the ejection current of kynurenic acid was 100 nA applied for 2 min. The neuron recovered after 2 min. The neuron was then inactivated a second time (2) with an ejection current of 200 nA. The higher ejection current suppressed the discharges and resulted in a longer recovery period. The recordings in both A and B were conducted in the same animal.
For DNLL inactivation experiments, the location of the DNLL was initially identified using a single-barrel electrode, using the criteria described above. After the DNLL was located with the single-barrel pipette, a multibarrel electrode was put in its place and was advanced while multiunit responses were monitored to ensure that the multibarrel electrode was in the DNLL. The inactivating electrode was positioned midway along the dorsoventral extent of the DNLL.
For each neuron we determined the percentage relief from inhibition because of DNLL inactivation. Spike counts evoked by binaural signals before inactivation were normalized in terms of the percentage change from the maximum response. Normalized spike counts were then determined during DNLL inactivation, and the difference between the two functions is given as the percentage relief from inhibition. For example, if contralaterally evoked discharges were completely suppressed at a given ipsilateral intensity, the suppression would be 100%. If, at the same ipsilateral intensity, the suppression was 60% during DNLL inactivation, the percentage relief at that ipsilateral intensity is 40%. Our criterion for “relief” was at least a 20% relief of the suppression produced at those ipsilateral intensities that, before inactivation, suppressed contralaterally evoked discharges by at least 50%.
Histological procedures. For localizing electrode locations in the DNLL after injections of rhodamine conjugated to dextran, animals were allowed to survive for 1 d. They then were deeply anesthetized with Metofane and perfused intracardially with 0.1m phosphate buffer followed by 4% paraformaldehyde in 0.1m phosphate buffer. Brains were removed, post-fixed for 4–24 hr, and then kept overnight in 30% sucrose. Sections were cut on a freezing microtome and mounted in Flouromount G (Southern Biotechnology, Birmingham, AL). Sections were viewed under a fluorescent microscope, and images of the DNLL were captured using a video frame grabber for archival purposes.
To determine the dimensions of the DNLL, bats were perfused as described above, and the brains were blocked and post-fixed for 24 hr before submersion in 30% sucrose. Sections (40 or 50 μm) were made through the portion of the brain containing the DNLL. Two brains were cut in the sagittal plane, and two others were cut in the transverse plane. Sections were processed using procedures described by Wong-Riley (1979). Sections were mounted and incubated in the dark at 37°C for 1–2 hr until brown reaction product was observed in the tissue. The incubation medium contained 50 mg of diaminobenzidine tetrahydrochloride (Sigma) in 90 ml of 0.1 m phosphate buffer, pH 7.4, 15 mg of cytochrome C type III (Sigma), and sucrose. Sections were imaged with a video frame grabber, and areas were computed with NIH Image software.
RESULTS
We report on 150 EI cells from the ICc and 113 EI neurons from the DNLL of Mexican free-tailed bats. The BFs, the frequencies to which each neuron was most sensitive, of the ICc neurons ranged from 19 to 36 kHz, and the BFs of the DNLL cells ranged from 18 to 79 kHz. The EI property of each neuron was determined by presenting a sound to the excitatory ear at a fixed intensity that was 10–30 dB above threshold and then presenting the same sound to the inhibitory ear at progressively increasing intensities. Because the intensity at the excitatory ear was fixed, each change in intensity at the inhibitory ear produced a different IID. The more intense inhibitory signals strongly suppressed discharges evoked by excitatory signals in most ICc cells. The degree of maximal suppression varied among the ICc population, although the maximal spike suppression in most cells (71%) was at least 90%. The average suppression was 91% and ranged from 30 to 100%.
The focus of this study was on the influences of the GABAergic projections from the DNLL to the opposite ICc and the functional significance of those influences. Below we first discuss some features of inhibition in DNLL neurons. We then show that many ICc neurons are influenced by GABAergic innervation from the opposite DNLL, and in the final sections we show the functional significance of those DNLL projections on the processing of IIDs in the ICc.
Persistent inhibition is a key feature of DNLL neurons
Previous studies in mustache bats have shown that inhibition in the DNLL, evoked by stimulation of the ear ipsilateral to it, is persistent, in that the inhibition often lasts for many milliseconds after the end of the signal that evoked it (Yang and Pollak, 1994a,b,1998). The duration of the inhibitory persistence, which can be as long as 80 msec, changes little with stimulus duration but increases with intensity and typically reaches a maximum duration 20–40 dB above the threshold for inhibition (Yang and Pollak, 1994a,c). Here we show that persistent inhibition is also a prominent feature of DNLL neurons in Mexican free-tailed bats. In a later section we demonstrate the functional impact that persistent inhibition in the DNLL has on many cells in the ICc.
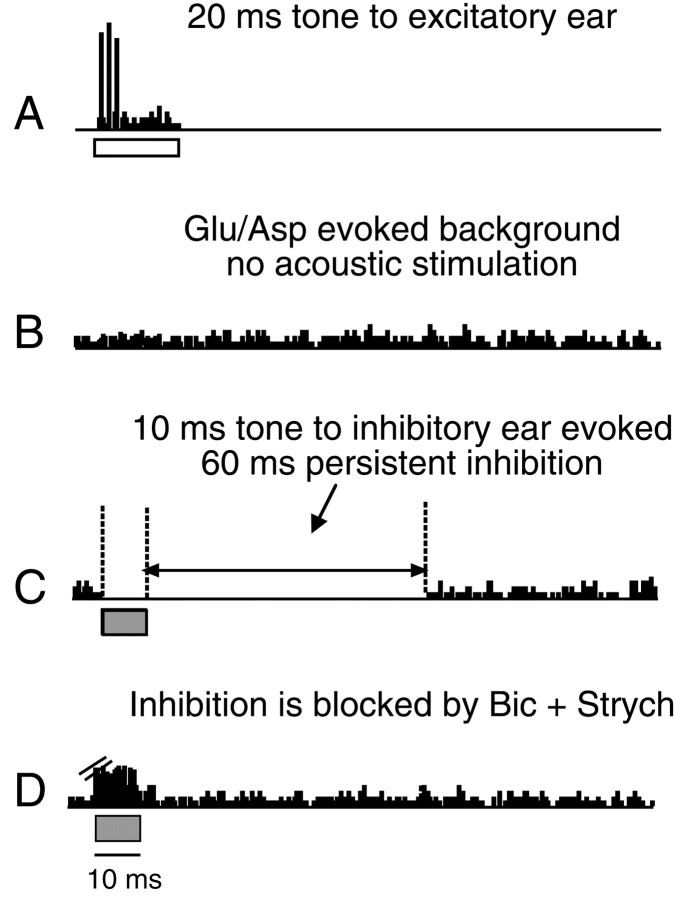
We tested 50 DNLL cells in Mexican free-tailed bats for persistent inhibition and illustrate this inhibition with the DNLL neuron in Figure 3. Figure 3A shows that the neuron was driven by tone bursts presented to the contralateral (excitatory) ear. Stimulation of the ipsilateral (inhibitory) ear evoked no responses (data not shown). To show that ipsilateral stimulation evoked an inhibition in the DNLL, we created background discharges by iontophoresing the excitatory transmitters glutamate and aspartate (Fig. 3B). Ipsilateral (inhibitory) tone bursts, at 40 dB sound pressure level (SPL) and 10 msec in duration, produced a gap in the glutamate-evoked discharges that lasted for 70 msec (Fig.3C). Thus, the inhibition persisted for 60 msec beyond the duration of the 10 msec tone that evoked it. That the inhibition occurred in the DNLL cell was confirmed by its elimination when bicuculline and strychnine were applied iontophoretically. The maximal periods of persistent inhibition varied among the 50 DNLL neurons that we tested and were typically <60 msec. The average period of the maximum persistent inhibition was 18 msec and ranged from 3 to 80 msec.
Fig. 3.
Excitation and inhibition in a DNLL neuron.A, Sustained response evoked by a BF tone burst (20 dB SPL; horizontal bar) presented to the ear contralateral to the DNLL is shown. B, Background activity evoked by iontophoretic application of glutamic and aspartic acids (Glu/Asp) is shown. C, A 10 msec BF tone burst (40 dB SPL) at the ear ipsilateral to the DNLL (inhibitory ear) inhibits Glu/Asp-evoked background activity. The inhibition, seen as thegap in the background activity, had a total duration of 70 msec. Thus, the inhibition persisted for 60 msec beyond the duration of the 10 msec tone burst that evoked the inhibition. D, Ipsilaterally evoked inhibition was abolished when a cocktail of bicuculline and strychnine was applied. The blockage of inhibition combined with the enhanced excitability caused by the Glu/Asp unmasked a subthreshold excitation. The excitation was not evoked in the absence of Glu/Asp, even when inhibition was blocked by bicuculline and strychnine. Not all DNLL cells displayed such a subthreshold excitation under these conditions. Ejection currents were as follows: Glu/Asp, 15 nA, and bicuculline and strychnine cocktail, 30 nA. Time bars under responses have been shifted to illustrate the relationship between the stimulus and response durations. BF was 35.2 kHz. Bic, Bicuculline; Strych, strychnine.
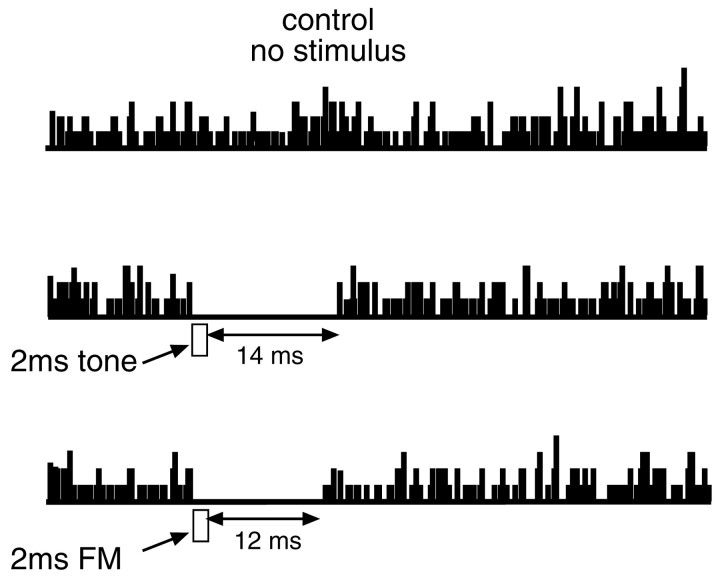
Persistent inhibition in the DNLL was evoked not only by tone bursts but also by brief FM sweeps, so long as the FM signal swept through the best frequency of the DNLL cells. The average persistent inhibition evoked by FM signals, measured in 21 DNLL neurons, was 13 msec and ranged from 4 to 38 msec. In 8 of the 21 neurons, we measured persistent inhibition evoked by both tone bursts and FM sweeps. As illustrated by the neuron in Figure 4, the two types of stimuli evoked inhibitory persistence of similar, but not identical, duration. Tone bursts usually caused a slightly longer persistent inhibition than did FM sweeps. In these eight neurons, the average FM-evoked inhibitory persistence was 12 msec, whereas the average tone-evoked inhibitory persistence was 17 msec. Previous studies reported comparable inhibitory periods evoked by tones and other signals in DNLL neurons of mustache bats (Yang and Pollak, 1998).
Fig. 4.
Persistent inhibition in the DNLL is evoked both by tone bursts and by FM sweeps. Top, Control showing background discharges evoked by iontophoresis of glutamic and aspartic acid when no stimulus was presented. Bottom, The gaps in the background evoked by 2 msec tone bursts or 2 msec FM sweeps. The tone burst evoked a total inhibitory period of 16 msec. The initial 2 msec of inhibition occurred over the duration of the tone burst, and the inhibition then persisted for an additional 14 msec. The 2 msec FM sweep evoked a persistent inhibition of 12 msec in the same DNLL neuron. The threshold at BF was −10 dB SPL. Time barsunder responses have been shifted to illustrate the relationship between the stimulus and response durations. Tone bursts were 40.4 kHz (BF) at 30 dB SPL. The FM was 30 dB SPL, the same intensity as the tone burst, and swept from 50 to 30 kHz and thus through the BF of the neuron.
