Abstract
Modulatory interneurons that can drive central pattern generators (CPGs) are considered as good candidates for decision-making roles in rhythmic behaviors. Although the mechanisms by which such neurons activate their target CPGs are known in detail in many systems, their role in the sensory activation of CPG-driven behaviors is poorly understood. In the feeding system of the molluscLymnaea, one of the best-studied rhythmical networks, intracellular stimulation of either of two types of neuron, the cerebral ventral 1a (CV1a) and the slow oscillator (SO) cells, leads to robust CPG-driven fictive feeding patterns, suggesting that they might make an important contribution to natural food-activated behavior. In this paper we investigated this contribution using a lip–CNS preparation in which feeding was elicited with a natural chemostimulant rather than intracellular stimulation. We found that despite their CPG-driving capabilities, neither CV1a nor SO were involved in the initial activation of sucrose-evoked fictive feeding, whereas a CPG interneuron, N1M, was active first in almost all preparations. Instead, the two interneurons play important and distinct roles in determining the characteristics of the rhythmic motor output; CV1a by modulating motoneuron burst duration and SO by setting the frequency of the ongoing rhythm. This is an example of a distributed system in which (1) interneurons that drive similar motor patterns when activated artificially contribute differently to the shaping of the motor output when it is evoked by the relevant sensory input, and (2) a CPG rather than a modulatory interneuron type plays the most critical role in initiation of sensory-evoked rhythmic activity.
Keywords: sensory-activated motor pattern, feeding, CPG, command-like neuron, mollusc, Lymnaea
Control of neuronal central pattern generators (CPGs) by modulatory interneurons appears to be an important common requirement in both invertebrates and vertebrates for optimizing CPG output to meet specific behavioral demands (Katz, 1995; Grillner et al., 1997; Selverston et al., 1997; Büschges and Manira, 1998;Kupfermann, 1998). Although intracellular stimulation and suppression experiments in isolated neuronal circuits have been very successful in revealing how individual modulatory neurons can activate rhythmic motor patterns or reconfigure neuronal networks (Marder and Calabrese, 1996), the role such neurons play in the natural activation of behavior is still poorly understood. A fuller understanding of this role can only be achieved by systematically analyzing the function of identified modulatory interneurons in the control of their target CPGs during sensory-activated motor patterns. Here we performed such an analysis in one of the best understood CPG-driven networks, the feeding system of the pond snail Lymnaea stagnalis.
Intracellular stimulation, suppression, and photoinactivation experiments already have previously suggested that theLymnaea feeding system has a distributed organization for motor pattern generation at both the interneuronal (Elliott and Benjamin, 1985a; McCrohan and Kyriakides, 1989; Kemenes and Elliott, 1994; Yeoman et al., 1995; Brierley et al., 1997; Vehovszky and Elliott, 2000) and motoneuronal levels (Staras et al., 1998a). An apparent inconsistency between this distributed organization and an earlier hierarchical model for sensory activation of feeding, based on cell stimulation experiments in isolated nervous systems (Benjamin, 1983), could only be resolved in semi-intact preparations in which natural feeding stimuli could be applied and neuronal responses could be recorded. Unlike many other distributed control systems in which it is difficult to experimentally address the problem of sensory-driven generation of behavior at the cellular level, the Lymnaeafeeding system offers an ideal experimental model for this type of investigation for two main reasons. First, semi-intact preparations already have been developed in which electrophysiological fictive feeding rhythms can be evoked by chemosensory stimuli applied to the lips (Kemenes et al., 1986; Staras et al., 1998b). Second, the feeding system is known in cellular detail (Benjamin et al., 2000), allowing the effect of sensory inputs to be studied simultaneously on modulatory neurons, CPG neurons, and motoneurons.
In Lymnaea, only two uniquely identifiable non-CPG cell types, the paired cerebral ventral 1a (CV1a) neurons and the single slow oscillator (SO), can drive fast rhythmic CPG activity, approaching the frequency of behavioral feeding, when activated intracellularly (Benjamin and Elliott, 1989). Therefore, these two cells, together with a CPG neuron, N1M, and motoneurons, were the targets for our investigations. We show here that despite their rhythm-driving capabilities, CV1a and SO are not necessary for the initial activation of fictive feeding by chemosensory inputs. Instead, these two cell types each control specific aspects of the fictive feeding pattern once it is activated by food; CV1a as a modulator of motoneuron burst duration and SO as a modulator of the frequency of the rhythm. In contrast, we demonstrate a pivotal role for the CPG neuron N1M in the decision-making process underlying feeding.
MATERIALS AND METHODS
Experimental animals, dissection procedures, and preparations. Wild-type specimens of adult Lymnaea stagnalis were obtained from animal suppliers (Blades Biological, Kent, UK). Animals were kept in groups in large holding tanks containing copper-free water at 18–20°C on a 12 hr light/dark regime and fed lettuce three times a week.
To produce semi-intact preparations for electrophysiological experiments, animals were dissected under a microscope in a dish containing HEPES-buffered snail saline (Benjamin and Winlow, 1981). The preparations used in these experiments consisted of the lips and the CNS and were described in detail in previous papers (Kemenes et al., 1986, 1997; Staras et al., 1998b, 1999a,b). After dissection, the preparations were transferred to a silicon elastomer (Sylgard)-lined electrophysiology chamber containing saline and pinned dorsal-side up. The outer ganglionic sheath of the cerebral and buccal ganglia was removed using a pair of fine forceps and the second, the inner sheath, was softened using a nonspecific solid protease (Sigma XIV; Sigma, Poole, UK).
Selection and identification of cell types for intracellular recording. The aim of the present electrophysiological experiments was to simultaneously monitor neuronal activity in previously identified motor, CPG, and modulatory neurons of the feeding system ofLymnaea (Fig.1A) while applying a food stimulus to the lips and/or manipulating interneuronal firing in reduced preparations. All the interneuron and motoneuron types recorded in these experiments were identified by their characteristic position, size, and color as well as by their firing patterns and connections to other cells in the feeding network (for recent overviews of the feeding system, see Brierley et al., 1997; Kemenes, 1997; Staras et al., 1998a;Benjamin et al., 2000). The rhythmic neuronal activity known to underlie feeding in intact animals is called fictive feeding, and it is generated by a set of premotor CPG interneurons (Rose and Benjamin 1981b; Elliott and Benjamin 1985a). These neurons belong to three main types, N1, N2, and N3, each active in one of the three behavioral phases of feeding, protraction (N1), rasp (N2), and swallow (N3). Fictive feeding was monitored directly by recording from one of the paired N1M protraction-phase CPG interneurons and/or indirectly by recording from identified modulatory interneurons and motoneurons (Fig.1A), which receive well characterized synaptic inputs from the CPG during each phase of fictive feeding (Benjamin and Elliott, 1989).
Fig. 1.
