Abstract
Several taste transduction mechanisms have been demonstrated in mammals, but little is known about their distribution within and across receptor cells. We recorded whole-cell responses of 120 taste cells of the rat fungiform papillae and soft palate maintained within the intact epithelium in a modified Ussing chamber, which allowed us to flow tastants across the apical membrane while monitoring the activity of the cell with a patch pipette. Taste stimuli were: 0.1m sucrose, KCl, and NH4Cl, 0.032 mNaCl, and 3.2 mm HCl and quinine hydrochloride (QHCl). When cells were held at their resting potentials, taste stimulation resulted in conductance changes; reversible currents >5 pA were considered reliable responses. Sucrose and QHCl produced a decrease in outward current and membrane conductance, whereas NaCl, KCl, NH4Cl, and HCl elicited inward currents accompanied by increased conductance. Combinations of responses to pairs of the four basic stimuli (sucrose, NaCl, HCl, and QHCl) across the 71–84 cells tested with each pair were predictable from the probabilities of responses to individual stimuli, indicating an independent distribution of sensitivities. Of 62 cells tested with all four basic stimuli, 59 responded to at least one of the stimuli; 16 of these (27.1%) responded to only one, 20 (33.9%) to two, 15 (25.4%) to three, and 8 (13.6%) to all of the basic stimuli. Cells with both inward (Na+) and outward (K+) voltage-activated currents were significantly more broadly tuned to gustatory stimuli than those with only inward currents.
Keywords: taste receptor cell, tongue epithelium, palate epithelium, gustatory sensitivity, breadth of tuning, sucrose, quinine, salt, acid, pattern coding
Taste transduction involves a variety of mechanisms, including direct permeation or block of ion channels and activation of metabotropic and ionotropic receptors (for review, see Lindemann, 1996; Herness and Gilbertson, 1999). There is little information, however, about how these mechanisms are distributed within and across taste receptor cells. Intracellular recording experiments have suggested that taste cells are broadly responsive to stimuli representing different taste qualities (Kimura and Beidler, 1961; Ozeki and Sato, 1972; Tonosaki and Funakoshi, 1984; Sato and Beidler, 1997). However, because of their relatively small membrane potentials and the possibility of leak currents associated with penetrating such small cells with sharp electrodes, many investigators have viewed these intracellular experiments with skepticism (Kinnamon, 1988; Avenet and Lindemann, 1989; Lindemann, 1996; Herness and Gilbertson, 1999). More recent experiments have used patch-clamp recording methods on isolated taste receptor cells (Avenet and Lindemann, 1987; Akabas et al., 1988; Kinnamon et al., 1988; Gilbertson et al., 1993; Herness and Sun, 1995; Chen et al., 1996; Cummings et al., 1996), but the range of stimuli that can be applied to an isolated cell preparation is limited and recording is hindered by having the apical and basolateral membranes in the same bathing medium.
In contrast, there is a great deal of information on the sensitivities of gustatory afferent fibers and central neurons. There is general consensus that afferent neurons, from the gustatory nerves to forebrain taste areas, show multiple sensitivity to stimuli representing different taste qualities (Pfaffmann, 1955, 1959; Ogawa et al., 1968;Smith et al., 1983; Yamamoto et al., 1984; Frank et al., 1988; Ninomiya and Funakoshi, 1988; Smith and Frank, 1993). Moreover, as information is passed from the gustatory afferent nerves to brainstem nuclei, the cells become more broadly tuned because of convergence at each successive stage (Smith and Travers, 1979; Travers and Smith, 1979; Van Buskirk and Smith, 1981; Sweazey and Smith, 1987; Frank et al., 1988). Thus, most levels of the gustatory system are characterized by broadly tuned afferent neurons.
To determine whether the taste receptor cells themselves contribute to this broad tuning and to examine the distribution of gustatory sensitivities across these cells, we have combined patch-clamp recording with apically restricted stimulus application. Whole-cell recordings were made from 120 receptor cells maintained in the intact epithelium of the soft palate or the anterior portion of the tongue. Up to six taste stimuli were applied to the apical membrane of each cell by perfusion through a closed mucosal chamber, which effectively separated the apical from the basolateral taste cell membranes. The data show that individual taste receptor cells often exhibit a range of chemical sensitivities. Almost three-quarters of the cells responded to more than one of four basic taste stimuli, although the receptor cells showed greater stimulus specificity than typically seen in first- or second-order afferent neurons. Thus, one source of the multiple sensitivity of peripheral and central gustatory neurons arises at the initial step of stimulus recognition by the taste receptor cells themselves.
Portions of these results have appeared in abstract form (Monroe et al., 1996; Gilbertson et al., 1999; Smith et al., 2000).
MATERIALS AND METHODS
Tissue preparation. Epithelial tissue was prepared from the anterior tongues or soft palates of 2- to 6-month old Sprague Dawley rats using established methods (Béhé et al., 1990b;Gilbertson, 1995) that have been adapted for intact epithelial preparations (Gilbertson and Zhang, 1998). Briefly, tongues or palates were injected between the epithelium and underlying tissue with ∼1.0 ml of Tyrode containing 0.5 mg/ml collagenase A, 2.5 mg/ml dispase (type II; Boehringer Mannheim, Indianapolis, IN), and 1 mg/ml trypsin inhibitor (type I-S; Sigma, St. Louis, MO). Once injected, tissues were incubated for ∼20 min at room temperature in Ca2+/Mg2+-free Tyrode and bubbled with O2. The lingual or palatal epithelia were peeled from the underlying tissue after incubation, rinsed several times with enzyme-free Tyrode, and pinned out in a Sylgard-lined Petri dish with the mucosal side down. Plastic coverslips (15 mm, Thermanox plastic; Nunc, Naperville, IL) with a 2–3 mm hole through the center were coated with a thin layer of cyanoacrylate glue and placed on both sides of the isolated epithelium. Generally, from two to six or more taste buds were accessible in the opening of the coverslip, as shown in Figure1A, which depicts the serosal side of the tongue epithelium. Under differential interference contrast (DIC) illumination, individual cells within the taste buds could be discerned (Fig. 1B). In this configuration, it was possible (see below) to deliver gustatory stimuli to the apical membranes of the cells via the taste pore, shown from the mucosal side of the epithelium in Figure 1C. The time course of solution change at the mucosal surface is shown in Figure1D, which depicts the current change caused by the liquid junction potential of a patch pipette (filled with 30 mm KCl) placed on the mucosal side of the chamber. When the mucosal solution was switched from 30 to 300 mm KCl, ∼10 sec were required to effect a complete change of stimulus solution.
Fig. 1.
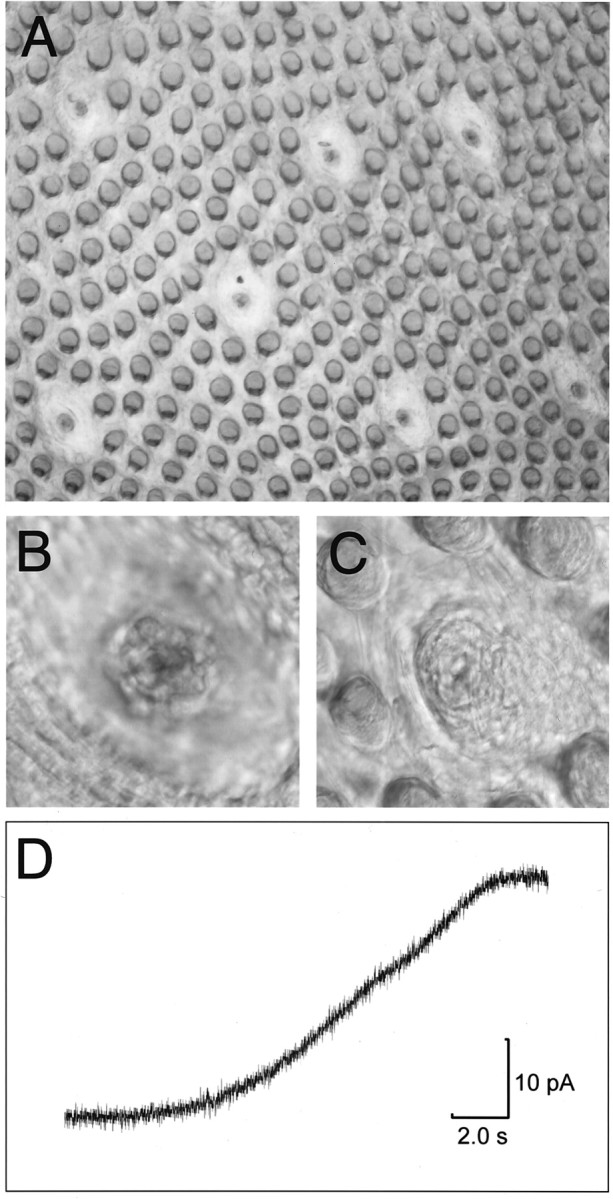
Photomicrographs of the lingual epithelium, after enzymatic removal from the anterior portion of the tongue.A, At low power, viewed from the serosal (basolateral) side of the epithelium, several taste buds within fungiform papillae can be seen amid the numerous filiform papillae in a freshly isolated strip of epithelium. B, At higher power and under DIC illumination, individual cells in the taste bud can be visualized.C, Taste pore of an individual taste bud, viewed from the mucosal side of the epithelium. Tastants applied to the mucosal surface have access to the taste bud only through the taste pore.D, Change in current produced by the liquid junction potential of a micropipette filled with 30 mm KCl when the mucosal solution was switched from 30 to 300 mm KCl.