Discharges evoked by contralateral stimulation are suppressed during periods of persistent inhibition
The persistence of inhibition suggests that a signal presented to the ipsilateral (inhibitory) ear should prevent DNLL neurons from responding to signals presented to the contralateral (excitatory) ear for a period of time after the inhibitory signal. To test this, we presented binaural signals in which the excitatory signals were presented at various times after the inhibitory signals. An example is shown in Figure 5. When the signals were presented simultaneously, the discharges evoked by stimulation of the excitatory ear were completely inhibited (data not shown). As the excitatory signal was delayed, the discharges continued to be suppressed. In this neuron, discharges began to appear at a delay of 10 msec, and full recovery occurred when the excitatory signal was presented 30 msec after the end of the inhibitory signal. This shows that the persistent inhibition generated by signals at the ipsilateral ear suppressed the excitation evoked by signals at the contralateral ear. The suppression of contralaterally evoked discharges during periods of persistent inhibition was seen in all 19 neurons tested in Mexican free-tailed bats. Moreover, it was seen previously in almost all neurons tested in the mustache bat DNLL, where it was also shown that the suppression of contralaterally evoked discharges during periods of persistent inhibition could be rescued by blocking inhibition at the DNLL with bicuculline and strychnine (Yang and Pollak, 1994c, 1998).
Fig. 5.
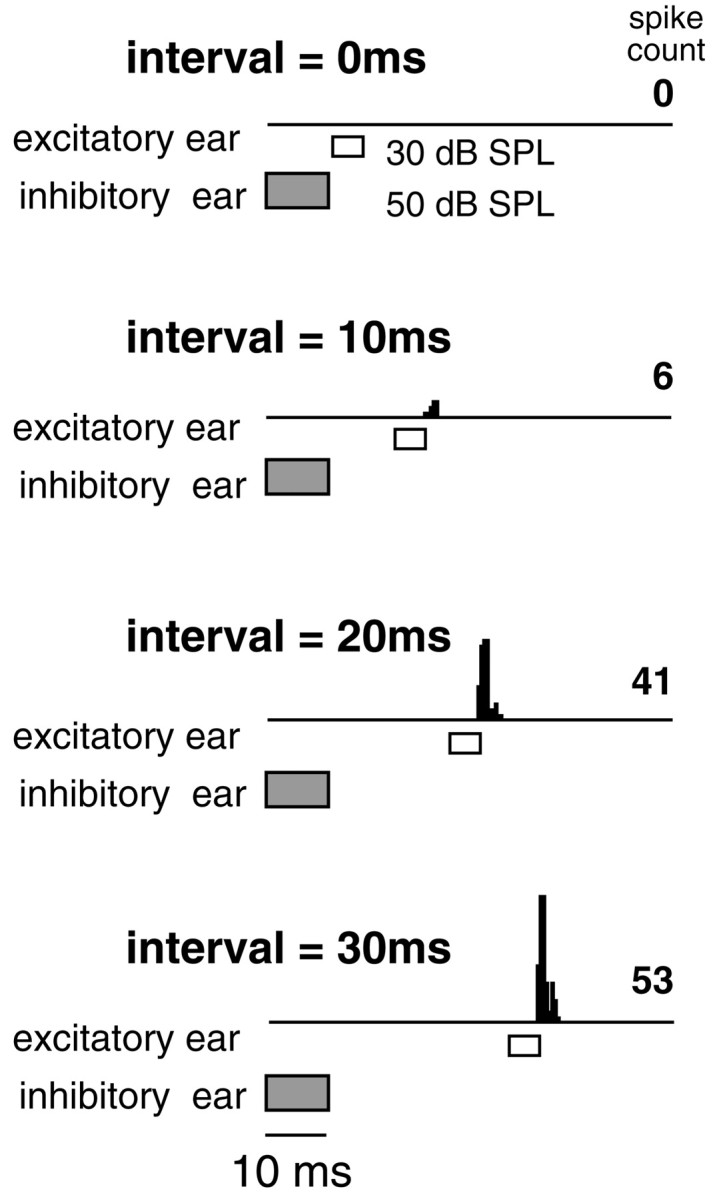
Persistent inhibition in DNLL evoked by an inhibitory signal at the ear ipsilateral to the DNLL suppresses discharges evoked by excitatory signals presented to the contralateral ear. Top, Complete suppression of contralateral (excitatory) signals by ipsilateral signals when the two were presented at an interval of 0 msec is shown. Bottom, Partial recovery occurred when the excitatory signal followed the inhibitory signals by 10–20 msec. Full recovery first occurred 30 msec after the inhibitory signal was over. The excitatory signals were 5 msec BF tone bursts and were 30 dB SPL. The inhibitory signals were 10 msec BF tone bursts at 50 dB SPL. BF was 36.1 kHz.
Inactivation of DNLL relieves inhibition in many ICc cells
The previous section dealt with stimuli that inhibited DNLL cells. In this section we deal with excitation of the DNLL and show that DNLL excitation provides inhibition to most, but not all, ICc neurons. We studied the effects of DNLL excitation in 78 ICc neurons by evaluating their EI properties before, during, and after DNLL inactivation produced by iontophoretic application of kynurenic acid. In 37% (29 of 78) of the ICc cells, inactivating the DNLL had no effect on their EI properties. In these neurons, the inhibition evoked by increasing the signal intensity to the ipsilateral ear was virtually the same during DNLL inactivation as it was before DNLL inactivation (Fig.6). This suggests that the DNLL played little or no role in the formation of the EI properties in these ICc cells.
Fig. 6.
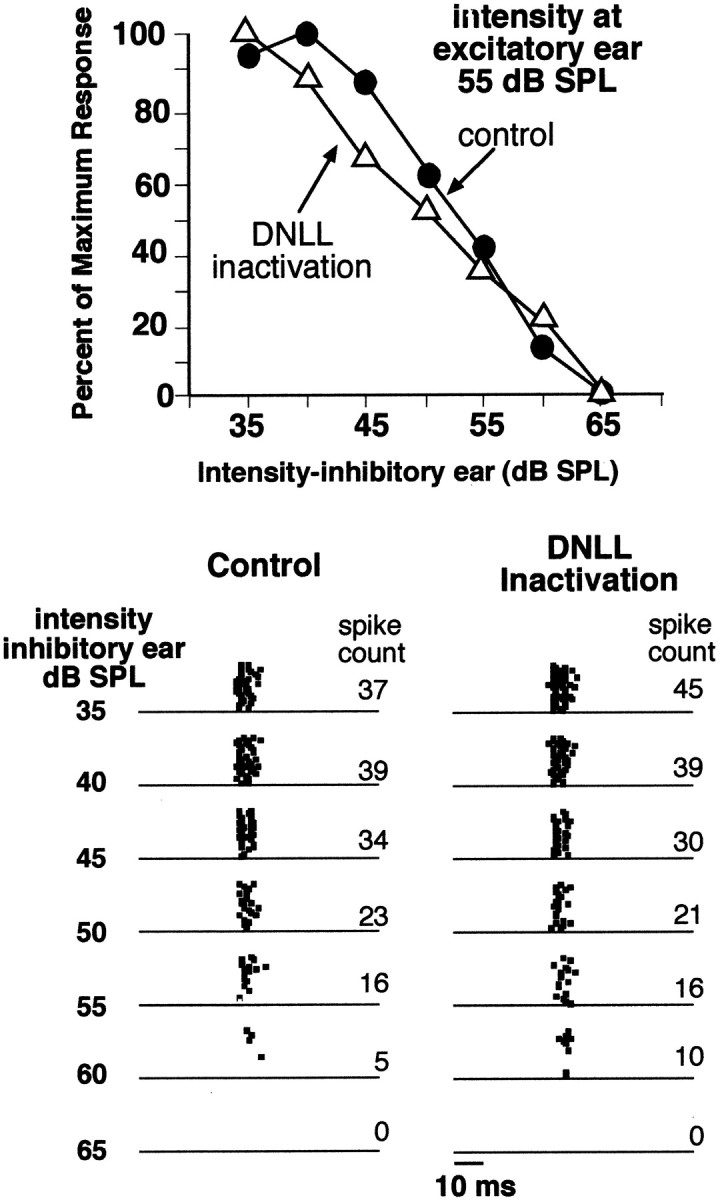
An ICc neuron that was not influenced by inactivation of the opposite DNLL. Bottom, Raster displays are shown of the responses evoked by binaural signals in which the intensity at the ear ipsilateral to the ICc (inhibitory ear) was increased by increments of 5 dB. Top, Suppression is plotted graphically. Here the suppression evoked by increasing ipsilateral intensities is plotted as the percentage of maximum response evoked. The functions obtained before (control) and during DNLL inactivation are similar, suggesting that the DNLL had little or no effect on the ipsilaterally evoked spike suppression in this cell. BF was 23.3 kHz.
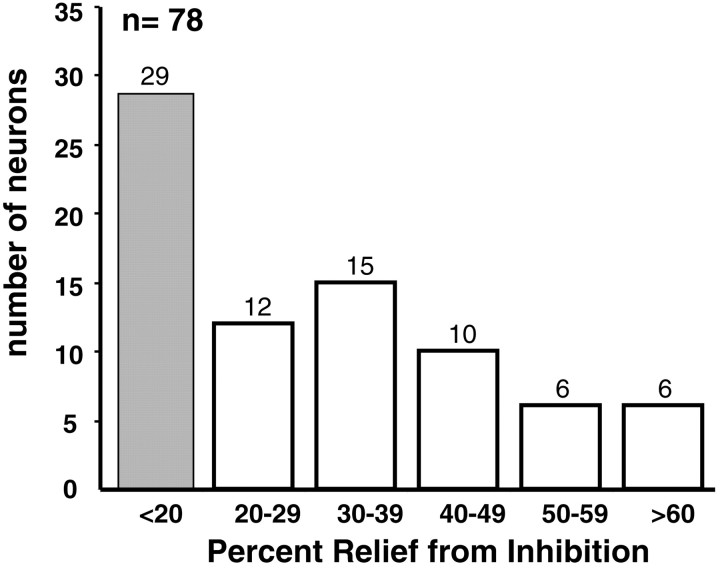
In most ICc neurons (49 of 78; 63%), however, DNLL inactivation reduced ipsilateral inhibition by at least 20%, indicating that the DNLL played a prominent role in the formation of their EI properties. On average, the maximum relief from inhibition among these 49 ICc cells was 41% and ranged from 20 to 100% (Fig.7). An example is shown in Figure8. This was a strongly inhibited EI neuron in which signal intensities of 30 and 40 dB SPL at the ipsilateral (inhibitory) ear completely suppressed discharges evoked by contralateral (excitatory) stimulation. During inactivation of the opposite DNLL, ipsilateral (inhibitory) signals produced a much smaller decline in spike count than the same signals did before inactivation. For example, with an ipsilateral intensity of 30 dB SPL, the spike count was 73% of the maximal count, a 27% suppression, whereas before inactivation the same ipsilateral intensity caused complete suppression. When the intensity at the inhibitory ear was increased to 40 dB SPL, which had also previously suppressed discharges completely, the spike count was reduced to 57% of maximum, a 43% suppression. The DNLL recovered within 10 min, and stimulation at the inhibitory ear again suppressed spike counts by 100%.
Fig. 7.
Maximum relief from inhibition by DNLL inactivation in 78 ICc neurons, the EI properties of which were tested before and during DNLL inactivation. Normalized spike counts to binaural signals were determined before and during DNLL inactivation. The difference between the value before and during inactivation is the percentage relief from inhibition. Shown here is the largest percentage relief for each cell. The average maximum relief was 41% among the 49 neurons that had relief of 20% or greater.
Fig. 8.
