Abstract
Dopamine is known to regulate several behavioral phenomena, including sensorimotor gating and aspects of motor activity. The roles of dopamine D1 and D2 receptors in these behaviors have been documented in the rat literature, but few reports exist on their role in mice. We used dopamine transporter (DAT) (−/−) mice to examine the behavioral consequences of a chronically hyperdopaminergic state, challenging them with the preferential dopamine D2 receptor antagonist raclopride and D1 receptor antagonist SCH23390. At baseline, DAT (−/−) mice exhibited deficient sensorimotor gating as measured by prepulse inhibition (PPI) of the startle response, exhibited nonfocal perseverative patterns of locomotion, and were hyperactive in a novel environment. Pretreatment with raclopride significantly increased PPI in the DAT (−/−) mice, whereas SCH23390 had no significant effect. Blockade of D2 receptors did not affect the predominantly straight patterns of motor behavior produced by the DAT (−/−) mice, but antagonism of D1 receptors significantly attenuated the perseverative patterns, producing more of a meandering behavior seen in the DAT (+/+) control mice. Both D1 and D2 receptor antagonists decreased the hyperactivity seen in the DAT (−/−) mice. These findings support the role of the D2, but not the D1, receptor in the modulation of PPI in mice. Furthermore, D1 receptor activation appears to be the critical substrate for the expression of perseverative patterns of motor behavior, whereas both D1 and D2 receptors appear to regulate the amount of motor activity.
Keywords: dopamine, prepulse inhibition, mice, behavior, dopamine transporter, locomotor activity, perseveration, D1 receptor, D2 receptor
Prepulse inhibition (PPI) of the startle response, an operational measure of sensorimotor gating, is a cross-species phenomenon in which the startle response is reduced when the startling stimulus is preceded by a low-intensity prepulse (Graham, 1975; Hoffman and Ison, 1980). Disruptions in PPI are produced in rodents using dopamine (DA) agonists such as amphetamine or apomorphine (Mansbach et al., 1988; Swerdlow et al., 1991). The DA D2 receptor appears to be a key modulator of these DA-stimulated disruptions of PPI because antagonism of D2 receptors will reverse an apomorphine-induced disruption of PPI (Mansbach et al., 1988; Swerdlow et al., 1991; Wan et al., 1996), whereas direct stimulation of D2 receptors produces disruptions in PPI (Peng et al., 1990; Wan et al., 1996). A more limited role of the D1 receptor has been implicated in the modulation of PPI, in which a synergistic relationship between D1 and D2 receptors is thought to exist (Peng et al., 1990; Wan et al., 1996). In studies using mice, a mixed D1/D2 agonist disrupts PPI (Dulawa and Geyer, 1996;Curzon and Decker, 1998), but the specific role of each receptor subtype remains unclear. Using genetically altered mice, we have shown that the D2 receptor is necessary for amphetamine to exert its disruptive effects on PPI (Ralph et al., 1999), but the role of the D1 receptor in PPI is yet to be examined in mice.
Along with modulating aspects of sensorimotor gating, DA has a demonstrated role in the expression of motor behavior. Both the D1 and D2 receptor systems are thought to regulate some of these behavioral processes because experiments have shown that antagonists of both systems will attenuate amphetamine- or cocaine-induced hyperactivity in rats and mice (Paulus and Geyer, 1991a; O'Neill and Shaw, 1999). Changes in the patterns of motor behavior have also been reported using DA agonists in which the direct D1/D2 agonist apomorphine produced perseverative motor patterns (Geyer et al., 1986, 1987; Paulus and Geyer, 1991b). Furthermore, D1, but not D2, receptor antagonism blocked perseverative locomotor behavior induced by amphetamine (Fritts et al., 1997). These findings suggest that there is a dissociation in the contributions of D1 and D2 receptors in the perseverative aspect of motor behavior that is produced by DA activation in which D2 receptors appear to control the amount, whereas D1 seems to influence the quality of the motor pattern.
Using gene-deletion technology, a dopamine transporter (DAT) null mutant mouse has been created, providing a unique opportunity to study the behavioral consequences of a chronically dysregulated DA system. We hypothesized that the DAT (−/−) mice, with their hyperdopaminergic tone, would have disrupted PPI and would be perseverative and hyperactive in their motor behavior. We report here on two separate DAT cohorts to confirm these behavioral phenotypes. Furthermore, we investigated the differential role of the DA D1 and D2 receptor subtypes in the modulation of these two behavioral phenotypes in the DAT mice.
MATERIALS AND METHODS
Subjects. The DAT mutant mice [cohort 1: 23 (+/+), 47 (+/−), and 9 (−/−) male and female mice; cohort 2: female, 20 (+/+), 22 (+/−), and 17 (−/−); male, 18 (+/+), 19 (+/−), and 18 (−/−)] were generated at University of California San Diego in an Association for Assessment and Accreditation of Laboratory Animal Care-approved animal facility using parental (+/−) mice from Duke University (Giros et al., 1996). The animal facility meets all federal and state requirements for animal care, and federal and state guidelines for the care and treatment of laboratory animals are followed. M.G.C. and R.J.R. performed the genotyping of the mice. Mouse pups were weaned at 4 weeks of age and were group-housed (segregated by sex) in a climate-controlled animal colony with a reversed day/night cycle (lights on at 7:00 P.M., off at 7:00 A.M.). All behavioral testing started at ∼8–9 weeks of age and occurred between 9:00 A.M. and 5:00 P.M. Food (Harlan Teklab, Madison, WI) and water were available throughout the experiments, except during behavioral testing.
Drugs. Raclopride HCl and SCH23390 were obtained from RBI/Sigma (St. Louis, MO). Raclopride was dissolved in 0.9% saline, and SCH23390 was dissolved in water. Free-base drug weights were used in all drug calculations. For PPI testing, injections of 3.0 mg/kg raclopride or saline were given intraperitoneally, and 1.0 mg/kg SCH23390 or water was injected subcutaneously immediately before behavioral testing. For locomotor pattern assessments, injections of 0.1 mg/kg raclopride or saline were given intraperitoneally, and injections of 0.01 mg/kg SCH23390 or water were given subcutaneously 10 min before behavioral testing. All injections were given at a volume of 5 ml/kg of body weight.
Apparatus. Startle reactivity was measured using four startle chambers (SR-LAB; San Diego Instruments, San Diego, CA). Each chamber consisted of a clear nonrestrictive Plexiglas cylinder resting on a platform inside a ventilated box. A high-frequency loudspeaker inside the chamber produced both a continuous background noise of 65 dB and the various acoustic stimuli. Vibrations of the Plexiglas cylinder caused by the whole-body startle response of the animal were transduced into analog signals by a piezoelectric unit attached to the platform. These signals were then digitized and stored by a computer. Sixty-five readings were taken at 1 msec intervals, starting at stimulus onset, and the average amplitude was used to determine the acoustic startle response. Sound levels in decibels (A) were measured as described previously (Dulawa et al., 1997), and the SR-LAB calibration unit was used routinely to ensure consistent stabilimeter sensitivities between test chambers and over time (Geyer and Swerdlow, 1998).
The video-tracker (VT) consisted of four adjacent white Plexiglas enclosures (41 × 41 × 34 cm) surrounded by a plastic curtain. Each mouse was tested individually in a separate enclosure. A video camera, mounted 158 cm above the enclosures, provided the signal for the Polytrack digitizer (San Diego Instruments). The signal was processed to obtain the left-uppermost coordinate for each of the four animals simultaneously. The signal was stored in a PC computer for further off-line processing. For this investigation, the position (x, y) (in pixels) of each animal was sampled at a rate of 18.18 Hz and used to generate a coordinate file (x, y, t) consisting of thex-location, the y-location, and the duration of time (t) spent at that location. The spatiotemporal resolution of each event recorded was 0.32 cm, 0.32 cm, and 55 msec, which corresponded to a maximum speed of 25 cm/sec.
