Abstract
The activation of δ-opioid receptors (DORs) in the caudate-putamen nucleus (CPN) produces regionally distinct changes in motor functions, many of which are also influenced by opioids active at μ-opioid receptors (MORs). These actions most likely occur in MOR-enriched patch compartments in the CPN. To determine the functional sites for DOR activation and potential interactions involving MOR in these regions, immunoperoxidase and immunogold–silver labeling methods were applied reversibly for the ultrastructural localization of DOR and MOR in single rat brain sections containing patches of the CPN. DOR immunoreactivity was commonly seen within the cytoplasm of spiny and aspiny neurons, many of which also expressed MOR. In dendrites and spines, DOR labeling was preferentially localized to membranes of the smooth endoplasmic reticulum and spine apparatus, whereas MOR showed a prominent plasmalemmal distribution. DOR- and/or MOR-labeled spines received asymmetric, excitatory synapses, some of which showed notable perforations, suggesting the involvement of these receptors in activity-dependent synaptic plasticity. DORs were more frequently detected than were MORs within axon terminals that formed either asymmetric synapses with spine heads or symmetric synapses with spine necks. Our results suggest that in striatal patches, DORs, often in cooperation with MORs, play a direct modulatory role in controlling the postsynaptic excitability of spines, whereas presynaptic neurotransmitter release onto spines is mainly influenced by DOR activation. In comparison with MOR, the prevalent association of DOR with cytoplasmic organelles that are involved in intracellular trafficking of cell surface proteins suggests major differences in availability of these receptors to extracellular opioids.
Keywords: electron microscopic immunocytochemistry, locomotor activity, opioid receptor, rat caudate-putamen nucleus, spine apparatus, synaptic plasticity
The caudate-putamen nucleus (CPN) is a vital area for the integration and regulation of motor and cognitive functions that are mediated by interactions involving afferents and local neurons that reside in patch or matrix compartments (Parent and Hazrati, 1995; Calabresi et al., 1997). Although both compartments contain mainly GABAergic spiny neurons, they show marked differences in intrinsic neurotransmitters and receptors (Angulo and McEwen, 1994; Holt et al., 1997), as well as cortical and subcortical connectivity (Gerfen, 1984, 1989). The patches are enriched in μ-opioid receptors (MORs). Within these regions, MORs are mainly localized in dendritic spines that are targeted by both dopaminergic and cortically derived terminals (Wang et al., 1997; Wang and Pickel, 1998). Together, these observations suggest that like dopamine (Dumartin et al., 1998; Arbuthnott et al., 2000), opiates active at MOR are modulators of the postsynaptic excitability of striatal dendritic spines.
In contrast with MOR, δ-opioid receptors (DORs) are present in both patch and matrix compartments (Mansour et al., 1987), suggesting partially overlapping but distinct roles for these receptors in the development and maintenance of opiate-mediated behaviors (Shippenberg et al., 1987; Bals-Kubik et al., 1990; Meyer and Meyer, 1993; Meyer et al., 1995; Negri et al., 1995). This conclusion is consistent with the fact that agonists selective for DOR and/or MOR, acting mainly in the CPN, produce similar locomotor activation but qualitatively different stereotypical behaviors (Michael-Titus et al., 1989; Mickley et al., 1990). Such differences may reflect particular regional and cellular targeting of DOR and MOR in the CPN.
The relatively low density of DOR in comparison with MOR in striatal patches, together with the small volume of patches (Johnston et al., 1990; Desban et al., 1993), has contributed to the limited knowledge of the specific consequences of DOR activation in patches. Microinjections of the DOR agonist d-Pen2,d-Pen5-enkephalin into the rat CPN result in large increases in motor behaviors related to reward (Johnson and Stellar, 1994). Similar reward-related motor functions also have been ascribed to the activation of neurons in the striatal patches (White and Hiroi, 1998), suggesting that in these regions DOR and MOR may play similar or complementary roles. A potentially additive interaction between DOR and MOR has been suggested in spinal-brainstem regions by the reduced δ-analgesia and the absence of δ-respiratory depression in MOR-deficient mice (Sora et al., 1997; Matthes et al., 1998). Moreover, in spinal cord, MOR and DOR are coexpressed in many of the same neurons (Cheng et al., 1997), but neither the subcellular distribution of DOR nor the relationship of DOR to MOR has been examined in striatal patches.
Thus, to determine the functional sites of DOR activation and potential interaction with MOR in striatal patches, we examined the electron microscopic immunocytochemical localization of sequence-specific antisera against DOR and MOR in the rat brain. The results provide the first evidence of preferential cytoplasmic distributions of DOR in comparison with MOR in spiny dendrites, suggesting interrelated but distinct roles of these two receptors in the responsiveness of striatal neurons.
MATERIALS AND METHODS
Antibodies. A guinea pig polyclonal antiserum against a peptide corresponding to the extracellular N terminal [amino acids (aa) 34–47] of the mouse DOR (Immuno-Dynamics, Inc., La Jolla, CA) and a rabbit polyclonal antiserum raised against a synthetic peptide corresponding to the intracellular C terminal (aa 384–398) of the rat MOR1 (DiaSorin, Stillwater, MN) were used in this study. The high specificities for antisera against DOR or MOR have been shown previously (Arvidsson et al., 1995; Cheng et al., 1995). The selective cellular distributions of these antisera also have been demonstrated in the rat CPN (Wang and Pickel, 1998; Wang et al., 1999) and in other brain regions (Commons and Milner, 1996, 1997; Svingos et al., 1998).
Tissue preparation. All procedures involving animals and their care were conducted in conformity with the National Institutes of Health Guidelines for the Care and Use of Laboratory Animals and were approved by the Research Animal Resource Center at Weill Medical College of Cornell University.
The methods used for fixation and dual immunolabeling were based on those described by Chan et al. (1990). Seven adult male Sprague Dawley rats (250–350 gm; Taconic, Germantown, NY) were anesthetized with sodium pentobarbital (100 mg/kg, i.p.). The anesthetized animals were perfused through the ascending aorta with 10–15 ml of heparin saline (1000 U/ml), followed by 50 ml of 3.8% acrolein and 2% paraformaldehyde in 0.1 m phosphate buffer (PB), pH 7.4, and then by 200 ml of 2% paraformaldehyde. The brains were removed and dissected into 5-mm-thick coronal blocks. The blocks containing the CPN were post-fixed in 2% paraformaldehyde for an additional 30 min. Sections of 40 μm thickness were cut on a Leica Vibratome VT1000 S (Leica Instruments GmbH, Nussloch, Germany) in chilled 0.1m PB. These sections were then incubated for 30 min in 1% sodium borohydride in 0.1 m PB to remove excess aldehydes and rinsed in 0.1 m PB until no bubbles emerged from the tissue. To enhance the penetration of immunoreagents, sections for electron microscopy were incubated for 15 min in a cryoprotectant (25% sucrose and 2.5% glycerol in 0.05 m PB), frozen rapidly in liquid Freon followed by liquid nitrogen, and thawed in PB at room temperature. The sections were incubated for 30 min in 0.5% bovine serum albumin (BSA) in 0.1 m Tris-buffered saline (TBS), pH 7.6, to reduce nonspecific staining and then processed for dual immunocytochemical labeling.
Dual immunocytochemical labeling. For immunocytochemical localization of DOR and MOR, previously prepared sections through the CPN were processed for combined immunoperoxidase and immunogold–silver labeling before plastic embedding. All incubations were performed with continuous agitation. Because of known differences in sensitivity and resolution, immunogold and immunoperoxidase were used reversibly for DOR and MOR labeling in sections from all seven rats. Sections were incubated in primary antisera solutions for 1 d at room temperature and the consecutive day at 4°C. The solutions were prepared in a 0.1% BSA and TBS solution containing (1) guinea pig polyclonal antiserum for DOR (1:1000 for immunoperoxidase; 1:500 for immunogold) and (2) rabbit polyclonal antiserum for MOR (1:12,000 for immunoperoxidase; 1:4000 for immunogold). After the primary antisera incubation, the sections were processed sequentially for immunoperoxidase and then for immunogold detection.