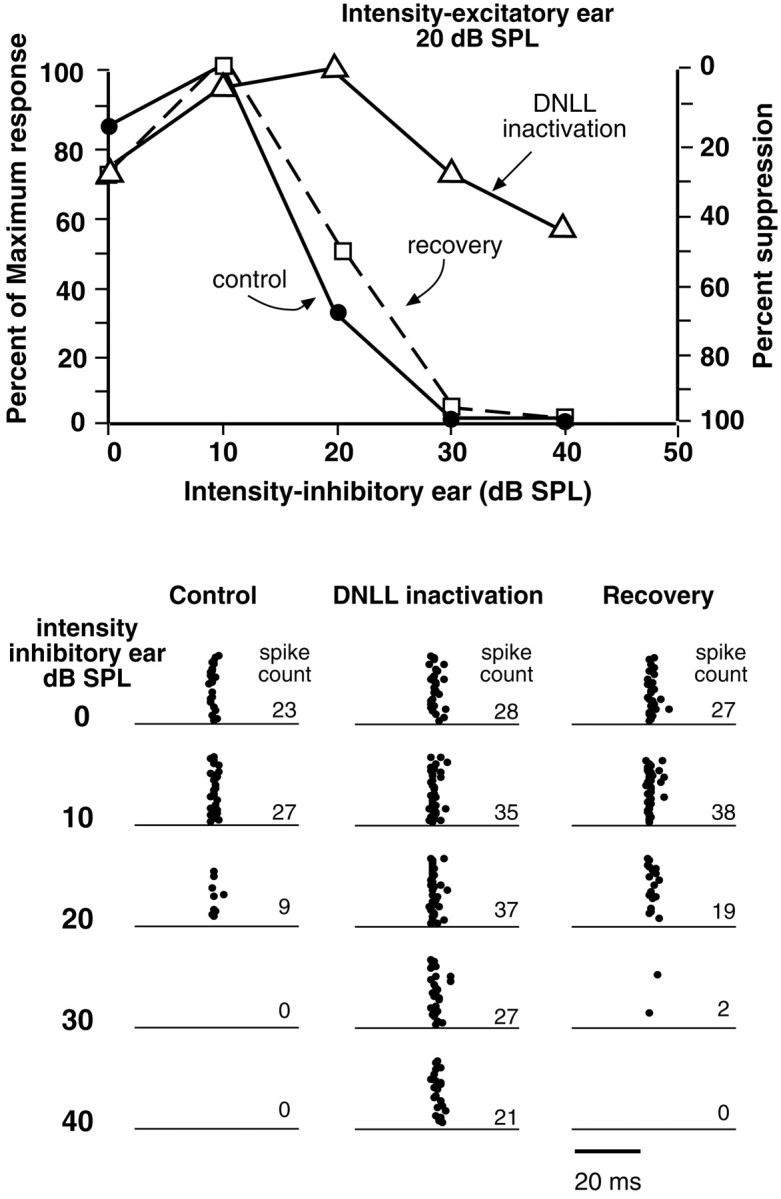
DNLL inactivation relieved ipsilaterally evoked spike suppression in an ICc neuron. Bottom, Raster displays are shown of spike suppression evoked by increasing intensity at the ear ipsilateral to the ICc (inhibitory ear) before (control), during, and after DNLL inactivation (recovery). The relief from inhibition caused by DNLL inactivation is especially apparent at ipsilateral intensities of 30–40 dB SPL. Top, The percentage of maximum response evoked by increasing ipsilateral intensities before and during DNLL inactivation and when the neuron recovered from inactivation is graphically plotted. BF was 29.5 kHz.
As with the cell in Figure 8, DNLL inactivation caused substantial, but not complete, relief from inhibition in most ICc cells. To evaluate whether DNLL inactivation eliminated most, if not all, ipsilaterally evoked GABAergic inhibition, we iontophoretically applied bicuculline to the ICc cell either during DNLL inactivation or after recovery from DNLL inactivation in 18 neurons. Bicuculline blocks not only the GABAergic innervation from the DNLL but other GABAergic inputs to the ICc cell as well. In general, relief of inhibition in the 18 cells was greater with bicuculline than with DNLL inactivation. Two representative examples are shown in Figure9. For the neuron in Figure9A, DNLL inactivation reduced inhibition from ∼98% of the maximum response before inactivation to ∼43% when intensity at the inhibitory ear was 50–60 dB SPL. However, when bicuculline was iontophoresed while the DNLL was inactivated, the inhibition was reduced even further and relieved almost 80% of the inhibition. For the neuron in Figure 9B, DNLL inactivation relieved ∼32% of the inhibition (at 50 dB SPL). After recovery from DNLL inactivation, bicuculline was applied and relieved almost all inhibition. Thus DNLL inactivation relieved inhibition, but with few exceptions, it alone did not eliminate all inhibition evoked by the ipsilateral (inhibitory) ear. This suggests either that there were, in addition to the DNLL, other sources of inhibition evoked by the inhibitory ear or that iontophoresis of kynurenic acid did not completely block DNLL activity. Although we cannot distinguish between these interpretations, the relevant point is that DNLL inactivation relieved at least 20% of the inhibition evoked by the ipsilateral (inhibitory) ear in 63% of the ICc neurons that we tested.
Fig. 9.
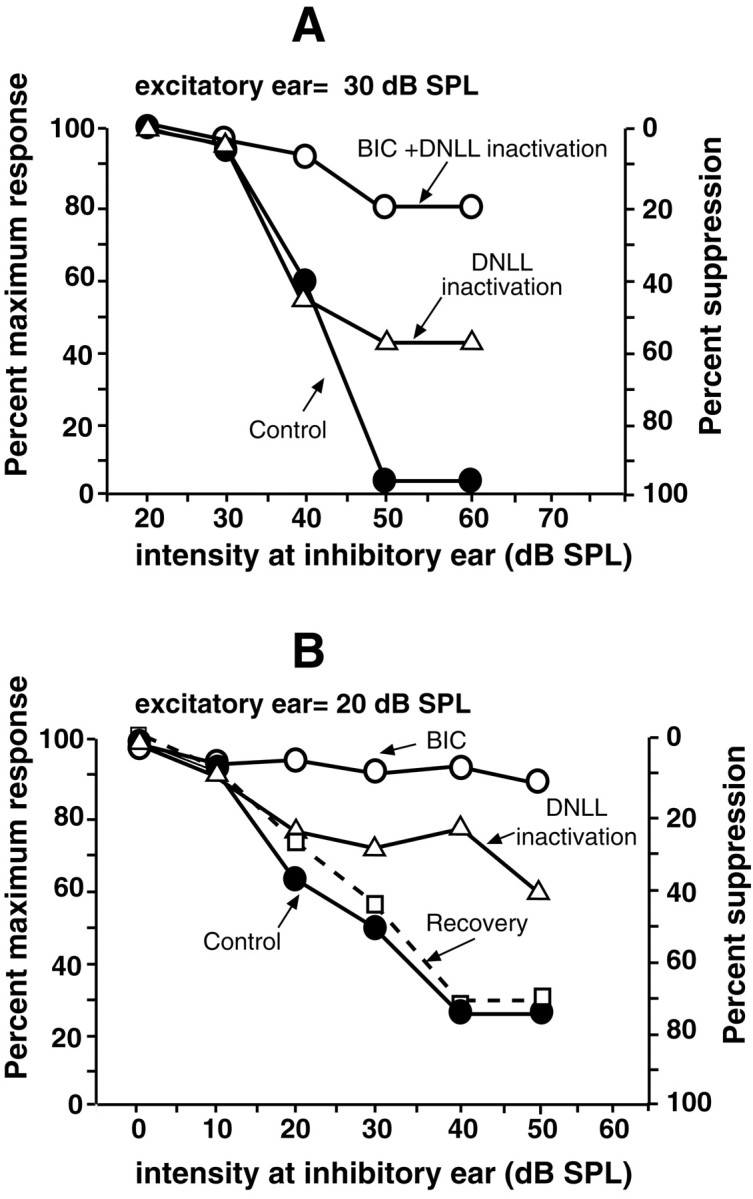
Two ICc neurons in which DNLL inactivation partially relieved ipsilaterally evoked inhibition but blocking GABAergic inhibition with bicuculline produced even greater relief. Signals were 10 msec tone bursts at the BF. BFs were 27.1 kHz (A) and 24.9 kHz (B). The ejection current for bicuculline was 60 nA in both A andB. BIC, Bicuculline.
Impact of DNLL persistent inhibition on ICc neurons
Here we test the hypothesis mentioned previously, that the functional impact of persistent inhibition in the DNLL on the opposite ICc is to influence the processing of multiple sound sources that emanate from different regions of space (Yang and Pollak, 1994c, 1998;Pollak, 1997). The hypothesis is shown diagrammatically in Figure10 and logically combines two principal features of the circuit. The first feature, shown in Figure10A, applies to a binaural signal that favors the ipsilateral (inhibitory) ear when presented alone. It shows that the excitation evoked by one ear can be strongly or completely suppressed by stimulation of the other ear and that the suppression at the ICc cell derives from the GABAergic inhibition provided by the opposite DNLL. The second feature is that a signal that favors the ear ipsilateral to the DNLL generates a persistent inhibition in DNLL neurons that innervate the opposite ICc (Fig. 10B). In Figure 10B we show this signal as a monaural signal or prepulse, although the same logic would apply to a binaural signal with an IID that favors the ear ipsilateral to the DNLL. By combining these features, the hypothesis predicts that presenting an initial signal, which is either monaural as shown here or binaural with an IID that produces DNLL persistent inhibition, should reconfigure the circuit and thereby change the way the ICc cell responds to a binaural trailing signal. Specifically, the initial presentation of a monaural prepulse (or a binaural signal) should drive the ICc, but it should also generate a persistent inhibition in the DNLL (Fig.10B). The key argument is that during the period when the DNLL is persistently inhibited, the ICc cell should be deprived of its ipsilaterally evoked inhibition, thereby allowing the ICc cell to respond to trailing binaural signals to which it previously responded poorly or not at all (Fig. 10C). We also emphasize that a corollary of the hypothesis applies to ICc cells that are not innervated by the opposite DNLL. In those cells, the hypothesis predicts that the introduction of a prepulse should have no effect on the trailing binaural signal; i.e., the ICc cell should respond to the trailing signals as it did when the same trailing signals were presented alone.
Fig. 10.
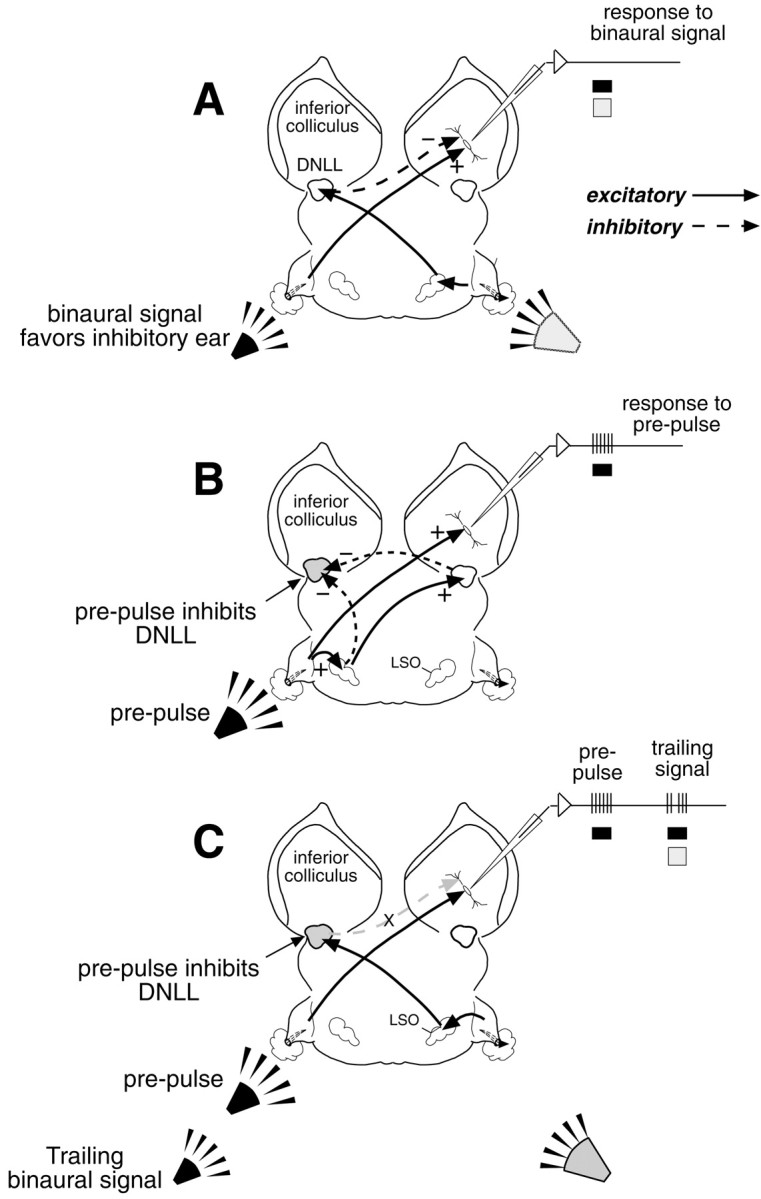
Schematic diagram of auditory pathways showing how a prepulse presented to the ear contralateral to the ICc could reconfigure the circuit and allow the ICc cell to respond to a trailing signal to which it was unresponsive when presented alone.A, A binaural signal with an IID that favors the ear ipsilateral to the ICc drives two projections. The first is an excitatory projection from a lower monaural nucleus (e.g., the cochlear nucleus) and a GABAergic inhibitory projection from the opposite DNLL. At this IID, the inhibitory projection from the DNLL suppresses the excitation from the contralateral ear. B, A monaural prepulse evokes a persistent inhibition in the ipsilateral DNLL (indicated by the stippling) and excites the contralateral ICc. Inhibition in the DNLL is evoked by a glycinergic projection from the ipsilateral LSO and a GABAergic inhibition from the opposite DNLL through the commissure of Probst (Yang and Pollak, 1994a,b). The excitation of the ICc is via an excitatory projection from a lower monaural nucleus, shown here generically as coming from the cochlear nucleus. C, The initial presentation of the monaural prepulse persistently inhibits the DNLL but excites the ICc, in the same way as shown in B. When a trailing binaural signal that favors the inhibitory ear follows shortly thereafter, the ICc neuron now responds to the trailing signal. The reason is that the prepulse generated a persistent inhibition in the DNLL that deprived the ICc cell of the inhibition that would be evoked by a binaural trailing signal if it were presented alone. Thus, the weaker stimulus at the contralateral (excitatory) ear evoked by the trailing signal is now free to drive the ICc cell.
