Abstract
Animals have evolved several chemosensory systems for detecting potentially dangerous foods in the environment. Activation of specific sensory cells within these chemosensory systems usually elicits an aversive behavioral response, leading to avoidance of the noxious foods. Although this aversive behavioral response can be adaptive, there are many instances in which it generates “false alarms,” causing animals to reject harmless foods. To minimize the number of false alarms, animals have evolved a variety of physiological mechanisms for selectively adapting their aversive behavioral response to harmless noxious compounds. We examined the mechanisms underlying exposure-induced adaptation to specific “bitter” compounds inManduca sexta caterpillars. M. sextaexhibits an aversive behavioral response to many plant-derived compounds that taste bitter to humans, including caffeine and aristolochic acid. This aversive behavioral response is mediated by three pairs of bitter-sensitive taste cells: one responds vigorously to aristolochic acid alone, and the other two respond vigorously to both caffeine and aristolochic acid. We found that 24 hr of exposure to a caffeinated diet desensitized all of the caffeine-responsive taste cells to caffeine but not to aristolochic acid. In addition, we found that dietary exposure to caffeine adapted the aversive behavioral response of the caterpillar to caffeine, but not to aristolochic acid. We propose that the adapted aversive response to caffeine was mediated directly by the desensitized taste cells and that the adapted aversive response did not generalize to aristolochic acid because the signaling pathway for this compound was insulated from that for caffeine.
Keywords: taste cell, plasticity, long-term adaptation, bitter taste, ingestive behavior, Manduca sexta
All animals have chemoreceptor cells that respond to potentially toxic compounds, and activation of these cells usually elicits an aversive behavioral response (Dethier, 1993). This aversive response is highly adaptive when animals encounter toxic substances (Garcia and Hankins, 1975). The problem is that many nontoxic substances stimulate the same chemoreceptor cells, causing “false alarms” (Harley and Thorsteinson, 1967; Glendinning, 1994). To overcome this problem, animals have evolved adaptation mechanisms that minimize the number of false alarms. For instance, chronic exposure to an oral irritant (Karrer and Bartoshuk, 1991), acrid odorant (Dalton et al., 1997; Wysocki et al., 1997), or “bitter” tastant (see references below) can adapt the aversive response to the same compound. Little is known, however, about what mediates this type of long-term adaptation. Here, we investigate how insects adapt to compounds that humans characterize as bitter.
It is known that dietary exposure to one bitter substance can adapt the aversive behavioral response to the same substance in rodents (Warren and Pfaffman, 1959; Zellner et al., 1985; Harder et al., 1989) and insects (Szentesi and Bernays, 1984; Usher et al., 1988; Glendinning and Gonzalez, 1995). This self-adaptation process could be mediated by at least three mechanisms: (1) the taste cells that respond to the bitter substance become progressively more desensitized with exposure, diminishing their ability to activate the aversive response; (2) repeated exposure to the bitter substance habituates the central pathways that trigger the aversive response; or (3) an association forms between the bitter taste stimulus and a positive postingestive effect, leading to a conditioned preference.
Another feature of the exposure-induced adaptation process is that it generalizes to some but not all bitter substances (McBurney et al., 1972; Glendinning and Gonzalez, 1995). The most parsimonious explanation for this cross-adaptation phenomenon is that animals have multiple signaling pathways for conveying information about bitter taste stimuli to the CNS, and each pathway has a different molecular receptive range. Accordingly, self-adaptation to one bitter substance would generalize to all other bitter substances that stimulate the same signaling pathway. In support of this hypothesis, there is evidence that different bitter taste stimuli may activate different subpopulations of bitter-sensitive taste cells in both insects (Glendinning et al., 1999a,b; van Loon and Schoonhoven, 1999) and mammals (Dahl et al., 1997; Danilova et al., 1999; Caicedo and Roper, 2001).
Here we evaluate the aforementioned mechanisms of self- and cross-adaptation in Manduca sexta caterpillars, using two compounds (caffeine and aristolochic acid) that taste bitter to humans and elicit an aversive behavioral response in these insects. The aversive behavioral response is mediated by three pairs of bitter-sensitive taste cells, each of which occurs in a different bilateral pair of gustatory sensilla (Fig.1). Here, we asked (1) whether dietary exposure to caffeine desensitizes all of the caffeine-responsive taste cells to caffeine and/or aristolochic acid; (2) whether the desensitization phenomenon is associated with an attenuated behavioral response to caffeine and/or aristolochic acid; and (3) whether the taste cells recover from desensitization.
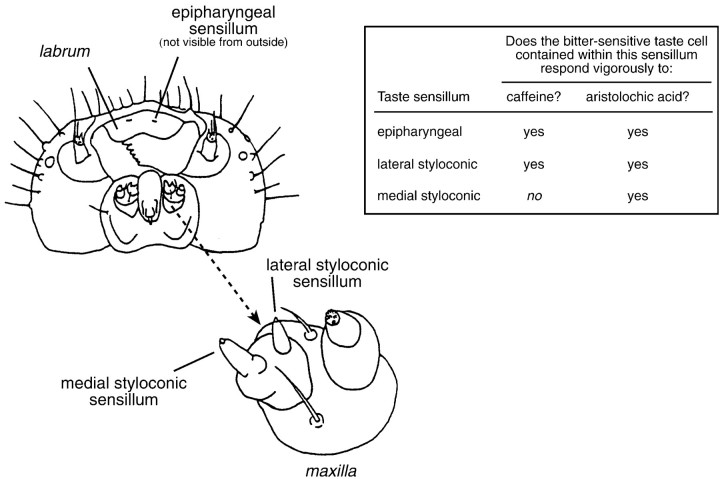
Fig. 1.
Diagram of the head of a caterpillar, as viewed from below. An enlargement of one maxilla (indicated with anarrow) is provided to show the locations of the medial and lateral styloconic sensilla. The epipharyngeal sensilla are located underneath the labrum and thus are not visible in this diagram. Each of these gustatory sensilla contains one bitter-sensitive taste cell. We indicate which bitter-sensitive taste cells exhibit a vigorous excitatory response to caffeine and aristolochic acid in theright panel; these latter data are fromGlendinning et al. (1999a). This illustration was adapted from Bernays and Chapman (1994, their Fig. 3.4).
MATERIALS AND METHODS
Caterpillar rearing procedure. In this and all subsequent experiments, we reared M. sexta caterpillars from eggs on a wheat germ-based artificial diet (Bell and Joachim, 1976), and maintained them in an environmental chamber on a 16/8 hr light/dark cycle (27.5°C). We began all experiments with caterpillars in the first day of their fifth instar (i.e., larval growth stage). All caterpillars were naive to the taste stimuli before testing. To control for any potential differences among caterpillars from different egg batches, we interspersed individuals from each batch across experimental treatments. Sample sizes for each experiment are provided in the figure legends.
Experiment 1: does dietary exposure to the caffeinated diet desensitize all caffeine-responsive taste cells? In this experiment, we used a noninvasive extracellular technique to record from the same caffeine-responsive taste cell, both before and after exposure to a caffeinated diet. We asked how exposure to the caffeinated diet altered the responsiveness of the entire population of caffeine-responsive taste cells. This taste cell population consists of two bilateral pairs of bitter-sensitive taste cells, one in the lateral styloconic sensilla and the other in the epipharyngeal sensilla (Fig.1). Previous work established that 48 hr of dietary exposure to caffeine desensitizes the bitter-sensitive taste cells in the lateral styloconic sensilla to caffeine (Glendinning et al., 1999b). The goals of this experiment were to determine whether desensitization developed in <48 hr and to determine whether it also developed in the caffeine-responsive taste cells within the epipharyngeal sensilla.