For immunoperoxidase labeling, the sections were incubated for 30 min in biotinylated IgG (1:400 in TBS and 0.1% BSA), either goat anti-guinea pig (Vector Laboratories, Burlingame, CA) or donkey anti-rabbit (Jackson ImmunoResearch, West Grove, PA), and then in avidin–biotin–peroxidase complex (Vectastain Elite kit; Vector Laboratories; 1:100 in TBS) for 30 min. The immunoperoxidase that was bound to the sections was visualized by an incubation for 6 min in 0.022% 3,3′diaminobenzidine (DAB; Aldrich, Milwaukee, WI) and 0.003% hydrogen peroxide in TBS. Sections were rinsed in TBS between incubations.
For immunogold–silver labeling, the sections were rinsed in 0.01m PBS, pH 7.4, blocked in 0.8% BSA and 0.1% gelatin in PBS for 10 min, and then incubated in either goat anti-guinea pig (for DOR) IgG conjugated with 0.8 nm gold particles (Electron Microscopy Sciences, Fort Washington, PA) or goat anti-rabbit (for MOR) IgG conjugated with 1 nm gold particles (AuroProbeOne; Amersham, Arlington Heights, IL), 1:50 in the BSA–gelatin blocking solution for 2–3 hr. Sections were rinsed in PBS and post-fixed in 2% glutaraldehyde in PBS for 10 min. The gold particles were silver enhanced by using the IntenSE-M kit (Amersham) for 7–9 min to obtain the most optimal visualization.
To test the specificity of the secondary antibodies in dual labeling, some of the sections incubated in guinea pig primary antiserum were treated with either biotinylated or gold-conjugated anti-rabbit secondary IgG, followed by immunoperoxidase or immunogold detection. Adjacent sections, which were incubated in rabbit primary antibody, were processed using either biotinylated or gold-conjugated anti-guinea pig secondary IgG before immunoperoxidase or immunogold detection. No immunoreaction product was detected after such incubations.
Metals such as zinc are present in the brain (Frederickson, 1989) and may potentially contribute to false-positive results with silver intensification. Thus, silver reactions were examined in the normal rat CPN and in the CPN of the animal that received a systemic injection (1 gm/kg, i.p.) of the heavy metal chelator sodium diethyldithiocarbamate trihydrate (Fluka, Milwaukee, WI) (Veznedaroglu and Milner, 1992). Electron microscopy revealed no silver deposits in the absence of primary and secondary antibodies in the CPN of untreated controls as well as those receiving the chelator.
Electron microscopy. Immunolabeled sections for electron microscopy were post-fixed for 1 hr with 2% osmium tetroxide in 0.1m PB, dehydrated through graded ethanols and propylene oxide, and incubated overnight in a 1:1 mixture of propylene oxide and Epon (EM bed-812; Electron Microscopy Sciences). The sections were transferred to 100% Epon for 2–3 hr and flat-embedded in Epon between two sheets of Aclar plastic film (Allied Signal, Pottsville, PA). In each animal, two to six flat-embedded sections were analyzed by electron microscopy at levels between 1.70 and 0.20 mm anterior to bregma (Paxinos and Watson, 1986). The region examined was located in the dorsal CPN and contained one dually labeled patch. Serial ultrathin sections (65 nm) were cut with a diamond knife (DiATOME U.S.) on an ultramicrotome (Leica Ultracut UCT; Leica, Wien, Austria). These sections were collected on 400 mesh copper grids and counterstained with 5% uranyl acetate followed by Reynolds lead citrate (Reynolds, 1963). The ultrathin sections were examined with a CM10 Philips (Mahwah, NJ) transmission electron microscope at 60 kV.
Electron microscopic data analyses. Because the purpose of this study was to compare the distribution of immunoreactivities of DOR with that of MOR in dually labeled tissues, single-section analysis was used. This analysis was performed on ultrathin sections collected near the surface of the tissue at the interface with Epon-embedding material. The classification of cellular elements was based on Peters et al. (1991). Neuronal perikarya were identified by the presence of a nucleus, Golgi apparatus, and endoplasmic reticulum. Dendrites usually contained abundant endoplasmic reticulum and were postsynaptic to axon terminals, which were distinguished by their content of synaptic vesicles. Unmyelinated axons had diameters of <0.1 μm and contained few synaptic vesicles. Asymmetric synapses showed thick postsynaptic membrane specializations, whereas symmetric synapses showed thin membrane specializations that were equally dense at presynaptic and postsynaptic sites. Nonsynaptic contacts (appositions) were defined by closely parallel plasma membranes, which lacked recognizable synaptic specializations but were not separated by glial processes.
A neuronal profile was considered to be selectively labeled with immunoperoxidase when cytoplasmic precipitates conferred an electron density greater than that of morphologically similar profiles observed within the same section. A profile was defined to be immunogold–silver labeled when two or more particles were seen in large profiles or a single particle was seen in small profiles, such as dendritic spines and unmyelinated small axons. The validity of this approach was confirmed by the fact that virtually no gold–silver deposits were seen in the plastic and tissue regions that were expected to be unlabeled, for example, the myelin of axons.
DOR- and MOR-labeled neuronal profiles were examined in single ultrathin sections taken from the surface of 25 coronal Vibratomesections of seven rats. These sections were scanned most frequently at 15,000× magnification. Criteria for selecting areas to scan included good morphological preservation, presence of immunolabeling, and proximity to the Epon–tissue interface (to minimize undercounting caused by limited immunoreagent penetration). The total scanned area was 15,622 μm2 from 513 electron micrographs. Labeled profiles were counted and classified by subcellular types as described above. The subcellular distributions of immunogold–silver particles for DOR and MOR were also examined in these animals, occupying total areas of 8662 μm2 from 12 Vibratome sections for DOR and 6959 μm2 from 13 Vibratome sections for MOR. Gold particles were counted and sorted according to their distribution relative to (1) synaptic and nonsynaptic plasmalemma, (2) nonmembranous cytoplasm, and (3) cytoplasmic and membranous spine apparatus or smooth endoplasmic reticulum. In each category, the number of gold particles for DOR or MOR was compared by χ2 test (implemented via StatView 5.0; Abacus Concepts, Berkeley, CA). This test normalized and expressed the particles as the proportions to avoid differences that are attributed to different antibody affinities and/or concentrations. The comparison was conducted in selected profiles such as dendrites, spines, axons, and terminals.
Electron micrographs used for illustrations were prepared by using desktop publishing software programs as described previously (Wang et al., 1999).
RESULTS
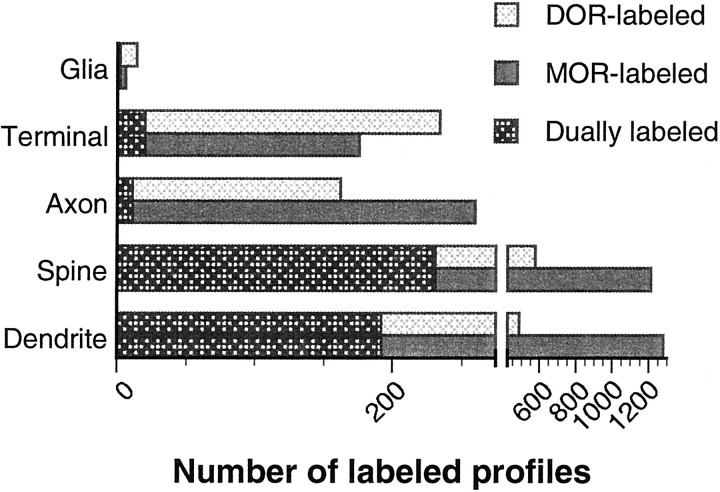
DOR immunoreactivity was seen by light microscopy as a homogeneous distribution with a slight lateral to medial decrease in density throughout the entire CPN. Ultrastructural localization of DOR was examined in MOR-enriched patch compartments by electron microscopy in single sections processed for DOR and MOR dual labeling. Although both DOR and MOR were frequently detected in dendrites and dendritic spines, relatively greater proportions of terminals and glial processes expressed DOR (Fig. 1). Immunogold–silver labeling for DOR and MOR in dendrites, spines, axons, and terminals also showed significant differences with respect to their association with synaptic or extrasynaptic plasma membranes and cytoplasmic organelles (Fig. 2). In addition, electron microscopy revealed that in a few sections from the matrix, the cellular and subcellular distribution pattern of DOR immunoreactivity was similar to that seen in the patches.
