Abstract
Phantom limb pain (PLP) in amputees is associated with reorganizational changes in the somatosensory system. To investigate the relationship between somatosensory and motor reorganization and phantom limb pain, we used focal transcranial magnetic stimulation (TMS) of the motor cortex and neuroelectric source imaging of the somatosensory cortex (SI) in patients with and without phantom limb pain. For transcranial magnetic stimulation, recordings were made bilaterally from the biceps brachii, zygomaticus, and depressor labii inferioris muscles. Neuroelectric source imaging of the EEG was obtained after somatosensory stimulation of the skin overlying face and hand. Patients with phantom limb pain had larger motor-evoked potentials from the biceps brachii, and the map of outputs was larger for muscles on the amputated side compared with the intact side. The optimal scalp positions for stimulation of the zygomaticus and depressor labii inferioris muscles were displaced significantly more medially (toward the missing hand representation) in patients with phantom limb pain only. Neuroelectric source imaging revealed a similar medial displacement of the dipole center for face stimulation in patients with phantom limb pain. There was a high correlation between the magnitude of the shift of the cortical representation of the mouth into the hand area in motor and somatosensory cortex and phantom limb pain. These results show enhanced plasticity in both the motor and somatosensory domains in amputees with phantom limb pain.
Keywords: cortical plasticity, sensorimotor reorganization, phantom limb pain, amputation, transcranial magnetic stimulation, neuroelectric source imaging
Amputation in humans leads to extensive reorganization in the somatosensory (Sica et al., 1984;Elbert et al., 1994; Yang et al., 1994; Flor et al., 1995; Reshetnyak et al., 1996; Birbaumer et al., 1997; Kew et al., 1997; Borsook et al., 1998; Davis et al., 1998; Flor et al., 1998; Montoya et al., 1998; Grüsser et al., 2001) and the motor cortex (Hall et al., 1990; Cohen et al., 1991; Fuhr et al., 1992; Kew et al., 1994; Ojemann and Silbergeld, 1995; Pascual-Leone et al., 1996; Chen et al., 1998;Röricht et al., 1999). Phantom limb pain (PLP) is a condition characterized by sensations of pain in the missing limb. It is usually more common in the initial stages after amputations (Jensen et al., 1985; Jensen and Rasmussen, 1995), but in some cases, it can remain present for many years (Sunderland, 1978; Sherman, 1989). Phantom limb pain is strongly correlated with representational plasticity in the somatosensory cortex (SI) (Flor et al., 1995, 1998; Birbaumer et al., 1997; Montoya et al., 1998; Grüsser et al., 2001).
Despite the extensive information on plastic changes in the human motor system after amputation (Hall et al., 1990; Cohen et al., 1991; Fuhr et al., 1992; Kew et al., 1994; Ojemann and Silbergeld, 1995;Pascual-Leone et al., 1996; Chen et al., 1998; Cohen et al., 1998), it is unclear, except for a single case report (Pascual-Leone et al., 1996), whether motor representations surrounding the representation of the missing limb expand over the deafferented cortex. It is also not known whether motor plasticity is related to phantom limb pain, a correlate of the somatosensory plasticity described previously by Flor et al. (1995). This form of representational plasticity in the motor domain can be studied using neuroimaging (Khorram-Sefat et al., 1997) and neurophysiological techniques such as transcranial magnetic stimulation (TMS) (Cohen et al., 1998).
The purpose of this study was to identify plastic changes in the motor and somatosensory domains in amputees with and without phantom limb pain.
MATERIALS AND METHODS
Participants. Five upper limb amputees with PLP and five pain-free amputees (non-PLP) participated in the study. Nine of the subjects were male. The groups were not significantly different regarding age, stump length, and handedness before the amputation (Table 1). All subjects gave written informed consent for the study, and the protocol was approved by the Clinical Research Review Committee of the University of Tübingen.
Table 1.
Demographic and clinical features of all participants
| Subject number | Amputation side1-a | Gender1-b | Age (years) | Time since amputation (years) | Age at the time of the amputation (years) | Stump length (cm) |
|---|---|---|---|---|---|---|
| Phantom limb pain patients | ||||||
| 1 | l | F | 43 | 38 | 5 | 21.0 |
| 2 | r | M | 61 | 41 | 20 | 62.0 |
| 3 | r | M | 39 | 31 | 8 | 45.0 |
| 4 | l | M | 38 | 26 | 12 | 52.5 |
| 5 | r | M | 77 | 24 | 53 | 21.0 |
| M/N | 3 r | 1 f | 51.60 | 32.00 | 19.60 | 40.30 |
| (SD)/N | 2 l | 4 m | (16.96) | (7.38) | (19.50) | (18.62) |
| Patients without phantom limb pain | ||||||
| 6 | r | M | 25 | 18 | 7 | 61.5 |
| 7 | l | M | 34 | 17 | 17 | 61.5 |
| 8 | r | M | 39 | 24 | 15 | 51.0 |
| 9 | r | M | 23 | 2 | 21 | 52.0 |
| 10 | r | M | 58 | 52 | 6 | 48.5 |
| M/N | 4 r | 0 f | 35.80 | 22.60 | 13.20 | 54.90 |
| (SD)/N | 1 l | 5 m | (14.02) | (18.32) | (6.50) | (6.16) |
| Handedness before the amputation1-a | Phantom limb pain (MPI-D, 0–6) | Stump pain (MPI-D, 0–6) | Pre-amputation pain | Nonpainful phantom sensations (VAS, 0–100) | Nonpainful stump sensations (VAS, 0–100) |
|---|---|---|---|---|---|
| r | 4.3 | 0 | yes | 56 | 0 |
| r | 4.0 | 4.0 | no | 0 | 0 |
| r | 1.7 | 0.7 | no | 0 | 0 |
| r | 3.7 | 0 | no | 34 | 0 |
| r | 3.0 | 3.0 | yes | 50 | 65 |
| 5 r | 3.34 | 1.54 | 2 yes | 28.00 | 13.00 |
| 01 | (1.04) | (1.85) | 3 no | (26.80) | (29.07) |
| r | 0 | 0 | yes | 89 | 26 |
| r | 0 | 0 | no | 60 | 25 |
| r | 0 | 0 | no | 26 | 77 |
| r | 0 | 0 | no | 0 | 0 |
| r | 0 | 0 | yes | 67 | 48 |
| 5 r | 0.00 | 0.00 | 2 yes | 48.40 | 35.20 |
| 0 l | (0.00) | (0.00) | 3 no | (35.26) | (2.89) |
r, Right; l, left.
M, Male; F, female.
Evaluation of phantom sensations. Duration, intensity, and frequency of phantom limb pain, nonpainful phantom sensations, stump pain, and stump sensations were investigated by a standardized interview (Flor et al., 1995) and the German version of the West Haven – Yale Multidimensional Pain Inventory (Kerns et al., 1985; Flor et al., 1990) modified to separately evaluate stump and phantom limb pain (Flor et al., 1995). In addition, referred sensations were tested by Q-tip and pin prick from 200 body sites as described previously (Flor et al., 1995).
Determination of motor reorganization. Focal TMS was delivered from a MagPro (Dantec Skovlunde, Copenhagen, Denmark) magnetic stimulator (study 1) through an 8-shaped magnetic coil (MC B70, 35 kt/sec, active pulse width 51 μsec, peak-B-field = 1.1 tesla). All 10 upper limb amputees participated in this study. Each wing of the coil had an 8 cm diameter. The magnetic coil, which delivers relatively focal stimuli (Cohen et al., 1990), was positioned to induce currents flowing approximately perpendicular to the central sulcus (Werhahn et al., 1994). The optimal scalp position was defined as the position that, on stimulation, elicited the largest peak-to-peak motor-evoked potential amplitude in each muscle. Motor thresholds were determined after stimulation of the optimal scalp position for each of two muscles: biceps brachii and zygomaticus. The motor threshold was defined as the lowest stimulus intensity that elicited motor-evoked potentials of at least 50 μV peak-to-peak amplitude in at least 5 of 10 trials at rest. Motor threshold provides indirect information about neuronal membrane excitability levels in the human motor cortex (Ziemann et al., 1996; Cohen et al., 1998) and possibly about excitability at subcortical sites. Motor threshold is elevated by sodium channel blockers such as carbamazepine and phenytoin (Mavroudakis et al., 1994; Ziemann et al., 1996; Chen et al., 1997) and is unchanged by GABAergic drugs such as benzodiazepines or vigabatrin (Inghilleri et al., 1996; Ziemann et al., 1996;Mavroudakis et al., 1997) and anti-glutamatergic drugs such as gabapentin or riluzole (Ziemann et al., 1996; Liepert et al., 1998b). Maps of output targeting each muscle were then obtained by applying single TMS stimuli at an intensity of 20% above the subject's motor threshold for each muscle. Intervals between stimuli were at least 5 sec. Each scalp position was stimulated three times. Electromyographic responses were recorded from surface electrodes overlying the muscle bellies of the first complete muscle above the stump (biceps brachii), amplified by Synamps amplifiers (Neuroscan Labs; bandpass 10 Hz to 2000 kHz), and stored for off-line analysis. A 7 × 9 cm grid of positions was randomly stimulated on each hemisphere, one at a time, with Cz as reference.