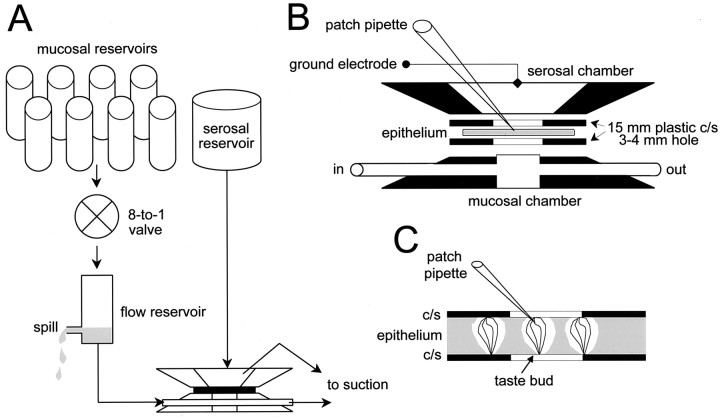
Modified Ussing chamber. The epithelial preparation was mounted in a bipartitioned chamber separating the mucosal and serosal surfaces of the epithelium (Fig.2). Both sides of the epithelium were initially perfused with Tyrode. This configuration is a variation of the classic Ussing chamber; it allows taste stimuli to be presented to the apical membranes of the taste cells via the closed mucosal chamber, while permitting access to the basolateral membranes with a patch electrode (Fig. 2B). Similar approaches have been attempted in the past with some success for intracellular, patch, and optical recording (Roper and McBride, 1989; Béhé et al., 1990a; Furue and Yoshii, 1997, 1998; Ohtubo et al., 2001). In the course of the present study, we have used several different designs for this modified Ussing (MU) chamber that all retain the same basic features (Fig. 2). For the taste solutions, as many as eight reservoirs could be connected to an 8-to-1 valve, the output of which was directed into a 5 ml flow reservoir, which in turn was connected to the input line of the mucosal chamber with PE-190 tubing (Becton Dickinson, Sparks, MD). The volume of the mucosal chamber was 65 μl, and the flow rate was 2.3 ml/min. The flow reservoir served to prevent epithelial movement during solution changes by keeping a constant head of pressure on the mucosal chamber (Furue and Yoshii, 1998). Stimulus solutions flowing into the reservoir replaced the distilled water in ∼10 sec, as reflected in the current changes shown in Figure1D. Solution output was collected passively and removed by suction. The serosal solution was provided by gravity flow from a 500 ml reservoir containing Tyrode. The ground electrode (150 mm NaCl in 4% agarose) was placed in the serosal chamber. Epithelia placed in the chamber and perfused with Tyrode in the serosal chamber were stable for a minimum of 3–4 hr without noticeable electrophysiological decrement.
Fig. 2.
Schematic diagrams of the MU chamber.A, The MU chamber consisted of two separate chambers, each with its own perfusion system. The mucosal (apical) chamber was fed by eight solution reservoirs connected via an eight-way solenoid valve to a flow reservoir and, in turn, to the chamber. The serosal (basolateral) chamber was perfused with Tyrode. B,C, Detailed views of the MU chamber. The lingual epithelium containing taste buds of the fungiform papillae was mounted with cyanoacrylate glue between two plastic coverslips (c/s) each of which had a ∼3 mm hole drilled through it. This hole permitted access to several taste buds per preparation. Orientation of the epithelium was such that the apical (chemoreceptive) ends of the taste cells faced into the stimulating mucosal solution, which accessed the apical membranes through the taste pore. The patch pipette had access to the basolateral regions of the cells via the serosal chamber.
The chemosensitivity of the cells was examined by flowing from one to six of the following taste stimuli through the mucosal chamber (in mm): sucrose, 100; quinine-HCl (QHCl), 3.2; KCl, 100; NaCl, 32; citric acid, 3.2; HCl, 3.2; and NH4Cl, 100. The mucosal epithelium was adapted to distilled H2O, as is typically done in electrophysiological experiments on peripheral gustatory nerve fibers or central neuronsin vivo. These solutions and their concentrations are similar to those used in earlier recordings of taste responses in chorda tympani fibers, the greater superficial petrosal nerve, and the nucleus of the solitary tract of the rat (Pfaffmann, 1955; Frank and Pfaffmann, 1969; Doetsch and Erickson, 1970; Contreras and Frank, 1979;Frank et al., 1983).
Solutions and recording conditions. Extracellular saline (Tyrode) contained (in mm): NaCl, 140; KCl, 5, CaCl2, 1; MgCl2, 1; HEPES, 10; glucose, 10; and Na+ pyruvate, 10. The pH was adjusted to 7.40 with NaOH. The only change made to prepare Ca2+/Mg2+-free Tyrode was to substitute 2 mm BAPTA (Molecular Probes, Eugene, OR) for the CaCl2 and MgCl2. The pipette solution contained (in mm): KCl, 140; CaCl2, 1; MgCl2, 2; HEPES, 10; EGTA, 11; Na2ATP, 5; GTP, 0.4. The pH was adjusted to 7.20 with KOH and the free Ca2+ was ∼10−8m
Recordings were made from individual taste receptor cells maintained in intact taste buds using the conventional whole-cell variation of the patch-clamp technique (Hamill et al., 1981). Patch pipettes were made from microhematocrit tubes (Scientific Products, McGaw Park, IL) pulled on a Flaming-Brown-type micropipette puller (model P-97; Sutter Instruments, Novato, CA) and fire-polished on a microforge (model MF-9; Narishige, Tokyo, Japan) to a resistance of 4–8 MΩ when filled with intracellular solution. Seal resistances were typically in the tens of GΩs. Series resistance and capacitance were compensated before recording.
Whole-cell membrane currents or voltages were recorded in voltage-clamp or current-clamp modes, respectively, by a high-impedance patch-clamp amplifier (Axopatch 1-D; Axon Instruments, Foster City, CA) interfaced to a computer (Pentium 90 MHz) by an analog-to-digital board (Digidata 1200A; Axon Instruments). Command potentials were delivered and currents recorded by computer-driven software (pClamp 6.0.3/7.0; Axon Instruments). In some experiments, steady-state current responses were recorded on VCR (44.1 kHz) and printed on a strip chart recorder (model RS3200; Gould Instrument Systems, Valley View, OH). Steady-state currents and voltage-activated currents were recorded at a sampling rate of 10 kHz. For analysis and presentation, data were low-pass filtered at 2 kHz. Once the whole-cell configuration was established, compensation for series resistance and cell capacitance was made. No records were leak-subtracted. Once in the whole-cell configuration, we tested every cell under voltage clamp for the evidence of voltage-activated Na+ and/or K+ current (Fig. 3) as an indicator that we were recording from receptor cells and not epithelial cells, which lack these currents (Akabas et al., 1990). In the initial experiments, we recorded the tastant-induced change in membrane potential in current-clamp mode with the cell held at or near its resting potential (Fig. 4). In these experiments, tetrodotoxin (0.5 mm) was included in the serosal chamber to inhibit action potential generation. However, we subsequently found that we had better success using voltage-clamp mode. In this configuration, the cell was held near its resting potential and we recorded the effects of tastants on the resting conductance (Fig.5). Data were consistent using both modes of recording.
Fig. 3.
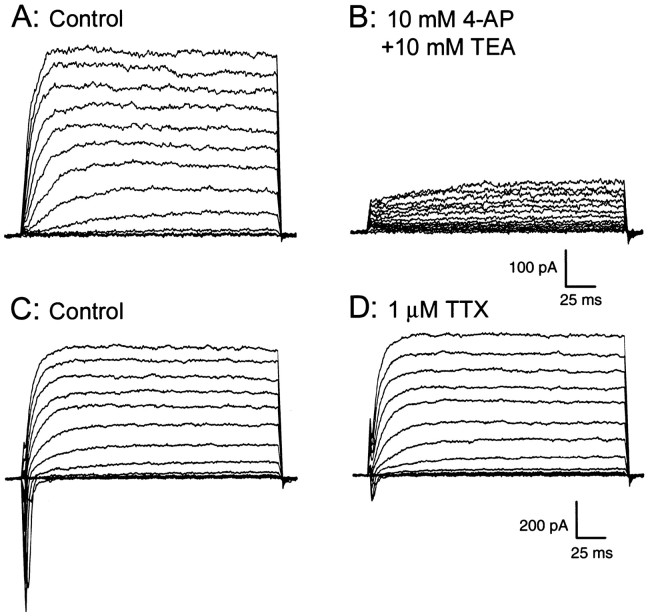
Voltage-activated currents recorded from two taste cells in the MU chamber. Cells were held at −80 mV and stepped to +40 mV in 10 mV increments. The cell in A showed only voltage-activated outward currents, which were reduced by the addition of 10 mm 4-AP and 10 mm TEA to the serosal bath (B). The cell in C showed both inward and outward currents; the inward currents were reduced by the addition of 1 μm TTX to the serosal bath (D).
Fig. 4.
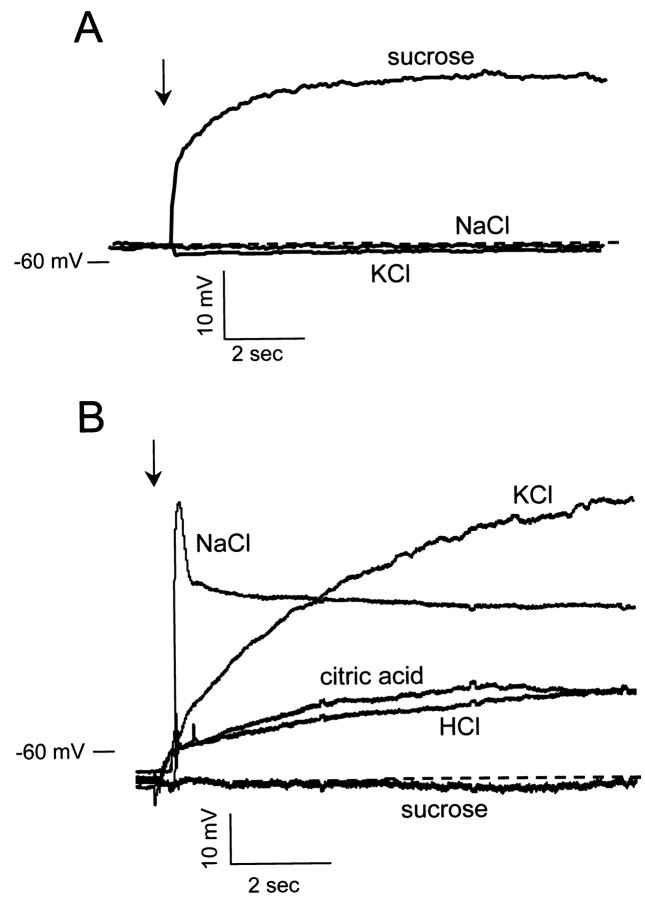
Current-clamp responses of taste receptor cells to various gustatory stimuli. The two cells were held near their respective resting potentials (−58 mV in A; −63 mV inB), and taste stimuli were perfused onto the mucosal surface of the epithelium. A, This cell was depolarized by 0.1 m sucrose, but not by 0.032 m NaCl or 0.1 m KCl. B, Voltage response of a broadly sensitive cell that was reversibly depolarized by 0.032 mNaCl, 0.1 m KCl, 3.2 mm citric acid, and 3.2 mm HCl, but not by 0.1 m sucrose. Stimulation began at the arrow and continued throughout the time period shown; 0.5 mm TTX was present in the serosal solution.
