Abstract
Agonist-induced internalization of G-protein-coupled receptors is an important mechanism for regulating receptor abundance and availability at the plasma membrane. In this study we have used immunolabeling techniques and confocal microscopy to investigate agonist-induced internalization and trafficking of CB1receptors in rat cultured hippocampal neurons. The levels of cell surface CB1 receptor immunoreactivity associated with presynaptic GABAergic terminals decreased markedly (by up to 84%) after exposure to the cannabinoid agonist (+)-WIN55212, in a concentration-dependent (0.1–1 μm) and stereoselective manner. Inhibition was maximal at 16 hr and abolished in the presence of SR141716A, a selective CB1 receptor antagonist. Methanandamide (an analog of an endogenous cannabinoid, anandamide) also reduced cell surface labeling (by 43% at 1 μm). Differential labeling of cell surface and intracellular pools of receptor demonstrated that the reduction in cell surface immunoreactivity reflects agonist-induced internalization and suggests that the internalized CB1 receptors are translocated toward the soma. The internalization process did not require activated G-protein α(i) or α(o) subunits. A different pattern of cell surface CB1 receptor expression was observed using an undifferentiated F-11 cell line, which had pronounced somatic labeling. In these cells substantial CB1 receptor internalization was also observed after exposure to (+)-WIN55212 (1 μm) for relatively short periods (30 min) of agonist exposure. In summary, this dynamic modulation of CB1 receptor expression may play an important role in the development of cannabinoid tolerance in the CNS. Agonist-induced internalization at presynaptic terminals has important implications for the modulatory effects of G-protein-coupled receptors on neurotransmitter release.
Keywords: internalization, cannabinoid, receptor trafficking, CB1, hippocampal, F-11
The effects of the major psychoactive constituent of cannabis, Δ9-tetrahydrocannabinol, are mediated by the CB1 subtype of cannabinoid receptor (Devane et al., 1992: Howlett, 1995), which is widely distributed throughout the CNS. High levels of CB1 receptor expression are found in the hippocampus, rivaling that of the classical neurotransmitters (Herkenham, 1992; Matsuda et al., 1993; Gatley et al., 1998;Tsou et al., 1998). The hippocampus also contains the highest levels of a putative endogenous ligand, arachidonoyl ethanolamide (anandamide;Felder et al., 1996). At the cellular level, CB1receptors are expressed on fine caliber axonal processes of cholecystokinin-containing neurons (Tsou et al., 1998; Katona et al., 1999) and are predominantly associated with GABAergic synaptic terminals (Katona et al., 1999; Hájos et al., 2000; Hoffman and Lupica, 2000; Irving et al., 2000).
Recent evidence suggests that the CB1 receptor, like many, but not all, G-protein-coupled, seven-transmembrane receptors, undergoes agonist-induced endocytosis (Garland et al., 1996;Roth et al., 1997; Zhang et al., 1997; Dumartin et al., 1998;Rinaldi-Carmona et al., 1998; Southwell et al., 1998; Doherty et al., 1999; Hsieh et al., 1999; Whistler et al., 1999). This process affects receptor abundance and availability and consequently the ability of agonists to generate an effective response. Receptor internalization also plays an important role in the processes of resensitization after prolonged agonist exposure (Garland et al., 1996; Zhang et al., 1997) and influences coupling to intracellular signaling pathways (Roche et al., 1999). Previous investigations of CB1receptor internalization have used transfected Chinese hamster ovary (CHO) or AtT20 cells (Rinaldi-Carmona et al., 1998; Hsieh et al., 1999), preparations that readily allow the visualization of changes in cellular localization with regard to the plasma membrane and cytoplasm. However, these cell lines may lack components in their signaling systems that affect the efficiency of the endocytotic process compared with native cells (Koenig and Edwardson, 1996). Thus, it is important both to demonstrate that these processes reflect events in native cells and to study the receptors at sites where they may exert a physiological role. However, in neurons it is more difficult to directly visualize receptor internalization, especially where the receptors are expressed on fine neurites or synaptic terminals.
In the present investigation laser-scanning confocal microscopy combined with the immunocytochemical labeling of a cell surface CB1 receptor epitope (Irving et al., 2000) was used to study the localization and endocytosis of CB1 receptors in cultured hippocampal neurons. Marked changes in the surface expression of CB1receptors after pre-exposure to cannabinoid agonists were observed. A new primary antibody prelabeling protocol demonstrated that this reflected agonist-induced internalization and suggest that the internalized receptors undergo retrograde translocation from axons toward somatodendritic regions. This protocol was also used to compare the CB1 receptor internalization process in a dorsal root ganglion (DRG) X mouse neuroblastoma hybrid cell line (F-11 cells), which are shown to naturally express CB1 receptors on their somata. These data suggest that the dynamic modulation of CB1 receptor expression could play an important role in the development of tolerance toward cannabinoids in the CNS.
MATERIALS AND METHODS
Materials. Triton X-100, paraformaldehyde, dialyzed fetal bovine serum, HEPES, protease type X and type XIV,l-glutamine, poly-d-lysine, cytosine arabinofuranoside, penicillin, EDTA, benzamidine, leupeptin, streptomycin, and nonenzymatic dissociation medium were obtained from Sigma-Aldrich (Dorset, UK). Minimal essential medium (MEM), fetal bovine serum (HyClone, Logan, UT), and HAT (100 μm hypoxanthine, 400 nmaminopterin, and 16 μm thymidine) supplement were from Life Technologies (Paisley, UK) and (R)-(+)-[2,3-dihydro-5-methyl-3-[(4-morpholino) methyl] pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthyl) methanone}((+)WIN55212), (R)-(−)-[2,3-dihydro-5-methyl-3-[(4-morpholino) methyl]pyrrolo-[1,2,3-de]-1,4-benzoxazin-6-yl](1-naphthyl) methanone} ((−)-WIN55212) and (R-(+)-arachidonoyl-1′-hydroxy-2′-propylamide (methanandamide) were from Research Biochemicals International (Hertfordshire, UK). Tris buffer came from Boehringer Mannheim (Lewes, East Sussex, UK).N-(piperidin-1-yl)-5-(4-chlorophenyl)-1-(2,4-dichlorophenyl)-4-methyl-1H-pyrazole-3-carboxamide hydrochloride (SR141716A) was gift from Sanofi Recherché(Montpellier, France). F-11 cells were purchased from Dr. Mark C. Fishman (Massachusetts General Hospital, Boston, MA). Stock solutions of cannabinoids and related compounds were made up in ethanol and kept at −20°C except for SR141716A stock solution, which was kept at 4°C.
Polyclonal and monoclonal antibodies. Rabbit polyclonal antibody raised against the N terminus (1–77 amino acid residues) of the cloned rat CB1 receptor was produced and characterized as described previously (Tsou et al., 1998; Katona et al., 1999). CB1 receptor (1–14 amino acid residues) polyclonal antiserum was supplied by Cayman Chemical (Ann Arbor, MI) and has also been extensively characterized (Howlett et al., 1998; McIntosh et al., 1998). Both N-terminal CB1receptor antibodies produced identical patterns of labeling. Mouse monoclonal anti-glutamic acid decarboxylase (GAD) antibody (clone 65) came from Boehringer Mannheim. Cy3-conjugated goat anti-rabbit and Cy5-conjugated goat anti-mouse secondary antibodies were obtained from Jackson ImmunoResearch (West Grove, PA). The Alexa 488 goat anti-mouse and Alexa 488 goat anti-rabbit secondary antibodies were obtained from Molecular Probes, Europe BV (Leiden, The Netherlands). Biotinylated anti-rabbit serum and streptavidin HRP-conjugated secondary antibodies were obtained from the Scottish Antibody Production Unit. For confocal microscopy studies, CB1 receptor antibodies were used at a final concentration of 1–10 μg/ml. Other antibodies were used at final concentrations of 2–6 μg/ml. In control experiments, immunostaining was blocked when either CB1 receptor antibody (1–14 and 1–77) was incubated with fusion protein for the 1–77 CB1 receptor epitope (100 μg/ml) for 1 hr before treatment with antibody. For immunoblots, CB1 receptor antibody (1–14) was used at a final concentration of 20 μg/ml.