To test this hypothesis, we recorded from 96 EI neurons in the ICc. In each neuron we presented a monaural signal, or prepulse, to the ear that both excited the ICc and generated a persistent inhibition in the DNLL. The prepulse was followed by binaural signals with different IIDs (Fig. 10C). We could not evaluate the effects of an initial signal (prepulse) in 42 of the 96 cells. In those cells, recovery was poor, and trailing signals evoked either no responses or very weak responses when they followed the first pulses by 15 msec or less. In these cases the prepulse presented to the contralateral ear apparently evoked both an excitation followed by a long-lasting inhibition at the ICc cell, which suppressed responses to the trailing signals for ≥15 msec. Long recovery times have been reported in previous IC studies concerned with the processing of multiple sound sources (Yin, 1994; Fitzpatrick et al., 1995). The functional significance of this contralaterally evoked persistent inhibition exhibited by many ICc neurons is unknown, and this puzzling feature of the IC has been discussed in a previous report (Bauer et al., 2000).
Of the 96 cells, 54 cells (56%) recovered quickly. As shown below, almost all of these neurons were consistent with the hypotheses presented above for ICc cells that are innervated by the DNLL and for ICc cells that are not. We turn first to 23 of the 54 ICc cells in which a monaural prepulse had no effect on the responses to the trailing binaural signal. The DNLL was inactivated while recording from 12 cells. In three of these cells, DNLL inactivation relieved ipsilateral inhibition although the prepulse did not. However, in 9 of 12 cells (75%), neither a prepulse nor DNLL inactivation relieved inhibition, and thus neither of the manipulations affected the EI properties evoked by the trailing binaural signals. Stated differently, these neurons responded to the trailing binaural signal as they did when same binaural signal (the trailing signal) was presented alone. A representative example is shown in Figure11. This neuron apparently was not innervated by the opposite DNLL, and its EI property may have been created in the LSO and then imposed on the ICc via the circuit shown in Figure 1B.
Fig. 11.
An ICc cell in which the responses evoked by a trailing binaural signal were not influenced either by a monaural prepulse or by DNLL inactivation. The percent-normalized responses for the prepulse and DNLL inactivation conditions are for the trailing binaural signals. Both prepulse and trailing binaural signals were 10 msec FM signals that swept from 40 to 20 kHz. The FM prepulse was 40 dB SPL.
In marked contrast were 31 cells in which a monaural prepulse substantially relieved ipsilateral inhibition. An example is shown in Figure 12. When a binaural signal was presented without a prepulse, the discharges evoked by the contralateral ear were inhibited by ipsilateral intensities of 40 dB SPL or greater (Fig. 12, bottom left). When a monaural prepulse was introduced and the same binaural signals followed the prepulse 1.0 msec later, there was only weak inhibition of the binaural signal, even at an ipsilateral intensity of 45 dB SPL, an intensity that completely inhibited the same binaural signal when there was no prepulse. The relief from inhibition afforded by the prepulse declined as the interval between the prepulse and trailing signals was lengthened (Fig. 12, top). When the interval was lengthened to 3 msec, the amount of relief was slightly reduced, and when the delay was lengthened further, to 5 msec, the prepulse no longer produced any relief from inhibition. Thus, the presentation of the prepulse at appropriate intervals relieved inhibition, allowing the ICc neuron to respond to the binaural trailing signal at IIDs that completely inhibited the cell when the trailing signal was presented alone. The intervals that were effective for relieving inhibition were very short in this ICc neuron. Our interpretation is that this ICc cell received innervation from DNLL cells that had very short periods of persistent inhibition. Because periods of persistent inhibition were longer in most DNLL cells, the effective intervals between the prepulse and trailing signals were also substantially longer in other ICc neurons, as we show below.
Fig. 12.
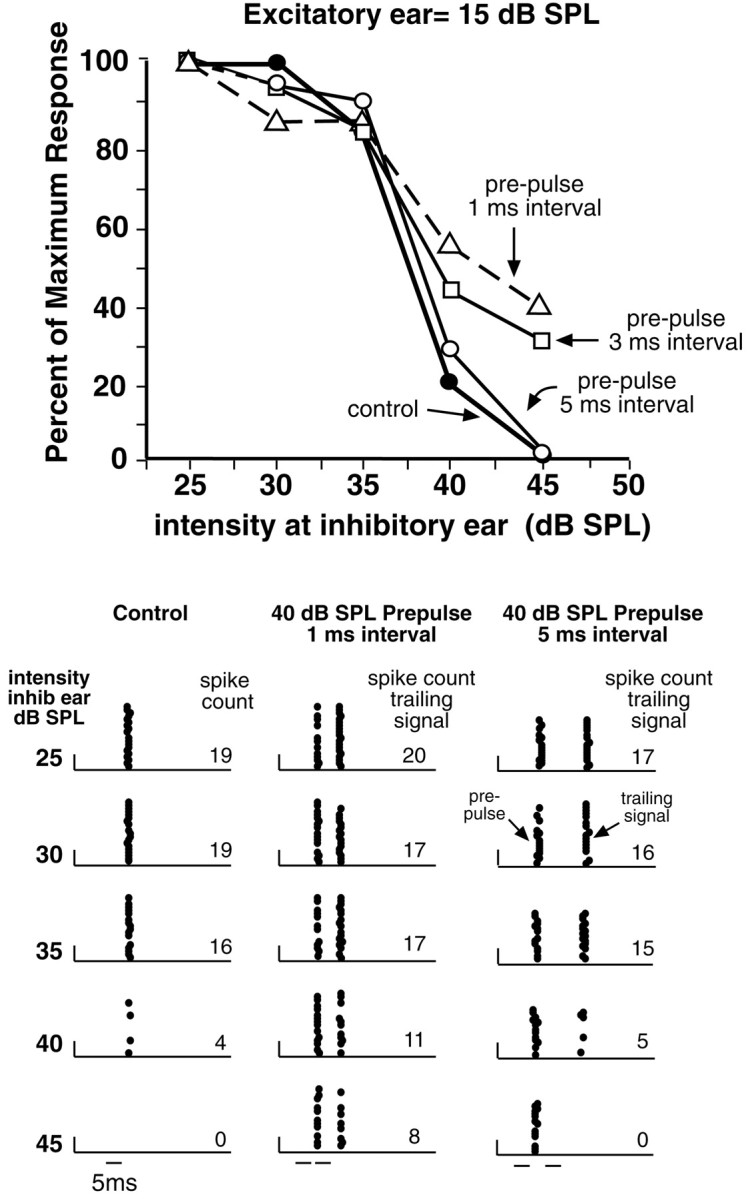
Prepulses relieved ipsilateral inhibition evoked by trailing binaural signals. Bottom left, Raster displays of binaural signals presented alone. Note the suppression of responses evoked by contralateral stimulation as the intensity at the ear ipsilateral to the ICc was increased. Bottom middle, right, Raster displays evoked by an FM prepulse at the ear contralateral to the ICc, followed 1.0 and 5.0 msec later by trailing binaural FM signals. Note the relief from inhibition afforded by the 1.0 msec interval when the ipsilateral intensities of the trailing signals were 40–45 dB SPL. Relief was over at the 5.0 msec interval, presumably because the persistent inhibition in the DNLL had ended at that time. FM signals swept from 40 to 15 kHz. Top, Suppression produced by ipsilateral intensities as a percentage of the maximum response. Included are data from the 3.0 msec interval between the prepulse and trailing signals, which are not shown in raster displays.
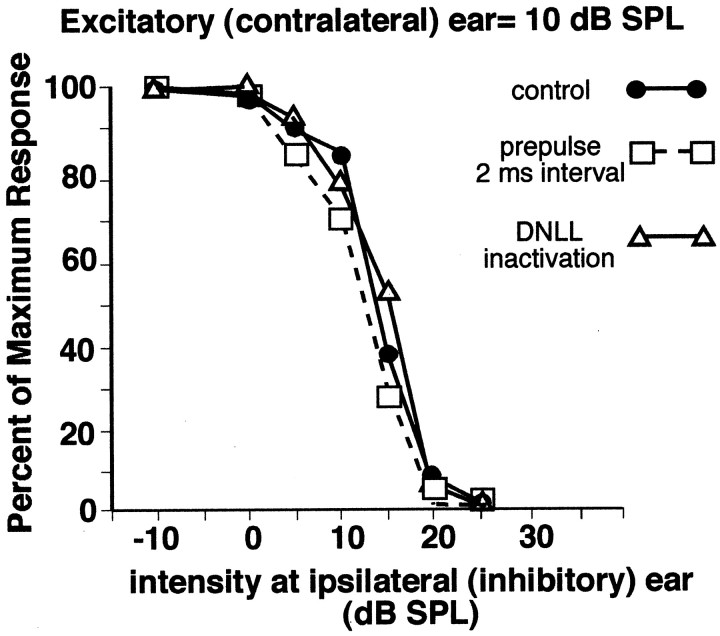
Although we did not inactivate the DNLL in the neuron shown above, we did inactivate the DNLL with kynurenic acid in 11 cells in which a prepulse relieved the inhibition produced by the trailing binaural signal. In all 11 cells, relief from inhibition was obtained from DNLL inactivation, and the relief was similar to the relief obtained with a prepulse. An example is shown in Figure13. Here we first selected an IID that produced a strong, but not complete, suppression of contralaterally evoked discharges and measured the relief from inhibition against that suppression. In this cell, the prepulse was an FM sweep that did not drive the ICc cell, although it swept through the BF of the ICc cells. The trailing binaural signals were BF tone bursts. Because we have shown that FM sweeps effectively generate persistent inhibition in the DNLL, we assume that if the DNLL innervated this ICc cell, the FM prepulse should inhibit those DNLL cells although the FM sweep did not drive the ICc cell. Figure 13A shows that a 15 dB SPL tone burst presented only to the excitatory ear evoked 39 spikes, but when the same excitatory signal was presented simultaneously with a 25 dB SPL signal at the inhibitory ear, only six spikes were evoked. When the DNLL was inactivated with kynurenic acid, the same binaural signal that evoked previously only six spikes now evoked 30 spikes, showing that the opposite DNLL provided most, if not all, of the ipsilateral inhibition evoked by the binaural signal. Introduction of the FM prepulse relieved the inhibition substantially. At an interval of 10 msec, the trailing binaural signal evoked 26 spikes (Fig.13B), whereas the same binaural signal presented alone evoked only six spikes. This amount of relief from inhibition was similar to the relief that occurred when the DNLL was pharmacologically inactivated. The amount of relief declined as the interval was lengthened to 20 msec (trailing signal evoked 17 spikes), and at an interval of 30 msec the trailing signal evoked only nine spikes, a spike count comparable with the six spikes obtained when the binaural trailing signal was presented without the prepulse. Thus, as with the cell in Figure 12, the prepulse not only relieved inhibition but its efficacy dissipated over time, as did the persistent inhibition in the DNLL (e.g., Fig. 5). Moreover, because most, if not all, of the ipsilateral inhibition in this cell derived from the opposite DNLL, the relief from inhibition afforded by the prepulse must have been caused by the generation of persistent inhibition in the DNLL by the prepulse.
