Abstract
Activity-dependent neuronal gene expression is thought to require activation of L-type calcium channels, a view based primarily on studies in which chronic potassium (K+) depolarization was used to mimic neuronal activity. However, N-type calcium channels are primarily inactivated during chronic depolarization, and their potential contribution to gene expression induced by physiological patterns of stimulation has not been defined. In the present study, electrical stimulation of dissociated primary sensory neurons at 5 Hz, or treatment with elevated K+, produced a large increase in the percentage of neurons that express tyrosine hydroxylase (TH) mRNA and protein. However, blockade of L-type channels, which completely inhibited K+-induced expression, had no effect on TH expression induced by patterned stimulation. Conversely, blockade of N-type channels completely inhibited TH induction by patterned stimulation, whereas K+-induced expression was unaffected. Similar results were obtained for depolarization-induced expression of the immediate early genes Nurr1 andNur77. In addition, TH induction by patterned stimulation was significantly reduced by inhibitors of PKA and PKC but was unaffected by inhibition of the mitogen-activated protein kinase (MAPK) pathway. On the other hand, K+-induced TH expression was significantly reduced by inhibition of the MAPK pathway but was unaffected by inhibitors of PKA or PKC. These results demonstrate that N-type calcium channels can directly link phasic membrane depolarization to gene expression, challenging the view that activation of L-type channels is required for nuclear responses to physiological patterns of activity. Moreover, our data show that phasic and chronic depolarizing stimuli act through distinct mechanisms to induce neuronal gene expression.
Keywords: activity-dependent gene expression, PKA, PKC, CREB, Nurr1, dopamine
Activity-dependent, calcium-regulated gene expression plays a critical role in diverse neural functions, including differentiation (Gu and Spitzer, 1997;Buonanno and Fields, 1999) and survival (Ghosh et al., 1994) of neurons, formation of synaptic connections (Lein and Shatz, 2000), and learning and memory (Svoboda and Mainen, 1999). The importance of calcium-regulated gene expression has led to numerous studies examining molecular mechanisms that link membrane depolarization, and subsequent elevation of intracellular calcium levels, with gene expression (Finkbeiner and Greenberg, 1998). One conclusion of these studies is that activation of L-type calcium channels is required for depolarization-mediated gene induction.
Most studies that have examined mechanisms of activity-dependent gene expression have used chronic membrane depolarization, induced by elevated K+ or glutamate, to raise intracellular calcium levels and stimulate gene expression. Although these methods are effective at elevating intracellular calcium, they produce a nonphysiological chronic membrane depolarization and tonic elevation of intracellular calcium, conditions that do not mimic cellular responses to physiological patterns of phasic neuronal activity. In fact, the importance of patterned stimulation, or oscillating levels of intracellular calcium, in mediating gene expression (Fields et al., 1997; Dolmetsch et al., 1998; Li et al., 1998), neurotrophin release (Balkowiec and Katz, 2000), and neuronal differentiation (Gu and Spitzer, 1997) is clear. For example, Fields and colleagues (1997) demonstrated that the level of c-fosexpression in primary sensory neurons is correlated with the time interval between bursts of action potentials rather than with the amplitude of calcium influx. In addition, Balkowiec and Katz (2000)demonstrated recently that, although short-term continuous membrane depolarization is completely ineffective at releasing brain-derived neurotrophic factor from primary sensory neurons, patterned stimulation for the same period of time increases release 20-fold. Thus, the use of chronic stimulation as a model for neuronal activity is unlikely to provide a complete understanding of mechanisms that underlie activity-dependent signaling events.
We demonstrated previously that activation of L-type calcium channels by chronic K+ depolarization alters short- and long-term regulation of tyrosine hydroxylase (TH) expression in primary sensory neurons, despite the fact that N-type calcium channels carry the majority of the voltage-activated calcium current in these cells (Brosenitsch et al., 1998). However, under conditions of sustained membrane depolarization, N-type calcium channels are inactivated (Nowycky et al., 1985; Hirning et al., 1988) and their potential contribution to activity-dependent events cannot be evaluated. Therefore, in the present study, we evaluated the role of N- and L-type calcium channels in activity-dependent gene expression by comparing the effects of K+ depolarization and patterned electrical field stimulation. Our findings demonstrate that, in sharp contrast to chronic membrane depolarization, patterned electrical stimulation acts through N-type calcium channels to induce neuronal gene expression. Moreover, we show that expression induced by electrical stimulation requires activation of intracellular protein kinase pathways distinct from those activated by chronic K+ depolarization.
MATERIALS AND METHODS
Cell culture. Pregnant dams (Sprague Dawley rat; Zivic-Miller, Zelienople, PA) were rapidly killed by exposure to carbon dioxide. The uterine horns were removed and placed into PBS containing 10% glucose, and the embryos were excised. To assign gestational ages, the day after mating was designated embryonic day (E) 0.5. E16.5 petrosal ganglia (PG) were digested in Dispase (Roche Molecular Biochemicals, Mannheim, Germany) for 30 min at 37°C, followed by trituration through siliconized, fire-polished Pasteur pipettes. Cells were plated onto glass coverslips coated with Growth Factor Reduced Matrigel Matrix (diluted 1:10; Becton Dickinson, Bedford, MA) at a density of one ganglion per well. Dissociate cultures were grown in Neurobasal medium supplemented with B-27 serum-free supplement, 1% penicillin–streptomycin–neomycin antibiotic mixture, and 0.5 mml-glutamine (Life Technologies, Gaithersburg, MD). All cultures were supplemented with recombinant human brain-derived neurotrophic factor (a gift from Regeneron Pharmaceuticals Inc., Tarrytown, NY) at a concentration of 10 ng/ml. Neurons were cultured for a total of 4 d before stimulation for 6 or 24 hr with either 40 mm KCl or patterned electrical impulses (see below). For experiments examining TH protein expression, the medium was replaced immediately after stimulation, and the cultures were grown for an additional 12 hr in control conditions to permit new protein synthesis. For experiments examining TH mRNA or Nur-related factor (Nurr) expression, cultures were fixed immediately after stimulation.
Stimulation protocols and reagents. PG cultures were electrically stimulated in 24-well plates fitted with a pair of platinum electrodes connected in parallel to a stimulator (MultiStim System; Digitimer, Hertfordshire, UK). Cultures were stimulated with 0.2 msec pulses of alternating polarity delivered at 5 Hz continuously or at 25 Hz in 2-sec-long bursts delivered once every 10 sec. These two stimulation paradigms produce an equivalent number of pulses over the 6 hr stimulation period. The drugs used are as follows. ω-Conotoxin GVIA (Sigma, St. Louis, MO) and tetrodotoxin (Sigma) were used at final concentrations of 1 and 1.5 μm, respectively. Nimodipine (Sigma), dissolved in methanol, was used at a final concentration of 2 μm. PD98059 (Calbiochem, La Jolla, CA) and H-89 (Calbiochem), dissolved in dimethylsulfoxide (DMSO), were used at final concentrations of 50 and 3 μm, respectively. Membrane-permeable forms of the protein kinase A (PKA) inhibitor PKI14–22amide-myristoylated (Calbiochem), the protein kinase C (PKC) inhibitor PKC-I19–27-myristoylated (Calbiochem), and the calcium/calmodulin kinase (CaMK) II inhibitor autocamtide-2 inhibitory peptide-myristoylated (Calbiochem) were used at a final concentration of 50 μm. These concentrations of kinase inhibitors have been shown previously to be efficacious and specific (Eichholtz et al., 1993; Alessi et al., 1995; Ishida et al., 1995;Harris et al., 1997). Actinomycin D (Sigma) was used at a final concentration of 0.5 μg/ml.d,l-2-Amino-5-phosphonovaleric acid (APV) (Sigma) was used at a concentration of 50 μm. 6-Cyano-7-nitroquinoxaline-2,3-(1H,4H)-dione (CNQX) (Sigma) was dissolved in DMSO and used at a final concentration of 10 μm. In each experiment, the final DMSO concentration never exceeded 0.02%. Pertussis toxin (Calbiochem) was used at a final concentration of 1 μg/ml, and cultures were preincubated with the toxin for 20 hr before the onset of stimulation.