Cell culture. Cultures of rat hippocampal neurons were prepared from neonatal Sprague Dawley rats as described previously (Irving et al., 2000). All efforts were made to minimize animal suffering and to keep the number of animals used to a minimum. Briefly, rat pups (1- to 3-d-old) were killed by cervical dislocation. The hippocampi were then removed, chopped, and treated with enzymes (protease types X and XIV, both at 0.5 mg/ml) for 40–50 min. The washed tissue was dissociated by trituration, centrifuged, and plated onto coverslips or plastic culture dishes (35 mm) that had been pretreated with poly d-lysine (0.01 mg/ml). Cultures were then incubated in a medium consisting of 90% MEM, supplemented with 10% dialyzed fetal bovine serum and 2 mml-glutamine and maintained in a humidified atmosphere of 5% CO2in air at 37°C. After 2–5 d cytosine arabinofuranoside (5 μm) was added to inhibit glial cell proliferation. Cells were described as mature after 6 d in culture. F-11 cells (a mouse N18TG2 neuroblastoma X rat dorsal root ganglion sensory neuron hybrid cell line; Platika et al., 1985) were grown either as monolayers in 75 cm2flasks (stock) or on glass coverslips in 35 mm dishes (for experiments). The cell culture medium was Ham's F-12 containing 2 mml-glutamine supplemented with 15% Hyclone fetal bovine serum, HAT supplement, 100 U/ml penicillin, and 100 μg/ml streptomycin. Cells were kept under 5% CO2 in air at 37°C and passaged twice per week using nonenzymatic cell dissociation solution. Passage numbers P1–P10 of undifferentiated cells were used for experiments.
Fluorescence procedures for laser-scanning confocal microscopy. Internalization of CB1 receptors was studied using two methods: (1) loss of cell surface immunoreactivity, with labeling performed after agonist pretreatment. Cells were incubated with cannabinoids and related compounds at 37°C in culture medium for varying periods of time. Cells were then transferred into HEPES-buffered saline (HBS), comprising (in mm): NaCl 130, d-glucose 25, HEPES 10, KCl 5.4, CaCl2 1.8, and MgCl2 1, pH 7.4, at room temperature and incubated for 40–60 min with CB1 receptor antibody. To minimize antibody capping, cells were fixed with either 4% paraformaldehyde for 10 min at room temperature or methanol for 5 min at −20°C before treatment with secondary antibody. Cell surface CB1receptor immunoreactivity was fluorescently labeled using a Cy3-conjugated goat anti-rabbit secondary antibody (40 min incubation). Because CB1receptors are expressed at the majority of GABAergic terminals (∼80%; Irving et al., 2000), CB1 receptor immunoreactivity was also compared with GAD labeling. Cells were permeabilized with 0.1% Triton X-100 (5 min) and then incubated with a mouse monoclonal antibody against GAD (60 min) followed by an Alexa 488-conjugated goat anti-mouse secondary antibody (40 min). In control experiments with nonpermeabilized cells, no detectable trapping of the mouse primary or secondary antibodies by CB1receptor immunolabeling was observed. Where necessary, with fixed and permeabilized cells, nonspecific antibody binding was blocked by incubation with goat serum or 10% fat-free milk protein. Internalization of CB1 receptors was also studied using: (2) effects on the cellular distribution of CB1 receptors, with cell surface receptors prelabeled with primary antibody before agonist exposure. After treatment with cannabinoids, exposure of living cells to a Cy3-conjugated anti-rabbit secondary antibody allowed the identification of primary antibody-labeled receptors that remained on the neuronal cell surface. After fixation and permeabilization of these cells, treatment with Alexa 488-conjugated anti-rabbit secondary antibody identified those receptors that had undergone internalization. Minimal trapping of Alexa 488-conjugated secondary antibody by cell surface CB1 receptor antibody/Cy3-conjugated secondary antibody was observed. Moreover, sites that expressed Alexa 488 labeling alone must reflect internalization of primary antibody-conjugated CB1 receptors. Although some antibody-induced clustering of cell surface receptors was observed with this protocol, the overall pattern of labeling was not affected.
Image acquisition and processing. A laser-scanning confocal imaging system, [MRC 1024, Bio-Rad (Hercules, CA) or MicroRadiance] and Olympus Optical (Tokyo, Japan) BX50WI microscope (60× objective) were used for image acquisition and processing. Cy3 was excited with a dedicated 543 nm line, and emitted light passed through an E570LP filter, whereas Alexa 488 was excited with a 488 nm line, and emitted light passed through an HQ515/30 filter. Images were obtained by Kalman averaging of seven individual scans, and in multiple-labeling experiments images were obtained sequentially and merged off-line. Lasersharp image-processing software (Bio-Rad) was used to determine labeling intensity. For quantification of immunolabeling the mean fiber fluorescence intensity level was measured. For each image, mean background intensity levels from three randomly selected regions were measured, and the average background intensity was determined. All pixels with intensity levels above this background were defined as specific labeling. Labeling intensity was determined from a minimum of nine randomly selected fibers, from three experiments, that exhibited both CB1 receptor and GAD immunostaining. In experiments where exposure to (+)-WIN55212 resulted in no detectable CB1 receptor staining, GAD immunoreactivity alone was used as a basis for the selection of fibers for analysis.N values refer to the number of fibers analyzed. In each experiment, the corrected mean fiber fluorescence intensity level determined after drug pretreatment was compared with that measured after pretreatment either with (−)-WIN55212, the inactive isomer of (+)-WIN55212, or vehicle alone. Pretreatment of neurons with (−)-WIN55212 had no significant effect by itself (see Results). To allow for the comparison of different experiments, data were normalized relative to the mean fiber fluorescence intensity level observed with (−)-WIN55212 or vehicle.
Quantification of internalized CB1receptors in F-11 cells. The proportion of CB1 receptor fluorescence on the surface and within F-11 cells was quantified with NIH Image software using a modification of a method previously described (Southwell et al., 1998). Kalman-averaged confocal images (seven scans; single Z-plane) of cells were obtained from at least three different experiments. In each experiment, the mean background intensity of fluorescence for each secondary antibody was determined from two cell-free areas in each image. A line was drawn round the outer surface of the cell membrane, and the total cell fluorescence (mean intensity per unit area × area) was determined for each secondary antibody and corrected for background labeling. A second concentric line was drawn along the intracellular side of the membrane, and the intracellular fluorescence was determined. A value for surface labeling alone was calculated as the difference between the total cell fluorescence and the intracellular fluorescence for each secondary antibody.
Immunoblots. Mature hippocampal cells were exposed to (+)WIN55212 (1 μm) or vehicle at 37°C for 16 hr. After incubation, cells were washed with cold glucose-free HBS and harvested with 2 ml of ice-cold homogenization buffer (1 mm EDTA, 5 mm Tris–HCl, pH 7.4, 0.25 m sucrose, 100 μm PMSF, 100 μmbenzamidin, and 10 μm leupeptin). Cells were homogenized with an ice-cold hand-held Teflon-on-glass homogenizer (60 strokes), and the homogenate was centrifuged at 100,000 ×g for 20 min at 4°C. The membrane pellet was resuspended in homogenization buffer, and the protein concentration was measured. Equivalent amounts of protein were incubated with 2% SDS supplemented with loading buffer (60% glycerol, 12.5% β-mercaptoethanol, and 1% bromophenyl blue) and boiled for 10 min to denature proteins and nucleic acids. Boiled samples were separated by SDS-PAGE (10% w/v acrylamide). Protein (40 μg) from the membrane fraction was electrophoresed and transferred to nitrocellulose membrane overnight, then CB1 receptor protein was identified on immunoblots that were blocked with 5% nonfat dried milk in PBS plus 0.1% Tween 80, and reacted with rabbit anti-CB1 receptor antibody (20 μg/ml in PBS plus 0.1% Tween 20) for 4 hr. Blots were then incubated with biotinylated anti-rabbit serum and streptavidin HRP-conjugated secondary antibodies (1:7000 in PBS plus 0.1% Tween 80, 1 hr in each). Color visualization of antisera-specific bands was performed by incubating the immunoblots in o-dianisidine (0.25 mg/ml) and 30% H2O2 solution (0.25 μl/ml) in PBS.