Startle and prepulse inhibition session. All PPI test sessions consisted of startle trials (PULSE-ALONE), prepulse trials (PREPULSE+PULSE), and no-stimulus trials (NOSTIM). The PULSE-ALONE trial consisted of a 40 msec 120 dB pulse of broadband noise. The PREPULSE+PULSE trials consisted of a 20 msec noise prepulse, a 100 msec delay, then a 40 msec 120 dB startle pulse (120 msec onset-to-onset interval). Prepulse intensities were 4, 8, and 16 dB above the 65 dB background noise. The NOSTIM trial consisted of background noise only. Each test session began and ended with five presentations of the PULSE-ALONE trial; in between, each trial type was presented 10 times in a pseudorandom order. The time between trials averaged 15 sec (range, 12–30 sec). After the mice were placed in the startle chambers, a 65 dB background noise level was presented for a 10 min acclimation period and continued throughout the test session. Each animal was always tested in the same startle chamber.
After the initial characterization of locomotor activity, mice were tested in a baseline session to determine PPI and startle reactivity levels. Mice were then assigned to receive either drug or vehicle (balanced for startle chamber assignment and treatment) and were tested in the PPI session. The amount of PPI was calculated as a percentage score for each prepulse trial type: % PPI = 100 − {[(startle response for PREPULSE+PULSE)/(startle response for PULSE-ALONE)] × 100}. Startle magnitude was calculated as the average response to all of the PULSE-ALONE trials, excluding the first and last blocks of five PULSE-ALONE trials. ANOVAs were used to compare means; where applicable, Tukey's tests were used for post hoc analysis. If there were no significant main effects or interactions with gender, data from males and females were combined for analysis. The computations were performed using the BMDP statistical package (SPSS, Inc., Chicago, IL).
For brevity, main effects of prepulse intensity (which were always significant) will not be discussed. PPI was also analyzed using difference scores, where PPI Difference = (PULSE-ALONE − PREPULSE+ PULSE) for each prepulse trial type. Using these difference scores, the same ANOVAs were performed, and the same effects of genotype and genotype by drug treatment interactions were found (data not shown). Data from the NOSTIM trials are not included in Results because the values were negligible relative to values on trials containing startle stimuli. The habituation of the startle response was investigated by grouping the startle trials into four blocks (five PULSE-ALONE trials each, in order of presentation) and measuring the decrease of startle magnitudes across blocks. ANOVAs revealed significant main effects of block, indicating normal habituation of the startle response, but there were no consistent interactions between block and genotype to suggest that the DAT (−/−) mice had any impairment in startle habituation (data not shown).
Locomotor pattern testing. The DAT mice were characterized initially in the VT enclosure before any PPI testing. Each mouse was placed in the bottom left-hand corner of an enclosure at the start of the test session. The movements of the mice were tracked for either 15 or 30 min, with data being stored in three or six 5 min blocks, respectively. Two categories of measures were obtained. First, the amount of locomotor activity was measured by the distance traveled, i.e., tracing the consecutive locations of the animal using the highest resolution of the video-tracker and calculating the distance between them. Second, the geometric patterns of locomotor activity were quantified by the spatial scaling exponent, d, as described in detail elsewhere (Paulus and Geyer, 1991a). Briefly, the spatial scaling exponent quantifies the extent to which a sequence of movements falls along a straight line (d = 1) or within a circumscribed area (d = 2). The locomotor activity data were analyzed based on a genotype-by-time or a genotype-by-drug-by-time ANOVA.
RESULTS
Prepulse inhibition
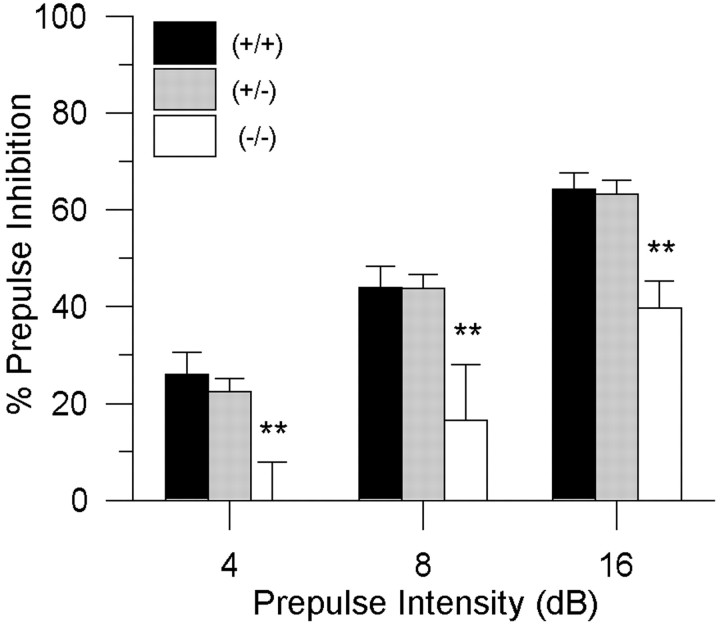
As hypothesized, the DAT (−/−) mice exhibited a consistent deficit in sensorimotor gating, as measured by the PPI of the startle response (Fig. 1). There was a main effect of genotype [F(2,76) = 8.3;p < 0.01] on PPI and no interaction with prepulse intensity [F(4,152) = 0.3; NS].Post hoc comparisons revealed that the DAT (−/−) group differed significantly from both the DAT (+/+) and DAT (+/−) groups of mice at the 4 dB [F(2,76) = 6.2;p < 0.01], 8 dB [F(2,76) = 6.2; p < 0.01], and 16 dB [F(2,76) = 6.8;p < 0.01] prepulse intensities. This deficit in PPI in the transporter knock-out mice was not associated with any significant alteration in startle reactivity (Table1).
Fig. 1.
Baseline PPI levels in DAT mice from the first cohort. PPI was significantly disrupted in the DAT knock-out (−/−) mice at each prepulse intensity, compared with both DAT (+/+) and DAT (+/−) mice (** p < 0.01). Values represent mean % PPI ± SEM.
Table 1.
Acoustic startle reactivity levels in the DAT mutant mice
| DAT (+/+) | DAT (+/−) | DAT (−/−) | |
|---|---|---|---|
| Baseline | |||
| Cohort 1 | 241.2 ± 32.1 | 203.2 ± 14.4 | 237.6 ± 33.9 |
| Cohort 2, female | 185.0 ± 18.0 | 186.6 ± 12.4 | 157.8 ± 19.2 |
| Cohort 2, male | 237.6 ± 23.6 | 302.4 ± 20.0* | 170.6 ± 15.8* |
| Raclopride | |||
| Vehicle | 270.7 ± 40.2 | 233.5 ± 17.1 | 233.8 ± 32.3 |
| 3.0 mg/kg | 263.4 ± 33.7 | 180.7 ± 14.2 | 218.6 ± 47.9 |
| SCH23390 | |||
| Vehicle | 232.6 ± 13.8 | 269.2 ± 15.3 | 210.2 ± 8.7 |
| 1.0 mg/kg | 223.8 ± 15.5 | 259.2 ± 13.0 | 199.8 ± 13.3 |
At baseline, there were no differences in startle reactivity between genotypes in Cohort 1, but the male DAT (+/−) mice in the second cohort had higher startle reactivity, and the (−/−) mice had lower startle reactivity compared with (+/+) controls (*p > 0.05). Treatment with 3.0 mg/kg raclopride or 1.0 mg/kg SCH23390 had no significant effect on the startle response in the DAT mice. Values (arbitrary units) represent mean startle magnitude ± SEM.