Immunocytochemistry. All cultures were fixed with 4% paraformaldehyde in 0.1 m sodium phosphate buffer (PFA), pH 7.4, for 30 min. The following antibodies were used for double-immunostaining: polyclonal anti-TH (Pel-Freez Biologicals, Rogers, AR), polyclonal anti-Nurr1/Nur77 (Santa Cruz Biotechnology, Santa Cruz, CA), polyclonal anti-phosphorylated cAMP response element-binding protein (pCREB) (Upstate Biotechnology, Lake Placid, NY), monoclonal anti-neurofilament (NF) protein (NF160,68; Sigma), goat anti-rabbit IgG-FITC (Roche Molecular Biochemicals), and goat anti-mouse IgG rhodamine (Cappel, Durham, NC). TH–NF immunostaining was performed as described previously (Brosenitsch et al., 1998). The protocol for Nurr and pCREB immunostaining was the same as for TH–NF, except that cells were incubated in 20% goat serum in PBS containing 0.5% Triton X-100 (PBS-Tx) before incubation in the primary antibodies, which was performed at 4°C in anti-Nurr1/Nur77 (1:4000) or anti-pCREB (1:2000) and anti-NF (1:100) diluted in PBS-Tx containing 2% goat serum.
In situ hybridization. The digoxigenin (DIG)-labeled TH sense and antisense RNA probes were prepared using as template a 280 bp fragment of TH cDNA (1240–1521) cloned into the EcoRI site of pGEM-3 phagemid [generated in the laboratory of A. William Tank, University of Rochester School of Medicine and Dentistry (Rochester, NY), and generously provided by Kumi Nagomoto-Combs, Case Western Reserve University]. Sense and antisense probes were synthesized with DIG-11-UTP (Roche Molecular Biochemicals) using an RNA transcription kit (Stratagene, La Jolla, CA). For hybridization, cultures were fixed in PFA for 30 min, rinsed twice in PBS, acetylated with 0.25% acetic anhydride in 10 mmtriethanolamine, pH 8.0, for 10 min, rinsed in 1× SSC for 10 min, and incubated in hybridization buffer (50% formamide, 5× SSC, 1 mg/ml yeast tRNA, 100 μg/ml heparin, 1× Denhardt's reagent, 0.1% Tween 20, 1 mg/ml 3-[(3-cholamidopropyl)dimethylammonio]-1-propanesulfonate, and 5 mm EDTA) without probe for 6 hr. Sense and antisense probes were diluted 1:40 in hybridization buffer, and cultures were hybridized for 16 hr at 50°C. After hybridization, cultures were washed in 1× SSC at 60°C for 10 min and again for 30 min, washed in 0.1× SSC for 1 hr at 60°C, incubated in 20 μg/ml RNase A at 37°C for 30 min, rinsed in 1 mmdithiothreitol at 37°C for 30 min, washed in 0.1× SSC for 1 hr at 65°C, and washed in 2× SSC for 30 min. For immunological detection of DIG-labeled hybrids, cultures were rinsed in PBS containing 0.1% Triton X-100 and 2 mg/ml BSA (PBT), blocked with 20% sheep serum in PBT for 6 hr, and incubated in sheep anti-DIG-alkaline phosphatase-conjugated Fab fragments (Roche Molecular Biochemicals) diluted 1:700 in PBT containing 20% sheep serum overnight at 4°C. The next day, cultures were rinsed four times in PBT for 10 min each, rinsed in buffer 2 (100 mm Tris, pH 9.5, 100 mm NaCl, and 50 mmMgCl2), rinsed in buffer 2 containing 5 mm levamisole (Sigma), and incubated in buffer 2 containing 5 mm levamisole, 340 μg/ml nitroblue tetrazolium (Roche Molecular Biochemicals), and 240 μg/ml 5-bromo-4-chloro-3-indolyl phosphate (Roche Molecular Biochemicals) for 30 hr. The reaction was stopped by rinsing the cultures with Tris-EDTA buffer, pH 8.0, and the cultures were subsequently processed for NF immunocytochemistry as described previously (Brosenitsch et al., 1998).
Cell counts and statistical analysis. The number of neurons in each culture was estimated by counting all cells within the central 10% of each coverslip. All cell counts were performed with the investigator blinded to the experimental treatment. Experiments were performed at least three times with at least two cultures per experimental group. At least 1200 neurons per experimental group were scored for TH, Nurr, or pCREB immunoreactivity. Data are presented as the percentage of total neurons that expressed TH protein, TH mRNA, Nurr, or pCREB. The intensity of TH immunoreactivity was measured from randomly chosen fields of cells using SimplePCI imaging software (Compix Inc., Cranberry, PA). Data are presented in arbitrary units with the background subtracted. Intensity was measured on ∼1700 neurons. For statistical analysis, percentages were normalized (arcsin transformation) and values were compared using ANOVA, followed by Duncan's multiple range test. p < 0.05 was considered significant.
RESULTS
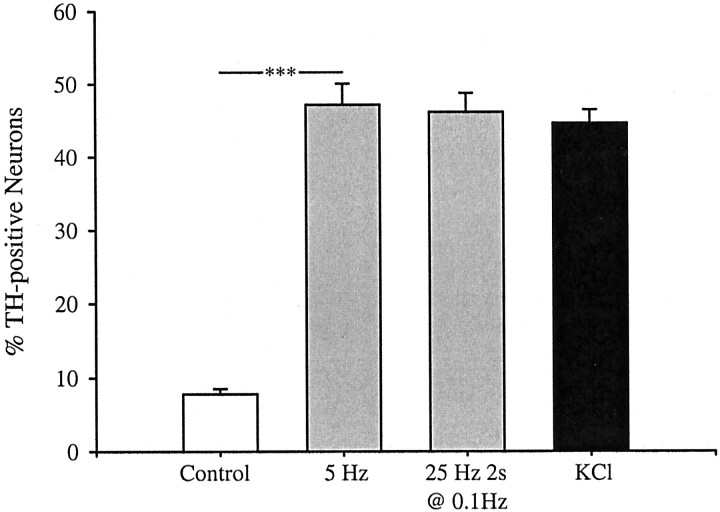
PG primary sensory neurons provide a robust model for studying activity-dependent neuronal gene expression. We found previously that exposure of fetal PG neurons to chronic depolarizing stimuli in culture leads to a fivefold increase in the percentage of ganglion cells that express dopaminergic traits, including TH, without affecting neuronal survival (Hertzberg et al., 1995; Brosenitsch et al., 1998). In the present study, therefore, we sought to compare induction of dopaminergic traits in response to chronic depolarization and more physiological, patterned, electrical stimuli. Normally, only 10–15% of PG neurons are dopaminergic (Katz et al., 1983; Finley et al., 1992). Field stimulation of E16.5 PG neurons for 6 hr at either 5 Hz (a physiological frequency; Bairam et al., 1993) continuously or 25 Hz for 2 sec, once every 10 sec, resulted in an approximate sixfold increase in the percentage of neurons expressing TH protein, an increase identical to that produced by 6 hr of chronic membrane depolarization with elevated K+ (Fig.1). Neuron survival was unaffected by either electrical stimulation or K+depolarization (control, 550 ± 57; 5 Hz, 428 ± 32; 25 Hz, 567 ± 83; KCl, 536 ± 37). In addition, stimulation at 5 Hz, or with elevated K+, for 24 hr significantly increased the percentage of neurons expressing TH mRNA as determined by in situ hybridization (see Fig. 4). The increase in TH protein expression after 6 hr of stimulation was prevented by inhibition of mRNA synthesis with 0.5 μg/ml actinomycin D (Fig. 2A), without affecting neuronal survival (control, 763 ± 106 neurons; 5 Hz, 662 ± 55; 5 Hz plus actinomycin, 594 ± 66). Moreover, the effect of 5 Hz stimulation, but not that of elevated K+, was blocked by the sodium channel antagonist tetrodotoxin (1.5 μm), indicating that activation of voltage-gated sodium channels is required for TH induction by electrical stimulation (Fig. 2B). These initial experiments demonstrated that patterned stimulation and chronic K+ depolarization are both effective at inducing TH mRNA and protein in fetal PG neurons.
Fig. 1.
TH induction by electrical stimulation and K+ depolarization. Dissociate cultures of E16.5 PG neurons were stimulated at 5 Hz continuously (0.2 msec alternating polarity pulses), at 25 Hz for 2 sec once every 10 sec, or chronically with 40 mm KCl. After 6 hr of stimulation, neurons were cultured for an additional 12 hr in control conditions and then processed for TH–NF immunocytochemistry. Each barrepresents the percentage of total neurons exhibiting TH immunoreactivity. Data are presented as the mean ± SEM. Comparisons among groups were made using ANOVA followed by Duncan's multiple range test; ***p ≤ 0.001.