Data analysis. Values are expressed as means and variability as SEM. Comparisons between pairs of treatments were determined using an unpaired Student's t test. Multiple treatments were compared by ANOVA with one-way ANOVA followed by Newman–Keuls analysis (GraphPad Prism). p values < 0.05 were considered to be significant.
RESULTS
Visualization of cell surface CB1receptor immunoreactivity
CB1 receptor immunoreactivity was detected on the surface of living hippocampal neurons with the N-terminal polyclonal antibody. Punctate CB1 receptor labeling was observed on fine axons and axonal growth cones, but was absent from somata, as described previously (Irving et al. 2000) (Fig.1A,B). However, when the cultures were fixed and permeabilized before labeling with primary antibody, a small proportion of neurons (10–20%) displayed considerable CB1 receptor immunoreactivity associated with putative intracellular sites, including the soma (Fig.1C,D). This pattern of labeling presumably reflects newly synthesized or recycled CB1 receptors (McIntosh et al., 1998; Katona et al., 1999). As with our previous investigation, there was a marked correspondence between CB1receptor (cell surface) and GAD immunolabeling (Fig.1E; Irving et al., 2000). Detailed anatomical and functional studies have shown that this distribution reflects presynaptic CB1 receptor clusters expressed on GABAergic terminals (Katona et al., 1999; Hájos et al., 2000;Hoffman and Lupica, 2000; Irving et al., 2000).
Fig. 1.
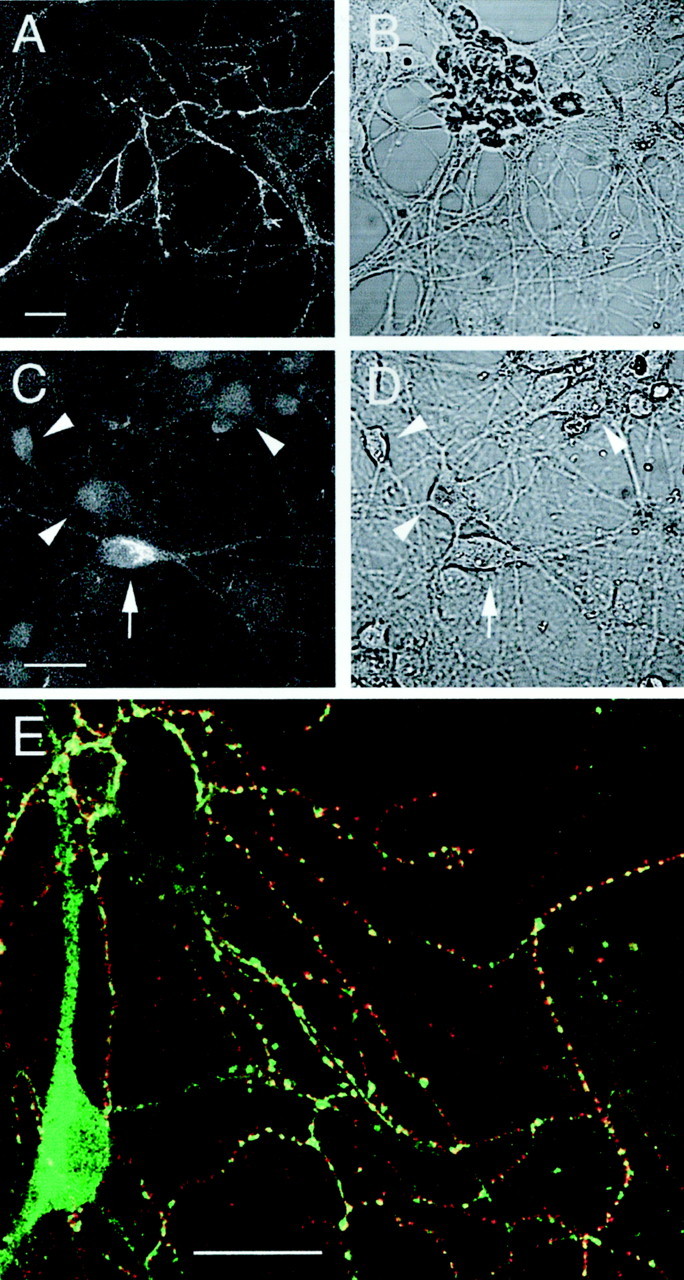
CB1 receptor immunoreactivity in cultured hippocampal neurons. Representative images depicting cell surface labeling of intact cells showing immunoreactivity associated with a network of fine fibers (A) and total labeling after fixation and permeabilization (C).B and D are corresponding bright-field images. Note the strong intracellular immunoreactivity associated with a neuronal somata (arrow). Neurons lacking somatic labeling are also indicated (arrowheads). Immunofluorescence images A and C arez projections of a series of confocal sections taken at 1–2 μm intervals. E shows merged, single plane confocal images from a dual-labeling experiment investigating the relationship between cell surface CB1 receptor clusters and inhibitory terminals, labeled with a monoclonal GAD antibody after permeabilization. Red corresponds to CB1receptor label (Cy3), green to GAD label (Alexa 488), and yellow to regions of overlap. Note the marked correspondence between CB1 receptor label and clusters of GAD immunoreactivity. Scale bars, 20 μm.
Agonist-induced loss of cell surface labeling
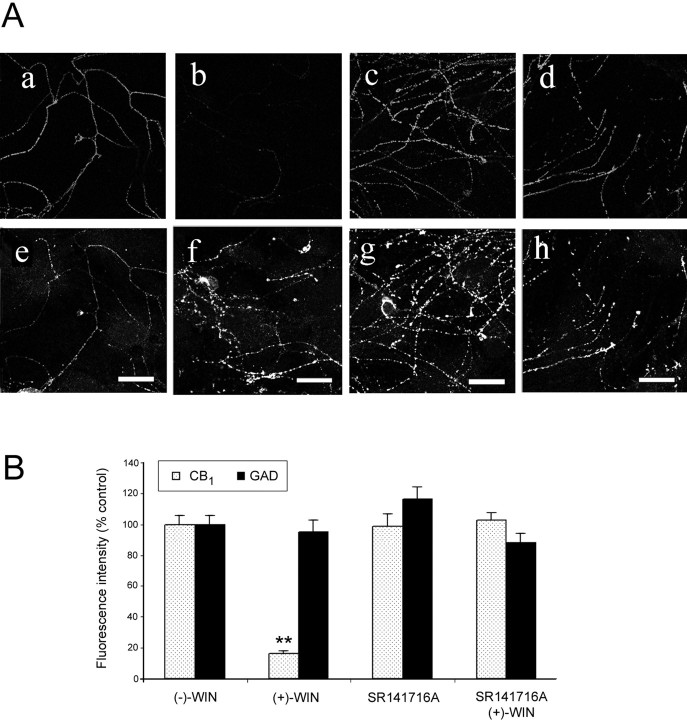
The effects of cannabinoid pretreatment on cell surface CB1 receptor immunoreactivity was investigated. (+)-WIN55212 is a potent synthetic cannabinoid receptor agonist, whereas its enantiomer, (−)-WIN55212 is inactive at CB1 receptors (Coutts and Pertwee, 1997; Pertwee, 1997). Mature hippocampal cells (6–18 d in culture) were kept for 16 hr in culture medium at 37°C containing either (+)-WIN55212, (−)-WIN55212, the CB1 receptor-selective antagonist, SR141716A, or (+)-WIN55212 in the presence of SR141716A, before the level of surface CB1 receptor immunoreactivity was measured. In these experiments, cells were colabeled with an antibody raised against GAD (Irving et al., 2000) to determine whether the cannabinoid pretreatment was selective for CB1 receptor immunostaining. In control experiments (data not shown), there was no significant effect of (−)-WIN55212 (1 μm) on the intensity of cell surface CB1 receptor labeling relative to vehicle controls (p > 0.05; n = 24 fibers). However, labeling was markedly reduced (by 84 ± 2% %) after incubation with (+)-WIN55212 (1 μm) compared with (−)-WIN55212 (1 μm). This effect was prevented by coincubation of the cells with the antagonist SR141716A (1 μm), which had no significant effect by itself. GAD immunostaining was not affected by pretreatment with cannabinoids. Images from these experiments and quantitative data, where the concentration of cannabinoids and related compounds was 1 μm, are summarized in Figure2. Similar observations were made with (+)-WIN55212 at 100 nm, however the reduction in cell surface labeling was less (Fig.3A).
