Abstract
Small diameter dorsal root ganglion (DRG) neurons, which include cells that transmit nociceptive information into the spinal cord, are known to express functional kainate receptors. It is well established that exposure to kainate will depolarize C-fiber afferents arising from these cells. Although the role of kainate receptors on sensory afferents is unknown, it has been hypothesized that presynaptic kainate receptors may regulate glutamate release in the spinal cord. Here we show that kainate, applied at low micromolar concentrations in the presence of the AMPA-selective antagonist (RS)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propyl-carbamoyl-6,7-methylenedioxyphthalazine, suppressed spontaneous NMDA receptor-mediated EPSCs in cultures of spinal dorsal horn neurons. In addition, kainate suppressed EPSCs in dorsal horn neurons evoked by stimulation of synaptically coupled DRG cells in DRG–dorsal horn neuron cocultures. Interestingly, although the glutamate receptor subunit 5-selective kainate receptor agonist (RS)-2-α-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA) (2 μm) was able to suppress DRG–dorsal horn synaptic transmission to a similar extent as kainate (10 μm), it had no effect on excitatory transmission between dorsal horn neurons. Agonist applications revealed a striking difference between kainate receptors expressed by DRG and dorsal horn neurons. Whereas DRG cell kainate receptors were sensitive to both kainate and ATPA, most dorsal horn neurons responded only to kainate. Finally, in recordings from dorsal horn neurons in spinal slices, kainate and ATPA were able to suppress NMDA and AMPA receptor-mediated EPSCs evoked by dorsal root fiber stimulation. Together, these data suggest that kainate receptor agonists, acting at a presynaptic locus, can reduce glutamate release from primary afferent sensory synapses.
Keywords: kainate, presynaptic, ATPA, glutamate receptor subunit 5, glutamate, autoreceptor, excitatory synaptic transmission, NMDA
Glutamate is the major excitatory transmitter at primary afferent synapses, at which it conveys sensory information to the CNS via postsynaptic AMPA, NMDA, and kainate (KA) receptors on spinal cord dorsal horn neurons (Yoshimura and Jessell, 1990; Li et al., 1999). In addition to postsynaptic receptors, many neurons express on their presynaptic terminals ionotropic receptors that are thought to regulate transmitter release (MacDermott et al., 1999), including receptors for heterologous transmitters as well as autoreceptors for the transmitter(s) released by the terminal itself.
Much recent effort has focused on kainate receptors as possible presynaptic regulators of transmission. In the hippocampus, for example, presynaptic kainate receptor activation appears to reduce release of both glutamate (Chittajallu et al., 1996; Kamiya and Ozawa, 1998) and GABA (Clarke et al., 1997; Rodriguez-Moreno et al., 1997) (but see Frerking et al., 1999). At primary afferent synapses in the spinal cord, in addition to the postsynaptic kainate receptors that contribute to EPSCs evoked by high-threshold dorsal root fiber stimulation (Li et al., 1999), there are kainate receptors expressed presynaptically by dorsal root ganglion (DRG) neurons.
It is well known that kainate can depolarize a subset of dorsal root fibers (Davies et al., 1979; Agrawal and Evans, 1986). In addition, the electrophysiological properties of kainate receptors were first described in acutely dissociated DRG neurons (Huettner, 1990). Defining a physiological role for these receptors has remained elusive, however, in part because of the slow development of selective agonists and antagonists. The observations that kainate receptor activation selectively depressed evoked C-fiber volleys (Agrawal and Evans, 1986) and caused action potential firing in cultured DRG cells (Lee et al., 1999) raised the possibility that, by depolarizing presynaptic fibers, kainate receptor agonists might regulate transmitter release at primary afferent synapses. In this study, we report that activation of presynaptic kainate receptors reduces glutamate release from DRG neurons onto their dorsal horn targets.
MATERIALS AND METHODS
Primary neuronal culture. Dorsal horn neurons were taken from young postnatal rats anesthetized with pentobarbital. The dorsal vertebral column was opened, and the cord was removed to a dish containing Earl's buffer. The cord was split down the midline, and each half was then divided longitudinally into dorsal and ventral strips. Dorsal strips from two animals were combined and incubated for 30–90 min at 30–35°C in oxygenated Earl's buffer containing papain (Huettner and Baughman, 1986; Wilding and Huettner, 1997). Cells were dissociated by trituration with a fire-polished Pasteur pipette, after rinsing several times with Earl's buffer containing BSA and ovomucoid, both at 1 mg/ml. Dissociated cells were plated onto 35 mm culture dishes coated with matrigel (Becton Dickinson, Mountain View, CA) or with a mixture of poly-d,l-ornithine (0.2 mg/ml) and laminin (6 μg/ml). In some cases, neurons were confined to small islands of ∼200 × 200 μm, which were created by drawing a grid of agarose (1.5 mg/ml) on the bottom of a culture dish. Cultures were maintained at 37°C in a humidified, 5% CO2 incubator in Eagle's minimal essential medium supplemented with 20 mm glucose, 0.5 mm glutamine, 100 U/ml penicillin, 0.1 mg/ml streptomycin, and 4% rat serum. Cultures were treated for 4 din vitro (DIV 4) with 100 μmcytosine β-d-arabinofuranoside and were used for experiments between DIV 7 and DIV 35.
DRG cell cultures were prepared as described above, except that freshly dissected ganglia were incubated for 20 min in 1 mg/ml protease type XXIII (Sigma, St. Louis, MO) before trituration. Dissociated DRG neurons were plated either alone or together with dorsal horn neurons.
Electrophysiology in cultured neurons. Culture dishes were placed on the stage of an Axiovert 25 inverted microscope (Zeiss, Oberkochen, Germany) and bath-perfused with Tyrode's solution, containing (in mm): 150 NaCl, 4 KCl, 2 MgCl2, 2 CaCl2, 10 glucose, and 10 HEPES, pH 7.4 with NaOH. In experiments testing NMDA receptor-mediated responses, a Tyrode's solution lacking MgCl2 was used. During recordings, neurons were under constant local perfusion using a gravity-fed multibarreled pipette as described previously (Wilding and Huettner, 1997). The local perfusion solutions consisted of Tyrode's solution plus various pharmacological agents, including bicuculline methoiodide (10 μm) and strychnine hydrochloride (1 μm), which were used in all experiments to eliminate inhibitory neurotransmission. Rapid agonist applications to characterize neuronal kainate receptors were made using the same local perfusion pipette, but in this case the solution reservoirs were maintained under 8–10 psi of static air pressure. Voltage-gated currents mediated by calcium channels were recorded with barium as the charge carrier in an external solution containing (in mm): 5 BaCl2, 150 tetraethylammonium (TEA) chloride, 2 MgCl2, 0.1 EGTA, 10 HEPES, and 1 μm TTX, pH 7.4 with TEA-OH.