Fig. 5.
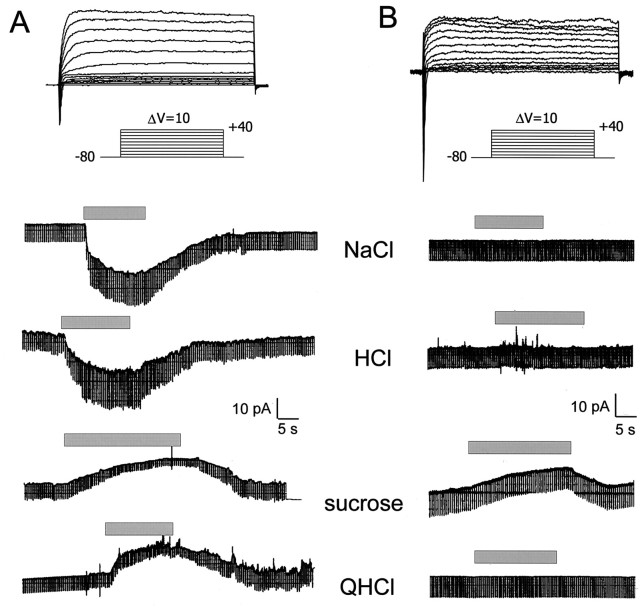
Voltage-clamp responses of taste cells to stimuli representing the four basic taste qualities. Top tracesshow the voltage-activated currents in two cells in response to voltage steps from −80 to +40 mV from a holding potential of −80 mV. These cells displayed both transient inward Na+ currents and sustained outward K+ currents; the difference between the voltage-activated currents shown in these cells is within normal variation. The bottom four sets of traces show the current responses to application of taste stimuli (0.032m NaCl, 3.2 mm HCl, 0.1 m sucrose, and 3.2 mm QHCl); the cells were held at their resting potential. Brief downward deflections in these records are the current responses to 10 mV hyperpolarizations used to monitor changes in conductance (e.g., larger deflections indicate a conductance increase and vice versa). The cell shown in A responded to all four stimuli, whereas that shown in B responded to only sucrose.
Data analysis. Stimulus-induced changes in voltage or current were recorded while the cells were held near their resting potentials. In the initial current-clamp experiments, a reversible change in membrane potential ≥5 mV that was time-locked to the stimulus application was considered a clearly discernable response. Most of the data were obtained in voltage-clamp mode, where a temporally associated reversible current ≥5 pA was the response criterion; for some analyses a more stringent 10 pA criterion was used. All stimulus-induced current changes ≥5 pA were recorded, and the actual current values for the 62 cells that were tested with all four of the basic stimuli (sucrose, NaCl, HCl and QHCl) are provided in Tables 1 and2. Although ramping through a series of voltages would have provided us with greater detail on the current–voltage relationships of these cells, we wished to determine whether taste stimuli would alter the conductance of these cells when they were at resting membrane potential.
Table 1.
Responses (in picoamperes) to four basic stimuli in 45 taste cells of the fungiform papillae
| Cell# | 0.1m sucrose | 0.032 m NaCl | 3.2 mmHCl | 3.2 mmQHCl | n1-a |
|---|---|---|---|---|---|
| 33 | — | — | −10 | — | 1 |
| 34 | — | — | — | 15 | 1 |
| 35 | — | — | — | — | 0 |
| 36 | 18 | — | — | — | 1 |
| 37 | — | −10 | −30 | — | 2 |
| 38 | — | — | — | — | 0 |
| 39 | 20 | −20 | −20 | 20 | 4 |
| 41 | 10 | — | — | — | 1 |
| 42 | — | −20 | −8 | 15 | 3 |
| 44 | — | — | −5 | — | 1 |
| 45 | 18 | — | — | — | 1 |
| 46 | 10 | −15 | — | — | 2 |
| 47 | 20 | — | — | — | 1 |
| 48 | — | −12 | — | 15 | 2 |
| 50 | 16 | — | — | — | 1 |
| 53 | — | — | −30 | 25 | 2 |
| 54 | — | −10 | −10 | — | 2 |
| 59 | 10 | — | −8 | — | 2 |
| 60 | 50 | — | −10 | 10 | 3 |
| 61 | 15 | — | −15 | 20 | 3 |
| 62 | 90 | — | — | — | 1 |
| 66 | 70 | −40 | — | — | 2 |
| 68 | — | −10 | — | — | 1 |
| 70 | — | −10 | — | — | 1 |
| 73 | 5 | — | — | — | 1 |
| 74 | 5 | −5 | — | 8 | 3 |
| 81 | 45 | −25 | — | 5 | 3 |
| 83 | 13 | −5 | — | — | 2 |
| 85 | 10 | −8 | −40 | 10 | 4 |
| 86 | 45 | — | −15 | — | 2 |
| 87 | — | — | −15 | 25 | 2 |
| 88 | 50 | — | −30 | 24 | 3 |
| 89 | 20 | — | — | — | 1 |
| 90 | 10 | −5 | −15 | 5 | 4 |
| 91 | — | −5 | — | 13 | 2 |
| 93 | 8 | −13 | — | — | 2 |
| 95 | 5 | −14 | — | 5 | 3 |
| 96 | 13 | −13 | — | 10 | 3 |
| 97 | 33 | −8 | −15 | 8 | 4 |
| 98 | — | −8 | — | 8 | 2 |
| 99 | 5 | −8 | −10 | — | 3 |
| 100 | 28 | — | −25 | — | 2 |
| 101 | 25 | −40 | — | 5 | 3 |
| 102 | — | −15 | −6 | 5 | 3 |
| 103 | — | −14 | — | 5 | 2 |
| n1-b | 28 | 24 | 19 | 21 | 92 |
| P1-c | 0.622 | 0.533 | 0.422 | 0.467 | |
Number of responses in each cell;
number of cells responding to each stimulus;
proportion of cells responding to each stimulus.
Table 2.
Responses (in picoamperes) to four basic stimuli in 17 taste cells of the soft palate
| Cell# | 0.1m sucrose | 0.032 m NaCl | 3.2 mmHCl | 3.2 mmQHCl | n2-a |
|---|---|---|---|---|---|
| 1 | — | — | −8 | — | 1 |
| 2 | — | — | — | — | 0 |
| 3 | 15 | — | — | — | 1 |
| 4 | 40 | −10 | −10 | 10 | 4 |
| 5 | — | −10 | — | 10 | 2 |
| 6 | 25 | — | — | 15 | 2 |
| 7 | 13 | −10 | −8 | — | 3 |
| 8 | — | −5 | — | 10 | 2 |
| 9 | — | — | −10 | — | 1 |
| 10 | 15 | −35 | −10 | 15 | 4 |
| 11 | 20 | −25 | −50 | — | 3 |
| 12 | 50 | −15 | −50 | — | 3 |
| 13 | 25 | −10 | — | — | 2 |
| 14 | 40 | — | −10 | — | 2 |
| 15 | 35 | −30 | −10 | 10 | 4 |
| 16 | 25 | −25 | −20 | 30 | 4 |
| 17 | 50 | — | −50 | 20 | 3 |
| n2-b | 12 | 10 | 11 | 8 | 41 |
| P2-c | 0.706 | 0.588 | 0.647 | 0.471 |
Number of responses in each cell;
number of cells responding to each stimulus;
proportion of cells responding to each stimulus.
The breadth of responsiveness of the cells was determined by using a criterion-free measure of breadth of tuning, which compares the relative magnitudes of responses within each cell. Breadth of tuning was quantified using the entropy equation first introduced for measuring breadth of gustatory sensitivity by Smith and Travers (1979). Entropy (H) is given by:
where H = breadth of responsiveness,K is a scaling constant (1.661 for four stimuli), andpi is the proportional response to each ofn stimuli. The pi for each cell are derived by converting the response profile of that cell to a proportional profile, the response to each stimulus being expressed as a proportion of the total current produced by all four stimuli. This measure takes the relative current magnitude (within a cell) into account in determining the breadth of responsiveness of the cell without imposing a response criterion beyond the minimum of 5 pA. In other words, this measure does not simply depend on whether a response occurs, but on the relative magnitude of the response to each stimulus (Smith and Travers, 1979).
Profiles of sensitivity for each cell were constructed by converting the current response of each cell to a relative (to the maximum current) response. Two multivariate procedures were used to investigate the underlying similarities and differences in these response profiles. First, a hierarchical cluster analysis was used to determine the extent to which the various response profiles fall into meaningful clusters (Everitt, 1980; Bieber and Smith, 1986). The clustering program (SPSS for Windows, version 9) processed the cell profiles based on a matrix of the Pearson correlation coefficients between all possible pairs of profiles and amalgamated the cells sequentially into the cluster solution using the average linkage method. The underlying structure of these data were further examined with multidimensional scaling (MDS) (Alscal, SPSS for Windows, version 9). For this analysis, the matrix of correlation coefficients among the response profiles of all possible pairs of cells was used as the input to produce a two-dimensional representation of the differences among the profiles. The MDS program places the cells into a spatial arrangement that reflects the correlations among them. The combination of these two multivariate procedures provides a view of the similarities (clustering) and dissimilarities (MDS) among the response profiles (Bieber and Smith, 1986), as has been frequently done for responses of gustatory afferent neurons (Frank et al., 1988).