Throughout this paper, we use the term “desensitization” to refer to the effect of caffeine exposure on taste cell responsiveness. This is done because previous work (Glendinning et al., 1999b) established that 48 hr of exposure to the caffeinated diet decreases the maximal response of the bitter-sensitive taste cells to all suprathreshold concentrations of caffeine by >50%, making the concentration–response curve virtually flat. Like Dalton (2000), we use the term “adaptation” to refer to any exposure-induced reduction in behavioral responsiveness to a chemical stimulus. By using this broad definition of adaptation, we can integrate a broad range of functionally related exposure effects, which are mediated by different physiological mechanisms. We avoided an exclusive term like habituation because it refers to a highly specific form of nonassociative sensory learning (Thompson and Spencer, 1966; Leibrecht and Askew, 1980).
The experimental protocol consisted of stimulating one lateral sensillum from individual caterpillars with 5 mm caffeine (in deionized water containing 0.1 M KCl, pH 5.7; Sigma-Aldrich, St. Louis, MO). We used this caffeine concentration because it produces maximal firing rates in all caffeine-responsive taste cells ofM. sexta (Glendinning et al., 1999a). We kept the caterpillar in the experiment if the bitter-sensitive taste cell in its lateral sensillum responded to the caffeine solution with a firing rate of 50 Hz; the caterpillar was discarded if the same taste cell responded with a firing rate of <50 Hz. Although this screening criterion caused us to discard 8% of the caterpillars, it increased our chances of detecting desensitization because we included only caterpillars with responsive taste cells. Next, we removed the caterpillar from the recording apparatus (see below for details), let it recover for 1 hr from immersion in the electrolyte solution, and then offered it a caffeinated diet for one of four exposure periods: 6, 12, 24, or 48 hr. During this exposure period, the caterpillar could sample and/or ingest the caffeinated disk ad libitum; it was motivated to do so because it did not have another source of food or water. At the end of the exposure period, we stimulated the same lateral sensillum (and hence, the same bitter-sensitive taste cell located within this sensillum) with the 5 mmcaffeine solution to determine the extent of desensitization. We did not need a control treatment in this experiment (i.e., one in which the caterpillar was offered a noncaffeinated diet) because we have shown previously that the responsiveness of the bitter-sensitive taste cell in the lateral styloconic sensillum to caffeine normally increases over the fifth instar when the caterpillars are maintained on a noncaffeinated diet (Glendinning et al., 1999b).
We stimulated one epipharyngeal sensillum from each caterpillar with the 5 mm caffeine solution (see below for details). If the response of the bitter-sensitive taste cell within this sensillum met the screening criteria outlined above for the lateral sensillum, we put the caterpillar on a caffeinated or noncaffeinated diet for 24 hr. After the exposure period, we restimulated the same epipharyngeal sensillum (and hence, the same bitter-sensitive taste cell located within this sensillum) a second time with the 5 mm caffeine solution to determine whether its responsiveness to caffeine was altered by the exposure diet. Note that we used different caterpillars in the tests for desensitization of taste cells within the lateral and epipharyngeal sensilla.
We recorded neural responses of individual taste sensilla using a noninvasive extracellular tip-recording technique (Gothilf and Hanson, 1994; Glendinning et al., 1998). In brief, we placed a glass electrode (containing a specific taste stimulus dissolved in 0.1 mKCl) over the tip of a lateral styloconic sensillum, or directly on top of an epipharyngeal sensillum (after deflecting the labrum back 90° from its normal position) (Fig. 1), and then recorded excitatory responses of taste cells within the sensillum (de Boer et al., 1977;Glendinning et al., 1999a). We were able to record from a bitter-sensitive taste cell, and then remove the caterpillar unharmed from the recording apparatus, within 20 min. The caterpillars invariably recovered from this procedure and began feeding normally within 45 min.
We recorded alternating current signals from individual taste sensilla with the Tasteprobe amplifier system (Syntech, Hilversum, The Netherlands) (Marion-Poll and Van der Pers, 1996). We preamplified each recording 10 times, ran it through a bandpass filter set at 100–1200 Hz, fed it into a computer through a 16-bit analog-to-digital converter board, and then analyzed it off-line with Autospike software (Syntech).
For each neural recording, we stimulated a sensillum for ∼2000 msec and quantified the number of action potentials generated 0–1000 msec after contact. We paused at least 3 min between each successive stimulation. To minimize the effects of solvent evaporation at the tip of the recording/stimulating electrode, we drew fluid from the tip with a piece of filter paper immediately before each stimulation. We tested only one member of each bilateral pair of gustatory sensilla per caterpillar.
Each taste sensillum contains three to four taste cells, and each taste cell within a sensillum exhibits a typical spike amplitude and temporal pattern of firing (Glendinning et al., 1999a, 2000b). We used the idiosyncratic response features of each taste cell as a basis for discriminating action potentials from different taste cells.
We used the rearing diet (see above) as the substrate for the caffeinated exposure diet. We established a 7.7 mm/kg caffeine concentration in the diet (fresh mass) by heating the agar-containing diet to ∼60°C, adding the appropriate quantity of caffeine, stirring vigorously for 3 min, and then pouring the diet into Plexiglas molds (2 × 3 × 1.5 cm). One diet block contained enough food to sustain a caterpillar for 24 hr. We used the 7.7 mm/kg concentration of caffeine because we have shown previously that 48 hr of exposure to a diet containing this concentration markedly desensitized the bitter-sensitive taste cell in the lateral styloconic sensilla to caffeine (Glendinning et al., 1999a,b). We prepared the noncaffeinated diet similarly, but neglected to add caffeine.
The diet exposure protocol consisted of placing a caterpillar in a sealed plastic deli-cup (160 ml volume with a vented lid) and then offering it the caffeinated or noncaffeinated diet for the predetermined period of time. In those instances in which we exposed a caterpillar to a diet for >24 hr, we gave it a fresh diet block each day.
To quantify the extent of desensitization in the lateral sensilla, we divided the response of a bitter-sensitive taste at the end of the exposure period by that obtained from the same taste cell before the exposure period; this value was then multiplied by 100 to yield the “percentage of initial response.” To determine the extent of desensitization in the epipharyngeal sensilla, we compared the neural response of individual taste cells to the 5 mm caffeine solution both before and after exposure to the caffeinated or noncaffeinated diet, using the Wilcoxon matched-pairs signed-rank test (α = 0.05). We concluded that desensitization occurred if exposure to the caffeinated diet (but not the noncaffeinated diet) significantly reduced the neural response.
Experiment 2: does exposure to the caffeinated diet alter the responsiveness of taste cells to aristolochic acid? Previous work in our laboratory established (1) that caffeine and aristolochic acid stimulate the same bitter-sensitive taste cell in both the lateral and epipharyngeal sensilla, through different transduction mechanisms (Glendinning and Hills, 1997, Glendinning et al., 1999a); but (2) that exposure to the caffeinated diet does not diminish the responsiveness of the bitter-sensitive taste cell in the lateral styloconic sensilla to aristolochic acid (Glendinning et al., 1999b). The goal of this experiment was to determine whether exposure to the caffeinated diet altered the response of the bitter-sensitive taste cell in the epipharyngeal sensilla to aristolochic acid (sodium salt; Sigma-Aldrich).
We used the same basic protocol described in experiment 1, with only minor differences. We stimulated the bitter-sensitive taste cell with 0.1 mm aristolochic acid (in deionized water containing 0.1m KCl), both before and after exposure to either the caffeinated diet or noncaffeinated diet. We selected the 0.1 mm concentration of aristolochic acid because it elicits a maximal excitatory response in the bitter-sensitive taste cell within the epipharyngeal sensilla (Glendinning et al., 1999a). We compared the response of each bitter-sensitive taste cell with the taste stimulus before and after the exposure period, separately for each diet treatment, using the Wilcoxon matched-pairs signed-rank test (α = 0.05).