Fig. 4.
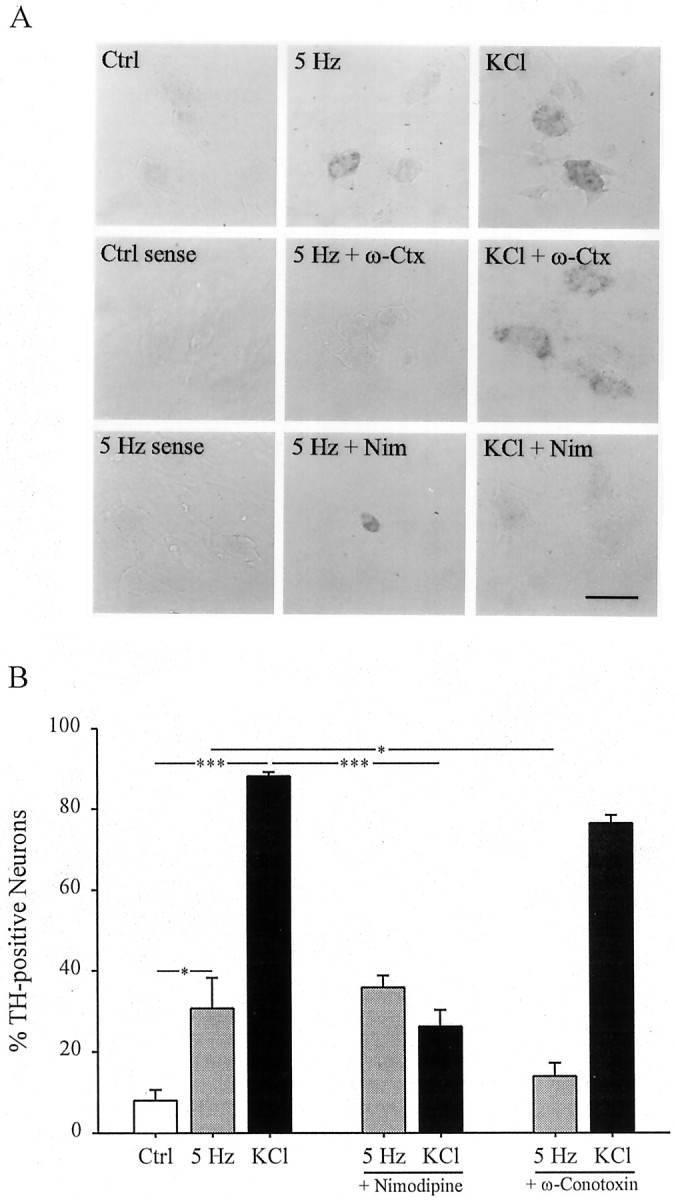
The effect of selective calcium channel antagonists on stimulation-induced expression of TH mRNA.A, TH mRNA expression in E16.5 PG cultures stimulated at 5 Hz or with 40 mm KCl for 24 hr in the presence or absence of 1 μm ω-conotoxin (ω-Ctx) or 2 μm nimodipine (Nim). Scale bar, 50 μm.B, Each bar represents the percentage of total neurons exhibiting TH mRNA by in situhybridization. Data are presented as the mean ± SEM. Comparisons among groups were made using ANOVA followed by Duncan's multiple range test; *p ≤ 0.05; ***p ≤ 0.001. Ctrl, Control.
Fig. 2.
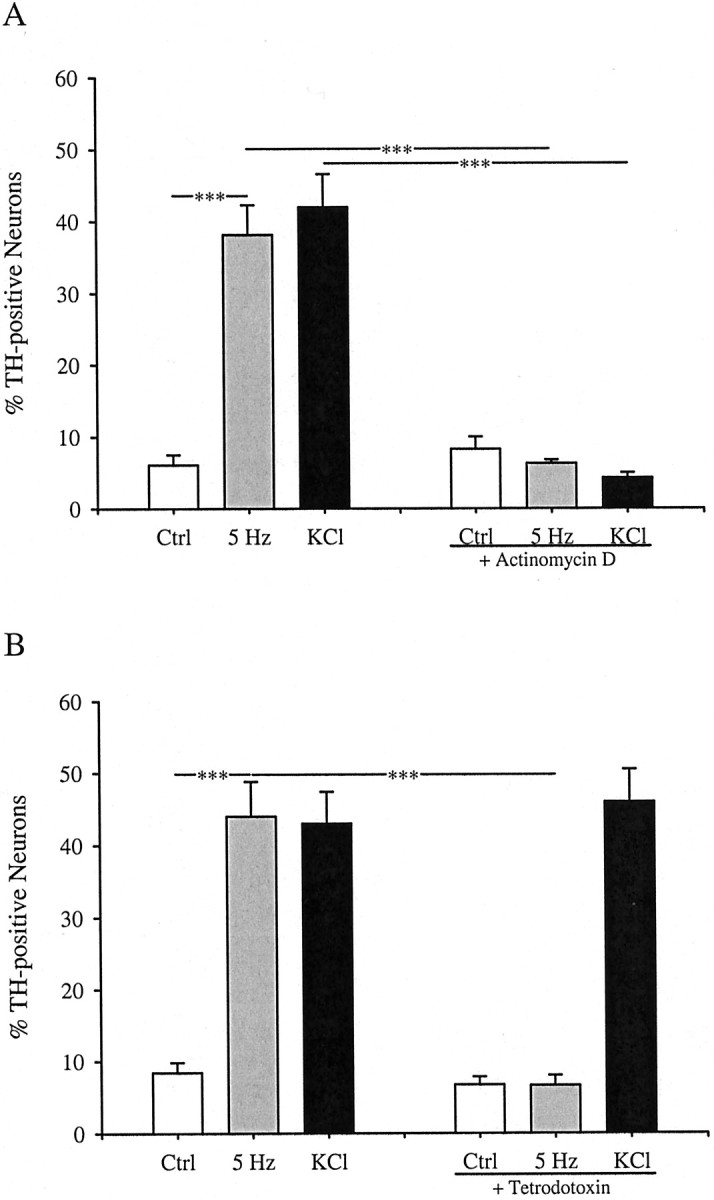
TH induction by electrical stimulation requires mRNA synthesis (A) and functional voltage-activated sodium channels (B). E16.5 PG neurons were stimulated at 5 Hz or with 40 mm KCl for 6 hr in the presence or absence of 0.5 μg/ml actinomycin D (A) or 1.5 μm tetrodotoxin (B). Each bar represents the percentage of total neurons exhibiting TH immunoreactivity. Data are presented as the mean ± SEM. Comparisons among groups were made using ANOVA followed by Duncan's multiple range test; ***p ≤ 0.001. Ctrl, Control.
To establish a semiquantitative measurement of the difference in TH expression between neurons classified as TH-positive and TH-negative, we measured their fluorescence intensity by digital imaging. In all experimental groups, the average fluorescence intensity of neurons classified as TH-positive was at least six times greater than the average intensity of neurons classified as TH-negative (Table1). Thus, the increase in the percentage of TH-positive neurons after 5 Hz stimulation and K+ depolarization reflects highly significant increases in TH expression per neuron. In addition, these data show that both 5 Hz stimulation and K+ depolarization induced, on average, a similar level of TH expression per neuron.
Table 1.
Fluorescence intensity measurements of neurons classified as TH-positive or TH-negative in control and stimulated cultures, in the absence or presence of calcium channel blockers
| Treatment | Fluorescence intensity (mean ± SEM) | |
|---|---|---|
| TH-Negative cells (n = 732) | TH-Positive cells (n = 823) | |
| Control | 5.85 ± 0.40 | 60.38 ± 5.49 |
| 5 Hz | 6.83 ± 0.33 | 45.72 ± 3.94 |
| KCl | 7.89 ± 0.45 | 48.55 ± 3.95 |
| Nimodipine | 9.39 ± 0.46 | 72.40 ± 8.31 |
| 5 Hz + nimodipine | 7.20 ± 0.48 | 46.86 ± 4.33 |
| KCl + nimodipine | 7.22 ± 0.39 | 68.43 ± 8.61 |
| ω-Conotoxin | 10.34 ± 0.45 | 63.89 ± 8.03 |
| 5 Hz + ω-conotoxin | 6.31 ± 0.41 | 49.42 ± 6.17 |
| KCl + ω-conotoxin | 7.33 ± 0.39 | 50.98 ± 4.18 |
E16.5 PG cultures were stimulated at 5 Hz or with 40 mm KCl for 6 hr in the presence or absence of 1 μm ω-conotoxin or 2 μm nimodipine. Fluorescence intensity measurements of single cells were obtained as described in Materials and Methods, and the data are expressed in arbitrary units (mean ± SEM). Between 50 and 135 neurons were analyzed per group. The average fluorescence intensity of cells classified as TH-positive was the same among all treatment groups and was at least sixfold higher than that of cells classified as TH-negative (p ≤ 0.001).
