Abstract
The cAMP response element-binding protein (CREB) is an evolutionarily conserved transcription regulator essential for long-term memory formation. It is not known, however, whether the molecular events downstream of CREB activation are also conserved. An early, cAMP-dependent event necessary for learning-related long-term synaptic plasticity in the invertebrate Aplysia californica is the induction of the transcription factor CCAAT enhancer-binding protein (C/EBP). Here we show that two homologs in the rat, C/EBPβ and C/EBPδ, are induced at discrete times after inhibitory avoidance learning and co-localize with phosphorylated CREB in the hippocampus. This induction is blocked by fornix lesions, which are known to disrupt activation of CREB in the hippocampus and to impair memory consolidation. These results indicate that C/EBPs are evolutionarily conserved components of the CREB-dependent gene cascade activated in long-term memory.
Keywords: C/EBP, CREB, learning and memory, inhibitory avoidance, fornix, lesion, hippocampus, rat
The requirement of gene expression for the formation of new memories is conserved from invertebrates to mammals. Several studies beginning in the 1960s have shown that the inhibition of mRNA or protein synthesis during or shortly after learning blocks long-term memory formation without interfering with memory acquisition, short-term memory, or the retrieval of previously stored information (Agranoff et al., 1965; Barondes, 1975; Davis and Squire, 1984). This suggested that evolutionarily conserved molecular mechanisms are necessary for the conversion or consolidation of short-term modifications into long-term memory. The last 10 years have witnessed great progress in the characterization of these mechanisms in several species. Specifically, members of the transcription factor family cAMP response element-binding proteins (CREBs) have been shown to possess an essential role in long-term memory formation (Dash et al., 1990; Bourtchouladze et al., 1994; Yin et al., 1994, 1995; Bartsch et al., 1995, 1998; Guzowski and McGaugh, 1997). Several recent studies in mammals have determined when and where in the brain CREB activity and CREB-dependent gene expression occur after learning. All report that a circumscribed and persistent CREB phosphorylation occurs in CA1 and dentate gyrus neurons of the hippocampal formation after inhibitory avoidance training (Bernabeu et al., 1997; Impey et al., 1998;Taubenfeld et al., 1999). In addition, lesions of the fornix, which produce a marked impairment in long-term memory consolidation, were found to completely prevent hippocampal CREB phosphorylation induced by training (Taubenfeld et al., 1999). This result suggests that inputs passing through the fornix, a massive fiber bundle connecting the hippocampus with the septum and hypothalamus, regulate CREB-dependent hippocampal gene expression required for memory consolidation. Crucial questions still remain, however, about which specific genes are regulated downstream of CREB in the hippocampus during long-term memory.
In the invertebrate Aplysia californica, an early event of gene expression occurring downstream of CREB is the induction of CCAAT enhancer-binding protein (ApC/EBP), a transcription factor regulated by cAMP. Like CREB, ApC/EBP is essential for long-term synaptic plasticity underlying memory in Aplysia. Thus, CREB controls the induction of regulatory immediate early genes (IEGs), which, in turn, regulate the transcription of more downstream target genes required for long-term memory (Alberini et al., 1994). To investigate whether the CREB-dependent gene cascade is conserved in mammalian memory, we analyzed endogenous C/EBP gene expression in normal and memory-impaired animals with fornix lesions after learning. A detailed time course study revealed that C/EBP induction is a conserved genetic correlate of memory formation. This model system has advantages over targeted gene disruption (knock-out) approaches, because it provides anatomical and temporal specificity of endogenous gene regulation critical for learning and memory studies and circumvents the problems of developmental defects and molecular compensation.
MATERIALS AND METHODS
Surgery. Long-Evans rats weighing between 200 and 250 gm were used in all experiments. Animals were housed in individual cages and maintained in a 12 hr light/dark cycle. All rats were allowedad libitum access to food and water. Rats were anesthetized with sodium pentobarbital (55 mg/kg, i.p.) and placed in a stereotaxic apparatus, where a midline incision was made and the scalp was retracted to expose the skull. Electrolytic lesions of the fornix were made by drilling holes through the skull at 0.4 and 1.4 mm posterior to bregma and 0.6 and 1.0 mm lateral to the midline. Monopolar electrodes (Teflon-coated wire, 125 μm in diameter) were lowered at each site to a depth of 4.4 mm measured from the surface of the skull. DC current at 1 mA was passed through the electrodes for a duration of 12 sec. The electrodes were then removed, and the wound was sutured. Postoperatively, the animals received a prophylactic dose of antibiotic (Claforan, 0.1 ml, i.m.) and were kept warm and monitored until spontaneous movement occurred. Once stabilized, they were returned to their home cages and left to recover for 7 d before training.
Inhibitory avoidance training. The inhibitory avoidance chamber consisted of a rectangular-shaped Perspex box, divided into a safe compartment and a shock compartment. The safe compartment was white and illuminated by a light fixture fastened to the cage lid. The shock compartment was dark and made of black Perspex.
Foot shocks were delivered to the grid floor of this chamber via a constant current scrambler circuit. The two compartments were separated by an automatically operated sliding door. The apparatus was located in a sound-attenuated, nonilluminated room.
During training sessions, each rat was placed in the safe compartment with its head facing away from the door. After 10 sec, the door was automatically opened, allowing the rat access to the shock chamber. The door closed 1 sec after the rat entered the shock chamber, and a brief foot shock (1.5 mA for 2 sec) was administered to the rat. The rat was then removed from the apparatus and either immediately anesthetized with sodium pentobarbital and killed (0 hr time point) or returned to its home cage and later anesthetized at a specific, post-training time point. Control groups consisted of (1) rats exposed to the inhibitory avoidance apparatus for the same duration as the trained animals without receiving a foot shock (no shock) and (2) rats placed directly on the metal grid and immediately foot-shocked (shock only). At each time point, brains were rapidly dissected and frozen for Western blot or Northern blot analysis or perfused for immunohistochemistry as described below.