Experiment 3: does the desensitization phenomenon adapt the aversive behavioral response to caffeine? This experiment asked whether desensitization phenomenon altered the behavioral response of caterpillars to caffeine. In particular, we asked whether 24 hr of exposure to the caffeinated diet attenuated their aversive response to caffeine.
We used a brief-access biting assay to assess the ingestive responses of individual caterpillars to 5 mm caffeine. Because this assay lasted only 2 min, we could be relatively confident that the ingestive responses of the caterpillars were mediated principally by the gustatory effects of caffeine and not by postingestive feedback. This interpretation is supported by the finding that ablation of all taste sensilla containing caffeine-responsive taste cells (i.e., the lateral styloconic and epipharyngeal sensilla) completely eliminates the aversive behavioral response to caffeine during this 2 min biting assay (Glendinning et al., 1999a).
Our brief-access biting assay consisted of the following steps. First, we placed a caterpillar in the “food-deprivation arena,” which consisted of a clean (inverted) Petri dish covered with a clear plastic cylinder (7.5 cm in diameter and 10 cm tall), and then fasted it for 30 min to standardize its “hunger” state. Next, we transferred the caterpillar to the “test arena,” which was identical to the food-deprivation arena in all respects except that a piece of cork (1 cm in diameter, 3–4 mm high) had been taped to the middle of the Petri dish. Immediately before a biting assay, we pinned a glass-fiber disk (Whatman GF/A, 4.25 cm diameter; Whatman International Ltd, Maidstone, UK) to the piece of cork and then moistened it with 400 μl of deionized water. Next, we placed the caterpillar on the edge of the disk, positioning it so that its legs and prolegs grasped the edge of the disk securely. Once the caterpillar brought its mouth parts into contact with the glass fiber disk and took a bite, we began the 2 min biting assay. We recorded the timing of each bite with a software-based event recorder. At the end of the assay, we removed the caterpillar from the disk, taking care to prevent the caterpillar from tearing the edge of the disk. If the caterpillar took <50 bites from the water-treated disk during the 2 min biting assay, we removed it from the experiment (this amounted to only 6% of the caterpillars). If the caterpillar took ≥50 bites, we gave it 30 min of ad libitumaccess to its exposure diet, food-deprived it for 30 min, and then ran it through a second 2 min biting assay, using a disk moistened with 400 μl of 5 mm caffeine (in deionized water). The observer was blind with respect to the nature of the exposure diet of the caterpillar.
Our rationale for this experimental design was as follows. The water-treated disk served as a positive control, ensuring that we included in the experiment only those caterpillars that fed readily on the disks in the absence of caffeine. Thus, whenever the caterpillar took ≥50 bites from the water-treated disk and <50 bites from the caffeine-treated disks, we assumed that they had accepted the former and rejected the latter.
Given that the disks constituted a novel food for the caterpillars, we took two precautions to minimize the chances that the caterpillars would reject the disks based solely on their novelty. First, 60 min before the biting assay of a caterpillar, we offered it a disk wetted with 400 μl of deionized water for 10 min. During this time, the caterpillar was free to investigate and/or ingest the disk ad libitum; we did not, however, record any of these behaviors. At the end of the 10 min period, we returned the caterpillar to its home cage with its exposure diet. Second, we treated all disks (both control and chemically treated, in this and all subsequent experiments) with 400 μl of leaf surface extract from tobacco leaves (Nicotiana tabacum, 35S-gus variety). We reasoned that this extract would promote consumption of the disks, because tobacco leaves are highly preferred foods for M. sexta caterpillars. To make the leaf surface extract, we immersed three large tobacco leaves (base to tip length: 25–30 cm) in 50 ml of chloroform for 30 sec, agitating each leaf gently.
We analyzed three aspects of the ingestive response of each caterpillar to the water- and caffeine-treated test disks during the 2 min biting assay: (1) total disk area eaten (in mm2) during the 2 min biting assay, using a digitization procedure described previously (Glendinning et al., 2000b); (2) total number of bites taken over the 2 min biting assay; and (3) bite size by dividing total area of disk eaten by total number of bites (units = mm2/bite).
To determine whether the exposure to the caffeinated diet adapted the aversive behavioral response to caffeine, we used a within-animal analysis. That is, we compared ingestive responses to the water- and caffeine-treated disks separately for individuals exposed to the caffeinated or noncaffeinated diet. To compare total intake, the number of bites during the initial 10 sec of the feeding test (i.e., initial biting activity), the total number of bites across the entire 2 min assay, and bite size, we made pairwise comparisons (separately for each response variable) between the response of each caterpillar to the water- and caffeine-treated disks, using Wilcoxon matched-pairs signed-rank tests (one-tailed; p ≤ 0.01).
Experiment 4: does the desensitization phenomenon adapt the aversive behavioral response to aristolochic acid? In this experiment, we asked whether exposure to the caffeinated diet alters the aversive behavioral response to aristolochic acid (sodium salt; Sigma-Aldrich). We used virtually the same experimental procedures outlined in the previous experiment. The only difference was that we compared the ingestive response of each caterpillar to a glass-fiber disk treated with 400 μl of deionized water versus one treated with 400 μl of 0.1 mm aristolochic acid (in deionized water, pH 5.7). We used the 0.1 mmconcentration of aristolochic acid because previous studies have established that it maximally stimulates the bitter-sensitive taste cells in the lateral and epipharyngeal sensilla (Glendinning et al., 1999a).
Experiment 5: could the bitter-sensitive taste cells in the lateral and epipharyngeal sensilla activate the aversive behavioral response after dietary exposure to caffeine? The previous experiment asked whether the exposure to the caffeine diet attenuated the aversive behavioral response to aristolochic acid. However, it did not enable us to address a more subtle question: are the bitter-sensitive taste cells in the lateral and epipharyngeal sensilla, after being desensitized to caffeine, still capable of eliciting an aversive behavioral response to aristolochic acid? The reason for the ambiguity is that M. sexta has a bilateral pair of bitter-sensitive taste cells in the medial styloconic sensilla that responds vigorously to aristolochic acid and only weakly to caffeine (Fig. 1). Given that the bitter-sensitive taste cells in the medial sensillum are sufficient to mediate the aversive behavioral response to aristolochic acid but not caffeine (Glendinning et al., 1999a), their presence could explain why the caterpillars (after dietary exposure to caffeine) still exhibited an aversive behavioral response to aristolochic acid. To resolve this issue, we repeated experiment 4 but surgically ablated the medial styloconic sensilla of the caterpillars before conducting the feeding tests.
We used the following procedure to make the ablations. We secured the head of each caterpillar with a latex gasket, inserted it backwards into a water-filled vial (which induced complete anesthesia within 5 min), and then, under a dissecting microscope, quickly removed the distal half of both medial sensilla with microdissection scissors. Within 1 hr of removing the caterpillar from the vial, all caterpillars were feeding and locomoting normally. At this point, we placed the caterpillar on its respective exposure diet (caffeinated or noncaffeinated) and let it ingest the diet ad libitum for 24 hr. At the end of the exposure period, we inspected the caterpillar for incomplete ablation or other signs of surgical complications. Because none of the caterpillars showed any such problems, we subjected all of them to the same feeding test described in experiment 4.
Experiment 6: do the bitter-sensitive taste cells recover from caffeine-induced desensitization? The goal of this experiment was to determine whether the bitter-sensitive taste cell in the lateral and epipharyngeal sensilla recovers from the caffeine-induced desensitization phenomenon, and if so, how long the recovery takes.