In two of the six subjects described above (one without, one with phantom limb pain), a modified procedure of transcranial magnetic stimulation mapping was used in an additional assessment (study 2). Stimuli were delivered from a Pro#4 high-speed stimulator (Cadwell Laboratories) through a smaller more focal figure-eight magnetic coil; each wing had a diameter of 5.4 cm. Stimulus intensities were adjusted for each individual muscle and set to 10% above motor threshold. The grid of stimulated positions was extended from 7 × 9 to 9 × 14 cm, and the number of stimulations per position was increased to 10. Recordings from face muscles were made from the less bilaterally represented muscle depressor labii inferioris instead of the muscle zygomaticus major (Meyer et al., 1994). The optimal scalp position and the motor threshold were determined as described above.
In study 1, motor-evoked potentials obtained from biceps brachii and zygomaticus (Table 1) after stimulation of each position were baseline corrected, rectified, and averaged. Subsequently, the maximal motor-evoked potential amplitude obtained by stimulation of any position was identified. For mapping purposes, the largest motor-evoked potential amplitude for each muscle was defined as 100%, and the motor-evoked potentials obtained after stimulation of other scalp positions were expressed as a percentage of this maximal response at the optimal scalp position.
In the two subjects of study 2 who were studied with the more focal coil and more detailed grid, motor-evoked potentials obtained from the biceps brachii and depressor labii inferioris after stimulation of each position were baseline corrected, rectified, and averaged. Maps of output were determined as described above, and centers of gravity were calculated. The size of motor maps determined in this way is influenced by a combination of factors that include excitability of the motor representation (Ridding and Rothwell, 1995, 1997; Thickbroom et al., 1998).
The center of gravity, an amplitude-weighted representative position of a motor map, was computed as follows. The coronal coordinate of the center of gravity is computed by multiplying the coronal coordinate of each position by the motor-evoked potential amplitude obtained after stimulation of that position and summing over all positions. The sagittal coordinate is computed in an analogous way. The center of gravity is usually more lateral for distal hand muscles and progressively more medial for forearm and upper arm muscles (Wassermann et al., 1992). The distance of the optimal scalp position and the lateral and medial border of each muscle representation from Cz were also determined. The evaluation of the center of gravity and the distance between optimal scalp position and the midline are useful because they allow identification of mediolateral map displacements (Cohen et al., 1995, 1996; Liepert et al., 1998a) and could reflect directionally selective expansion of the motor representation (Cohen et al., 1998).
Determination of somatosensory reorganization. In six subjects (three with and three without phantom limb pain), neuroelectric source imaging of the somatosensory cortex was performed (study 3). Light superficial pressure stimuli were applied to each of the following four sites: first and fifth digit of the intact hand, and corner of the lower lip on both the intact and the amputation side. At each location, 1000 pneumatic stimuli were applied (Elbert et al., 1994; Yang et al., 1994; Flor et al., 1995). The four blocks of 1000 stimuli with a duration of 50 msec were delivered in random order to the four sites with interstimulus intervals of 705 msec. Somatosensory evoked potentials (SEPs) were obtained from 60 electrodes that were affixed to an elastic cap and spaced 3 cm apart (center to center) in a 6 × 10 rectangular array centered over Cz using a linked ear reference. All signals were sampled at a rate of 1 kHz with a bandpass from 0.1 to 200 Hz using a 64-channel Synamps amplifier.
Trials that exceeded 200 μV in any channel were excluded from further analysis (median rejection rate: 11.2%). Eye movement artifacts, determined from vertical and horizontal electro-oculographic recordings, were corrected using the algorithm incorporated in the Neuroscan software (Semlitsch et al., 1986). SEPs were filtered off-line using a low-frequency cutoff (8 Hz) and then transformed to a common average reference. For each somatosensory evoked field, a principal component analysis (PCA) was performed to achieve an improved signal-to-noise ratio. The PCA was computed for a time window ranging from 40 msec prestimulus baseline through 85 msec after stimulus onset (125 data points), which was chosen on the basis of previous findings concerning the approximate time window for primary SI activity (Elbert et al., 1994, 1995; Flor et al., 1995).
To increase the signal-to-noise ratio for the dipole fitting in the contralateral hemisphere, the overall weight of the SEPs from the hemisphere ipsilateral to the site of stimulation (which receives a lesser input from the stimulated side) was decreased (Birbaumer et al., 1997). The amplitudes of the SEPs from the hemisphere ipsilateral to the site of stimulation were weighted in an exponential fashion, with maximal input from the more medial electrodes and minimal input from the temporal locations. The amplitudes of the SEPs from the hemisphere contralateral to the site of stimulation were used in an unweighted fashion. The three-dimensional location of each dipole was then computed based on three-dimensional magnetic resonance coordinates (determined for each individual) of the 60 electrode positions, which had been marked individually with vitamin E capsules (Siemens Vision MR scanner: 198 slices; field of view = 230 mm; 3-D Flash; repetition time = 20 msec; echo time = 6 msec; α = 30). For each electrical field distribution, a spherical four-shell model of the head was fitted using a standard least squares fit algorithm of the electrode coordinates; the radii from the center to the scalp, skull, liquid, and cortex surface were estimated according to standard ratios (Cuffin and Cohen, 1979). A coordinate system was used that had its origin in the center of the sphere, its z-axis pointing toward Cz, its x-axis oriented in the medial-lateral direction, and its y-axis pointed in the anterior-posterior direction. A larger polar angle denotes a more lateral and inferior position of the respective location; a smaller polar angle is achieved by more medial and superior locations (Birbaumer et al., 1997). Cortical reorganization was determined by computing the polar angle (referred to as Cz) of the dipole locations of the finger and lip representations on the postcentral gyrus. The polar angle of the (absent) finger on the amputation side was computed by projecting the finger representation of the intact side across the midline onto the hemisphere contralateral to the amputation stump, thus creating a “mirror” finger location of the amputation side (Elbert et al., 1994).
Coregistration of motor and somatosensory representations. To compare representational changes in motor and somatosensory systems, the six subjects who participated in SEP recordings (three with and three without phantom limb pain) also received transcranial magnetic stimulation. The somatosensory data were then overlaid on magnetic resonance images (study 3). The location of the dipole (computed as described above) of the somatosensory representation of the lips (amputated and intact side) and intact hand as well as that of the mirrored location of the intact lip was then superimposed on the magnetic resonance image.
For coregistration purposes, the center of gravity for the motor maps was calculated using the following formula with x andy signifying the lateral-medial and anterior-posterior distance from Cz and f referring to the amplitude of the motor-evoked potential:
The size of the circles in Figures 2 and 3 represents 1 SD around the center of gravity as calculated by the following formula:
This information was then projected onto the same individual's magnetic resonance image.
Fig. 2.
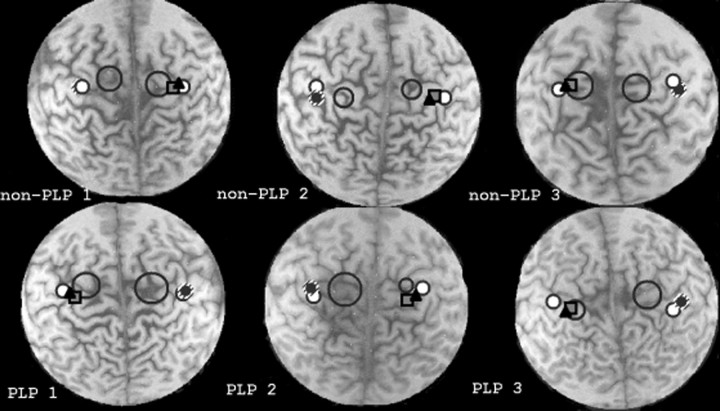
Superimposition of somatosensory representations of the first digit (D1) (square) and of the fifth digit (D5) (filled black triangle) of the intact hand and motor representation of the biceps brachii (large circles) on each subjects' MRI. Data from three patients with phantom limb pain (PLP, bottom) and three without PLP (non-PLP, top) are shown (patients non-PLP 1, non-PLP 2, andPLP 2 had right arm amputations; patients PLP 1, PLP 3, and non-PLP 3 had left arm amputations). The somatosensory representations of the lips on the amputated and intact sides are indicated by the white dot with the black edge. The lip representation corresponding to the intact side was projected over the representation corresponding to the amputated side (Elbert et al., 1994) and is displayed as small black dot overlaid on a larger hatched circle. Note the larger size of the biceps representations in PLP patients but not in non-PLP patients.