RESULTS
Basic response properties of taste cellsin situ
We recorded stimulus-induced responses from 120 taste receptor cells maintained in taste buds in the intact epithelium of the anterior tongue (n = 103) and soft palate (n = 17) using the whole-cell patch-clamp configuration. With the pipette containing 140 mm KCl and the bath Tyrode, cells of the fungiform papillae had an input slope resistance between 0.59 and 2.11 GΩ [mean, 1.28 ± 0.37 (SD) GΩ] and a zero current potential ranging between −71 and −29 mV (mean, −53.4 ± 10.2 mV). These values are in the same range as those recorded from dissociated taste cells from rat fungiform (Béhé et al., 1990b; Gilbertson et al., 1997) or vallate (Herness and Sun, 1995) papillae. Similar values were obtained from the 17 cells in the soft palate. Zero current potentials for palatal taste cells ranged from −67 to −39 mV [mean, −52.4 ± 9.3 (SD) mV] and input resistance ranged from 0.78 to 2.49 GΩ (mean, 1.64 ± 0.51 GΩ). All of the recorded cells showed voltage-activated outward (K+) currents and a subset of these additionally exhibited transient voltage-activated inward (Na+) currents (29 of 120 = 24.2%).
The currents evoked in two cells recorded in the MU chamber in response to a voltage-step protocol are shown in Figure 3. These cells were held at −80 mV and stepped to +40 mV in 10 mV increments. The cell inA showed only voltage-activated outward currents, which were reduced (B) by the addition of the K+ channel blockers 4-aminopyridine (4-AP; 10 mm) and tetraethylammonium chloride (TEA; 10 mm) to the serosal bath. The cell in Cshowed both inward and outward currents in response to the voltage protocol; the inward currents were reduced (D) by the Na+ channel blocker tetrodotoxin (TTX; 1 μm).
Taste responses: current-clamp experiments
Experiments on taste responses were conducted in one of two ways. In an early series of experiments, taste cells of the fungiform papillae were held near their resting potentials in current-clamp mode (i.e., zero current level), and tastant-induced changes in membrane potential were recorded. A cell was considered responsive to one of the taste stimuli if it induced a reversible change in membrane potential that was at least 5 mV from resting levels and time-locked to the stimulus flow; such a change was a clearly discernable response. Figure4 shows taste responses of two cells in this configuration. One of these cells (Fig. 4A) was tested with sucrose, NaCl and KCl and showed a depolarization only to sucrose. The other cell (Fig. 4B) responded to NaCl, KCl, citric acid, and HCl, but not to sucrose; it was not tested with QHCl. Although the response of this cell to NaCl had a much more sudden onset than those to KCl and the two acids, this was not a consistent observation across cells. The weakest response (to HCl) was two or three times the response criterion of 5 mV. In these current-clamp experiments, all responses were depolarizing in nature, no cells responded to any of the six stimuli with a hyperpolarizing response.
Data were obtained from 21 cells in current-clamp mode. Because we were unable to hold any of these cells long enough to apply all six stimuli, it was not possible to determine precisely the breadth of their chemical sensitivities. Some of the cells were tested with only one or two stimuli. Nevertheless, 11 of the 21 cells (52.4%) responded to more than one stimulus, and 5 (23.8%) responded to three or four stimuli. Three cells were tested with both of the acids (citric acid and HCl), which elicited similar responses in each cell (see also the acid responses in Fig. 4B). In this part of the study, we were unable to record from any cells during application of all six stimuli, but these data are combined with the voltage-clamp data presented below to determine the overall distributions of responsiveness to pairs of stimuli (Table3).
Table 3.
Distribution of sensitivities to pairs of four basic stimuli in rat taste cells (based on all possible combinations across 120 cells)
| Combination (x, y) | No. cells tested | (px)(py)3-a | Predicted | Observed | P3-b |
|---|---|---|---|---|---|
| S, N | 84 | (0.643)(0.488) = 0.314 | 26.4 | 25 | >0.65 |
| S, H | 71 | (0.648)(0.507) = 0.329 | 23.3 | 23 | >1.00 |
| S, Q | 71 | (0.634)(0.493) = 0.313 | 22.2 | 21 | >0.63 |
| N, H | 72 | (0.542)(0.514) = 0.279 | 20.1 | 21 | >0.81 |
| N, Q | 73 | (0.479)(0.507) = 0.243 | 17.8 | 22 | >0.06 |
| H, Q | 72 | (0.514)(0.458) = 0.235 | 16.9 | 19 | >0.36 |
Proportion of tested cells responding to each member of the pair; response criterion: ≥5 pA or 5 mV;
statistical difference tested using the Fisher exact probability test (SigmaStat version 2.0). S, Sucrose; N, NaCl; H, HCl; Q, QHCl.
Taste responses: voltage-clamp studies
Because we had better success using voltage clamp to record stimulus-induced changes in whole-cell currents, the majority of experiments were performed using the voltage-clamp recording mode. Similar to the results using current-clamp recording, we found that cells displayed a range of chemical sensitivities. Figure5 shows current responses of two taste cells of the fungiform papillae to stimuli representing the four basic taste qualities. In these experiments, the criterion for the occurrence of a response was a reversible change in current ≥5 pA from resting current level; the response to sucrose in Figure 5B was about twice this criterion. When reversible and time-locked to the stimulus application (as in Fig. 5), a 5 pA current change is an unmistakable response. Some analyses were also conducted using a more stringent (≥10 pA) criterion. Many of the cells responded to more than one class of taste stimulus; the cell depicted in Figure 5Aresponded to all four of the basic taste stimuli. Responses to the six stimuli used in these experiments took one of two forms. Cells responded to NaCl, HCl, KCl, and NH4Cl with a conductance increase (increase in inward current), whereas responses to sucrose and QHCl showed a decrease in cellular conductance (decrease in outward current), as seen in Figure 5A. Both response types would lead to depolarization of a taste cell, consistent with the responses recorded under current clamp (Fig. 4). These response types were consistent across all cells of fungiform papillae and palate, as may be seen below in Tables 1 and 2. Many cells showed greater specificity, such as the sucrose-responsive cell in Figure5B (compare Fig. 4A), which did not respond to NaCl, HCl, or QHCl.
Responses to taste stimulation were recorded in voltage-clamp mode from 82 cells of the fungiform papillae and 17 of the soft palate. Of these 99 cells, 67 (67.7%) responded (≥5 pA) to more than one stimulus; if the criterion for a response was set at a more stringent level (≥10 pA), then 87 of these cells responded to at least one stimulus, and 51 of these 87 (58.6%) to more than one. Over all 120 cells (including the 21 recorded in current-clamp mode), 78 (65.0%) responded (≥5 pA or 5 mV) to more than one stimulus, although it should be noted that a number of these cells were only tested with one or two stimuli. Responses to different stimuli in the same cell were often similarly robust. Across the 67 cells recorded in voltage-clamp mode that responded to more than one stimulus, the response to the second most effective stimulus averaged 63.9% of the current change induced by the most effective (Fig. 5A, Tables 1, 2).
Across both the fungiform papillae and palate, 62 cells were tested with each of the four basic stimuli (sucrose, NaCl, HCl, and QHCl); 24 of the fungiform cells were also tested with KCl and NH4Cl. Of the 62 cells tested with the four basic stimuli, 59 responded to at least one of them. Of these 59 cells, 16 (27.1%) responded to only one of the four, whereas the remaining cells responded to two or more stimuli (43 cells; 72.9%). Two of the cells responding to none of these four stimuli responded to KCl and/or NH4Cl; the third was not tested with these compounds. Responses to sucrose, NaCl, HCl and QHCl in the 45 cells of the fungiform papillae are shown in Table 1, and those of the 17 palatal cells are depicted in Table 2. These tables also depict the proportion (P) of cells in these samples responding to each stimulus. Altogether, there were 92 reliable responses (i.e., reversible responses ≥5 pA) evoked in the 43 responsive fungiform cells by these four stimuli, averaging 2.14 responses per cell. The mean response to all second, third, and fourth responses in these 43 cells was 56.0% of the magnitude of the response to the most effective stimulus (Table 1).
The responses of the 17 cells of the soft palate are shown in Table 2. Within the 16 cells responsive to at least one of the four basic stimuli, there were 41 responses (≥5 pA), averaging 2.56 responses per cell. The mean response to all second, third, and fourth responses in these 16 cells was 53.6% of the magnitude of the response to the most effective stimulus (Table 2).
To determine the breadth of sensitivity of the cells that were tested with all four stimuli, their breadth of tuning (H) was determined using the entropy measure (Smith and Travers, 1979). Over all 59 responsive cells, the mean H value was 0.462 ± 0.042 (SEM). This value is smaller than what has been reported for fibers of the rat chorda tympani nerve (0.561) (Travers, 1993) or nucleus of the solitary tract (0.790) (Giza and Scott, 1991). For the 43 cells of the fungiform papillae that were responsive to at least one of the four basic stimuli, the mean entropy was 0.429 ± 0.049. The breadth of tuning of the 16 palatal cells (0.552 ± 0.083) was not significantly different from that of the cells of the fungiform papillae (t = 1.302; df = 57; p > 0.1).
Among the 59 responsive cells tested with all four stimuli in both the fungiform papillae and palate, 13 exhibited both inward (Na+) and outward (K+) currents in response to the voltage-step protocol (Figs. 3C, 5A,B). The cells with both Na+ and K+ currents had significantly greater breadth of responsiveness [mean H, 0.660 ± 0.078 (SEM); n = 13] than those with only K+ currents (mean, 0.406 ± 0.047;n = 46; two-tailed t test; t= 2.614; df = 57; p < 0.02).
Taste responses: independent sensitivities
Among the four basic stimuli, as many as 84 cells and no fewer than 71 cells were tested with two members of each possible pair of stimuli. The distributions of sensitivities to pairs of the four basic stimuli are shown in Table 3. Here, all data for each pair of tastants (x and y), from both the voltage-clamp and the earlier current-clamp experiments were combined to assess the relative distributions of sensitivities. The number of cells tested with each member of the pair is given in Table 3, along with the proportions of cells responding to each member of the pair (px andpy), and the number of predicted and observed responses to both members of the pair. If the distributions of these sensitivities were not independent, we would expect that certain combinations would occur more or less often than predicted. For each possible pair of the four basic stimuli, however, the number of cells showing sensitivity to both members of the pair is no different than predicted by chance (all Ps > 0.05; Fisher exact probability test); i.e., the number of cells responding to both is predicted by the product of the probabilities of the response to each member of the pair. Increasing the response criterion to ≥10 pA resulted in fewer responses (as can be appreciated by examination of Tables 1 and 2), but an analysis of the sensitivities to pairs of the four stimuli still shows them to be independent of one another, except for sucrose and HCl, which occurred slightly more often than expected by chance. With a response criterion of ≥10 pA, sensitivities to sucrose and HCl occurred together in 20 cells, although an independent distribution would predict only 15.8 cells with joint sensitivity to these two stimuli (Fisher exact probability test, p = 0.042). All other pairs of sensitivities were not different from chance occurrence, even with a response criterion of ≥10 pA. Thus, using a more stringent criterion does not reduce the number of joint occurrences among these sensitivities.