Our four-step experimental protocol was as follows. (1) We recorded the baseline response of the bitter-sensitive taste cell in either the lateral styloconic or epipharyngeal sensilla to 5 mmcaffeine. If the response was <50 Hz, the caterpillar was discarded. (This screening criteria caused us to reject 4% of the caterpillars.) If the response was ≥50 Hz, we extracted the caterpillar from the recording apparatus, let it recover for 1 hr, and then offered it the caffeinated diet for 24 hr. (2) After this exposure period, we recorded the response of the same bitter-sensitive taste cell to 5 mm caffeine a second time. If the bitter-sensitive taste cell was desensitized (i.e., its response to 5 mm caffeine was ≤50% of its baseline response), the caterpillar was kept in the experiment; otherwise, the caterpillar was discarded. (This screening criterion caused us to reject 10% of the caterpillars.) We then offered the noncaffeinated diet to the caterpillar for 24 hr. (3) After this exposure period, we recorded the response of the same bitter-sensitive taste cell to 5 mm caffeine for a third time, and then returned the caterpillar to the noncaffeinated diet for another 24 hr. (4) After this final exposure period, we recorded the response of the same bitter-sensitive taste cell to 5 mmcaffeine for the fourth and last time. We considered a desensitized taste cell to have “recovered” if its responsiveness to caffeine returned to the level observed before exposure to the caffeinated diet.
RESULTS
Experiment 1: does dietary exposure to the caffeinated diet desensitize all caffeine-responsive taste cells?
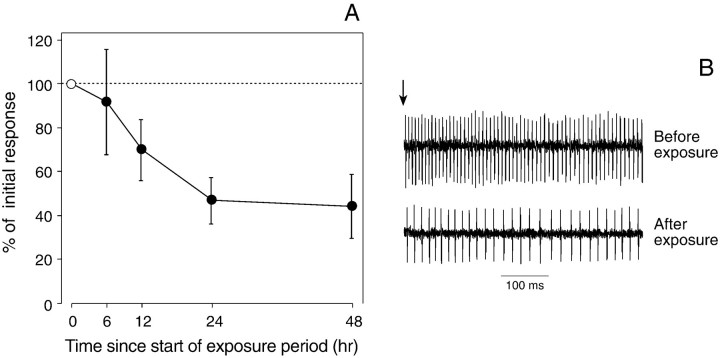
Exposure to the caffeinated diet substantially reduced the responsiveness of the bitter-sensitive taste cell in the lateral styloconic sensillum to 5 mm caffeine (Fig.2). The extent of this desensitization increased steadily over the initial 24 hr of exposure but leveled off at ∼45% of the baseline response, after additional exposure (Fig.1). Based on these results, we used a 24 hr exposure period for all subsequent experiments.
Fig. 2.
Change in the responsiveness of the bitter-sensitive taste cell within the lateral styloconic sensillum to caffeine after 6, 12, 24, or 48 hr of exposure to the caffeinated diet. We recorded the excitatory response (impulses per second) of a single taste cell to 5 mm caffeine, both before and after each dietary exposure period. A, To quantify the extent of desensitization after each exposure period, we divided the response at the end of the exposure period by that obtained from the same taste cell before the exposure period; this value was then multiplied by 100 to yield the percentage of initial response. All data are presented as median ± median absolute deviation. The number of caterpillars subjected to each exposure period ranged from 14 to 19.B, Representative responses of a lateral styloconic sensillum to 5 mm caffeine before and after 24 hr of exposure to the caffeinated diet. In both traces, only the bitter-sensitive taste cell is firing. The vertical arrow above the top trace indicates the onset of stimulation.
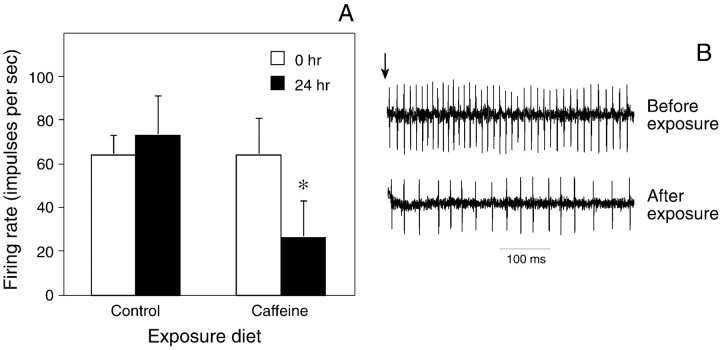
We found that 24 hr of exposure to the caffeinated diet also significantly desensitized (p ≤ 0.05) the bitter-sensitive taste cell in the epipharyngeal sensillum to 5 mm caffeine (Fig.3). In contrast, exposure to the noncaffeinated diet did not produce any systematic changes in responsiveness of the same taste cell to 5 mmcaffeine.
Fig. 3.
Excitatory response (impulses per second) of the bitter-sensitive taste cell in the epipharyngeal sensillum to 5 mm caffeine, both before and after 24 hr of exposure to the noncaffeinated or caffeinated diet. A, Median responsiveness (± median absolute deviation) of the epipharyngeal sensilla to 5 mm caffeine both before and after exposure to the noncaffeinated or caffeinated diet. For each diet treatment, we tested a total of 11 epipharyngeal sensilla, each from different caterpillars. We determined whether either exposure diet altered the responsiveness of the taste cells to caffeine with the Wilcoxon matched-pairs signed-rank test (*p < 0.05). B, Representative responses of an epipharyngeal sensillum to 5 mm caffeine both before and after exposure to the caffeinated diet. In bothtraces, only the bitter-sensitive taste cell is firing. The vertical arrow above the top traceindicates the onset of stimulation.
Experiment 2: does the desensitization phenomenon alter the responsiveness of taste cells to aristolochic acid?
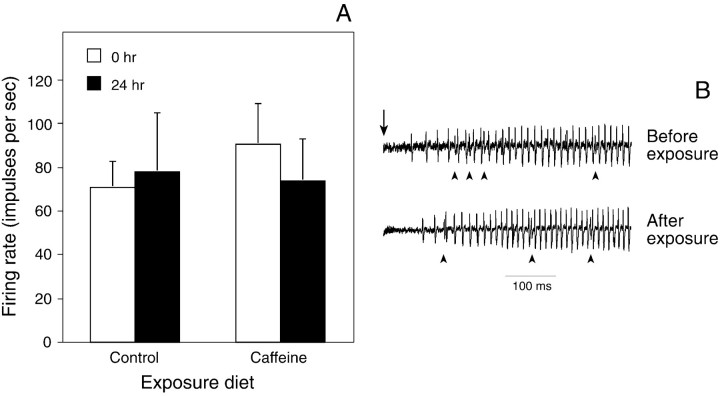
We have shown previously that exposure to the caffeinated diet does not desensitize the bitter-sensitive taste cell in the lateral styloconic sensillum to aristolochic acid (Glendinning et al., 1999b). Here, we found that 24 hr of dietary exposure to the caffeinated (or noncaffeinated) diet failed to produce any significant (p > 0.05) changes in responsiveness to 0.1 mm aristolochic acid (Fig.4). Thus, the caffeine-induced desensitization phenomenon does not generalize to aristolochic acid.
Fig. 4.
Excitatory response (impulses per second) of the bitter-sensitive taste cell in the epipharyngeal sensillum to 0.1 mm aristolochic acid, both before and after 24 hr of exposure to the noncaffeinated or caffeinated diet.A, Median responses (± median absolute deviation) of the epipharyngeal sensilla to 0.1 mm aristolochic acid before and after exposure to the noncaffeinated or caffeinated diet. For each diet treatment, we tested a total of 11 epipharyngeal sensilla, each from different caterpillars. Neither exposure diet altered the responsiveness of taste cells to aristolochic acid (Wilcoxon matched-pairs signed-rank test; p > 0.05). B, Representative responses of an epipharyngeal sensillum to 0.1 mm aristolochic acid before and after exposure to the caffeinated diet. In both traces, one taste cell is firing at a consistent rate (the bitter-sensitive taste cell) and others (indicated by arrowheads) are firing irregularly and less frequently; the latter taste cells are responding to the KCl in the stimulating solution. The vertical arrow above the top trace indicates the onset of stimulation.