Western blot analysis. Extracts from rat hippocampi were obtained by Polytron homogenization in cold lysis buffer with protease inhibitors (0.2 m NaCl, 0.1 m HEPES, 10% glycerol, 2 mm NaF, 2 mmNa4P2O7, 5 mm EDTA, 1 mm EGTA, 2 mm DTT, 0.5 mm PMSF, 1 mm benzamidine, 10 μg/ml leupeptin, 400 U/ml aprotinin, and 1 μm microcystin). After 10 min on ice, the samples were centrifuged at 16,000 ×g for 15 min at 4°C. The supernatants were collected, and their total protein concentration was determined using the BioRad (Hercules, CA) protein assay. The lysates were then aliquoted and stored at −80°C. Equal amounts of total protein corresponding to 25 μg/lane were resolved on denaturing 10% SDS-PAGE gels and transferred to Immobilon-P (polyvinylidene difluoride) transfer membranes (Millipore, Bedford, MA) by electroblotting. Membranes were pretreated with 5% BLOTTO buffer and then incubated with anti-phosphorylated CREB (PCREB, 1:2000; Upstate Biotechnology, Lake Placid, NY) or anti-C/EBPβ (1:2000; Santa Cruz Biotechnology, Santa Cruz, CA) antisera in Tris-buffered saline overnight at 4°C. The membranes were then washed, treated with a secondary HRP-labeled donkey anti-rabbit antibody (1:4000) for 1 hr, washed again, and incubated with HRP–streptavidin complex and ECL detection reagents (Amersham Pharmacia Biotech, Arlington Heights, Illinois). Membranes were exposed to ECL Hyperfilm (Amersham), and quantitative densitometric analysis was performed using NIH Image. Statistical analysis was performed using one-way ANOVA followed by Dunnett's multiple comparison test.
RNA extraction and Northern blot analysis. Total RNA was extracted following the method of Chomczynski and Sacchi (1987). One milliliter of solution D (4 m guanidinium thiocyanate, 25 mm sodium citrate, pH 7.0, 0.1 m2-mercaptoethanol, and 0.5% sarcosyl) was added per 100 mg of tissue. The samples were Polytron-homogenized, extracted with 1 volume of phenol-chloroform, and precipitated with 1 volume of isopropanol. Ten micrograms of total RNA sample were electrophoresed on 1.2% agarose gels, transferred to Hybond-N+ nylon membranes (Amersham), and UV-cross-linked. The membranes were hybridized overnight at 42°C with specific probes in 50% formamide, 5× SSPE, 0.1% SDS, 2× Denhardt's solution, 0.1 mg/ml tRNA, and 0.1 mg/ml salmon sperm DNA. Probes were labeled with random oligonucleotide primers (Prime-It II kit; Stratagene, Cedar Creek, TX) and [α-32P]dCTP (Amersham). At the end of the hybridization, the membranes were washed and exposed to BioMax MS film (Eastman Kodak, Rochester, NY), and quantitative densitometric analysis was performed using NIH Image. Statistical analysis was performed using one-way ANOVA followed by Dunnett's post hoc analysis. The same membrane was stripped and rehybridized with different probes as described in Results. The following probes were used: The rat C/EBPβ probe included the last 400 bpSmaI–PstI fragment of the 3′ untranslated region. The rat C/EBPδ probe carried the 493 bp region beginning from base 13 of the open reading frame. A full-length rat cyclophilin cDNA was used as a control probe to which both C/EBP hybridizations were normalized.
Immunohistochemistry. Animals were perfused transcardially with cold PBS containing 20 U/ml heparin (Sigma, St. Louis, MO) followed by cold 4% paraformaldehyde in PBS. Brains were post-fixed overnight in the same fixative with 30% sucrose and then cryoprotected overnight in 30% sucrose and PBS. Fourty micrometer sections were cut in the coronal plane on a freezing microtome. Immunostaining was performed on free-floating slices using the streptavidin–biotin complex immunoperoxidase technique according to manufacturer's instructions (ImmunoPure ABC peroxidase rabbit IgG staining kit; Pierce, Rockford, IL). Briefly, sections underwent a series of preincubations in 0.3% hydrogen peroxide, 0.3% Triton X-100, and 10% normal goat serum. The slices were then incubated with anti-PCREB antibody diluted at 1:1000 or anti-C/EBPβ or -δ (Santa Cruz Biotechnology) antibodies diluted at 1:1500 for 48 hr at 4°C, washed three times with PBS, and then treated with a 1:400 dilution of biotinylated goat anti-rabbit IgG in PBS for 30 min at room temperature. Slices were finally washed three times in PBS and incubated with avidin-biotinylated HRP. Staining was revealed by incubating the slices in 0.25 mg/ml diaminobenzidene (Sigma) at room temperature for 5–10 min. After washing with water, the slices were mounted on gelatin-coated slides, air-dried, and counterstained with cresyl violet.
RESULTS
Inhibitory avoidance training induces a long-lasting phosphorylation of CREB at Ser-133
The activation of CREB as a transcription regulator requires the phosphorylation of Ser-133, which mediates the binding to the transcriptional activator CREB-binding protein and, subsequently, recruits the general transcription machinery (Montminy, 1997). Thus, detection of sites of Ser-133 CREB phosphorylation in the brain can be used to reveal the neural circuits in which CREB-mediated gene expression underlies the formation of specific types of memory. In a previous study, we showed that, immediately after inhibitory avoidance (IA) learning, hippocampal CREB phosphorylation at Ser-133 (PCREB) is significantly increased and remains elevated for at least 6 hr after training compared with controls that were exposed to the training apparatus without receiving a foot shock and immediately killed (0 h −). We extended this time course using quantitative Western blot analysis and measured post-training PCREB levels at 12 and 20 hr using a Ser-133-specific PCREB antiserum. Figure1 depicts previously reported data (open symbols) together with new time points (closed symbols), which show a sustained increase in PCREB in the hippocampi of trained animals at both 12 hr (141.1 ± 8.8%) and 20 hr (146.7 ± 9.2%) after training. A one-way ANOVA revealed a significant main effect of time (F = 9.906;p < 0.006), and Dunnett's post hoccomparisons confirmed that PCREB was significantly greater at both 12 hr (p < 0.05) and 20 hr (p < 0.01) compared with the 0 h − control group. Therefore, an increase in PCREB, lasting nearly 1 day, accompanies IA consolidation. This suggests that during this period an extended phase of CREB-dependent gene expression may occur in the hippocampus.
Fig. 1.
Inhibitory avoidance-related hippocampal PCREB is sustained beyond 9 hr after training. Data up to 9 hr (open symbols) were previously reported by Taubenfeld et al. (1999).Closed symbols, Densitometric analysis of PCREB Western blot immunostaining of hippocampal extracts taken from rats at 12 and 20 hr after training. A significant increase in PCREB was detected at 12 hr (n = 4; p < 0.0.5) and 20 hr (n = 4; p < 0.01) compared with 0 h − control levels immediately after training. Data are expressed as mean percentage ± SEM of the 0 h − control mean values.