Whole-cell recordings were established using heat-polished pipettes pulled from borosilicate capillary tubes (Warner Instruments, Hamden, CT) with a tip resistance of 4–8 MΩ when filled with a solution containing (in mm): 140 Cs-MeSO3, 10 EGTA, 10 HEPES, 5 CsCl, 5 MgCl2, 5 Mg-ATP, and 1 Li-GTP, pH 7.4 with CsOH. (Potassium currents were recorded using an internal solution in which 140 mm K-glucuronate replaced Cs-MeSO3.) Cells were voltage-clamped at −70 mV. Series resistance (15–40 MΩ) was monitored throughout the experiments. Recorded currents were filtered at 2 kHz, digitized at 10 kHz, and stored in a personal computer for display and analysis with an Axopatch 200B amplifier, Digidata 1200 analog-to-digital converter interface, and the pClamp 6.0 software suite (all from Axon Instruments, Foster City, CA) Stimulation of synaptic currents was achieved with the S48 single-channel stimulator and SIU5 stimulus isolation unit (Grass Instruments, Quincy, MA) connected to a bipolar stimulating electrode, constructed with two Ag/AgCl wires immersed in Tyrode's solution within a θ glass electrode, pulled to a final tip diameter of ∼20 μm. We included only experiments in which evoked EPSCs occurred at a fixed latency after stimulation.
Data are presented as mean ± SEM, and statistical analysis was performed as described in the text and figure legends. The noise-free coefficient of variation (CV) was calculated as
where ς2 (EPSC) and ς2 (baseline) are the variance of the EPSC amplitude and baseline, respectively, and Amplitude(EPSC) is the mean amplitude of the synaptic current (Bekkers and Stevens, 1990;Clements, 1990; Malinow and Tsien, 1990; Rodriguez-Moreno et al., 1997). All compounds were obtained from Sigma except (2S)-3-{[(15)-1-(3,4-dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid (CGP55845),(RS)-4-(4-aminophenyl)-1,2-dihydro-1-methyl-2-propylcarbamoyl-6,7-meth-ylenedioxyphthalazine (SYM2206), and (RS)-2-α-amino-3-(3-hydroxy-5-tert-butylisoxazol-4-yl) propanoic acid (ATPA), which were obtained from Tocris Cookson (Ballwin, MO).
Electrophysiology in spinal cord slices. Spinal cord slices were prepared from postnatal day 4 (P4) to P21 rats as described previously (Li and Zhuo, 1998) and superfused constantly with a solution containing (in mm): 113 NaCl, 3 KCl, 25 NaHCO3, 1 NaH2PO3, 2 CaCl2, 1 MgCl2, and 25d-glucose (equilibrated with 95% O2–5% CO2, pH 7.3). Whole-cell recordings were established from lamina I–II neurons using unpolished 5–10 MΩ electrodes filled with a solution containing (in mm): 110 Cs-MeSO3, 5 MgCl2, 1 EGTA, 40 Na-HEPES, 2 Mg-ATP, and 0.1 Na3GTP, pH 7.2 (osmolarity adjusted to 295–300 mOsm). Recordings were performed as described above, except that EPSCs were evoked at 0.05–0.02 Hz with a bipolar tungsten electrode placed near dorsal rootlets or in the dorsal root entry zone, producing a stimulus width of 0.1–0.4 msec.
RESULTS
Kainate suppresses spontaneous NMDA receptor-mediated EPSCs in cultured dorsal horn neurons
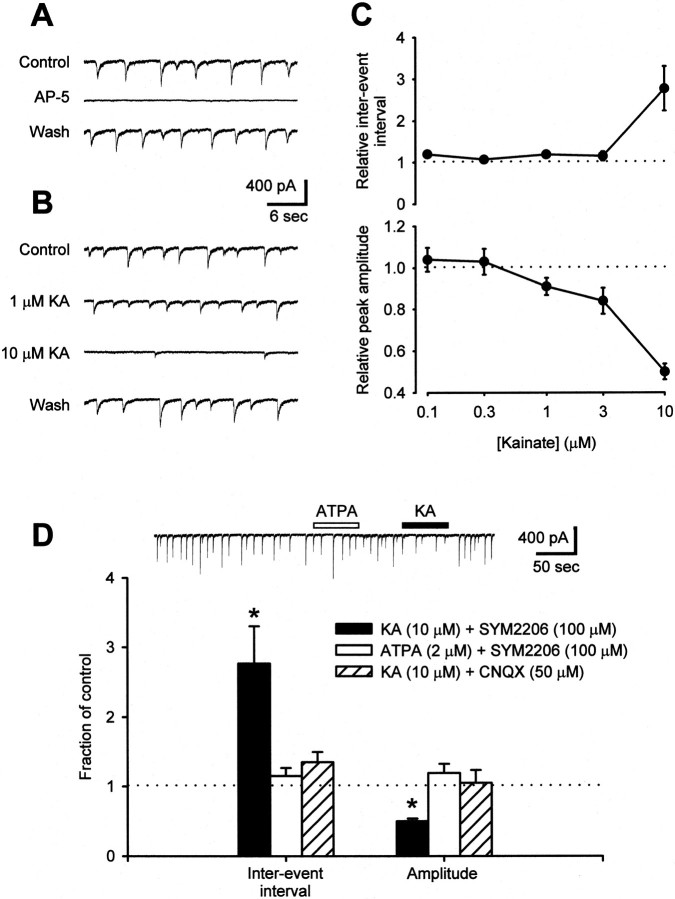
To test whether presynaptic kainate receptors may serve to regulate spinal sensory transmission, we initially investigated the effect of low kainate concentrations on dorsal horn neurons in dissociated cell cultures. EPSCs mediated by NMDA receptors were studied by removing Mg2+ from the perfusion medium (Nowak et al., 1984) and by adding bicuculline (10 μm) and strychnine (1 μm) to eliminate inhibitory neurotransmission. Because kainate can activate neuronal AMPA receptors (Patneau and Mayer, 1991), we added the noncompetitive AMPA receptor-selective antagonist SYM2206 (100 μm) (Pelletier et al., 1996). Under these conditions, spontaneous firing of neurons in mass cultures evoked spontaneous, AP-5-sensitive EPSCs (Fig.1A). Addition of kainate produced a dose-dependent decrease in the amplitude of these events (Fig. 1B,C). For low doses of kainate (0.3–3 μm), there was little or no change in the frequency of spontaneous EPSCs (sEPSCs); however, at a higher dose (10 μm), kainate significantly depressed both the amplitude and frequency of spontaneous events (Fig.1B,C). All effects of kainate on sEPSCs were readily reversible after reperfusion with control solution, and kainate had no effect on sEPSC amplitude or frequency when the nonselective AMPA/kainate receptor antagonist CNQX (50 μm) was substituted for SYM2206 (Fig.1D). In principal, these changes in sEPSCs could result from either a presynaptic or postsynaptic action of kainate. However, for most of the cells that we tested, the slow application of 10 μm kainate caused little change in holding current and no change in input or series resistance (Fig.2B and below), suggesting that the action of kainate was largely presynaptic. In contrast to kainate, which activates all kainate receptor subtypes, the glutamate receptor subunit 5 (GluR5) selective kainate receptor agonist ATPA (2 μm) (Clarke et al., 1997) did not affect spontaneous synaptic transmission between dorsal horn neurons (Fig. 1D).