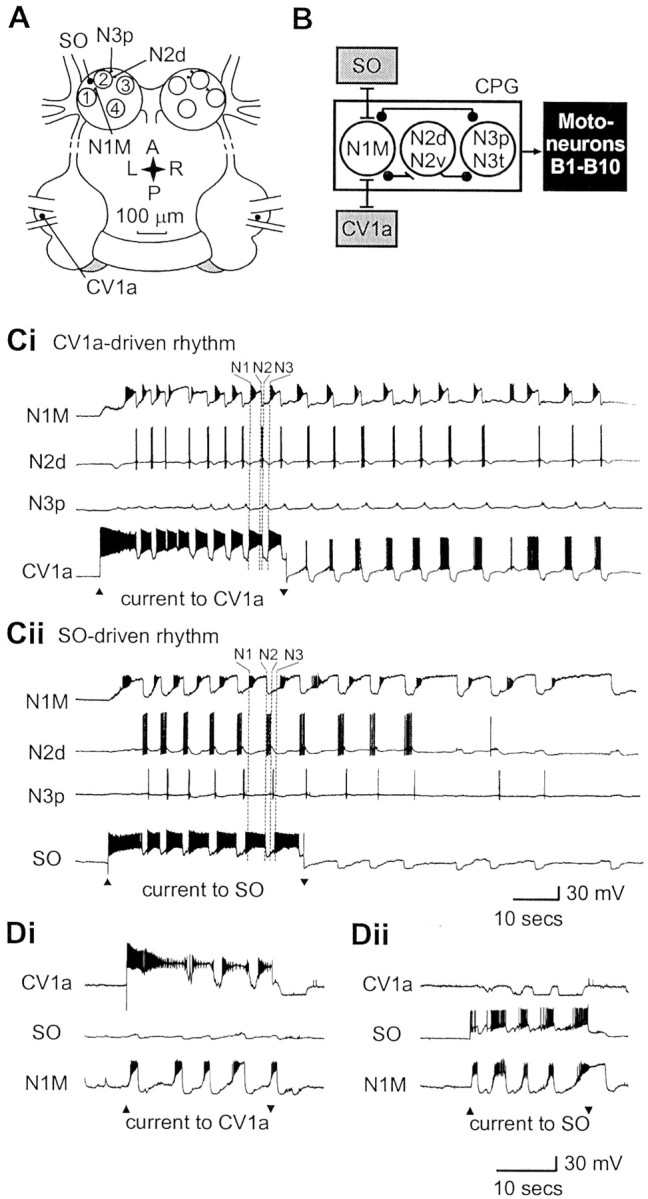
Evidence for a command-like function for two interneurons in the Lymnaea feeding CPG.A, Location of neurons of the feeding network that were recorded in the present study. Modulatory interneurons:CV1a, cerebral ventral 1a; SO, slow oscillator. CPG interneurons: N1M, medial N1;N2d, dorsal N2; N3p, N3 phasic. Motoneurons: B3 and B4 are shown as numbered circles. B1 and B2 are only shown as landmarks. B, Synaptic connections between SO and N1M and CV1a and N1M and a summary of CPG connectivity based on published results (Elliott and Benjamin, 1985a,b;McCrohan and Kyriakides, 1989; Brierley et al., 1997). For N2 and N3 types, which were not considered in detail in these experiments, only a generalized representation of their connectivity is shown here for clarity. The N1L (lateral N1), a hybrid CPG–modulatory neuron (Yeoman et al., 1995), is not shown here. Bar, Excitatory connection; filled circle, inhibitory connection.Ci, Cii, The CV1a and SO neurons, when activated by steady depolarizing current injection, drive the same set of CPG interneurons (N1M, N2d, N3p) to produce a fictive feeding rhythm consisting of cycles of protraction (N1), rasp (N2), and swallow (N3) phase activity. Di, Dii, When activated by steady depolarizing current, both CV1a and SO independently drive the N1M CPG neuron to produce rhythmical activity without activating one another.Ci and Cii and Di andDii, respectively, are from the same preparations.
The two phasic modulatory interneuron types recorded in these experiments were the paired CV1a cells (McCrohan, 1984; McCrohan and Kyriakides, 1989) in the cerebral ganglia and the single SO cell (Rose and Benjamin, 1981a) in the buccal ganglia (Fig. 1A). Although depolarization of another cerebral interneuron type, the CV1b cell, can also lead to activation of slow fictive feeding (McCrohan and Kyriakides, 1989), CV1a and SO are the only non-CPG interneurons that can initiate fast fictive feeding patterns in isolated brain preparations when activated by intracellular current injection (CV1a:McCrohan, 1984; McCrohan and Kyriakides, 1989; SO: Rose and Benjamin, 1981a; Elliott and Benjamin, 1985b). Both CV1a and SO are known to drive fictive feeding by activating the same set of CPG interneurons (Fig. 1B). In the same preparation, depolarization of either CV1a (Fig. 1Ci) or SO (Fig. 1Cii) activated exactly the same individual interneurons belonging to the three main types of CPG interneuron, N1, N2, and N3. Both CV1a and SO are known to generate 1:1 EPSPs in the N1M, leading to spike activity, and this is thought to be the main mechanism by which they drive theLymnaea feeding CPG (Rose and Benjamin, 1981a; Elliott and Benjamin, 1985b; McCrohan and Kyriakides, 1989). The N2d and N3p, two types of retraction and swallow phase CPG interneurons, are subsequently activated in the feeding cycle by synaptic connections between N1M and the other CPG interneurons (Fig. 1B) (for review, see Brierley et al., 1997). Previous work using isolated brains showed that there were no direct synaptic connections between CV1a and SO (McCrohan, 1984), and the main mechanism by which they drove fictive feeding was via direct but independent pathways to the N1M (Rose and Benjamin, 1981a; Elliott and Benjamin, 1985b; McCrohan and Kyriakides, 1989) (Fig. 1B,D). Both SO and CV1a are independently capable of driving a fictive feeding rhythm in an N1M cell without spiking activity in the alternative cell type (Fig.1Di,Dii). The CV1a and SO, unlike CV1b, show strong rhythmic activity during ongoing fictive feeding, phase-locked to activity in the CPG network. Thus, both cells are excited during the N1 (protraction) phase but strongly inhibited during N2 (rasp) and less strongly inhibited during N3 (swallow) (Elliott and Benjamin, 1985b;McCrohan and Kyriakides, 1989; Yeoman et al., 1995) (N1, N2, N3 phases in one fictive feeding cycle in CV1a and SO activity are marked in Fig.1, Ci and Cii, respectively).
Of the two different types of motoneurons used to monitor sucrose-evoked feeding motor output in the present experiments, the B3 cell is inhibited during N1 but excited during N2 and N3, and the B4 cells are inhibited during N1 and the first phase of N2 but excited during the second phase of N2 and during N3 (Rose and Benjamin, 1979;1981a,b). In suppression experiments the B3 motoneuron was used as a monitor of the effect of suppressing activity in CV1a/SO and N1M on the final motor output of the feeding system. This motoneuron type shows reliable bursting activity during fictive feeding cycles in semi-intact preparations and is therefore suitable for statistical analyses of fictive feeding activity (Staras et al., 1999a).
The intracellular recording and stimulation techniques used in these experiments were described in detail in previous papers (Kemenes et al., 1997; Staras et al., 1998b, 1999a,b). In the suppression experiments, hyperpolarization of interneurons was achieved by passing current through the recording electrode and this, together with the fact that recording from the small CV1a and N1M interneurons required the use of sharp electrodes, often resulted in bridge imbalance in the hyperpolarized CV1a and N1M traces. These traces were often outside the recording range of our recording device, a DAT recorder (Biological DTR 1801; Biological Science Instruments, Claix, France). However, the traces were also monitored on an oscilloscope (Gould 1604; Gould Instrument Systems, Hainault, UK) with a much wider display range than those of the DAT recorder and at a lower gain, and this allowed us to establish the minimum level of hyperpolarization required for suppression of spike generation in the cells. This method of monitoring of the effect of hyperpolarization through a single electrode on the spike generation of neurons of the Lymnaea feeding system was described in a previous paper (Perry et al., 1998).
Chemical stimulation of the lips in reduced preparations. In the reduced preparations, we applied the same chemosensory food stimulus that had been shown to evoke the strongest feeding responses in intact animals (Kemenes et al., 1986; Staras et al., 1998b). The chemostimulant, sucrose solution at 0.01 mconcentration, was released from the end of a thin plastic tube and diffused passively across the lip chemosensory structures. In this way the tactile component of sucrose application was minimized. The sucrose solution was completely removed from the lips within 2 min by rapid perfusion with fresh saline.
RESULTS
CV1a and SO are not involved in the initial activation of fictive feeding by sucrose
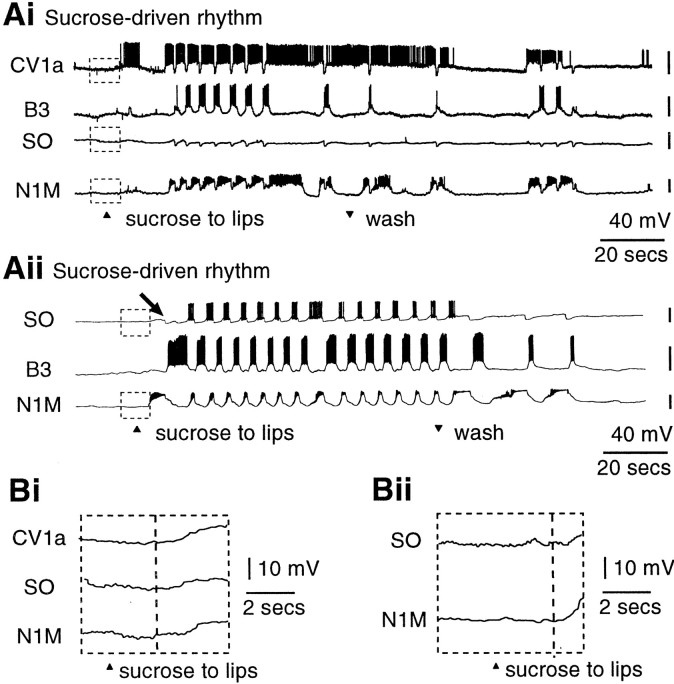
To examine whether CV1a and SO were important for the normal chemosensory activation of feeding, sucrose was applied to the lips of semi-intact preparations. The N1M protraction-phase CPG interneuron was always recorded as a direct monitor of CPG activation with various combinations of CV1a, SO, and B3 or B4 motoneurons. In preparations in which sucrose was effective in driving a fictive feeding rhythm (n = 32), fictive feeding was usually maintained for as long as the chemostimulant was applied (Figs.2Aii,Bii, 3Ai,Aii), and feeding bursts often continued for several cycles beyond the end of sucrose application (Figs. 2Bii, 3Ai,Aii). The phases of activity and synaptic inputs in sucrose-evoked fictive feeding rhythms (Fig. 2Aii,Bii) resembled those in CV1a or SO-driven patterns in the same preparations (Fig.2Ai,Bi) (Elliott and Benjamin, 1985a,b; Yeoman et al., 1995; Brierley et al., 1997).