C/EBPβ and -δ are induced after inhibitory avoidance learning
Because high levels of PCREB persist for many hours, we set out to determine whether the expression of C/EBPβ and -δ were changing during the potentially wide time window of CREB-mediated gene transcription. We performed Northern blot analyses of C/EBPβ and -δ at 3, 6, 9, 20, and 72 hr and 1 week after training. Three to four animals per time point were investigated, and hybridizations were normalized using the internal reference gene cyclophilin to correct for loading differences (Fig. 2, Table1). The same membrane was sequentially hybridized with all the probes. As shown in Table 1 and Figure 2A and B, we found that the mRNA levels of C/EBPβ at 3 hr and 6 hr after IA training remained similar to those of control rats (0 h −). However, C/EBPβ mRNA levels were selectively increased in all animals at 9 hr (144.5 ± 11.8%) and 20 hr (196.0 ± 20.1%) and returned to control levels at 72 hr and 1 week. A one-way ANOVA showed a significant main effect of time (F = 9.567; p < 0.0001), and Dunnett's post hoc comparisons revealed that, compared with controls, C/EBPβ mRNA levels were significantly greater at both 9 hr (p < 0.01) and 20 hr (p< 0.01) and not at the other time points.
Fig. 2.
C/EBPβ and -δ mRNA levels after inhibitory avoidance training. Northern blot analyses were performed of hippocampal extracts taken from rats immediately (0 h), 3, 6, 9, 20, and 72 hr, and 1 week after training. A, Two independent autoradiographs show mRNA levels of individual animals that (1) underwent full training (+), (2) entered the IA apparatus but received no shock (−), or (3) received the shock without exposure to the apparatus [shock only (SO)]. Cyclophilin was used as a control probe for normalization of both C/EBP hybridizations.B, Densitometric analysis of C/EBPβ autoradiographs described in A. Trained animals showed a significant induction of C/EBPβ mRNA at 9 hr (p < 0.01) and 20 hr (p < 0.01) compared with control rats immediately killed after training (0 h −). In contrast, no significant changes in C/EBPβ were found in any of the control no shock or shock only groups. Data are expressed as mean percentage ± SEM of the 0 h − (100%) control mean values. C, Densitometric analysis of C/EBPδ Northern blots described in A. C/EBPδ mRNA is significantly induced in rats 20 hr (p < 0.01) after training compared with 0 h − control levels. No significant changes were detected in any of the controls groups. Data are expressed as mean percentage ± SEM of the0 h − (100%) control mean values.
Table 1.
C/EBP mRNA and protein levels after inhibitory avoidance training
| 0 hr | 3 hr | 6 hr | 9 hr | 20 hr | 28 hr | 48 hr | 72 hr | 1 week | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| − | − | + | − | + | − | + | SO | − | + | SO | − | + | − | + | − | + | − | + | |
| mRNA | |||||||||||||||||||
| C/EBPβ | 100 ± 4.8 | 98.0 ± 5.4 | 94.0 ± 16.5 | 88.5 ± 9.8 | 86.1 ± 7.0 | 105.6 ± 8.1 | 144.5 ± 11.8 | 90.8 ± 6.9 | 101.9 ± 4.3 | 196.0 ± 20.1 | 100.6 ±3.5 | 112.8 ± 10.4 | 104.1 ± 12.3 | 97.3 ± 7.2 | 78.6 ± 7.1 | ||||
| p | <0.01 | <0.01 | |||||||||||||||||
| CEBPδ | 100 ± 3.6 | 88.1 ± 2.8 | 107.8 ± 12.4 | 96.0 ± 17.4 | 111.3 ± 4.5 | 110.1 ± 17.2 | 105.9 ± 3.7 | 109.9 ± 17.2 | 92.4 ± 5.8 | 146.6 ± 14.6 | 99.1 ± 1.9 | 102.1 ± 3.3 | 100.2 ± 7.8 | 93.5 ± 5.6 | 88.0 ± 5.6 | ||||
| p | <0.01 | ||||||||||||||||||
| Protein | |||||||||||||||||||
| C/EBPβ | 100 ± 4.2 | 90.2 ± 3.4 | 127.3 ± 3.6 | 110.4 ± 3.0 | 159.7 ± 6.6 | 104.1 ± 6.1 | 154.8 ± 11.9 | 111.3 ± 1.8 | 90.7 ± 6.1 | 109.2 ± 4.2 | 106.7 ± 3.5 | ||||||||
| p | <0.05 | <0.01 | <0.01 | ||||||||||||||||
Concentrations are expressed as mean percentage ± SEM of the 0 hr − (100%) control mean values. Groups of animals were fully trained (+), entered the training apparatus without receiving foot shock (−), or received the shock without exposure to the apparatus [shock only (SO)] and killed at assigned time points. Concentrations are expressed as mean percentage ± SEM of the 0 hr − (100%) control mean values. Groups of animals were fully trained (+), entered the training apparatus without receiving foot shock (−), or received the shock without exposure to the apparatus [shock only (SO)] and killed at assigned time points.
As shown in Table 1 and Figure 2, A and C, C/EBPδ mRNA levels were significantly increased relative to controls (0 h −) only at 20 hr (146.6 ± 14.6%) after training (ANOVA, F = 1.902; p < 0.04; Dunnett's post hoc, p < 0.01) but not at any other time points. In parallel, we determined whether the levels of hippocampal C/EBPβ or -δ mRNAs changed at the same post-training time points in control animals that (1) only received the foot shock without contextual learning or (2) walked through the inhibitory avoidance apparatus without receiving the foot shock in the dark chamber. As shown in Table 1 and Figure 2, no significant changes were measured under any of these conditions. These data demonstrate that the increase observed in C/EBPβ and δ expression is related to pairing a context with the foot shock rather than exploring a new environment or receiving a foot shock alone. Therefore, this gene induction in response to IA training is specific for memory consolidation of the task and not attributable to other stimuli evoked by the training.
To determine whether C/EBPβ protein levels correspondingly changed with mRNA induction, we performed quantitative Western blot analysis of C/EBPβ on hippocampal protein extracts. We limited our analysis to C/EBPβ, because anti-C/EBPδ antiserum gave nearly undetectable signals in Western blot. Groups of rats received IA training and C/EBPβ protein concentrations were measured 9, 20, 28, 48, and 72 hr later and compared with controls (0 h −). As shown in Table1 and Figure 3, hippocampi of trained animals showed a significant induction of C/EBPβ protein at 9 hr (127.3 ± 3.6%). The increase was sustained at 20 hr (159.7 ± 6.6%) and 28 hr (154.8 ± 11.9%) and returned to control levels at 48 and 72 hr. A one-way ANOVA revealed a significant main effect of time (F = 17.96; p < 0.0001), and Dunnett's post hoc comparisons confirmed that this increase was significant at 9 hr (p < 0.05), 20 hr (p < 0.01), and 28 hr (p < 0.01) but not at 48 and 72 hr. Time-paired no shock control groups did not show any significant increase of C/EBPβ.