Fig. 3.
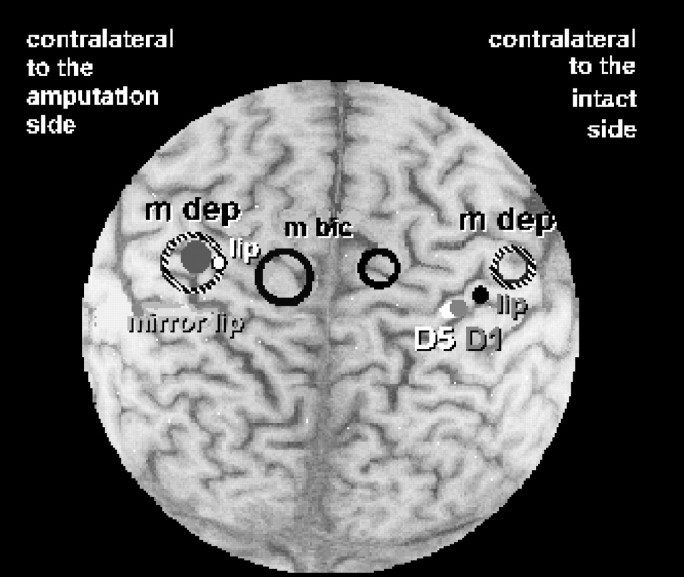
Superimposition of somatosensory representations of the first digit (D1) (gray filled dot) and of the fifth digit (D5) (white filled dot) of the intact hand and motor representation of the biceps brachii and depressor labii inferioris (circle outlined inblack, m bic; hatched open circle, m dep) in a patient with PLP tested in study 2 to obtain a more precise motor map using a more focal coil (Cadwell 8-shaped coil). The somatosensory representation of the lip on the amputated side is indicated by the white dot with the black border and that of the intact side is indicated by the black dot. The lip representation corresponding to the intact side was projected over the representation corresponding to the amputated side (Elbert et al., 1994) and displayed as a large filled gray circle. Note the larger size of the biceps and depressor labii inferioris representations in these patients and particularly the smaller distance between biceps and depressor labii inferioris representations in the hemisphere contralateral to the amputation.
Statistical analysis of the data. Analyses were performed with hypothesis-guided one-tailed t tests for independent and paired samples. In the case of non-normal distribution the data were log-transformed. The relationship between the hemispheric difference of the motor threshold and the time since the amputation was computed by Pearson-Bravais correlation. The relationship between motor and somatosensory reorganization was computed by Spearman rank correlations using the distance of the optimal scalp position from Cz on the hemisphere representing the amputation side and the Euclidean distance measure in the somatosensory domain.
RESULTS
Transcranial magnetic stimulation
Study 1
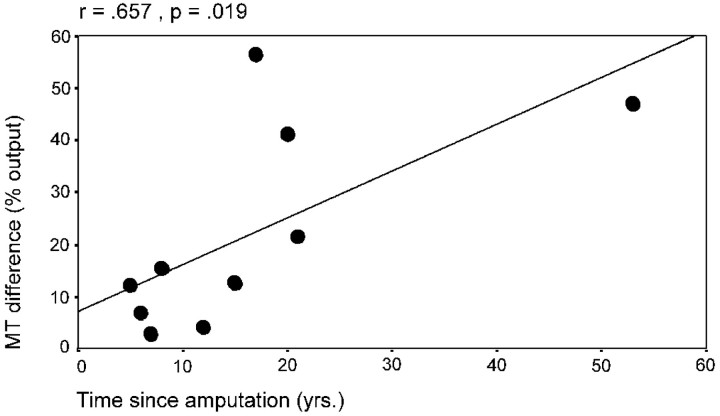
For the 10 subjects of study 1, motor threshold was lower for the biceps muscle on the amputated side (75.18 ± 12.98 A/μsec) than for that on the intact side (87.57 ± 30.17 A/μsec), although this difference did not reach statistical significance, as was revealed by repeated measures ANOVA including “phantom limb pain” as between-subjects factor and “stimulation side” (contralateral to the amputated/intact side;) as within-subjects factor (phantom limb pain: F(1,8) = 0.64; NS; stimulation side: F(1,8) = 1.34; NS; interaction phantom limb pain × stimulation side:F(1,8) = 0.01; NS). There was a positive correlation, however, between the interhemispheric differences in motor threshold for the biceps muscle and the time since amputation (r = 0.657; p < 0.05; Pearson-Bravais correlation) (Fig. 1).
Fig. 1.
Relationship between the time since amputation and the hemispheric difference of the motor threshold (MT) of the arm muscle.
Amplitudes of the motor-evoked potentials were determined as another indicator of motor cortex excitability. One subject without phantom limb pain was excluded as an outlier from this analysis (Table2). Repeated measures ANOVA for the motor-evoked potential of the biceps including phantom limb pain as between-subjects factor and stimulation side (contralateral to the amputated/intact side) revealed a weak tendency for the interaction between phantom limb pain and stimulation side (F(1,7) = 2.98; p = 0.12), but no effects for phantom limb pain (F(1,7) = 1.10; NS) and stimulation side (F(1,7) = 0.07; NS). Post hoc t tests revealed that only patients with phantom limb pain had larger amplitudes of the motor-evoked potentials for the biceps brachii on the amputated side as compared with the intact side (t(4) = 1.85; p = 0.06), whereas no side difference was found within the patients without phantom limb pain (t(4) = −0.93; NS).Post hoc t tests revealed further that the motor-evoked potentials at the amputated side differed significantly between the groups with the patients with phantom limb pain (158.32 ± 94.97 μV), showing a higher amplitude than the patients without phantom limb pain (54.02 ± 14.60 μV) (t(4.23) = 2.42; p < 0.05). Repeated measures ANOVA for the motor-evoked potential of the zygomaticus did not indicate statistically significant effects (phantom limb pain: F(1,7) = 0.11; NS; stimulation side: F(1,7) = 0.29; NS; phantom limb pain × stimulation side:F(1,7) = 0.57; NS).
Table 2.
Results obtained in the entire sample (studies 1, 2, and 3)
| Subject number | Study 1 | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Biceps mean MEP (μV) | Biceps max MEP (μV) | Zygomaticus mean MEP (μV) | Zygomaticus max MEP (μV) | Biceps COG (cm) | Biceps OSP (cm) | |||||||
| Phantom limb pain patients | ||||||||||||
| amp | int | amp | int | amp | int | amp | int | amp | int | amp | int | |
| 1 | 111.60 | 27.50 | 271.60 | 92.00 | 146.80 | 35.20 | 267.30 | 104.90 | 3.77 | 4.94 | 6 | 6 |
| 2 | 53.60 | 114.10 | 171.20 | 332.50 | 51.10 | 62.60 | 214.80 | 197.40 | 2.62 | 3.28 | 1 | 2 |
| 3 | 224.50 | 40.10 | 553.50 | 135.90 | 93.80 | 199.90 | 226.60 | 315.30 | 2.79 | 4.98 | 2 | 5 |
| 4 | 114.60 | 69.60 | 147.40 | 243.20 | 101.20 | 122.40 | 315.70 | 338.40 | 5.20 | 4.02 | 4 | 6 |
| 5 | 287.30 | 31.60 | 658.50 | 76.40 | 48.10 | 60.00 | 110.20 | 102.10 | 2.86 | 2.84 | 3 | 6 |
| M | 158.32 | 56.58 | 360.44 | 176.00 | 88.20 | 96.02 | 226.92 | 211.62 | 3.45 | 4.01 | 3.20 | 5.00 |
| (SD) | 94.97 | 36.12 | 231.95 | 109.08 | 40.68 | 66.33 | 76.29 | 112.26 | (1.08) | (0.96) | (1.92) | (1.73) |
| Patients without phantom limb pain | ||||||||||||
| 6 | (336.20)2-a | (252.80)2-a | (1091.40)2-a | (579.80)2-a | 84.30 | 42.30 | 246.80 | 87.10 | 3.48 | 4.88 | 4 | 5 |
| 7 | 42.00 | 42.00 | 141.10 | 131.50 | 58.20 | 110.40 | 140.80 | 223.40 | 2.40 | 5.07 | 1 | 7 |
| 8 | 69.40 | 52.50 | 171.50 | 193.60 | 114.00 | 111.30 | 383.90 | 385.80 | 3.89 | 3.04 | 2 | 2 |
| 9 | 41.10 | 163.80 | 137.20 | 364.90 | 53.10 | 141.90 | 112.30 | 328.80 | 3.33 | 3.03 | 2 | 1 |
| 10 | 63.60 | 75.50 | 151.10 | 180.30 | 56.60 | 67.10 | 156.50 | 127.70 | 4.80 | 4.20 | 5 | 5 |
| M | 54.03 | 83.45 | 150.23 | 217.58 | 73.24 | 94.60 | 208.06 | 230.56 | 3.58 | 4.04 | 2.80 | 4.00 |
| (SD) | 14.60 | 55.36 | 15.34 | 101.78 | 25.94 | 39.55 | 110.42 | 127.44 | (0.87) | (0.98) | (1.64) | (2.45) |
| Study 2 | Study 3 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Zygomaticus OSP (cm) | Biceps max MEP (μV) | Depressor labii inferioris max MEP (μV) | Biceps COG (cm) | Depressor labii inferioris COG (cm) | Somatosensory Reorganization (cm) | Motor Reorganization (cm) | |||||
| amp | int | amp | int | amp | int | amp | int | amp | int | ||
| 7 | 8 | 0.62 | 0.27 | ||||||||
| 7 | 9 | 79.60 | 42.80 | 110.80 | 264.40 | 2.39 | 3.10 | 9.19 | 11.30 | ||
| 7 | 8 | 0.62 | 1.16 | ||||||||
| 7 | 9 | 1.22 | 0.64 | ||||||||
| 7 | 8 | ||||||||||
| 7.00 | 8.40 | 0.82 | 0.69 | ||||||||
| (0.00) | (0.55) | (0.35) | (0.45) | ||||||||
| 9 | 9 | ||||||||||
| 8 | 9 | 0.54 | 0.03 | ||||||||
| 9 | 7 | 0.02 | 0.01 | ||||||||
| 9 | 8 | 78.40 | 81.20 | 124.10 | 233.00 | 5.03 | 6.56 | 7.53 | 9.83 | ||
| 8 | 7 | 0.02 | 0.01 | ||||||||
| 8.60 | 8.00 | 0.19 | 0.01 | ||||||||
| (0.55) | (1.00) | (0.30) | (0.02) | ||||||||
amp, Hemisphere contralateral to the amputation side; int, hemisphere contralateral to the intact side; MEP, motor-evoked potential; OSP, optimal scalp position; COG, center of gravity.