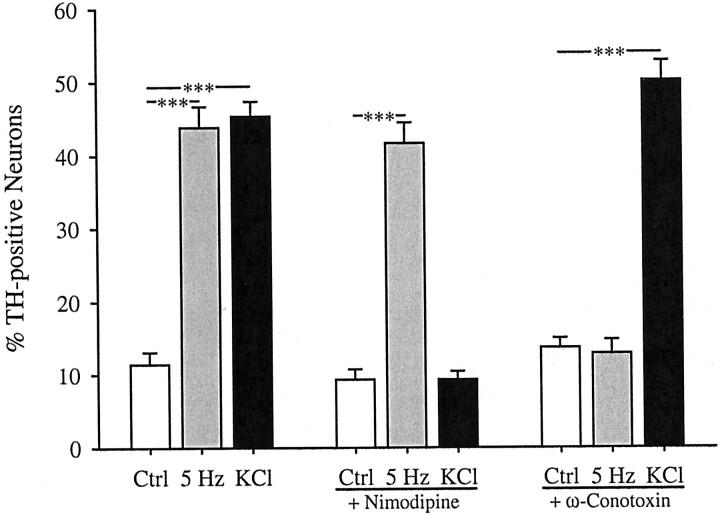
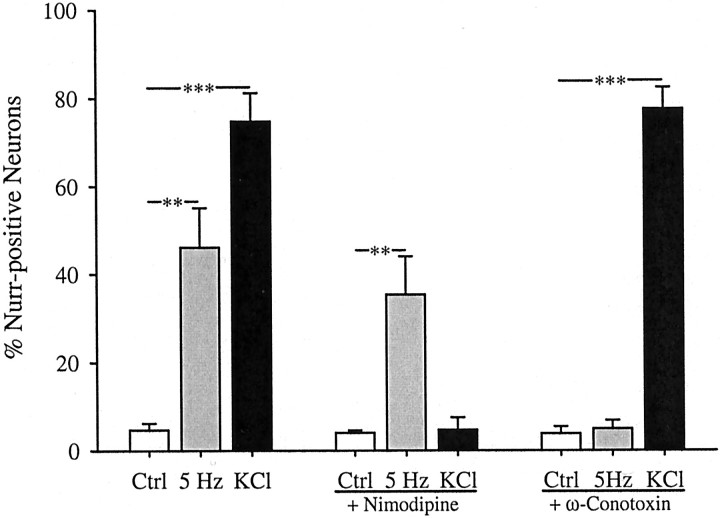
We found previously that 68% of the high voltage-activated calcium current in E16.5 PG neurons is blocked by the N-type channel antagonist ω-conotoxin, whereas only 16% is blocked by the L-type channel antagonist nimodipine (Brosenitsch et al., 1998). In the present study, we used these antagonists to determine the role of high voltage-activated calcium channels in gene expression induced by patterned electrical stimulation. Inhibition of L-type channels had no effect on the induction of TH protein (Fig.3) or mRNA (Fig.4) in response to 5 Hz stimulation but completely blocked induction by chronic K+depolarization, as described previously (Brosenitsch et al., 1998). Conversely, N-type channel blockade completely abolished both TH protein (Fig. 3) and mRNA (Fig. 4) induction after 5 Hz stimulation and had no effect on induction by chronic K+depolarization. In addition, nimodipine had no effect on the average fluorescence intensity of TH-positive neurons in 5 Hz stimulated cultures, and ω-conotoxin had no effect on the average fluorescence intensity of TH-positive neurons in K+-depolarized cultures (Table 1). Thus, nimodipine and ω-conotoxin were not only ineffective in reducing the percentage of TH-positive neurons in 5 Hz stimulated and K+-depolarized cultures, respectively, but also did not decrease the average level of TH-expression per neuron in these groups. To determine whether N-type calcium channels are also required for activity-dependent regulation of other dopaminergic traits, we examined expression of the Nurr family of orphan nuclear receptors using a pan-Nurr antibody that recognizes Nurr1 and Nur77 [nerve growth factor inducible-B (NGFI-B)]. Electrical stimulation (5 Hz) induced a 10-fold increase in the percentage of Nurr-immunoreactive neurons that was abolished by N-type channel blockade and unaffected by inhibition of L-type channels (Fig.5). Elevated K+, on the other hand, produced a 16-fold increase in the percentage of Nurr-positive cells that was prevented by blockade of L-type calcium channels and unaffected by blockade of N-type channels (Fig. 5). These data indicate, therefore, that patterned electrical stimulation and chronic membrane depolarization, respectively, induce TH and Nurr expression by activating distinct populations of voltage-activated calcium channels.
Fig. 3.
Electrical stimulation (5 Hz) and K+ depolarization induce TH expression by activating distinct calcium channel subtypes. Each bar represents the percentage of total neurons in stimulated and unstimulated (Ctrl) cultures exhibiting TH immunoreactivity in the absence or presence of 2 μm nimodipine, an L-type calcium channel antagonist, or 1 μm ω-conotoxin, an N-type calcium channel antagonist. Data are presented as the mean ± SEM. Comparisons among groups were made using ANOVA followed by Duncan's multiple range test; ***p ≤ 0.001.
Fig. 5.
Electrical stimulation (5 Hz) and K+ depolarization induce Nurr expression by activating distinct calcium channel subtypes. Each barrepresents the percentage of total neurons in stimulated and unstimulated (Ctrl) cultures exhibiting Nurr immunoreactivity in the absence or presence of 2 μmnimodipine or 1 μm ω-conotoxin. Data are presented as the mean ± SEM. Comparisons among groups were made using ANOVA followed by Duncan's multiple range test; **p ≤ 0.01; ***p ≤ 0.001.
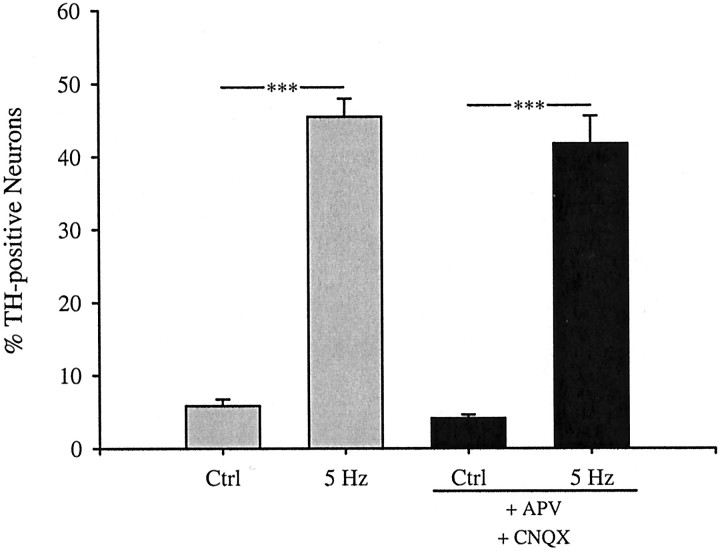
N-type calcium channels have been associated primarily with neurotransmitter release at synaptic sites (Dunlap et al., 1995). This raised the possibility that the TH and Nurr induction we observed after 5 Hz stimulation was secondary to electrically evoked transmitter release at synaptic sites that may have developed between PG neurons in our cultures. We considered this unlikely, because primary sensory neurons do not form synapses in vitro in the presence of ganglionic satellite cells (Cooper, 1984; Zhong et al., 1997), as in our cultures. Nonetheless, we compared the effect of 5 Hz stimulation on TH expression in the absence and presence of pharmacological blockers of glutamate receptors, because glutamate is the principal excitatory transmitter of PG neurons (Mizusawa et al., 1994). Addition of the NMDA receptor antagonist APV (50 μm) and the non-NMDA receptor antagonist CNQX (10 μm) had no effect on TH induction by 5 Hz stimulation (Fig.6). In addition, application of pertussis toxin (1 μg/ml), to prevent potential transmitter actions through pertussis toxin-sensitive G-protein-linked receptors, had no effect on stimulation-induced TH expression (data not shown). These data argue strongly against a role for indirect effects of N-type calcium channel activation on activity-dependent TH and Nurr induction in our cultures.