Fig. 2.
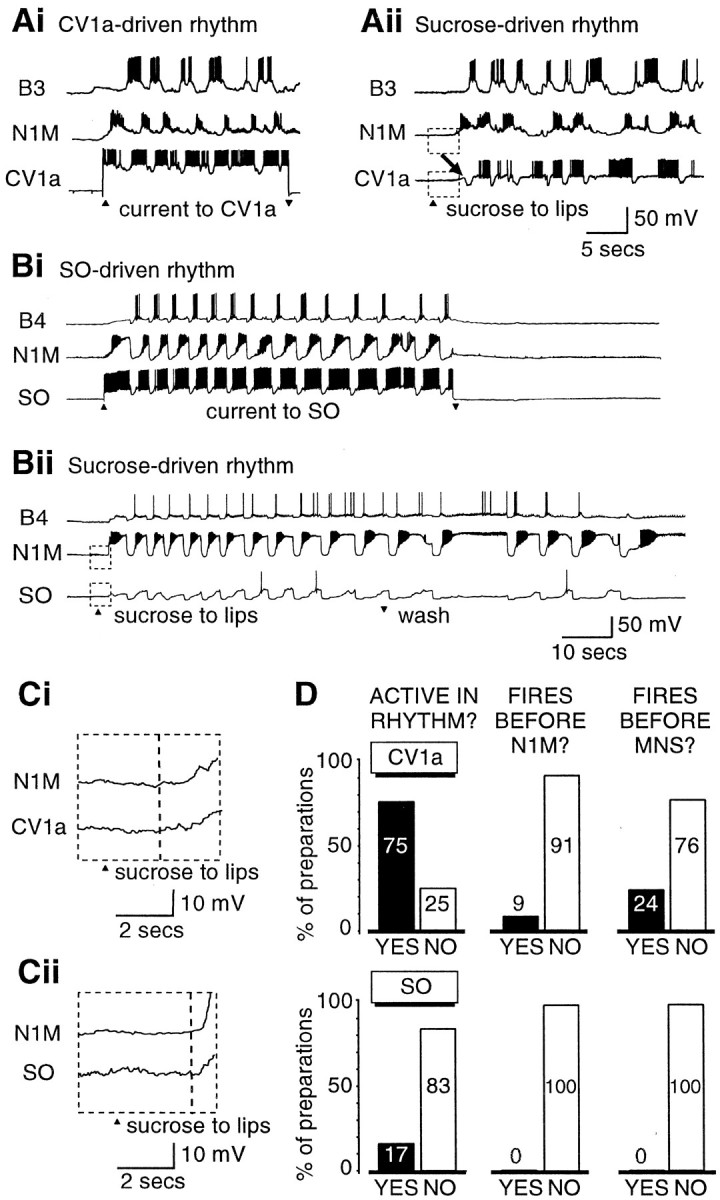
Activity in the CV1a and SO cells is not essential for the chemosensory activation of fictive feeding. Ai, Activation of CV1a by current injection drives a full fictive feeding rhythm in the N1M CPG interneuron and the B3 motoneuron.Aii, Sucrose applied to the lips in the same semi-intact preparation leads to rhythmical activation of the CV1a, but only after a full cycle of bursting has already occurred in N1M and B3, during which CV1a only shows subthreshold CPG inputs (arrow).Bi, Like the CV1a, the SO cell is similarly capable of driving a full feeding rhythm. Bii, In the same preparation, however, sucrose evokes a feeding rhythm without rhythmical activation of the SO. Ci, Details of initial chemosensory inputs on N1M and CV1a on an expanded time and voltage scale (Aii, boxed area). Cii,Boxed areas of Bii shown in one box on an expanded time and voltage scale. D, Summary histograms comparing the percentage of preparations in which the modulatory neurons CV1a or SO were rhythmically bursting (black bars) or not (white bars) in a sucrose-driven fictive feeding rhythm. The order of firing relative to N1M and motoneurons is also shown (black bar, cell fires before N1M/motoneurons; white bar, cell fires after N1M/motoneurons).
The first important issue we examined was whether CV1a or SO were consistently active in sucrose-driven rhythms. When injected with intracellular current, CV1a could drive a fictive feeding rhythm (Fig.2Ai) and, as would be predicted from this and its previously proposed putative role as a command-like neuron (Benjamin, 1983), it was also active in and phase-locked to a sucrose-driven fictive feeding rhythm in the same preparation (Fig.2Aii). In contrast, the SO, the other proposed putative command-like neuron (Benjamin, 1983), behaved very differently to CV1a. This is illustrated in Figure 2B, which shows an example of an experiment in which SO, N1M, and a motoneuron (B4) were recorded together in the same preparation. As expected, the SO was capable of driving fictive feeding when injected with a depolarizing current (Fig. 2Bi). In the same preparation when fictive feeding was activated by sucrose, the SO did not show rhythmic bursting activity (Fig. 2Bii), although it did fire occasional spikes in some of the fictive feeding cycles. However, this was unlikely to have contributed significantly to rhythm generation because it has been shown previously that a series of facilitating spike-initiated EPSPs on the N1M cells are necessary for the SO to influence the CPG rhythm (Elliott and Benjamin, 1985b).
The consistency of chemosensory activation of CV1a and SO, relative to N1M, was assessed quantitatively by an overall analysis of the 32 preparations in which sucrose-evoked fictive feeding was seen. As predicted from the previously described key role for N1M in pattern generation in the isolated CNS (Kemenes and Elliott, 1994), this protraction phase CPG interneuron always fired in response to sucrose applied to the lips (100%; n = 32). The CV1a was active in the majority of the experiments (75%; n = 12 from 16 preparations in which it was recorded) (Fig.2D). In contrast, in only the minority of preparations (17%; n = 3 from the 18 preparations in which it was recorded) did the SO (Fig. 2D) fire regular bursts (Fig. 3Aii), although in all the preparations it was receiving characteristic CPG-driven inputs in each phase of the fictive feeding cycles (Figs. 2Bii, 3Ai). The proportion of cells firing in a sucrose-driven rhythm was significantly greater for the CV1a cells (12 of 16) than for the SO cells (3 of 15) (p ≪ 0.01).
Fig. 3.
Chemosensory activation of CV1a and SO cells.Ai, In this preparation, CV1a fired a burst of spikes before N1M in response to sucrose presentation. The SO neuron receives only subthreshold CPG inputs during the fictive feeding pattern.Aii, An example of one of the three preparations in which the SO was activated by sucrose. The SO fires after a single full cycle in N1M and the motoneuron B3, producing a robust fictive feeding rhythm (compare to Ai). Arrow points at the series of subthreshold CPG inputs on SO preceding its activation.Bi, Bii, Details of initial chemosensory inputs onto the interneurons. Expanded boxed portions ofAi and Aii, respectively.
Even when the CV1a or the SO cells were active in a sucrose-driven rhythm, their activity was almost always preceded by spike activity in the N1M at the beginning of the pattern [CV1a, 10 of 11 (91%); SO, 3 of 3 (100%)] (Fig. 2D). The first sucrose-triggered burst of spikes in N1M occurred within 5 sec of the beginning of sucrose application to the lips (Figs. 2Aii,Bii, 3Aii), with the exception of the one preparation in which it fired after CV1a (Fig. 3Ai). The motoneurons also usually started firing before CV1a/SO in sugar-driven rhythms [CV1a, 10 of 13 (76%); SO, 3 of 3 (100%)] (Fig. 2D).