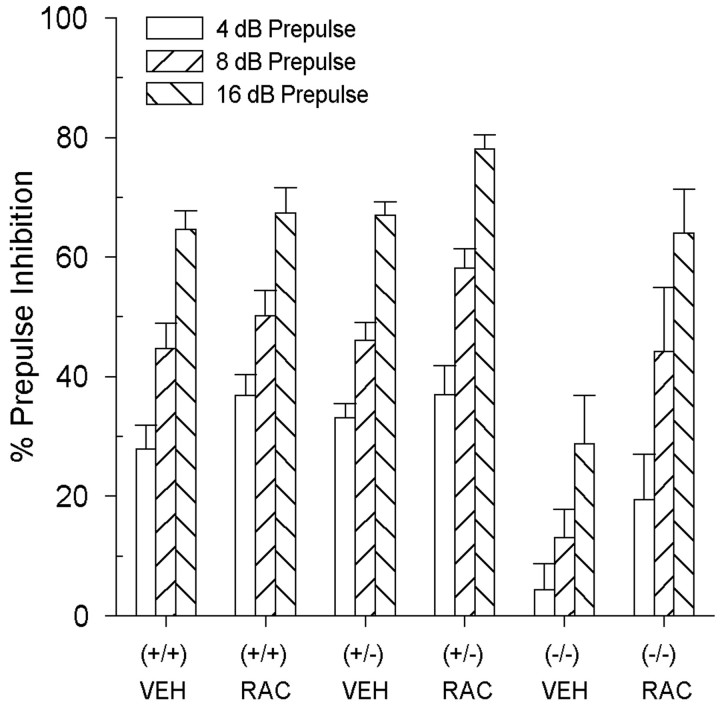
When the three groups of DAT mice were treated with the DA D2 receptor antagonist, raclopride, PPI was increased significantly in the DAT (−/−) and (+/−) mice, but not in the (+/+) mice (Fig.2). There were main effects of genotype [F(2,75) = 10.0; p < 0.01] and drug treatment [F(1,75) = 30.0; p < 0.01], as well as a significant genotype by treatment interaction [F(2,75) = 4.7;p < 0.01]. To determine the source of this interaction, data were segregated by genotype, and two-way ANOVAs were completed with drug treatment and prepulse intensity being within-subjects factors. As expected from studies in rats (Swerdlow et al., 1991), raclopride had no effect in the DAT (+/+) mice (Fig. 2). In contrast, the DAT (−/−) mice showed a significant main effect of drug treatment [F(1,8) = 12.8;p < 0.01], reflecting a raclopride-induced increase in PPI (Fig. 2). In addition, the DAT (+/−) mice also showed significant increases in PPI after raclopride treatment [F(1,45) = 12.6; p < 0.01] (Fig. 2). There were no significant effects of genotype or raclopride treatment on startle reactivity in the DAT mice (Table1).
Fig. 2.
PPI levels in DAT mice after pretreatment with 3.0 mg/kg raclopride. Raclopride had no effect on PPI in the DAT (+/+) mice; however, raclopride significantly increased in both the DAT (−/−) and (+/−) mice (p < 0.01). Values represent mean % PPI ± SEM.
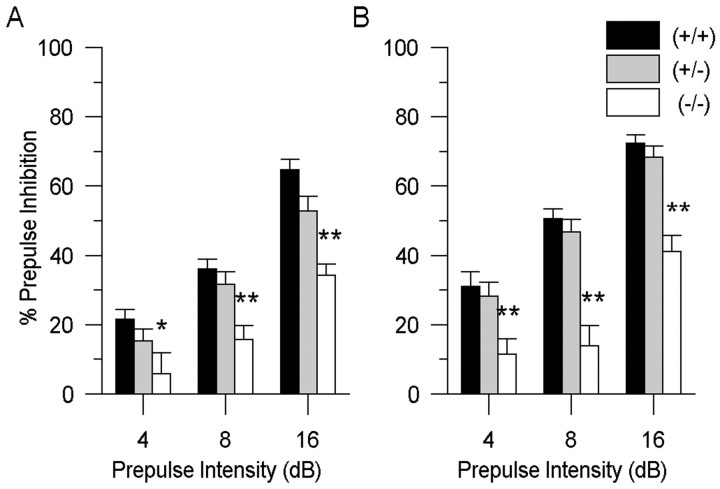
To determine the reliability of these phenotypic differences, a problem that has been raised as a general concern for all studies with mutant mice (Crabbe et al., 1999), a second cohort of DAT mice was examined. In this cohort, equivalent deficiencies in PPI were found in the DAT (−/−) mice. The large sample size of this cohort enabled us to determine that gender significantly affected startle plasticity. Because there was a significant main effect of gender on PPI [F(1,95) = 7.1; p < 0.01] and startle reactivity [F(1,95) = 10.1; p < 0.01], we considered the startle data from the female and male mice separately. Regardless of these gender differences, both the female and male DAT (−/−) mice still displayed decreased levels of PPI. There was a significant main effect of genotype on PPI in both female [F(2,56) = 10.0; p < 0.01] and male [F(2,52) = 20.5;p < 0.01] mice (Fig.3A,B). Similar to the previous cohort, further analysis revealed that female DAT (−/−) mice had significantly lower PPI compared with (+/+) controls at the 4 dB [F(2,56) = 3.5;p < 0.05], 8 dB [F(2,56) = 8.5; p < 0.01], and 16 dB [F(2,56) = 13.6;p < 0.01] prepulse intensities; comparable disruptions in PPI were also found in the male DAT (−/−) mice at the 4 dB [F(2,52) = 6.4;p < 0.01], 8 dB [F(2,52) = 22.2; p < 0.01], and 16 dB [F(2,52) = 22.5;p < 0.01] prepulse intensities. Although female DAT mice had comparable levels of startle reactivity, there was a significant main effect of genotype on startle reactivity in the male DAT mice [F(2,52) = 10.9;p < 0.01], in which startle reactivity tended to be higher for the DAT (+/−) and lower for the DAT (−/−), compared with (+/+) controls (p < 0.1) (Table 1).
Fig. 3.
Baseline PPI levels in the second cohort of female and male DAT mice. Despite a gender effect, PPI was significantly disrupted in the female (A) and male (B) DAT knock-out (−/−) mice at each prepulse intensity compared with both DAT (+/+) and (+/−) mice (*p < 0.05, ** p < 0.01). Values represent mean % PPI ± SEM.
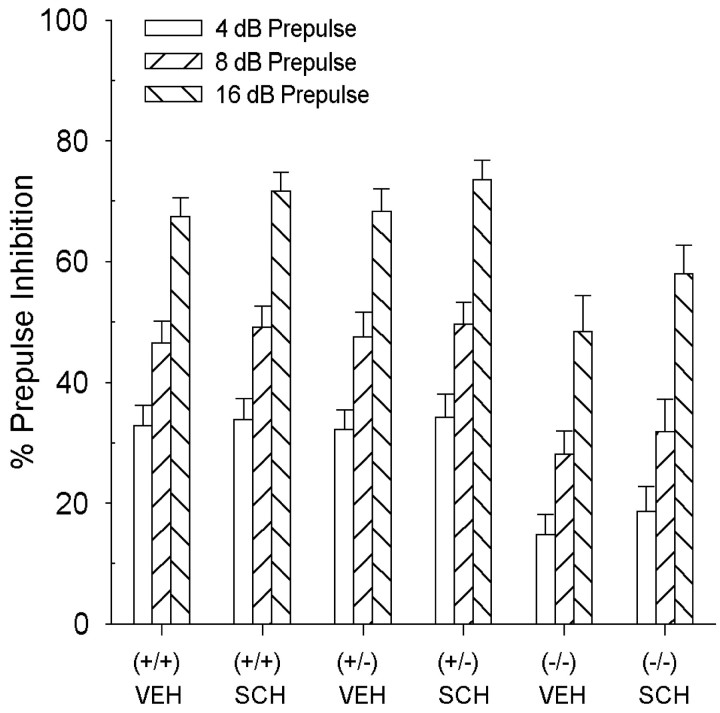
In an attempt to restore PPI and further characterize the role of the D1 receptor in the mediation of PPI, the male DAT mice were tested with the DA D1 receptor antagonist, SCH23390. The DAT (−/−) mice, however, continued to have lower levels of PPI compared with (+/+) mice, evidenced by a main effect of genotype on PPI [F(2,52) = 12.9; p < 0.01] (Fig. 4). There were no main effects of genotype or drug treatment on startle reactivity in the male DAT mice (Table 1).