Excluded from statistical analysis (outlier).
The topographic motor representations of the biceps and zygomaticus muscles were determined by assessing the optimal scalp position (the scalp position with the highest motor evoked potential measured in centimeters from the midline) and by determining the center of gravity for the biceps brachii. Because the lateral border of the zygomaticus map could not be properly defined given the relatively large Dantec coil used in study 1, the center of gravity of the face muscles was computed only in study 2.
Repeated measures ANOVA for the optimal scalp position of the biceps brachii with phantom limb pain as between-subjects factor and stimulation side (contralateral to the amputated/intact side) as within-subjects factor tended to be significant for stimulation side (F(1,8) = 4.79; p = 0.06; phantom limb pain: F(1,8) = 0.46; NS; interaction phantom limb pain × stimulation side:F(1,8) = 0.19; NS). The biceps representation of the amputated as compared with the intact side was more medial in all patients (t(9) = −2.29;p < 0.05), with a significant side difference only in the group with phantom limb pain (t(4)= −3.09; p < 0.05; without phantom limb pain:t(4) = −0.97; NS). For the center of gravity of the biceps brachii, the repeated measures ANOVA revealed no statistically significant effects (phantom limb pain:F(1,8) = 0.04; NS; stimulation side:F(1,8) = 1.36; NS; interaction phantom limb pain × stimulation side:F(1,8) = 0.01; NS).
A repeated measures ANOVA for the optimal scalp position for stimulation of the zygomaticus muscle with phantom limb pain as between-subjects factor and stimulation side (contralateral to the amputated/intact side) as within-subjects factor was significant for the interaction phantom limb pain × stimulation side (F(1,8) = 12.50; p < 0.01; stimulation side: F(1,8) = 2.00; NS). Post hoc t tests revealed that the optimal scalp position of the zygomaticus muscle representation in the hemisphere contralateral to the amputated side was located more medially in patients with phantom limb pain than in those without phantom limb pain (t(4) = −6.53; p < 0.01). Only in patients with phantom limb pain did post hoct tests reveal a significant hemispheric difference with a more medial zygomaticus representation at the hemisphere contralateral to the amputation as compared with the intact side (t(4) = −5.72; p < 0.01; patients without phantom limb pain:t(4) = 1.18; NS).
Study 2
For the two subjects of study 2, the motor threshold was lower for the biceps brachii and the depressor labii inferioris on the amputated as compared with the intact side, regardless of the presence of phantom limb pain. For the patient with phantom limb pain, motor thresholds of 56 and 60% were obtained for the biceps of the amputated versus intact side. Motor threshold was 70% for the depressor labii inferioris of the amputated and 85% for the intact side. For the patient without phantom limb pain, the motor threshold was 73% for the biceps of the amputated and 85% for the intact side. For the depressor labii inferioris motor threshold was 85% of the amputated and of 89% for the intact side. The patient with phantom limb pain displayed generally lower motor thresholds than the patient without phantom limb pain.
In the patient with phantom limb pain, the motor-evoked potential of the biceps brachii was larger on the amputated (79.6 μV) than the intact (42.8 μV) side. The motor-evoked potential of the depressor labii inferioris was smaller on the amputated than on the intact side in both patients. For the phantom limb pain patient, the motor-evoked potential of the depressor labii inferioris of the amputated side was 110.8 μV compared with 264.4 μV on the intact side. For the patient without phantom limb pain, the motor-evoked potential for the depressor labii inferioris of the amputated side was 124.1 μV; for the intact side, it was 232.0 μV.
The centers of gravity of the depressor labii inferioris and of the biceps brachii of the amputated side were located more medially than in the intact side in both patients. In the phantom limb pain patient, the center of gravity of the depressor labii inferioris of the amputated side was 9.19 cm compared with 11.30 cm on the intact side. The center of gravity of the biceps brachii of the amputated side was 2.39 cm from the midline compared with 3.10 cm on the intact side. In the patient without phantom limb pain, the center of gravity of the depressor labii inferioris of the amputated side was 7.43 cm from the midline; that of the intact side was 9.83 cm. In the same patient, the center of gravity of the biceps brachii of the amputated side was 5.03 cm from the midline, whereas that of the intact side was 6.56 cm.
Combination of neuroelectric source imaging and transcranial magnetic stimulation data (study 3)
Neuroelectric source imaging revealed a significantly larger shift (indicated by a larger difference of the polar angles of the amputated and intact representations) of the mouth area into the hand area on the somatosensory cortex contralateral to the amputation side in the phantom limb pain subjects as compared with the pain-free amputees (t(4) = −2.37; p < 0.05) (see Table 3, Fig. 3) as demonstrated in previous studies (Flor et al., 1995).
Table 3.
Coregistration data (study 3)
| Amputees with phantom limb pain (n = 3) | Amputees without phantom limb pain (n = 3) | Group difference (t tests) | |||
|---|---|---|---|---|---|
| M | SD | M | SD | ||
| Amount of somatosensory reorganization (difference of the polar angle of the dipole locations of finger and lip representation referred to Cz, in degrees and [mm]) | 4.10 | (1.73) | 0.96 | (1.50) | 3-150 |
| [8.20 | (3.46)] | [1.93 | (3.01)] | ||
| Difference in excitability between biceps motor representations of amputated and intact side (SD; see coregistration of transcranial magnetic stimulation and neuroelectric source imaging) | 0.69 | (0.45) | 0.01 | (0.02) | 3-150 |
The larger the difference of the polar angle, the more medial the lip somatosensory representation is (see Materials and Methods).
F3-150: p < 0.05.
The medial displacement of the somatosensory representation of the mouth (Table 2, Figs. 2, 3) in patients with phantom limb pain was paralleled by the medial displacement of the motor mouth representation (Table 2, Fig. 3) in the transcranial magnetic stimulation paradigm. In one phantom limb pain patient of study 2 (Fig. 3), both the somatosensory representation of the lower lip and the motor representation of the depressor labii inferioris as determined by transcranial magnetic stimulation were co-registered. These data show that the shift of the somatosensory motor representation was paralleled by a similar shift of the depressor labii inferioris representation. In addition, the size of the motor maps (indicated by 1 SD of the center of gravity of that muscle and superimposed on the magnetic resonance image) of both the biceps (Figs. 2, 3) and the depressor labii inferioris (Fig. 3) was larger contralateral to the amputation side as compared with the intact side. Contrary to expectations, the center of gravity of the biceps in this patient was displaced more medially rather than laterally. The difference of the size of the motor maps (calculated as the SD of the center of gravity of the biceps brachii) between the amputated and the intact side was significantly larger for the amputees with pain compared with the pain-free amputees (t(4) = 2.58; p < 0.05), suggesting increased excitability of the biceps cortical representation contralateral to the amputation side in patients with phantom limb pain (Figs. 2, 3).