Fig. 1.
Comparative distributions of neuronal and glial processes containing δ- and/or μ-opioid receptors (DORs; MORs) in patches of the caudate-putamen nucleus. Bar graphs show the number of singly and dually labeled profiles that were determined by the use of immunogold–silver and immunoperoxidase labeling methods for individual receptors. Data were obtained from 25 Vibratome sections of seven rats, representing a total area of 15,622 μm2.
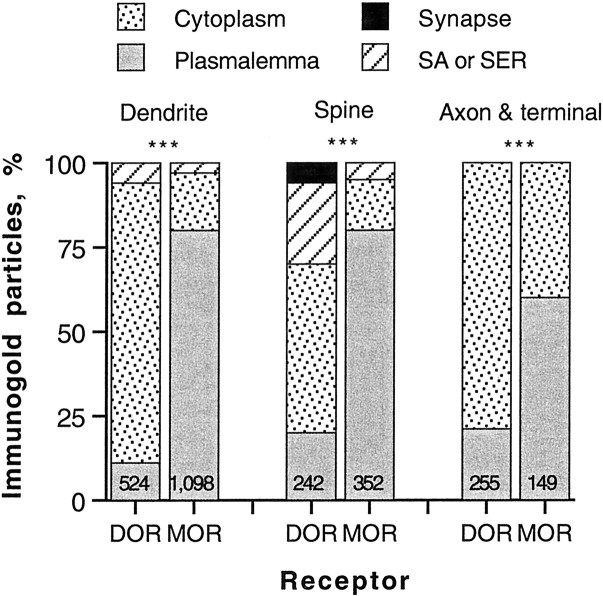
Fig. 2.
Bar graphs show a comparison of the subcellular distributions of immunogold–silver particles for δ- and μ-opioid receptors (DORs; MORs) in striatal patches. The particles in dendrites, spines, axons, and terminals were counted from 25 Vibratome sections of seven rats. A χ2 test showed significant differences (***p < 0.0001) between DOR and MOR in the proportion of immunogold particles associated with the plasmalemma, cytoplasm, spine apparatus (SA) or smooth endoplasmic reticulum (SER), or synaptic specializations in all three subcellular profiles. The numbers in eachcolumn indicate the total number of immunogold–silver particles for DOR or MOR. The total number of labeled profiles included the following: 214 DOR- and 331 MOR-labeled dendrites, 169 DOR- and 225 MOR-labeled spines, and 189 DOR- and 108 MOR-labeled axons and terminals.
Somatodendritic localization of DOR
Immunogold–silver particles for DOR were readily detected by electron microscopy in many neuronal perikarya. Most of these perikarya had ultrastructural features characteristic of striatal spiny neurons showing a round or oval unindented nuclear membrane and a modest amount of cytoplasm (Fig. 3A). A few DOR-labeled perikarya contained a large indented nucleus (Fig.3B) that is typical of aspiny interneurons (DiFiglia et al., 1980). Gold–silver particles were mainly, but not exclusively, seen in the cytoplasm, where they were associated with cytoplasmic organelles such as Golgi lamellae and endoplasmic reticulum. These DOR-labeled perikarya also were sometimes contacted by dendrites or spines that were DOR and/or MOR immunoreactive (Fig. 3).
Fig. 3.
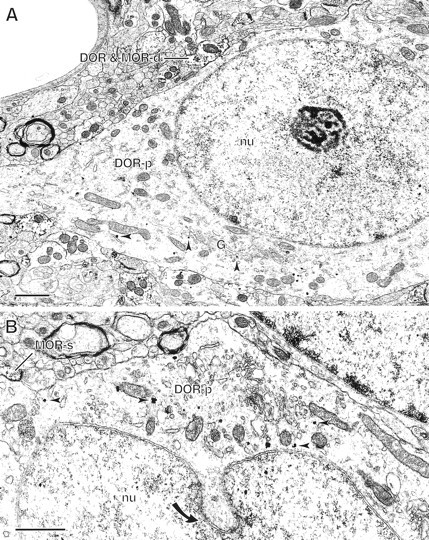
Immunogold–silver labeling for the δ-opioid receptor (DOR) in different types of neuronal perikarya.A, A perikaryon (DOR-p) with a round nucleus (nu) that is characteristic of spiny neurons contains immunogold–silver particles for DOR. These particles are preferentially distributed in the cytoplasm, and some of them (arrowheads) are associated with Golgi lamellae (G) and endoplasmic reticulum. This labeled perikaryon is in contact with a dendrite (DOR & MOR-d) containing immunogold particles for DOR and immunoperoxidase labeling for MOR. B, A perikaryon (DOR-p) with an indented nucleus (nu; solid curved arrow) that is characteristic of aspiny interneurons contains cytoplasmic immunogold–silver particles for DOR (arrowheads). A spine (MOR-s) containing immunoperoxidase labeling for MOR apposes this perikaryon. Scale bars, 1 μm.
Both immunoperoxidase and immunogold–silver labeling revealed a similar localization pattern for DOR in dendrites, many of which were spiny. The labeling was more commonly associated with cytoplasmic organelles and was only occasionally seen on the plasma membrane. Gold–silver particles identifying DORs were mainly associated with larger tubulovesicles, resembling smooth endoplasmic reticulum (SER; see Fig. 5C). DOR labeling was also seen in the spine apparatus, a specialized organelle containing stacks of SER (Spacek and Harris, 1997) (Fig.4A). This subcellular distribution was markedly different from that of the gold–silver deposits for MOR (Fig. 4B), which were predominantly located along the extrasynaptic plasma membrane of dendrites and spines.
Fig. 5.
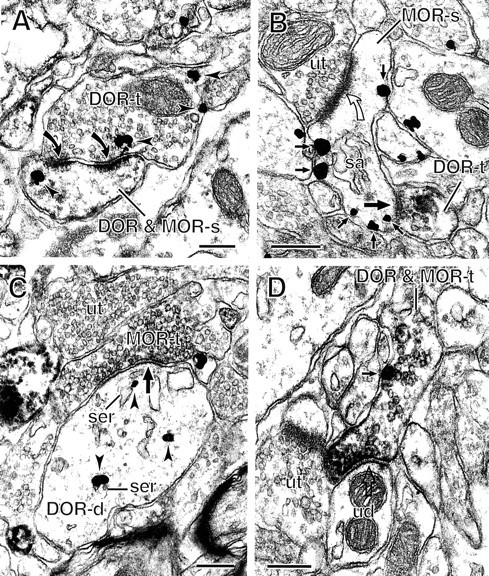
Axon terminals containing δ- and/or μ-opioid receptors (DORs; MORs) form synaptic contacts with spines and dendrites. A, Immunogold particles for DOR (arrowheads) are seen in an axon terminal (DOR-t) that forms an asymmetric, perforated synapse (solid curved arrows) with a spine (DOR & MOR-s) that contains immunogold–silver particles for DOR and immunoperoxidase for MOR. B, A small axon terminal containing peroxidase reaction product for DOR (DOR-t) forms a symmetric synapse (large solid arrow) on the neck of a dendritic spine. The spine (MOR-s) shows plasmalemmal immunogold–silver particles for MOR (small solid arrows) and receives an asymmetric synapse (open curved arrow) from an unlabeled terminal (ut).C, An axon terminal containing immunoperoxidase labeling for MOR (MOR-t) forms a symmetric synapse (large arrow) with a dendrite (DOR-d). This dendrite shows immunogold–silver particles for DOR (arrowheads) located in the cytoplasm, some of which are associated with smooth endoplasmic reticulum (ser). D, An axon terminal (DOR & MOR-t) is dually labeled with immunogold–silver for MOR (small solid arrow) and immunoperoxidase for DOR. The terminal forms a symmetric synapse (large open arrow) with an unlabeled dendrite (ud). sa, Spine apparatus. Scale bars, 0.25 μm.
Fig. 4.