Fig. 3.
C/EBPβ protein is selectively induced after inhibitory avoidance training. Western blot analyses were performed with anti-C/EBPβ antiserum. A, Western blot immunostaining of hippocampal extracts from rats killed immediately (0 h) and 9, 20, 28, 48, and 72 hr after training. Groups of animals either (1) underwent full training (+) or (2) entered the IA apparatus but received no shock (−). B, Densitometric analysis of C/EBPβ Western blot depicted inA revealed a significant increase in C/EBPβ protein at 9 hr (p < 0.05), 20 hr (p < 0.01), and 28 hr (p < 0.01) compared with 0 h − control rats. There were no significant differences in any of the time-paired no shock control groups. Data are expressed as mean percentage ± SEM of the 0 h − (100%) control mean values.
Fornix lesions block the induction of C/EBPβ and -δ after inhibitory avoidance learning
Previously we have shown that fornix lesions selectively blocked hippocampal CREB phosphorylation induced after IA learning (Taubenfeld et al., 1999). Here, we addressed the question of whether the C/EBPβ and -δ induction that occurred after IA training also depended on the integrity of the fornix. In striking contrast to unoperated animals, the mRNA levels of C/EBPβ (Fig.4A,B) and -δ (Fig. 4A,C) in hippocampi of rats with fornix lesions did not significantly increase at 9 hr (β, 113.3 ± 3.8%; δ, 90.0 ± 5.3%) or 20 hr (β, 100.7 ± 10.1%; δ, 98.7 ± 2.9%) after training.
Fig. 4.
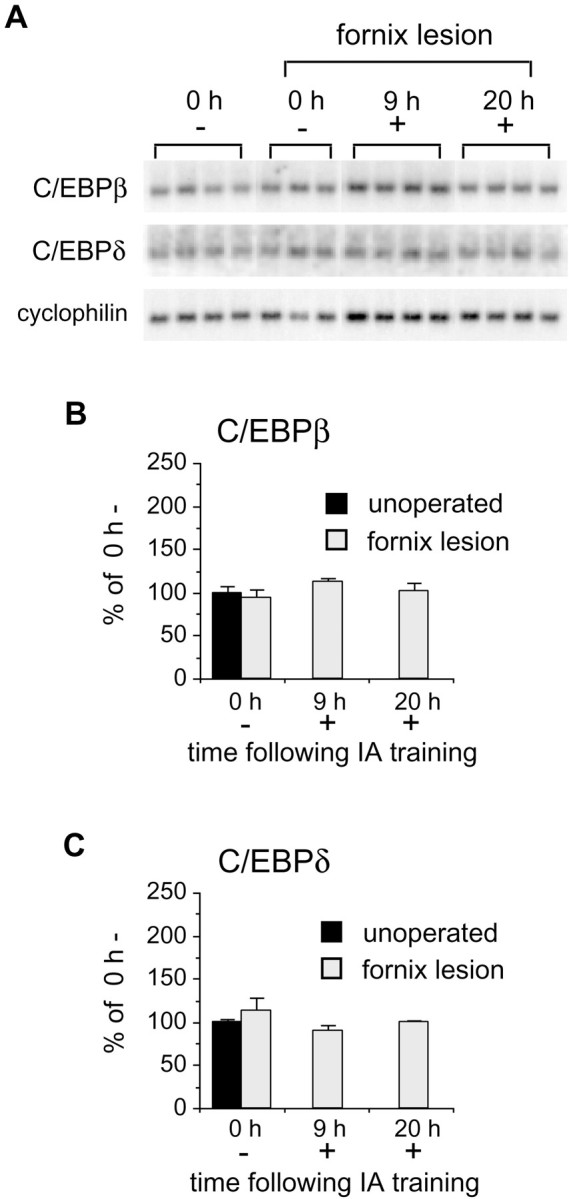
Memory-impaired animals with lesions of the fornix fail to induce C/EBPβ or -δ. A, Northern blot analyses of hippocampal extracts taken from rats with fornix lesions immediately (0 h) or 9 and 20 hr after training. Autoradiograph shows mRNA levels of individual animals that either (1) underwent full training (+) or (2) entered the IA apparatus but received no shock (−). Cyclophilin was used as a control probe for normalization of both C/EBP hybridizations. B, Densitometric analysis of C/EBPβ autoradiographs shown inA. Rats with fornix lesions did not exhibit IA learning-related induction of C/EBPβ at 9 or 20 hr after training compared with unoperated, 0 h − control rats. Data are expressed as mean percentage ± SEM of the unoperated,0 h − (100%) control mean values.C, Densitometric analysis of C/EBPδ Northern blots shown in A. C/EBPδ mRNA is not induced in rats with fornix lesions after IA training compared with unoperated, 0 h − controls. Data are expressed as mean percentage ± SEM of the unoperated, 0 h − (100%) control mean values.
Importantly, lesioning the fornix did not interfere with basal levels of hippocampal C/EBPβ and -δ mRNAs. In fact, rats with fornix lesions that were exposed to the training box without foot shock and immediately killed exhibited mRNA levels comparable with those of unoperated, 0 h − controls (β, 94.3 ± 9.1%; δ, 113.0 ± 14.5%; Fig. 4). This finding demonstrates that an intact fornix is required for the C/EBPβ and -δ gene response to IA training and strengthens the hypothesis that the observed changes are selectively related to memory consolidation.
Learning-related induction of C/EBPβ and -δ follows CREB phosphorylation in the same subset of hippocampal neurons
Results from our Northern and Western analyses suggested that C/EBPβ and -δ expression temporally follows CREB phosphorylation in the hippocampi of trained animals. Because the rat C/EBPβ gene contains CRE sites within its promoter region (Niehof et al., 1997), and its expression can be modulated by cAMP in the hippocampus (Yukawa et al., 1998), we next examined whether C/EBPβ and -δ are induced downstream of PCREB after IA training. Using antisera specific for PCREB and C/EBPβ and -δ, we performed immunohistochemistry on adjacent hippocampal sections from control rats (0 h −) and trained rats killed immediately or 24 hr later. Confirming our earlier data (Taubenfeld et al., 1999), we found that control rats (0 h −) displayed variable staining for PCREB in the neurons of the dentate gyrus and CA3, whereas CA1 neurons were generally PCREB-negative. This PCREB immunoreactivity co-localized remarkably well with C/EBPβ and -δ immunostaining (data not shown).