Fig. 4.
PPI levels in DAT mice after pretreatment with 1.0 mg/kg SCH23390. SCH23390 had no effect on PPI in the DAT (+/+), (+/−), or (−/−) mice. Values represent mean % PPI ± SEM.
Motor behavior analysis
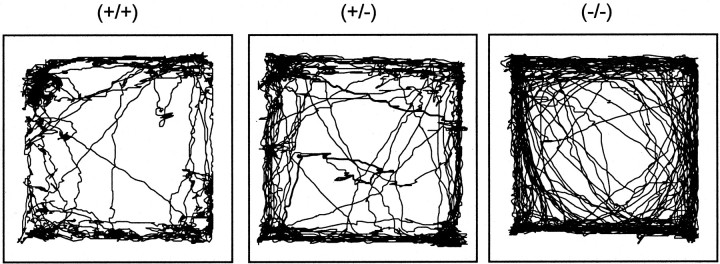
Figure 5 shows a 5 min sample of the movement patterns from each of the three genotypes during the test session in the video-tracker enclosure from the first cohort of DAT mice. The movement patterns of the DAT (+/+) and (−/−) mice reflect the profound differences in the sequential organization of their behavior. The wild-type mouse exhibited a mixture of straight, meandering, and circumscribed movements around the enclosure. In contrast, the homozygous mouse engaged almost exclusively in repetitive straight movements around the perimeter of the enclosure. The heterozygous mutant exhibited bouts of both repetitive straight movement and varied movement patterns. These differences were confirmed quantitatively by the spatial scaling exponent, d, a measure based on fractal geometry, which quantifies the degree to which sequences of movements are straight (d = 1) or circumscribed (d = 2) (Paulus and Geyer, 1991b). The significant effect of genotype on the d measure [F(2,74) = 8.1; p < 0.01] confirmed the reliability of the observed pattern differences. There was a significant time-by-genotype interaction [F(4,148) = 2.5; p < 0.05]; post hoc analysis revealed that the average pattern did not differ across genotype during the first 5 min of testing. In contrast, during the second and third 5 min blocks, both the DAT (+/−) and (−/−) mice exhibited significantly straighter movements (lower spatial d values) when compared with (+/+) controls (p < 0.05 and p < 0.01, respectively), with the (+/−) being significantly higher than the (−/−) (p < 0.05). Thus, the interaction between genotype and time is attributable to the fact that the DAT (+/−) and (−/−) mice, relative to DAT (+/+) mice, show an attenuated transition from predominantly straight movements to predominantly circumscribed movements that typically accompanies the habituation of locomotor activity in a novel environment (Paulus et al., 1999). Confirming previous reports (Giros et al., 1996), a significant main effect of genotype on locomotor activity was found [F(2,74) = 26.8; p < 0.01]. DAT (−/−) mice were more active than the DAT (+/+) and (+/−) mice in the open field (p < 0.01), with the DAT (+/−) levels of locomotor activity falling between the (+/+) and (−/−) levels.
Fig. 5.
Baseline characterization of the patterns of locomotor activity. The DAT (+/+) mouse (left panel) explored all parts of the video-tracker enclosure and engaged in both circumscribed and straight movements, particularly near the corners of the enclosure. In contrast, the DAT (−/−) mouse (right panel) engaged in repetitive straight movements along the walls of the enclosure and rarely ventured into the center region. The DAT (+/−) mouse (center panel) showed a pattern that contained elements of occasional meandering and circumscribed movements and some repetitive straight movements in the periphery of the enclosure. Each pattern was reconstructed from a representative mouse from each genotype using the first 5 min of data.
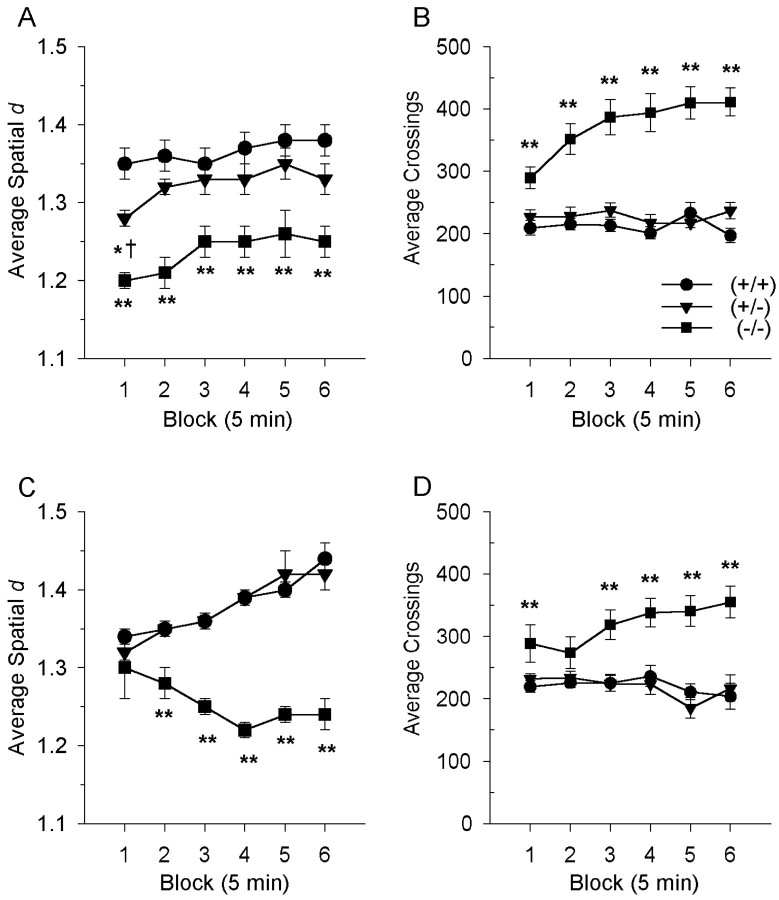
The same perseverative motor phenotype was found in the second cohort of DAT mice, in which the DAT (−/−) mice had markedly different patterns of motor activity as measured by spatial d at baseline testing. The larger sample size of the second cohort enabled us to examine whether the sequential organization of behavior differed across gender. Indeed, male and female mice differed in their patterns of locomotor behavior [F(1,98) = 6.3;p < 0.01]. Specifically, female mice, when compared with males, showed straighter movement sequences in the test enclosures. In addition, the spatial d values for the heterozygous (+/−) female mice showed an intermediate level of motor patterning, composed of circumscribed and meandering movements as well as straight movement sequences. Consequently, the motor activity data were separated by gender for further analysis. As seen in Figure6A, the female DAT (−/−) mice were more perseverative in their motor behavior than (+/+) controls, with significant main effects of genotype [F(2,52) = 18.2; p < 0.01] and time [F(5,260) = 6.3;p < 0.01] on spatial d. The female DAT (−/−) mice moved in straighter sequences (lower spatial dvalues) than the DAT (+/+) (p < 0.01) or the (+/−) (p < 0.05) mice in each block of time. The female DAT (−/−) mice were also hyperactive, supported by a significant main effect of genotype on the amount of activity in the female DAT mice [F(2,49) = 19.7;p < 0.01] and a time-by-genotype interaction [F(10,245) = 2.9; p< 0.01]. The female DAT (−/−) mice were more active compared with either the (+/+) or (+/−) mice in each block (p< 0.01), except for the second block in which there was a trend toward the (−/−) mice traveling more than the (+/+) mice (Fig.6B).