The medial displacement of the somatosensory representation of the lip on the amputated side was significantly positively correlated with the medial displacement of the optimal scalp position of the zygomaticus (r = −0.939; p < 0.005). Somatosensory reorganization correlated well with the magnitude of phantom limb pain (r = 0.832; p < 0.05). Additionally, the more intense the phantom limb pain, the more medial was the optimal scalp position of the zygomaticus (r = −0.939; p < 0.005). Nonpainful phantom sensations, including referred sensations (present in three subjects, one with topographic remapping), were not significantly correlated with any of the reorganization measures (r< 0.080; NS).
DISCUSSION
Our results indicate a different magnitude of motor and somatosensory cortical plasticity in amputees with and without phantom limb pain
Sensorimotor plasticity after amputation
In the human somatosensory system, plasticity after deafferentation has been detected at cortical (Ramachandran, 1993;Elbert et al., 1994; Yang et al., 1994; Flor et al., 1995, 1998;Birbaumer et al., 1997; Borsook et al., 1998; Montoya et al., 1998;Grüsser et al., 2001) and thalamic levels (Davis et al., 1998), and it correlates with phantom limb pain. In this study we found that the medial displacement of the face somatosensory representation (lip) was correlated positively with the medial displacement of the face motor representation (zygomaticus muscle) and with the magnitude of phantom limb pain. Additionally, the more intense the phantom limb pain, the more medial the displacement of the face motor representation (zygomaticus muscle). Related studies after amputations in monkeys in whom phantom limb pain was not assessed have not identified a displacement of the mouth motor representation (Schieber and Deuel, 1997; Wu and Kaas, 1999). Nonpainful phantom phenomena such as topographically referred phantom sensations have been described (Ramachandran et al., 1992a,b; Halligan et al., 1993; Aglioti et al., 1994, 1995, 1997; Elbert et al., 1994; Halligan and Marshall, 1994; Flor et al., 1995, 1998, 2000; Knecht et al., 1995, 1996, 1998;Montoya et al., 1998; Grüsser et al., 2001) in some amputees. Although some of our subjects also described these and related nonpainful phantom phenomena, nonpainful sensations were not significantly correlated with measures of motor or somatosensory plasticity. Although the link between phantom limb pain and sensorimotor plasticity is still unclear, evidence from stimulation of motor cortex suggests that it can modify pain related to deafferentation (Saitoh et al., 2000). Overall, these findings support the association of somatosensory and motor reorganization after amputations and phantom limb pain.
Given the high concordance between motor and somatosensory plasticity found in this study, it is reasonable to assume that reorganization of the somatosensory cortex can play a role in the motor plasticity described here. In fact, the somatosensory cortex has projections to layers II/III of the motor cortex that are closely connected to motor output neurons in layer V (Kaneko et al., 1994a,b). Stimulation of the somatosensory cortex is known to induce long-term potentiation in the motor cortex (Sakamoto et al., 1987). Additionally, projections from the somatosensory cortex to the motor cortex are important in the acquisition of motor skills (Pavlides et al., 1993). Therefore, reorganization in the motor cortex may be secondary to changes in the somatosensory cortex. An alternative possibility would be that reorganization in the thalamus modulates motor plasticity (Jones and Pons, 1998).
Site of plasticity after amputation
Previous reports demonstrated increased excitability of the motor cortex contralateral to an upper arm amputation (Hall et al., 1990;Cohen et al., 1991, 1993; Fuhr et al., 1992; Kew et al., 1994; Ojemann and Silbergeld, 1995; Pascual-Leone et al., 1996). Our results here expand previous findings by identifying a differential magnitude of plastic changes according to the presence or absence of phantom limb pain. Motor reorganization in amputees takes place predominantly at the cortical level (Brasil-Neto et al., 1992, 1993; Fuhr et al., 1992; Chen et al., 1998). It is likely that cortical mechanisms are also responsible for the differences in reorganization observed in both patient groups. Three lines of evidence point to this site. (1) The excitability of the motor neuron pool in amputees remains unchanged, whereas the motor-evoked potentials to transcranial magnetic stimulation show substantial differences between the amputated and intact side, pointing to a suprasegmental site (Fuhr et al., 1992). (2) Intracortical inhibition and excitation are substantially abnormal on the side of the amputation (Chen et al., 1998). (3) Other studies demonstrated that reversible or transient deafferentation is associated with reorganization at cortical levels (Brasil-Neto et al., 1993). Our results, however, do not rule out the possibility of additional subcortical reorganization.
Measures of cortical plasticity and implications for mechanisms
In this study, motor threshold tended to be lower for arm muscles ipsilateral to the amputation, a finding consistent with previous reports (Hall et al., 1990; Cohen et al., 1991; Chen et al., 1998) that could reflect changes in sodium channels, already implicated in other forms of plasticity (Carp and Wolpaw, 1994; Halter et al., 1995). Interestingly, motor thresholds do not change after short-term deafferentation (Brasil-Neto et al., 1992, 1993; Ridding and Rothwell, 1997; Ziemann et al., 1998), but decrease after long-term amputations (Cohen et al., 1991; Chen et al., 1998), as also reported in animal models (Sanes et al., 1990; Huntley, 1997). These findings are consistent with our result reported here that a higher hemispheric difference in motor threshold is correlated with a longer time period between amputation and testing. In addition to changes in the motor threshold, motor-evoked potential amplitudes from biceps ipsilateral to the stump were larger in patients with phantom limb pain than in pain-free patients, further suggesting enhanced cortical excitability of the stump representation in patients with phantom limb pain.
Motor map size is influenced by changes in excitability of the motor representations (Ridding and Rothwell, 1997; Thickbroom et al., 1998). On the other hand, optimal positions for stimulation and centers of gravity could reflect directionally selective expansions of the motor representations or regional changes in excitability within the same motor representation (Cohen et al., 1995, 1996, 1998; Liepert et al., 1998a). In our study, we found medial displacements of the optimal position for stimulation for mouth muscles ipsilateral to the stump in patients with phantom limb pain in study 1. In study 2, the medial displacement of the center of gravity of the depressor labii inferioris was detected regardless of phantom limb pain. We interpret these findings as reflecting directionally selective expansion of the mouth representation over the deafferented hand representation or increased excitability of the part of the mouth representation bordering the deafferented hand representation. This form of motor plasticity across limb representation boundaries has been demonstrated before in animals (Huntley, 1997) and in humans with facial palsy (Rijntjes et al., 1997), but it has not been associated with painful conditions. Results obtained from biceps recordings are consistent with an increased excitability of the biceps motor representation contralateral to the amputated limb. Contrary to our expectation, the biceps representation did not shift laterally, indicating that the representational plasticity detected when testing mouth muscles was absent in upper arm muscles. Although the upper arm motor representation is intertwined with the hand representation in monkeys (Donoghue et al., 1992) and humans (Rao et al., 1995), the face representation has a relatively clear boundary with the hand representation. It is conceivable that the medial expansion of the face representation across a well defined boundary will be more easily identified in the motor maps. On the other hand, displacements of the upper arm muscles over hand muscles are of the order of only 0.5 cm (Cohen et al., 1995, 1996) because both representations overlap substantially. Another reason for the lack of lateral displacement of the upper arm representation may be the fact that three of the five subjects with phantom limb pain also complained of stump pain. Flor et al. (1997) reported expansion of the body region affected by chronic pain in SI over neighboring representations. Therefore, one would expect that stump pain might lead to an enlarged representation of the stump area that might interfere with the reorganizational changes related to the original deafferentation. Another factor could be that use of the stump might also have prevented the deafferentation-induced changes from occurring (Jenkins et al., 1990; Lotze et al., 1999).
Functional relevance of cortical plasticity
Results from our study further add to an accumulating literature that suggests that some measures of cortical reorganization are associated with aversive perceptual experiences such as phantom limb pain (Flor et al., 1995), chronic pain (Flor et al., 1997), or tinnitus (Mühlnickel et al., 1998; Elbert and Flor, 1999), defining what can be called “maladaptive” plasticity. In contrast to previous reports, this study included only patients whose amputations had occurred several years before the investigation, and a substantial number of the patients suffered from chronic pain before the amputation. It is possible that different relationships between pain and cortical reorganization exist in recently amputated subjects or subjects with long-standing pain as compared with no long-standing pain before the amputation (Pascual-Leone et al., 1996; Flor and Birbaumer, 2000). Other forms of plasticity can play a clearly compensatory and beneficial role, as in the case of cross-modal plasticity in blind humans (Cohen et al., 1997), or a likely beneficial role, as in the case of recovery of motor function after stroke (Hamdy et al., 1996; Kopp et al., 1999). It is important to define in each specific setting of plasticity to which extent it plays a beneficial role and to which extent it is maladaptive. This is the precondition for designing appropriate therapeutic strategies to enhance it (e.g., by using transcranial magnetic stimulation) when beneficial or to downregulate it when maladaptive (Ziemann et al., 1998).