Fig. 2.
The effects of cannabinoid pretreatment on cell surface CB1 receptor labeling. A, Immunolabeling of cells pretreated for 16 hr with (−)-WIN55212 [(−)-WIN; a, e], (+)-WIN55212[(+)-WIN; b, f], (+)-WIN55212 with SR141716A (c, g), and SR141716A alone (d, h), all at 1 μm. Representative confocal images (single section) of CB1 receptor (a–d) and corresponding GAD (e–h) immunolabeling are depicted for each treatment. Scale bars, 20 μm.B, Quantitative histogram showing the effects of cannabinoid pretreatment on CB1 receptor labeling. Values are mean ± SEM of normalized relative to control fluorescence intensities obtained with (−)-WIN55212. For each paradigm 27 fibers from a minimum of three independent experiments were analyzed (**p < 0.01). Note that (+)-WIN55212 markedly inhibited the labeling of CB1 receptor immunoreactivity in the absence, but not in the presence, of SR141716A. GAD immunolabeling was unaffected by cannabinoid pretreatment.
Fig. 3.
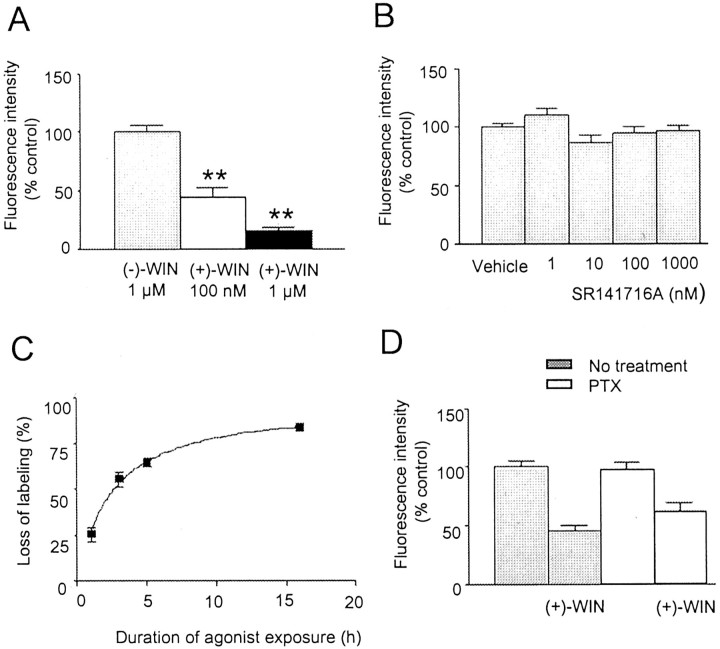
Further characterization of the effects of cannabinoids on cell surface CB1 receptor expression.A, Histogram showing the inhibition of CB1receptor immunofluorescence on cells preincubated for 16 hr with (+)-WIN55212 (100 nm and 1 μm) compared with control cells preincubated with (−)-WIN55212 (1 μm).B, Histogram showing the mean fluorescence intensity of fibers after preincubation with SR141716A (1–1000 nm) compared with vehicle (control). C, Graph showing the effects of incubation time in (+)-WIN55212 (1 μm) on cell surface labeling. The mean level of fluorescence at each time interval was compared with that of cells treated with vehicle (control).D, Histogram showing the effects of overnight pretreatment of hippocampal cells with PTX (100 ng/ml) on the agonist-induced loss of cell surface labeling (expressed relative to vehicle controls). Neither the level of CB1 receptor expression nor the loss of cell surface labeling caused by treatment with (+)-WIN55212 (1 μm; 16 hr) were significantly affected by PTX (p > 0.05). Values are mean ± SEM; ** p < 0.01.
To test for potential inverse agonist actions of SR141716A (Bouaboula et al., 1997; Rinaldi-Carmona et al., 1998), a range of doses were tested (1–1000 nm), however no significant effects were observed (Fig. 3B). The action of methanandamide, a hydrolysis-resistant analog of the putative endogenous CB1 receptor ligand, anandamide, on labeling intensity was also determined. Pretreatment of cells for 16 hr at 37°C with methanandamide (1 μm) reduced labeling by 43 ± 5% relative to vehicle control (p < 0.01; data not shown).
Effects of agonist incubation time on CB1receptor labeling
To determine the rate at which surface CB1receptors internalized, hippocampal neurons were exposed to (+)-WIN55212 (1 μm) at 37°C for different incubation periods. The results of these experiments are summarized in Figure3C, in which the intensity of immunostaining is compared with that of vehicle control. After a 1 hr incubation with (+)-WIN55212, the labeling intensity was significantly reduced, reaching a maximum of 84 ± 2% at 16 hr. A further increase in the incubation time to 72 hr resulted in no additional loss of immunoreactivity (p > 0.05; data not shown).
Effect of pertussis toxin on CB1 receptor labeling
Many of the receptor-mediated actions of cannabinoids (activation of mitogen-activated protein kinase, inhibition of adenylate cyclase, and ion channel modulation) are mediated by pertussis toxin (PTX)-sensitive G-proteins (Pertwee, 1997). To determine whether the agonist-induced loss of surface CB1 receptor immunoreactivity observed in our studies was also sensitive to PTX, cells were incubated overnight with PTX (100 ng/ml) before pretreatment with either (+)-WIN55212 (1 μm) or vehicle. Under these conditions the inhibition of CB1 receptor immunofluorescence caused by treatment with (+)-WIN55212 was not blocked (Fig. 3D). In parallel experiments using the same pretreatment schedule as for hippocampal cells, pertussis toxin completely blocked the inhibition by the cannabinoid agonist CP55940 of forskolin-stimulated cAMP in CHO cells transfected with CB2 receptors (data not shown).
Actions of (+)-WIN55212 on immature hippocampal neurons
The ability of prolonged exposure to (+)-WIN55212 to inhibit cell surface CB1 receptor labeling in immature cells, at an age corresponding with the onset of synapse formation (Fletcher et al., 1991), was also investigated. Cells that had been cultured in the presence of (+)-WIN55212 for 3 d from seeding (1 μm) showed a significant reduction in CB1 receptor labeling (73 ± 5%) compared with untreated cells (p < 0.01) or vehicle-treated cells (95 ± 7%) (Fig.4A). A direct comparison of the surface CB1 receptor labeling between immature cells (2 d in culture) and mature cells (9–11 d in culture) also demonstrated a significant increase in expression with time in culture (p < 0.0001; Fig.4B). This increased labeling reflected both an increase in the number of puncta and an increase in the mean intensity of fluorescence at each punctum, suggesting that both the number of synapses and the number of CB1 receptors per cluster increase as the cultures mature.
Fig. 4.