Activity in motoneurons started immediately after N1M activity (Figs.2Aii,Bii, 3Ai,Aii). In fact, a sequence of synaptic inputs that are known to arise from the N1, N2, and N3 cells (Elliott and Benjamin, 1985a; Brierley et al., 1997) occur on CV1a and SO before they start to fire (Figs. 2Aii, 3Aii, arrows), indicating that a cycle of CPG activity has occurred before they fire their first burst. Importantly, the initial subthreshold excitatory chemosensory inputs appeared to reach all the interneuron types at approximately the same time indicated by a depolarizing waveform shared by the N1M, CV1a, and SO cells (Figs.2Ci,Cii, 3Bi,Bii). This initial depolarization occurred within the first 2–4 sec after the application of sucrose in all preparations, but its rate and peak amplitude were highly variable both within and between preparations, making a quantitative analysis of its contribution to firing in the cells difficult. However, of the three cell types the N1M CPG cells appeared to be the most excitable by chemosensory inputs. Unlike CV1a and SO, the N1Ms always fired in response to these inputs, and they were the first of the three cell types to reach firing threshold in almost all preparations (31 of 32).
From these experiments it was clear that rhythmic activity can be initiated in the whole CPG without any spike activity occurring in the CV1a or SO cells. Sucrose-driven rhythms were possible without activation of either of the previously proposed command-like cell types (Benjamin, 1983), and this was particularly true in the case of the SO.
CV1a and SO have distinct alternative roles in sucrose-evoked CPG-driven fictive feeding
In preparations in which CV1a or SO were active in sucrose-driven rhythms, it was possible to show that they could modulate various features of the feeding program once it was activated by a natural chemosensory stimulus.
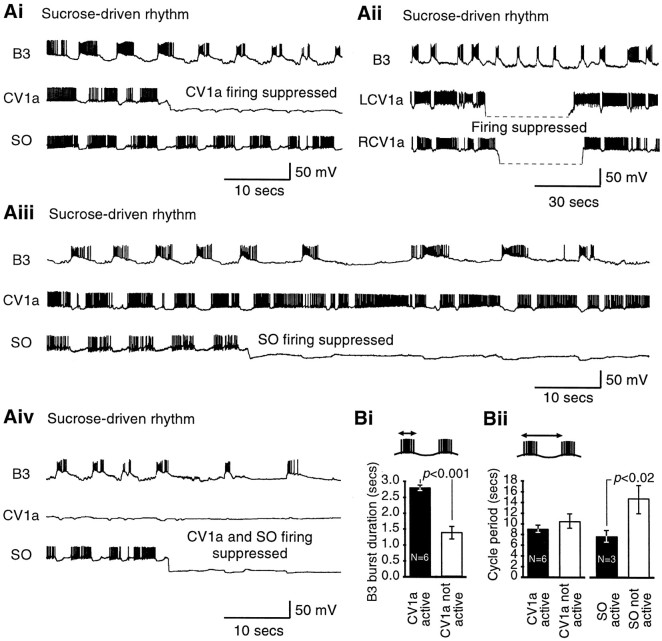
Suppression of firing in either one or both CV1a neurons (n = 6 and 3 preparations, respectively) or the SO (n = 3 preparations) or both a CV1a and an SO together (n = 2 preparations) never simply resulted in the cessation of fictive feeding (Fig. 4). Instead, specific changes in the detailed features of the feeding motor pattern occurred, which were monitored on motoneuron B3 in all these preparations.
Fig. 4.
CV1a and SO modulate the ongoing chemosensory-activated rhythm. A, Effect of suppression of CV1a and SO on sucrose-activated rhythms. Ai, Suppression of a single CV1a cell reduces the burst duration of B3 but does not influence the frequency of the rhythm. Aii, Left and right CV1a cells fire together in a sucrose-driven rhythm, and suppression of both CV1a cells has the same effect as suppression of a single CV1a cell (Ai). In this record the hyperpolarized CV1a traces could not be bridge-balanced and were outside the recording range. Aiii, Even prolonged suppression of SO only slows but does not stop the rhythm. Aiv, Suppression of CV1a and SO together reduces the B3 burst duration and the cycle frequency, although CPG-driven fictive feeding cycles still occur. Ai, Aiii, and Aiv are recordings from one experiment (the trace in Aiv is an overlapping continuation of the trace in Ai), and Aii is a recording from a different experiment. B, Quantitative analysis of CV1a and SO suppression experiments. Bi, Histogram of B3 burst duration (see schematic) for four cycles before and after suppression of CV1a in ongoing sucrose-driven rhythms.Bii, Histogram of cycle period (see schematic) for four cycles before and after suppression of CV1a (left panel) or SO (right panel) in ongoing rhythms. Histograms show mean ± SE. pvalues are results of paired t tests (see Results).
If in an ongoing sucrose-activated fictive feeding rhythm spike activity in a single CV1a was suppressed by the injection of a steady hyperpolarizing current (Fig. 4Ai) while the SO continued to fire, bursts in the B3 motoneuron became shorter in duration without any change in the frequency of the rhythm. Left and right CV1a neurons fired together in sucrose-activated rhythms (n = 3 preparations). In these preparations suppressing both CV1a neurons (Fig. 4Aii) had the same effect as suppressing a single CV1a in the same (data not shown) or in other preparations (n = 6) (Fig.4Ai), namely a shortening of B3 burst duration without a decrease in cycle period. A quantitative analysis of single CV1a suppression experiments (Fig. 4Bi) showed that the mean B3 burst duration was 2.8 sec (±0.1 SE) during CV1a firing, but it dropped significantly, to 1.4 ± 0.2 sec, after firing had been suppressed (n = 6; paired t test;p < 0.001). However, the mean period of the fictive feeding cycles before (9.0 ± 0.8 sec) and after (10.5 ± 1.4 sec) CV1a spike suppression (Fig. 4Bii) was not significantly different (p = 0.07).
In contrast to CV1a (Fig. 4Ai), the alternative suppression of firing in SO in the same preparation resulted in a slowing of the rhythm, which however was still present during even a long (lasting in excess of a minute) suppression of this cell (Fig.4Aiii). A quantitative analysis (Fig.4Bii) showed that this caused a significant increase in the cycle period (from 7.7 ± 1.1 to 14.7 ± 2.6 sec;n = 3 preparations; paired t test;p < 0.02).
When firing in both CV1a and SO were suppressed (Fig.4Aiv, from the same experiment as in Aiand Aiii), the B3 burst duration and the frequency of the rhythm were reduced (for a comparison, see initial portions of Fig. 4,Ai and Aiii), but nevertheless CPG-driven fictive feeding bursts still occurred.
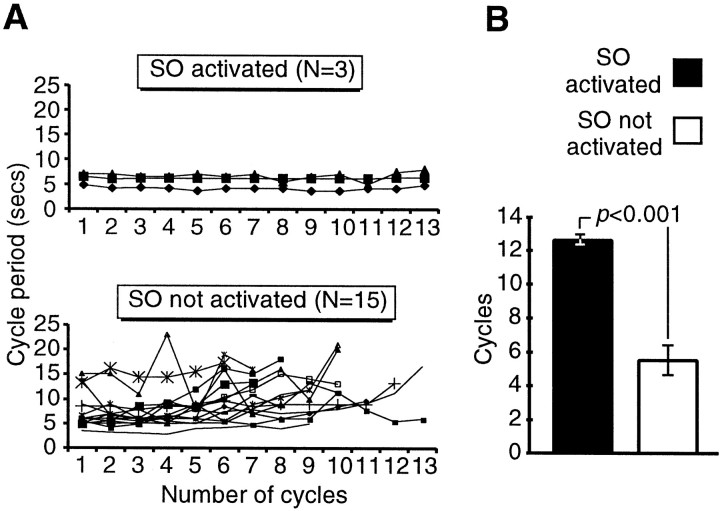
The results of a further type of analysis, involving the SO, were consistent with the notion that this cell is important in maintaining the long-term frequency of the feeding pattern. Plotting the cycle period for successive cycles of fictive feeding with (n= 3 preparations) or without (n = 15 preparations) SO firing showed that the fictive feeding rhythm was more constant over a 100 sec period after sucrose application with SO activation than without (Fig. 5A). A quantitative analysis (Fig. 5B) showed that although the mean initial cycle period was not significantly different between the two types of preparation (5.9 ± 0.7 vs 7.5 ± 0.9 sec; unpaired t test; p = 0.4), it was maintained through significantly more successive cycles in preparations in which the SO was activated by sucrose (12.7 ± 0.3 cycles) (Fig.3Aii) than in preparations in which it was not (5.5 ± 2.1 cycles) (Fig. 2Bii) (unpaired t test;p ≪ 0.001).