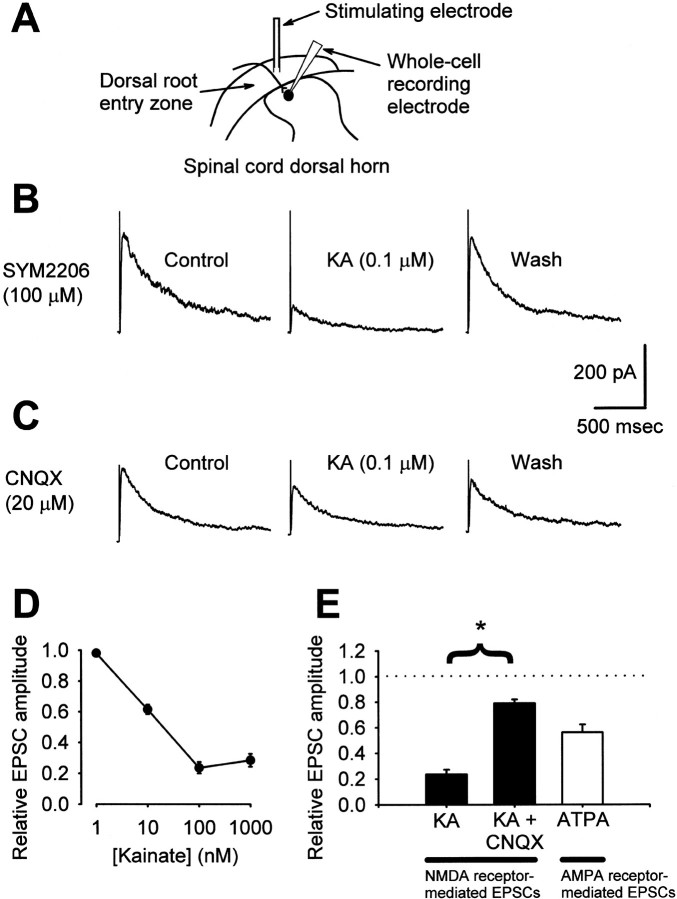
Fig. 1.
Kainate suppresses spontaneous excitatory transmission between cultured dorsal horn neurons. A, In the presence of 100 μm SYM2206 and no Mg2+, sEPSCs were recorded from dorsal horn neurons in mass culture. These events were silenced in the presence of 25 μm AP-5. B, Addition of kainate altered the characteristics of sEPSCs. At 1 μm, kainate reduced sEPSC amplitude without affecting frequency. Both parameters were decreased by 10 μm kainate. C, Summary of the effects of various doses of kainate on sEPSC interevent interval and peak amplitude (0.1 μm KA,n = 2 cells; 0.3 μm ka,n = 6; 1 μm ka, n= 8; 3 μm ka, n = 6; 10 μm ka; n = 8). D, Whereas 10 μm kainate altered both the amplitude and frequency of spontaneous NMDA receptor-mediated EPSCs (n = 7 cells), neither 2 μm ATPA (n = 5) nor 10 μm kainate plus 50 μm CNQX (n = 3) had any effect. *p < 0.05 indicates significant difference from control; two-way ANOVA with Tukey's test for post hoccomparison.
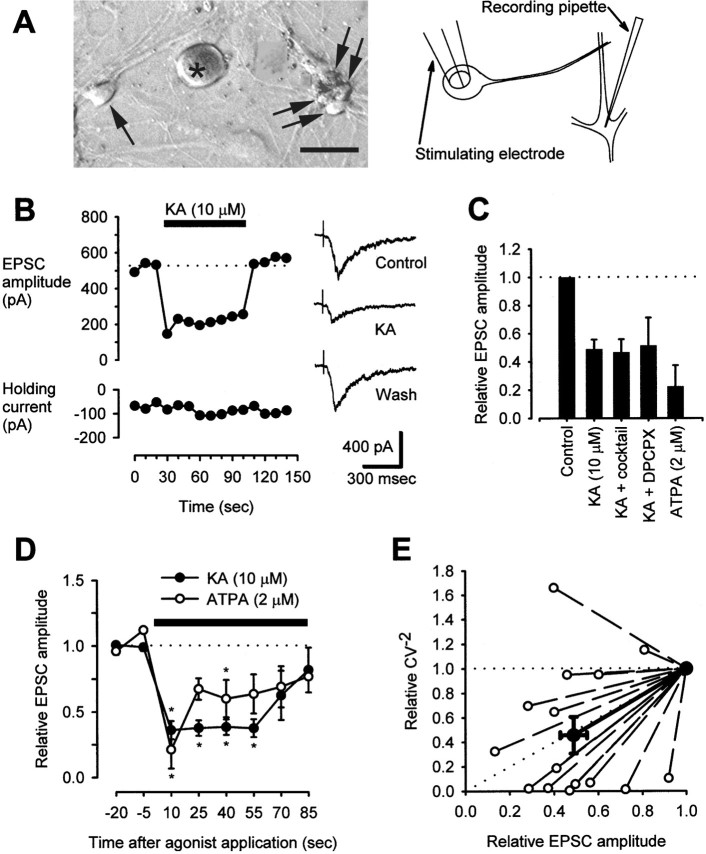
Fig. 2.
Kainate suppresses evoked excitatory transmission between DRG neurons and dorsal horn neurons in coculture.A, A photograph of cocultured DRG (asterisk) and dorsal horn neurons (arrows) and a diagram showing placement of recording and stimulating electrodes illustrate the experimental system. Scale bar, 20 μm. B, In a representative neuron, 10 μm kainate reversibly reduced NMDA receptor-mediated EPSC amplitude. Traces at the right show the shapes of EPSCs before, during, and after KA treatment. This recording was from a neuron treated with the antagonist cocktail described inC. The dotted line indicates the baseline level. C, Pooled data illustrate the relative amplitudes of NMDA receptor-mediated EPSCs in control conditions and after treatment with 10 μm KA (n = 8) or 2 μm ATPA (n = 6) alone; reduction of EPSC amplitude in both cases was statistically significant (p < 0.05; paired t test comparing absolute EPSC amplitudes in control and test conditions). In addition, the effect of KA was tested in some cells that were exposed continuously to a cocktail (containing 50 μm atropine, 100 μm naloxone, 2 mm AIDA, 500 μm CPPG, and 10 μm CGP55845;n = 4) or 1 μm DPCPX (n = 3). The effect of KA in these instances was indistinguishable from and did not differ statistically from the effect of KA alone (one-way ANOVA, comparing the percentage of EPSC depression in each condition). D, The effects of a continuous administration of 10 μm kainate (n = 8) or 2 μm ATPA (n = 6) on EPSC amplitude are plotted relative to baseline values. *p < 0.05 indicates significant difference between relative EPSC amplitude in kainate- or ATPA-treated cells at the indicated time points compared with baseline; Kruskal–Wallis one-way ANOVA on ranks with Dunn's test for post hoccomparison. The dotted line indicates the baseline level. E, Relative values for the statistic CV−2 (see Materials and Methods) are plotted against relative values for mean EPSC amplitude in cells before and after treatment with 10 μm KA. Data are included from all 15 KA-treated cells described in C, including experiments using various receptor antagonists. The bold line and bold points represent the mean relationship. The diagonal dotted line indicates the predicted relationship if only presynaptic phenomena underlie a change in EPSC amplitude; the horizontal dotted line indicates the relationship predicted by purely postsynaptic effects (Bekkers and Stevens, 1990; Clements, 1990; Malinow and Tsien, 1990).