Analysis of the distributions of the responses to KCl and NH4Cl also suggest stochastic independence between these sensitivities and among KCl, NH4Cl, and the other four stimuli. For example, KCl and NH4Cl were tested together on 35 receptor cells (data not shown). KCl produced a reversible change in membrane current in 18 cells, NH4Cl in 16 cells, and both produced responses in 11 of the same cells. An independent distribution of these sensitivities predicts that 8.2 cells should respond to both; this difference was not statistically significant (Fisher exact probability test, p = 0.729). Similarly, KCl and NH4Cl responses were not associated with responses to sucrose, NaCl, HCl, or QHCl to a greater or lesser extent than predicted by chance. In some instances (e.g., NaCl and KCl), as many as 48 cells and no fewer than 30 cells (NH4Cl and HCl) were tested with pairs of these stimuli.
Taste responses: multivariate analyses
For the 59 cells (of both the fungiform papillae and palate) responding to at least one of the four basic stimuli, the magnitudes of the current responses (shown in Tables 1 and 2) were converted to proportions of the maximum response for each cell. These relative current values were then entered into a hierarchical cluster analysis (SPSS for Windows, version 9) to examine the similarities in their response patterns to the four stimuli. The results of this analysis are depicted in the dendrogram of Figure 6, which shows the ordering of the cells from those with the most similar response profiles to those that are least similar in their responses. Along the ordinate, the response profiles are indicated by letters [sucrose (S), NaCl (N), HCl (H), and QHCl (Q)] arranged from left to right in order of response magnitude within the cell. Capital letters indicate either the maximum response (on the left) or other responses that were at least half the value of the maximum. Responses smaller than half the maximum are shown as lowercase letters. The cluster analysis arranged the cells into three major clusters of response patterns, indicated by the cluster distances depicted by the horizontal and vertical lines. These three groups, labeled H/Q, S, and N, were characterized by their common response to one or more of the four basic stimuli. For example, all the cells in the S group (except one) responded to sucrose, although many of these cells also responded to other stimuli; sucrose often produced responses also in the other groups of cells (and was even the maximum response for some other cells). The other two groups (H/Q and N) were characterized by their common responses to HCl and/or QHCl and NaCl, respectively. Although each of these groups is characterized by its common response to one or two stimuli, that stimulus did not necessarily produce the best (maximum) response in each member of the group. Thus, although the responses to these four stimuli are independent of one another, there is some order to their patterns of sensitivity when response magnitude is considered.
Fig. 6.
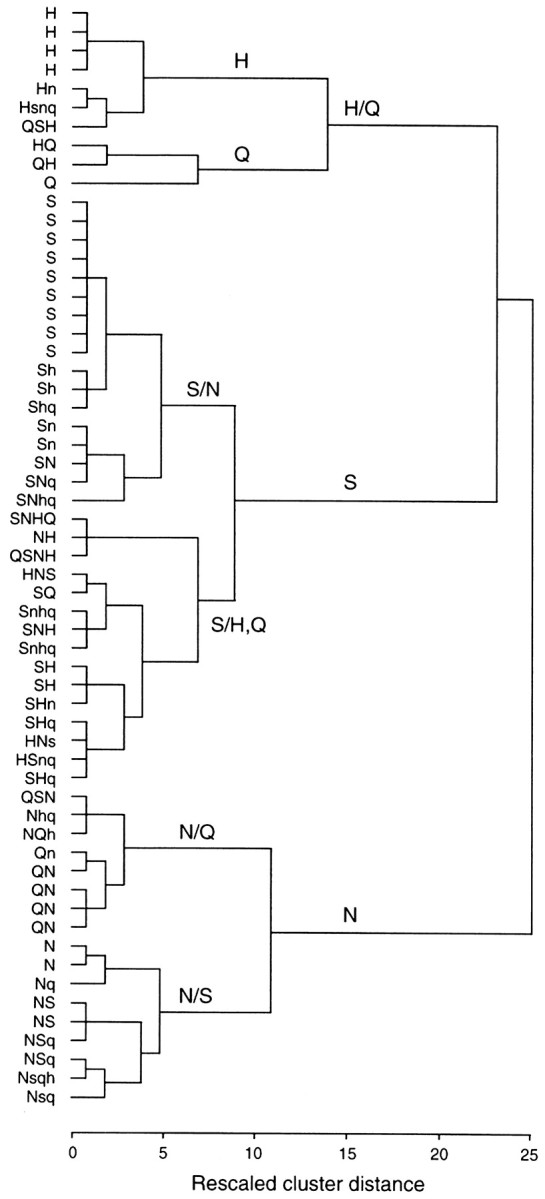
Cluster dendrogram showing the relationships among response profiles of rat taste receptor cells. Input was the normalized (to the maximum response) current produced in each cell by the four stimuli. The response profile of each cell is indicated on the left.Capital letters indicate the stimulus producing the maximum response (shown first) and all others with responses ≥50% of maximum. Lowercase letters indicate responses <50% of the maximum; the order of the letters indicates the relative magnitude of the response to each stimulus. The three clusters, which are not sharply differentiated, are labeled H/Q, S, and N according to which stimulus was common to all members of the group (but not necessarily the best stimulus for every member of the group). The common stimuli for two subclusters of each major cluster are also indicated on the dendrogram.
To further examine the relationships among the response profiles of these cells, the relative current responses were analyzed using MDS. For this analysis, the matrix of correlation coefficients among the response profiles of all possible pairs of cells was used as the data for an MDS analysis, the results of which are shown in Figure7. This two-dimensional solution accounted for 95.4% of the data variance. The proximity of cells in this figure depicts the similarity in their response profiles; symbols represent different subclusters in the dendrogram of Figure 6, and dashed lines indicate the three major clusters. That is, triangles represent the cells of the H/Q cluster, with the H subcluster shown as gray and the Q as black triangles. The cells of the N cluster are depicted as squares, with the open squares the N/S subcluster and the black squares the N/Q subcluster. The cells of the S cluster are shown as circles, with the open circles the S/N subcluster and the gray circles the S/H,Q subcluster. The labels S, N, H, and Q indicate the cells responding exclusively to one of the four stimuli, which are maximally separated within this space. The position of these cells in this two-dimensional space reflects the similarities and differences in their response profiles, which directly reflects the results of the hierarchical cluster analysis shown in Figure 6. The one exception is the gray circle shown in the H/Q cluster, which was a cell of the S cluster that responded strongly to both NaCl and HCl; it was the only cell of its kind. The positions of these subclusters in multidimensional space indicate that the profiles of sensitivity are representative of very loosely defined cell types. That is, although there are orderly groupings of these receptor cells on the basis of their response profiles, the distinctions among these groups are not striking, with almost all combinations of sensitivities occurring together across this sample of cells. Similar analyses at higher levels of the rat gustatory system also show relatively loosely defined clusters of cells (Chang and Scott, 1984; Giza and Scott, 1991).
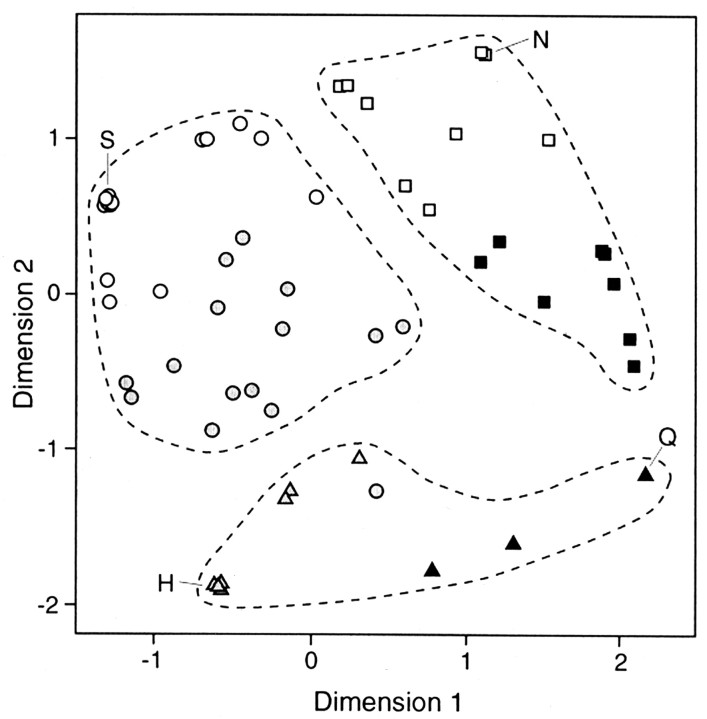
Fig. 7.
Two-dimensional space showing the relationships among response profiles of rat taste receptor cells, derived from multidimensional scaling. Input was the complete correlation matrix among the normalized currents produced in each cell by the four stimuli. The three clusters of cells identified by the hierarchical cluster analysis of Figure 6 are delineated by dashed lines and also indicated by different symbols: H/Q cluster, triangles; S cluster,circles, and N cluster, squares. These three groups are further delineated in the figure by symbol shading, which depicts the subclusters shown in Figure 6 (see Results). The letters S, N, H, and Q indicate the positions of the cells responding exclusively to each one of the four stimuli; all other cells were more broadly responsive, as indicated in the dendrogram of Figure6.