Fig. 13.
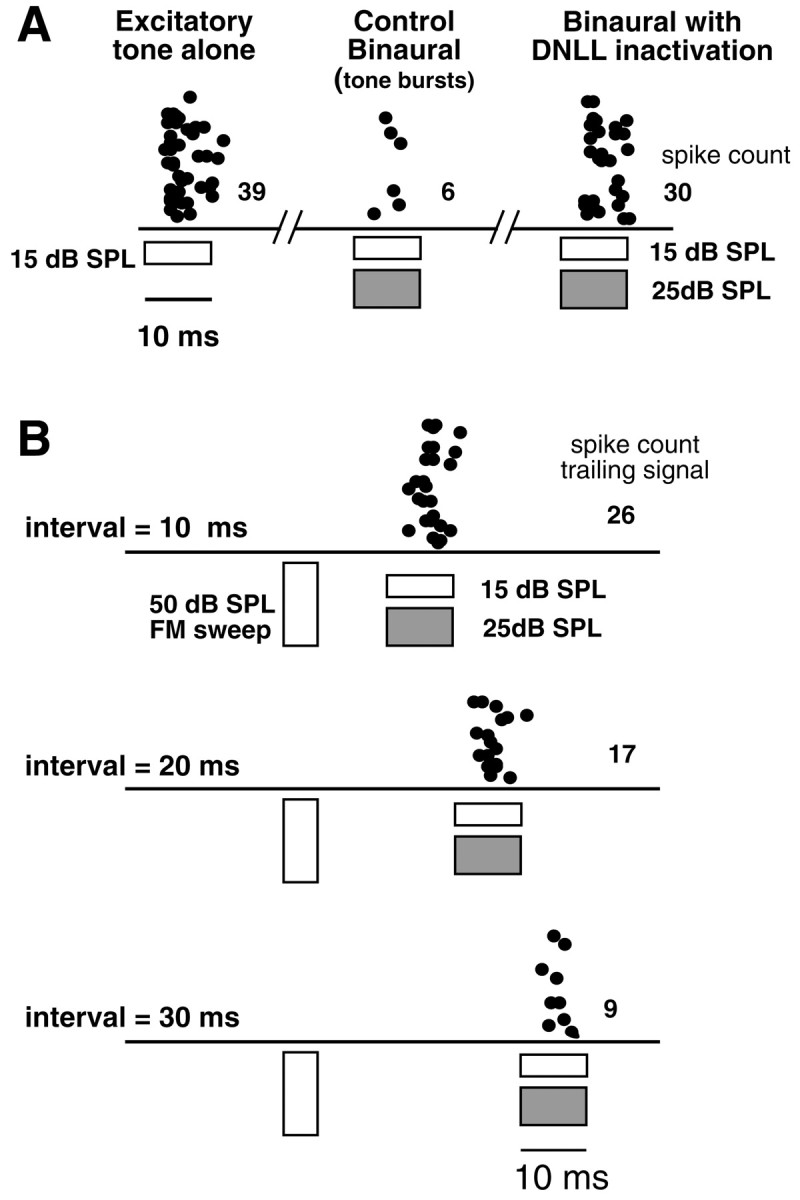
DNLL inactivation mimicked the relief of inhibition produced by a monaural prepulse. A, Control responses evoked without a prepulse. Signals presented to the ear contralateral to the ICc (the excitatory ear) are shown asunfilled bars, whereas signals presented to the ear ipsilateral to the ICc (the inhibitory ear) are shown as hatched bars. BF tone bursts (30 kHz) presented to only the contralateral (excitatory) ear at 15 dB SPL evoked 39 spikes. Binaural tone bursts, having the same contralateral intensity (15 dB SPL) and an ipsilateral intensity of 25 dB SPL, evoked only six discharges, an 85% suppression. When the DNLL was inactivated, the same binaural signal evoked 30 spikes, a 23% suppression. Thus, inactivating the DNLL relieved ∼62% of the ipsilaterally evoked inhibition. Thediagonal slash marks on the horizontal line indicate that tone alone, binaural, and DNLL inactivation records were each obtained separately and independently.B, Relief from inhibition afforded by a 5.0 msec FM prepulse presented to the ear contralateral to the ICc (the excitatory ear). The FM swept from 40 to 20 kHz. The neuron was unresponsive to the FM signal but responded briskly to the trailing binaural tone bursts. The trailing binaural signal evoked 26 spikes when it followed the prepulse by 10 msec (a 33% suppression). Thus the prepulse relieved ∼52% of the ipsilaterally evoked inhibition, a value close to the 62% relieved by DNLL inactivation. Less relief was achieved when the interval was lengthened to 20 msec, and there was no relief at 30 msec, the longest interval.
Initial binaural signals also relieve inhibition generated by trailing signals in the ICc
In the above sections we showed that a monaural signal relieved the inhibition generated by a trailing binaural signal. Those conditions were artificial in that sounds almost always stimulate both ears. Here we show that relief from inhibition was also produced with two binaural signals, in which the first had an IID that favored the contralateral ear and therefore drove the ICc cell and inhibited the DNLL, whereas the second, or trailing signal, had an IID that favored the ipsilateral ear, which drove the DNLL and inhibited the ICc cell. This stimulus arrangement simulates the reception of two signals that emanate from different regions of space. As with the results shown in the previous section, the relief from inhibition was obtained in ICc cells that were innervated by the DNLL, and no relief was observed in ICc cells that were not innervated by the DNLL.
We tested 12 ICc cells with two binaural signals. Two of the 12 cells showed no inhibitory relief from the initial signal, and in both of those cells DNLL inactivation also provided no relief. In 10 cells, however, initial signals relieved inhibition evoked by the trailing signals. In four of those cells, we also inactivated the DNLL with kynurenic acid. In all four neurons, the relief achieved with the initial binaural signal was mimicked by pharmacological inactivation of the DNLL. As with the neurons in Figures 12 and 13, we also show that the relief afforded by the initial binaural signal is effective only for a limited time window that presumably corresponds to the periods of persistent inhibition in the DNLL.
We illustrate relief of inhibition with the two ICc cells in Figures14 and15. For the neuron in Figure 14, the IID of the trailing signal was 0 dB, and responses to this binaural signal presented alone are shown in the top panel. The IID of the initial signal was +20 dB (excitatory ear more intense). When the initial and trailing signals were presented at intervals ranging from 2 to 20 msec, the spike counts evoked by the trailing binaural signal were much larger than were the spike counts evoked by the same binaural signal when it was presented alone. The enhanced spike counts at the ICc evoked by the trailing signal were presumably a consequence of the persistent inhibition at the DNLL that was generated by the initial binaural signal and that deprived the ICc cell its inhibitory input from the ipsilateral ear. The strength of the persistent inhibition at the DNLL was greatest during the times immediately after the initial signal and declined over time, as shown previously. Thus, the highest spike count was evoked at the shortest delay (2 msec) and then declined as the delay was lengthened to 10 and 20 msec. At the longest delays, of 30 and 40 msec, the spike counts evoked by the trailing signal were comparable with the spike counts evoked by the same signal when presented alone. It appears, therefore, that the persistent inhibition at the DNLL was over at the longest intervals, thereby allowing the ipsilateral tone of the trailing signal to again inhibit the discharges evoked by the contralateral tone.
Fig. 14.
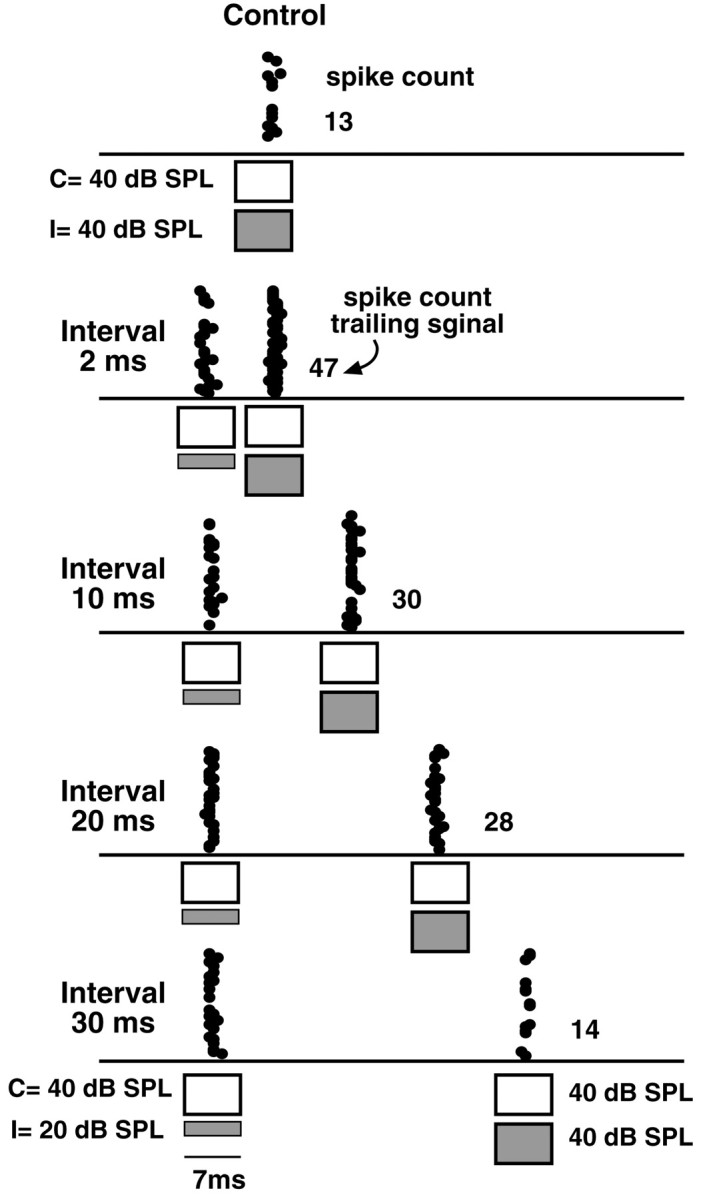
Initial binaural signals that favor the ear contralateral to the ICc (the excitatory ear) relieve ipsilateral inhibition evoked by trailing signals. All signals were 7.0 msec FM signals that swept from 45 to 20 kHz. Signals presented to the ear contralateral to the ICc (the excitatory ear) are shown asunfilled bars, whereas signals presented to the ear ipsilateral to the ICc (the inhibitory ear) are shown as hatched bars. Top, Control showing that 13 spikes were evoked by binaural signals presented with equal intensities at the two ears (IID, 0 dB). Bottom, An initial binaural signal, having an IID of +20 dB (excitatory ear louder), relieved ipsilateral inhibition evoked by the trailing binaural signal. When the interval was 2.0 msec, the trailing signal evoked 47 spikes, whereas it evoked only six spikes when presented alone. Increasing the interval between the prepulse and trailing signal caused progressively less relief. Relief was over at 30 msec, because the trailing signal evoked almost the same spike count as it did when presented alone. C, Contralateral; I, ipsilateral.
Fig. 15.