Fig. 6.
TH induction by 5 Hz stimulation is unaffected by treatment with ionotropic glutamate receptor antagonists. E16.5 PG neurons were stimulated at 5 Hz for 6 hr in the presence or absence of the NMDA receptor antagonist APV (50 μm) and the non-NMDA receptor antagonist CNQX (10 μm). Each barrepresents the percentage of total neurons exhibiting TH immunoreactivity presented as the mean ± SEM. Comparisons were made using ANOVA followed by Duncan's multiple range test; ***p ≤ 0.001. Ctrl, Control.
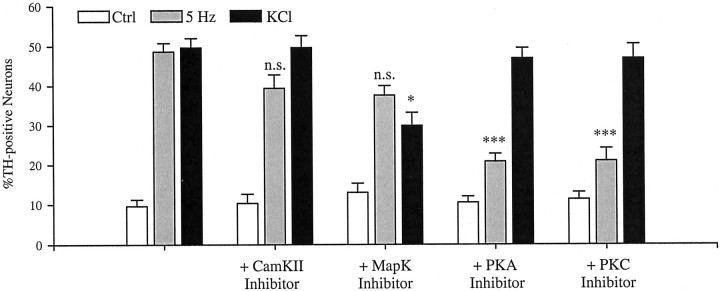
Previous studies demonstrated that different calcium influx sites mediate neuronal gene expression through distinct protein kinase pathways and promoter elements. For example, in hippocampal neurons, calcium entry through either NMDA or L-type channels increases expression of the immediate early response gene c-fos; however, CaMK II is required only for c-fos induction after activation of L-type channels (Bading et al., 1993). To begin examining whether patterned electrical stimulation and activation of N-type channels induce gene expression through intracellular signaling pathways distinct from those recruited in response to chronic K+ depolarization and L-type channel activation, we compared TH induction by these two protocols in the absence or presence of specific protein kinase inhibitors. Inhibition of PKA with the specific peptide inhibitor PKI14–22 (50 μm) (Harris et al., 1997) or PKC with the specific peptide inhibitor PKC-I19–27 (50 μm) (Eichholtz et al., 1993) resulted in a significant 57% reduction in the percentage of TH-positive neurons induced by 5 Hz stimulation but had no effect on K+-induced TH expression (Fig. 7). A similar result was observed with the structurally distinct PKA antagonist H89 (3 μm; data not shown). Conversely, the mitogen-activated protein kinase (MAPK) pathway inhibitor PD98059 (50 μm) (Alessi et al., 1995) did not significantly alter TH induction by 5 Hz stimulation but reduced TH induction by chronic K+ depolarization by 41.5% (Fig.7). Inhibition of CaMK II with the specific peptide inhibitor autocamtide-2 inhibitory peptide (50 μm) (Ishida et al., 1995) had no effect on TH expression after either 5 Hz stimulation or K+ depolarization (Fig. 7). To rule out the possibility that the PKA and PKC inhibitors decreased the level of TH expression per neuron in K+-depolarized cultures without affecting the percentage of TH-positive neurons, we measured the fluorescence intensity of TH-positive neurons in these groups. Neither inhibitor significantly altered the average fluorescence intensity of TH-positive neurons in K+-depolarized cultures (KCl, 54.3 ± 5.8, n = 56 neurons; KCl plus PKA inhibitor, 64.5 ± 8.3, n = 38 neurons; KCl plus PKC inhibitor, 51.0 ± 6.03, n = 37 neurons). Similarly, PD98059 did not significantly alter the average fluorescence intensity of TH-positive neurons in 5 Hz-stimulated cultures (5 Hz, 56.7 ± 9.9, n = 25 neurons; 5 Hz plus PD98059, 64.7 ± 10.1, n = 34 neurons). Moreover, none of the kinase inhibitors significantly affected neuronal survival (Table2). Together, these data indicate that TH expression is regulated by distinct protein kinase pathways after patterned electrical stimulation and chronic K+ depolarization, respectively.
Fig. 7.
TH induction by 5 Hz stimulation and potassium depolarization, respectively, requires activation of distinct intracellular kinase pathways. E16.5 PG neurons were stimulated for 6 hr at 5 Hz (gray bars) or with 40 mmKCl (black bars) in the presence or absence of the PKA antagonist PKI14–22 amide-myristoylated, the PKC antagonist PKC-I19–27-myristoylated, the CaMK II inhibitor autocamtide-2 inhibitory peptide-myristoylated, or the MAPK pathway inhibitor PD98059. Cultures were incubated for 1 hr with each drug (50 μm) before stimulation. After stimulation, cultures were grown an additional 12 hr in control conditions. Eachbar represents the percentage of total neurons exhibiting TH immunoreactivity presented as the mean ± SEM. Comparisons were made using ANOVA followed by Duncan's multiple range test; *p ≤ 0.05 compared with KCl alone; ***p ≤ 0.001 compared with 5 Hz stimulation alone;n.s., not significantly different from 5 Hz stimulation alone. Ctrl, Control.
Table 2.
Neuronal survival in vitro is unaffected by treatment with protein kinase inhibitors
| Treatment | Survival/ganglion |
|---|---|
| Control | 624 ± 43 |
| 5 Hz | 609 ± 46 |
| KCl | 613 ± 40 |
| AIP | 615 ± 49 |
| 5 Hz + AIP | 607 ± 28 |
| KCl + AIP | 664 ± 23 |
| PD98059 | 585 ± 54 |
| 5 Hz + PD98059 | 591 ± 55 |
| KCl + PD98059 | 639 ± 57 |
| PKI14–22 | 650 ± 40 |
| 5 Hz + PKI14–22 | 596 ± 42 |
| KCl + PKI14–22 | 584 ± 48 |
| PKC-I19–27 | 611 ± 34 |
| 5 Hz + PKC-I19–27 | 563 ± 48 |
| KCl + PKC-I19–27 | 614 ± 53 |
Numbers represent the total number of NF-immunoreactive cells per cultured ganglion (mean ± SEM). AIP, Autocamtide-2 inhibitory peptide.
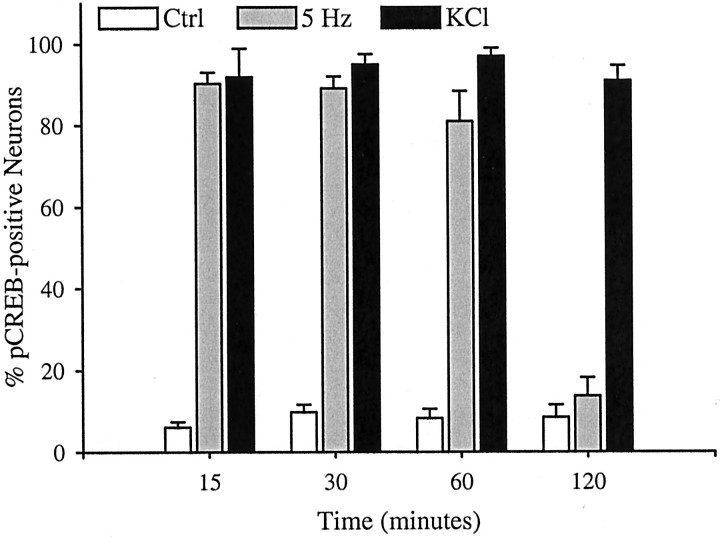
The time course of calcium-induced CREB phosphorylation has been shown previously to be dependent on the route by which calcium enters a neuron (Hardingham et al., 1999). To determine whether K+-induced activation of L-type calcium channels and 5 Hz activation of N-type channels lead to distinct profiles of CREB phosphorylation, we examined expression of pCREB in PG neurons after 5 Hz stimulation and K+depolarization for varying periods of time. Both 5 Hz stimulation and K+ depolarization led to a marked increase in the percentage of pCREB-expressing neurons after 15 min exposure to either protocol (Fig. 8). However, K+ depolarization produced a sustained increase in the percentage of pCREB-positive neurons that could still be observed after 2 hr of continuous depolarization, whereas pCREB was almost undetectable after 2 hr of continuous 5 Hz stimulation (Fig. 8). These data demonstrate a dynamic regulation of pCREB during patterned stimulation, possibly by phosphatase activity (Bito et al., 1996), which is not seen in response to chronic membrane depolarization.