Fig. 6.
Measures of locomotor behavior in the DAT mice. The spatial scaling exponent, d, is based on the concept of fractal geometry and describes the roughness or straightness of the path by relating the number of movements to the distance between these movements. Specifically, a d value of 1 indicates that successive movements follow a straight line, whereas a dvalue of 2 signifies a highly circumscribed movement pattern.A, In the female mice, the movement patterns of DAT (−/−) mice were characterized by a significantly reducedd value in each block, indicating a predominance of repetitive straight movement sequences (**p < 0.01). DAT (+/−) mice exhibited locomotor patterns that were intermediate to the DAT (+/+) and (−/−) mice in block 1 [*p < 0.05, compared with DAT (+/+) mice;†p < 0.05, compared with DAT (−/−) mice]. C, In the male mice, the DAT (−/−) mice also displayed significantly straighter sequences of motor behavior, indicated by lower spatial d values compared with (+/+) control mice (**p < 0.01). Both the female (B) and male (D) DAT (−/−) mice were significantly more active than the DAT (+/+) and (+/−) mice across the test session (**p < 0.01). Values represent means ± SEM for six 5 min blocks.
Similar findings of perseverative and hyperactive motor behavior were also found in the male DAT mice. There were significant main effects of gene [F(2,46) = 36.9;p < 0.01] and time [F(5,230) = 3.2; p < 0.01] on spatial d, with interactions between genotype and time [F(10,230) = 4.0;p < 0.01]. There were no significant differences between genotypes in the first 5 min block, but the DAT (−/−) mice moved in significantly straighter sequences (lower spatial dvalues) than either the (+/+) or (+/−) mice (p< 0.01) for the rest of the test session (Fig. 6C). The male DAT (−/−) also displayed increases in activity, confirmed by main effects of genotype [F(2,54) = 38.8; p < 0.01] and time [F(5,270) = 7.6; p < 0.01], as well as a time-by-genotype interaction [F(10,270) = 7.7; p< 0.01]. Like the female mice, the male DAT (−/−) mice were hyperactive compared with either the DAT (+/+) or (+/−) mice in each block of time (p < 0.01) (Fig.6D).
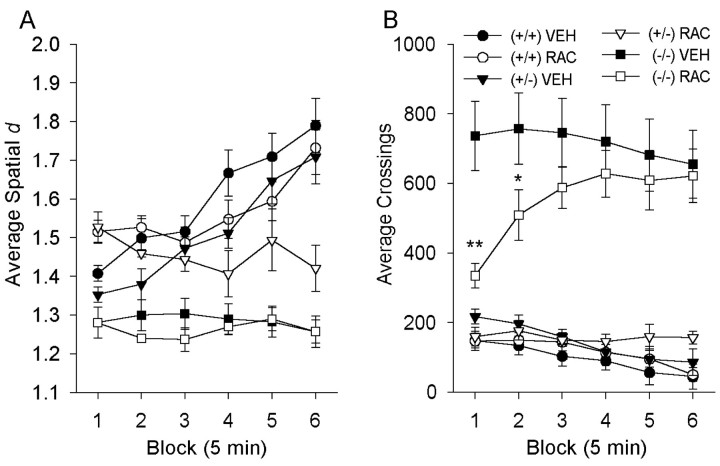
To examine whether the changes in locomotor patterns induced by the removal of the DA transporter were related to an increased occupancy of the D2 receptor, a DA D2 receptor antagonist was administered before testing. As seen in Figure 7, raclopride significantly affected the amount of locomotor activity in the DAT (−/−) mice, but these effects were not parallel to those observed for the patterns of locomotor behavior. In heterozygous (+/−) mice, however, raclopride differentially and time-dependently affected the locomotor patterns. Specifically, although neither (+/+) nor DAT (−/−) mice exhibited changes in locomotor patterns, DAT (+/−) showed a significant increase in spatial d, indicating a more circumscribed movement pattern (Figs. 7,8A). There were significant main effects of genotype [F(2,43) = 45.5; p < 0.01] and time [F(5,215) = 16.4;p < 0.01] on spatial d, with interactions between block and genotype [F(10,215)= 6.1; p < 0.01], block and drug treatment [F(5,215) = 7.8; p < 0.01], and time and genotype and drug treatment [F(10,215) = 4.0; p< 0.01]. There was no effect of raclopride in either the (+/+) or (−/−) mice, but raclopride made the DAT (+/−) mice more local in their motor behavior (increased spatial d) in blocks 1, 2, and 6 (p < 0.01) (Fig. 8A). As during baseline testing, the vehicle-treated DAT (−/−) mice moved in significantly straighter sequences (lower spatial dvalues) at blocks 2–6 (p < 0.01), with a trend toward straighter movements in block 1 (p < 0.10), when compared with (+/+) controls (Fig. 8A). The vehicle-treated (−/−) mice were also more active than the (+/+) controls, and, as expected from previous studies (Paulus and Geyer, 1991a), the D2 receptor antagonist significantly reduced the hyperactivity in the DAT (−/−) mice. There were significant main effects of genotype [F(2,43) = 89.7;p < 0.01] and time [F(5,215) = 4.0; p < 0.01] on the amount of motor activity, with block-by-genotype [F(10,215) = 6.4; p< 0.01], block-by-drug [F(5,215) = 10.7; p < 0.01], and block-by-genotype-by-drug [F(10,215) = 3.8; p< 0.01] interactions, as well as a nearly significant genotype by drug [F(2,43) = 3.0;p = 0.06] interaction. Raclopride significantly reduced motor activity in the DAT (−/−) mice in block 1 (p < 0.01) and block 2 (p < 0.05), although they were still more active than the (+/+) controls (p < 0.01) (Fig.8B). In contrast, raclopride had no effect in either the (+/+) or (+/−) mice.
Fig. 7.
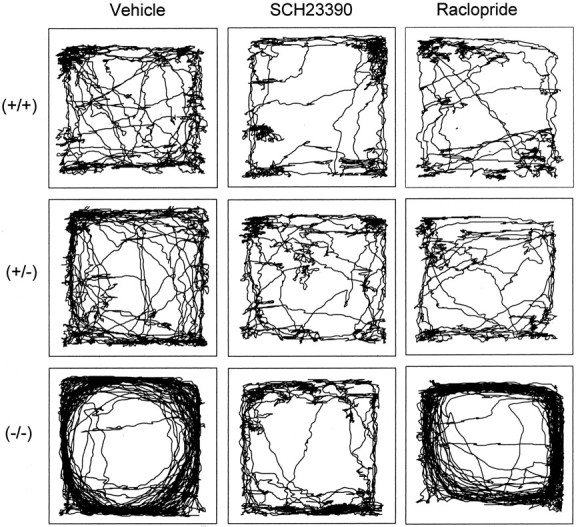
Five min sampling of patterns of locomotor activity in DAT mice treated with vehicle, SCH23390, or raclopride.
Fig. 8.
Effects of 0.1 mg/kg raclopride on spatiald and amount of locomotor activity. A, In the female mice, the movement patterns of DAT (−/−) mice were not affected by pretreatment with the D2 antagonist raclopride.B, The amount of locomotor activity was significantly reduced, however, in the DAT (−/−) mice pretreated with raclopride in blocks 1 and 2 (*p < 0.05, **p< 0.01), while having no effect in either the DAT (+/+) or (+/−) mice. Values represent means ± SEM for six 5 min blocks.