Kainate and ATPA suppress excitatory transmission between cocultured DRG and dorsal horn neurons
Next, we examined the synapses formed by DRG neurons onto dorsal horn neurons (DRG → spinal synapses) in coculture. Excitatory neurotransmission was monitored by recording NMDA receptor-mediated EPSCs in dorsal horn neurons as described above, and DRG → spinal synapses were activated with a bipolar stimulating electrode in a θ glass pipette, placed against the cell body of a nearby, synaptically coupled DRG neuron (Fig. 2A). DRG cells chosen for stimulation were typically of the size shown in Figure2A (<20 μm diameter). Fixed-latency, NMDA receptor-mediated EPSCs could be evoked in these conditions (Fig.2B). Consistent with its effects at synapses between dorsal horn neurons (spinal → spinal synapses), kainate (10 μm) reversibly suppressed evoked EPSC amplitude at DRG → spinal synapses (Fig. 2B). Of 15 cell pairs tested, 14 exhibited only a suppression of EPSC amplitude (Fig.2B), and one gave a transient enhancement (lasting <25 sec) before a sustained suppression ensued. Only a suppression is evident in the pooled data (Fig. 2D). The ability of kainate to suppress NMDA receptor-mediated EPSCs was not altered by addition of the GABAB receptor antagonist CGP55845 (10 μm), the group I metabotropic glutamate receptor antagonist 1-aminoindan-1,5-dicarboxylic acid (AIDA) (2 mm), the group II/III metabotropic glutamate receptor antagonist (RS)-α-cyclopropyl-4-phosphonophenylglycine (CPPG) (500 μm), the opioid receptor antagonist naloxone (100 μm), the cholinergic receptor antagonist atropine (50 μm), or the adenosine receptor antagonist 8-cyclopentyl-1,3-dipropylxanthine (DPCPX) (1 μm) to the extracellular medium (Fig.2C), suggesting that these receptors did not contribute to the observed depression of excitatory transmission.
Interestingly, whereas 2 μm ATPA had no effect on spinal → spinal excitatory transmission, it did inhibit DRG → spinal transmission. Acute (10 sec) application of 10 μm kainate or 2 μm ATPA suppressed evoked DRG → spinal EPSCs to a similar extent (Fig. 2C,D), but during a prolonged application of these agonists, the effect of kainate waned more slowly (Fig. 2D), a difference that is consistent with the stronger desensitization produced by ATPA relative to kainate on kainate receptors in DRG cells (Fig.3).
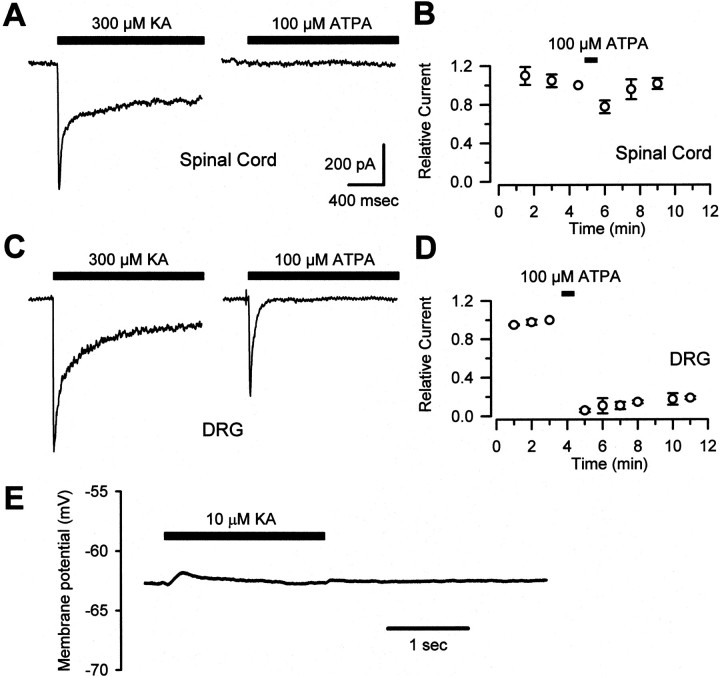
Fig. 3.
ATPA activates kainate receptors on DRG but not dorsal horn neurons. A, In cultured spinal cord dorsal horn neurons, fast application of 300 μm kainate but not 100 μm ATPA evoked a fast, incompletely desensitizing current. B, A 30 sec application of ATPA exerted little or no effect on the amplitude of peak currents evoked by 300 μm KA in spinal neurons (n = 11).C, In cultured DRG neurons, fast currents were elicited by both 300 μm KA and 100 μm ATPA.D, In experiments performed as in B, but using DRG neurons, ATPA caused a prolonged desensitization of peak kainate-evoked currents (n = 9). E, In a representative current-clamp recording from a cultured DRG neuron, 10 μm KA induced little somatic depolarization.
Consistent with a presynaptic locus of action of kainate receptor agonists, the inverse square of the coefficient of variation of EPSC amplitude (CV−2; see Materials and Methods), measured in the presence and absence of kainate, was proportional to EPSC amplitude (Fig. 2E). In addition, neither 10 μm kainate nor 2 μm ATPA substantially affected the passive membrane properties of the voltage-clamped dorsal horn neurons in these experiments (in 10 μm kainate, input resistance was 99 ± 5% of control, holding current changed by +26 ± 20 pA; in 2 μm ATPA, input resistance was 96 ± 10%, holding current changed by +1 ± 3 pA;n = 6–8 cells per measurement). Series resistance also remained constant throughout experiments (in 10 μm kainate or 2 μmATPA, series resistance was 100 ± 1% of control;n = 7–8).
ATPA selectively activates kainate receptors on DRG but not dorsal horn neurons
If kainate receptor agonists suppress excitatory transmission in the spinal cord by activating presynaptic receptors, then the differential effect of ATPA to inhibit DRG → spinal but not spinal → spinal transmission may be because of differential agonist sensitivities of the two cell types. To test this hypothesis, we compared the ability of kainate and ATPA to activate kainate receptors in cultured DRG and dorsal horn neurons. ATPA has been shown to be a potent and selective ligand for the kainate receptors that include the GluR5 subunit (Clarke et al., 1997; Hoo et al., 1999). Previous studies of mRNA levels in vivo have shown that the GluR5 subunit is expressed strongly in DRG neurons (Partin et al., 1993; Sato et al., 1993) but is much less prevalent in the spinal cord (Tölle et al., 1993). Consistent with this differential distribution of GluR5 and with the selective action of ATPA on DRG → spinal synapses, we observed a dramatic difference in the ability of ATPA to activate kainate receptors in DRG cells versus dorsal horn neurons. In contrast to 300 μm kainate, which elicited desensitizing currents in both cell types (in the presence of 100 μm SYM2206), 100 μmATPA evoked current when applied to DRG cells but was much less effective when applied to cultured spinal neurons. Many spinal neurons did not exhibit any response to ATPA (20 of 38 cells) (Fig.3A,C), and those cells that did displayed much smaller currents than could be elicited by kainate. Moreover, exposure to 100 μm ATPA for 30–60 sec had little effect on currents evoked by kainate in cultured dorsal horn neurons but produced a long-lasting cross-desensitization of kainate receptors in DRG cells (t1/2of recovery, >15 min; n = 9) (Fig.3B,D). Recovery from cross-desensitization in DRG cells was faster after treatment with lower doses of ATPA.