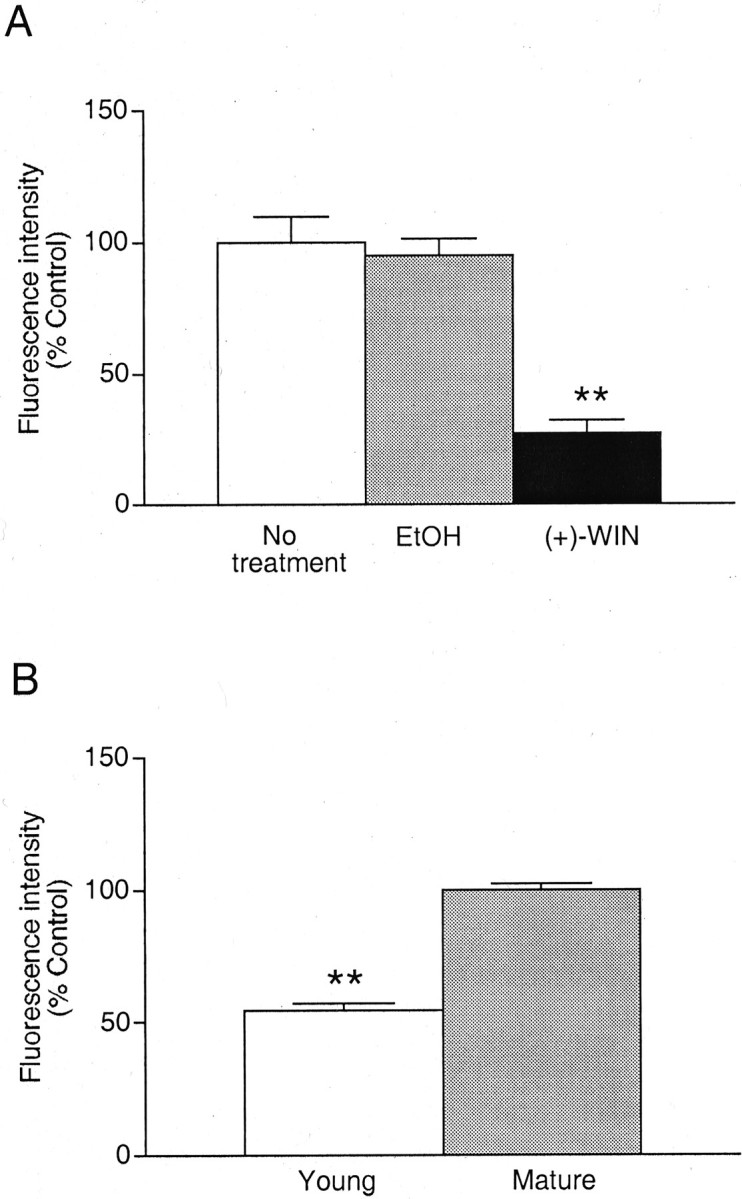
Actions of cannabinoids on immature hippocampal neurons. A, Histogram showing the inhibition of cell surface CB1 receptor immunofluorescence on fibers after exposure to (+)-WIN55212 (1 μm) or the equivalent concentration of vehicle (EtOH) for 72 hr immediately after plating and compared with untreated (control) cells. B, Histogram showing the relative level of CB1 receptor immunoreactivity expressed in young cultures (2 d) compared with control values obtained with mature neurons (9–14 d). Values are mean ± SEM (**p < 0.01).
Visualization and translocation of internalized receptors
The visualization of subtle differences in the cellular localization of CB1 receptors at the presynaptic terminal is not practical at the light microscopy level. In addition, permeabilization of hippocampal neurons before labeling for CB1 receptor immunoreactivity reveals intracellular receptors in the absence of agonist pretreatment that would obscure changes induced during receptor endocytosis. Thus, we devised a protocol to directly visualize internalized receptors involving prelabeling with primary antibody alone before agonist treatment. Cell surface and internalized receptors were then labeled with separate secondary antibodies (Cy3 or Alexa 488 conjugates) at the end of the experiment. The data obtained with hippocampal neurons were compared with similar studies using undifferentiated F-11 cells, which exhibited CB1receptor labeling on their somatic membrane in a manner similar to that of transfected cells. After labeling with primary antibody, cells were incubated with either (+)-WIN55212, (-)-WIN55212, SR141716A, a combination of (+)-WIN55212 and SR141716A, or vehicle alone for 16 hr (hippocampal neurons) or 30 min (F-11 cells). Cannabinoids and related compounds were applied at a concentration of 1 μm. The CB1 receptor immunoreactivity that remained on the cell surface was then visualized with the Cy3-conjugated secondary antibody. After fixation and permeabilization, the Alexa 488-conjugated secondary antibody identified CB1 receptor-primary antibody labeling that had undergone endocytosis during incubation with cannabinoids or vehicle. The levels of surface labeling in both hippocampal and F-11 cells were markedly reduced after pretreatment with (+)-WIN55212, and this effect was accompanied by the appearance of internalized receptors (Figs. 5,6). In a subpopulation of hippocampal neurons, internalized receptor labeling was detected in bright vesicles within the cytosol of somatodendritic regions (Fig.5E,H). In addition, internalized clusters of CB1 receptor label were present within putative axons (data not shown). These findings suggest that the CB1 receptors undergo retrograde translocation along axons toward somatodendritic areas. In contrast, in vehicle-pretreated hippocampal cells there was no direct correspondence between CB1 receptor labeling and MAP-2, although cell surface CB1 receptor-positive fibers were intertwined with, and often ran along, MAP-2-positive dendrites (Fig.5G). This pattern of labeling is consistent with the axonal localization of CB1 receptors reported previously (Irving et al., 2000). In F-11 cells, internalized CB1 receptor labeling was also detected as discrete puncta within perinuclear regions of the cytosol (Fig. 6). The pattern of labeling observed in the F-11 cells allowed for the quantitative analysis of the cannabinoid effects, where the relative intensity of cell surface and intracellular immunolabeling could be compared at individual somata (Table 1). Little internalized receptor labeling was detected in vehicle-treated hippocampal and F-11 cells, suggesting that, in the absence of agonist, receptor turnover rates are relatively slow.
Fig. 5.
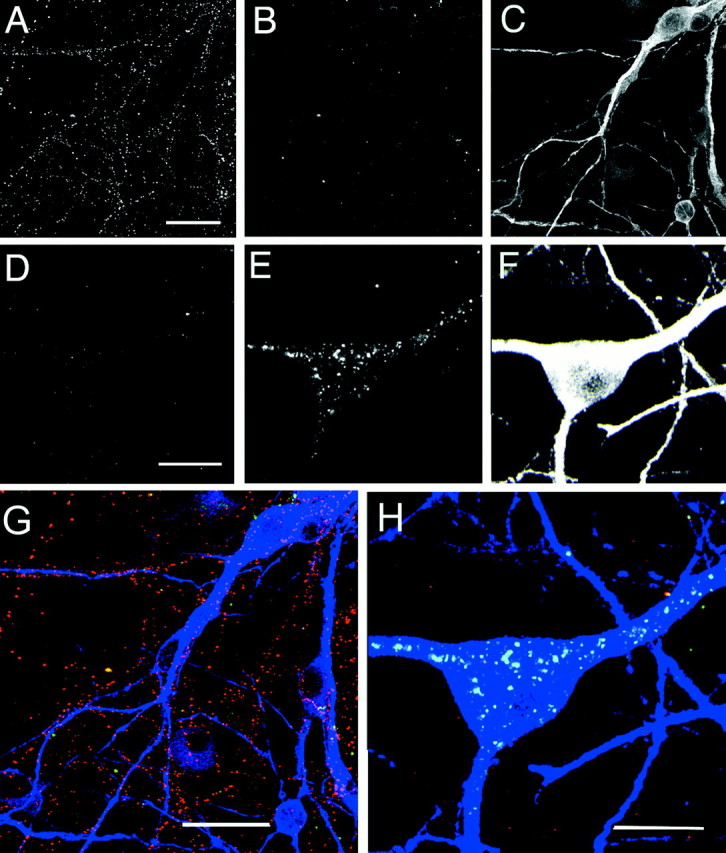
Visualizing cell surface and internalized CB1 receptors in cultured hippocampal neurons using primary antibody prelabeling. Corresponding images from triple-labeling experiments comparing vehicle (A–C) with (+)-WIN55212 (1 μm; D–F) on cell surface and intracellular CB1 receptor immunofluorescence.A and D show surface CB1receptors; B and E show internalized receptors. In C and F the cell soma and proximal dendrites have been labeled with MAP-2 antibody. Images arez projections of a series of 11 confocal sections taken at 2 μm intervals. G and H show the corresponding merged color images for vehicle and (+)-WIN55212 treatment. Red corresponds to cell surface label (Cy3), green to internalized receptor (Alexa 488), and blue to MAP-2 (Cy5). Note how cell surface CB1 receptor-positive fibers are intertwined with and track the MAP-2-positive dendrites. The figure shows a representative experiment from seven determinations with similar findings, and in three of these, cells were subsequently colabeled with MAP-2. Scale bars: A, G, 25 μm; D, H, 15 μm.