Fig. 8.
Electrical stimulation (5 Hz) and potassium depolarization lead to distinct temporal patterns of CREB phosphorylation. E16.5 PG neurons were stimulated at 5 Hz or with 40 mm KCl for 15, 30, 60, or 120 min. Immediately after the stimulation period, the cultures were fixed and processed for pCREB–NF immunocytochemistry. Each bar represents the percentage of total neurons exhibiting pCREB immunoreactivity. Data are presented as the mean ± SEM. At each time point, except 120 min, 5 Hz and KCl are significantly different from corresponding controls;p ≤ 0.001. At 120 min, only KCl is significantly different from control; p ≤ 0.001.
DISCUSSION
The present findings demonstrate that calcium influx through N-type channels can directly link phasic electrical stimulation to changes in neuronal gene expression. Although N-type calcium channels have been associated predominately with transmitter release (Dunlap et al., 1995), our results are consistent with the fact that N-type calcium channels carry the majority of the high voltage-activated calcium current in many populations of cells, including primary sensory (Nowycky et al., 1985; Mendelowitz and Kunze, 1992; Brosenitsch et al., 1998) and sympathetic (Hirning et al., 1988; Plummer et al., 1989) neurons, as well as pheochromacytoma (PC12) cells (Plummer et al., 1989). Moreover, although N-type calcium channels are localized in discrete patches, presumably at synaptic sites, in mature neurons (Westenbroek et al., 1992, 1998; Haydon et al., 1994; Mills et al., 1994), this is not true during early development. Before synaptogenesis, N-type channels are diffusely expressed over the entire neuronal surface (Jones et al., 1989; Bahls et al., 1998). Thus, in immature neurons, N-type calcium channels are required for multiple cellular events, including neuronal migration (Komuro and Rakic, 1992), axon outgrowth (Doherty et al., 1991), and, as indicated by the present findings, activity-dependent gene expression.
In other neuron types, action potentials, in the absence of synaptic activity, are ineffective at triggering specific intracellular responses, such as TH enzyme activation (Chalazonitis and Zigmond, 1980), CREB phosphorylation (Deisseroth et al., 1996), orc-fos expression (Luckman et al., 1994). For example,Deisseroth and colleagues (1996) demonstrated that CREB phosphorylation induced by patterned stimulation of hippocampal neurons absolutely requires NMDA receptor activation, despite the fact that bulk intracellular and nuclear calcium levels were increased by stimulation alone. This requirement for transynaptic activation has been linked to the fact that L-type calcium channels have relatively slow activation kinetics and are only fully activated by sustained, EPSP-like depolarizations (Nakazawa and Murphy, 1999; Mermelstein et al., 2000), whereas N-type channels are rapidly activating and inactivating (Nowycky et al., 1985; Hirning et al., 1988). In the present study, exposure of sensory neurons to sustained high K+, or rapid, phasic electrical stimuli, induced gene expression by activating L- and N-type channels, respectively. These findings are consistent with the distinct biophysical properties of L- and N-type channels and supports the hypothesis that their differential activation in vivo allows neurons to distinguish between different types of depolarization (Mermelstein et al., 2000). Specifically, N-type calcium channels could transduce rapid depolarizations, such as those resulting from action potentials, whereas L-type channels would preferentially respond to slow, EPSP-like depolarizations. Our findings demonstrate that these two different temporal patterns of depolarization can be encoded by activation of distinct intracellular signaling pathways. Although our data show that dopaminergic traits are induced in sensory neurons by both chronic and phasic depolarizing stimuli, it is possible that expression of other genes is differentially regulated by activation of L- and N-type channels, respectively.
Based on previous studies, in which K+depolarization was used to model neuronal activity, the prevailing view has been that activation of L-type calcium channels links membrane depolarization to calcium-induced gene expression. For example, K+-induced expression of immediate early genes, such as c-fos, requires calcium influx through L-type calcium channels (Morgan and Curran, 1986). In addition, regulation of late response genes, such as transmitter receptor subunits (De Koninck and Cooper, 1995; Gault and Siegel, 1997), transmitter-related proteins (Ai et al., 1998; Brosenitsch et al., 1998; Cigola et al., 1998), ion channels (Schorge et al., 1999), and growth factors (Zafra et al., 1990; Ghosh et al., 1994) by chronic depolarization has been attributed to calcium influx exclusively through L-type calcium channels. However, in these models, N-type channels were most likely inactivated by the level of sustained depolarization produced by chronic K+ treatment and thus unable to flux calcium. For example, we found previously that primary sensory neurons treated with 40 mm KCl exhibit membrane potentials of approximately −20 mV, a voltage at which 72% of the total calcium current is inactivated after a 2 sec conditioning pulse (Brosenitsch et al., 1998). This finding suggests that the use of chronic elevated K+ as a model of neuronal activity may generally preclude the ability to assess the role of N-type calcium channels in gene expression. On the other hand, post-translational events, such as TH enzyme activation by short-term (90 sec) exposure to elevated K+, have been shown to require N-type channels (Rittenhouse and Zigmond, 1991,1999).
Our data indicate that the intracellular signaling cascades required for gene expression in response to patterned electrical stimulation are distinct from those recruited by chronic membrane depolarization. This differential requirement for specific intracellular protein kinases could arise from differences in the amplitude of the cytosolic or nuclear calcium signal (Hardingham et al., 1997; Chawla et al., 1998), the frequency or kinetics of calcium influx (Buonanno and Fields, 1999), or the fact that the route of calcium entry activated by these two stimulation paradigms are distinct (Finkbeiner and Greenberg, 1998). Over the past few years, evidence has accumulated that the route by which calcium enters the cytosol is important for determining which intracellular signaling pathways are recruited and, subsequently, which genes are expressed (Bading et al., 1993; Lerea and McNamara, 1993;Deisseroth et al., 1996; Hardingham et al., 1999; Hu et al., 1999). For example, K+-induced L-type calcium channel activation in hippocampal neurons leads to sustained pCREB and activation of CREB-binding protein-dependent transcription through a CaMK IV-dependent mechanism, whereas activation of NMDA receptors results in transient pCREB and CamK IV-independent transcription (Hardingham et al., 1999). In addition, K+-induced L-type channel activation in cortical neurons results in transient CaMK II activity and weak PKA activity, whereas NMDA receptor activation produces more sustained CaMK II activity and no PKA activation (Hu et al., 1999). These studies demonstrate clear differences in the intracellular response to activation of two distinct classes of calcium channels, i.e., ligand-gated ionotropic glutamate receptors and voltage-gated L-type calcium channels. Our data indicate that activation of distinct intracellular signaling pathways also occurs when calcium enters through different subtypes of voltage-activated calcium channels. An additional possibility, however, is that the requirement for distinct kinases results from different levels or spatial patterns of calcium influx produced by each stimulation paradigm. For example, Hardingham and colleagues (1997) demonstrated that an increase in nuclear calcium is required for CRE-mediated transcription in response to L-type channel activation, whereas an increase in cytoplasmic calcium, in the absence of nuclear calcium elevation, is not sufficient to induce CRE-mediated transcription, although serum response element-dependent transcription is maintained. Thus, stimuli sufficient to elevate nuclear calcium could induce gene expression through mechanisms that are distinct from stimuli that produce only local cytoplasmic elevations in intracellular calcium.
Previous studies examining the role of kinases in calcium-activated gene expression have implicated numerous intracellular cascades in this process depending on the cell type, stage of development, and type of stimulation. Our data indicate that at least two kinases, PKA and PKC, are required for TH induction by patterned stimulation of fetal primary sensory neurons. PKA can be activated by intracellular calcium influx and has been implicated previously in calcium-induced gene expression in primary neurons, as well as PC12 cells (Ginty et al., 1991; Thompson et al., 1995). K+-induced TH expression, on the other hand, was independent of PKA and PKC and was only partially attenuated by a MAPK pathway inhibitor, suggesting that kinases other than MAPK, PKA, PKC, and CaMK II are involved. Alternatively, inhibition of one kinase could be compensated for by activation of multiple cascades by calcium influx. For example, depolarization of hippocampal neurons leads to parallel activation of the MAPK pathway, as well as CaMK, each of which is sufficient for maximum CREB phosphorylation (Hardingham et al., 1999). In addition, extensive cross talk between the PKA, PKC, and MAPK pathways has been demonstrated (Grewal et al., 1999; Impey et al., 1999). Finally, although our data suggest that CaMK II is not required for activity-dependent TH induction in sensory neurons, we cannot exclude a role for CaMK IV, which has been localized to the nucleus and can mediate calcium-induced gene expression (Bito et al., 1996; Chawla et al., 1998).