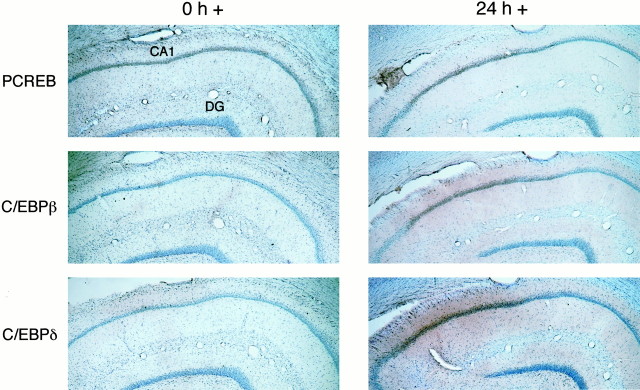
In contrast, immediately after IA training (0 h +), the characteristic induction of PCREB in the CA1 subregion of the hippocampus did not co-localize with either C/EBPβ or -δ (Fig.5). However, at 24 hr, both C/EBPβ and -δ were present in CA1 and completely overlapped the PCREB immunostaining pattern (Fig. 5). These data confirm the Northern blot analysis results and support the hypothesis that CREB regulates the induction of C/EBPs during the consolidation phase of IA memory.
Fig. 5.
Learning-related induction of C/EBPβ and -δ follows CREB phosphorylation in the same subset of hippocampal neurons. Examples of CA1 immunohistochemical staining using anti-PCREB and anti-C/EBPβ and -δ antisera on adjacent brain slices from rats killed immediately (0 h +; n = 3) and 1 d (24 h +; n = 3) after IA training are shown. PCREB but not C/EBPβ or -δ was induced in CA1 neurons immediately after foot shock. In contrast, PCREB and C/EBPβ and -δ were all induced in the same subpopulation of CA1 neurons at 24 hr. Forty micrometer sections magnified at 20× are shown. CA1 and dentate gyrus (DG) subregions are indicated.
It is interesting to note that, whereas PCREB staining was always confined to the nuclei, C/EBPβ and -δ were both evident in the nuclei and processes of hippocampal neurons.
DISCUSSION
Our studies provide evidence that long-term memory formation is accompanied by a co-localized induction of C/EBPβ and -δ and PCREB in hippocampal neuronal populations at discrete times after training. The induction of both C/EBPβ and -δ occurs after CREB phosphorylation and depends on the integrity of the fornix, which is essential for the hippocampal CREB response required for memory consolidation (Taubenfeld et al., 1999). Therefore, mammalian memory formation appears to use an evolutionarily conserved cascade of genetic events characterized by fornix-dependent CREB activation, induction of C/EBPs, and consequently, regulation of additional downstream genes.
Selectivity and temporal profile of hippocampal gene expression underlying memory consolidation
In this paper, we demonstrate that hippocampal induction of C/EBPs after IA training is selective for the shock–context association and is not an effect of shock or novelty alone. These findings agree with previous studies showing that the hippocampal PCREB response is also selective for learning (Bernabeu et al., 1997; Izquierdo and Medina, 1997; Impey et al., 1998; Taubenfeld et al., 1999). Together, these results indicate that fear-based contextual learning involves selective CREB–C/EBP activation within restricted neuronal populations in the hippocampus.
The temporal profiles of PCREB and C/EBPβ and -δ induction after IA training exhibit two important features: first, the changes are sustained for at least 1 d, and second, the onset of C/EBP induction occurs in a delayed manner. This late C/EBPβ and -δ induction may explain why Hall et al. (2000) found no selective changes of C/EBPβ expression 30 min after contextual fear conditioning. Results obtained in Aplysia (Alberini et al., 1994) and in numerous cell lines or culture systems, including neurons (Cardinaux and Magistretti, 1996; Yukawa et al., 1998), indicate that C/EBPs are induced as immediate early genes via the cAMP–PKA pathway, and, therefore, their expression increases rapidly within minutes after stimulation. Here, we have shown that although CREB phosphorylation occurs immediately after training (Bernabeu et al., 1997; Taubenfeld et al., 1999), the induction of C/EBPβ and -δ is delayed and, in fact, first becomes detectable at 9 or 20 hr. One possible explanation for this delay is that the changes we observed may have a temporal profile of expression very different from that of pharmacologically treatedin vitro systems, because they are associated with a behavioral response. Alternatively, the slow response may be characteristic of particular tissues or functions. Similar to our findings, kinetics characterized by elevations of C/EBPβ and -δ with a delayed onset have been described in human endometrial stromal cells during decidualization (Pohnke et al., 1999) and after rat brain injury (S. M. Taubenfeld and C. M. Alberini, unpublished results). Another possibility is that, in mammalian memory, C/EBPβ and -δ are induced as late response genes. In fact, the delayed increase of C/EBPs observed in our paradigm does not exclude the possibility that other IEGs are induced more rapidly after training. For example, hippocampal induction of c-fos, zif 268, and Arc seems to occur immediately after training (Hess et al., 1995; Grimm and Tischmeyer, 1997; Guzowski et al., 1999; Cammarota et al., 2000).
Our results indicate that gene expression underlying memory consolidation may last several hours or, more likely, days. Studies conducted in many different species over the last 40 years have delineated the transcription–translation-dependent phases of memory formation. Although controversial (Squire and Barondes, 1970; Daniels, 1971), it was concluded that inhibitors were most effective when given before, during, or immediately after training (Davis and Squire, 1984;Meiri and Rosenblum, 1998). Thus, it has been generally believed that the protein and RNA syntheses essential for memory consolidation occur within a very short time after learning. However, recent studies based on more detailed time courses have shown that long-term memory requires more than one time window of gene expression. In several species, including chick, mouse, and rat, memory consolidation requires a second time window of protein synthesis starting at 3–5 or 6–7 hr after training and lasting for several hours (Grecksch and Matthies, 1980;Chew et al., 1995; Freeman et al., 1995; Bourtchouladze et al., 1998;Tiunova et al., 1998; Quevedo et al., 1999). In the zebra finch, maintenance of a memory-related, experience-dependent neural plasticity requires multiple episodes of gene expression at discrete times (Chew et al., 1996). Our data reveal that the induction of C/EBPβ and -δ begins between 6 and 9 hr after training, which overlaps with the second time window of protein synthesis described above. Why do separate phases of protein synthesis appear be required for memory consolidation? A possible working hypothesis is that long-term memory forms as a result of the integration of combinatorial events that originate in distinct neuronal compartments at different times. These localized events may contribute to different phases of the consolidation process. Perhaps memory induction first occurs at specific synapses with the translation of localized mRNAs. This protein synthesis, together with post-translational modifications (Frey and Morris, 1997), could send signals to the nucleus that subsequently, after integration with other modulatory inputs, might activate a cell-wide form of gene expression involving transcription and translation. This late phase of gene expression may stabilize the early changes occurring at specific synapses. In agreement with this hypothesis, in Aplysia and Hermissenda, short, intermediate, and long-term memory require post-translational modification, translation, and transcription–translation, respectively (Ghirardi et al., 1995; Crow et al., 1997, 1999; Sherff and Carew, 1999). In Aplysia, a cell-wide transient long-term form of CREB-mediated facilitation can be stabilized at specific synapses by local protein synthesis (Martin et al., 1997; Casadio et al., 1999).