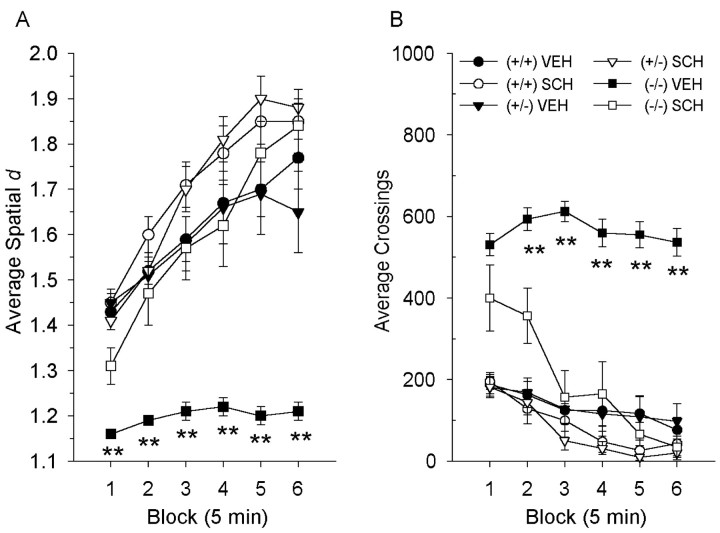
In a separate experiment, the effect of reducing DA occupancy at the D1 receptor was examined by pretreatment with SCH23390. If D1 receptors are critical for the distance-covering movements, which has been hypothesized for rats previously (Paulus et al., 1993), one would expect an increase in spatial d regardless of changes in locomotor activity in mice that exhibit an increased dopaminergic tone. Specifically, it was hypothesized that DAT (−/−) and DAT (+/−) but not (+/+) mice should show an increase in spatial d after the administration of SCH 23390. When the male DAT mice from the second cohort were challenged with DA D1 receptor antagonist SCH23390, the DAT (−/−) mice were less perseverative and less active in their motor behavior (Fig. 7). Main effects of genotype [F(2,43) = 27.1; p < 0.01], drug [F(1,43) = 38.0;p < 0.01], and block [F(5,215) = 94.3; p< 0.01] were found on spatial d, with interactions between genotype and drug [F(2,43) = 9.2;p < 0.01], time and drug [F(10,215) = 16.1; p< 0.01], and time and genotype and drug [F(10,215) = 3.1; p< 0.01]. The vehicle-treated DAT (−/−) mice maintained more straight patterns of motor behavior (lower spatial d values) in blocks 1–6 compared with both (+/+) and (+/−) mice (p < 0.01) (Fig.9A). SCH23390 significantly increased spatial d in the DAT (−/−) mice in each block (p < 0.01), inducing more local and meandering behavior, while having no effect in either the (+/+) or (+/−) mice (Fig. 9A). D1 receptor antagonism also reduced the DAT (−/−) hyperactivity, confirmed by significant main effects of genotype [F(2,43) = 61.7;p < 0.01], drug treatment [F(1,43) = 47.0; p < 0.01], and time [F(5,215) = 40.8;p < 0.01] on the amount of activity displayed by the DAT mice, with genotype-by-drug treatment [F(2,43) = 21.0; p < 0.01], time-by-drug treatment [F(5,215) = 14.9; p< 0.01], and time-by-genotype-by-drug [F(10,215) = 4.9; p< 0.01] interactions. The vehicle-treated DAT (−/−) mice continued to show hyperactivity compared with both the vehicle-treated (+/+) and (+/−) mice in each block (p < 0.01), whereas SCH23390 significantly reduced the amount of activity in the DAT (−/−) mice in blocks 2–6 (p < 0.01) (Fig.9B).
Fig. 9.
Effects of 0.01 mg/kg SCH23390 on spatiald and amount of locomotor activity. A, In the male mice, the D1 antagonist SCH23390 significantly attenuated the perseverative motor patterns displayed by the DAT (−/−) mice across the test session (**p < 0.01). B, D1 receptor antagonism also reduced the amount of locomotor activity in the DAT (−/−) mice in blocks 2–6 (**p < 0.01). Values represent means ± SEM for six 5 min blocks.
DISCUSSION
In this study, two separate cohorts of DAT (−/−) mice exhibited two behavioral phenotypes: a profound disruption of sensorimotor gating and highly perseverative locomotor patterns. Unlike either DAT (+/+) or (+/−) mice, DAT (−/−) mice had disrupted PPI, which was reversed significantly by pretreatment with the preferential D2 receptor antagonist raclopride. This result supports the hypothesis that D2 receptors are necessary for the dopaminergic modulation of PPI and is in line with previous findings in both rats and mice (Mansbach et al., 1988; Peng et al., 1990; Swerdlow et al., 1991; Ralph et al., 1999). The selective DA D1 receptor antagonist, SCH23390, however, was ineffective at increasing DAT (−/−) PPI, suggesting that D1 receptors are not necessary for the dopaminergic modulation of PPI. The DAT (−/−) mice also displayed nonfocal perseverative motor patterns of behavior, repeatedly moving in straight sequences, compared with DAT (+/+) control mice. The DAT (−/−) patterns of motor behavior were affected differentially by DA receptor antagonists: D2 receptor blockade had no effect on the perseverative patterns, whereas D1 receptor antagonism augmented the perseverative nature of the motor behavior. These results demonstrate that D1 receptors are critically involved in the expression of repetitive perseverative movements in DAT (−/−) mice. As previously reported (Giros et al., 1996), DAT (−/−) mice were also found to be hyperactive, and both D1 and D2 receptor blockade significantly reduced the amount of motor behavior produced by the (−/−) mice.
Stimulation of the dopaminergic system via blockade of the transporter or by direct agonists disrupts PPI in rodents (Mansbach et al., 1988;Dulawa and Geyer, 1996). Accordingly, DAT (−/−) mice exhibited profound deficits in PPI, presumably because of their hyperdopaminergic tone. Of note was the lack of disruption in PPI in DAT (+/−) mice, which have an intermediate expression of the DAT and a corresponding intermediate elevation of extracellular DA levels [twofold increase compared with fivefold in DAT (−/−)], as well as decreases in DA release, uptake, and tissue concentrations relative to DAT (+/+) and (−/−) mice (Jones et al., 1998). Hence, a gene-dosage effect does not appear to be present in the PPI phenotype in these mice. Instead, profound changes in the DA system in DAT (−/−) mice appear to be necessary to produce a disruption in sensorimotor gating in mice. These results are consistent with pharmacological studies which have shown that high doses of DA agonists are necessary to disrupt PPI in mice (Dulawa and Geyer, 1996; Ralph et al., 1999). Although no gene-dosage effect was present in the PPI phenotype, it should be noted that others have reported the presence of gene-dosage effects in other phenotypes not involving behavioral measures (Jones et al., 1999).