Experiment 3: does the desensitization phenomenon adapt the aversive behavioral response to caffeine?
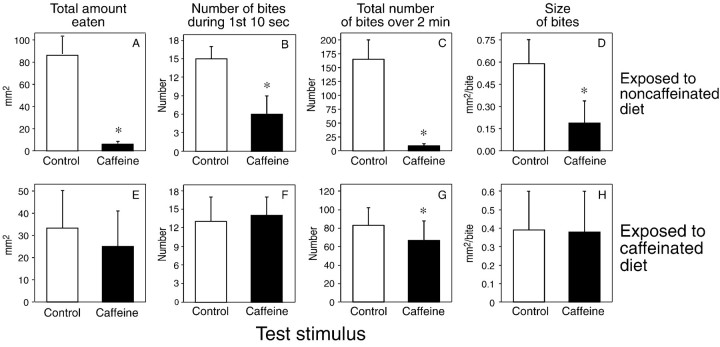
The caterpillars exposed to the noncaffeinated diet fed readily on the water-treated (i.e., control) disk but exhibited an aversive behavioral response to the caffeine-treated disk (Fig.5A–D). This aversive response was manifested as a significant reduction in overall consumption, initial biting rate, total number of bites, and size of individual bites (across the 2 min test). Thus, the aversive response was robust and rapid.
Fig. 5.
Ingestive responses of caterpillars to disks treated with water alone (control) or 5 mm caffeine after 24 hr of exposure to a noncaffeinated (n = 17) or caffeinated (n = 21) diet. We determined four ingestive parameters from the 2 min, brief-access biting assay: total intake (A, E), number of bites during the initial 10 sec of the assay (B, F), total number of bites over the 2 min assay (C, G), and bite size (D, H). We compare the median (± median absolute deviation) values within each panel using the Wilcoxon matched-pairs signed-rank test (*p < 0.05).
The caterpillars exposed to the caffeinated diet fed with equal vigor on the water- and caffeine-treated disks, indicating that the aversive behavioral response to caffeine was adapted by 24 hr of exposure to the caffeine diet (Fig. 5E–H). There was no significant difference in overall consumption, initial biting rate, or bite size on the two type of disks. The caterpillars took significantly fewer total bites on the caffeine-treated disk (across the 2 min test), but this difference was small compared with that observed in the caterpillars exposed to the noncaffeinated diet (Fig. 5, compare C andG).
It is notable that the caterpillars exposed to the caffeinated diet took fewer overall bites from both the water- and caffeine-treated disks than did the caterpillars exposed to the noncaffeinated diet (Fig. 5C,G). When we subjected the caterpillars from both diet treatments to gross tests of motor function (e.g., laid them on their back and observed how long they took to right themselves, and then observed them locomoting), they all righted themselves and locomoted in a similar manner, implying that exposure to the caffeinated diet did not impair gross motor function. The only obvious difference between to two groups was that the caterpillars on the caffeinated diet weighed less than those on the noncaffeinated diet (2.82 ± 0.07 vs 4.00 ± 0.10 gm, respectively).
Experiment 4: does the desensitization phenomenon adapt the aversive behavioral response to aristolochic acid?
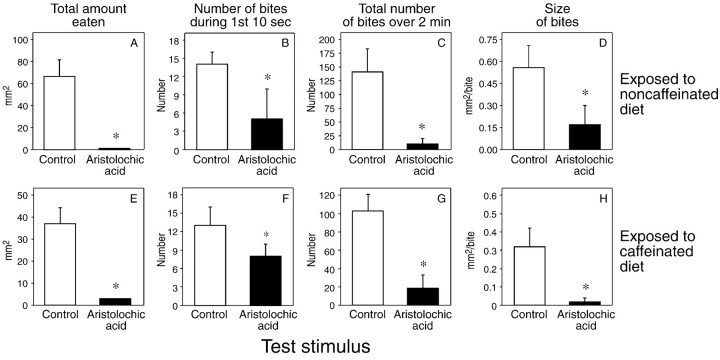
The caterpillars exposed to the noncaffeinated diet fed readily on the water-treated (i.e., control) disk but exhibited an aversive behavioral response to the aristolochic acid-treated disks (Fig.6A–D). This aversive response was manifested as a significant reduction in overall consumption, initial biting rate, total number of bites, and bite size.
Fig. 6.
Ingestive responses of caterpillars to disks treated with water alone (control) or a 0.1 mmaristolochic acid solution after 24 hr of exposure to a noncaffeinated (n = 18) or caffeinated (n = 19) diet. We determined four ingestive parameters from the 2 min, brief-access biting assay: total intake (A, E), number of bites during the initial 10 sec of the assay (B, F), total number of bites over the 2 min assay (C, G), and bite size (D, H). We compare the median (± median absolute deviation) values within eachpanel using the Wilcoxon matched-pairs signed-rank test (*p < 0.05).
The caterpillars exposed to the caffeinated diet also exhibited a robust aversive response to the aristolochic acid-treated disk (Fig.6E–H), demonstrating that the caffeine-induced adaptation phenomenon does not generalize to aristolochic acid. The caterpillars fed less vigorously on the aristolochic acid-treated diet across the entire 2 min feeding test.
Experiment 5: could the bitter-sensitive taste cells in the lateral and epipharyngeal sensilla activate the aversive behavioral response after dietary exposure to caffeine?
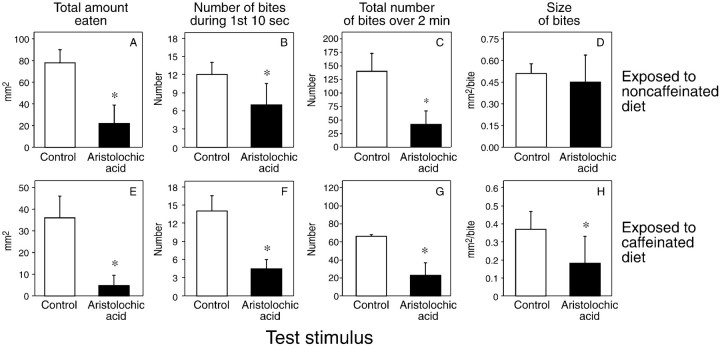
Despite the loss of the bitter-sensitive taste cells in the medial styloconic sensilla, the caterpillars nevertheless exhibited a robust aversive behavioral response to the aristolochic acid-treated disk, irrespective of whether they had been exposed to the caffeinated or noncaffeinated diets (Fig.7A–H). The only notable effect of the medial sensilla ablations was in the caterpillars exposed to the noncaffeinated diet: the size of the bites on the water- and aristolochic acid-treated disks was statistically indistinguishable. Thus, sensory input from the bitter-sensitive taste cell in the lateral and epipharyngeal sensilla is sufficient for activation of the aversive behavioral response to aristolochic acid in caterpillars exposed to the caffeinated diet.
Fig. 7.
Ingestive responses of caterpillars, lacking their medial styloconic sensilla, to disks treated with water alone (control) or a 0.1 mm aristolochic acid solution after 24 hr of exposure to a noncaffeinated (n = 14) or caffeinated (n = 15) diet. We determined four ingestive parameters from the 2 min, brief-access biting assay: total intake (A, E), number of bites during the initial 10 sec of the assay (B, F), total number of bites over the 2 min assay (C, G), and bite size (D, H). We compare the median (± median absolute deviation) values within each panel using the Wilcoxon matched-pairs signed-rank test (*p < 0.05).
Experiment 6: do the bitter-sensitive taste cells recover from caffeine-induced desensitization?