Is the fornix modulating a wide time window of memory consolidation?
Damage to the fornix causes a severe memory deficit (Aggleton and Saunders, 1997). We previously reported that this deficit is significant when memory is tested ≥1 d after training but not at early time points, such as 3 or 6 hr (Taubenfeld et al., 1999). Therefore, the fornix is involved in a phase of the consolidation process that begins a few hours after training. Interestingly, the same time window is required for the fornix-dependent induction of C/EBPβ and -δ. Perhaps the role of the fornix is to modulate a cascade of gene expression that, over time, will stabilize the synaptic changes that occurred early during the fornix-independent phase of memory consolidation.
The fornix does not appear to be required for the basal expression of PCREB or C/EBPβ or -δ, because untrained rats with lesions displayed levels of PCREB or C/EBPβ and -δ mRNAs comparable with those of untrained, unoperated animals. Thus, fornix inputs seem to be necessary for the sustained, training-dependent increase of CREB and C/EBP responses underlying memory consolidation. Among the fornix inputs, serotonin, noradrenaline, and dopamine can stimulate the cAMP- and CREB-mediated gene response. In remarkable parallel with the temporal profile of C/EBP induction, Bernabeu et al. (1997) showed that, beginning at 3–6 hr after training, IA memory consolidation requires the activation of the cAMP-coupled D1 and D5 dopamine receptors. Bevilaqua et al. (1997) found that the activation of the cAMP/PKA pathway coupled to D1, β-adrenergic, and 5-HT1A receptors enhances memory consolidation only when the pharmacological stimulus is applied into the hippocampus 3 or 6 but not 1.5 or 9 hr after training. Finally, in agreement with our results and hypothesis, hippocampal late long-term potentiation, a sustained synaptic response thought to underlie memory, requires a late protein synthesis-dependent phase mediated by cAMP and D1 and D5 activation (Frey et al., 1993; Huang et al., 1996).
C/EBPs are conserved molecules of long-term memory
The first evidence that members of the C/EBP family were required for long-term memory comes from the invertebrate Aplysia. In this system, C/EBP induction is necessary for the consolidation phase of long-term facilitation of the sensorimotor synapses, an in vitro model of memory (Alberini et al., 1994). Our findings show that, in mammals, memory formation is accompanied by the induction of two C/EBP isoforms in the same neuronal population and that this induction follows CREB activation. This implies possible functional cooperation or redundancy of both C/EBPβ and -δ within the CREB-dependent pathway. In agreement, several studies in different cell types, including neurons, report that C/EBPβ and -δ are co-expressed and interact in regulating gene expression via cAMP and PKA signaling (Cardinaux and Magistretti, 1996; Sterneck and Johnson, 1998; Sterneck et al., 1998; Yukawa et al., 1998; Gretchen, 1999; Lane et al., 1999; Pohnke et al., 1999). In addition, others have shown that both C/EBPs can compete or cooperate with members of the CREB family in regulating gene expression and that C/EBPβ transcription is controlled by CREB (Vallejo et al., 1993, 1995; Inoue et al., 1995;Niehof et al., 1997; Yukawa et al., 1998; Yamada et al., 1999).
Like members of the CREB family (Yin and Tully, 1996; Bartsch et al., 1998), different C/EBP isoforms may have different transcriptional regulatory actions. A recent report by Sterneck et al. (1998) showed that C/EBPδ knock-out mice have a selective enhancement in contextual fear conditioning but not in Morris water maze spatial learning or auditory cue conditioning. This suggests a repressor role for C/EBPδ. Anatomically and temporally selective expression or disruption of this gene will help confirm this hypothesis.
Footnotes
This work was supported by Whitehall Foundation Grant F97-07, the Rhode Island Foundation, and the Howard Hughes Medical Institute. C.M.A. is on a leave of absence from Dipartimento Materno Infantile e Tecnologie Biomediche, University of Brescia, Brescia, Italy. B.M. was a recipient of a Human Frontier and Science Program Organization short-term fellowship. We thank Mark Bear for helpful scientific discussions, Rebecca Burwell and Kelsey Martin for their comments on this manuscript, Valeria Poli for generously providing the rat C/EBPβ clone, and Erik Sklar, Ann Beauregard-Young, and Jim Harper for technical assistance.
Correspondence should be addressed to Cristina M. Alberini, Department of Physiology and Biophysics, Mount Sinai School of Medicine, New York, NY 10029-6574. E-mail: Cristina.Alberini@inka.mssm.edu.