Fig. 6.
Localization of cell surface and internalized CB1 receptors in F-11 cells. A–D, Confocal images of F-11 cells that had been incubated with vehicle alone (A, B) were compared with cells that had been treated with (+)-WIN55212 (1 μm; C, D). Cells were labeled for surface CB1 receptors (A, C) and internalized receptors after cell permeabilization (B, D). Note the loss of cell surface labeling and the appearance of internalized receptors after exposure to (+)-WIN55212. Scale bars, 50 μm.
Table 1.
Effects of cannabinoids on the internalization of CB1 receptors in F-11 cells
| Treatment | F-11 surface labeling (Cy3) | F-11 intracellular labeling (Alexa 488) |
|---|---|---|
| (+)-WIN55212 (30) | 0.39 ± 0.071-160 | 1.73 ± 0.33* |
| (+)-WIN-55212/SR141716A (29) | 0.77 ± 0.13 | 0.66 ± 0.09 |
| (−)-WIN55212 (28) | 0.77 ± 0.11 | 1.18 ± 0.19 |
| Vehicle (30) | 1.00 ± 0.10 | 1.00 ± 0.11 |
| SR141716A (31) | 1.12 ± 0.33 | 0.96 ± 0.14 |
The Cy3-conjugated secondary antibody labeled receptors remained on the cell surface after cannabinoid incubation, whereas the Alexa 488-conjugated secondary antibody-labeled receptors were internalized. Values are mean (±SEM) fluorescence intensity levels measured from defined intracellular and plasma membrane regions and normalized relative to vehicle controls. The number of cells are given in parentheses and were taken from four independent experiments. Cannabinoids and related compounds were applied at 1 μm. Multiple comparisons between groups within each column was made with ANOVA with Dunnett's post hoc test comparing results with those for vehicle alone.
p < 0.05;
F1-160: p < 0.01.
Immunoblots
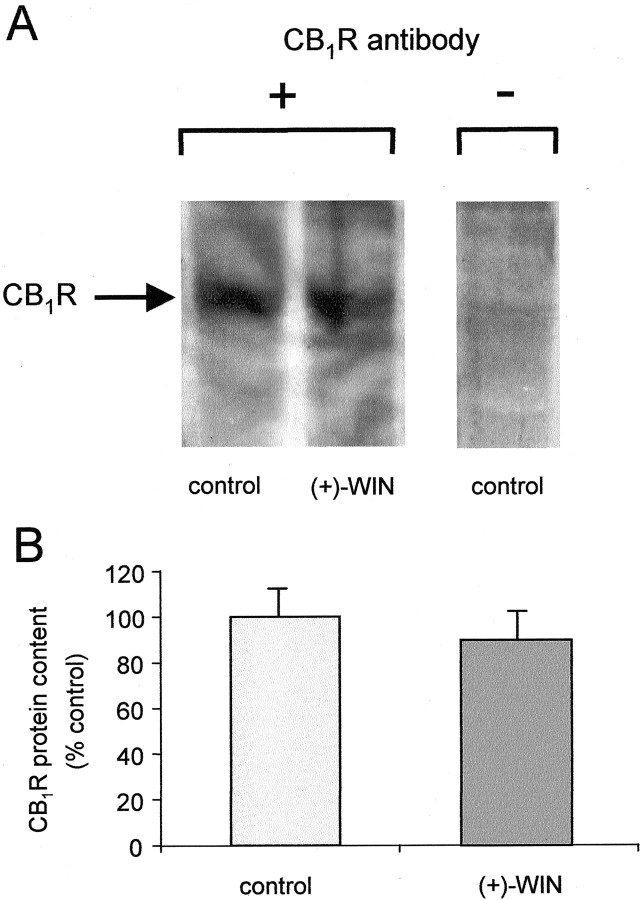
Further evidence in support of agonist induced-internalization of CB1 receptors in hippocampal neurons was obtained using immunoblots. CB1 receptor protein was identified in cells pretreated with either (+)-WIN55212 (1 μm) or vehicle for 16 hr (Fig.7). In both treatments the antibody directed against the CB1 receptor epitope recognized a specific band of 61 kDa. This molecular weight is similar to the expected molecular weight of the CB1receptor (Song and Howlett, 1995; McIntosh et al., 1998). Using densitometry measurements, no significant difference was observed between blots from the two treatments, suggesting that total receptor protein levels are similar (p > 0.05;n = 3) (Fig 7b).
Fig. 7.
Western analysis of CB1 receptor immunoreactivity in rat cultured hippocampal cells. Cells were pretreated with (+)-WIN55212 or vehicle (control) at 37°C for 16 hr.A, The membrane proteins were immunostained with (+) or without (−) exposure to CB1 receptor primary antibody before secondary antisera. In both treatments a specific band of 61 kDa (CB1R) was detected. This band was not markedly altered by (+)-WIN55212 treatment. B, Densitometric analysis of the 61 kDa band (CB1R) from Western blots of rat cultured hippocampal cells as described in (A). Mean ± SEM; p > 0.05; n = 3.
DISCUSSION
In this paper, we have demonstrated using immunohistochemistry and laser-scanning confocal microscopy the internalization and trafficking of CB1 receptors in hippocampal neurons.
Agonist-induced internalization
In both immature and mature cells, the level of cell surface CB1 receptor immunoreactivity decreased significantly after the pharmacological activation of CB1 receptors. The prelabeling protocol, together with data from immunoblot experiments suggests that this effect primarily reflects CB1 receptor internalization. This supports observations made in transfected cells where the endocytosis of CB1 receptors occurs without a concomitant decrease in receptor number as measured by radioligand binding (Rinaldi-Carmona et al., 1998). However, with longer periods of agonist exposure (up to 2 weeks) variable reductions in CB1 receptorBmax in brain have been observed (Matsuda, 1997). Agonist-induced endocytosis has been described for CB1 receptors in transfected cells (Rinaldi-Carmona et al., 1998; Hsieh et al., 1999; Roche et al., 1999), but only now in neurons where receptors are targeted to sites linked to their physiological role. Methanandamide, a more metabolically stable analog of anandamide, was also effective in reducing CB1-selective surface immunolabeling, consistent with findings using transfected cells (Hsieh et al., 1999).
A surprising observation in the present study was the relatively long time course required for the internalization process in the hippocampal neurons to reach its maximal effect. The rate of internalization described for many receptors, including CB1receptors expressed on F-11 cells in this study, is of the order of 10–30 min to achieve maximal levels, whereas this was between 5 and 16 hr for CB1 receptors expressed on hippocampal neurons. Previous studies with muscarinic receptors also indicate that rates of internalization can vary between different cell types (Koenig and Edwardson, 1996). These observations might reflect differences in the internalization machinery expressed between cell populations and/or within particular neuronal compartments.
Although many of the physiological actions of cannabinoids are mediated by pertussis toxin-sensitive G-proteins, under the present experimental conditions pertussis toxin did not block CB1receptor internalization. These findings are consistent with studies using CB1 receptor-transfected cells (Hsieh et al., 1999) and for IL-8 or somatostatin receptors (Feniger-Barish et al., 2000; Hipkin et al., 2000).