Fig. 5.
Spike activity in SO during chemosensory-activated fictive feeding helps to maintain a long-term, high-frequency rhythm.A, Top, Cycle period for 13 successive sucrose-activated fictive feeding cycles in which the SO was active. The period remains largely constant through all 13 cycles of CPG activity. A, Bottom, Cycle period for sucrose-activated fictive feeding rhythms in which the SO was not active. The period was highly variable with the most marked increases after between three and eight CPG feeding cycles. B, Histogram summary showing the number of subsequent cycles with cycle periods not exceeding the averaged period of the first two cycles of activity (initial cycle period). Data are shown for sucrose-driven preparations in which the SO was active (black bar) versus those in which SO was not active (white bar). Histograms show mean ± SE. p value shows result of unpaired t test (see Results).
The N1M CPG interneuron is necessary for sucrose-activated fictive feeding
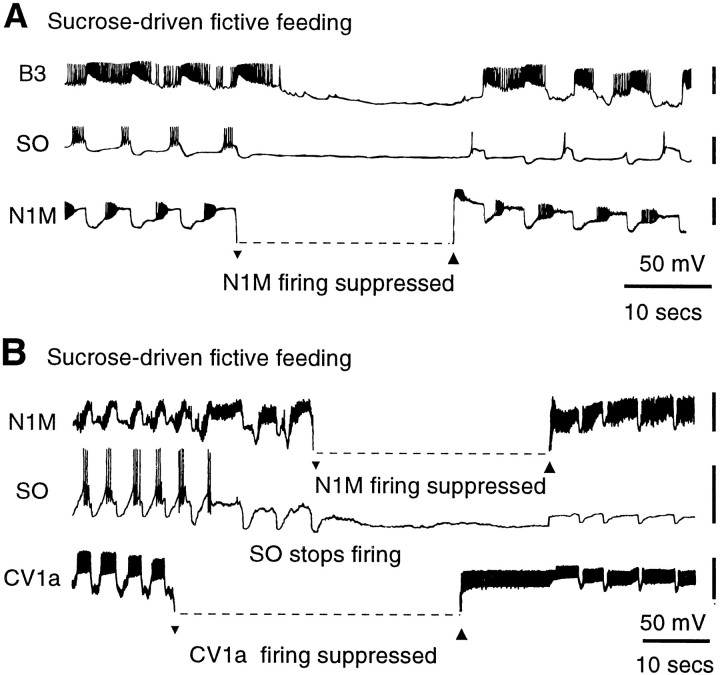
In contrast to the CV1a and SO cells, removal of N1M activity by hyperpolarization immediately resulted in a cessation of sucrose-driven fictive feeding in all semi-intact preparations in which this was performed (n = 12). This is illustrated in the experiment shown in Figure6A, in which suppression of N1M spike activity effectively stopped fictive feeding activity in both the B3 motoneuron and the SO recorded at the same time. When N1M was released from suppression, it could drive a rhythm, although the SO cell was only firing occasional single spikes. Figure6B shows another experiment in which the effects of N1M, SO, and CV1a suppression were examined in the same preparation. As expected, suppression of firing in CV1a did not lead to cessation of fictive feeding. The SO also stopped firing during the sucrose-driven rhythm. This was unlikely to be a direct effect of CV1a suppression because it only occurred two cycles after CV1a hyperpolarization. When both the SO and CV1a were silent, the feeding rhythm, monitored directly on the N1M, still continued, although at a lower rate (Fig.4Aiv). In contrast, suppression of firing in N1M led to an abrupt cessation of all fictive feeding activity. When CV1a was allowed to fire again for a brief period, the pattern was still absent and only recommenced when N1M was allowed to fire again.
Fig. 6.
Activity in individual N1M CPG interneurons is necessary for maintained chemosensory-activated fictive feeding patterns. A, Simultaneous recording of an N1M, the SO, and a B3 motoneuron during a sucrose-evoked fictive feeding rhythm. Suppression of N1M using a maintained hyperpolarizing current leads to complete cessation of fictive feeding cycles. Repolarization of N1M immediately restores CPG-driven rhythmic activity. B, Testing the relative contributions of N1M, SO, and CV1a during sucrose-evoked fictive feeding. Neither suppression of CV1a by hyperpolarizing current nor spontaneous cessation of spike activity in the SO two cycles later results in a cessation of CPG-driven fictive feeding. However, subsequent suppression of N1M causes an abrupt termination of fictive feeding, as seen by the lack of inhibitory N2 cycles on the SO. Repolarization of CV1a does not lead to fictive feeding cycles, but the release of N1M from inhibition fully restores CPG-driven rhythmic activity. In the records shown in this figure the hyperpolarized N1M and CV1a traces could not be bridge-balanced and were outside the recording range.
The two CV1a neurons are not coupled to each other or any other known interneurons of the feeding system, and although the paired protraction-phase N1M interneurons are coupled to each other (Kemenes and Elliott, 1994), they are not coupled to other interneurons. The effects of hyperpolarization of these two cell types were likely to be limited to just one or very few cells of the same type, and therefore the effects seen on the fictive feeding pattern were likely to be specific for the suppression of N1M and CV1a, respectively. However, it could not be ruled out entirely that suppression of these cells also affected the feeding system more indirectly through as yet unidentified interneurons that might be electrotonically coupled to CV1a/N1M.
DISCUSSION
We analyzed the cellular processes leading to the activation of a rhythmic motor pattern by a natural sensory stimulus in a model experimental system. We demonstrated that two interneuron types, the CV1a and SO cells, which are capable of driving the Lymnaeafeeding CPG when activated intracellularly, were not necessary for the initiation of CPG-driven fictive feeding by chemosensory input. In contrast, a CPG interneuron, N1M, was a critical component of the decision-making process leading to the initiation of fictive feeding.
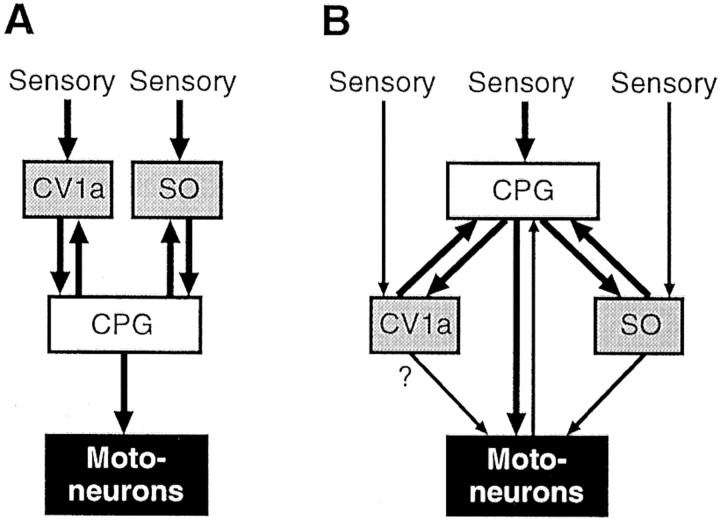
A model of the chemosensory activation of feeding behavior inLymnaea based on the results of present work is shown in Figure 7B, and it is compared with a previous, hierarchical, activation model based on intracellular stimulation experiments in isolated CNS preparations (Fig.7A) (Benjamin, 1983). In our new model the N1M CPG neurons can be regarded as the primary central decision-making cells in the feeding network because they show the most consistent short-latency phasic activation by lip chemosensory inputs. Also, they contribute to rhythm generation in the entire CPG network through their endogenous bursting properties, providing synaptic inputs to other CPG neurons and feedback to CV1a and SO and entraining them to the feeding rhythm (Benjamin and Elliott, 1989). Despite this, N1M cells alone can only drive slow fictive feeding rhythms even when directly activated by intracellular depolarization (Elliott and Benjamin, 1985b). This clearly shows that activity in N1M cells is not sufficient to support the fast and regular feeding rhythms measured in intact animals (Kemenes et al., 1986; Jansen et al., 1999). Activation of modulatory neurons such as CV1a/SO increases the robustness of the primarily N1M-driven CPG activity, resulting in a faster and more regular rhythm. Intact animals show an initial slow followed by a subsequent fast sucrose-evoked feeding rhythm (Tuersley and McCrohan, 1987). Prolonged slow feeding can be evoked by suboptimal concentrations of sucrose (Kemenes et al., 1986). It is plausible that slower rhythms are driven by the N1Ms alone but, in addition to modulating fast feeding, CV1a may also be involved in slow feeding evoked by suboptimal stimuli. The reciprocal excitatory N1M–SO/CV1a connections (Figs.1B, 7B), together with the convergent excitation by the chemosensory input (Figs. 2Ci,Cii, 3Bi,Bii, 7B), are likely to contribute significantly to a more regular and higher frequency feeding rhythm. In addition, SO provides inputs to N2 (rasp-phase) CPG interneurons (Elliott and Benjamin, 1985b), and this further contributes to the maintenance of a prolonged CPG-driven rhythm. Unlike SO, CV1a is only known to have synaptic connections with the N1M neurons, and this may explain its weak influence on the frequency of sucrose-driven rhythms. The mechanism by which CV1a modulates motoneuronal firing is unclear because no data are available yet on possible CV1a–motoneuron synaptic connections (Fig. 7B).