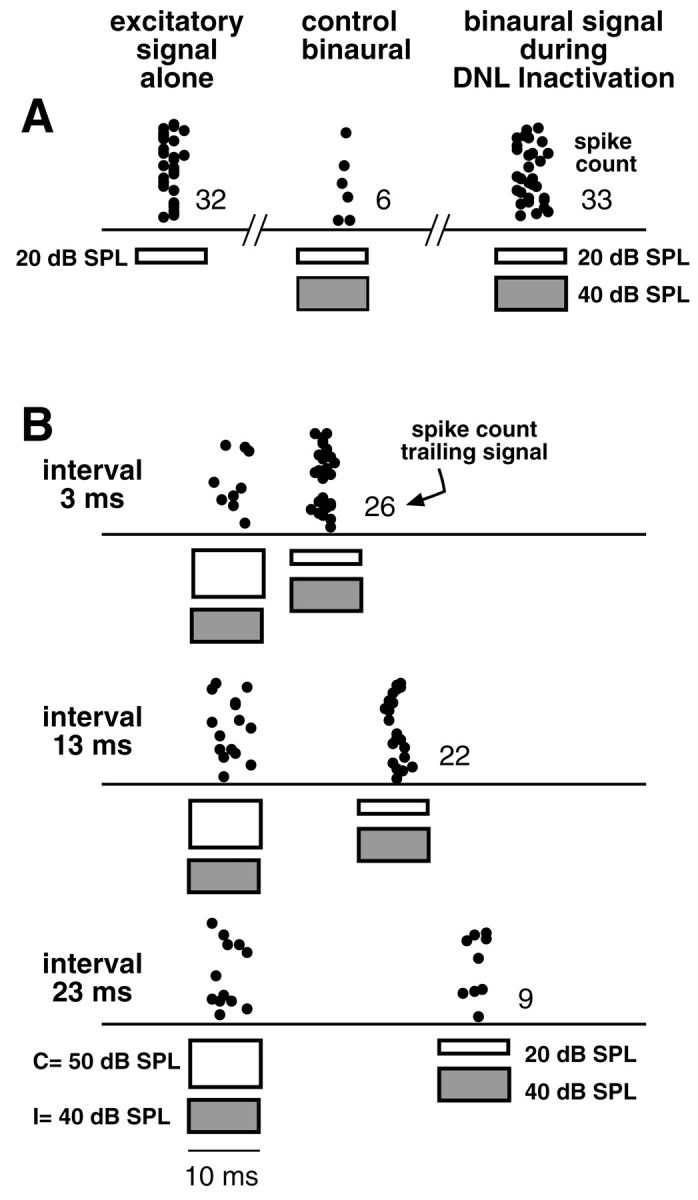
DNLL inactivation mimicked the relief of inhibition produced by an initial binaural signal. All signals were 10 msec FMs that swept from 40 to 20 kHz. Signals presented to the ear contralateral to the ICc (the excitatory ear) are shown asunfilled bars, whereas signals presented to the ear ipsilateral to the ICc (the inhibitory ear) are shown as hatched bars. A, Control responses evoked without a prepulse. Tone bursts presented to only the contralateral (excitatory) ear at 20 dB SPL evoked 32 spikes. A binaural signal, having the same contralateral intensity (20 dB SPL) but an ipsilateral intensity of 40 dB SPL, evoked only six discharges, an 82% suppression. When the DNLL was inactivated, the same binaural signal evoked no suppression. Thus inactivating the DNLL relieved 100% of the ipsilaterally evoked inhibition. B, Relief from inhibition afforded by a binaural FM prepulse. The IID of the prepulse was +10 dB, and the excitatory ear was more intense. The trailing binaural signal evoked 26 spikes when it followed the prepulse by 3.0 msec (a suppression of 19%). Thus the prepulse relieved ∼81% of the ipsilaterally evoked inhibition. Slightly less relief was achieved when the interval was lengthened to 13 msec, and relief was over when the interval was 23 msec.
A similar result is shown for the neuron in Figure 15. Here the first signal relieved most of the ipsilateral inhibition at delays of 3 and 13 msec, and relief was over when the interval was 23 msec. As shown in Figure 15A, inactivation of the DNLL with kynurenic acid eliminated all of the ipsilateral inhibition, suggesting that GABAergic innervation from the DNLL could account for all of the ipsilateral inhibition at this ICc cell.
The time periods over which relief from inhibition dissipated varied among the cells to which we presented first and trailing signals at a variety of delays. In some ICc cells, the relief afforded by the first signal was over at short delays (e.g., Fig. 12), whereas in other neurons the relief lasted for >15 msec (e.g., Fig. 14). We obtained an estimate for how long, after an initial signal, the population of ICc neurons might be relieved. To do this, in each neuron we measured the percentage relief at the shortest delay, which almost always produced the greatest relief, and the first delay at which relief was no longer present. On average, the relief dissipated by 9%/ms. Thus, on average, relief from inhibition attributable to an initial signal was over after ∼10 msec.
DISCUSSION
Here we showed that the DNLL provides a potent GABAergic inhibition to the opposite ICc. The inhibition shapes the EI properties of most ICc cells, a finding consistent with numerous previous studies (Faingold et al., 1991, 1993; Li and Kelly, 1992; Park and Pollak, 1993, 1994; Klug et al., 1995; Kidd and Kelly, 1996; van Adel et al., 1999; Kelly and Kidd, 2000). One EI property shaped in the ICc is the IID at which the cell is maximally inhibited. For convenience, we refer to this IID as the IID sensitivity of the neuron. Because of DNLL influences, IID sensitivities among the ICc population range from approximately −30 dB (ipsilateral ear more intense) to approximately +10 dB (contralateral ear more intense) (Wenstrup et al., 1988a; Park and Pollak, 1993; Park, 1998).
The significance of this distribution of IID sensitivities is that it provides the substrate for the population coding of IIDs generated by high-frequency sounds (Fuzessery and Pollak, 1984; Fuzessery et al., 1985; Wenstrup et al., 1986, 1988b). Each IID sensitivity of each neuron determines its spatial receptive field, the regions of space from which sounds evoke discharges (Fuzessery and Pollak, 1984, 1985;Wenstrup et al., 1988b). Assuming that a wide range of IID sensitivities is represented in isofrequency contours of the ICc, as occurs in the mustache bat (Wenstrup et al., 1986), the arrangement translates into a representation of the IID generated by the frequency to which the neurons in that contour are tuned. The idea is that when a complex sound emanates from a location in space, each frequency in the spectrum of the sound generates a particular IID. The IID generated by each frequency is then coded by the EI population response in the corresponding frequency contour, where some EI neurons with particular IID sensitivities are inhibited whereas other neurons, with different IID sensitivities, are excited. Changing the location of the sound changes the IID generated by each frequency and, thus, the population response in each frequency contour. In this way, the EI populations in the frequency contours of the ICc can collectively encode the location of a sound source in both azimuth and elevation from most locations in the frontal hemifield (Fuzessery and Pollak, 1984, 1985; Pollak et al., 1986).
The importance of collicular EI neurons for sound localization is illustrated both by the disruption in localization acuity caused by lesions of the DNLL and by sectioning of the commissure of Probst (Ito et al., 1996; Kelly et al., 1996). The DNLL, however, not only shapes the IID sensitivities of the ICc population, but it also changes the IID sensitivities of many ICc cells to trailing sounds. With only a single sound source, all ICc neurons with a given IID sensitivity respond to a frequency from a particular location in the same way, regardless of whether or not their IID sensitivity is shaped by the DNLL. However, an initial signal that persistently inhibits the DNLL will degrade the spatial selectivity of those ICc cells for a trailing signal by expanding the regions of space from which the trailing sound can drive those cells. Our results show that this general rule is applicable to most EI collicular neurons that are innervated by the opposite DNLL.
The persistent inhibition in the DNLL may have consequences for other aspects of binaural processing
The effects of DNLL persistent inhibition on the ICc should be especially significant for those signals that generate IIDs that change over time, such as moving sound sources or multiple sounds that emanate from different regions of space. That the DNLL may influence motion sensitivity of ICc cells is suggested by the differential influence that positive and negative IIDs of initial sounds have on the responses to subsequent sounds. These features suggest that a moving sound source that begins in the contralateral sound field and moves with a certain velocity into the ipsilateral sound field should evoke more vigorous responses in an ICc cell than would be evoked when the sound source moved from the ipsilateral into the contralateral sound field. This type of sensitivity for the direction of movement has been reported for neurons in the IC of several mammals, including bats (Spitzer and Semple, 1993; Kleiser and Schuller, 1995; Wilson and O'Neill, 1998;McAlpine et al., 2000). Moreover, Keller and Takahashi (1996) proposed a similar mechanism to explain directionally selective motion sensitivity in IC neurons of the barn owl.
The demonstration that an initial signal can change the spatial selectivity of ICc cells to a trailing signal suggests that the DNLL circuitry could contribute to a precedence-like effect. The precedence effect was discovered in human psychophysical studies and is caused by a mechanism that suppresses the directional information carried by echoes. It explains how, in a reverberant room, a listener can hear only a single sound and not the sequence of separate sounds produced by the echoes reflected from the various surfaces and objects in the room (Wallach et al., 1949; Blauert, 1983; Zurek, 1987; Litovsky et al., 1999).
Precedence is classically demonstrated with two speakers, separated along the same plane in space (Wallach et al., 1949; Litovsky et al., 1999). The speakers emit identical sounds, but the sound from one speaker is presented a few milliseconds before the sound from the other speaker. Under these conditions, normal listeners hear a single composite sound and perceive the composite sound as originating from the leading speaker. The second sound is heard by the listener but is not perceived as a separate sound, nor does it influence the perceived location of the composite sound. Rather the second sound fuses with the first sound and contributes to the overall volume and timbre of the fused sound. Whether the listener hears a single, fused sound or two separate sounds depends on the delay between the two sounds, as well as on the duration and the complexity of the sound. If the interval between the first and second sounds exceeds an upper limit, the two sounds are no longer heard as a single sound but as two separate sounds in succession, each with a perceived location in space.
One significant feature of precedence, that a trailing sound is heard but not localized, correlates closely with the DNLL influences that we observed. Specifically, persistent inhibition in DNLL evoked by the first sound does not suppress the activity of ICc neurons to the trailing sound. Rather, it allows many ICc cells to fire to trailing signals to which they were previously poorly responsive or unresponsive, thereby expanding the regions of space from which sound could drive those ICc cells. Thus, the activity in the ICc should allow the animal to hear trailing sounds, but DNLL innervated cells should also degrade the accuracy with which the ICc population codes for their locations because these cells constitute a large proportion of the EI population in the IC. The degradation of the population code, in turn, should impair the localization of the trailing signal.
Another feature of agreement is the time course of precedence and the time course of relief from inhibition at the ICc afforded by an initial signal. Precedence persists from a few to 20–40 msec, depending on the experiment and type of stimulus. The average period during which a first signal relieved the inhibition evoked by a trailing signal in the ICc is ∼1–20 msec, which falls between the short and long periods estimated in psychophysical experiments (Blauert, 1983; Litovsky et al., 1999).
We point out that the mechanisms we showed here can only account for the precedence of high-frequency sounds, which are initially processed binaurally in the LSO. Moreover, the first sound has to emanate from a location off the midline, which generates IIDs that evoke a persistent inhibition in the DNLL, followed by sounds that emanate from the other acoustic hemifield. If the first sounds are located at or around the midline and produce IIDs at or around 0 dB, persistent inhibition would not be generated in the DNLL, and thus the spatial selectivity of ICc cells for trailing sounds would not be degraded.
Precedence, however, also occurs with low frequencies, which are initially processed binaurally in the medial superior olive. Furthermore, precedence is not limited to initial and trailing sounds that emanate from opposite regions of the frontal sound field. These features, together with the results from previous studies (Wickesberg and Oertel, 1990; Yin, 1994; Fitzpatrick et al., 1995;Litovsky and Yin, 1998a,b), suggest that precedence is almost certainly the consequence of several mechanisms that act at a variety of levels along the auditory pathway and that the relief from DNLL inhibition at the ICc shown here may be but one of those mechanisms.