Although these high doses of kainate receptor agonists could induce whole-cell currents in somatic recordings from DRG neurons, much lower doses had been used to modulate excitatory transmission at DRG → spinal synapses (see above). To investigate the effect of 10 μm kainate on cultured DRG neurons, these neurons were current-clamped, and somatic membrane potential was monitored before and during agonist exposure. In nine cells tested (resting membrane potential of 64 ± 2 mV), this dose of kainate induced little or no somatic depolarization and no action-potential firing in eight cells (Fig. 3E). In one cell, a single action potential fired at the onset of agonist exposure. In contrast, 10 μm kainate depressed excitatory transmission at every DRG → spinal neuron pair tested.
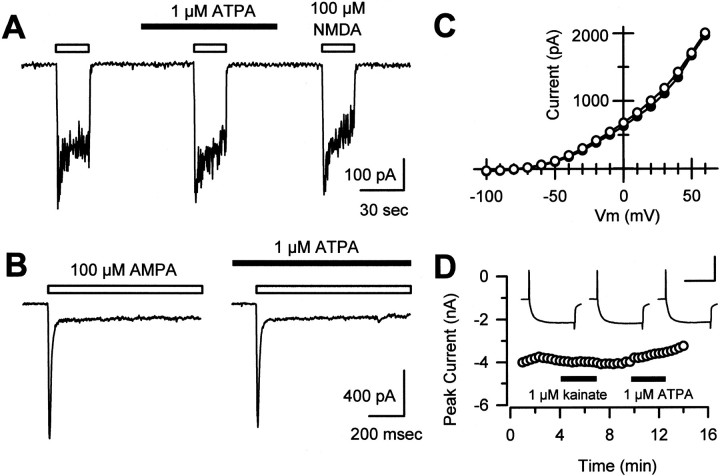
As a further control for the selectivity of agonist action, we tested whether kainate or ATPA had any effect on whole-cell currents evoked by direct activation of NMDA or AMPA receptors in dorsal horn neurons. Currents evoked by 100 μm NMDA in the presence of 1 or 10 μm glycine were not affected by coapplication of 1 μm ATPA (current amplitude, 97 ± 1% of control;n = 11) (Fig.4A) or 10 μm kainate (current amplitude, 93 ± 3% of control, n = 4). Similarly, 1 μm ATPA had no effect on currents evoked by 100 μm AMPA (current amplitude, 89 ± 6% of control; n = 9) (Fig. 4B). These results indicate that kainate and ATPA are unlikely to reduce transmission by a direct inhibition of postsynaptic NMDA or AMPA receptors. In addition, exposure to ATPA or kainate caused little or no change in voltage-gated K+ or Ca2+ currents (Fig.4C,D) that were recorded in isolated, voltage-clamped DRG cell bodies. Although these results cannot rule out the possibility that kainate or ATPA might modulate voltage-gated currents at DRG cell terminals, they do not provide any evidence that DRG cells express a mechanism for such modulation to occur.
Fig. 4.
ATPA and kainate do not affect AMPA- or NMDA-evoked currents in postsynaptic cells or affect Ca2+ or K+ channels in presynaptic cells. A, In cultured dorsal horn neurons, application of 100 μm NMDA and 1 μm glycine evoked inward currents that were insensitive to the presence of 1 μm ATPA. B, As in A, ATPA did not affect currents evoked by 100 μm AMPA.C, In cultured DRG neurons, the I–Vrelationship of voltage-gated K+ channel currents was identical in the absence (open circles) or presence (filled circles) of 1 μm ATPA. Steady-state currents are plotted at each potential. Similar results were obtained in 13 other cells. D, Voltage-gated Ca2+ channel currents in DRG neurons were not affected by application of 1 μm kainate (98 ± 1% of control; n = 23) or 1 μm ATPA (98 ± 1% of control; n = 10). Peak current evoked by stepping from −80 to 0 mV is plotted as a function of time.Inset, Individual traces recorded in control, kainate, and ATPA. Calibration: 4 nA, 50 msec.
Kainate receptor activation suppresses primary afferent neurotransmission in spinal slices
The selective block of DRG → spinal synapses in culture by ATPA suggested that exposure to agonists acting at presynaptic kainate receptors also might inhibit primary afferent transmission in a more intact preparation. To test this possibility, we performed whole-cell recordings from dorsal horn neurons in rat spinal cord slices. NMDA receptor-mediated EPSCs were evoked by dorsal root fiber stimulation in the presence of SYM2206 (100 μm) and blockers of inhibitory transmission (Fig.5A,B). Under these conditions, low concentrations of kainate produced a dose-dependent and reversible decrease in EPSC amplitude (Fig.5B,D). Maximal inhibition of NMDA receptor-mediated EPSCs (∼80%) was obtained with application of 100 nm kainate. This action of kainate was mostly blocked by substituting 20 μm CNQX for SYM2206 in the bath solution (Fig. 5C,E); 100 μm CNQX did not provide any additional blockade (n = 3; data not shown).
Fig. 5.
Kainate receptor activation suppresses primary afferent neurotransmission in spinal cord slices. A, A diagram illustrates the placement of stimulating and recording electrodes in the dorsal horn of a spinal slice. B, NMDA receptor-mediated EPSCs were isolated at a holding potential of +40 mV in the presence of 100 μm SYM2206. Traces from a representative neuron show reversible inhibition of EPSCs by 0.1 μm KA. C, When 20 μm CNQX replaced SYM2206 in experiments, as in B, 0.1 μm KA had less of an effect. D, Kainate exerted a dose-dependent inhibition of NMDA receptor-mediated EPSCs (n = 3–4 cells per concentration).E, Summarized data showing inhibition of NMDA receptor-mediated EPSCs by 0.1 μm KA in the presence of 100 μm SYM2206 (KA; n= 3) that was sensitive to 20 μm CNQX (KA+ CNQX; n = 3). Treatment with 100 μm CNQX (n = 3) afforded no additional blockade of the effect of kainate (data not shown). In addition, AMPA receptor-mediated EPSCs were suppressed by application of 2 μm ATPA (n = 5). ATPA- and KA-induced EPSC suppression were both statistically significant (p < 0.05; paired t test comparing absolute EPSC amplitudes in control and test conditions). *p < 0.05 indicates a significant difference in EPSC reduction by KA in the presence of SYM2206 versus CNQX;t test comparing the percentage of EPSC suppression in each condition.
We further tested whether kainate receptor agonists could inhibit presynaptic release of glutamate from primary afferent terminals by recording AMPA receptor-mediated EPSCs in spinal slices. As summarized in Figure 5E, exposure to ATPA reduced the peak amplitude of AMPA receptor-mediated EPSCs by ∼50% (Fig. 5E). Neither kainate nor ATPA had any significant effect on the holding current of cells voltage-clamped at +40 mV (for NMDA receptor-mediated currents) or −70 mV (for AMPA receptor-mediated currents), respectively (in 1 μm kainate, holding current changed by +7 ± 4 pA; in 2 μm ATPA, holding current changed by +9 ± 7 pA; n = 4–5 cells per condition).
DISCUSSION
In this study, we provide evidence that presynaptic kainate receptors regulate transmitter release from DRG cells onto dorsal horn spinal neurons. Evoked transmission was inhibited by exposure to kainate or ATPA, in both dissociated cell cultures and intact slices. In addition, our results demonstrate a significant difference in the pharmacology of kainate receptors expressed by DRG cells and spinal cord neurons. DRG cell kainate receptors were sensitive to ATPA (cf.Clarke et al., 1997), whereas ATPA produced little or no response in dorsal horn neurons and was unable to cross-desensitize the currents evoked by kainate. This difference provides one of the key pieces of evidence that kainate receptor agonists act presynaptically to reduce evoked transmission. Kainate, but not ATPA, was effective at suppressing spontaneous action potential-dependent transmission at excitatory spinal → spinal synapses, whereas both agonists suppressed DRG → spinal transmission. Thus, the selectivity of ATPA for DRG cell kainate receptors strongly indicates a presynaptic locus of action in reducing evoked transmission at DRG → spinal synapses (Fig.6).