Fig. 7.
Alternative schemes for the organization of neurons for higher-order control of the Lymnaea feeding CPG. A, In a previously proposed hierarchical activation model, the modulatory neurons CV1a and SO were believed to be responsible for activation of the CPG after presentation of a sensory stimulus. Activity in the CPG then drives motoneurons to elicit the motor pattern. B, Our current model suggests that the sensory activation of the system is organized in a more distributed manner, and the CV1a and SO, although possessing potential command-like capabilities for activation of the CPG, are in reality, involved in modulation of the ongoing rhythm rather than its initiation. Thus, the CPG itself and particularly the N1M interneuron seem to be the pivotal components in determining whether a sensory-evoked motor pattern occurs. The distributed nature of the network has been furthered by the findings that motoneurons have a feedback connection with the CPG (Staras et al., 1999a) and that the SO (Elliott and Benjamin, 1985b), and perhaps also the CV1a, have direct connections with motoneurons.Thin arrows, Weaker connections; thick arrows, stronger connections.
Unlike semi-intact preparations, sucrose always maintains a regular feeding rhythm in intact animals (Kemenes et al., 1986; Staras et al., 1998a). This is likely to depend on both initial activation of external chemoreceptors and subsequent activation of internal chemoreceptors, some of which were absent in the semi-intact preparations. Activation of internal chemoreceptors (e.g., in the alimentary tract) by sucrose may also result in a more regular activation of the SO, and this may contribute to the stable feeding motor outputs observed in intact animals. An experimental verification of this hypothesis would require more complex preparations, including stable recordings of small interneurons, which are very difficult to obtain (G. Kemenes, unpublished observations). The CV1a neurons are known to receive strong excitatory chemosensory inputs from the lips even in isolated lip–cerebral ganglia preparations (Whelan and McCrohan, 1992), and this can account for the more consistent activation of these cells in most (75%) of the lip–CNS preparations.
Although CV1a/SO are not necessary for the initial activation of the rhythm by chemosensory inputs, they both contribute to the dynamic properties of the fictive feeding rhythm once it has been evoked by sucrose. Suppression of CV1a leads to a decrease in the duration, but not the frequency, of bursts in a motoneuron that fires predominantly in the rasp (N2) phase of feeding. The length of this phase is an important factor in determining the actual food intake during each feeding cycle. This is also the least variable of the three phases of feeding (Rose and Benjamin, 1979; Elliott and Andrew, 1991), and activity in CV1a might be an important factor contributing to this stability. Unlike CV1a, suppression of SO leads to a significant decrease in the frequency of sucrose-activated fictive feeding, consistent with the observation that the frequency of the fictive feeding rhythm in isolated CNS preparations could be controlled by varying the level of intracellular current injection in the SO (Rose and Benjamin, 1981a). Because CV1a and SO can influence the CPG separately, this provides a mechanism for independent modulation of rasp duration and frequency, perhaps to allow the animal to feed effectively on different types of food material. In contrast to CV1a and SO, suppression of N1M leads to a cessation of the fictive feeding pattern, showing that in addition to being the most important cell type for feeding initiation, this CPG neuron plays a key role in the maintenance of sucrose-evoked fictive feeding.
Although we cannot rule out that the suppression of identified interneurons can also have a more indirect effect on the feeding CPG by affecting other, unidentified, cells, our results support the notion that CV1a and SO each has a distinct modulatory rather than a narrowly defined command-like decision-making role in feeding. These modulatory roles may be important for optimizing the output of the feeding CPG to meet specific behavioral demands during feeding in a natural environment.
Comparisons with other systems
Cerebral to buccal interneurons, thought to be homologous to CV1a in Lymnaea, have been identified in other molluscs, including Aplysia (CBI2; Rosen et al., 1991),Limax (CB1; Delaney and Gelperin, 1990a,b,c),Pleurobranchaea (phasic paracerebral neurons;Gillette et al., 1982), and Achatina (C1; Yoshida and Kobayashi, 1992). Like CV1a, these neurons can drive feeding rhythms when activated intracellularly and can respond to food stimuli, but chemosensory inputs can initiate fictive feeding without these cells becoming active (Gillette et al., 1982; Delaney and Gelperin, 1990b;Rosen et al., 1991). The lack of quantitative data in the other systems makes direct comparisons with Lymnaea difficult, but the contribution these interneurons make to the initiation of fictive feeding appears to be at least qualitatively similar to that of CV1a.
Like the SO in Lymnaea, a variety of buccal neurons are capable of driving rhythmic fictive feeding in Aplysia(Susswein and Byrne, 1988; Kirk, 1989; Teyke et al., 1993; Hurwitz and Susswein, 1996; Kabotyanski et al., 1998), Helisoma(Quinlan et al., 1997), Clione (Arshavsky et al., 1989),Planorbis (Arshavsky et al., 1988a,b), andAchatina (Yoshida and Kobayashi, 1992). However, these cells are not thought to be homologous to SO. Two of these cell types, the B1 cells of Achatina (Yoshida and Kobayashi, 1992) and the N1a cells of Helisoma (Quinlan et al., 1997), were tested for chemosensory responses, and it was shown that activity in these neurons was not necessary for fictive feeding to occur. N1M-type buccal CPG interneurons also have been found in other molluscan feeding systems (Aplysia, Susswein and Byrne, 1988; Teyke et al., 1993; Clione, Arshavsky et al., 1989; Planorbis,Arshavsky et al., 1988a,b), but their role in the sensory activation of feeding has not yet been investigated.
The stomatogastric systems of decapod crustaceans have provided important models for extrinsic neuromodulatory control over pattern generation (for review, see Katz, 1995). Despite the observation that some stomatogastric CPGs can be almost continuously active in freely behaving animals (Clemens et al., 1998) and therefore do not appear to be gated by peripheral sensory inputs in the same way as other CPGs, multiple higher-order control by a variety of extrinsic modulatory interneurons is very important in shaping the motor output of all known stomatogastric CPGs (Marder and Calabrese, 1996; Harris-Warrick et al., 1997; Blitz and Nusbaum, 1999). It has been shown that many of these interneurons receive mechanosensory inputs from the stomach (Sigvardt and Mulloney, 1982; Simmers and Moulins, 1988), which can profoundly alter ongoing motor patterns (Hooper et al., 1990; Katz and Harris-Warrick, 1991; Nargeot and Moulins, 1997; Combes et al., 1999).
Only a few cell types in rhythmically active networks, such as the dorsal ramp interneurons in the Tritonia swim system (Frost and Katz, 1996) or the pyloric suppressor interneuron of the lobster (Meyrand et al., 1991), satisfy all the defined criteria for command neuron function (Kupfermann and Weiss, 1978). The two likely candidates for a command role in feeding initiation in Lymnaea, CV1a and SO, fail to meet these criteria, but interestingly, N1M appears to satisfy them. It is conceivable that, unusually for a CPG neuron, N1M may have preferential (but not exclusive) access to chemosensory inputs in addition to its activity being both sufficient and necessary for fictive feeding to occur. However, this potential command role is clearly restricted to the initial phase of feeding and does not extend to the whole of the feeding motor pattern. To achieve and maintain a fast and robust rhythm, activity in N1M needs to be supported by other interneurons, a characteristic shared by the vast majority of modulatory interneurons identified in other systems (Marder and Calabrese, 1996).