In summary, we report here differential motor and somatosensory reorganization in patients with and without phantom limb pain and provide further evidence for reorganizational plasticity in the human somatosensory and motor cortex after amputations.
Footnotes
The completion of this study was supported by the Deutsche Forschungsgemeinschaft (Fl 156/16, Bi 195/24) and the Humboldt Foundation.
Correspondence should be addressed to Dr. Herta Flor, Department of Clinical and Cognitive Neuroscience at the University of Heidelberg, Central Institute of Mental Health, J5, D-68159 Mannheim, Germany, E-mail: flor@as200.zi-mannheim.de; or Dr. Leonardo G. Cohen, Human Cortical Physiology Section, National Institute of Neurological Disorders and Stroke, National Institutes of Health, Bethesda, MD 20892. E-mail: lcohen@codon.nih.gov.
REFERENCES
- 1.Aglioti S, Cortese F, Franchini C. Rapid sensory remapping in the adult human brain as inferred from phantom breast perception. NeuroReport. 1994;5:473–476. doi: 10.1097/00001756-199401120-00026. [DOI] [PubMed] [Google Scholar]
- 2.Aglioti S, Bonazzi A, Cortese F. Phantom lower limb as a perceptual marker of neural plasticity in the mature human brain. Proc R Soc Lond B Biol Sci. 1995;255:273–278. doi: 10.1098/rspb.1994.0039. [DOI] [PubMed] [Google Scholar]
- 3.Aglioti S, Smania N, Atzei BA, Berlucchi G. Spatio-temporal pattern of evoked phantom sensations in a left index amputee patient. Behav Neurosci. 1997;111:867–872. doi: 10.1037//0735-7044.111.5.867. [DOI] [PubMed] [Google Scholar]
- 4.Birbaumer N, Lutzenberger W, Montoya P, Larbig W, Unertl K, Töpfner S, Taub E, Flor H. Effects of regional anesthesia on phantom limb pain are mirrored in changes in cortical reorganization. J Neurosci. 1997;17:5503–5508. doi: 10.1523/JNEUROSCI.17-14-05503.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Borsook D, Becerra L, Fishman S, Edwards A, Jennings CL, Stojanovic M. Acute plasticity in the human somatosensory cortex following amputation. NeuroReport. 1998;9:1013–1017. doi: 10.1097/00001756-199804200-00011. [DOI] [PubMed] [Google Scholar]
- 6.Brasil-Neto JP, Cohen LG, Pascual-Leone A, Jabir FK, Wall RT, Hallett M. Rapid reversible modulation of human motor outputs after transient deafferentation of the forearm: a study with transcranial magnetic stimulation. Neurology. 1992;42:1302–1306. doi: 10.1212/wnl.42.7.1302. [DOI] [PubMed] [Google Scholar]
- 7.Brasil-Neto JP, Valls-Solè J, Pascual-Leone A, Cammarota A, Amassian VE, Cracco R. Rapid modulation of human cortical motor outputs following ischaemic nerve block. Brain. 1993;116:511–525. doi: 10.1093/brain/116.3.511. [DOI] [PubMed] [Google Scholar]
- 8.Carp JS, Wolpaw JR. Motoneuron plasticity underlying operantly conditioned decrease in primate H-reflex. J Neurophysiol. 1994;72:431–442. doi: 10.1152/jn.1994.72.1.431. [DOI] [PubMed] [Google Scholar]
- 9.Chen R, Samii A, Canos M, Wassermann EM, Hallett M. Effects of phenytoin on cortical excitability in humans. Neurology. 1997;49:881–883. doi: 10.1212/wnl.49.3.881. [DOI] [PubMed] [Google Scholar]
- 10.Chen R, Corwell B, Yaseen Z, Hallett M, Cohen LG. Mechanisms of cortical reorganization in lower-limb amputees. J Neurosci. 1998;18:3443–3450. doi: 10.1523/JNEUROSCI.18-09-03443.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cohen LG, Roth BJ, Nilsson J, Dang N, Panizza M, Bandinelli S. Effects of coil design on delivery of focal magnetic stimulation. Technical considerations. Electroencephalogr Clin Neurophysiol. 1990;75:350–357. doi: 10.1016/0013-4694(90)90113-x. [DOI] [PubMed] [Google Scholar]
- 12.Cohen LG, Bandinelli S, Findley TW, Hallett M. Motor reorganization after upper limb amputation in man. Brain. 1991;114:615–627. doi: 10.1093/brain/114.1.615. [DOI] [PubMed] [Google Scholar]
- 13.Cohen LG, Brasil-Neto JP, Pascual-Leone A, Hallett M. Plasticity of cortical motor output organization following deafferentation, cerebral lesions, and skill acquisition. Adv Neurol. 1993;63:187–200. [PubMed] [Google Scholar]
- 14.Cohen LG, Gerloff C, Ikoma K, Hallett M. Plasticity of motor cortex elicited by training of synchronous movements of hand and shoulder. Soc Neurosci Abstr. 1995;21:517. [Google Scholar]
- 15.Cohen LG, Gerloff C, Faiz L, Uenishi N, Hallett M. Directional modulation of motor cortex plasticity induced by synchronicity of motor outputs in humans. Soc Neurosci Abstr. 1996;22:1452. [Google Scholar]
- 16.Cohen LG, Celnik P, Pascual-Leone A, Corwell B, Falz L, Dambrosia J, Honda M, Sadato N, Gerloff C, Catala MD, Hallett M. Functional relevance of cross-modal plasticity in blind humans. Nature. 1997;389:180–183. doi: 10.1038/38278. [DOI] [PubMed] [Google Scholar]
- 17.Cohen LG, Ziemann U, Chen R, Classen J, Hallett M, Gerloff C. Studies of neuroplasticity with transcranial magnetic stimulation. Clin Neurophysiol. 1998;15:305–324. doi: 10.1097/00004691-199807000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Cuffin BN, Cohen D. Comparison of the magnetoencephalogram and electroencephalogram. Electroencephalogr Clin Neurophysiol. 1979;47:132–146. doi: 10.1016/0013-4694(79)90215-3. [DOI] [PubMed] [Google Scholar]
- 19.Davis KD, Kiss ZHT, Luo L, Tasker RR, Lozano AM, Dostrovsky JOP. Phantom sensations generated by thalamic microstimulation. Nature. 1998;391:385–387. doi: 10.1038/34905. [DOI] [PubMed] [Google Scholar]
- 20.Donoghue JP, Leibovic S, Sanes JN. Organization of the forelimb area in squirrel monkey motor cortex: representation of digit, wrist, and elbow muscles. Exp Brain Res. 1992;89:1–19. doi: 10.1007/BF00228996. [DOI] [PubMed] [Google Scholar]
- 21.Elbert T, Flor H. Magnetoencephalographic investigations of cortical reorganization in humans. Electroencephalogr Clin Neurophysiology [Suppl] 1999;49:284–291. [PubMed] [Google Scholar]
- 22.Elbert T, Flor H, Birbaumer N, Knecht S, Hampson S, Larbig W. Extensive reorganization of the somatosensory cortex in adult humans after nervous system injury. NeuroReport. 1994;5:2593–2597. doi: 10.1097/00001756-199412000-00047. [DOI] [PubMed] [Google Scholar]
- 23.Elbert T, Junghöfer M, Scholz B, Schneider S. The separation of overlapping neuromagnetic sources in first and second somatosensory cortices. Brain Topogr. 1995;7:275–282. doi: 10.1007/BF01195253. [DOI] [PubMed] [Google Scholar]
- 24.Flor H, Birbaumer Phantom limb pain: cortical plasticity and novel therapeutic approaches. Curr Opin Anesthesiol. 2000;13:561–564. doi: 10.1097/00001503-200010000-00013. [DOI] [PubMed] [Google Scholar]
- 25.Flor H, Rudy TE, Birbaumer N, Streit B, Schugens MM. Zur Anwendbarkeit des West Haven – Yale Multidimensional Pain Inventory im deutschen Sprachraum: Daten zur Reliabilität und Validität des MPID. Der Schmerz. 1990;4:82–87. doi: 10.1007/BF02527839. [DOI] [PubMed] [Google Scholar]
- 26.Flor H, Elbert T, Knecht S, Wienbruch C, Pantev C, Larbig W. Phantom limb pain as a perceptual correlate of massive cortical reorganization in upper extremity amputees. Nature. 1995;375:482–484. doi: 10.1038/375482a0. [DOI] [PubMed] [Google Scholar]
- 27.Flor H, Braun C, Elbert T, Birbaumer N. Extensive reorganization of primary somatosensory cortex in chronic back pain patients. Neurosci Lett. 1997;224:5–8. doi: 10.1016/s0304-3940(97)13441-3. [DOI] [PubMed] [Google Scholar]