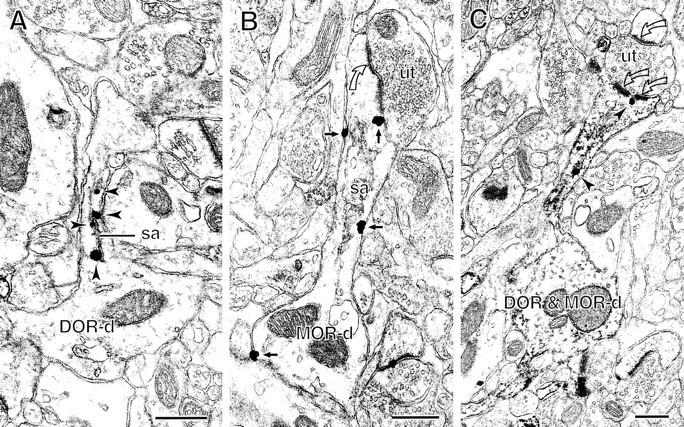
Comparison of labeling for δ- and μ-opioid receptor (DOR; MOR) in spiny dendrites. A, B, Immunogold–silver particles show distinct patterns of subcellular distributions for DOR and MOR. In A, gold particles for DOR (arrowheads) are exclusively located in the spine neck of a dendrite (DOR-d) in association with the spine apparatus (sa). In B, gold particles for MOR (small solid arrows) are distributed at extrasynaptic sites on the plasmalemma of a spiny dendrite (MOR-d). C, A spiny dendrite (DOR & MOR-d) shows immunogold–silver particles for DOR (arrowheads) and diffuse immunoperoxidase labeling for MOR. One gold–silver particle is located in the cytoplasm of the spine neck, and the other is close to the perforated portion of an asymmetric synapse (double open curved arrows). The unlabeled terminal (ut) also forms an asymmetric synapse (single open curved arrow) with an unlabeled spine. Scale bars, 0.4 μm.
Colocalization of DOR and MOR in dendrites and spines
Single dendrites and spines were the profiles that most commonly contained both DOR and MOR immunoreactivities (Fig. 1). The dually labeled dendrites and spines constituted 39% (192/487) and 40% (231/578) of DOR-labeled dendrites and spines, respectively. In contrast, dually labeled profiles comprised only 15% (192/1281) of MOR-labeled dendrites and 19% (231/1214) of MOR-labeled spines. Regardless of whether immunogold or immunoperoxidase markers were used for detection of the respective antisera, DOR immunoreactivity was often located in spine necks in association with the spine apparatus (Fig. 4C). Gold–silver particles for DOR occasionally were in contact with postsynaptic densities at asymmetric axospinous synapses. In contrast, MOR immunoreactivity was distributed along the cytoplasmic surface of the plasma membrane of spiny dendrites. Dually labeled spiny dendrites often received synaptic input from unlabeled axon terminals that formed asymmetric synapses with the spine heads (Fig. 4C). A few unlabeled terminals also formed synaptic but mainly symmetric junctions with the dually labeled dendritic shafts.
The localization pattern of DOR immunoreactivity in dually labeled spines was similar to that seen in single-labeled ones and was quite different from that of MOR. In large and mushroom-like spines, DOR immunoreactivity was frequently seen on membranes of the spine apparatus, indicated by either peroxidase or gold–silver labeling. Occasionally, peroxidase reaction product for DOR was seen on membranes of endocytotic vesicles in spines. In contrast, MOR immunoreactivity was exclusively localized along extrasynaptic membranes of dendrites and spines, as observed previously (Wang et al., 1996, 1997,1999). Both singly and dually labeled spines often received asymmetric, excitatory synapses (Figs. 4C, 5A,B). Some synaptic membrane specializations showed a prominent discontinuity, typical of perforated synapses (Figs. 4C, 5A). The site of the perforation sometimes protruded into the presynaptic terminal and coated evaginations of the membrane. Of the total of 178 dually labeled spines receiving asymmetric synapses, >90% (162/178) received asymmetric synapses from unlabeled terminals (Fig. 4), and <10% (16/178) were contacted by DOR- or MOR-labeled terminals (Fig.5A).
Axonal and terminal localization of DOR and MOR
DOR immunoreactivity was seen in a morphologically heterogeneous population of axon terminals. These included large axon terminals forming asymmetric, excitatory synapses, as well as relatively small terminals showing appositions or symmetric junctions on the lateral head and neck of dendritic spines. The peroxidase and gold–silver labeling for DOR was only occasionally seen on the plasma membrane and more commonly was associated with membranes of synaptic vesicles (Fig.2). This cytoplasmic distribution of DOR was significantly different from that of MOR (Fig. 2). When axospinous contacts were observed between immunoreactive profiles, >90% (61/67) showed presynaptic DOR and postsynaptic MOR or MOR and DOR immunoreactivities (Fig.5A). Conversely, <10% (6/67) involved MOR-labeled terminals and DOR-labeled or dually labeled spines. None of the dually labeled axon terminals formed asymmetric synapses with DOR- or MOR-labeled spines. Several small axon terminals containing DOR or both receptors, however, showed either symmetric synapses (Fig.5B) or nonsynaptic junctional contacts with MOR-labeled or dually labeled spines. All of these terminals contacted the side or necks of spines that also received asymmetric synapses from unlabeled terminals.
As compared with spines, dendrites immunoreactive for DOR and/or MOR less frequently received synaptic input from labeled or unlabeled terminals. Of the total of 25 immunoreactive axodendritic synapses, 84% (21/25) were symmetric, and 16% (4/25) were asymmetric. Nearly half of the total symmetric synapses (9/21) were formed by MOR-labeled terminals (Fig. 5C), 28% (6/21) were formed by unlabeled terminals, 19% (4/21) were formed by DOR-labeled terminals, and <10% (2/21) were formed by dually labeled terminals (Fig.5D).
DISCUSSION
Our results show that in patch compartments of the CPN, DOR and MOR are strategically positioned for dual involvement in modulation of the excitatory postsynaptic responses of spiny projection neurons, some of which contain both receptors. In contrast with MOR, DOR has a preferential cytoplasmic distribution in spiny dendrites and a more prominent presynaptic location (Fig. 6), suggesting major differences in intracellular targeting and transport of these receptor proteins. In addition, a specific role for DOR in activity-dependent synaptic plasticity is supported by our observed preferential association of DOR with the spine apparatus, an organelle that is critically involved in calcium homeostasis and remodeling of dendritic spines (Jones and Harris, 1995; Mattson et al., 2000). Many of these spines also show extrasynaptic plasma membrane labeling for MOR and prominent synaptic perforations that are associated with synaptic efficacy (Neuhoff et al., 1999).
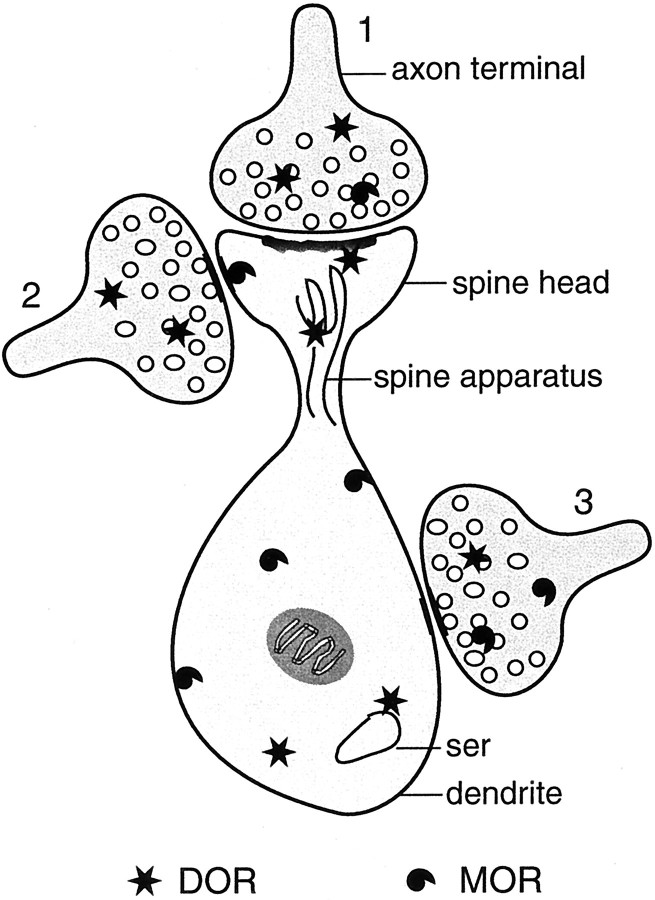
Fig. 6.
