Abstract
The brain contains an intrinsic vasopressin fiber system the function of which is unknown. It has been demonstrated recently that astrocytes express high levels of a water channel, aquaporin-4 (AQP4). Because vasopressin is known to regulate aquaporin expression and translocation in kidney collecting ducts and thereby control water reabsorption, we hypothesized that vasopressin might serve a similar function in the brain. By recording intrinsic optical signals in an acute cortical slice preparation we showed that evoked neuronal activity generates a radial water flux in the neocortex. The rapid onset and high capacity of this flux suggest that it is mediated through the AQP4-containing astrocytic syncytium that spans the entire thickness of the neocortical mantle. Vasopressin and vasopressin receptor V1a agonists were found to facilitate this flux. V1a antagonists blocked the facilitatory effect of vasopressin and reduced the water flux even in the absence of any exogenous agonist. V2 agonists or antagonists had no effect. These data suggest that vasopressin and V1a receptors play a crucial role in the regulation of brain water and ion homeostasis, most probably by modulating aquaporin-mediated water flux through astrocyte plasma membranes.
Keywords: vasopressin, aquaporin-4, volume changes, spatial buffer, V1a receptor, intrinsic optical signals, brain water homeostasis, extracellular space
Small vasopressin-containing neurons in the hypothalamus are known to give rise to a fiber system that extends throughout the brain and spinal cord (de Vries and Miller, 1998). The functions of this intrinsic fiber system are largely unknown. One possibility is that vasopressin controls or modulates water fluxes in the brain, similar to its role in the kidney.
Water homeostasis in the brain is of central clinical and physiological importance. Cerebral edema is a final common path of a number of neurological conditions and may rapidly become life threatening because of the rigid encasement of the brain. Furthermore, water and ion homeostasis are inextricably coupled. For example, the clearance of K+ from areas of high neuronal activity is contingent on a concomitant water flux (Dietzel et al., 1980; Holthoff and Witte, 2000). Rapid transmembrane movement of water is mediated by a distinct family of proteins, the aquaporins, and one member of this family [aquaporin-4 (AQP4)] is expressed in high amounts in brain neuropil (Nielsen et al., 1997). Knockout of AQP4 was recently found to reduce the extent of postischemic brain edema and to reduce glial swelling secondary to hypo-osmotic stress (Manley et al., 2000).
Could vasopressin modulate aquaporin-mediated water flux in the brain? If so, this would be parallel to the situation in the kidney, where vasopressin controls water flux by regulating aquaporin-2 expression and translocation. To test this hypothesis we chose a cortical slice model in which water fluxes are elicited by focal electrical stimulation (Holthoff and Witte, 1996, 2000). These water fluxes are extremely rapid, indicating that they are mediated by aquaporins.
Here we show that vasopressin modulates this radial, activity-dependent water flux in the cerebral cortex and that this effect is mediated through V1a receptors.
MATERIALS AND METHODS
Animals. The experiments were performed on 14-d-old male Wistar rats. Animals had free access to food and water, and institutional guidelines for animal safety and comfort were adhered to.
Optical recording. Male Wistar rats, 14-d-old, were anesthetized and decapitated. Brains were removed rapidly and cooled down to 4°C. Coronal neocortical slices (400 μm thick) (bregma −5.8 mm to bregma −6.3 mm, occipital cortex) were prepared (VT1000S, Leica) and stored at room temperature in artificial CSF (aCSF) containing (in mm): NaCl 124, NaHCO3 26, KCl 3, CaCl2 2, MgSO4 2, NaH2PO4 1.25, and glucose 10, equilibrated with 95% O2 and 5% CO2 to pH 7.4. In all experiments extracellular chloride concentration was reduced to 17 mm to reduce net uptake of KCl via the glial NaKCl2cotransporter (Dietzel et al., 1980; Holthoff and Witte, 1996). This procedure ensures that most of the K+uptake occurs by way of spatial buffering (Holthoff and Witte, 2000). Low chloride solution contained (in mm): NaCl 10, Na-gluconate 111, Ca1/2-gluconate 4, NaHCO3 26, KCl 3, CaCl2 2, MgSO4 2, NaH2PO4 1.25, and glucose 10, equilibrated with 5% CO2 in O2 to pH 7.4, and was washed in low chloride solution for at least 50 min before an experiment was started. In aCSF containing tetramethylammoniumchloride (TMA-Cl), equimolar amounts of NaCl were omitted. Slices were stored in the recording chamber submerged at 32°C.
Brain slices were illuminated in the dark-field configuration of an upright microscope (Axioskop FS, Zeiss) with near-infrared light (750 ± 50 nm) (Holthoff et al., 1994). Intrinsic optical signals (IOSs) (transmitted scattered light) were recorded using a CCD camera (C2400–77, Hamamatsu). Camera signal was contrast enhanced, and shading was corrected by the CCD camera control device (Hamamatsu). The slices were electrically stimulated (concentric stimulation electrode, tip 100 μm; Science Products Trading) with stimulus trains (pulses of 200 μsec at 3–6 V in a train of 50 Hz for 2 sec) every 10 min. Before each stimulation, background intensity was captured and subtracted from actual video signal, and difference images were digitally enhanced on-line using a video processing unit (DVS 3000, Hamamatsu). The resulting video signal was stored on an S-VHS video recorder and analyzed off-line with a Macintosh Computer equipped with a frame grabber card using NIH Image Software.