In summary, our findings demonstrate that phasic and chronic depolarizing stimuli induce neuronal gene expression through distinct signaling mechanisms, including activation of different calcium channel subtypes and intracellular kinases. It is possible that the role of N-type calcium channels in gene induction by patterned stimulation is unique to immature neurons in general or to fetal sensory neurons in particular. Nonetheless, the present findings challenge the prevailing view that activation of L-type calcium channels is required for nuclear responses to physiological patterns of neuronal stimulation.
Footnotes
This work was supported by National Institutes of Health Grants HL25830 (Project 2; to D.M.K.) and 5T32NS07118. We thank Drs. Lynn Landmesser, Gary Landreth, Evan Deneris, Vance Lemmon, and Karl Herrup for their very helpful discussions of this work. We also thank Drs. R. Douglas Fields and Agnieszka Balkowiec for advice regarding electrical field stimulation of neuronal cultures.
Correspondence should be addressed to Dr. David M. Katz, Department of Neurosciences, Case Western Reserve University School of Medicine, 10900 Euclid Avenue, Cleveland, OH 44106. E-mail: dmk4@po.cwru.edu.
REFERENCES
- 1.Ai X, MacPhedran SE, Hall AK. Depolarization stimulates initial calcitonin gene-related peptide expression by embryonic sensory neurons in vitro. J Neurosci. 1998;18:9294–9302. doi: 10.1523/JNEUROSCI.18-22-09294.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 3.Bading H, Ginty DD, Greenberg ME. Regulation of gene expression in hippocampal neurons by distinct calcium signaling pathways. Science. 1993;260:181–186. doi: 10.1126/science.8097060. [DOI] [PubMed] [Google Scholar]
- 4.Bahls FH, Lartius R, Trudeau LE, Doyle RT, Fang Y, Witcher D, Campbell K, Haydon PG. Contact-dependent regulation of N-type calcium channel subunits during synaptogenesis. J Neurobiol. 1998;35:198–208. doi: 10.1002/(sici)1097-4695(199805)35:2<198::aid-neu6>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 5.Bairam A, Hannhart B, Chone C, Marchal F. Effects of dopamine on the carotid chemosensory response to hypoxia in newborn kittens. Respir Physiol. 1993;94:297–307. doi: 10.1016/0034-5687(93)90025-6. [DOI] [PubMed] [Google Scholar]
- 6.Balkowiec A, Katz DM. Activity-dependent release of endogenous brain-derived neurotrophic factor from primary sensory neurons detected by ELISA in situ. J Neurosci. 2000;20:7417–7423. doi: 10.1523/JNEUROSCI.20-19-07417.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bito H, Deisseroth K, Tsien RW. CREB phosphorylation and dephosphorylation: a Ca2+- and stimulus duration-dependent switch for hippocampal gene expression. Cell. 1996;87:1203–1214. doi: 10.1016/s0092-8674(00)81816-4. [DOI] [PubMed] [Google Scholar]
- 8.Brosenitsch TA, Salgado-Commissariat D, Kunze DL, Katz DM. A role for L-type calcium channels in developmental regulation of transmitter phenotype in primary sensory neurons. J Neurosci. 1998;18:1047–1055. doi: 10.1523/JNEUROSCI.18-03-01047.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buonanno A, Fields RD. Gene regulation by patterned electrical activity during neural and skeletal muscle development. Curr Opin Neurobiol. 1999;9:110–120. doi: 10.1016/s0959-4388(99)80014-2. [DOI] [PubMed] [Google Scholar]
- 10.Chalazonitis A, Zigmond RE. Effects of synaptic and antidromic stimulation on tyrosine hydroxylase activity in the rat superior cervical ganglion. J Physiol (Lond) 1980;300:525–538. doi: 10.1113/jphysiol.1980.sp013177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chawla S, Hardingham GE, Quinn DR, Bading H. CBP: a signal-regulated transcriptional coactivator controlled by nuclear calcium and CaM kinase IV. Science. 1998;281:1505–1509. doi: 10.1126/science.281.5382.1505. [DOI] [PubMed] [Google Scholar]
- 12.Cigola E, Volpe BT, Lee JW, Franzen L, Baker H. Tyrosine hydroxylase expression in primary cultures of olfactory bulb: role of L-type calcium channels. J Neurosci. 1998;18:7638–7649. doi: 10.1523/JNEUROSCI.18-19-07638.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cooper E. Synapse formation among developing sensory neurones from rat nodose ganglia grown in tissue culture. J Physiol (Lond) 1984;351:263–274. doi: 10.1113/jphysiol.1984.sp015244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.De Koninck P, Cooper E. Differential regulation of neuronal nicotinic ACh receptor subunit genes in cultured neonatal rat sympathetic neurons: specific induction of α7 by membrane depolarization through a Ca2+/calmodulin-dependent kinase pathway. J Neurosci. 1995;15:7966–7978. doi: 10.1523/JNEUROSCI.15-12-07966.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 16.Doherty P, Ashton SV, Moore SE, Walsh FS. Morphoregulatory activities of NCAM and N-cadherin can be accounted for by G protein-dependent activation of L- and N-type neuronal Ca2+ channels. Cell. 1991;67:21–33. doi: 10.1016/0092-8674(91)90569-k. [DOI] [PubMed] [Google Scholar]
- 17.Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- 18.Dunlap K, Luebke JI, Turner TJ. Exocytotic Ca2+ channels in mammalian central neurons. Trends Neurosci. 1995;18:89–98. [PubMed] [Google Scholar]
- 19.Eichholtz T, de Bont DB, de Widt J, Liskamp RM, Ploegh HL. A myristoylated pseudosubstrate peptide, a novel protein kinase C inhibitor. J Biol Chem. 1993;268:1982–1986. [PubMed] [Google Scholar]
- 20.Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Finkbeiner S, Greenberg ME. Ca2+ channel-regulated neuronal gene expression. J Neurobiol. 1998;37:171–189. [PubMed] [Google Scholar]
- 22.Finley JC, Polak J, Katz DM. Transmitter diversity in carotid body afferent neurons: dopaminergic and peptidergic phenotypes. Neuroscience. 1992;51:973–987. doi: 10.1016/0306-4522(92)90534-9. [DOI] [PubMed] [Google Scholar]
- 23.Gault LM, Siegel RE. Expression of the GABAA receptor δ subunit is selectively modulated by depolarization in cultured rat cerebellar granule neurons. J Neurosci. 1997;17:2391–2399. doi: 10.1523/JNEUROSCI.17-07-02391.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ghosh A, Carnahan J, Greenberg ME. Requirement for BDNF in activity-dependent survival of cortical neurons. Science. 1994;263:1618–1623. doi: 10.1126/science.7907431. [DOI] [PubMed] [Google Scholar]
- 25.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- 26.Grewal SS, York RD, Stork PJ. Extracellular-signal-regulated kinase signalling in neurons. Curr Opin Neurobiol. 1999;9:544–553. doi: 10.1016/S0959-4388(99)00010-0. [DOI] [PubMed] [Google Scholar]
- 27.Gu X, Spitzer NC. Breaking the code: regulation of neuronal differentiation by spontaneous calcium transients. Dev Neurosci. 1997;19:33–41. doi: 10.1159/000111183. [DOI] [PubMed] [Google Scholar]
- 28.Hardingham GE, Chawla S, Johnson CM, Bading H. Distinct functions of nuclear and cytoplasmic calcium in the control of gene expression. Nature. 1997;385:260–265. doi: 10.1038/385260a0. [DOI] [PubMed] [Google Scholar]
- 29.Hardingham GE, Chawla S, Cruzalegui FH, Bading H. Control of recruitment and transcription-activating function of CBP determines gene regulation by NMDA receptors and L-type calcium channels. Neuron. 1999;22:789–798. doi: 10.1016/s0896-6273(00)80737-0. [DOI] [PubMed] [Google Scholar]