Precedence occurs in many animals
Mechanisms that suppress the localization of reverberations must be under strong selective pressures because precedence is a widespread, if not universal, feature of auditory systems. Precedence has been found in insects (Wyttenbach and Hoy, 1993), birds (Keller and Takahashi, 1996), and a variety of mammals including rodents (Kelly, 1974; Wickesberg and Oertel, 1990), carnivores (Cranford and Oberholtzer, 1976; Yin, 1994; Litovsky and Yin, 1998a,b), rabbits (Fitzpatrick et al., 1995), and humans (Litovsky et al., 1999). We suggest that bats should be added to this list.
Bats have to cope extensively with acoustic reverberations in their daily lives. The Mexican free-tailed bats used in this study, for example, live in caves where they congregate in large colonies that often number in the millions. They use a rich repertoire of communication calls that they use for a wide variety of social interactions (Balcombe, 1990; Balcombe and McCracken, 1992; French and Lollar, 2000). Caves are highly reverberant, and in this environment precedence would facilitate the perception of communication signals from other bats by allowing the bat to localize the first sound received. Precedence would thus prevent the bat from perceiving each reverberation as a separate sound with its own location, similar to the way that precedence in humans prevents the potential confusion from the multiple reflections in a reverberant room. Precedence might also be advantageous for echolocation. While flying either in caves or among the vegetation outside, orientation calls are emitted that are reflected as echoes from objects ahead. The sound reflected from a given object not only reflects directly back to the bat as a primary echo but it also reflects or scatters in a variety of directions. The sound scattered from the first object may then be reflected again, but now from other objects, producing secondary, weaker echoes that are also reflected back to the bat. Similar to the argument for communication sounds, precedence could be advantageous in allowing the bat to localize and focus on primary objects without the confusion that would occur if each of the secondary echoes were perceived as separate targets with their own locations.
Concluding comments
Here we showed that both the excitation and persistent inhibition in the DNLL are key factors that influence binaural processing in the ICc. The significance of the DNLL innervation is to create an emergent property in the ICc, a property that is not possessed by LSO neurons or by IC cells that are not innervated by the DNLL. That property is a change in the binaural responsiveness of the ICc cell, a change produced by the reception of an earlier sound the IID of which is strongly excitatory to the ICc cell. These features suggest that the circuitry linking the DNLL with the contralateral ICc is important for the processing of signals the IIDs of which change over time, such as the IIDs that would be generated by moving stimuli or by multiple sound sources that emanate from different regions of space.
Footnotes
This work was supported by Grant RO1 DC 00268-16 from the the National Institute on Deafness and Other Communicative Disorders, National Institutes of Health. We thank Eric Bauer, Jane Lubischer, Jeff Wenstrup, Achim Klug, Walt Wilczynski, and David Ryugo for their comments and suggestions, Linslee Luke for her assistance with the histology, and Carl Resler for technical support.
Correspondence should be addressed to Dr. George D. Pollak at the above address. E-mail: gpollak@mail.utexas.edu.
R. M. Burger's present address: Virginia Merrill Bloedel Hearing Research Center, University of Washington, Seattle, WA 98195-7923.
REFERENCES
- 1.Adams JC, Mugniani E. Dorsal nucleus of the lateral lemniscus: a nucleus of GABAergic projection neurons. Brain Res Bull. 1984;13:585–590. doi: 10.1016/0361-9230(84)90041-8. [DOI] [PubMed] [Google Scholar]
- 2.Aitkin L. The auditory midbrain: structure and function of the central auditory pathway. Humana; Clifton, NJ: 1986. [Google Scholar]
- 3.Balcombe JP. Vocal recognition of pups by mother Mexican free-tailed bats. Anim Behav. 1990;39:960–966. [Google Scholar]
- 4.Balcombe JP, McCracken GF. Vocal recognition in Mexican free-tailed bats: do pups recognize mothers? Anim Behav. 1992;43:79–87. [Google Scholar]
- 5.Bauer EE, Klug A, Pollak GD. Features of contralaterally evoked inhibition in the inferior colliculus. Hear Res. 2000;141:80–96. doi: 10.1016/s0378-5955(99)00206-3. [DOI] [PubMed] [Google Scholar]
- 6.Blauert J. Spatial hearing: the psychophysics of human sound localization. MIT; Cambridge, MA: 1983. [Google Scholar]
- 7.Borman J. Electrophysiology of GABAA and GABAB receptor subtypes. Trends Neurosci. 1988;11:112–116. doi: 10.1016/0166-2236(88)90156-7. [DOI] [PubMed] [Google Scholar]
- 8.Boudreau JC, Tsuchitani C. Binaural interaction in the cat superior olive S segment. J Neurophysiol. 1968;31:442–454. doi: 10.1152/jn.1968.31.3.442. [DOI] [PubMed] [Google Scholar]
- 9.Brugge JF, Anderson DJ, Aitkin LM. Responses of neurons in the dorsal nucleus of the lateral lemniscus of cat to binaural tonal stimulation. J Neurophysiol. 1970;33:441–458. doi: 10.1152/jn.1970.33.3.441. [DOI] [PubMed] [Google Scholar]
- 10.Collingridge GL, Lester RA. Excitatory amino acid receptors in the vertebrate central nervous system. Pharmacol Rev. 1989;41:143–210. [PubMed] [Google Scholar]
- 11.Cooper JR, Bloom FE, Roth RH. The biochemical basis of neuropharmacology, 4th edition. Oxford UP; New York: 1982. [Google Scholar]
- 12.Covey E. Response properties of single units in the dorsal nucleus of the lateral lemniscus and paralemniscal zone of an echolocating bat. J Neurophysiol. 1993;69:842–859. doi: 10.1152/jn.1993.69.3.842. [DOI] [PubMed] [Google Scholar]
- 13.Cranford JL, Oberholtzer M. Role of neocortex in binaural hearing in the cat. II. The “precedence effect” in sound localization. Brain Res. 1976;111:225–239. doi: 10.1016/0006-8993(76)90768-x. [DOI] [PubMed] [Google Scholar]
- 14.Erulkar S. Comparative aspects of sound localization. Physiol Rev. 1972;52:237–360. doi: 10.1152/physrev.1972.52.1.237. [DOI] [PubMed] [Google Scholar]
- 15.Faingold CL, Boersma Anderson CA, Caspary DM. Involvement of GABA in acoustically-evoked inhibition in inferior colliculus neurons. Hear Res. 1991;52:201–216. doi: 10.1016/0378-5955(91)90200-s. [DOI] [PubMed] [Google Scholar]
- 16.Faingold CL, Anderson CA, Randall ME. Stimulation or blockade of the dorsal nucleus of the lateral lemniscus alters binaural and tonic inhibition in contralateral inferior colliculus neurons. Hear Res. 1993;69:98–106. doi: 10.1016/0378-5955(93)90097-k. [DOI] [PubMed] [Google Scholar]
- 17.Finlayson PG, Caspary DM. Low-frequency neurons in the lateral superior olive exhibit phase-sensitive binaural inhibition. J Neurophysiol. 1991;65:598–605. doi: 10.1152/jn.1991.65.3.598. [DOI] [PubMed] [Google Scholar]
- 18.Fitzpatrick DC, Kuwada S, Batra R, Trahiotis C. Neural responses to simple simulated echoes in the auditory brain stem of the unanesthetized rabbit. J Neurophysiol. 1995;74:2469–2486. doi: 10.1152/jn.1995.74.6.2469. [DOI] [PubMed] [Google Scholar]
- 19.French B, Lollar A. Communication among Mexican free-tailed bats. Bats: Bat Conservation International. 2000;18:1–4. [Google Scholar]
- 20.Fuzessery ZM, Pollak GD. Neural mechanisms of sound localization in an echolocating bat. Science. 1984;225:725–728. doi: 10.1126/science.6463649. [DOI] [PubMed] [Google Scholar]
- 21.Fuzessery ZM, Pollak GD. Determinants of sound location selectivity in bat inferior colliculus: a combined dichotic and free-field stimulation study. J Neurophysiol. 1985;54:757–781. doi: 10.1152/jn.1985.54.4.757. [DOI] [PubMed] [Google Scholar]
- 22.Fuzessery ZM, Wenstrup JJ, Pollak GD. A representation of horizontal sound location in the inferior colliculus of the mustache bat (Pteronotus p. parnellii). Hear Res. 1985;20:85–89. doi: 10.1016/0378-5955(85)90061-9. [DOI] [PubMed] [Google Scholar]
- 23.Glendenning KK, Brunso-Bechtold JK, Thompson GC, Masterton RB. Ascending auditory afferents to the nuclei of the lateral lemniscus. J Comp Neurol. 1981;197:673–703. doi: 10.1002/cne.901970409. [DOI] [PubMed] [Google Scholar]
- 24.Glendenning KK, Baker BN, Hutson KA, Masterton RB. Acoustic chiasm V: inhibition and excitation in the ipsilateral and contralateral projections of LSO. J Comp Neurol. 1992;319:100–122. doi: 10.1002/cne.903190110. [DOI] [PubMed] [Google Scholar]
- 25.Gonzalez-Hernandez T, Mantolan-Sarmiento B, Gonzalez-Gonzalez B, Perez-Gonzalez H. Sources of GABAergic input to the inferior colliculus of the rat. J Comp Neurol. 1996;372:309–326. doi: 10.1002/(SICI)1096-9861(19960819)372:2<309::AID-CNE11>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 26.Grothe B, Park TJ. Sensitivity to interaural time differences in the medial superior olive of a small mammal, the Mexican free-tailed bat. J Neurosci. 1998;18:6608–6622. doi: 10.1523/JNEUROSCI.18-16-06608.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Grothe B, Schweizer H, Pollak GD, Schuller G, Rosemann C. Anatomy and projection patterns of the superior olivary complex in the Mexican free-tailed bat, Tadarida brasiliensis mexicana. J Comp Neurol. 1994;343:630–646. doi: 10.1002/cne.903430412. [DOI] [PubMed] [Google Scholar]
- 28.Havey DC, Caspary DM. A simple technique for constructing “piggy back” multibarrel microelectrodes. Electroencephalogr Clin Neurophysiol. 1980;48:249–251. doi: 10.1016/0013-4694(80)90313-2. [DOI] [PubMed] [Google Scholar]
- 29.Ito M, van Adel B, Kelly JB. Sound localization after transection of the commissure of Probst in the albino rat. J Neurophysiol. 1996;76:3493–3502. doi: 10.1152/jn.1996.76.5.3493. [DOI] [PubMed] [Google Scholar]
- 30.Keller CH, Takahashi TT. Responses to simulated echoes by neurons in the barn owl's auditory space map. J Comp Physiol [A] 1996;178:499–512. doi: 10.1007/BF00190180. [DOI] [PubMed] [Google Scholar]
- 31.Kelly JB. Localization of paired sound sources in the rat: small time differences. J Acoust Soc Am. 1974;55:1277–1284. doi: 10.1121/1.1914697. [DOI] [PubMed] [Google Scholar]
- 32.Kelly JB, Kidd SA. NMDA and AMPA receptors in the dorsal nucleus of the lateral lemniscus shape binaural response in rat inferior colliculus. J Neurophysiol. 2000;83:1403–1414. doi: 10.1152/jn.2000.83.3.1403. [DOI] [PubMed] [Google Scholar]
- 33.Kelly JB, Li L, van Adel B. Sound localization after kainic acid lesions of the dorsal nucleus of the lateral lemniscus in the albino rat. Behav Neurosci. 1996;110:1445–1455. doi: 10.1037//0735-7044.110.6.1445. [DOI] [PubMed] [Google Scholar]
- 34.Kelly JB, Buckthought AD, Kidd SA. Monaural and binaural response properties of single neurons in the rat's dorsal nucleus of the lateral lemniscus. Hear Res. 1998;122:25–40. doi: 10.1016/s0378-5955(98)00082-3. [DOI] [PubMed] [Google Scholar]