REFERENCES
- 1.Aggleton JP, Saunders RC. The relationships between temporal lobe and diencephalic structures implicated in anterograde amnesia. Memory. 1997;5:49–71. doi: 10.1080/741941143. [DOI] [PubMed] [Google Scholar]
- 2.Agranoff BW, Davis RE, Brink JJ. Memory fixation in the goldfish. Proc Natl Acad Sci USA. 1965;54:788–793. doi: 10.1073/pnas.54.3.788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alberini CM, Ghirardi M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76:1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- 4.Barondes SH. Protein synthesis dependent and protein synthesis independent memory storage processes. In: Deutsch D, Deutsch JA, editors. Short-term memory. Academic; New York: 1975. pp. 379–390. [Google Scholar]
- 5.Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural changes. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 6.Bartsch D, Casadio A, Karl KA, Serodio P, Kandel ER. CREB1 encodes a nuclear activator, a repressor, and a cytoplasmic modulator that form a regulatory unit critical for long-term facilitation. Cell. 1998;95:211–223. doi: 10.1016/s0092-8674(00)81752-3. [DOI] [PubMed] [Google Scholar]
- 7.Bernabeu R, Bevilaqua L, Ardenghi P, Bromberg E, Schmitz P, Bianchin M, Izquierdo I, Medina JH. Involvement of hippocampal cAMP/cAMP-dependent protein kinase signaling pathways in a late memory consolidation phase of aversively motivated learning in rats. Proc Natl Acad Sci USA. 1997;94:7041–7046. doi: 10.1073/pnas.94.13.7041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bevilaqua L, Ardenghi P, Schroder N, Bromberg E, Schmitz PK, Schaeffer E, Quevedo J, Bianchin M, Walz R, Medina JH, Izquierdo I. Drugs acting upon the cyclic adenosine monophosphate/protein kinase A signalling pathway modulate memory consolidation when given late after training into rat hippocampus but not amygdala. Behav Pharmacol. 1997;8:331–338. doi: 10.1097/00008877-199708000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Bourtchouladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP-responsive element binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 10.Bourtchouladze R, Abel T, Berman N, Gordon R, Lapidus K, Kandel ER. Different training procedures recruit either one or two critical periods for contextual memory consolidation, each of which requires protein synthesis and PKA. Learn Mem. 1998;5:365–374. [PMC free article] [PubMed] [Google Scholar]
- 11.Cammarota M, Bevilaqua LRM, Ardenghi P, Paratcha G, Levi de Stein M, Izquierdo I, Medina JH. Learning-associated activation of nuclear MAPK, CREB and Elk-1, along with Fos production, in the rat hippocampus after a one-trial avoidance learning: abolition by NMDA receptor blockade. Mol Brain Res. 2000;76:36–46. doi: 10.1016/s0169-328x(99)00329-0. [DOI] [PubMed] [Google Scholar]
- 12.Cardinaux JR, Magistretti PJ. Vasoactive intestinal peptide, pituitary adenylate cyclase-activating peptide, and noradrenaline induce the transcription factors CCAAT/enhancer binding protein (C/EBP)-β and C/EBP δ in mouse cortical astrocytes: involvement in cAMP-regulated glycogen metabolism. J Neurosci. 1996;16:919–929. doi: 10.1523/JNEUROSCI.16-03-00919.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Casadio A, Martin KC, Giustetto M, Zhu H, Chen M, Bartsch D, Bailey CH, Kandel ER. A transient, neuron-wide form of CREB-mediated long-term facilitation can be stabilized at specific synapses by local protein synthesis. Cell. 1999;99:221–237. doi: 10.1016/s0092-8674(00)81653-0. [DOI] [PubMed] [Google Scholar]
- 14.Chew SJ, Mello C, Nottebohm F, Jarvis E, Vicario DS. Decrements in auditory responses to a repeated conspecific song are long-lasting and require two periods of protein synthesis in the songbird forebrain. Proc Natl Acad Sci USA. 1995;92:3406–3410. doi: 10.1073/pnas.92.8.3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chew SJ, Vicario DS, Nottebohm F. Quantal duration of auditory memories. Science. 1996;274:1909–1914. doi: 10.1126/science.274.5294.1909. [DOI] [PubMed] [Google Scholar]
- 16.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chlorophorm extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 17.Crow T, Siddiqi V, Dash PK. Long-term enhancement but not short-term in Hermissenda is dependent upon mRNA synthesis. Neurobiol Learn Mem. 1997;68:343–350. doi: 10.1006/nlme.1997.3779. [DOI] [PubMed] [Google Scholar]
- 18.Crow T, Xue-Bian JJ, Siddiqi V. Protein synthesis-dependent and mRNA synthesis-independent intermediate phase of memory in Hermissenda. J Neurophysiol. 1999;82:495–500. doi: 10.1152/jn.1999.82.1.495. [DOI] [PubMed] [Google Scholar]
- 19.Daniels D. Acquisition, storage and recall of memory for brightness discrimination by rats following intracerebral infusion of acetoxycycloheximide. Comp Physiol Psychol. 1971;76:110–118. doi: 10.1037/h0031044. [DOI] [PubMed] [Google Scholar]
- 20.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 21.Davis HP, Squire LR. Protein synthesis and memory: a review. Psychol Bull. 1984;96:518–559. [PubMed] [Google Scholar]
- 22.Freeman FM, Rose SP, Scholey AB. Two time windows of anisomycin-induced amnesia for passive avoidance training in the day-old chick. Neurobiol Learn Mem. 1995;63:291–295. doi: 10.1006/nlme.1995.1034. [DOI] [PubMed] [Google Scholar]
- 23.Frey U, Morris RG. Synaptic tagging and long-term potentiation. Nature. 1997;385:533–536. doi: 10.1038/385533a0. [DOI] [PubMed] [Google Scholar]
- 24.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 25.Ghirardi M, Montarolo PG, Kandel ER. A novel intermediate stage in the transition between short- and long-term facilitation in the sensory to motor neuron synapse of aplysia. Neuron. 1995;14:413–420. doi: 10.1016/0896-6273(95)90297-x. [DOI] [PubMed] [Google Scholar]
- 26.Grecksch G, Matthies H. Two sensitive periods for the amnesic effect of anisomycin. Pharmacol Biochem Behav. 1980;12:663–665. doi: 10.1016/0091-3057(80)90145-8. [DOI] [PubMed] [Google Scholar]
- 27.Gretchen JD. Molecular mechanisms of liver development and differentiation. Curr Opin Cell Biol. 1999;11:678–682. doi: 10.1016/s0955-0674(99)00035-6. [DOI] [PubMed] [Google Scholar]
- 28.Grimm R, Tischmeyer W. Complex pattern of immediate-early gene induction in rat brain following brightness discrimination training and pseudotraining. Behav Brain Res. 1997;84:109–116. doi: 10.1016/s0166-4328(97)83330-x. [DOI] [PubMed] [Google Scholar]