Footnotes
This work was supported by a Biotechnology and Biological Sciences Research Council Grant (United Kingdom) to P.R.B., G.K., and K.S. and a Medical Research Council Grant (United Kingdom) to G.K. and K.S.
Correspondence should be addressed to Dr. György Kemenes, Sussex Centre for Neuroscience, School of Biological Sciences, University of Sussex, Falmer, Brighton, UK, BN1 9QG. E-mail: G.Kemenes@sussex.ac.uk.
This work was supported by a Biotechnology and Biological Sciences Research Council Grant (United Kingdom) to P.R.B., G.K., and K.S. and a Medical Research Council Grant (United Kingdom) to G.K. and K.S.
Correspondence should be addressed to Dr. György Kemenes, Sussex Centre for Neuroscience, School of Biological Sciences, University of Sussex, Falmer, Brighton, UK, BN1 9QG. E-mail: G.Kemenes@sussex.ac.uk.
REFERENCES
- 1.Arshavsky YI, Deliagina TG, Meizerov ES, Orlovsky GN, Panchin YV. Control of feeding movements in the freshwater snail Planorbis corneus. I. Rhythmical neurons of buccal ganglia. Exp Brain Res. 1988a;70:310–322. doi: 10.1007/BF00248356. [DOI] [PubMed] [Google Scholar]
- 2.Arshavsky YI, Deliagina TG, Orlovsky GN, Panchin YV. Control of feeding movements in the freshwater snail Planorbis corneus. III. Organization of the feeding rhythm generator. Exp Brain Res. 1988b;70:332–341. doi: 10.1007/BF00248358. [DOI] [PubMed] [Google Scholar]
- 3.Arshavsky YI, Deliagina TG, Orlovsky GN, Panchin YV. Control of feeding movements in pteropod mollusc Clione limacina. Exp Brain Res. 1989;78:387–397. doi: 10.1007/BF00228911. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin PR. Gastropod feeding: behavioral and neural analysis of a complex multicomponent system. In: Roberts A, Roberts B, editors. Neural origin of rhythmic movements. Cambridge UP; Cambridge, UK: 1983. [PubMed] [Google Scholar]
- 5.Benjamin PR, Elliott CJH. Snail feeding oscillator: The central pattern generator and its control by modulatory interneurons. In: Jacklet J, editor. Neuronal and cellular oscillators. Dekker; New York: 1989. pp. 173–214. [Google Scholar]
- 6.Benjamin PR, Winlow W. The distribution of three wide-acting synaptic inputs to identified neurons in the isolated brain of Lymnaea. Comp Biochem Physiol [A] 1981;70:293–307. [Google Scholar]
- 7.Benjamin PR, Staras K, Kemenes G. A systems approach to the cellular analysis of associative learning in the pond snail Lymnaea. Learn Mem. 2000;7:124–131. doi: 10.1101/lm.7.3.124. [DOI] [PubMed] [Google Scholar]
- 8.Blitz DM, Nusbaum MP. Distinct functions for cotransmitters mediating motor pattern selection. J Neurosci. 1999;19:6774–6783. doi: 10.1523/JNEUROSCI.19-16-06774.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Brierley MJ, Yeoman MS, Benjamin PR. Glutamatergic N2v cells are central pattern generator interneurons of the Lymnaea feeding system: a new model for rhythm generation. J Neurophysiol. 1997;78:3396–3407. doi: 10.1152/jn.1997.78.6.3396. [DOI] [PubMed] [Google Scholar]
- 10.Büschges A, Manira AE. Sensory pathways and their modulation in the control of locomotion. Curr Opin Neurobiol. 1998;8:733–739. doi: 10.1016/s0959-4388(98)80115-3. [DOI] [PubMed] [Google Scholar]
- 11.Clemens S, Combes D, Meyrand P, Simmers J. Long-term expression of two interacting motor pattern-generating networks in the stomatogastric system of freely behaving lobster. J Neurophysiol. 1998;79:1396–1408. doi: 10.1152/jn.1998.79.3.1396. [DOI] [PubMed] [Google Scholar]
- 12.Combes D, Meyrand P, Simmers J. Dynamic restructuring of a rhythmic motor program by a single mechanoreceptor neuron in lobster. J Neurosci. 1999;19:3620–3628. doi: 10.1523/JNEUROSCI.19-09-03620.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Delaney K, Gelperin A. Cerebral interneurons controlling fictive feeding in Limax maximus. I. Anatomy and criteria for re-identification. J Comp Physiol. 1990a;166:297–310. [Google Scholar]
- 14.Delaney K, Gelperin A. Cerebral interneurons controlling fictive feeding in Limax maximus. II. Initiation and modulation of fictive feeding. J Comp Physiol. 1990b;166:311–326. [Google Scholar]
- 15.Delaney K, Gelperin A. Cerebral interneurons controlling fictive feeding in Limax maximus. III. Integration of sensory inputs. J Comp Physiol. 1990c;166:327–343. [Google Scholar]
- 16.Elliott CJH, Andrew T. Temporal analysis of snail feeding rhythms: a three-phase relaxation oscillator. J Exp Biol. 1991;157:391–408. [Google Scholar]
- 17.Elliott CJH, Benjamin PR. Interactions of pattern-generating interneurons controlling feeding in Lymnaea stagnalis. J Neurophysiol. 1985a;54:1396–1411. doi: 10.1152/jn.1985.54.6.1396. [DOI] [PubMed] [Google Scholar]
- 18.Elliott CJH, Benjamin PR. Interactions of the slow oscillator interneuron with feeding pattern-generating interneurons in Lymnaea stagnalis. J Neurophysiol. 1985b;54:1412–1421. doi: 10.1152/jn.1985.54.6.1412. [DOI] [PubMed] [Google Scholar]
- 19.Frost WN, Katz PS. Single neuron control over a complex motor program. Proc Natl Acad Sci USA. 1996;93:422–426. doi: 10.1073/pnas.93.1.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gillette R, Kovac MP, Davis WJ. Control of feeding motor output by paracerebral neurons in brain of Pleurobranchaea californica. J Neurophysiol. 1982;47:885–908. doi: 10.1152/jn.1982.47.5.885. [DOI] [PubMed] [Google Scholar]
- 21.Grillner S, Georgopoulus AP, Jordan LM. Selection and initiation of motor behavior. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks and motor behavior. MIT; Cambridge, MA: 1997. pp. 3–20. [Google Scholar]
- 22.Harris-Warrick RM, Baro DJ, Coniglio LM, Johnson BJ, Levini RM, Peck JH, Zhang B. Chemical modulation of crustacean stomatogastric pattern generator networks. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks and motor behavior. MIT; Cambridge, MA: 1997. pp. 209–216. [Google Scholar]
- 23.Hooper SL, Moulins M, Nonnotte L. Sensory input induces long-lasting changes in the output of the lobster pyloric network. J Neurophysiol. 1990;64:1555–1573. doi: 10.1152/jn.1990.64.5.1555. [DOI] [PubMed] [Google Scholar]
- 24.Hurwitz I, Susswein AJ. B64, a newly identified central pattern generator element producing a phase switch from protraction to retraction in buccal motor programs of Aplysia californica. J Neurophysiol. 1996;75:1327–1344. doi: 10.1152/jn.1996.75.4.1327. [DOI] [PubMed] [Google Scholar]
- 25.Jansen RF, Pieneman AW, Maat AT. Pattern generation in the buccal system of freely behaving Lymnaea stagnalis. J Neurophysiol. 1999;82:3378–3391. doi: 10.1152/jn.1999.82.6.3378. [DOI] [PubMed] [Google Scholar]
- 26.Kabotyanski EA, Baxter DA, Byrne JH. Identification and characterization of catecholaminergic neuron B65, which initiates and modifies patterned activity in the buccal ganglia of Aplysia. J Neurophysiol. 1998;79:605–621. doi: 10.1152/jn.1998.79.2.605. [DOI] [PubMed] [Google Scholar]
- 27.Katz PS. Intrinsic and extrinsic neuromodulation of motor circuits. Curr Opin Neurobiol. 1995;5:799–808. doi: 10.1016/0959-4388(95)80109-x. [DOI] [PubMed] [Google Scholar]
- 28.Katz PS, Harris-Warrick RM. Recruitment of crab gastric mill neurons into the pyloric motor pattern by mechanosensory afferent stimulation. J Neurophysiol. 1991;65:1442–1451. doi: 10.1152/jn.1991.65.6.1442. [DOI] [PubMed] [Google Scholar]