- 35.Kidd SA, Kelly JB. Contributions of the dorsal nucleus of the lateral lemniscus to binaural response properties in the inferior colliculus of the rat: interaural time delays. J Neurosci. 1996;16:7390–7397. doi: 10.1523/JNEUROSCI.16-22-07390.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kleiser A, Schuller G. Responses of collicular neurons to acoustic motion in the horseshoe bat Rhinolophus rouxi. Naturwissenschaften. 1995;82:337–340. [Google Scholar]
- 37.Klug A, Park TJ, Pollak GD. Glycine and GABA influence binaural processing in the inferior colliculus of the mustache bat. J Neurophysiol. 1995;74:1701–1713. doi: 10.1152/jn.1995.74.4.1701. [DOI] [PubMed] [Google Scholar]
- 38.Klug A, Bauer EE, Pollak GD. Multiple components of ipsilaterally evoked inhibition in the inferior colliculus. J Neurophysiol. 1999;82:593–610. doi: 10.1152/jn.1999.82.2.593. [DOI] [PubMed] [Google Scholar]
- 39.Li L, Kelly JB. Inhibitory influence of the dorsal nucleus of the lateral lemniscus on binaural responses in the rat's inferior colliculus. J Neurosci. 1992;12:4530–4539. doi: 10.1523/JNEUROSCI.12-11-04530.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Litovsky RY, Yin TCT. Physiological studies of the precedence effect in the inferior colliculus of the cat. I. Correlates of psychophysics. J Neurophysiol. 1998a;80:1285–1301. doi: 10.1152/jn.1998.80.3.1285. [DOI] [PubMed] [Google Scholar]
- 41.Litovsky RY, Yin TCT. Physiological studies of the precedence effect in the inferior colliculus of the cat. II. Neural mechanisms. J Neurophysiol. 1998b;80:1302–1316. doi: 10.1152/jn.1998.80.3.1302. [DOI] [PubMed] [Google Scholar]
- 42.Litovsky RY, Colburn HS, Yost WA, Guzman SJ. The precedence effect. J Acoust Soc Am. 1999;106:1633–1654. doi: 10.1121/1.427914. [DOI] [PubMed] [Google Scholar]
- 43.Markovitz NS, Pollak GD. The dorsal nucleus of the lateral lemniscus in the mustache bat: monaural properties. Hear Res. 1993;71:51–63. doi: 10.1016/0378-5955(93)90020-2. [DOI] [PubMed] [Google Scholar]
- 44.Markovitz NS, Pollak GD. Binaural processing in the dorsal nucleus of the lateral lemniscus. Hear Res. 1994;73:121–140. doi: 10.1016/0378-5955(94)90290-9. [DOI] [PubMed] [Google Scholar]
- 45.McAlpine D, Jiang D, Shackleton TM, Palmer AR. Responses of neurons in the inferior colliculus to dynamic interaural phase cues: evidence for a mechanism of binaural adaptation. J Neurophysiol. 2000;83:1356–1365. doi: 10.1152/jn.2000.83.3.1356. [DOI] [PubMed] [Google Scholar]
- 46.Mills AW. Auditory localization. In: Tobias JV, editor. Foundations in modern auditory theory. Academic; New York: 1972. pp. 303–348. [Google Scholar]
- 47.Oliver DL, Huerta MF. Inferior and superior colliculi. In: Webster DB, Popper AN, Fay RR, editors. The mammalian auditory pathway: neuroanatomy. Springer; New York: 1992. pp. 168–222. [Google Scholar]
- 48.Park TJ. IID sensitivity differs between two principal centers in the interaural intensity difference pathway: the LSO and the IC. J Neurophysiol. 1998;79:2416–2431. doi: 10.1152/jn.1998.79.5.2416. [DOI] [PubMed] [Google Scholar]
- 49.Park TJ, Pollak GD. GABA shapes sensitivity to interaural intensity disparities in the mustache bat's inferior colliculus: implications for encoding sound location. J Neurosci. 1993;13:2050–2067. doi: 10.1523/JNEUROSCI.13-05-02050.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Park TJ, Pollak GD. Azimuthal receptive fields are shaped by GABAergic inhibition in the inferior colliculus of the mustache bat. J Neurophysiol. 1994;72:1080–1102. doi: 10.1152/jn.1994.72.3.1080. [DOI] [PubMed] [Google Scholar]
- 51.Park TJ, Grothe B, Pollak GD, Schuller G, Koch U. Neural delays shape selectivity to interaural intensity differences in the lateral superior olive. J Neurosci. 1996;16:6554–6566. doi: 10.1523/JNEUROSCI.16-20-06554.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Pollak G, Marsh D, Bodenhamer R, Souther A. Echo-detecting characteristics of neurons in inferior colliculus of unanesthetized bats. Science. 1977;196:675–678. doi: 10.1126/science.857318. [DOI] [PubMed] [Google Scholar]
- 53.Pollak GD. Roles of GABAergic inhibition for the binaural processing of multiple sound sources in the inferior colliculus. Ann Otol Rhinol Laryngol. 1997;106:44–54. [PubMed] [Google Scholar]
- 54.Pollak GD, Marsh DS, Bodenhamer R, Souther A. Characteristics of phasic on neurons in inferior colliculus of unanesthetized bats with observations relating to mechanisms for echo ranging. J Neurophysiol. 1977;40:926–942. doi: 10.1152/jn.1977.40.4.926. [DOI] [PubMed] [Google Scholar]
- 55.Pollak GD, Wenstrup JJ, Fuzessery ZM. Auditory processing in the mustache bat's inferior colliculus. Trends Neurosci. 1986;9:556–561. [Google Scholar]
- 56.Ross LS, Pollak GD, Zook JM. Origin of ascending projections to an isofrequency region of the mustache bat's inferior colliculus. J Comp Neurol. 1988;270:488–505. doi: 10.1002/cne.902700403. [DOI] [PubMed] [Google Scholar]
- 57.Saint Marie RL, Baker RA. Neurotransmitter-specific uptake and retrograde transport of [3H]glycine from the inferior colliculus by ipsilateral projections of the superior olivary complex and nuclei of the lateral lemniscus. Brain Res. 1990;524:244–253. doi: 10.1016/0006-8993(90)90698-b. [DOI] [PubMed] [Google Scholar]
- 58.Saint Marie RL, Ostapoff EM, Morest DK, Wenthold RJ. Glycine-immunoreactive projection of the cat lateral superior olive: possible role in midbrain ear dominance. J Comp Neurol. 1989;279:382–396. doi: 10.1002/cne.902790305. [DOI] [PubMed] [Google Scholar]
- 59.Schuller G, Radtke-Schuller S, Betz M. A stereotaxic method for small animals using experimentally determined reference profiles. J Neurosci Methods. 1986;18:339–350. doi: 10.1016/0165-0270(86)90022-1. [DOI] [PubMed] [Google Scholar]
- 60.Shneiderman A, Oliver DL, Henkel CK. Connections of the dorsal nucleus of the lateral lemniscus: an inhibitory parallel pathway in the ascending auditory system? J Comp Neurol. 1988;276:188–208. doi: 10.1002/cne.902760204. [DOI] [PubMed] [Google Scholar]
- 61.Shneiderman A, Stanforth DA, Henkel CK, Saint Marie RL. Input-output relationships of the dorsal nucleus of the lateral lemniscus: possible substrate for the processing of dynamic spatial cues. J Comp Neurol. 1999;410:265–276. [PubMed] [Google Scholar]
- 62.Spitzer MW, Semple MN. Responses of inferior colliculus neurons to time-varying interaural phase disparity: effects of shifting the locus of virtual motion. J Neurophysiol. 1993;69:1245–1263. doi: 10.1152/jn.1993.69.4.1245. [DOI] [PubMed] [Google Scholar]
- 63.van Adel BA, Kidd SA, Kelly JB. Contribution of the commissure of Probst to binaural evoked responses in the rat's inferior colliculus: interaural time differences. Hear Res. 1999;130:115–130. doi: 10.1016/s0378-5955(98)00226-3. [DOI] [PubMed] [Google Scholar]
- 64.Vater M, Habbicht H, Kossl M, Grothe B. The functional role of GABA and glycine in monaural and binaural processing in the inferior colliculus of horseshoe bats. J Comp Physiol [A] 1992;171:541–553. doi: 10.1007/BF00194587. [DOI] [PubMed] [Google Scholar]
- 65.Wallach H, Newman EB, Rosenzweig MR. The precedence effect in sound localization. Am J Psychol. 1949;52:315–336. [PubMed] [Google Scholar]
- 66.Wenstrup JJ, Ross LS, Pollak GD. Binaural response organization within a frequency-band representation of the inferior colliculus: implications for sound localization. J Neurosci. 1986;6:962–973. doi: 10.1523/JNEUROSCI.06-04-00962.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wenstrup JJ, Fuzessery ZM, Pollak GD. Binaural neurons in the mustache bat's inferior colliculus. I. Responses of 60-kHz EI units to dichotic sound stimulation. J Neurophysiol. 1988a;60:1369–1383. doi: 10.1152/jn.1988.60.4.1369. [DOI] [PubMed] [Google Scholar]
- 68.Wenstrup JJ, Fuzessery ZM, Pollak GD. Binaural neurons in the mustache bat's inferior colliculus. II. Determinants of spatial responses among 60-kHz EI units. J Neurophysiol. 1988b;60:1384–1404. doi: 10.1152/jn.1988.60.4.1384. [DOI] [PubMed] [Google Scholar]
- 69.Wickesberg RE, Oertel D. Delayed, frequency-specific inhibition in the cochlear nuclei of mice: a mechanism for monaural echo suppression. J Neurosci. 1990;10:1762–1768. doi: 10.1523/JNEUROSCI.10-06-01762.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wilson WW, O'Neill WE. Auditory motion induces directionally dependent receptive field shifts in inferior colliculus neurons. J Neurophysiol. 1998;79:2040–2062. doi: 10.1152/jn.1998.79.4.2040. [DOI] [PubMed] [Google Scholar]
- 71.Winer JA, Larue DT, Pollak GD. GABA and glycine in the central auditory system of the mustache bat: structural substrates for inhibitory neuronal organization. J Comp Neurol. 1995;355:317–353. doi: 10.1002/cne.903550302. [DOI] [PubMed] [Google Scholar]
- 72.Wong-Riley M. Changes in the visual system of monocularly sutured or enucleated cats demonstrable with cytochrome oxidase histochemistry. Brain Res. 1979;171:11–28. doi: 10.1016/0006-8993(79)90728-5. [DOI] [PubMed] [Google Scholar]
- 73.Wyttenbach RA, Hoy RR. Demonstration of the precedence effect in an insect. J Acoust Soc Am. 1993;94:777–784. doi: 10.1121/1.408207. [DOI] [PubMed] [Google Scholar]
- 74.Yang L, Pollak GD. The roles of GABAergic and glycinergic inhibition on binaural processing in the dorsal nucleus of the lateral lemniscus of the mustache bat. J Neurophysiol. 1994a;71:1999–2013. doi: 10.1152/jn.1994.71.6.1999. [DOI] [PubMed] [Google Scholar]
- 75.Yang L, Pollak GD. GABA and glycine have different effects on monaural response properties in the dorsal nucleus of the lateral lemniscus of the mustache bat. J Neurophysiol. 1994b;71:2014–2024. doi: 10.1152/jn.1994.71.6.2014. [DOI] [PubMed] [Google Scholar]
- 76.Yang L, Pollak G. Binaural inhibition in the dorsal nucleus of the lateral lemniscus of the mustache bat affects responses for multiple signals. Auditory Neuroscience. 1994c;1:1–17. [Google Scholar]
- 77.Yang L, Pollak GD. Features of ipsilaterally evoked inhibition in the dorsal nucleus of the lateral lemniscus. Hear Res. 1998;122:125–141. doi: 10.1016/s0378-5955(98)00088-4. [DOI] [PubMed] [Google Scholar]
- 78.Yang L, Liu Q, Pollak GD. Afferent connections to the dorsal nucleus of the lateral lemniscus of the mustache bat: evidence for two functional subdivisions. J Comp Neurol. 1996;373:575–592. doi: 10.1002/(SICI)1096-9861(19960930)373:4<575::AID-CNE7>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 79.Yin TC. Physiological correlates of the precedence effect and summing localization in the inferior colliculus of the cat. J Neurosci. 1994;14:5170–5186. doi: 10.1523/JNEUROSCI.14-09-05170.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zurek PM. The precedence effect. In: Yost WA, Gourevitch G, editors. Directional hearing. Springer; New York: 1987. pp. 85–105. [Google Scholar]