- 29.Guzowski JF, McGaugh JL. Anti-sense oligodeoxynucleotide-medi-ated disruption of hippocampal CREB protein levels impairs memory of a spatial task. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 31.Hall J, Thiomas KL, Everitt BJ. Rapid and selective induction of BDNF expression in the hippocampus during contextual learning. Nat Neurosci. 2000;3:533–535. doi: 10.1038/75698. [DOI] [PubMed] [Google Scholar]
- 32.Hess US, Lynch G, Gall CM. Changes in c-fos mRNA expression in rat brain during odor discrimination learning: differential involvement of hippocampal subfields CA1 and CA3. J Neurosci. 1995;15:4786–4795. doi: 10.1523/JNEUROSCI.15-07-04786.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang YY, Nguyen PV, Abel T, Kandel ER. Long-lasting forms of synaptic potentiation in the mammalian hippocampus. Learn Mem. 1996;3:74–85. doi: 10.1101/lm.3.2-3.74. [DOI] [PubMed] [Google Scholar]
- 34.Impey S, Smith DM, Obrietan K, Donahue R, Wade C, Storm DR. Stimulation of cAMP response element (CRE)-mediated transcription during contextual learning. Nat Neurosci. 1998;1:595–601. doi: 10.1038/2830. [DOI] [PubMed] [Google Scholar]
- 35.Inoue H, Yokoyama C, Hara S, Tone Y, Tanabe T. Transcriptional regulation of human prostaglandin-endoperoxide synthase-2 gene by lipopolysaccharide and phorbol ester in vascular endothelial cells. Involvement of both nuclear factor for interleukin-6 expression site and cAMP response element. J Biol Chem. 1995;270:24965–24971. doi: 10.1074/jbc.270.42.24965. [DOI] [PubMed] [Google Scholar]
- 36.Izquierdo I, Medina JH. Memory formation: the sequence of biochemical events in the hippocampus and its connection to activity in other brain areas. Neurobiol Learn Mem. 1997;68:285–316. doi: 10.1006/nlme.1997.3799. [DOI] [PubMed] [Google Scholar]
- 37.Lane MD, Tang QQ, Jiang MS. Role of the CCAAT enhancer binding proteins (C/EBPs) in adipocyte differentiation. Biochem Biophys Res Commun. 1999;266:677–683. doi: 10.1006/bbrc.1999.1885. [DOI] [PubMed] [Google Scholar]
- 38.Martin KC, Casadio A, Zhu H, Yaping E, Rose JC, Chen M, Bailey CH, Kandel ER. Synapse-specific, long-term facilitation of Aplysia sensory to motor synapses: a function for local protein synthesis in memory storage. Cell. 1997;91:927–938. doi: 10.1016/s0092-8674(00)80484-5. [DOI] [PubMed] [Google Scholar]
- 39.Meiri N, Rosenblum K. Lateral ventricle injection of the protein synthesis inhibitor anisomycin impairs long-term memory in a spatial memory task. Brain Res. 1998;789:48–55. doi: 10.1016/s0006-8993(97)01528-x. [DOI] [PubMed] [Google Scholar]
- 40.Montminy M. Transcriptional regulation by cAMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 41.Niehof M, Manns MP, Trautwein C. CREB controls LAP/C/EBP beta transcription. Mol Cell Biol. 1997;17:3600–3613. doi: 10.1128/mcb.17.7.3600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pohnke Y, Kempf R, Gellersen B. CCAAT/enhancer-binding proteins are mediators in the protein kinase A-dependent activation of the decidual prolactin promoter. J Biol Chem. 1999;274:24808–24818. doi: 10.1074/jbc.274.35.24808. [DOI] [PubMed] [Google Scholar]
- 43.Quevedo J, Vianna MRM, Roesler R, de-Paris F, Izquierdo I, Rose SPR. Two time windows of anisomycin-induced amnesia for inhibitory avoidance training in rats: protection from amnesia by pretraining but not pre-exposure to the task apparatus. Learn Mem. 1999;6:600–607. doi: 10.1101/lm.6.6.600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sherff CM, Carew TJ. Coincident induction of long-term facilitation in Aplysia: cooperativity between cell bodies and remote synapses. Science. 1999;285:1911–1914. doi: 10.1126/science.285.5435.1911. [DOI] [PubMed] [Google Scholar]
- 45.Squire L, Barondes S. Actinomycin-D: effects on memory at different times after training. Nature. 1970;225:649–650. doi: 10.1038/225649a0. [DOI] [PubMed] [Google Scholar]
- 46.Sterneck E, Johnson PF. CCAAT/enhancer binding protein beta is a neuronal transcriptional regulator activated by nerve growth factor receptor signaling. J Neurochem. 1998;70:2424–2433. doi: 10.1046/j.1471-4159.1998.70062424.x. [DOI] [PubMed] [Google Scholar]
- 47.Sterneck E, Paylor R, Jackson-Lewis V, Libbey M, Przedborski S, Tessarollo L, Crawley JN, Johnson PF. Selective enhanced contextual fear conditioning in mice lacking the transcriptional regulator CCAAT/enhancer binding protein δ. Proc Natl Acad Sci USA. 1998;95:10908–10913. doi: 10.1073/pnas.95.18.10908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Taubenfeld SM, Wiig KA, Bear MF, Alberini CM. A molecular correlate of memory and amnesia in the hippocampus. Nat Neurosci. 1999;2:309–310. doi: 10.1038/7217. [DOI] [PubMed] [Google Scholar]
- 49.Tiunova AA, Anokhin KV, Rose SP. Two critical periods of protein and glycoprotein synthesis in memory consolidation for visual categorization learning in chick. Learn Mem. 1998;4:401–410. doi: 10.1101/lm.4.5.401. [DOI] [PubMed] [Google Scholar]
- 50.Vallejo M, Ron D, Miller CP, Habener JF. C/ATF, a member of the activating transcription factor family of DNA-binding proteins, dimerizes with CAAT/enhancer-binding proteins and directs their binding to cAMP response elements. Proc Natl Acad Sci USA. 1993;90:4679–4683. doi: 10.1073/pnas.90.10.4679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vallejo M, Gosse ME, Beckman W, Habener JF. Impaired cyclic AMP-dependent phosphorylation renders CREB a repressor of C/EBP-induced transcription of the somatostatin gene in an insulinoma cell line. Mol Cell Biol. 1995;15:415–424. doi: 10.1128/mcb.15.1.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Yamada K, Duong DT, Scott DK, Wang JC, Granner DK. CCAAT/enhancer-binding protein beta is an accessory factor for the glucocorticoid response from the cAMP response element in the rat phosphoenolpyruvate carboxykinase gene promoter. J Biol Chem. 1999;274:5880–5887. doi: 10.1074/jbc.274.9.5880. [DOI] [PubMed] [Google Scholar]
- 53.Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- 54.Yin JCP, Wallach JS, Del Vecchio M, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 55.Yin JCP, Del Vecchio M, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]
- 56.Yukawa K, Tanaka T, Tsuji S, Akira S. Expressions of CCAAT/enhancer-binding proteins beta and delta and their activities are intensified by cAMP signaling as well as Ca2+/calmodulin kinases activation in hippocampal neurons. J Biol Chem. 1998;273:31345–31351. doi: 10.1074/jbc.273.47.31345. [DOI] [PubMed] [Google Scholar]