- 29.Kemenes G. In vivo neuropharmacological and in vitro laser ablation techniques as tools in the analysis of neuronal circuits underlying behavior in a molluscan model system. Gen Pharmacol. 1997;29:7–15. doi: 10.1016/s0306-3623(96)00520-4. [DOI] [PubMed] [Google Scholar]
- 30.Kemenes G, Elliott CJH. Analysis of the feeding motor pattern in the pond snail, Lymnaea stagnalis: photoinactivation of axonally stained pattern-generating interneurons. J Neurosci. 1994;14:153–166. doi: 10.1523/JNEUROSCI.14-01-00153.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kemenes G, Elliott CJH, Benjamin PR. Chemical and tactile inputs to the Lymnaea feeding system: effects on behaviour and neural circuitry. J Exp Biol. 1986;122:113–137. [Google Scholar]
- 32.Kemenes G, Staras K, Benjamin PR. In vitro appetitive classical conditioning of the feeding response in the pond snail Lymnaea stagnalis. J Neurophysiol. 1997;78:2351–2362. doi: 10.1152/jn.1997.78.5.2351. [DOI] [PubMed] [Google Scholar]
- 33.Kirk MD. Premotor neurons in the feeding system of Aplysia californica. J Neurobiol. 1989;20:497–512. doi: 10.1002/neu.480200516. [DOI] [PubMed] [Google Scholar]
- 34.Kupfermann I. The role of modulatory systems in optimizing behavior: Studies of feeding in the mollusc Aplysia californica. Zoology. 1998;100:235–243. [Google Scholar]
- 35.Kupfermann I, Weiss KR. The command neuron concept. Behav Brain Sci. 1978;1:3–39. [Google Scholar]
- 36.Marder E, Calabrese L. Principles of rhythmic motor pattern generation. Physiol Rev. 1996;76:687–717. doi: 10.1152/physrev.1996.76.3.687. [DOI] [PubMed] [Google Scholar]
- 37.McCrohan CR. Initiation of feeding motor output by an identified interneurone in the snail Lymnaea stagnalis. J Exp Biol. 1984;113:351–366. [Google Scholar]
- 38.McCrohan CR, Kyriakides MA. Cerebral interneurones controlling feeding motor output in the snail Lymnaea stagnalis. J Exp Biol. 1989;147:361–374. [Google Scholar]
- 39.Meyrand P, Simmers J, Moulins M. Construction of pattern generating circuit with neurons of different networks. Nature. 1991;351:60–63. doi: 10.1038/351060a0. [DOI] [PubMed] [Google Scholar]
- 40.Nargeot R, Moulins M. Sensory-induced plasticity of motor pattern selection in the lobster stomatogastric nervous system. Eur J Neurosci. 1997;8:1636–1645. doi: 10.1111/j.1460-9568.1997.tb01521.x. [DOI] [PubMed] [Google Scholar]
- 41.Perry SJ, Straub VA, Kemenes G, Santama N, Worster BM, Burke JF, Benjamin PR. Neural modulation of gut motility by myomodulin peptides and acetylcholine in the snail Lymnaea. J Neurophysiol. 1998;79:2460–2474. doi: 10.1152/jn.1998.79.5.2460. [DOI] [PubMed] [Google Scholar]
- 42.Quinlan EM, Arnett BC, Murphy AD. Feeding stimulants activate an identified dopaminergic interneuron that induces the feeding motor program in Helisoma. J Neurophysiol. 1997;78:812–824. doi: 10.1152/jn.1997.78.2.812. [DOI] [PubMed] [Google Scholar]
- 43.Rose RM, Benjamin PR. The relationship of the central motor pattern of the feeding cycle of Lymnaea stagnalis. J Exp Biol. 1979;80:137–163. doi: 10.1242/jeb.80.1.137. [DOI] [PubMed] [Google Scholar]
- 44.Rose RM, Benjamin PR. Interneuronal control of feeding in Lymnaea stagnalis. I. Initiation of feeding by a single buccal interneuron. J Exp Biol. 1981a;92:187–201. [Google Scholar]
- 45.Rose RM, Benjamin PR. Interneuronal control of feeding in Lymnaea stagnalis. II. The interneuronal mechanisms generating feeding cycles. J Exp Biol. 1981b;92:203–228. [Google Scholar]
- 46.Rosen SC, Teyke T, Miller MW, Weiss KR, Kupfermann I. Identification and characterization of cerebral-to-buccal interneurons implicated in the control of motor programs associated with feeding in Aplysia. J Neurosci. 1991;11:3630–3655. doi: 10.1523/JNEUROSCI.11-11-03630.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Selverston AI, Panchin YV, Arshavsky YI, Orlovsky GN. Shared features of invertebrate central pattern generators. In: Stein PSG, Grillner S, Selverston AI, Stuart DG, editors. Neurons, networks and motor behavior. MIT; Cambridge, MA: 1997. [Google Scholar]
- 48.Sigvardt KA, Mulloney B. Sensory alteration of motor patterns in the stomatogastric nervous system of the spiny lobster Panulirus interruptus. J Exp Biol. 1982;97:137–152. doi: 10.1242/jeb.97.1.137. [DOI] [PubMed] [Google Scholar]
- 49.Simmers J, Moulins M. A disynaptic sensorimotor pathway in the lobster stomatogastric system. J Neurophysiol. 1988;59:740–756. doi: 10.1152/jn.1988.59.3.740. [DOI] [PubMed] [Google Scholar]
- 50.Staras K, Kemenes G, Benjamin PR. Pattern-generating role for motoneurons in a rhythmically active neuronal network. J Neurosci. 1998a;18:3669–3688. doi: 10.1523/JNEUROSCI.18-10-03669.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Staras K, Kemenes G, Benjamin PR. Neurophysiological correlates of unconditioned and conditioned feeding behavior in the pond snail Lymnaea stagnalis. J Neurophysiol. 1998b;79:3030–3040. doi: 10.1152/jn.1998.79.6.3030. [DOI] [PubMed] [Google Scholar]
- 52.Staras K, Kemenes G, Benjamin PR. Cellular traces of behavioral classical conditioning can be recorded at several specific sites in a simple nervous system. J Neurosci. 1999a;19:347–357. doi: 10.1523/JNEUROSCI.19-01-00347.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Staras K, Kemenes G, Benjamin PR. Electrophysiological and behavioral analysis of lip touch as a component of the food stimulus in the snail Lymnaea. J Neurophysiol. 1999b;81:1261–1273. doi: 10.1152/jn.1999.81.3.1261. [DOI] [PubMed] [Google Scholar]
- 54.Susswein AJ, Byrne JH. Identification and characterization of neurons initiating patterned neural activity in the buccal ganglia of Aplysia. J Neurosci. 1988;8:2049–2061. doi: 10.1523/JNEUROSCI.08-06-02049.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Teyke T, Rosen SC, Weiss KR, Kupfermann I. Dopaminergic neuron B20 generates rhythmic neuronal activity in the feeding motor circuitry of Aplysia. Brain Res. 1993;630:226–237. doi: 10.1016/0006-8993(93)90661-6. [DOI] [PubMed] [Google Scholar]
- 56.Tuersley MD, McCrohan CR. Food arousal in the pond snail, Lymnaea stagnalis. Behav Neural Biol. 1987;48:222–236. doi: 10.1016/s0163-1047(87)90780-1. [DOI] [PubMed] [Google Scholar]
- 57.Vehovszky Á, Elliott CJH. The octopamine-containing buccal neurons are a new group of feeding interneurons in the pond snail Lymnaea stagnalis. Acta Biol Hung. 2000;51:165–176. [PubMed] [Google Scholar]
- 58.Whelan HA, McCrohan CR. Effect of sensory stimuli on synaptic input to an identified interneurone and on feeding behaviour in Lymnaea stagnalis. J Physiol (Lond) 1992;446:159. [Google Scholar]
- 59.Yeoman MS, Vehovszky Á, Kemenes G, Elliott CJH, Benjamin PR. Novel interneuron having hybrid modulatory-central pattern generator-properties in the feeding system of the snail, Lymnaea stagnalis. J Neurophysiol. 1995;73:112–124. doi: 10.1152/jn.1995.73.1.112. [DOI] [PubMed] [Google Scholar]
- 60.Yoshida M, Kobayashi M. Identified neurons involved in the control of rhythmic buccal motor activity in the snail Achatina fulica. J Exp Biol. 1992;164:117–133. [Google Scholar]