- 30.Harris TE, Persaud SJ, Jones PM. Pseudosubstrate inhibition of cyclic AMP-dependent protein kinase in intact pancreatic islets: effects on cyclic AMP-dependent and glucose-dependent insulin secretion. Biochem Biophys Res Commun. 1997;232:648–651. doi: 10.1006/bbrc.1997.6344. [DOI] [PubMed] [Google Scholar]
- 31.Haydon PG, Henderson E, Stanley EF. Localization of individual calcium channels at the release face of a presynaptic nerve terminal. Neuron. 1994;13:1275–1280. doi: 10.1016/0896-6273(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 32.Hertzberg T, Brosenitsch T, Katz DM. Depolarizing stimuli induce high levels of dopamine synthesis in fetal rat sensory neurons. NeuroReport. 1995;7:233–237. [PubMed] [Google Scholar]
- 33.Hirning LD, Fox AP, McCleskey EW, Olivera BM, Thayer SA, Miller RJ, Tsien RW. Dominant role of N-type Ca2+ channels in evoked release of norepinephrine from sympathetic neurons. Science. 1988;239:57–61. doi: 10.1126/science.2447647. [DOI] [PubMed] [Google Scholar]
- 34.Hu SC, Chrivia J, Ghosh A. Regulation of CBP-mediated transcription by neuronal calcium signaling. Neuron. 1999;22:799–808. doi: 10.1016/s0896-6273(00)80738-2. [DOI] [PubMed] [Google Scholar]
- 35.Impey S, Obrietan K, Storm DR. Making new connections: role of ERK/MAP kinase signaling in neuronal plasticity. Neuron. 1999;23:11–14. doi: 10.1016/s0896-6273(00)80747-3. [DOI] [PubMed] [Google Scholar]
- 36.Ishida A, Kameshita I, Okuno S, Kitani T, Fujisawa H. A novel highly specific and potent inhibitor of calmodulin-dependent protein kinase II. Biochem Biophys Res Commun. 1995;212:806–812. doi: 10.1006/bbrc.1995.2040. [DOI] [PubMed] [Google Scholar]
- 37.Jones OT, Kunze DL, Angelides KJ. Localization and mobility of ω-conotoxin-sensitive Ca2+ channels in hippocampal CA1 neurons. Science. 1989;244:1189–1193. doi: 10.1126/science.2543080. [DOI] [PubMed] [Google Scholar]
- 38.Katz DM, Markey KA, Goldstein M, Black IB. Expression of catecholaminergic characteristics by primary sensory neurons in the normal adult rat in vivo. Proc Natl Acad Sci USA. 1983;80:3526–3530. doi: 10.1073/pnas.80.11.3526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Komuro H, Rakic P. Selective role of N-type calcium channels in neuronal migration. Science. 1992;257:806–809. doi: 10.1126/science.1323145. [DOI] [PubMed] [Google Scholar]
- 40.Lein ES, Shatz CJ. Rapid regulation of brain-derived neurotrophic factor mRNA within eye-specific circuits during ocular dominance column formation. J Neurosci. 2000;20:1470–1483. doi: 10.1523/JNEUROSCI.20-04-01470.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lerea LS, McNamara JO. Ionotropic glutamate receptor subtypes activate c-fos transcription by distinct calcium-requiring intracellular signaling pathways. Neuron. 1993;10:31–41. doi: 10.1016/0896-6273(93)90239-n. [DOI] [PubMed] [Google Scholar]
- 42.Li W, Llopis J, Whitney M, Zlokarnik G, Tsien RY. Cell-permeant caged InsP3 ester shows that Ca2+ spike frequency can optimize gene expression. Nature. 1998;392:936–941. doi: 10.1038/31965. [DOI] [PubMed] [Google Scholar]
- 43.Luckman SM, Dyball RE, Leng G. Induction of c-fos expression in hypothalamic magnocellular neurons requires synaptic activation and not simply increased spike activity. J Neurosci. 1994;14:4825–4830. doi: 10.1523/JNEUROSCI.14-08-04825.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Mendelowitz D, Kunze DL. Characterization of calcium currents in aortic baroreceptor neurons. J Neurophysiol. 1992;68:509–517. doi: 10.1152/jn.1992.68.2.509. [DOI] [PubMed] [Google Scholar]
- 45.Mermelstein PG, Bito H, Deisseroth K, Tsien RW. Critical dependence of cAMP response element-binding protein phosphorylation on L-type calcium channels supports a selective response to EPSPs in preference to action potentials. J Neurosci. 2000;20:266–273. doi: 10.1523/JNEUROSCI.20-01-00266.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mills LR, Niesen CE, So AP, Carlen PL, Spigelman I, Jones OT. N-type Ca2+ channels are located on somata, dendrites, and a subpopulation of dendritic spines on live hippocampal pyramidal neurons. J Neurosci. 1994;14:6815–6824. doi: 10.1523/JNEUROSCI.14-11-06815.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mizusawa A, Ogawa H, Kikuchi Y, Hida W, Kurosawa H, Okabe S, Takishima T, Shirato K. In vivo release of glutamate in nucleus tractus solitarii of the rat during hypoxia. J Physiol (Lond) 1994;478:55–66. doi: 10.1113/jphysiol.1994.sp020229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 49.Nakazawa H, Murphy TH. Activation of nuclear calcium dynamics by synaptic stimulation in cultured cortical neurons. J Neurochem. 1999;73:1075–1083. doi: 10.1046/j.1471-4159.1999.0731075.x. [DOI] [PubMed] [Google Scholar]
- 50.Nowycky MC, Fox AP, Tsien RW. Three types of neuronal calcium channel with different calcium agonist sensitivity. Nature. 1985;316:440–443. doi: 10.1038/316440a0. [DOI] [PubMed] [Google Scholar]
- 51.Plummer MR, Logothetis DE, Hess P. Elementary properties and pharmacological sensitivities of calcium channels in mammalian peripheral neurons. Neuron. 1989;2:1453–1463. doi: 10.1016/0896-6273(89)90191-8. [DOI] [PubMed] [Google Scholar]
- 52.Rittenhouse AR, Zigmond RE. ω-conotoxin inhibits the acute activation of tyrosine hydroxylase and the stimulation of norepinephrine release by potassium depolarization of sympathetic nerve endings. J Neurochem. 1991;56:615–622. doi: 10.1111/j.1471-4159.1991.tb08194.x. [DOI] [PubMed] [Google Scholar]
- 53.Rittenhouse AR, Zigmond RE. Role of N- and L-type calcium channels in depolarization-induced activation of tyrosine hydroxylase and release of norepinephrine by sympathetic cell bodies and nerve terminals. J Neurobiol. 1999;40:137–148. doi: 10.1002/(sici)1097-4695(199908)40:2<137::aid-neu1>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- 54.Schorge S, Gupta S, Lin Z, McEnery MW, Lipscombe D. Calcium channel activation stabilizes a neuronal calcium channel mRNA. Nat Neurosci. 1999;2:785–790. doi: 10.1038/12153. [DOI] [PubMed] [Google Scholar]
- 55.Svoboda K, Mainen ZF. Synaptic [Ca2+]: intracellular stores spill their guts. Neuron. 1999;22:427–430. doi: 10.1016/s0896-6273(00)80698-4. [DOI] [PubMed] [Google Scholar]
- 56.Thompson MA, Ginty DD, Bonni A, Greenberg ME. L-type voltage-sensitive Ca2+ channel activation regulates c-fos transcription at multiple levels. J Biol Chem. 1995;270:4224–4235. doi: 10.1074/jbc.270.9.4224. [DOI] [PubMed] [Google Scholar]
- 57.Westenbroek RE, Hell JW, Warner C, Dubel SJ, Snutch TP, Catterall WA. Biochemical properties and subcellular distribution of an N-type calcium channel α1 subunit. Neuron. 1992;9:1099–1115. doi: 10.1016/0896-6273(92)90069-p. [DOI] [PubMed] [Google Scholar]
- 58.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Zafra F, Hengerer B, Leibrock J, Thoenen H, Lindholm D. Activity dependent regulation of BDNF and NGF mRNAs in the rat hippocampus is mediated by non-NMDA glutamate receptors. EMBO J. 1990;9:3545–3550. doi: 10.1002/j.1460-2075.1990.tb07564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhong H, Zhang M, Nurse CA. Synapse formation and hypoxic signalling in co-cultures of rat petrosal neurones and carotid body type 1 cells. J Physiol (Lond) 1997;503:599–612. doi: 10.1111/j.1469-7793.1997.599bg.x. [DOI] [PMC free article] [PubMed] [Google Scholar]