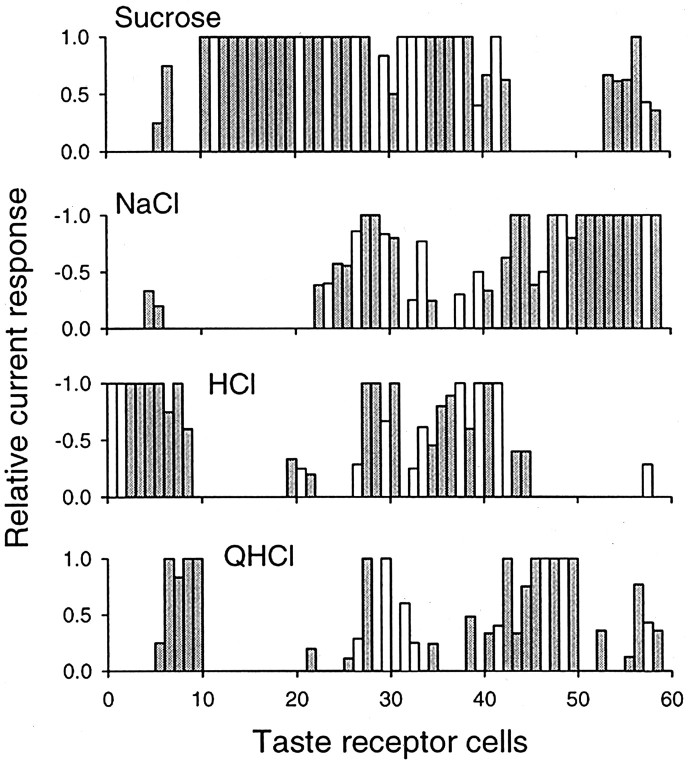
Across the 59 cells responding to at least one of the four basic stimuli, each stimulus elicited a unique pattern of responses, as shown in Figure 8. In this figure, responses of each cell that reached the 5 pA criterion are shown as relative (to the maximum response) current; the cells are arranged in order of the results of the hierarchical cluster analysis (Fig. 6). That is, cells on the extreme left are those at the top of the dendrogram of Figure 6, and those at the extreme right are those at the bottom of the dendrogram. Cells recorded from the fungiform papillae are shown as shaded bars and those from the palate as open bars. As noted above, most of these 59 cells (72.9%) responded to more than one of these stimuli, but there is a unique pattern of activity generated by each stimulus across these cells. Many of these cells responded maximally to more than one stimulus (such as the cell shown in Fig. 5A). Differential input from these broadly tuned receptor cells serves to establish unique across-fiber patterns in the chorda tympani or greater superficial petrosal nerves; such patterns may underlie the ability of rats to discriminate among these basic tastes (Pfaffmann, 1959;Erickson et al., 1965; Erickson, 1968; Smith and St. John, 1999).
Fig. 8.
Response patterns to four basic taste stimuli evoked across 59 rat taste receptor cells of the fungiform papillae and palate. Responses above criterion (a reversible current ≥5 pA) were converted to proportions of the maximum response for each cell (actual current values are given in Tables 1 and 2). The cells are arranged along the abscissa in the order given by the hierarchical cluster analysis of Figure 6. Thus, the leftmost cells (cells 1–10) are characterized by their common response to HCl and/or QHCl, the next group of cells (11–42) all (except one) responded to sucrose, and the last group (43–59) to NaCl. Many of these cells responded maximally to several of the stimuli. Sixteen cells were responsive to only one of the four stimuli; the other 43 cells responded to more than one. Although these receptor cells are largely responsive to more than one stimulus, the patterns of activity across the cells provides differential input to first-order afferent neurons that can serve as a substrate for gustatory discrimination.
DISCUSSION
Apical chemical stimulation of taste receptor cells
Recent studies of taste receptor cell physiology have used patch-clamp recording methods on isolated cells (Avenet and Lindemann, 1987; Akabas et al., 1988; Kinnamon et al., 1988; Gilbertson et al., 1993; Herness and Sun, 1995; Chen et al., 1996; Cummings et al., 1996), but the range of gustatory stimuli that can be applied to isolated cells is restricted. Although other laboratories have used an intact epithelial preparation (Roper and McBride, 1989; Béhé et al., 1990a; Furue and Yoshii, 1997, 1998; Ohtubo et al., 2001), there has been limited success in applying a range of stimuli to a sizeable number of cells. For example, Furue and Yoshii (1997) recorded from only five cells in mouse fungiform taste buds that were responsive to one or more of three taste stimuli applied to the apical membrane andOhtubo et al. (2001) applied only NaCl or a taste mixture. The present data are the first to show the distribution of gustatory sensitivities across a large number of mammalian taste receptor cells using patch-clamp methods and apically restricted stimuli.
The increased conductance and inward current produced by NaCl in the present experiment are compatible with its passage through both apically located amiloride-sensitive Na+channels and basolateral ion channels (Boughter and Gilbertson, 1999). Indeed, the rapid depolarization seen to NaCl in Figure4B could be caused by the passage of Na+ through the amiloride-sensitive channel, although this possibility was not tested nor was this rapid time course consistent across the few cells tested in the current-clamp experiments. The mechanisms underlying the inward current produced by HCl in these experiments, however, are less clear. Acids have been shown to depolarize mammalian taste cells by proton permeation of apical amiloride-sensitive sodium channels under conditions of low mucosal sodium (Gilbertson et al., 1992, 1993; Harris et al., 1994), which is consistent with the responses seen in the present experiments. However, acids have been shown to block apical K+ channels in mudpuppy (Kinnamon et al., 1988) and in mammals a Cl− conductance may also be involved in acid transduction (Miyamoto et al., 1998). Without exception, both NaCl and HCl produced inward currents accompanied by increased conductance, suggesting that acids do not depolarize rat taste cells by blocking channels.
In the present experiment, both sucrose and QHCl decreased an outward conductance. Sucrose and other sweeteners have been shown to block a basolateral K+ channel as a consequence of second-messenger activation (Cummings et al., 1996), but the conductance decrease produced by QHCl in these cells is not so readily explained. If QHCl stimulates via a gustducin-mediated pathway, one would expect an increased conductance resulting from opening of basolateral cyclic nucleotide-gated channels; activation via the IP3 pathway would also not be expected to produce a decreased membrane conductance (Herness and Gilbertson, 1999). In patch-clamp experiments on isolated mouse taste cells, quinine produced an inward current and increased conductance when the cells were held at negative potentials (Seto et al., 1999), even when quinine was restricted to the apical membrane (Furue and Yoshii, 1998). On the other hand, QHCl has been shown to block an outward K+ conductance in isolated rat taste cells (Akabas et al., 1990; Chen and Herness, 1997). However, there is no evidence for apically localized K+channels on mammalian taste cells. The present results show that QHCl always produces a decrease in outward current in cells held at resting potential, suggesting that either apically applied QHCl is able to block K+ channels or it may lead to a conductance decrease through less frequent opening of a basolateral cyclic nucleotide-gated channel (Yan et al., 2000).
Although there have been a few reports of hyperpolarizing responses to taste stimuli in intracellular studies of mammalian taste cells [Sato and Beidler, 1982 (in rat); Tonosaki and Funakoshi, 1984 (in mouse)], we saw no such responses in either fungiform or palatal taste cells. In these earlier studies, there were fewer hyperpolarizing responses when the cells were adapted to distilled water, as in the present experiments. Most other studies of mammalian taste cells, however, have reported only depolarizing responses (Ozeki and Sato, 1972; Tonosaki and Funakoshi, 1984; Sato and Beidler, 1997).
Multiple gustatory sensitivities
The data presented here demonstrate that taste receptor cells are often responsive to stimuli representing more than one of the classic four taste qualities (sucrose, NaCl, HCl, and QHCl). Measures of the breadth of tuning show that taste receptor cells are slightly less broadly tuned to these stimuli than fibers of the chorda tympani nerve (Travers, 1993), suggesting some convergence onto first-order neurons. Furthermore, the number of cells responding to each of the six possible pairs of these four stimuli is predictable from an assumption of four sensitivities independently distributed across receptor cells. A similar result has been shown previously for the distribution of sensitivities across single fibers of the rat chorda tympani and glossopharyngeal nerves (Frank and Pfaffmann, 1969). An earlier intracellular recording experiment on rat taste cells (Ozeki and Sato, 1972) also found an independent distribution of sensitivities to sucrose, NaCl, HCl, and QHCl. Independence among sensitivities to several bitter stimuli was reported in a recent calcium imaging study of rat lingual slices (Caicedo and Roper, 2001). These previous data and the present results suggest strongly, on the basis of different recording methods, that taste sensitivities to stimuli representing the human qualities of sweet, salty, sour, and bitter are not restricted to separate, specifically tuned cell types.
This broad responsiveness could result from an overlap in the transduction mechanisms for different classes of stimuli within single receptor cells, as reported in hamster taste cells for sodium salts and acids, which both use the amiloride-sensitive Na+ channel (Gilbertson et al., 1992,1993). Alternatively, multiple receptors and transduction cascades could be present within a single cell (Herness and Gilbertson, 1999) or there could be some form of cell-to-cell communication within the taste bud (Roper, 1993). Recent data showing the coexpression of several members of a family of putative bitter taste receptors in single receptor cells (Adler et al., 2000; Chandrashekar et al., 2000) are not incompatible with the present results, which suggest that these same cells could possibly express other receptors as well. Further molecular studies should be able to provide definitive evidence for the origin of the multiple sensitivities shown in the present experiment. Although it is likely that testing these cells with additional stimuli and a broader range of concentrations would more clearly reveal the extent of this multiple sensitivity than can be seen with only four stimuli, the present results clearly show that these cells are, for the most part, not specific to a single stimulus.
We observed that cells exhibiting voltage-activated Na+ currents (13/62, 21%) were significantly more broadly tuned than those with only K+ currents. Since the generation of action potentials, which depend on voltage-activated Na+ channels, may be necessary for transmitter release (Roper, 1983; Avenet and Lindemann, 1989;Béhé et al., 1990b), it is likely that these broadly tuned cells are more mature than those without Na+ currents. In the mudpuppy, mature cells with apical processes reaching the taste pore show large inward and outward currents, whereas those that have not yet reached the pore have only outward currents (Mackay-Sim et al., 1996). Previous studies of isolated cells in the rat have also shown that only subsets of cells have inward currents, ranging from only 10% (Akabas et al., 1990) to 50–75% (Béhé et al., 1990b; Chen et al., 1996).