Schematic summary of the localization of δ- and μ-opioid receptor (DOR; MOR) immunoreactivities in patch compartments of the CPN. DOR labeling is commonly seen within the cytoplasm of spiny dendrites and associated with the spine apparatus and asymmetric postsynaptic densities on spine heads. Within the same or separate spiny dendrites, MOR labeling shows a prominent plasmalemmal distribution. In addition, DOR is present in morphologically heterogeneous axon terminals (1, 2) that are presynaptic to spines. Axon terminal 1 is a putative corticostriatal afferent that forms an asymmetric synapse with the spine head. Terminal 2 has morphological similarity to dopaminergic terminals and forms a symmetric synapse with the side of the spine head. Terminal 3 is a typical GABAergic terminal, containing mainly MOR, sometimes also DOR, and forming a symmetric synapse with the dendritic shaft. The small open circles in terminals represent synaptic vesicles. ser, Smooth endoplasmic reticulum.
Cytoplasmic DOR distribution in dendrites with or without plasmalemmal MOR
The colocalization of DOR and MOR in dendrites and spines provides the first direct evidence that these opioid receptors are targeted to single neurons in striatal patches. In addition, although previous studies suggested the existence of a DOR–MOR complex in the cellular membranes of the rat striatum (Schoffelmeer et al., 1990), we observed differential plasmalemmal and cytoplasmic distribution of these receptors within individual neurons in the striatal patches. Although these differences could be methodological, this is unlikely, because each antigen has equal access to immunoreagents at the tissue surface. Conceivably, the formation of DOR–MOR complexes is dependent on extracellular availability of agonists, as has been shown for DOR dimerization (Cvejic and Devi, 1997). The consequence of dual activation of DOR and MOR in single neurons is, however, more likely to involve interactions with their respective second messengers affecting levels of cyclic nucleotides and ion channel permeability (Stefani et al., 1994; Acosta and López, 1999).
In this study, DOR immunoreactivity was detected mainly in association with cytoplasmic organelles such as Golgi lamellae and SER that are involved in the delivery and/or return of receptor proteins to the cell surface (Rivera et al., 2000). In contrast to DOR, MOR was preferentially localized to the extrasynaptic plasma membrane of dendrites and spines, as shown previously (Wang et al., 1996, 1997,1999). Differential targeting of DOR and MOR in dendrites and spines may, in part, result from their different molecular structures. The C terminal of the rat MOR contains a domain of Ser–Thr residues that is absent in DOR (Evans et al., 1992; Thompson et al., 1993). Truncation of the Ser–Thr domain in the C terminal results in constitutive activation of internalization and recycling of MORs (Segredo et al., 1997). Different mechanisms that are unrelated to the C terminal are observed, however, in the agonist-induced downregulation and internalization of DOR (Afify et al., 1998). In Neuro2A cells, uncoupling from G-proteins not only inactivates MORs but also prevents them from agonist-induced receptor downregulation, whereas DORs retain high-affinity binding after uncoupling from G-proteins, permitting internalization and downregulation (Chakrabarti et al., 1997). Furthermore, in vitro studies have demonstrated that unlike MOR, overexpression of G-protein-coupled receptor kinase does not result in the enhancement of DOR internalization (Zhang et al., 1998, 1999). Therefore, DOR and MOR internalizations are likely to be regulated by different mechanisms, which may also contribute to their distinct subcellular distributions.
We observed DOR labeling mainly in association with the spine apparatus and SER in striatal spiny dendrites. These cytoplasmic organelles have been implicated in calcium storage (Korkotian and Segal, 1998; Mattson et al., 2000), prevention of excitotoxicity (Harris and Kater, 1994;Segal, 1995), and synaptic plasticity (Jones and Harris, 1995). The plasmalemmal availability of receptor proteins may primarily account for their involvement in synaptic plasticity. The export of DOR from SER is the limiting step in the maturation and cell surface expression of the receptor (Petäjä-Repo et al., 2000). The frequent association of DOR with the SER may thus reflect local availability of the receptor proteins that can be rapidly inserted into the plasma membrane in response to calcium influx, thus permitting adaptive adjustments to excitatory inputs. Such local translocation of plasmalemmal proteins in dendritic spines has been revealed recently in the rat hippocampus (Pierce et al., 2000).
The involvement of DOR in the regulation of postsynaptic excitation is supported by the localization of DOR not only to the spine apparatus but also to asymmetric excitatory postsynaptic membrane specializations in striatal patches. The apparent association of DOR with postsynaptic densities may be, in part, caused by the greater association of DOR with the spine apparatus. The electron-dense material between the lamellae in the spine apparatus is divided into an “inner dense plate” and an “outer dense plate,” the latter being contiguous with the postsynaptic density (Spacek, 1985a). Thus, these structures are likely to play a role in activity-dependent synaptic plasticity and postsynaptic protein synthesis (Westrum et al., 1980; Spacek, 1985a,b). This hypothesis is supported by the prominence of perforated, asymmetric postsynaptic densities on spines immunoreactive to DOR and/or MOR. Perforated synapses are believed to occur in parallel with changes in synaptic activity and efficacy and are intimately associated with the spine apparatus (Spacek and Hartmann, 1983). Thus, the presently observed association of DOR with the spine apparatus and asymmetric postsynaptic densities suggests a major involvement of the receptor in opioid-induced long-term adaptive changes in corticostriatal transmission. Such a role for DOR has been suggested previously in other excitatory pathways (Bramham et al., 1991). In contrast, MOR distribution on plasma membranes of these dendritic spines is more consistent with rapid activation after acute opiate administration.
Preferential presynaptic distribution of DOR
We observed significantly more axon terminals immunolabeled for DOR than for MOR in striatal patches, and many of these terminals formed asymmetric axospinous synapses. Excitatory synaptic plasticity in the CPN is mainly regulated by the interactions between glutamate and dopamine systems (Calabresi et al., 1997; Ingham et al., 1998). The possibility that DOR-labeled terminals forming asymmetric axospinous synapses are cortical in origin is supported by the expression of moderate levels of DOR mRNA and binding sites in the cortex (Mansour et al., 1994, 1995). In contrast, MOR mRNA expression in the cortex is comparatively low, which is consistent with our infrequent detection of MOR in presynaptic axon terminals forming excitatory synapses on spine heads. This does not, however, preclude the possibility of direct presynaptic actions of MOR agonists on corticostriatal terminals as suggested by previous physiological (Jiang and North, 1992) and anatomical (Wang and Pickel, 1998) studies.
Our results also indicate that DOR, as compared with MOR, is more often expressed in small axon terminals that form symmetric synapses with the necks of spines. This type of terminal is typical of cholinergic interneurons (Izzo and Bolam, 1988; Calabresi et al., 2000), as well as dopaminergic nigrostriatal neurons (Bouyer et al., 1984; Freund et al., 1984; Wang et al., 1997). The presence of DOR in acetylcholine- and/or dopamine-containing terminals is consistent with (1) light microscopic studies showing DOR localization within cholinergic interneurons in the CPN (Mansour et al., 1994) and (2) electron microscopic evidence that DOR and the dopamine transporter are colocalized in axon terminals in the ventral striatum (Svingos et al., 1999). DOR agonists also are more efficacious than are MOR agonists in modulating the release of these neurotransmitters within the CPN (Wichmann and Starke, 1990; Dourmap et al., 1992). Together, these observations suggest that in striatal patches DOR plays an important role in modulating the presynaptic release of excitatory amino acids, as well as acetylcholine and/or dopamine.
Unlike the prominent localization of DOR in axon terminals presynaptic to spines, we observed MOR in terminals that mainly formed symmetric synapses on dendrites, some of which contained DOR and/or MOR. These MOR-labeled terminals are morphologically similar to those that have been described for local axon collaterals of GABAergic spiny neurons in the CPN (Smith and Bolam, 1990). Recently, MOR has been shown to be present in ventral striatal GABAergic neurons and axon terminals forming mainly symmetric axodendritic synapses (Svingos et al., 1997). Thus, we suggest that activation of MOR is likely to have a more direct effect than is activation of DOR on modulating the presynaptic release of GABA onto projection neurons in striatal patches.