Double-barreled ion-selective microelectrodes. Ion-selective microelectrodes (ISMEs) were prepared with a liquid potassium ion exchanger (IE 190, WPI) and calibrated in conventional fashion. In addition to its selectivity for potassium ions, the ion exchanger is highly selective for quaternary ammonium ions (Hansen and Olsen, 1980; Huang and Karwoski, 1992). Changes in extracellular space (ECS) volume can be detected by adding 10 mm TMA-Cl to extracellular fluid (Ransom et al., 1985; Huang and Karwoski, 1992). Because TMA is largely restricted to the extracellular compartment (Nicholson and Phillips, 1981), changes in extracellular TMA concentration can be interpreted as alterations in ECS volume. In the presence of quaternary ammonium ions, the ISMEs used are virtually blind to potassium ions.
Test agents. The following test agents were dissolved in phosphate buffer: (Arg8)-vasopressin (AVP; 500 nm; Bachem); [Phe2, Ile3, Orn8]-vasopressin ([Phe2, Orn8]-vasotocin) (500 nm; Peninsula Laboratories, Belmont, CA); (Phenylac1,d-Tyr(Me)2, Arg6,8, Lys-NH29)-vasopressin (500 nm; Bachem); (deamino-Cys1,d-Arg8)-vasopressin (500 nm, Bachem); and (d(CH2)51,d-Ile4, Arg8, Ala-NH29)-vasopressin (500 nm; Bachem). The following test agents were dissolved in dimethylsulfoxide (Merck, Darmstadt, Germany): bisindolylmaleimide I (BIS I) (300 nm, 2 μm; Calbiochem, La Jolla, CA); and thapsigargin (1 μm; ICN Biochemicals, Costa Mesa, CA).
Antibodies. Two different antibodies against AQP4 were used in this study. One was provided by Nielsen's lab in Aarhus (LL182) (Nielsen et al., 1997; Wen et al., 1999), and one was commercially available (AQP4 1A; Alpha Diagnostics International).
Immunocytochemistry. The animals were anesthetized and decapitated, and neocortical slices were obtained as described above. The slices were fixed by immersion, after incubation in standard aCSF, or standard aCSF followed by aCSF with low chloride. Some slices of the latter group were exposed to 500 nm vasopressin for 1 hr (vasopressin was added to the low-chloride aCSF). Two fixatives were used: 4% formaldehyde (for light microscopy) or 4% formaldehyde/0.1% glutaraldehyde (for electron microscopy).
Light microscopic immunocytochemistry was performed using a method of indirect fluorescence described elsewhere (Nagelhus et al., 1999). The concentrations of antibodies were 1 or 2 μg/ml for both antibodies. Antibodies were diluted in phosphate buffer containing 3% normal goat serum, 1% bovine serum albumin, 0.5% Triton X-100, and 0.05% sodium azide, pH 7.4. The primary antibodies were revealed by a carboxymethylindocyanine-coupled secondary antibody (1:1000; Jackson ImmunoResearch Laboratories, West Grove, PA). The secondary antibody was diluted in the same solution as the primary antibodies, with the omission of sodium azide. The sections were viewed and photographed with a Leica Microscope equipped with epifluorescence optics. For control the AQP4 antibodies were omitted or preadsorbed with the immunizing peptide.
For electron microscopic immunocytochemistry, small blocks of the cortical slices were subjected to freeze substitution (Van Lookeren et al., 1991). Ultrathin sections were cut with a Reichert ultramicrotome, mounted on nickel grids, and processed for immunogold cytochemistry as described previously (Takumi et al., 1998; Wen et al., 1999). In brief, the sections were treated with a saturated solution of NaOH in absolute ethanol (2–3 sec), rinsed in distilled water, and incubated sequentially in the following solutions: (1) 0.1% sodium borohydride and 50 mm glycine in Tris buffer (5 mm) containing 0.01% Triton X-100 and 0.3% NaCl (TBST, 10 min); (2) 2% human serum albumin in TBST (10 min); (3) primary antibody (2.0 μg/ml both antibodies) diluted in the solutions mentioned in step 2 (overnight); (4) TBST (10 min); (5) same solution as step 2 (10 min); and (6) gold-conjugated Fab fragments (10 or 20 nm; EM.GFAR; BioCell Research Laboratories, Cardiff, UK), diluted 1:20 in TBST containing 2% human serum albumin and polyethylene glycol (0.5 mg/ml, 2 hr). Finally, the sections were examined and micrographs were taken in a Philips CM10 transmission electron microscope.
Statistics. Data are given as mean ± SD. For calculation of differences, Student's t test was used. If not indicated otherwise, number of experiments equals number of animals.