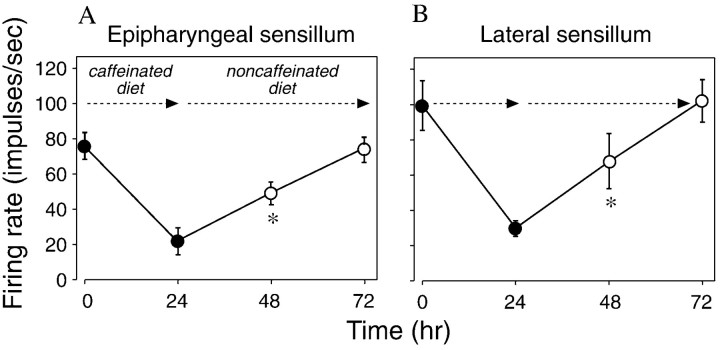
Our results indicate that the bitter-sensitive taste cells in the epipharyngeal and lateral styloconic sensilla recover fully from the desensitization phenomenon, once the caterpillar is returned to a noncaffeinated diet (Fig.8A,B). This recovery process, however, takes almost twice as long as the onset process (i.e., 48 vs 24 hr, respectively). As can be seen in Figure 8, the responsiveness of the bitter-sensitive taste cells to caffeine 24 hr after being transferred to the noncaffeinated diet (i.e., at 48 hr), was still significantly below that observed at the beginning of the experiment (i.e., at 0 hr). In contrast, the responsiveness of the bitter-sensitive taste cells to caffeine 48 hr after being transferred to the noncaffeinated diet (i.e., at 72 hr), was statistically indistinguishable from that at 0 hr.
Fig. 8.
Recovery from desensitization in the bitter-sensitive taste cell within the epipharyngeal sensilla (A) or lateral styloconic sensilla (B). We offered each caterpillar the caffeinated diet for the initial 24 hr and then the noncaffeinated diet over the next 48 hr. We recorded the response of the epipharyngeal (n = 4) or lateral (n = 20) sensilla of a caterpillar to 5 mm caffeine four times: at the onset of the experiment (0 hr), at the end of the caffeine exposure period (at 24 hr), and then twice after the caterpillar was returned to the noncaffeinated diet (at 48 and then 72 hr). We made paired comparisons between the neural response at 0 hr and that at 48 and 72 hr, using the Wilcoxon matched-pairs signed-rank test (*p < 0.025).
DISCUSSION
We found that dietary exposure to the caffeinated diet desensitized all caffeine-responsive taste cells to caffeine. The desensitization developed gradually over time, reaching its maximum 24 hr after the onset of dietary exposure. Once we returned the caterpillars to the noncaffeinated diet, their caffeine-responsive taste cells gradually recovered their responsiveness to caffeine over a period of 48 hr, revealing the plastic nature of this phenomenon. Although other studies have documented exposure-induced desensitization of bitter-sensitive taste cells (Schoonhoven, 1969, 1976; Simmonds and Blaney, 1983; Blaney and Simmonds, 1987), none have done so for the entire population of taste cells that responds to the desensitizing bitter substance, and none have documented that the taste cells can recover from the exposure-induced desensitization.
We proposed three hypothetical mechanisms to explain how exposure to a caffeinated diet could adapt the aversive behavioral response to caffeine. The first was that the caffeine-responsive taste cells could become progressively more desensitized with repeated exposure, diminishing their ability to elicit the aversive behavioral response. We found clear support for this mechanism: the desensitization phenomenon reduced the responsiveness of all caffeine-responsive taste cells by >55%. We believe that this level of desensitization was sufficient to adapt the aversive behavioral response because we have shown previously (Glendinning et al., 1999a) that firing rates in the bitter-sensitive taste cells, comparable with those produced by the desensitized taste cells in this study (i.e., <40 spikes/sec), did not elicit an aversive behavioral response in M. sexta that had been maintained on a noncaffeinated diet.
The second hypothetical mechanism was that dietary exposure to caffeine could have habituated the central pathways that trigger the aversive behavioral response. Although we cannot reject this hypothesis, we think the magnitude of the peripheral desensitization phenomenon was so great that it would have either prevented habituation from taking place or rendered it unnecessary. The third hypothetical mechanism was that the caterpillars could learn to associate the sensory input provided by caffeine with a positive postingestive effect and thereby develop a preference for caffeine. This type of conditioning has been observed in rats and humans for several bitter taste stimuli (Naim et al., 1977;Zellner et al., 1985; Sclafani, 1991; Falk et al., 1999), including caffeine (Kuznicki and Turner, 1986). However, the caffeine-exposed caterpillars never developed a preference for caffeine (i.e., they did not bite more vigorously on the caffeine-treated disk than on the water-treated disk). Instead, their ingestive response to caffeine changed from aversion to indifference over the exposure period, implying that they simply lost their ability to taste the caffeine.
Lack of cross-adaptation to aristolochic acid
We found that adaptation of the aversive behavioral response to caffeine did not generalize to aristolochic acid. This lack of cross-adaptation between bitter taste stimuli has been reported previously for humans (McBurney et al., 1972) and insects (Glendinning and Gonzalez, 1995), but virtually nothing was known about the underlying physiological mechanisms. Our findings indicate that the specificity of the adaptation process in M. sexta occurs because exposure to the caffeinated diet selectively desensitized the bitter-sensitive taste cells to caffeine, leaving their responsiveness to aristolochic acid unaltered. As a result, the bitter-sensitive taste cells in the medial, epipharyngeal, and/or lateral styloconic sensilla were able to elicit a vigorous aversive behavioral response to aristolochic acid.
In the final experiment, we sought to confirm that the bitter-sensitive taste cells in the lateral and epipharyngeal sensilla were capable of activating an aversive behavioral response to aristolochic acid, even after they had been desensitized to caffeine. To answer this question, we ablated the medial sensilla (which contains a bitter-sensitive taste cell that responds vigorously to aristolochic acid but not caffeine) from a group of caterpillars and subsequently recorded their aversive behavioral response to aristolochic acid after 24 hr of exposure to the caffeinated diet. We found that these ablated caterpillars exhibited a normal aversive behavioral response to aristolochic acid, demonstrating that desensitizing the taste cells to caffeine does not impair their ability to elicit an aversive behavioral response to aristolochic acid. If follows, therefore, that desensitization of the signaling pathway for caffeine does not diminish the ability of the signaling pathway for aristolochic acid to elicit an aversive response. Thus, the signaling pathway for aristolochic acid appears to be functionally insulated from that for caffeine.
The prevalence of insulated signaling pathways in the chemosensory cells of other animal taxa is unclear. The only other documented example involves the nematode, Caenorhabditis elegans, which has chemosensory cells that express at least two signaling pathways. These chemosensory cells can be desensitized (through chronic exposure) to ligands that stimulate one signaling pathway and yet retain sensitivity to ligands that stimulate a different signaling pathway (Colbert and Bargmann, 1995; Carlson, 2000; L'Etoile and Bargmann, 2000). The situation in vertebrates, however, is less well understood. For example, although it is known that individual taste cells contain several bitter receptors (Adler et al., 2000; Chandrashekar et al., 2000) and/or signaling pathways (Bernhardt et al., 1996; Rössler et al., 2000), the question of whether these different receptors or signaling pathways can be desensitized independently of one another has apparently not been examined. In addition, a variety of signaling pathways for bitter taste stimuli have been discovered in vertebrate taste cells (for review, see Glendinning et al., 2000a), but it is unclear whether any of these pathways (1) are coexpressed within the same taste cell, or (2) can be desensitized through chronic exposure.
We should note that some animal species use “cross talk” between coexpressed signaling pathways as a mechanism for peripheral signal processing. For instance, many lobster olfactory cells express at least two transduction pathways; in some cases, these pathways act antagonistically (i.e., one depolarizes and the other hyperpolarizes the cell) (Ache and Zhainazarov, 1995), and in other cases they act additively (i.e., they both depolarize the cell) (Cromarty and Derby, 1997).