In summary, our results identify dendritic spines in patch compartments of the CPN as major sites where activation of DOR may directly affect excitatory corticostriatal transmission. The long-term activation of DOR together with MOR within the same spiny neurons is likely to contribute to reward-related motor responses that are seen in opiate addiction (White and Hiroi, 1998). The differential subcellular distributions of DOR and MOR suggest, however, fundamental differences in their functions even when coexpressed in single neurons. This may directly relate to agonist binding and involvement in distinct aspects of activity-dependent synaptic plasticity. In addition, axon terminals are more frequently immunolabeled for DOR than for MOR, suggesting a primary role for DOR in opioid modulation of presynaptic transmitter release. These observations have important implications for our understanding of the role of DOR in adaptive behaviors and drug addition (Quock et al., 1999).
Footnotes
This work was supported by National Institute on Drug Abuse Grant DA046000 to V.M.P. and by National Institute of Mental Health Grant MH00078 to V.M.P. We thank Drs. Adena L. Svingos and Joseph Pierce for their helpful comments on this manuscript.
Correspondence should be addressed to Dr. Hong Wang, Division of Neurobiology, Department of Neurology and Neuroscience, Weill Medical College of Cornell University, 411 East 69th Street, New York, NY 10021. E-mail: hwang@mail.med.cornell.edu.
REFERENCES
- 1.Acosta CG, López HS. δ Opioid receptor modulation of several voltage-dependent Ca2+ currents in rat sensory neurons. J Neurosci. 1999;19:8337–8348. doi: 10.1523/JNEUROSCI.19-19-08337.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Afify EA, Law PY, Riedl M, Elde R, Loh HH. Role of carboxyl terminus of mu- and delta-opioid receptor in agonist-induced down-regulation. Brain Res Mol Brain Res. 1998;54:24–34. doi: 10.1016/s0169-328x(97)00315-x. [DOI] [PubMed] [Google Scholar]
- 3.Angulo JA, McEwen BS. Molecular aspects of neuropeptide regulation and function in the corpus striatum and nucleus accumbens. Brain Res Rev. 1994;19:1–28. doi: 10.1016/0165-0173(94)90002-7. [DOI] [PubMed] [Google Scholar]
- 4.Arbuthnott GW, Ingham CA, Wickens JR. Dopamine and synaptic plasticity in the neostriatum. J Anat. 2000;196:587–596. doi: 10.1046/j.1469-7580.2000.19640587.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Arvidsson U, Riedl M, Chakrabarti S, Lee J-H, Nakano AH, Dado RJ, Loh HH, Law P-Y, Wessendorf MW, Elde R. Distribution and targeting of a μ-opioid receptor (MOR1) in brain and spinal cord. J Neurosci. 1995;15:3328–3341. doi: 10.1523/JNEUROSCI.15-05-03328.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bals-Kubik R, Shippenberg TS, Herz A. Involvement of central mu and delta opioid receptors in mediating the reinforcing effects of beta-endorphin in the rat. Eur J Pharmacol. 1990;175:63–69. doi: 10.1016/0014-2999(90)90153-w. [DOI] [PubMed] [Google Scholar]
- 7.Bouyer JJ, Park DH, Joh TH, Pickel VM. Chemical and structural analysis of the relation between cortical inputs and tyrosine hydroxylase-containing terminals in rat neostriatum. Brain Res. 1984;302:267–275. doi: 10.1016/0006-8993(84)90239-7. [DOI] [PubMed] [Google Scholar]
- 8.Bramham CR, Milgram NW, Srebro B. Delta opioid receptor activation is required to induce LTP of synaptic transmission in the lateral perforant path in vivo. Brain Res. 1991;567:42–50. doi: 10.1016/0006-8993(91)91433-2. [DOI] [PubMed] [Google Scholar]
- 9.Calabresi P, De Murtas M, Bernardi G. The neostriatum beyond the motor function: experimental and clinical evidence. Neuroscience. 1997;78:39–60. doi: 10.1016/s0306-4522(96)00556-8. [DOI] [PubMed] [Google Scholar]
- 10.Calabresi P, Centonze D, Gubellini P, Pisani A, Bernardi G. Acetylcholine-mediated modulation of striatal function. Trends Neurosci. 2000;23:120–126. doi: 10.1016/s0166-2236(99)01501-5. [DOI] [PubMed] [Google Scholar]
- 11.Chakrabarti S, Yang W, Law PY, Loh HH. The mu-opioid receptor down-regulates differently from the delta-opioid receptor: requirement of a high affinity receptor/G protein complex formation. Mol Pharmacol. 1997;52:105–113. [PubMed] [Google Scholar]
- 12.Chan J, Aoki C, Pickel VM. Optimization of differential immunogold-silver and peroxidase labeling with maintenance of ultrastructure in brain sections before plastic embedding. J Neurosci Methods. 1990;33:113–127. doi: 10.1016/0165-0270(90)90015-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cheng PY, Svingos AL, Wang H, Clarke CL, Jenab S, Beczkowska IW, Inturrisi CE, Pickel VM. Ultrastructural immunolabeling shows prominent presynaptic vesicular localization of δ-opioid receptor within both enkephalin- and nonenkephalin-containing axon terminals in the superficial layers of the rat cervical spinal cord. J Neurosci. 1995;15:5976–5988. doi: 10.1523/JNEUROSCI.15-09-05976.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cheng PY, Liu-Chen LY, Pickel VM. Dual ultrastructural immunocytochemical labeling of mu and delta opioid receptors in the superficial layers of the rat cervical spinal cord. Brain Res. 1997;778:367–380. doi: 10.1016/s0006-8993(97)00891-3. [DOI] [PubMed] [Google Scholar]
- 15.Commons KG, Milner TA. Cellular and subcellular localization of delta opioid receptor immunoreactivity in the rat dentate gyrus. Brain Res. 1996;738:181–195. doi: 10.1016/s0006-8993(96)00774-3. [DOI] [PubMed] [Google Scholar]
- 16.Commons KG, Milner TA. Localization of delta opioid receptor immunoreactivity in interneurons and pyramidal cells in the rat hippocampus. J Comp Neurol. 1997;381:373–387. [PubMed] [Google Scholar]
- 17.Cvejic S, Devi LA. Dimerization of the delta opioid receptor: implication for a role in receptor internalization. J Biol Chem. 1997;272:26959–26964. doi: 10.1074/jbc.272.43.26959. [DOI] [PubMed] [Google Scholar]
- 18.Desban M, Kemel ML, Glowinski J, Gauchy C. Spatial organization of patch and matrix compartments in the rat striatum. Neuroscience. 1993;57:661–671. doi: 10.1016/0306-4522(93)90013-6. [DOI] [PubMed] [Google Scholar]
- 19.DiFiglia M, Pasik T, Pasik P. Ultrastructure of Golgi-impregnated and gold-toned spiny and aspiny neurons in the monkey neostriatum. J Neurocytol. 1980;9:471–492. doi: 10.1007/BF01204837. [DOI] [PubMed] [Google Scholar]
- 20.Dourmap N, Michael-Titus A, Costentin J. Differential effect of intrastriatal kainic acid on the modulation of dopamine release by mu- and delta-opioid peptides: a microdialysis study. J Neurochem. 1992;58:709–713. doi: 10.1111/j.1471-4159.1992.tb09775.x. [DOI] [PubMed] [Google Scholar]
- 21.Dumartin B, Caille I, Gonon F, Bloch B. Internalization of D1 dopamine receptor in striatal neurons in vivo as evidence of activation by dopamine agonists. J Neurosci. 1998;18:1650–1661. doi: 10.1523/JNEUROSCI.18-05-01650.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Evans CJ, Keith DE, Jr, Morrison H, Magendzo K, Edwards RH. Cloning of a delta opioid receptor by functional expression. Science. 1992;258:1952–1955. doi: 10.1126/science.1335167. [DOI] [PubMed] [Google Scholar]