CB1 receptor trafficking
In mature cultured hippocampal neurons, which are highly differentiated compared with the F-11 cells, cell surface CB1 receptor immunolabeling is present in high levels on GABAergic synaptic terminals (Irving et al., 2000). The changes in surface levels of CB1 receptor labeling and the concurrent appearance of vesicles of internalized receptor/primary antibody complex within the perikarya of hippocampal neurons and perinuclear region of F-11 cells suggests that (+)-WIN55212 causes CB1 receptors to translocate centripetally toward these areas. Although it is possible that the linkage of primary antibody to the CB1 receptor could alter the trafficking of CB1 receptor protein within the cell, other studies suggest that the translocation of internalized receptors toward somatic or perinuclear endosomes is a common feature of many neuronal G-protein-coupled receptors (Faure et al., 1995;Bernard et al., 1998; Dumartin et al., 1998). Moreover, the presence of primary antibody did not appear to affect the internalization process itself, because the agonist-induced loss of cell surface receptors measured with the two labeling protocols was similar.
Antagonist, but not inverse agonist actions of SR141716A
SR141716A has been described as both a competitive antagonist and an inverse agonist at CB1 receptors (Bouaboula et al., 1997; Coutts and Pertwee, 1997; Coutts et al., 2000). In CB1 receptor-transfected CHO cells, treatment with SR141716A results in an increased expression of CB1 receptors, which is ascribed to its inverse agonist properties (Bouaboula et al., 1997; Rinaldi-Carmona et al., 1998). However, in our studies, the intensity of CB1 receptor staining was not significantly affected by preincubation with SR141716A over a wide range of concentrations. One explanation for this discrepancy is that the level of immunolabeling in our cells may be sufficiently high that a marginal increase in receptor expression may not be detectable using the current techniques. A more likely explanation is that there are a greater number of constitutively active, precoupled receptors in the transfected cells, hence the potential for inverse agonism. The majority of studies of inverse agonism use systems that have been manipulated to increase constitutive receptor activity, which is much less pronounced in naturally expressing cells (MacEwan and Milligan, 1996; Stevens and Milligan, 1998). However, SR141716A can exert inverse agonist properties in some native cells, including neurons of the rat pelvic ganglion (Pan et al., 1998).
CB1 receptor expression on F-11 cells
The presence of CB1 receptor expression on the surface of F-11 cells is a new, but not surprising observation because CB1 receptors are present on both parental cell lines (Howlett et al., 1991; Hohmann and Herkenham, 1999; Ross et al., 2001), and these cells display many of the characteristics of the parent cells (Francel et al., 1987; McIntosh et al., 1998). Recent studies also indicate the presence of cell surface CB1receptors on the soma of cultured DRG neurons (Ross et al., 2001). The expression of CB1 receptors or CB1 receptor mRNA in F-11 and DRG cells (Hohmann and Herkenham, 1999; Ross et al., 2001) is of particular interest with regard to the physiology and pathology of pain pathways, in which DRG cells are the means of primary sensory afferent transmission from the periphery to the dorsal horn of the spinal cord. Thus, F-11 cells provide a useful in vitro substrate in which to study CB1 receptor mechanisms in which the pattern of labeling is similar to that of the parental cells.
Conclusion
We have shown, for the first time, agonist-induced internalization of cannabinoid CB1 receptors in hippocampal neurons and F-11 cells. This process may be characteristic of nonclassical, intercellular transmitters that act presynaptically as neuromodulators. In addition, the dynamic modulation of CB1 receptor expression by cannabinoids could also influence the patterns of tolerance that develops toward this class of compounds in the CNS.
Footnotes
This work was supported by Wellcome Trust Grants 47368 and 055291 and Medical Research Council Grant G9901500.
Correspondence should be addressed to Dr. Angela Coutts, Department of Biomedical Sciences, University of Aberdeen, Scotland, AB25 2ZD, UK. E-mail: a.a.coutts@abdn.ac.uk.
REFERENCES
- 1.Bernard V, Laribi O, Levey AI, Bloch B. Subcellular redistribution of m2 muscarinic acetylcholine receptors in striatal interneurons in vivo after acute cholinergic stimulation. J Neurosci. 1998;18:10207–10218. doi: 10.1523/JNEUROSCI.18-23-10207.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouaboula M, Perrachon S, Milligan L, Canat X, Rinaldi-Carmona M, Portier M, Barth F, Calandra B, Pecceu F, Lupker J, Maffrand J-P, Le Fur G, Casellas P. A selective inverse agonist for central cannabinoid receptor inhibits mitogen-activated protein kinase activation stimulated by insulin or insulin-like growth factor 1. Evidence for a new model of receptor/ligand interactions. J Biol Chem. 1997;272:22330–22339. doi: 10.1074/jbc.272.35.22330. [DOI] [PubMed] [Google Scholar]
- 3.Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Coutts AA, Brewster N, Ingram T, Razdan RK, Pertwee RG. Comparison of novel cannabinoid partial agonists and SR141716A in the guinea-pig small intestine. Br J Pharmacol. 2000;129:645–652. doi: 10.1038/sj.bjp.0703094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 6.Doherty AJ, Coutinho V, Collingridge GL, Henley JM. Rapid internalization and surface expression of a functional, fluorescently tagged G-protein-coupled glutamate receptor. Biochem J. 1999;341:415–422. [PMC free article] [PubMed] [Google Scholar]
- 7.Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Faure MP, Nouel D, Beaudet A. Axonal and dendritic transport of internalized neurotensin in rat mesostriatal dopaminergic neurons. Neurosci. 1995;68:519–529. doi: 10.1016/0306-4522(95)00145-9. [DOI] [PubMed] [Google Scholar]
- 9.Felder CC, Nielsen A, Briley EM, Palkovits M, Priller J, Axelrod J, Nguyen DN, Richardson JM, Riggin RM, Koppel GA, Paul SM, Becker GW. Isolation and measurement of the endogenous cannabinoid receptor agonist, anandamide, in brain and peripheral tissues of human and rat. FEBS Lett. 1996;393:231–235. doi: 10.1016/0014-5793(96)00891-5. [DOI] [PubMed] [Google Scholar]
- 10.Feniger-Barish R, Belkin D, Zaslaver A, Gal S, Dori M, Ran M, Ben-Baruch A. GCP-2-induced internalization of IL-8 receptors: hierarchical relationships between GCP-2 and other ELR+-CXC chemokines and mechanisms regulating CXCR2 internalization and recycling. Blood. 2000;95:1551–1559. [PubMed] [Google Scholar]
- 11.Fletcher TL, Cameron P, Decamilli P, Banker G. The distribution of synapsin-I and synaptophysin in hippocampal-neurons developing in culture. J Neurosci. 1991;11:1617–1626. doi: 10.1523/JNEUROSCI.11-06-01617.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Francel PC, Harris K, Smith M, Fishman MC, Dawson G, Miller RJ. Neurochemical characteristics of a novel dorsal-root ganglion X neuroblastoma hybrid cell-line, F-11. J Neurochem. 1987;48:1624–1631. doi: 10.1111/j.1471-4159.1987.tb05711.x. [DOI] [PubMed] [Google Scholar]