- 28.Flor H, Elbert T, Mühlnickel W, Pantev C, Wienbruch C, Taub E. Cortical reorganization and phantom phenomena in congenital and traumatic upper extremity amputees. Exp Brain Res. 1998;119:205–212. doi: 10.1007/s002210050334. [DOI] [PubMed] [Google Scholar]
- 29.Flor H, Mühlnickel W, Karl A, Denke C, Fritzsche K, Grüsser S, Taub E. A neural substrate of nonpainful phantom limb phenomena. NeuroReport. 2000;11:1407–1411. doi: 10.1097/00001756-200005150-00011. [DOI] [PubMed] [Google Scholar]
- 30.Fuhr P, Cohen LG, Dang N, Findley T, Haghighi S, Oro J. Physiological analysis of motor reorganization following lower limb amputation. Electroencephalogr Clin Neurophysiol. 1992;85:53–60. doi: 10.1016/0168-5597(92)90102-h. [DOI] [PubMed] [Google Scholar]
- 31.Grüsser S, Mühlnickel W, Denke C, Karl A, Flor H. The relationship of phantom phenomena and cortical reorganization. Neuroscience. 2001;102:263–272. doi: 10.1016/s0306-4522(00)00491-7. [DOI] [PubMed] [Google Scholar]
- 32.Hall EJ, Flament D, Fraser C, Lemon RN. Non-invasive brain stimulation reveals reorganized cortical outputs in amputees. Neurosci Lett. 1990;116:379–386. doi: 10.1016/0304-3940(90)90105-i. [DOI] [PubMed] [Google Scholar]
- 33.Halligan PW, Marshall JC. Sensory disorganization and perceptual plasticity after limb amputation: a follow-up study. NeuroReport. 1994;27:1341–1345. [PubMed] [Google Scholar]
- 34.Halligan PW, Marshall JC, Wade DT, Davey J, Morrison D. Thumb in cheek? Sensory reorganization and perceptual plasticity after limb amputation. NeuroReport. 1993;4:233–236. doi: 10.1097/00001756-199303000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Halter JA, Carp JS, Wolpaw JR. Operantly conditioned motoneuron plasticity: possible role of sodium channels. J Neurophysiol. 1995;73:867–871. doi: 10.1152/jn.1995.73.2.867. [DOI] [PubMed] [Google Scholar]
- 36.Hamdy S, Aziz Q, Rothwell JC, Singh KD, Barlow J, Hughes DG. The cortical topography of human swallowing musculature in health and disease. Nat Med. 1996;2:1217–1224. doi: 10.1038/nm1196-1217. [DOI] [PubMed] [Google Scholar]
- 37.Huntley GW. Correlation between patterns of horizontal connectivity and the extent of short-term representational plasticity in rat motor cortex. Cereb Cortex. 1997;7:143–156. doi: 10.1093/cercor/7.2.143. [DOI] [PubMed] [Google Scholar]
- 38.Inghilleri M, Berardelli A, Marchetti P, Manfredi M. Effects of diazepam, baclofen and thiopental on the silent period evoked by transcranial magnetic stimulation in humans. Exp Brain Res. 1996;109:467–472. doi: 10.1007/BF00229631. [DOI] [PubMed] [Google Scholar]
- 39.Jenkins WM, Merzenich MM, Ochs MT, Allert T, Guic-Robles E. Functional reorganization of primary somatosensory cortex in adult owl monkeys after behaviorally controlled tactile stimulation. J Neurophysiol. 1990;63:82–104. doi: 10.1152/jn.1990.63.1.82. [DOI] [PubMed] [Google Scholar]
- 40.Jensen TS, Rasmussen P. Phantom limb pain and related phenomena after amputation. In: Wall PD, Melzack R, editors. Textbook of pain. Churchill Livingstone; New York: 1995. pp. 651–665. [Google Scholar]
- 41.Jensen TS, Krebs B, Nielsen J, Rasmussen P. Immediate and long-term phantom limb pain in amputees: incidence, clinical characteristics and relationship to pre-amputation limb pain. Pain. 1985;21:267–278. doi: 10.1016/0304-3959(85)90090-9. [DOI] [PubMed] [Google Scholar]
- 42.Jones TE, Pons TP. Thalamic and brainstem contributions to large-scale plasticity of primate somatosensory cortex. Science. 1998;282:1121–1125. doi: 10.1126/science.282.5391.1121. [DOI] [PubMed] [Google Scholar]
- 43.Kaas J. Phantoms of the brain. Nature. 1998;391:331–333. doi: 10.1038/34782. [DOI] [PubMed] [Google Scholar]
- 44.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. I. Responses of morphologically identified motor cortical cells to stimulation of the somatosensory cortex. J Comp Neurol. 1994a;345:161–171. doi: 10.1002/cne.903450202. [DOI] [PubMed] [Google Scholar]
- 45.Kaneko T, Caria MA, Asanuma H. Information processing within the motor cortex. II. Intracortical connections between neurons receiving somatosensory cortical input and motor output neurons of the cortex. J Comp Neurol. 1994b;345:172–184. doi: 10.1002/cne.903450203. [DOI] [PubMed] [Google Scholar]
- 46.Kerns RD, Turk DC, Rudy TE. The West Haven – Yale Multidimensional Pain Inventory (MPI). Pain. 1985;23:345–356. doi: 10.1016/0304-3959(85)90004-1. [DOI] [PubMed] [Google Scholar]
- 47.Kew JJ, Halligan PW, Marshall JC, Passingham RE, Rothwell JC, Ridding MC, Passingham RE, Rothwell JC, Ridding MC, Marsden CD, Brooks DJ. Abnormal access of axial vibrotactile input to deafferented somatosensory cortex in human upper limb amputees. J Neurophysiol. 1997;77:2753–2764. doi: 10.1152/jn.1997.77.5.2753. [DOI] [PubMed] [Google Scholar]
- 48.Kew JJM, Ridding MC, Rothwell JC, Passingham RE, Leigh PN, Sooriakumaran S. Reorganization of cortical blood flow and transcranial magnetic stimulation maps in human subjects after upper limb amputation. J Neurophysiol. 1994;72:2517–2524. doi: 10.1152/jn.1994.72.5.2517. [DOI] [PubMed] [Google Scholar]
- 49.Khorram-Sefat D, Nitschke MF, Frahm J, Hacker H. Functional MRI demonstrates expanded somatotopic representation in congenital and traumatic amputees. NeuroImage. 1997;4:S390. [Google Scholar]
- 50.Knecht S, Henningsen H, Elbert T, Flor H, Hoehling C, Pantev C, Birbaumer N, Taub E. Cortical reorganization in human amputees and mislocalization of painful stimuli to the phantom limb. Neurosci Lett. 1995;201:262–264. doi: 10.1016/0304-3940(95)12186-2. [DOI] [PubMed] [Google Scholar]
- 51.Knecht S, Henningsen H, Elbert T, Flor H, Hoehling C, Pantev C, Taub E. Reorganizational and perceptual changes after amputation. Brain. 1996;119:1213–1219. doi: 10.1093/brain/119.4.1213. [DOI] [PubMed] [Google Scholar]
- 52.Knecht S, Henningsen H, Hoehling C, Elbert T, Flor H, Pantev C, Taub E. Plasticity of plasticity? Changes in the pattern of perceptual correlates of reorganization after amputation. Brain. 1998;121:717–724. doi: 10.1093/brain/121.4.717. [DOI] [PubMed] [Google Scholar]
- 53.Kopp B, Kunkel A, Mühlnickel W, Villringer K, Taub E, Flor H. Plasticity in the motor system related to therapy-induced improvement of movement after stroke. NeuroReport. 1999;10:807–810. doi: 10.1097/00001756-199903170-00026. [DOI] [PubMed] [Google Scholar]
- 54.Liepert J, Classen J, Cohen LG, Hallett M. Task-dependent changes of intracortical inhibition. Exp Brain Res. 1998a;118:421–426. doi: 10.1007/s002210050296. [DOI] [PubMed] [Google Scholar]
- 55.Liepert J, Schwenkreis P, Tegenthoff M, Malin JP. The glutamate antagonist Riluzole suppresses intracortical facilitation. J Neural Transm. 1998b;104:1207–1214. doi: 10.1007/BF01294721. [DOI] [PubMed] [Google Scholar]
- 56.Lotze M, Grodd W, Birbaumer N, Erb M, Huse E, Flor H. Does use of a myoelectric prosthesis prevent cortical reorganization and phantom limb pain? Nat Neurosci. 1999;2:501–502. doi: 10.1038/9145. [DOI] [PubMed] [Google Scholar]
- 57.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of diphenylhydantoin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1994;93:428–433. doi: 10.1016/0168-5597(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 58.Mavroudakis N, Caroyer JM, Brunko E, Zegers de Beyl D. Effects of vigabatrin on motor potentials evoked with magnetic stimulation. Electroencephalogr Clin Neurophysiol. 1997;105:124–127. doi: 10.1016/s0924-980x(96)96607-2. [DOI] [PubMed] [Google Scholar]