Mechanisms underlying the desensitization phenomenon
We determined previously that the caffeine-induced desensitization phenomenon is produced by a local effect of caffeine on the bitter-sensitive taste cells, rather than, for instance, through a centrifugal neural mechanism (Glendinning et al., 1999b). This was accomplished by dripping a 5 mm caffeine solution directly onto a single lateral sensillum intermittently for 24 hr and showing that the desensitization phenomenon did not transfer to the contralateral bitter-sensitive taste cell. In addition, by preventing the caterpillar from ingesting the caffeine solution as it dripped onto the sensillum, we eliminated the possibility that the desensitization stemmed from a systemic effect of ingested caffeine in the blood on the bitter-sensitive taste cell.
Although little is known about how caffeine actually produces this desensitization phenomenon, we can draw several inferences based on results from this study and others (Glendinning and Hills, 1997;Glendinning et al., 1999b). First, the fact the bitter-sensitive taste cell adapted to caffeine without adapting to aristolochic acid strongly suggests that it expresses two kinds of bitter receptors, which couple to different signaling pathways. Second, our results suggest that these bitter receptors, or their downstream transduction pathways, can be desensitized individually. Third, although receptor phosphorylation could have produced the desensitization (Dawson et al., 1993), a reduction in receptor expression is more likely because of the slow rate of adaptation. It is also possible that caffeine accumulated within the taste cell and interfered with its own signaling pathway. A general inhibition of the taste cell by hyperpolarization is an unlikely mechanism because it would have affected both the caffeine- and aristolochic acid-activated transduction pathways.
Clearly, more work is needed to explain the desensitization phenomenon. Two important questions to examine would be: why does desensitization take 24 hr to develop and 48 hr to recover, and why does it only produce a 55% (as opposed to a 100%) reduction in responsiveness to caffeine?
Conclusion
When M. sexta is exposed chronically to an unpalatable caffeinated diet, we found that it gradually adapts its aversive behavioral response to the diet, enabling it to consume the diet and meet its nutritional needs. The adaptation process, however, does not render M. sexta unresponsive to all bitter and potentially toxic bitter compounds. It retains its responsiveness to another bitter compound, aristolochic acid, which is substantially more toxic than caffeine to M. sexta (J. Glendinning, unpublished data).
We have also established previously that when M. sexta is exposed to a toxic aristolochic acid diet, its bitter-sensitive taste cells do not become desensitized to aristolochic acid (Glendinning et al., 1999b), and it does not experience any behavioral adaptation to the diet (Glendinning, unpublished data). This latter finding establishes that long-term adaptation mechanisms are not activated by all noxious compounds. Instead, they appear to be activated selectively by relatively harmless compounds and enable insects like M. sexta to minimize the number of false alarms that they exhibit toward foods containing bitter but harmless compounds.
Footnotes
This project was supported in part by Research Grant 5 R29 DC 02416 from the National Institute on Deafness and Other Communication Disorders, National Institutes of Health (J.I.G.), and by a grant from the Howard Hughes Medical Institute (to Barnard College). We also thank two anonymous reviewers for helpful comments.
Correspondence should be addressed to John I. Glendinning, Department of Biological Science, Barnard College, Columbia University, 3009 Broadway, New York, NY 10027. E-mail: jglendinning@barnard.edu.
REFERENCES
- 1.Ache BW, Zhainazarov A. Dual second-messenger pathways in olfactory transduction. Curr Opin Neurobiol. 1995;5:461–466. doi: 10.1016/0959-4388(95)80006-9. [DOI] [PubMed] [Google Scholar]
- 2.Adler E, Hoon MA, Mueller KL, Chandrashekar J, Ryba NJP, Zucker CS. A novel family of mammalian bitter taste receptors. Cell. 2000;100:693–702. doi: 10.1016/s0092-8674(00)80705-9. [DOI] [PubMed] [Google Scholar]
- 3.Bell RA, Joachim FA. Techniques for rearing laboratory colonies of tobacco hornworms and pink bollworms. Ann Entomol Soc Am. 1976;69:365–373. [Google Scholar]
- 4.Bernays EA, Chapman RF. Hostplant selection by phytophagous insects. Kluwer Academic; Norwell, MA: 1994. [Google Scholar]
- 5.Bernhardt SJ, Naim M, Zehavi U, Lindemann B. Changes in IP3 and cytosolic Ca2+ in response to sugars and non-sugar sweeteners in transduction of sweet taste in the rat. J Physiol (Lond) 1996;490:325–336. doi: 10.1113/jphysiol.1996.sp021147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Blaney WM, Simmonds MSJ. Experience: a modifier of neural and behavioural sensitivity. In: Labeyrie V, Fabres G, Lachaise D, editors. Insects–plants. Dr. W. Junk Publishers; Dordrecht, Netherlands: 1987. pp. 237–241. [Google Scholar]
- 7.Caicedo A, Roper SD. Taste receptor cells that discriminate between bitter stimuli. Science. 2001;291:1557–1560. doi: 10.1126/science.291.5508.1557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Carlson JR. Insulation of signaling pathways: odor discrimination via olfactosomes? Neuron. 2000;25:503–504. doi: 10.1016/s0896-6273(00)81052-1. [DOI] [PubMed] [Google Scholar]
- 9.Chandrashekar J, Mueller J, Hoon MA, Adler E, Feng L, Guo W, Zucker CS, Ryba NJP. T2Rs function as bitter taste receptors. Cell. 2000;100:703–711. doi: 10.1016/s0092-8674(00)80706-0. [DOI] [PubMed] [Google Scholar]
- 10.Colbert HA, Bargmann CI. Odorant-specific adaptation pathways generate olfactory plasticity in C. elegans. Neuron. 1995;14:803–812. doi: 10.1016/0896-6273(95)90224-4. [DOI] [PubMed] [Google Scholar]
- 11.Cromarty SI, Derby CD. Multiple excitatory receptor types on individual olfactory neurons: implications for coding of mixtures in the spiny lobster. J Comp Physiol [A] 1997;180:481–491. doi: 10.1007/s003590050065. [DOI] [PubMed] [Google Scholar]
- 12.Dahl M, Erickson RP, Simon SA. Neural responses to bitter compounds in rats. Brain Res. 1997;756:22–34. doi: 10.1016/s0006-8993(97)00131-5. [DOI] [PubMed] [Google Scholar]
- 13.Dalton P. Psychophysical and behavioral characteristics of olfactory adaptation. Chem Senses. 2000;25:487–492. doi: 10.1093/chemse/25.4.487. [DOI] [PubMed] [Google Scholar]
- 14.Dalton P, Wysocki CJ, Brody MJ, Lawley HJ. Perceived odor, irritation, and health symptoms following short-term exposure to acetone. Am J Ind Med. 1997;31:558–569. doi: 10.1002/(sici)1097-0274(199705)31:5<558::aid-ajim10>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 15.Danilova V, Roberts T, Hellekant G. Responses of single taste fibers and whole chorda tympani and glossopharyngeal nerve in the domestic pig, Sus scrofa. Chem Senses. 1999;24:301–316. doi: 10.1093/chemse/24.3.301. [DOI] [PubMed] [Google Scholar]