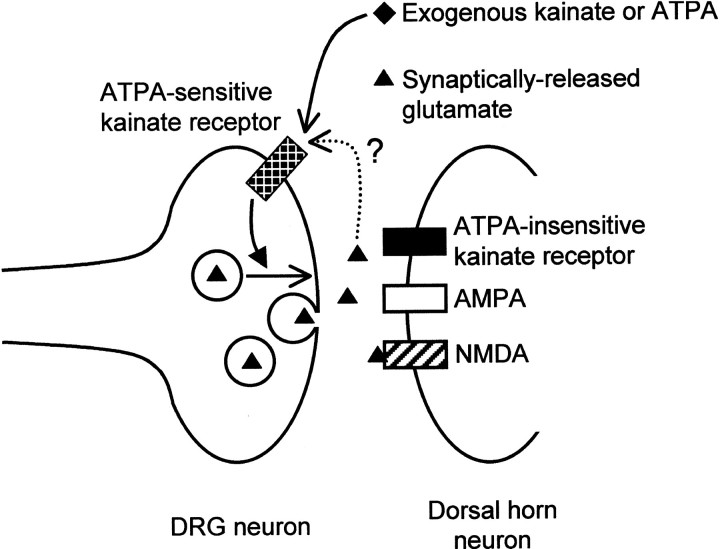
Fig. 6.
Kainate autoreceptors at primary afferent synapses. A model illustrates the hypothesis that kainate receptors, in addition to residing in the postsynaptic membrane of dorsal horn neurons, are also present on the presynaptic terminals of DRG neurons. Presynaptic and postsynaptic kainate receptors could be distinguished in this study by their sensitivity to the GluR5-selective agonist ATPA, and activation of presynaptic kainate receptors, at least by exogenous agonists, inhibited evoked release of glutamate. Unresolved is whether the presynaptic receptors can be activated by synaptically released glutamate (dotted line), and, if so, whether subsequent glutamate release would be inhibited or enhanced.
Pharmacological discrimination of kainate receptors on DRG and dorsal horn neurons: ATPA and the GluR5 subunit
Although the absolute selectivity of ATPA for all of the possible kainate receptor subunit combinations remains to be established, previous studies provide strong evidence that ATPA interacts preferentially with receptors that include the GluR5 subunit. Clarke et al. (1997) showed that ATPA potently elicits current in HEK293 cells expressing the GluR5 subunit (EC50 of 2.1 μm) and in isolated DRG neurons (EC50 of 600 nm), which had been treated with Con A to block kainate receptor desensitization (Huettner, 1990). In contrast, EC50 values for ATPA at other kainate receptor subunits and at AMPA receptors are in the hundreds of micromolar or higher (Clarke et al., 1997). More recent work (Bortolotto et al., 1999; Cui and Mayer, 1999; Paternain et al., 2000) has demonstrated that heteromeric receptors formed by coexpression of GluR5 and GluR6 or GluR7 retain sensitivity to ATPA. In addition, Lerma's group has published evidence (Paternain et al., 2000) that ATPA can activate heteromeric receptors formed by coexpression of the GluR6 and KA2 subunits in HEK293 cells, albeit with lower potency than for receptors that include GluR5. In the present study, we confirmed the activation of DRG cell kainate receptors by ATPA and showed that, in cells that were not treated with Con A, ATPA induced strong desensitization of these receptors similar to that observed with glutamate (Huettner, 1990) or 2S,3R-4-methyl-glutamate (Jones et al., 1997). Previous studies have highlighted the greater abundance of GluR5 message in DRG cells (Partin et al., 1993) compared with dorsal horn neurons (Tölle et al., 1993). In particular, expression of GluR5 is high in the small-diameter cells that are likely to carry nociceptive input (Sato et al., 1993). Moreover, radiolabeled binding studies (Hoo et al., 1999) have demonstrated a selective, high-affinity interaction between [3H]ATPA and membranes containing recombinant GluR5 (Kd ∼13 nm), as well as membranes from native rat DRG cells (Kd ∼4 nm). Whether the small currents we observed with ATPA in some of the spinal cord neurons were because of low-level expression of GluR5 (Tölle et al., 1993) or were caused by a low-efficacy action of ATPA at receptors lacking GluR5 (Paternain et al., 2000) remains to be determined. Nevertheless, the majority of receptors expressed by spinal neurons are either weakly sensitive or insensitive to ATPA, as evidenced by the lack of cross-desensitization of current evoked by kainate.
Presynaptic kainate receptor-mediated regulation of transmitter release
This study presents strong evidence that presynaptic kainate receptors modulate sensory neurotransmission in the dorsal horn. Because we used superfusion with exogenous kainate receptor agonists to achieve modulation of presynaptic glutamate release in our experiments, it is difficult to be certain where on presynaptic cells the relevant kainate receptors are located. It has been proposed that kainate receptors are preferentially distributed to the central terminals of DRG neurons, on which they would be poised to act as true autoreceptors (Agrawal and Evans, 1986; Lee et al., 1999). Previous work (Agrawal and Evans, 1986) has demonstrated particularly strong physiological responses when kainate was applied to centrally projecting axons between the ganglia and the cord. Evidence for modest expression on DRG cell bodies (Huettner, 1990) and on peripheral fibers and peripheral axon terminals (Ault and Hildebrand, 1993) has also been reported. In our spinal slice experiments, DRG cell soma are absent, leaving only the short segments of dorsal root fibers that traverse the dorsal root entry zone before terminating on neurons in the superficial laminas; our data indicate that kainate receptors capable of regulating primary afferent neurotransmission are located within that span. Evidence for a true autoreceptor function for sensing endogenously released glutamate at synaptic terminals might come from experiments with recently described antagonists selective for the GluR5 subunit (Bortolotto et al., 1999), once these compounds become generally available. Such experiments will be crucial to establishing whether activation of these receptors by synaptically released glutamate serves to inhibit subsequent release, as suggested by our experiments with exogenous agonists, or whether physiological activation of these receptors actually facilitates succeeding transmission events.