- 23.Frederickson CJ. Neurobiology of zinc and zinc-containing neurons. Int Rev Neurobiol. 1989;31:145–238. doi: 10.1016/s0074-7742(08)60279-2. [DOI] [PubMed] [Google Scholar]
- 24.Freund TF, Powell JF, Smith AD. Tyrosine hydroxylase-immunoreactive boutons in synaptic contact with identified striatonigral neurons, with particular reference to dendritic spines. Neuroscience. 1984;13:1189–1215. doi: 10.1016/0306-4522(84)90294-x. [DOI] [PubMed] [Google Scholar]
- 25.Gerfen CR. The neostriatal mosaic: compartmentalization of corticostriatal input and striatonigral output systems. Nature. 1984;311:461–464. doi: 10.1038/311461a0. [DOI] [PubMed] [Google Scholar]
- 26.Gerfen CR. The neostriatal mosaic: striatal patch-matrix organization is related to cortical lamination. Science. 1989;246:385–388. doi: 10.1126/science.2799392. [DOI] [PubMed] [Google Scholar]
- 27.Harris KM, Kater SB. Dendritic spines: cellular specializations imparting both stability and flexibility to synaptic function. Annu Rev Neurosci. 1994;17:341–371. doi: 10.1146/annurev.ne.17.030194.002013. [DOI] [PubMed] [Google Scholar]
- 28.Holt DJ, Graybiel AM, Saper CB. Neurochemical architecture of the human striatum. J Comp Neurol. 1997;384:1–25. doi: 10.1002/(sici)1096-9861(19970721)384:1<1::aid-cne1>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 29.Ingham CA, Hood SH, Taggart P, Arbuthnott GW. Plasticity of synapses in the rat neostriatum after unilateral lesion of the nigrostriatal dopaminergic pathway. J Neurosci. 1998;18:4732–4743. doi: 10.1523/JNEUROSCI.18-12-04732.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Izzo PN, Bolam JP. Cholinergic synaptic input to different parts of spiny striatonigral neurons in the rat. J Comp Neurol. 1988;269:219–234. doi: 10.1002/cne.902690207. [DOI] [PubMed] [Google Scholar]
- 31.Jiang ZG, North RA. Pre- and postsynaptic inhibition by opioids in rat striatum. J Neurosci. 1992;12:356–361. doi: 10.1523/JNEUROSCI.12-01-00356.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Johnson PI, Stellar JR. Comparison of delta opiate receptor agonist induced reward and motor effects between the ventral pallidum and dorsal striatum. Neuropharmacology. 1994;33:1171–1182. doi: 10.1016/s0028-3908(05)80007-3. [DOI] [PubMed] [Google Scholar]
- 33.Johnston JG, Gerfen CR, Haber SN, van der Kooy D. Mechanisms of striatal pattern formation: conservation of mammalian compartmentalization. Brain Res Dev Brain Res. 1990;57:93–102. doi: 10.1016/0165-3806(90)90189-6. [DOI] [PubMed] [Google Scholar]
- 34.Jones DG, Harris RJ. An analysis of contemporary morphological concepts of synaptic remodelling in the CNS: perforated synapses revisited. Rev Neurosci. 1995;6:177–219. doi: 10.1515/revneuro.1995.6.3.177. [DOI] [PubMed] [Google Scholar]
- 35.Korkotian E, Segal M. Fast confocal imaging of calcium released from stores in dendritic spines. Eur J Neurosci. 1998;10:2076–2084. doi: 10.1046/j.1460-9568.1998.00219.x. [DOI] [PubMed] [Google Scholar]
- 36.Mansour A, Khachaturian H, Lewis ME, Akil H, Watson SJ. Autoradiographic differentiation of μ, δ, and κ opioid receptors in the rat forebrain and midbrain. J Neurosci. 1987;7:2445–2464. [PMC free article] [PubMed] [Google Scholar]
- 37.Mansour A, Fox CA, Burke S, Meng F, Thompson RC, Akil H, Watson SJ. Mu, delta, and kappa opioid receptor mRNA expression in the rat CNS: an in situ hybridization study. J Comp Neurol. 1994;350:412–438. doi: 10.1002/cne.903500307. [DOI] [PubMed] [Google Scholar]
- 38.Mansour A, Watson SJ, Akil H. Opioid receptors: past, present and future. Trends Neurosci. 1995;18:69–70. [PubMed] [Google Scholar]
- 39.Matthes HWD, Smadja C, Valverde O, Vonesch J-L, Foutz AS, Boudinot E, Denavit-Saubié M, Severini C, Negri L, Roques BP, Maldonado R, Kieffer B. Activity of the δ-opioid receptor is partially reduced, whereas activity of the κ-receptor is maintained in mice lacking the μ-receptor. J Neurosci. 1998;18:7285–7295. doi: 10.1523/JNEUROSCI.18-18-07285.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mattson MP, La Ferla FM, Chan SL, Leissring MA, Shepel PN, Geiger JD. Calcium signaling in the ER: its role in neuronal plasticity and neurodegenerative disorders. Trends Neurosci. 2000;23:222–229. doi: 10.1016/s0166-2236(00)01548-4. [DOI] [PubMed] [Google Scholar]
- 41.Meyer ME, Meyer ME. Behaval effects of the mu-opioid peptide agonists DAMGO, DALDA, and PL017 on locomotor activities. Pharmacol Biochem Behav. 1993;46:391–395. doi: 10.1016/0091-3057(93)90369-5. [DOI] [PubMed] [Google Scholar]
- 42.Meyer ME, McLaurin BI, Meyer ME. DALDA (H-Tyr-d-Arg-Phe-Lys-NH2), a potent μ-opioid peptide agonist, affects various patterns of locomotor activities. Pharmacol Biochem Behav. 1995;51:149–151. doi: 10.1016/0091-3057(94)00308-6. [DOI] [PubMed] [Google Scholar]
- 43.Michael-Titus A, Dourmap N, Costentin J. Mu and delta opioid receptors control differently the horizontal and vertical components of locomotor activity in mice. Neuropeptides. 1989;13:235–242. doi: 10.1016/0143-4179(89)90076-0. [DOI] [PubMed] [Google Scholar]
- 44.Mickley GA, Mulvihill MA, Postler MA. Brain mu and delta opioid receptors mediate different locomotor hyperactivity responses of the C57BL/6J mouse. Psychopharmacology (Berl) 1990;101:332–337. doi: 10.1007/BF02244050. [DOI] [PubMed] [Google Scholar]
- 45.Negri L, Improta G, Lattanzi R, Potenza RL, Luchetti F, Melchiorri P. Interaction between the mu-agonist dermorphin and the delta-agonist [d-Ala2, Glu4]deltorphin in supraspinal antinociception and delta-opioid receptor binding. Br J Pharmacol. 1995;116:2931–2938. doi: 10.1111/j.1476-5381.1995.tb15947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Neuhoff H, Roeper J, Schweizer M. Activity-dependent formation of perforated synapses in cultured hippocampal neurons. Eur J Neurosci. 1999;11:4241–4250. doi: 10.1046/j.1460-9568.1999.00856.x. [DOI] [PubMed] [Google Scholar]
- 47.Parent A, Hazrati LN. Functional anatomy of the basal ganglia. I. The cortico-basal ganglia-thalamo-cortical loop. Brain Res Brain Res Rev. 1995;20:91–127. doi: 10.1016/0165-0173(94)00007-c. [DOI] [PubMed] [Google Scholar]
- 48.Paxinos G, Watson C. The rat brain in stereotaxic coordinates, Second edition. Academic; Orlando, FL: 1986. [Google Scholar]
- 49.Petäjä-Repo UE, Hogue M, Laperriere A, Walker P, Bouvier M. Export from the endoplasmic reticulum represents the limiting step in the maturation and cell surface expression of the human delta opioid receptor. J Biol Chem. 2000;275:13727–13736. doi: 10.1074/jbc.275.18.13727. [DOI] [PubMed] [Google Scholar]
- 50.Peters A, Palay SL, Webster H deF. The fine structure of the nervous system. Oxford UP; New York: 1991. [Google Scholar]
- 51.Pierce JP, van Leyen K, McCarthy JB. Translocation machinery for synthesis of integral membrane and secretory proteins in dendritic spines. Nat Neurosci. 2000;3:311–313. doi: 10.1038/73868. [DOI] [PubMed] [Google Scholar]
- 52.Quock RM, Burkey TH, Varga E, Hosohata Y, Hosohata K, Cowell SM, Slate CA, Ehlert FJ, Roeske WR, Yamamura HI. The delta-opioid receptor: molecular pharmacology, signal transduction, and the determination of drug efficacy. Pharmacol Rev. 1999;51:503–532. [PubMed] [Google Scholar]