- 13.Garland AM, Grady EF, Lovett M, Vigna SR, Frucht MM, Krause JE, Bunnett NW. Mechanisms of desensitization and resensitization of G protein-coupled neurokinin1 and neurokinin 2 receptors. Mol Pharmacol. 1996;49:438–446. [PubMed] [Google Scholar]
- 14.Gatley SJ, Lan R, Volkow ND, Pappas N, King P, Wong CT, Gifford AN, Pyatt B, Dewey SL, Makriyannis A. Imaging the brain marijuana receptor: development of a radioligand that binds to cannabinoid CB1 receptors in vivo. J Neurochem. 1998;70:417–423. doi: 10.1046/j.1471-4159.1998.70010417.x. [DOI] [PubMed] [Google Scholar]
- 15.Hájos N, Katona I, Naiem SS, Mackie K, Ledent C, Mody I, Freund T. Cannabinoids inhibit hippocampal GABAergic transmission and network oscillations. Eur J Neurosci. 2000;12:3239–3249. doi: 10.1046/j.1460-9568.2000.00217.x. [DOI] [PubMed] [Google Scholar]
- 16.Herkenham M. Cannabinoid receptor localization in brain-relationship to motor and reward systems. In: Kalivas PW, Samson HH, editors. Neurobiology of drug and alcohol addiction. New York Academy of Sciences; New York: 1992. pp. 19–32. [DOI] [PubMed] [Google Scholar]
- 17.Hipkin RW, Wang YN, Schonbrunn A. Protein kinase C activation stimulates the phosphorylation and internalization of the sst2A somatostatin receptor. J Biol Chem. 2000;275:5591–5599. doi: 10.1074/jbc.275.8.5591. [DOI] [PubMed] [Google Scholar]
- 18.Hoffman AF, Lupica CR. Mechanisms of cannabinoid inhibition of GABA(A) synaptic transmission in the hippocampus. J Neurosci. 2000;20:2470–2479. doi: 10.1523/JNEUROSCI.20-07-02470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neuroscience. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- 20.Howlett AC. Pharmacology of cannabinoid receptors. Annu Rev Pharmacol. 1995;33:607–634. doi: 10.1146/annurev.pa.35.040195.003135. [DOI] [PubMed] [Google Scholar]
- 21.Howlett AC, Championdorow TM, Mcmahon LL, Westlake TM. The cannabinoid receptor-biochemical and cellular properties in neuroblastoma cells. Pharmacol Biochem Behav. 1991;40:565–569. doi: 10.1016/0091-3057(91)90364-8. [DOI] [PubMed] [Google Scholar]
- 22.Howlett AC, Song C, Berglund BA, Wilken GA, Pigg JJ. Characterization of CB1 cannabinoid receptors using receptor peptide fragments and site-directed antibodies. Mol Pharmacol. 1998;53:504–510. doi: 10.1124/mol.53.3.504. [DOI] [PubMed] [Google Scholar]
- 23.Hsieh C, Brown S, Derleth C, Mackie K. Internalization and recycling of the CB1 cannabinoid receptor. J Neurochem. 1999;73:493–501. doi: 10.1046/j.1471-4159.1999.0730493.x. [DOI] [PubMed] [Google Scholar]
- 24.Irving AJ, Coutts AA, Harvey J, Rae MG, Mackie K, Bewick GS, Pertwee RG. Functional expression of cell surface cannabinoid CB1 receptors on presynaptic inhibitory terminals in cultured rat hippocampal neurons. Neuroscience. 2000;98:253–262. doi: 10.1016/s0306-4522(00)00120-2. [DOI] [PubMed] [Google Scholar]
- 25.Katona I, Sperlágh B, Sik A, Käfalvi A, Vizi ES, Mackie K, Freund TF. Presynaptically located CB1 cannabinoid receptors regulate GABA release from axon terminals of specific hippocampal interneurons. J Neurosci. 1999;19:4544–4558. doi: 10.1523/JNEUROSCI.19-11-04544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Koenig JA, Edwardson JM. Intracellular trafficking of the muscarinic acetylcholine receptor: importance of subtype and cell type. Mol Pharmacol. 1996;49:351–359. [PubMed] [Google Scholar]
- 27.MacEwan DJ, Milligan G. Inverse agonist-induced up-regulation of the human beta(2)-adrenoceptor in transfected neuroblastoma X glioma hybrid cells. Mol Pharmacol. 1996;50:1479–1486. [PubMed] [Google Scholar]
- 28.Matsuda LA. Molecular aspects of cannabinoid receptors. Crit Rev Neurobiol. 1997;11:143–166. doi: 10.1615/critrevneurobiol.v11.i2-3.30. [DOI] [PubMed] [Google Scholar]
- 29.Matsuda LA, Bonner TI, Lolait SJ. Localization of cannabinoid receptor messenger RNA in rat brain. J Comp Neurol. 1993;327:535–550. doi: 10.1002/cne.903270406. [DOI] [PubMed] [Google Scholar]
- 30.McIntosh HH, Song C, Howlett AC. CB1 cannabinoid receptor: cellular regulation and distribution in N18TG2 neuroblastoma cells. Mol Brain Res. 1998;53:163–173. doi: 10.1016/s0169-328x(97)00294-5. [DOI] [PubMed] [Google Scholar]
- 31.Pan XH, Ikeda SR, Lewis DL. SR 141716A acts as an inverse agonist to increase neuronal voltage-dependent Ca2+ currents by reversal of tonic CB1 cannabinoid receptor activity. Mol Pharmacol. 1998;54:1064–1072. doi: 10.1124/mol.54.6.1064. [DOI] [PubMed] [Google Scholar]
- 32.Pertwee RG. Pharmacology of cannabinoid CB1 and CB2 receptors. Pharmacol Ther. 1997;74:129–180. doi: 10.1016/s0163-7258(97)82001-3. [DOI] [PubMed] [Google Scholar]
- 33.Platika D, Boulos MH, Baizer L, Fishman MC. Neuronal traits of clonal cell-lines derived by fusion of dorsal-root ganglia neurons with neuroblastoma cells. Proc Natl Acad Sci USA. 1985;82:3499–3503. doi: 10.1073/pnas.82.10.3499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rinaldi-Carmona M, Le Duigou A, Oustric D, Barth F, Bouaboula M, Carayon P, Casellas P, Le Fur G. Modulation of CB1 cannabinoid receptor functions after a long-term exposure to agonist or inverse agonist in the Chinese hamster ovary cell expression system. J Pharmacol Exp Ther. 1998;287:1038–1047. [PubMed] [Google Scholar]
- 35.Roche JP, Bounds S, Brown S, Mackie K. A mutation in the second transmembrane region of the CB1 receptor selectively disrupts G protein signaling and prevents receptor internalization. Mol Pharmacol. 1999;56:611–618. doi: 10.1124/mol.56.3.611. [DOI] [PubMed] [Google Scholar]
- 36.Ross RA, Coutts AA, McFarlane SM, Anavi-Goffer S, Irving AJ, Pertwee RG, MacEwan DJ, Scott RH. Actions of cannabinoid ligands on rat cultured sensory neurons: implications for antinociception. Neuropharmacology. 2001;40:221–232. doi: 10.1016/s0028-3908(00)00135-0. [DOI] [PubMed] [Google Scholar]
- 37.Roth A, Kreienkamp HJ, Meyerhof W, Richter D. Phosphorylation of four amino acid residues in the carboxyl terminus of the rat somatostatin receptor subtype 3 is crucial for its desensitization and internalization. J Biol Chem. 1997;272:23769–23774. doi: 10.1074/jbc.272.38.23769. [DOI] [PubMed] [Google Scholar]
- 38.Song C, Howlett AC. Rat brain cannabinoid receptors are N-linked glycosylated proteins. Life Sci. 1995;56:1983–1989. doi: 10.1016/0024-3205(95)00179-a. [DOI] [PubMed] [Google Scholar]
- 39.Southwell BR, Seybold VS, Woodman HL, Jenkinson KM, Furness JB. Quantitation of neurokinin 1 receptor internalization and recycling in guinea-pig myenteric neurons. Neuroscience. 1998;87:925–931. doi: 10.1016/s0306-4522(98)00176-6. [DOI] [PubMed] [Google Scholar]
- 40.Stevens PA, Milligan G. Efficacy of inverse agonists in cells overexpressing a constitutively active beta(2)-adrenoceptor and type II adenylyl cyclase. Br J Pharmacol. 1998;123:335–343. doi: 10.1038/sj.bjp.0701600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83:393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 42.Whistler JL, Chuang HH, Chu P, Jan LY, von Zastrow M. Functional dissociation of mu opioid receptor signaling and endocytosis: implications for the biology of opiate tolerance and addiction. Neuron. 1999;23:737–746. doi: 10.1016/s0896-6273(01)80032-5. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]