The mechanism by which kainate receptor activation exerts these effects, whether at primary afferent synapses or other central synapses, remains unclear. Studies of the effects of kainate receptor agonists on glutamate release from synaptosomes have produced divergent results (Zhou et al., 1995; Chittajallu et al., 1996; Perkinton and Sihra, 1999). One hypothesis, that kainate receptors regulate transmitter release by a mechanism involving direct depolarization of axons or axon terminals, is supported by the observation that in some CA1 neurons, kainate application induced a transient facilitation of NMDA receptor-mediated EPSCs before a prolonged depression ensued (Chittajallu et al., 1996), a phenomenon we observed in 1 of 15 experiments. More direct evidence that kainate receptors mediate axonal depolarization comes from studies of mossy fiber synapses, showing that presynaptic kainate receptor-mediated suppression of synaptic transmission was accompanied by enhanced mossy fiber excitability (Kamiya and Ozawa, 2000; Schmitz et al., 2000), a phenomenon reproduced when synaptically released glutamate from mossy fibers or associational–commissural fibers was used in place of kainate as an agonist (Schmitz et al., 2000). A potential link between axonal depolarization and suppression of glutamate release has been proposed by Kamiya and Ozawa (1998, 2000), who demonstrated reduced action potential-triggered Ca2+ influx into mossy fiber terminals with presynaptic kainate receptor activation. In the spinal cord, Lee et al. (1999) showed that kainate application increased the frequency of spontaneous, tetrodotoxin-insensitive postsynaptic currents, although the synapses responsible for these currents (excitatory vs inhibitory; primary afferent synapses vs local synapses) were not identified. Although we do not provide direct evidence that presynaptic kainate receptor activation suppresses primary afferent transmission by depolarization of presynaptic fibers, our data are at least consistent with such a model.
As an alternative to an ionotropic effect of kainate on presynaptic sites, some evidence supports a possible G-protein-mediated action of presynaptic kainate receptor stimulation at GABAergic terminals in the hippocampus (Rodriguez-Moreno and Lerma, 1998; Rodriguez-Moreno et al., 2000), although this proposed mechanism remains controversial and may depend, at least in part, on indirect effects (Frerking et al., 1999). A number of studies (Cossart et al., 1998; Frerking et al., 1998;Rodriguez-Moreno et al., 2000; Schmitz et al., 2000) have demonstrated depolarization and repetitive firing of action potentials by hippocampal interneurons after activation of postsynaptic kainate receptors by kainate or ATPA. In addition, Lee et al. (1999) observed that 100 μm kainate was sufficient to cause some action potential firing in DRG neurons. The dose of kainate (10 μm) used to affect DRG → spinal transmission in our study, however, did not induce somatic depolarization or action potential firing in the majority of DRG or spinal neurons and reduced (rather than enhanced) sEPSC frequency among dorsal horn neurons. Although it is possible that kainate receptor activation leads indirectly to activation of other receptors, such as GABAB (Frerking et al., 1999; Schmitz et al., 2000) or adenosine receptors (Chergui et al., 2000), we found no evidence of a role for those receptors or for opioid, muscarinic, or metabotropic glutamate receptors in our experiments. Nevertheless, further studies will be needed to elucidate in full the mediators that link kainate receptor activation to presynaptic alterations in synaptic transmission in the spinal cord and elsewhere.
In summary, our data provide new evidence that presynaptic kainate receptors on small-diameter DRG cells can regulate glutamate release at primary afferent synapses. Thus, kainate receptors, in addition to mediating postsynaptic responses at those synapses (Li et al., 1999), may also modulate somatosensory input into the spinal cord by acting on primary afferent fibers themselves. This study provides evidence of a functional role for the kainate receptors that have long been known to reside on a subset of DRG neurons. In addition, because the DRG → spinal synapse is a critical target for clinical treatment of pain, we suggest that selective activation of DRG kainate receptors with appropriate agonists may represent a novel strategy for pain control.
Footnotes
This work was supported by National Institutes of Health Grants DA10833, NS38680, and NS30888. We are grateful to Brian Schlag and Susan Kim for critical reading of this manuscript.
Correspondence should be addressed to Dr. James E. Huettner, Department of Cell Biology and Physiology, Washington University School of Medicine, Campus Box 8228, 660 South Euclid Avenue, St. Louis, MO 63110, E-mail: huettner@cellbio.wustl.edu; or Dr. Min Zhuo, Department of Anesthesiology, Washington University School of Medicine, Campus Box 8054, 660 South Euclid Avenue, St. Louis, MO 63110, E-mail:zhuom@morpheus.wustl.edu.
REFERENCES
- 1.Agrawal SG, Evans RH. The primary afferent depolarizing action of kainate in the rat. Br J Pharmacol. 1986;87:345–355. doi: 10.1111/j.1476-5381.1986.tb10823.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ault B, Hildebrand LM. Activation of nociceptive reflexes by peripheral kainate receptors. J Pharmacol Exp Ther. 1993;265:927–932. [PubMed] [Google Scholar]
- 3.Bekkers JM, Stevens CF. Presynaptic mechanism for long-term potentiation in the hippocampus. Nature. 1990;346:724–729. doi: 10.1038/346724a0. [DOI] [PubMed] [Google Scholar]
- 4.Bortolotto ZA, Clarke VR, Delany CM, Parry MC, Smolders I, Vignes M, Ho KH, Miu P, Brinton BT, Fantaske R, Ogden A, Gates M, Ornstein PL, Lodge D, Bleakman D, Collingridge GL. Kainate receptors are involved in synaptic plasticity. Nature. 1999;402:297–301. doi: 10.1038/46290. [DOI] [PubMed] [Google Scholar]
- 5.Chergui K, Bouron A, Normand E, Mulle C. Functional GluR6 kainate receptors in the striatum: indirect downregulation of synaptic transmission. J Neurosci. 2000;20:2175–2182. doi: 10.1523/JNEUROSCI.20-06-02175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chittajallu R, Vignes M, Dev KK, Barnes JM, Collingridge GL, Henley JM. Regulation of glutamate release by presynaptic kainate receptors in the hippocampus. Nature. 1996;379:78–81. doi: 10.1038/379078a0. [DOI] [PubMed] [Google Scholar]
- 7.Clarke VR, Ballyk BA, Hoo KH, Mandelzys A, Pellizzari A, Bath CP, Thomas J, Sharpe EF, Davies CH, Ornstein PL, Schoepp DD, Kamboj RK, Collingridge GL, Lodge D, Bleakman D. A hippocampal GluR5 kainate receptor regulating inhibitory synaptic transmission. Nature. 1997;389:599–603. doi: 10.1038/39315. [DOI] [PubMed] [Google Scholar]
- 8.Clements JD. A statistical test for demonstrating a presynaptic site of action for a modulator of synaptic amplitude. J Neurosci Methods. 1990;31:75–88. doi: 10.1016/0165-0270(90)90012-5. [DOI] [PubMed] [Google Scholar]
- 9.Cossart R, Esclapez M, Hirsch JC, Bernard C, Ben-Ari Y. GluR5 kainate receptor activation in interneurons increases tonic inhibition of pyramidal cells. Nat Neurosci. 1998;1:470–478. doi: 10.1038/2185. [DOI] [PubMed] [Google Scholar]