- 53.Reynolds ES. The use of lead citrate and high pH as an electron-opaque stain in electron microscopy. J Cell Biol. 1963;17:208–212. doi: 10.1083/jcb.17.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Rivera VM, Wang X, Wardwell S, Courage NL, Volchuk A, Keenan T, Holt DA, Gilman M, Orci L, Cerasoli F, Jr, Rothman JE, Clackson T. Regulation of protein secretion through controlled aggregation in the endoplasmic reticulum. Science. 2000;287:826–830. doi: 10.1126/science.287.5454.826. [DOI] [PubMed] [Google Scholar]
- 55.Schoffelmeer AN, Yao YH, Gioannini TL, Hiller JM, Ofri D, Roques BP, Simon EJ. Cross-linking of human [125I]beta-endorphin to opioid receptors in rat striatal membranes: biochemical evidence for the existence of a mu/delta opioid receptor complex. J Pharmacol Exp Ther. 1990;253:419–426. [PubMed] [Google Scholar]
- 56.Segal M. Dendritic spines for neuroprotection: a hypothesis. Trends Neurosci. 1995;18:468–471. doi: 10.1016/0166-2236(95)92765-i. [DOI] [PubMed] [Google Scholar]
- 57.Segredo V, Burford NT, Lameh J, Sadée W. A constitutively internalizing and recycling mutant of the μ-opioid receptor. J Neurochem. 1997;68:2395–2404. doi: 10.1046/j.1471-4159.1997.68062395.x. [DOI] [PubMed] [Google Scholar]
- 58.Shippenberg TS, Bals-Kubik R, Herz A. Motivational properties of opioids: evidence that an activation of delta-receptors mediates reinforcement processes. Brain Res. 1987;436:234–239. doi: 10.1016/0006-8993(87)91667-2. [DOI] [PubMed] [Google Scholar]
- 59.Smith AD, Bolam JP. The neural network of the basal ganglia as revealed by the study of synaptic connections of identified neurones. Trends Neurosci. 1990;13:259–265. doi: 10.1016/0166-2236(90)90106-k. [DOI] [PubMed] [Google Scholar]
- 60.Sora I, Funada M, Uhl GR. The mu-opioid receptor is necessary for [d-Pen2,d-Pen5]enkephalin-induced analgesia. Eur J Pharmacol. 1997;324:R1–R2. doi: 10.1016/s0014-2999(97)10016-4. [DOI] [PubMed] [Google Scholar]
- 61.Spacek J. Three-dimensional analysis of dendritic spines. II. Spine apparatus and other cytoplasmic components. Anat Embryol (Berl) 1985a;171:235–243. doi: 10.1007/BF00341418. [DOI] [PubMed] [Google Scholar]
- 62.Spacek J. Relationships between synaptic junctions, puncta adhaerentia and the spine apparatus at neocortical axo-spinous synapses. A serial section study. Anat Embryol (Berl) 1985b;173:129–135. doi: 10.1007/BF00707311. [DOI] [PubMed] [Google Scholar]
- 63.Spacek J, Harris KM. Three-dimensional organization of smooth endoplasmic reticulum in hippocampal CA1 dendrites and dendritic spines of the immature and mature rat. J Neurosci. 1997;17:190–203. doi: 10.1523/JNEUROSCI.17-01-00190.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Spacek J, Hartmann M. Three-dimensional analysis of dendritic spines. I. Quantitative observations related to dendritic spine and synaptic morphology in cerebral and cerebellar cortices. Anat Embryol (Berl) 1983;167:289–310. doi: 10.1007/BF00298517. [DOI] [PubMed] [Google Scholar]
- 65.Stefani A, Surmeier DJ, Bernardi G. Opioids decrease high-voltage activated calcium currents in acutely dissociated neostriatal neurons. Brain Res. 1994;642:339–343. doi: 10.1016/0006-8993(94)90940-7. [DOI] [PubMed] [Google Scholar]
- 66.Svingos AL, Moriwaki A, Wang JB, Uhl GR, Pickel VM. μ-Opioid receptors are localized to extrasynaptic plasma membranes of GABAergic neurons and their targets in the rat nucleus accumbens. J Neurosci. 1997;17:2585–2594. doi: 10.1523/JNEUROSCI.17-07-02585.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Svingos AL, Clarke CL, Pickel VM. Cellular sites for activation of d-opioid receptors in the rat nucleus accumbens shell: relationship with Met5-enkephalin. J Neurosci. 1998;18:1923–1933. doi: 10.1523/JNEUROSCI.18-05-01923.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Svingos AL, Clarke CL, Pickel VM. Localization of the d-opioid receptor and dopamine transporter in the nucleus accumbens shell: implications for opiate and psychostimulant cross-sensitization. Synapse. 1999;34:1–10. doi: 10.1002/(SICI)1098-2396(199910)34:1<1::AID-SYN1>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 69.Thompson RC, Mansour A, Akil H, Watson SJ. Cloning and pharmacological characterization of a rat mu opioid receptor. Neuron. 1993;11:903–913. doi: 10.1016/0896-6273(93)90120-g. [DOI] [PubMed] [Google Scholar]
- 70.Veznedaroglu E, Milner TA. Elimination of artifactual labeling of hippocampal mossy fibers seen following pre-embedding immunogold-silver technique by pretreatment with zinc chelator. Microsc Res Tech. 1992;23:100–101. doi: 10.1002/jemt.1070230110. [DOI] [PubMed] [Google Scholar]
- 71.Wang H, Pickel VM. Dendritic spines containing μ-opioid receptors in rat striatal patches receive asymmetric synapses from prefrontal corticostriatal afferents. J Comp Neurol. 1998;396:223–237. [PubMed] [Google Scholar]
- 72.Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of μ opioid receptors and Leu5-enkephalin in the patch compartment of the rat caudate-putamen nucleus. J Comp Neurol. 1996;375:659–674. doi: 10.1002/(SICI)1096-9861(19961125)375:4<659::AID-CNE7>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 73.Wang H, Moriwaki A, Wang JB, Uhl GR, Pickel VM. Ultrastructural immunocytochemical localization of μ-opioid receptors in dendritic targets of dopaminergic terminals in the rat caudate-putamen nucleus. Neuroscience. 1997;81:757–771. doi: 10.1016/s0306-4522(97)00253-4. [DOI] [PubMed] [Google Scholar]
- 74.Wang H, Gracy KN, Pickel VM. μ-Opioid and NMDA-type glutamate receptors are often colocalized in spiny neurons within patches of the caudate-putamen nucleus. J Comp Neurol. 1999;412:132–146. doi: 10.1002/(sici)1096-9861(19990913)412:1<132::aid-cne10>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 75.Westrum LE, Jones DH, Gray EG, Barron J. Microtubules, dendritic spines and spine apparatuses. Cell Tissue Res. 1980;208:171–181. doi: 10.1007/BF00234868. [DOI] [PubMed] [Google Scholar]
- 76.White NM, Hiroi N. Preferential localization of self-stimulation sites in striosomes/patches in the rat striatum. Proc Natl Acad Sci USA. 1998;95:6486–6491. doi: 10.1073/pnas.95.11.6486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Wichmann T, Starke K. Modulation by muscarine and opioid receptors of acetylcholine release in slices from striato-striatal grafts in the rat. Brain Res. 1990;510:296–302. doi: 10.1016/0006-8993(90)91380-y. [DOI] [PubMed] [Google Scholar]
- 78.Zhang J, Ferguson SS, Barak LS, Bodduluri SR, Laporte SA, Law PY, Caron MG. Role for G protein-coupled receptor kinase in agonist-specific regulation of mu-opioid receptor responsiveness. Proc Natl Acad Sci USA. 1998;95:7157–7162. doi: 10.1073/pnas.95.12.7157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhang J, Ferguson SS, Law PY, Barak LS, Caron MG. Agonist-specific regulation of delta-opioid receptor trafficking by G protein-coupled receptor kinase and beta-arrestin. J Recept Signal Transduct Res. 1999;19:301–313. doi: 10.3109/10799899909036653. [DOI] [PubMed] [Google Scholar]