- 16.Dawson TM, Arriza JL, Jaworsky DE, Borisy FF, Attramadal H, Lefkowitz RJ, Ronnet GV. β-Adrenergic receptor kinase-2 and β-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993;259:825–828. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- 17.de Boer G, Dethier VG, Schoonhoven LM. Chemoreceptors in the preoral cavity of the tobacco hornworm, Manduca sexta, and their possible function in feeding behavior. Entomol Exp Appl. 1977;21:287–298. [Google Scholar]
- 18.Dethier VG. The role of taste in food intake: a comparative view. In: Simon SA, Roper SD, editors. Mechanisms of taste transduction. CRC; Boca Raton, FL: 1993. pp. 3–25. [Google Scholar]
- 19.Falk JL, Yosef E, Schwartz A, Lau CE. Establishing oral preference for quinine, phencyclidine, and caffeine solutions in rats. Behav Pharmacol. 1999;10:27–38. doi: 10.1097/00008877-199902000-00003. [DOI] [PubMed] [Google Scholar]
- 20.Garcia J, Hankins WG. The evolution of bitter and the acquisition of toxiphobia. In: Denton DA, Coghlan JP, editors. Olfaction and taste. V. Proceedings of the 5th international symposium in Melbourne, Australia. Academic; New York: 1975. pp. 39–45. [Google Scholar]
- 21.Glendinning JI. Is the bitter rejection response always adaptive? Physiol Behav. 1994;56:1217–1227. doi: 10.1016/0031-9384(94)90369-7. [DOI] [PubMed] [Google Scholar]
- 22.Glendinning JI, Gonzalez NA. Gustatory habituation to deterrent compounds in a grasshopper: concentration and compound specificity. Anim Behav. 1995;50:915–927. [Google Scholar]
- 23.Glendinning JI, Hills TT. Electrophysiological evidence for two transduction pathways within a bitter-sensitive taste receptor. J Neurophysiol. 1997;78:734–745. doi: 10.1152/jn.1997.78.2.734. [DOI] [PubMed] [Google Scholar]
- 24.Glendinning JI, Valcic S, Timmermann BN. Maxillary palps can mediate taste rejection of plant allelochemicals by caterpillars. J Comp Physiol [A] 1998;183:35–44. doi: 10.1007/s003590050232. [DOI] [PubMed] [Google Scholar]
- 25.Glendinning JI, Tarre M, Asaoka K. Contribution of different bitter-sensitive taste cells to feeding inhibition in a caterpillar. Behav Neurosci. 1999a;113:840–854. [PubMed] [Google Scholar]
- 26.Glendinning JI, Ensslen S, Eisenberg ME, Weiskopf P. Diet-induced plasticity in the taste system of an insect: localization to a single transduction pathway in an identified taste cell. J Exp Biol. 1999b;202:2091–2102. doi: 10.1242/jeb.202.15.2091. [DOI] [PubMed] [Google Scholar]
- 27.Glendinning JI, Chaudhari N, Kinnamon SC. Taste transduction and molecular biology. In: Finger T, Silver WL, Restrepo D, editors. The neurobiology of taste and smell, Ed 2. Wiley-Liss; New York: 2000a. pp. 315–351. [Google Scholar]
- 28.Glendinning JI, Nelson N, Bernays EA. How do inositol and glucose modulate feeding in Manduca sexta caterpillars? J Exp Biol. 2000b;203:1299–1315. doi: 10.1242/jeb.203.8.1299. [DOI] [PubMed] [Google Scholar]
- 29.Gothilf S, Hanson FE. A technique for electrophysiologically recording from chemosensory organs of intact caterpillars. Entomol Exp Appl. 1994;72:304–310. [Google Scholar]
- 30.Harder DB, Maggio JC, Whitney G. Assessing gustatory detection capabilities using preference procedures. Chem Senses. 1989;14:547–564. [Google Scholar]
- 31.Harley KLS, Thorsteinson AJ. The influence of plant chemicals on the feeding behavior, development, and survival of the two-striped grasshopper, Melanoplus bivittatus (Say), Acrididae: Orthoptera. Can J Zool. 1967;45:305–319. [Google Scholar]
- 32.Karrer T, Bartoshuk L. Capsaicin desensitization and recovery on the human tongue. Physiol Behav. 1991;49:757–764. doi: 10.1016/0031-9384(91)90315-f. [DOI] [PubMed] [Google Scholar]
- 33.Kuznicki JT, Turner LS. The effects of caffeine on caffeine users and non-users. Physiol Behav. 1986;37:397–408. doi: 10.1016/0031-9384(86)90197-6. [DOI] [PubMed] [Google Scholar]
- 34.Leibrecht BC, Askew HR. Habituation from a comparative perspective. In: Denny MR, editor. Comparative psychology: an evolutionary analysis of animal behavior. Wiley; New York: 1980. pp. 208–229. [Google Scholar]
- 35.L'Etoile ND, Bargmann CI. Olfaction and odor discrimination are mediated by the C. elegans guanylyl cyclase ODR-1. Neuron. 2000;25:575–586. doi: 10.1016/s0896-6273(00)81061-2. [DOI] [PubMed] [Google Scholar]
- 36.Marion-Poll F, Van der Pers J. Un-filtered recordings from insect taste sensilla. Entomol Exp Appl. 1996;80:113–115. [Google Scholar]
- 37.McBurney DH, Smith DV, Shick TR. Gustatory cross adaptation: sourness and bitterness. Percept Psychophys. 1972;11:228–232. [Google Scholar]
- 38.Naim M, Kare MR, Ingle DE. Sensory factors which affect the acceptance of raw and heated defatted soybeans by rats. J Nutr. 1977;107:1653–1658. doi: 10.1093/jn/107.9.1653. [DOI] [PubMed] [Google Scholar]
- 39.Rössler P, Boekhoff I, Tareilus E, Beck S, Breer H, Freitag J. G protein β-γ complexes in circumvallate taste cells involved in bitter transduction. Chem Senses. 2000;25:413–421. doi: 10.1093/chemse/25.4.413. [DOI] [PubMed] [Google Scholar]
- 40.Schoonhoven LM. Sensitivity changes in some insect chemoreceptors and their effect on food selection behavior. Proc Koninkl Ned Akad Wetensh (Amsterdam) C. 1969;72:491–498. [Google Scholar]
- 41.Schoonhoven LM. On the variability of chemosensory information. Symp Biol Hung. 1976;16:261–266. [Google Scholar]
- 42.Sclafani A. Conditioned food preferences. Bull Psychon Soc. 1991;29:256–260. [Google Scholar]
- 43.Simmonds MSJ, Blaney WM. Some neurophysiological effects of azadirachtin on lepidopterous larvae and their feeding response. In: Schmutterer H, Ascher KRS, editors. Proceedings of the Second International Neem Conference. Deutsche Gesellschaft für Technische Zusammenarbeit (GTZ); Eschborn: 1983. pp. 163–180. [Google Scholar]
- 44.Szentesi A, Bernays EA. A study of behavioural habituation to a feeding deterrent in nymphs of Schistocerca gregaria. Physiol Entomol. 1984;9:329–340. [Google Scholar]
- 45.Thompson RF, Spencer WA. Habituation: a model phenomenon for the study of neuronal substrates of behavior. Psychol Rev. 1966;73:16–43. doi: 10.1037/h0022681. [DOI] [PubMed] [Google Scholar]
- 46.Usher BF, Bernays EA, Barbehenn RV. Antifeedant tests with larvae of Pseudaletia unipuncta: variability of behavioral response. Entomol Exp Appl. 1988;48:203–212. [Google Scholar]
- 47.van Loon JJA, Schoonhoven LM. Specialist deterrent chemoreceptors enable Pieris caterpillars to discriminate between chemically different deterrents. Entomol Exp Appl. 1999;91:29–35. [Google Scholar]
- 48.Warren RP, Pfaffman C. Early experience and taste aversion. J Comp Physiol Psychol. 1959;52:263–266. doi: 10.1037/h0047655. [DOI] [PubMed] [Google Scholar]
- 49.Wysocki CJ, Dalton P, Brody MJ, Lawley HJ. Acetone odor and irritation thresholds obtained from acetone-exposed factory workers and from control (occupationally unexposed) subjects. Am Ind Hyg Assoc J. 1997;58:704–712. doi: 10.1080/15428119791012342. [DOI] [PubMed] [Google Scholar]
- 50.Zellner DA, Berridge KC, Grill HJ, Ternes JW. Rats learn to like the taste of morphine. Behav Neurosci. 1985;99:290–300. doi: 10.1037//0735-7044.99.2.290. [DOI] [PubMed] [Google Scholar]