- 10.Cui C, Mayer ML. Heteromeric kainate receptors formed by the coassembly of GluR5, GluR6, and GluR7. J Neurosci. 1999;19:8281–8291. doi: 10.1523/JNEUROSCI.19-19-08281.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Davies J, Evans RH, Francis AA, Watkins JC. Excitatory amino acid receptors and synaptic excitation in the mammalian central nervous system. J Physiol (Paris) 1979;75:641–654. [PubMed] [Google Scholar]
- 12.Frerking M, Malenka RC, Nicoll RA. Synaptic activation of kainate receptors on hippocampal interneurons. Nat Neurosci. 1998;1:479–486. doi: 10.1038/2194. [DOI] [PubMed] [Google Scholar]
- 13.Frerking M, Petersen CC, Nicoll RA. Mechanisms underlying kainate receptor-mediated disinhibition in the hippocampus. Proc Natl Acad Sci USA. 1999;96:12917–12922. doi: 10.1073/pnas.96.22.12917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hoo K, Legutko B, Rizkalla G, Deverill M, Hawes CR, Ellis GJ, Stensbol TB, Krogsgaard-Larsen P, Skolnick P, Bleakman D. [3H]ATPA: a high affinity ligand for GluR5 kainate receptors. Neuropharmacology. 1999;38:1811–1817. doi: 10.1016/s0028-3908(99)00133-1. [DOI] [PubMed] [Google Scholar]
- 15.Huettner JE. Glutamate receptor channels in rat DRG neurons: activation by kainate and quisqualate and blockade of desensitization by Con A. Neuron. 1990;5:255–266. doi: 10.1016/0896-6273(90)90163-a. [DOI] [PubMed] [Google Scholar]
- 16.Huettner JE, Baughman RW. Primary culture of identified neurons from the visual cortex of postnatal rats. J Neurosci. 1986;6:3044–3060. doi: 10.1523/JNEUROSCI.06-10-03044.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones KA, Wilding TJ, Huettner JE, Costa AM. Desensitization of kainate receptors by kainate, glutamate, and diastereomers of 4-methylglutamate. Neuropharmacology. 1997;36:853–863. doi: 10.1016/s0028-3908(97)00066-x. [DOI] [PubMed] [Google Scholar]
- 18.Kamiya H, Ozawa S. Kainate receptor-mediated inhibition of presynaptic Ca2+ influx and EPSP in area CA1 of the rat hippocampus. J Physiol (Lond) 1998;509:833–845. doi: 10.1111/j.1469-7793.1998.833bm.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kamiya H, Ozawa S. Kainate receptor-mediated presynaptic inhibition at the mouse hippocampal mossy fibre synapse. J Physiol (Lond) 2000;523:653–665. doi: 10.1111/j.1469-7793.2000.t01-1-00653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee CJ, Engelman HS, MacDermott AB. Activation of kainate receptors on rat sensory neurons evokes action potential firing and may modulate transmitter release. Ann NY Acad Sci. 1999;868:546–549. doi: 10.1111/j.1749-6632.1999.tb11325.x. [DOI] [PubMed] [Google Scholar]
- 21.Li P, Zhuo M. Silent glutamatergic synapses and nociception in mammalian spinal cord. Nature. 1998;393:695–698. doi: 10.1038/31496. [DOI] [PubMed] [Google Scholar]
- 22.Li P, Wilding TJ, Kim SJ, Calejesan AA, Huettner JE, Zhuo M. Kainate-receptor-mediated sensory synaptic transmission in mammalian spinal cord. Nature. 1999;397:161–164. doi: 10.1038/16469. [DOI] [PubMed] [Google Scholar]
- 23.MacDermott AB, Role LW, Siegelbaum SA. Presynaptic ionotropic receptors and the control of transmitter release. Annu Rev Neurosci. 1999;22:443–485. doi: 10.1146/annurev.neuro.22.1.443. [DOI] [PubMed] [Google Scholar]
- 24.Malinow R, Tsien RW. Presynaptic enhancement shown by whole-cell recordings of long-term potentiation in hippocampal slices. Nature. 1990;346:177–180. doi: 10.1038/346177a0. [DOI] [PubMed] [Google Scholar]
- 25.Nowak L, Bregestovski P, Ascher P, Herbet A, Prochiantz A. Magnesium gates glutamate-activated channels in mouse central neurones. Nature. 1984;307:462–465. doi: 10.1038/307462a0. [DOI] [PubMed] [Google Scholar]
- 26.Partin KM, Patneau DK, Winters CA, Mayer ML, Buonanno A. Selective modulation of desensitization at AMPA versus kainate receptors by cyclothiazide and concanavalin A. Neuron. 1993;11:1069–1082. doi: 10.1016/0896-6273(93)90220-l. [DOI] [PubMed] [Google Scholar]
- 27.Paternain AV, Herrera MT, Nieto MA, Lerma J. GluR5 and GluR6 kainate receptor subunits coexist in hippocampal neurons and coassemble to form functional receptors. J Neurosci. 2000;20:196–205. doi: 10.1523/JNEUROSCI.20-01-00196.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Patneau DK, Mayer ML. Kinetic analysis of interactions between kainate and AMPA: evidence for activation of a single receptor in mouse hippocampal neurons. Neuron. 1991;6:785–798. doi: 10.1016/0896-6273(91)90175-y. [DOI] [PubMed] [Google Scholar]
- 29.Pelletier JC, Hesson DP, Jones KA, Costa A-M. Substituted 1,2-dihydrophthalazines: potent, selective, and non-competitive inhibitors of the AMPA receptor. J Med Chem. 1996;39:343–346. doi: 10.1021/jm950740w. [DOI] [PubMed] [Google Scholar]
- 30.Perkinton MS, Sihra TS. A high-affinity presynaptic kainate-type glutamate receptor facilitates glutamate exocytosis from cerebral cortex nerve terminals (synaptosomes). Neuroscience. 1999;90:1281–1292. doi: 10.1016/s0306-4522(98)00573-9. [DOI] [PubMed] [Google Scholar]
- 31.Rodriguez-Moreno A, Lerma J. Kainate receptor modulation of GABA release involves a metabotropic function. Neuron. 1998;20:1211–1218. doi: 10.1016/s0896-6273(00)80501-2. [DOI] [PubMed] [Google Scholar]
- 32.Rodriguez-Moreno A, Herreras O, Lerma J. Kainate receptors presynaptically downregulate GABAergic inhibition in the rat hippocampus. Neuron. 1997;19:893–901. doi: 10.1016/s0896-6273(00)80970-8. [DOI] [PubMed] [Google Scholar]
- 33.Rodriguez-Moreno A, Lopez-Garcia JC, Lerma J. Two populations of kainate receptors with separate signaling mechanisms in hippocampal interneurons. Proc Natl Acad Sci USA. 2000;97:1293–1298. doi: 10.1073/pnas.97.3.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA, and NMDA receptors are expressed in the rat DRG neurones. NeuroReport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- 35.Schmitz D, Frerking M, Nicoll RA. Synaptic activation of presynaptic kainate receptors on hippocampal mossy fiber synapses. Neuron. 2000;27:327–338. doi: 10.1016/s0896-6273(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 36.Tölle TR, Berthele A, Zieglgansberger W, Seeburg PH, Wisden W. The differential expression of 16 NMDA and non-NMDA receptor subunits in the rat spinal cord and in periaqueductal gray. J Neurosci. 1993;13:5009–5028. doi: 10.1523/JNEUROSCI.13-12-05009.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wilding TJ, Huettner JE. Activation and desensitization of hippocampal kainate receptors. J Neurosci. 1997;17:2713–2721. doi: 10.1523/JNEUROSCI.17-08-02713.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yoshimura M, Jessell T. Amino acid-mediated EPSPs at primary afferent synapses with substantia gelatinosa neurones in the rat spinal cord. J Physiol (Lond) 1990;430:315–335. doi: 10.1113/jphysiol.1990.sp018293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou M, Peterson CL, Lu YB, Nadler JV. Release of glutamate and aspartate from CA1 synaptosomes: selective modulation of aspartate release by ionotropic glutamate receptor ligands. J Neurochem. 1995;64:1556–1566. doi: 10.1046/j.1471-4159.1995.64041556.x. [DOI] [PubMed] [Google Scholar]