Disruptions in PPI produced by DA agonists are reversed by DA D2 receptor antagonists (Mansbach et al., 1988; Swerdlow et al., 1991). Consistent with these previous reports, the D2 receptor antagonist raclopride reversed the PPI deficits in the DAT (−/−) mice without affecting startle reactivity. Furthermore, the dose of raclopride used to restore PPI in DAT (−/−) mice had no effect on PPI in the DAT (+/+) mice. In preliminary studies, the clinically effective antipsychotic haloperidol, which has a high affinity for the D2 receptor, also significantly reversed deficient PPI observed in a separate cohort of DAT (−/−) mice (Ralph et al., 1997). The fact that the DAT (−/−) has 50% downregulation of D2 receptors (Giros et al., 1996) and loss of autoreceptor function (Jones et al., 1999) did not appear to affect the ability of raclopride to reverse the PPI deficit in the mutants, indicating that the D2 receptor system is still functional despite the profound changes found in the DAT (−/−) mice. Although DA D2 receptor antagonism significantly increased PPI in the DAT (−/−) mice, the preferential DA D1 receptor antagonist, SCH23390, had no effect on PPI. Previous studies have suggested that the DA D1 receptor does not have an independent role in the modulation of PPI in rats but rather acts together with the DA D2 receptor (Peng et al., 1990; Wan et al., 1996). Our data suggest that a similar synergistic mechanism may be present in mice, although further pharmacological studies are necessary to confirm this conclusion. In sum, the improvement in sensorimotor gating in the DAT (−/−) mice by D2 receptor antagonists corroborates evidence that the PPI-disruptive effects of amphetamine are absent in D2, but not D3 or D4, receptor knock-out mice (Ralph et al., 1999) and further supports the specific role of D2 receptors in the modulation of PPI.
The locomotor activity of DAT (−/−) mice was characterized by repetitive, perseverative straight movements in the periphery of the enclosure. In contrast to both DAT (+/+) and (+/−) mice, these animals did not sample the entire enclosure; rather, the DAT (−/−) showed a restricted repertoire of locomotor behavior. This restriction of the behavioral repertoire is similar to the behavioral patterns that are observed in rats treated with the direct D1/D2 agonist apomorphine (Geyer et al., 1986, 1987; Paulus and Geyer, 1991a). The D2 receptor antagonist raclopride attenuated the locomotor hyperactivity but did not significantly alter the perseverative movement patterns of DAT (−/−) mice. Interestingly, raclopride produced more perseverative patterns in the DAT (+/−) mice compared with vehicle-treated (+/−) mice but not in DAT (+/+) or (−/−) mice. Thus, blocking the D2 receptor in a mouse with mild hyperdopaminergia, which may result in a relative increase of D1 occupancy, increased perseverative locomotor patterns. This finding is consistent with the effect of the D1 antagonist SCH23390 to reduce the perseverative locomotor patterns in the hyperdopaminergic DAT (−/−) mouse. Therefore, with increased dopaminergic tone, occupancy of D1 receptors may be critical in regulating the perseverative aspect of locomotor behavior in mice. These findings are also consistent with pharmacological evidence that antagonism of DA D1 receptors, but not D2 receptors, blocks the perseverative locomotor behavior induced by amphetamine (Fritts et al., 1997). Furthermore, the motor patterns in rats treated with the D2/D3 receptor agonist, quinpirole, become straighter when coadministered with the D1 agonist, SKF38393 (Eilam et al., 1991). Taken together, these findings provide further evidence that D1 receptor activation is a critical substrate for the expression of perseverative movement patterns. Although both receptors appear to regulate how frequently behaviors are expressed, the D1 receptor may be critical to how behaviors are organized. Indeed, it has been suggested that perseverations are linked to prefrontal modulation of ongoing behaviors (Crider, 1997), a hypothesis that may be related to the important role of D1 receptors in prefrontal cortical function. Therefore, prefrontal D1 receptors may provide a molecular target to adjust the extent or degree of perseverative behavior.
Although there has been some recent criticism of the reliability of behavioral phenotypes in genetically altered mice (Crabbe et al., 1999), we have now observed these two striking behavioral phenotypes in three separate cohorts of DAT mice (Paulus et al. 1997; Ralph et al., 1997). Although deficits in sensorimotor gating and perseverative patterns of motor behavior were seen consistently in both the female and male DAT (−/−), some gender differences were found. When a large cohort of DAT mice was tested, we identified significant gender differences in both PPI and startle reactivity. Male DAT mice had higher PPI and startle reactivity than female mice. The female DAT (−/−) mice also moved in straighter sequences (lower spatiald values) compared with male DAT (−/−) mice. In contrast to the PPI phenotype, there was a gene-dosage effect on the patterns of locomotor activity. Specifically, female (+/−) mice displayed an intermediate alteration of movement patterns characterized by bouts of both repetitive straight movements and meandering or circumscribed movements. Although further experiments will need to clarify the role of gender in the gene-dosage effect on patterns of locomotor behavior, these results underscore the importance of examining the behavioral phenotype of a mutant mouse in both genders. Although we used a relatively large number of mice in these experiments, which provided sufficient power to reliably detect phenotypic differences between DAT (+/−) and (−/−) female mice, some of the phenotypic differences may be too small to be detected reliably. Therefore, a differential effect size may provide an alternative explanation for the gender-dependent gene-dosage effect.
When the DA system is functioning normally, as in the (+/+) mice, behavioral organization is intact, and normal motor activity and gating can occur. If, however, the DA system is perturbed, in this case through the hyperdopaminergic state produced by the DAT mutation, there appears to be a disruption in the processes that are involved in both the modulation of incoming stimuli and the appropriate expression of motor responses. Furthermore, there seems to be a differential DA receptor modulation of these behavioral phenomena, in which the D2 receptor is a key modulator of deficits in PPI and the D1 receptor modulates the perseverative motor patterns in the DAT (−/−) mice. Several psychiatric populations display both deficits in PPI and perseverative behaviors, including schizophrenia, schizotypal personality disorder, obsessive–compulsive disorder, Tourette's syndrome, and attention deficit hyperactivity disorder (Braff et al., 1992; Cadenhead et al., 1993; Swerdlow et al., 1993; Castellanos et al., 1996; Swerdlow, 1998). By revealing aspects of the mechanisms that control the behavioral deficits seen in the DAT (−/−) mice, critical information that is necessary to further understand the pathophysiologies of these disorders and their therapeutic profiles may be provided.
Footnotes
This work was supported by the Veterans Affairs VISN 22 Mental Illness Research, Education, and Clinical Center, National Institute on Drug Abuse Grants DA02925 and DA11277, and National Institute of Mental Health Grants F31-MH12806, MH61326, and MH42228. M. A. Geyer holds an equity interest in San Diego Instruments. M. G. Caron is an investigator for the Howard Hughes Medical Institute. We thank Beth Gregersen-Coates, Darlene Giracello, Jason Holt, and Susan Suter for excellent technical assistance.
Correspondence should be addressed to Dr. Mark A. Geyer, Department of Psychiatry, University of California San Diego, La Jolla, CA 92093-0804. E-mail: mgeyer@ucsd.edu.