- 59.Meyer BU, Werhahn K, Rothwell JC, Röricht S, Fauth C. Functional organisation of corticonuclear pathways to motoneurons of lower facial muscles in man. Exp Brain Res. 1994;101:465–472. doi: 10.1007/BF00227339. [DOI] [PubMed] [Google Scholar]
- 60.Montoya P, Ritter K, Huse E, Larbig W, Braun C, Töpfner S, Birbaumer N. The cortical somatotopic map and phantom phenomena in subjects with congenital limb atrophy and traumatic amputees with phantom limb pain. Eur J Neurosci. 1998;10:1095–1102. doi: 10.1046/j.1460-9568.1998.00122.x. [DOI] [PubMed] [Google Scholar]
- 61.Mühlnickel W, Elbert T, Taub E, Flor H. Reorganization of auditory cortex in tinnitus. Proc Natl Acad Sci USA. 1998;95:10340–10343. doi: 10.1073/pnas.95.17.10340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ojemann JG, Silbergeld DL. Cortical stimulation mapping of phantom limb rolandic cortex. Case report. J Neurosurg. 1995;82:641–644. doi: 10.3171/jns.1995.82.4.0641. [DOI] [PubMed] [Google Scholar]
- 63.Pascual-Leone A, Peris M, Tormos JM, Pascual-Leone Pascual A, Catala MD. Reorganization of human cortical motor output maps following traumatic forearm amputation. NeuroReport. 1996;7:2068–2070. doi: 10.1097/00001756-199609020-00002. [DOI] [PubMed] [Google Scholar]
- 64.Pavlides C, Miyashita E, Asanuma H. Projection from the sensory to the motor cortex is important in learning motor skills in the monkey. J Neurophysiol. 1993;70:733–741. doi: 10.1152/jn.1993.70.2.733. [DOI] [PubMed] [Google Scholar]
- 65.Ramachandran VS. Behavioral and magnetoencephalographic correlates of plasticity in the adult human brain. Proc Natl Acad Sci USA. 1993;90:10413–10420. doi: 10.1073/pnas.90.22.10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ramachandran VS, Rogers-Ramachandran DC, Stewart M. Perceptual correlates of massive cortical reorganization. Science. 1992a;258:1159–1160. doi: 10.1126/science.1439826. [DOI] [PubMed] [Google Scholar]
- 67.Ramachandran VS, Stewart M, Rogers-Ramachandran DC. Perceptual correlates of massive cortical reorganization. NeuroReport. 1992b;3:583–586. doi: 10.1097/00001756-199207000-00009. [DOI] [PubMed] [Google Scholar]
- 68.Rao SM, Binder JR, Hammeke TA, Bandettini PA, Bobholz JA, Frost JA, Myklebust BM, Jacobson RD, Hyde JS. Somatotopic mapping of the human primary motor cortex with functional magnetic resonance imaging. Neurology. 1995;45:919–924. doi: 10.1212/wnl.45.5.919. [DOI] [PubMed] [Google Scholar]
- 69.Reshetnyak VK, Kukushkin ML, Ovechkin AM, Smirnova VS, Gnezdilow AV. Ossobjenosti ismenenija somatosensornych wyswannych potentijalow u patijentow s amputierowannymi konjetschnostjami pri nalitschij ili otsutstwij u nich phantomno-bolewogo syndroma. Anesteziol Reanimatol. 1996;4:4–7. [Google Scholar]
- 70.Ridding MC, Rothwell JC. Reorganization in human motor cortex. Can J Physiol Pharmacol. 1995;73:218–222. doi: 10.1139/y95-032. [DOI] [PubMed] [Google Scholar]
- 71.Ridding MC, Rothwell JC. Stimulus/response curves as a method of measuring motor cortical excitability in man. Electroencephalogr Clin Neurophysiol. 1997;105:340–344. doi: 10.1016/s0924-980x(97)00041-6. [DOI] [PubMed] [Google Scholar]
- 72.Rijntjes M, Tegenthoff M, Liepert J, Leonhardt G, Kotterba S, Müller S. Cortical reorganization in patients with facial palsy. Ann Neurol. 1997;41:621–630. doi: 10.1002/ana.410410511. [DOI] [PubMed] [Google Scholar]
- 73.Röricht S, Meyer BU, Niehaus L, Brandt SA. Long-term reorganization of motor cortex outputs after arm amputation. Neurology. 1999;53:106–111. doi: 10.1212/wnl.53.1.106. [DOI] [PubMed] [Google Scholar]
- 74.Saitoh Y, Shibata M, Hirano S, Hirata M, Mashimo T, Yoshimine T. Motor cortex stimulation for central and peripheral deafferentation pain. Report of eight cases. J Neurosurg. 2000;92:150–155. doi: 10.3171/jns.2000.92.1.0150. [DOI] [PubMed] [Google Scholar]
- 75.Sakamoto T, Porter LL, Asanuma H. Long-lasting potentiation of synaptic potentials in the motor cortex produced by stimulation of the sensory cortex in the cat: a basis of motor learning. Brain Res. 1987;413:360–364. doi: 10.1016/0006-8993(87)91029-8. [DOI] [PubMed] [Google Scholar]
- 76.Sanes JN, Suner S, Donoghue JP. Dynamic organization of primary motor cortex output to target muscles in adult rats. I. Long-term patterns of reorganization following motor or mixed peripheral nerve lesions. Exp Brain Res. 1990;79:479–491. doi: 10.1007/BF00229318. [DOI] [PubMed] [Google Scholar]
- 77.Schieber MH, Deuel RK. Primary motor cortex reorganization in a long-term monkey amputee. Somatosen Motor Res. 1997;14:157–167. doi: 10.1080/08990229771024. [DOI] [PubMed] [Google Scholar]
- 78.Semlitsch HV, Anderer P, Schuster P, Presslich O. A solution for reliable and valid reduction of ocular artifacts, applied to the P300 ERP. Psychophysiology. 1986;23:695–703. doi: 10.1111/j.1469-8986.1986.tb00696.x. [DOI] [PubMed] [Google Scholar]
- 79.Sica REP, Sanz OP, Cohen LG. Changes in the N1–P1 component of the somatosensory cortical evoked response in patients with partial limb amputation. Electromyogr Clin Neurophysiol. 1984;24:415–427. [PubMed] [Google Scholar]
- 80.Sherman RA. Stump and phantom limb pain. Neurol Clin. 1989;7:249–264. [PubMed] [Google Scholar]
- 81.Sunderland S. Nerves and nerve injuries, Ed 2. Churchill Livingstone; Edinburgh: 1978. [Google Scholar]
- 82.Thickbroom GW, Sammut R, Mastaglia FL. Magnetic stimulation mapping of motor cortex: factors contributing to map area. Electroencephalogr Clin Neurophysiol. 1998;109:79–84. doi: 10.1016/s0924-980x(98)00006-x. [DOI] [PubMed] [Google Scholar]
- 83.Wassermann EM, McShane LM, Hallett M, Cohen LG. Noninvasive mapping of muscle representations in human motor cortex. Electroencephalogr Clin Neurophysiol. 1992;85:1–8. doi: 10.1016/0168-5597(92)90094-r. [DOI] [PubMed] [Google Scholar]
- 84.Werhahn K, Fong JKY, Meyer BU, Priori A, Rothwell JC, Day BL. The effect of magnetic coil orientation on the latency of surface EMG and single motor unit responses in the first dorsal interosseus muscle. Electroencephalogr Clin Neurophysiol. 1994;93:138–146. doi: 10.1016/0168-5597(94)90077-9. [DOI] [PubMed] [Google Scholar]
- 85.Wu CWH, Kaas JH. Reorganization in primary motor cortex of primates with long-standing therapeutic amputations. J Neurosci. 1999;19:7679–7997. doi: 10.1523/JNEUROSCI.19-17-07679.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Yang TT, Gallen CC, Ramachandran VS, Cobb S, Schwartz BJ, Bloom FE. Noninvasive detection of cerebral plasticity in adult human somatosensory cortex. NeuroReport. 1994;5:701–704. doi: 10.1097/00001756-199402000-00010. [DOI] [PubMed] [Google Scholar]
- 87.Ziemann U, Lönnecker S, Steinhoff BJ, Paulus W. Effects of antiepileptic drugs on motor cortex excitability in humans: a transcranial magnetic stimulation study. Ann Neurol. 1996;40:367–378. doi: 10.1002/ana.410400306. [DOI] [PubMed] [Google Scholar]
- 88.Ziemann U, Corwell B, Cohen LG. Modulation of plasticity in human motor cortex after forearm ischemic nerve block. J Neurosci. 1998;18:1115–1123. doi: 10.1523/JNEUROSCI.18-03-01115.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]