Information transmission
At first glance, an independent distribution of gustatory sensitivities seems counterintuitive. What possible advantage could there be to such an arrangement? One possibility lies in the greater capacity of such a system for transmitting information. A basic tenet of information theory is that multicomponent messages convey maximum information only when the individual components are independent (Shannon and Weaver, 1959). This means that sensory systems that encode information by the patterns of activity across broadly tuned neurons are inherently capable of transmitting more information than systems using specifically tuned cells (Pfaff, 1975). In general, greater information capacity means that finer discriminations can be made on the basis of sensory input. Even assuming only four taste qualities, the hundreds of potential gustatory stimuli would be composed of subtle combinations of these four. The known ability of rats to make behavioral discriminations between, for example, the taste of sucrose and maltose (Spector et al., 1997) or QHCl and KCl (St. John and Spector, 1998), depends on a system with subtle discriminatory capabilities. Thus, the independent distribution of taste sensitivities across receptor cells and the resulting broadly tuned afferent neurons provide the substrate for an across-neuron pattern code capable of relatively subtle behavioral discriminations (Pfaff, 1975).
An independent distribution of taste sensitivities raises interesting questions about the synaptic relationships between taste receptor cells and first-order neurons. Input from the taste receptors must generate a unique, recognizable pattern of activity in the CNS. However, taste receptor cells turn over with a life span of 9 or 10 d (Beidler and Smallman, 1965; Farbman, 1980), necessitating the continual formation of new synaptic connections between emerging receptor cells and afferent nerve fibers. To maintain a constant neural code for sensory quality, either the nerve fibers must impart the sensitivities to the developing receptor cells or they must seek out particular types of cells with which to make synaptic contact. Cross-reinnervation experiments, in which the IXth nerve is made to reinnervate the anterior tongue, demonstrate that neither the gustatory sensitivities nor the molecular phenotypes of taste cells in fungiform papillae are influenced by the innervating nerve (Oakley, 1967; Smith et al., 1999). In contrast, the several branches of a peripheral axon that innervate different fungiform papillae have been shown to have similar gustatory sensitivities (Oakley, 1975). Taken together, these data imply that gustatory afferent fibers are guided to particular taste receptor cells during cell turnover and synaptogenesis. A major challenge is to determine the molecular signals that underlie the anatomical relationships between taste receptor cells and their innervating axons.
Footnotes
This work was supported in part by National Institute on Deafness and Other Communication Disorders Grants DC00353 (D.V.S.) and DC02507 (T.A.G.). We thank W. Todd Monroe for help in the early phases of design and testing of the chamber used in this study and the expert technical assistance of Nikki D. Siears, Alicia Lumpkin, and Holly Lively.
Correspondence should be addressed to Dr. David V. Smith, Department of Anatomy and Neurobiology, University of Maryland School of Medicine, 685 West Baltimore Street, Baltimore, MD 21201-1509. E-mail:dvsmith@umaryland.edu.
T. A. Gilbertson's present address: Department of Biology, Utah State University, Logan, UT 84322-5305.
REFERENCES
- 1.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zuker CS. A novel family of mammalian taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 2.Akabas MH, Dodd J, Al-Awqati Q. A bitter substance induces a rise in intracellular calcium in a subpopulation of rat taste cells. Science. 1988;242:1047–1050. doi: 10.1126/science.3194756. [DOI] [PubMed] [Google Scholar]
- 3.Akabas MH, Dodd J, Al-Awqati Q. Identification of electrophysiologically distinct subpopulations of rat taste cells. J Membr Biol. 1990;114:71–78. doi: 10.1007/BF01869386. [DOI] [PubMed] [Google Scholar]
- 4.Avenet P, Lindemann B. Patch-clamp study of isolated taste receptor cells of the frog. J Membr Biol. 1987;97:223–240. doi: 10.1007/BF01869225. [DOI] [PubMed] [Google Scholar]
- 5.Avenet P, Lindemann B. Perspectives of taste reception. J Membrane Biol. 1989;112:1–8. doi: 10.1007/BF01871158. [DOI] [PubMed] [Google Scholar]
- 6.Béhé P, DeSimone JA, Avenet P, Lindemann B. Patch-clamp recording from taste buds of maintained epithelial polarity: a novel approach. In: Doving KB, editor. ISOT X. Proceedings of the Tenth International Symposium on Olfaction and Taste. Graphic Communication System A/S; Oslo: 1990a. p. 271. [Google Scholar]
- 7.Béhé P, DeSimone JA, Avenet P, Lindemann B. Membrane currents in taste cells of the rat fungiform papillae: evidence for two types of Ca currents and inhibition of K currents by saccharin. J Gen Physiol. 1990b;96:1061–1084. doi: 10.1085/jgp.96.5.1061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Beidler LM, Smallman R. Renewal of cells within taste buds. J Cell Biol. 1965;27:263–272. doi: 10.1083/jcb.27.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bieber SL, Smith DV. Multivariate analysis of sensory data: a comparison of methods. Chem Senses. 1986;11:19–47. [Google Scholar]
- 10.Boughter JD, Jr, Gilbertson TA. From channels to behavior: an integrative model of NaCl taste. Neuron. 1999;22:213–215. doi: 10.1016/s0896-6273(00)81082-x. [DOI] [PubMed] [Google Scholar]
- 11.Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chandrashekar J, Mueller KL, Hoon MA, Adler E, Feng L, Guo W, Zuker CS, Ryba JP. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 13.Chang F-CT, Scott TR. Conditioned taste aversions modify neural responses in the rat nucleus tractus solitarius. J Neurosci. 1984;4:1850–1862. doi: 10.1523/JNEUROSCI.04-07-01850.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Y, Herness MS. Electrophysiological actions of quinine on voltage-dependent currents in dissociated rat taste cells. Pflügers Arch. 1997;434:215–226. doi: 10.1007/s004240050388. [DOI] [PubMed] [Google Scholar]
- 15.Chen Y, Sun X-D, Herness MS. Characteristics of action potentials and their underlying outward currents in rat taste receptor cells. J Neurophysiol. 1996;75:820–831. doi: 10.1152/jn.1996.75.2.820. [DOI] [PubMed] [Google Scholar]
- 16.Contreras R, Frank M. Sodium deprivation alters neural responses to gustatory stimuli. J Gen Physiol. 1979;73:569–594. doi: 10.1085/jgp.73.5.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cummings TA, Daniels C, Kinnamon SC. Sweet taste transduction in hamster: sweeteners and cyclic nucleotides depolarize taste cells by reducing a K+ current. J Neurophysiol. 1996;75:1256–1263. doi: 10.1152/jn.1996.75.3.1256. [DOI] [PubMed] [Google Scholar]
- 18.Doetsch GS, Erickson RP. Synaptic processing of taste-quality information in the nucleus tractus solitarius of the rat. J Neurophysiol. 1970;23:490–507. doi: 10.1152/jn.1970.33.4.490. [DOI] [PubMed] [Google Scholar]
- 19.Erickson RP. Stimulus coding in topographic and non-topographic afferent modalities: on the significance of the activity of individual sensory neurons. Psychol Rev. 1968;75:447–465. doi: 10.1037/h0026752. [DOI] [PubMed] [Google Scholar]
- 20.Erickson RP, Doetsch GS, Marshall DA. The gustatory neural response function. J Gen Physiol. 1965;49:247–263. doi: 10.1085/jgp.49.2.247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Everitt B. Cluster analysis. Halsted; New York: 1980. [Google Scholar]
- 22.Farbman AI. Renewal of taste bud cells in rat circumvallate papillae. Cell Tissue Kinet. 1980;13:349–357. doi: 10.1111/j.1365-2184.1980.tb00474.x. [DOI] [PubMed] [Google Scholar]
- 23.Frank M, Pfaffmann C. Taste nerve fibers: a random distribution of sensitivities to four tastes. Science. 1969;164:1183–1185. doi: 10.1126/science.164.3884.1183. [DOI] [PubMed] [Google Scholar]
- 24.Frank ME, Contreras RJ, Hettinger TP. Nerve fiber sensitivities to ionic taste stimuli in chorda tympani of the rat. J Neurophysiol. 1983;50:941–960. doi: 10.1152/jn.1983.50.4.941. [DOI] [PubMed] [Google Scholar]
- 25.Frank ME, Bieber SL, Smith DV. The organization of taste sensibilities in hamster chorda tympani nerve fibers. J Gen Physiol. 1988;91:861–896. doi: 10.1085/jgp.91.6.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Furue H, Yoshii K. In situ tight-seal recordings of taste substance-elicited action currents and voltage-gated Ba currents from single taste bud cells in the peeled epithelium of mouse tongue. Brain Res. 1997;776:133–139. doi: 10.1016/s0006-8993(97)00974-8. [DOI] [PubMed] [Google Scholar]
- 27.Furue H, Yoshii K. A method for in-situ tight-seal recordings from single taste bud cells of mice. J Neurosci Methods. 1998;84:109–114. doi: 10.1016/s0165-0270(98)00104-6. [DOI] [PubMed] [Google Scholar]
- 28.Gilbertson TA. Patch-clamping of taste cells in hamster and rat. In: Spielman AI, Brand JG, editors. Experimental cell biology of taste and olfaction: current techniques and protocols. CRC; Boca Raton, FL: 1995. pp. 317–328. [Google Scholar]
- 29.Gilbertson TA, Zhang H. Characterization of sodium transport in gustatory epithelia from the hamster and rat. Chem Senses. 1998;23:283–293. doi: 10.1093/chemse/23.3.283. [DOI] [PubMed] [Google Scholar]