REFERENCES
- 1.Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch Gen Psychiatry. 1992;49:206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- 2.Cadenhead KS, Geyer MA, Braff DL. Impaired startle prepulse inhibition and habituation in patients with schizotypal personality disorder. Am J Psychiatry. 1993;150:1862–1867. doi: 10.1176/ajp.150.12.1862. [DOI] [PubMed] [Google Scholar]
- 3.Castellanos FX, Fine EJ, Kaysen D, Marsh WL, Rapoport JL, Hallett M. Sensorimotor gating in boys with Tourette's syndrome and ADHD: preliminary results. Biol Psychiatry. 1996;39:33–41. doi: 10.1016/0006-3223(95)00101-8. [DOI] [PubMed] [Google Scholar]
- 4.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 5.Crider A. Perseveration in schizophrenia. Schizophr Bull. 1997;23:63–74. doi: 10.1093/schbul/23.1.63. [DOI] [PubMed] [Google Scholar]
- 6.Curzon P, Decker MW. Effects of phencyclidine (PCP) and (+)MK-801 on sensorimotor gating in CD-1 mice. Prog Neuropsychopharmacol Biol Psychiatry. 1998;22:129–146. doi: 10.1016/s0278-5846(97)00184-x. [DOI] [PubMed] [Google Scholar]
- 7.Dulawa SC, Geyer MA. Psychopharmacology of prepulse inhibition in mice. Chin J Physiol. 1996;39:139–146. [PubMed] [Google Scholar]
- 8.Dulawa SC, Hen R, Scearce-Levie K, Geyer MA. Serotonin1B receptor modulation of startle reactivity, habituation, and prepulse inhibition in wild-type and serotonin1B knockout mice. Psychopharmacology (Berl) 1997;132:125–134. doi: 10.1007/s002130050328. [DOI] [PubMed] [Google Scholar]
- 9.Eilam D, Clements KV, Szechtman H. Differential effects of D1 and D2 dopamine agonists on stereotyped locomotion in rats. Behav Brain Res. 1991;45:117–124. doi: 10.1016/s0166-4328(05)80077-4. [DOI] [PubMed] [Google Scholar]
- 10.Fritts ME, Mueller K, Morris L. Amphetamine-induced locomotor stereotypy in rats is reduced by a D1 but not a D2 antagonist. Pharmacol Biochem Behav. 1997;58:1015–1019. doi: 10.1016/s0091-3057(97)00308-0. [DOI] [PubMed] [Google Scholar]
- 11.Geyer MA, Swerdlow NR. Measurement of the startle response and its use in preclinical measures of prepulse inhibition and habituation. In: Crawley JN, Skolnick P, editors. Current protocols in neuroscience. Wiley; New York: 1998. pp. 8.7.1–8.7.15. [DOI] [PubMed] [Google Scholar]
- 12.Geyer MA, Russo PV, Masten VL. Multivariate assessment of locomotor behavior: pharmacological and behavioral analyses. Pharmacol Biochem Behav. 1986;25:277–288. doi: 10.1016/0091-3057(86)90266-2. [DOI] [PubMed] [Google Scholar]
- 13.Geyer MA, Russo PV, Segal DS, Kuczenski R. Effects of apomorphine and amphetamine on patterns of locomotor and investigatory behavior in rats. Pharmacol Biochem Behav. 1987;28:393–399. doi: 10.1016/0091-3057(87)90460-6. [DOI] [PubMed] [Google Scholar]
- 14.Giros B, Jaber M, Jones SR, Wightman RM, Caron MG. Hyperlocomotion and indifference to cocaine and amphetamine in mice lacking the dopamine transporter. Nature. 1996;379:606–612. doi: 10.1038/379606a0. [DOI] [PubMed] [Google Scholar]
- 15.Graham FK. Presidential Address 1974: the more or less startling effects of weak prestimulation. Psychophysiology. 1975;12:238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- 16.Hoffman HS, Ison JR. Reflex modification in the domain of startle: I. Some empirical findings and their implications for how the nervous system processes sensory input. Psychol Rev. 1980;87:175–189. [PubMed] [Google Scholar]
- 17.Jones SR, Gainetdinov RR, Jaber M, Giros B, Wightman RM, Caron MG. Profound neuronal plasticity in response to inactivation of the dopamine transporter. Proc Natl Acad Sci USA. 1998;95:4029–4034. doi: 10.1073/pnas.95.7.4029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jones SR, Gainetdinov RR, Hu XT, Cooper DC, Wightman RM, White FJ, Caron MG. Loss of autoreceptor functions in mice lacking the dopamine transporter. Nat Neurosci. 1999;2:649–655. doi: 10.1038/10204. [DOI] [PubMed] [Google Scholar]
- 19.Mansbach RS, Geyer MA, Braff DL. Dopaminergic stimulation disrupts sensorimotor gating in the rat. Psychopharmacology (Berl) 1988;94:507–514. doi: 10.1007/BF00212846. [DOI] [PubMed] [Google Scholar]
- 20.O'Neill MF, Shaw G. Comparison of dopamine receptor antagonists on hyperlocomotion induced by cocaine, amphetamine, MK-801 and the dopamine D1 agonist C-APB in mice. Psychopharmacology (Berl) 1999;145:237–250. doi: 10.1007/s002130051055. [DOI] [PubMed] [Google Scholar]
- 21.Paulus MP, Geyer MA. A scaling approach to find order parameters quantifying the effects of dopaminergic agents on unconditioned motor activity in rats. Prog Neuropsychopharmacol Biol Psychiatry. 1991a;15:903–919. doi: 10.1016/0278-5846(91)90018-v. [DOI] [PubMed] [Google Scholar]
- 22.Paulus MP, Geyer MA. A temporal and spatial scaling hypothesis for the behavioral effects of psychostimulants. Psychopharmacology (Berl) 1991b;104:6–16. doi: 10.1007/BF02244547. [DOI] [PubMed] [Google Scholar]
- 23.Paulus MP, Callaway CW, Geyer MA. Quantitative assessment of the microstructure of rat behavior: II. Distinctive effects of dopamine releasers and uptake inhibitors. Psychopharmacology (Berl) 1993;113:187–198. doi: 10.1007/BF02245696. [DOI] [PubMed] [Google Scholar]
- 24.Paulus MP, Ralph R, Jones SR, Fumagalli F, Geyer MA, Caron MG. Dopamine transporter (DAT) (+/−) and (−/−) mice exhibit graded changes in geometric patterns of locomotor behavior. Soc Neurosci Abstr. 1997;23:722.1. [Google Scholar]
- 25.Paulus MP, Dulawa SC, Ralph RJ, Mark AG. Behavioral organization is independent of locomotor activity in 129 and C57 mouse strains. Brain Res. 1999;835:27–36. doi: 10.1016/s0006-8993(99)01137-3. [DOI] [PubMed] [Google Scholar]
- 26.Peng RY, Mansbach RS, Braff DL, Geyer MA. A D2 dopamine receptor agonist disrupts sensorimotor gating in rats. Implications for dopaminergic abnormalities in schizophrenia. Neuropsychopharmacology. 1990;3:211–218. [PubMed] [Google Scholar]
- 27.Ralph RJ, Varty GB, Jones SR, Fumagalli F, Caron MG, Geyer MA. Prepulse inhibition deficits in dopamine transporter (DAT) knockout mice. Soc Neurosci Abstr. 1997;23:722.3. [Google Scholar]
- 28.Ralph RJ, Varty GB, Kelly MA, Wang YM, Caron MG, Rubinstein M, Grandy DK, Low MJ, Geyer MA. The dopamine D2, but not D3 or D4, receptor subtype is essential for the disruption of prepulse inhibition produced by amphetamine in mice. J Neurosci. 1999;19:4627–4633. doi: 10.1523/JNEUROSCI.19-11-04627.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Swerdlow NR. Startle in Tourette's syndrome. Biol Psychiatry. 1998;44:935–936. doi: 10.1016/s0006-3223(98)00066-3. [DOI] [PubMed] [Google Scholar]
- 30.Swerdlow NR, Keith VA, Braff DL, Geyer MA. Effects of spiperone, raclopride, SCH 23390 and clozapine on apomorphine inhibition of sensorimotor gating of the startle response in the rat. J Pharmacol Exp Ther. 1991;256:530–536. [PubMed] [Google Scholar]
- 31.Swerdlow NR, Benbow CH, Zisook S, Geyer MA, Braff DL. A preliminary assessment of sensorimotor gating in patients with obsessive compulsive disorder. Biol Psychiatry. 1993;33:298–301. doi: 10.1016/0006-3223(93)90300-3. [DOI] [PubMed] [Google Scholar]
- 32.Wan FJ, Taaid N, Swerdlow NR. Do D1/D2 interactions regulate prepulse inhibition in rats? Neuropsychopharmacology. 1996;14:265–274. doi: 10.1016/0893-133X(95)00133-X. [DOI] [PubMed] [Google Scholar]