- 30.Gilbertson TA, Avenet P, Kinnamon SC, Roper SD. Proton currents through amiloride-sensitive Na+ channels in hamster taste cells: role in acid transduction. J Gen Physiol. 1992;100:803–824. doi: 10.1085/jgp.100.5.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gilbertson TA, Roper SD, Kinnamon SC. Proton currents through amiloride-sensitive Na+ channels in isolated hamster taste cells: enhancement by vasopressin and cAMP. Neuron. 1993;10:931–942. doi: 10.1016/0896-6273(93)90208-9. [DOI] [PubMed] [Google Scholar]
- 32.Gilbertson TA, Fontenot DT, Zhang H, Liu L, Monroe WT. Fatty acid modulation of K+ channels in taste receptor cells: gustatory cues for dietary fat. Am J Physiol. 1997;272:C1203–C1210. doi: 10.1152/ajpcell.1997.272.4.C1203. [DOI] [PubMed] [Google Scholar]
- 33.Gilbertson TA, Zhang H, Boughter JD, Jr, Smith DV. Multiple sensitivity of rat fungiform taste cells: whole cell responses to apical chemical stimulation. Chem Senses. 1999;24:569. [Google Scholar]
- 34.Giza BK, Scott TR. The effect of amiloride on taste-evoked activity in the nucleus tractus solitarius of the rat. Brain Res. 1991;550:247–256. doi: 10.1016/0006-8993(91)91325-u. [DOI] [PubMed] [Google Scholar]
- 35.Hamill OP, Marty A, Neher E, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflügers Arch. 1981;391:561–577. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 36.Harris DE, Gilbertson DM, Monroe WT, Kinnamon SC, Gilbertson TA. Contribution of amiloride-sensitive pathways to acid transduction in rats. Chem Senses. 1994;19:481–482. [Google Scholar]
- 37.Herness MS, Gilbertson TA. Cellular mechanisms of taste transduction. Annu Rev Physiol. 1999;61:873–900. doi: 10.1146/annurev.physiol.61.1.873. [DOI] [PubMed] [Google Scholar]
- 38.Herness MS, Sun X-D. Voltage-dependent sodium currents recorded from dissociated rat taste cells. J Membr Biol. 1995;146:73–84. doi: 10.1007/BF00232681. [DOI] [PubMed] [Google Scholar]
- 39.Kimura K, Beidler LM. Microelectrode study of taste receptor of rat and hamster. J Cell Comp Physiol. 1961;58:131–140. doi: 10.1002/jcp.1030580204. [DOI] [PubMed] [Google Scholar]
- 40.Kinnamon S. Taste transduction: a diversity of mechanisms. Trends Neurosci. 1988;11:491–496. doi: 10.1016/0166-2236(88)90010-0. [DOI] [PubMed] [Google Scholar]
- 41.Kinnamon SC, Dionne VE, Beam KG. Apical localization of K+ channels in taste cells provides the basis for sour transduction. Proc Natl Acad Sci USA. 1988;85:7023–7027. doi: 10.1073/pnas.85.18.7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lindemann B. Taste reception. Physiol Rev. 1996;76:719–766. doi: 10.1152/physrev.1996.76.3.719. [DOI] [PubMed] [Google Scholar]
- 43.Mackay-Sim A, Delay RJ, Roper SD, Kinnamon SC. Development of voltage-dependent currents in taste receptor cells. J Comp Neurol. 1996;365:278–288. doi: 10.1002/(SICI)1096-9861(19960205)365:2<278::AID-CNE6>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 44.Miyamoto T, Fujiyama R, Okada Y, Sato T. Sour transduction involves activation of NPPB-sensitive conductance in mouse taste cells. J Neurophysiol. 1998;80:1852–1859. doi: 10.1152/jn.1998.80.4.1852. [DOI] [PubMed] [Google Scholar]
- 45.Monroe WT, Smith DV, Gilbertson TA. The MU chamber: a new method to record electrophysiological responses of taste receptor cells to gustatory stimuli. Chem Senses. 1996;21:644–645. [Google Scholar]
- 46.Ninomiya Y, Funakoshi M. Amiloride inhibition of responses of rat single chorda tympani fibers to chemical and electrical tongue stimulations. Brain Res. 1988;451:319–325. doi: 10.1016/0006-8993(88)90777-9. [DOI] [PubMed] [Google Scholar]
- 47.Oakley B. Altered taste responses from cross-regenerated taste nerves in the rat. In: Hayashi T, editor. Olfaction and taste II. Pergamon; London: 1967. pp. 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oakley B. Receptive fields of cat taste fibers. Chem Senses Flav. 1975;1:431–442. [Google Scholar]
- 49.Ogawa H, Sato M, Yamashita S. Multiple sensitivity of chorda tympani fibres of the rat and hamster to gustatory and thermal stimuli. J Physiol (Lond) 1968;199:223–240. doi: 10.1113/jphysiol.1968.sp008650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ohtubo Y, Suemitsu T, Shobara S, Matsumoto T, Kumazawa T, Yoshii K. Optical recordings of taste responses from fungiform papillae of mouse in situ. J Physiol (Lond) 2001;530:287–293. doi: 10.1111/j.1469-7793.2001.0287l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ozeki M, Sato M. Responses of gustatory cells in the tongue of rat to stimuli representing four taste qualities. Comp Biochem Physiol [A] 1972;41:391–407. doi: 10.1016/0300-9629(72)90070-9. [DOI] [PubMed] [Google Scholar]
- 52.Pfaff DW. Theoretical consideration of cross-fiber pattern coding in the neural signalling of pheromones and other chemical stimuli. Psychoneuroendocrinology. 1975;1:79–93. [Google Scholar]
- 53.Pfaffmann C. Gustatory nerve impulses in rat, cat and rabbit. J Neurophysiol. 1955;18:429–440. doi: 10.1152/jn.1955.18.5.429. [DOI] [PubMed] [Google Scholar]
- 54.Pfaffmann C. The afferent code for sensory quality. Am Psychol. 1959;14:226–232. [Google Scholar]
- 55.Roper SD. Regenerative impulses in taste cells. Science. 1983;220:1311–1312. doi: 10.1126/science.6857254. [DOI] [PubMed] [Google Scholar]
- 56.Roper SD. Synaptic interactions in taste buds. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. CRC; Boca Raton, FL: 1993. pp. 275–293. [Google Scholar]
- 57.Roper SD, McBride DW., Jr The distribution of ion channels on taste cells and its relationship to chemosensory transduction. J Membr Biol. 1989;109:29–39. doi: 10.1007/BF01870788. [DOI] [PubMed] [Google Scholar]
- 58.Sato T, Beidler LM. The response characteristics of rat taste cells to four basic taste stimuli. Comp Biochem Physiol A. 1982;73:1–10. doi: 10.1016/0300-9629(82)90083-4. [DOI] [PubMed] [Google Scholar]
- 59.Sato T, Beidler LM. Broad tuning of rat taste cells to four basic taste stimuli. Chem Senses. 1997;22:287–293. doi: 10.1093/chemse/22.3.287. [DOI] [PubMed] [Google Scholar]
- 60.Seto E, Hayashi Y, Mori T. Patch clamp recording of the responses to three bitter stimuli in mouse taste cells. Cell Mol Biol. 1999;45:317–325. [PubMed] [Google Scholar]
- 61.Shannon CE, Weaver W. The mathematical theory of communication. University of Illinois; Urbana: 1959. [Google Scholar]
- 62.Smith DV, Frank ME. Sensory coding by peripheral taste fibers. In: Simon SA, Roper SD, editors. Mechanisms of Taste Transduction. CRC; Boca Raton, FL: 1993. pp. 295–338. [Google Scholar]
- 63.Smith DV, St John SJ. Neural coding of gustatory information. Curr Opin Neurobiol. 1999;9:427–435. doi: 10.1016/S0959-4388(99)80064-6. [DOI] [PubMed] [Google Scholar]
- 64.Smith DV, Travers JB. A metric for the breadth of tuning of gustatory neurons. Chem Senses Flav. 1979;4:215–229. [Google Scholar]
- 65.Smith DV, Van Buskirk RL, Travers JB, Bieber SL. Coding of taste stimuli by hamster brainstem neurons. J Neurophysiol. 1983;50:541–558. doi: 10.1152/jn.1983.50.2.541. [DOI] [PubMed] [Google Scholar]
- 66.Smith DV, Som J, Boughter JD, St. John SJ, Jr, Yu C, Christy RC. Cellular expression of α-gustducin and the A blood group antigen in rat fungiform taste buds cross-reinnervated by the IXth nerve. J Comp Neurol. 1999;409:118–130. doi: 10.1002/(sici)1096-9861(19990621)409:1<118::aid-cne9>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- 67.Smith DV, Zhang H, Boughter JD, Jr, St. John SJ, Gilbertson TA. Random distribution of gustatory sensitivities across rat taste receptor cells and brainstem neurons. Chem Senses. 2000;25:661. [Google Scholar]
- 68.Spector AC, Markison S, St. John SJ, Garcea M. Sucrose vs. maltose taste discrimination by rats depends on the input of the seventh cranial nerve. Am J Physiol. 1997;272:R1210–R1218. doi: 10.1152/ajpregu.1997.272.4.R1210. [DOI] [PubMed] [Google Scholar]
- 69.St. John SJ, Spector AC. Behavioral discrimination between quinine and KCl is dependent on input from the seventh cranial nerve: implications for the functional roles of the gustatory nerves in rats. J Neurosci. 1998;18:4353–4362. doi: 10.1523/JNEUROSCI.18-11-04353.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sweazey RD, Smith DV. Convergence onto hamster medullary taste neurons. Brain Res. 1987;408:173–184. doi: 10.1016/0006-8993(87)90369-6. [DOI] [PubMed] [Google Scholar]
- 71.Tonosaki K, Funakoshi M. Intracellular taste cell responses of mouse. Comp Biochem Physiol [A] 1984;78:651–656. doi: 10.1016/0300-9629(84)90611-x. [DOI] [PubMed] [Google Scholar]
- 72.Travers JB, Smith DV. Gustatory sensitivities in neurons of the hamster nucleus tractus solitarius. Sens Processes. 1979;3:1–26. [PubMed] [Google Scholar]
- 73.Travers SP. Orosensory processing in neural systems of the nucleus of the solitary tract. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. CRC; Boca Raton, FL: 1993. pp. 339–394. [Google Scholar]
- 74.Van Buskirk RL, Smith DV. Taste sensitivity of hamster parabrachial pontine neurons. J Neurophysiol. 1981;45:144–171. doi: 10.1152/jn.1981.45.1.144. [DOI] [PubMed] [Google Scholar]
- 75.Yamamoto T, Yuyama N, Kato T, Kawamura Y. Gustatory responses of cortical neurons in rats. I. Response characteristics. J Neurophysiol. 1984;51:616–635. doi: 10.1152/jn.1984.51.4.616. [DOI] [PubMed] [Google Scholar]
- 76.Yan W, Rosenzweig S, Brand JG, Spielman AI. Bitter taste transduction uses two second messenger systems. Chem Senses. 2000;25:687–688. [Google Scholar]