RESULTS
Brain cortical slices were stimulated electrically every 10 min through an electrode in layer VI, after 50 min preincubation with low-chloride aCSF (to prevent Cl−-coupled K+ transport) (Holthoff and Witte, 1996). The ensuing water flux was assessed by recording IOSs, indicative of changes in extracellular volume (Holthoff and Witte, 2000). Although some components of the IOS do not seem to correlate to the changes of extracellular space size in extreme situations (such as spreading depression) (Aitken et al., 1999), the IOSs correlate very well with changes of extracellular space volume in the present recording and stimulus conditions (Holthoff and Witte, 1996, 2000).
The electrical stimulation caused a dark IOS (hereafter referred to as a “black wave”) in the slice (Fig.1a). Recordings of TMA activity with ion-sensitive microelectrodes confirmed that the black wave corresponded to a widening of the extracellular space (Fig.1c) [also see Holthoff and Witte (2000)]. The wave had a time to peak of 1.9 ± 0.4 sec (n = 9) and recovered in the course of the following 1–2 min. The signal started in the superficial cortical layers (I–III) perpendicular to the stimulation electrode (Fig. 1a) and expanded in the tangential plane with a velocity of 115 ± 13 μm/sec (n = 5). The area of the black wave under control conditions varied from slice to slice (probably because of differences in the basal level of vasopressin) with a mean ± SD of 281.53 ± 230.73 μm2(n = 14). The signal size was measured at its maximum after each stimulus train. Layer IV displayed a bright signal that corresponds to a reduction of the extracellular space (as judged by TMA activity recordings) (Holthoff and Witte, 1996, 2000). This pattern of changes is assumed to reflect water redistribution secondary to an activation of layer IV neurons through direct stimulation of ascending fiber systems.
Fig. 1.
Effect of vasopressin on darkening of the intrinsic optical signal (black wave) corresponding to extracellular space widening. The top panelsshow the IOSs and field potentials evoked by afferent stimulation in the neocortical slice. Slices were electrically stimulated every 10 min with stimulus trains lasting 2 sec. Extracellular field potentials shown were recorded in layer IV and depict the response to the first stimulus within the stimulus train. IOSs were captured 6 sec after stimulation was started. The stimulation electrode in layer VI is shown schematically in this Figure.a, IOS and field potentials under control conditions.b, Increased IOS but similar field potentials after 30 min superfusion with AVP. c, Widening of extracellular space: correlation between TMA+ activity (left panel) and IOS (right panel). d, Statistics of field potentials under control conditions, in the presence ofAVP and in the presence of aV1-antagonist. FP, Field potentials. The differences do not reach statistical significance. Scale bar, 200 μm.
AVP-treated slices
Superfusion of the slice with AVP strongly increased the areal extent of the black wave (Fig. 1b). The increase occurred in the tangential direction; only a slight increase could be observed along the radial axis of the cortex. AVP did not change the amplitude of the extracellular volume increase, when assessed by TMA measurements in the superficial cortical layers perpendicular to the stimulation electrode. Thus the TMA concentration (Fig. 1c) (baseline, 10 mm) decreased by 0.56 ± 0.4 mm in control solution (10 recordings in slices from three animals) and by 0.68 ± 0.4 mmafter AVP superfusion (9 recordings from three animals; difference not statistically significant). The increase of the black wave was already apparent with the first stimulation after solution exchange (e.g., after 10 min), but reached its maximum after ∼30 min. This delayed response is probably attributable to a slow diffusion of the drug into the slice.
The areal extent of the bright signal in layer IV (which corresponds to a reduction of the extracellular space) increased to 368 ± 157% of control (p < 0.001; n = 9) after AVP treatment.
Field potentials
The AVP-induced widening of the extracellular space could be secondary to changes in neuronal excitability. This was ruled out by use of extracellular field potential recordings (Fig.1a,b,d) (n = 3). Likewise, field potentials remained unaltered after application of the V1a receptor antagonist (Phenylac1,d-Tyr(Me)2, Arg6,8, Lys-NH29)-vasopressin (Fig. 1d) (n = 3).
Measurement of potassium ion concentration
After electrical stimulation in layer VI, the K+ ion concentration increased in layer IV (where the extracellular space decreased) as well as in the superficial layers (where the extracellular space increased) (Fig.2a). A similar increase in K+ concentration was observed in slices that were stimulated in the presence of AVP (Fig. 2b). There was no significant correlation between the maximal amplitude of potassium increase and the spatial extent of the black wave. The K+ signal had a longer duration in layer IV (24.6 ± 5.4 sec) than in layer I (13.9 ± 4.6 sec;p < 0.02; n = 5). This duration did not change significantly in layer I (13.5 ± 1.4 sec) or layer IV (27.1 ± 4.2 sec; recordings from five slices of three animals) after superfusion with AVP.
Fig. 2.
Changes of potassium ion activity accompanying black waves. IOS and measurements of extracellular potassium ion activity in layers I and IV of the cortex under control conditions (a) and after 30 min superfusion with AVP (b). Slices were electrically stimulated every 10 min with stimulus trains lasting 2 sec. The site where the potassium activity was recorded is indicated by schematic electrodes. Scale bar, 200 μm.
V1 and V2 receptors
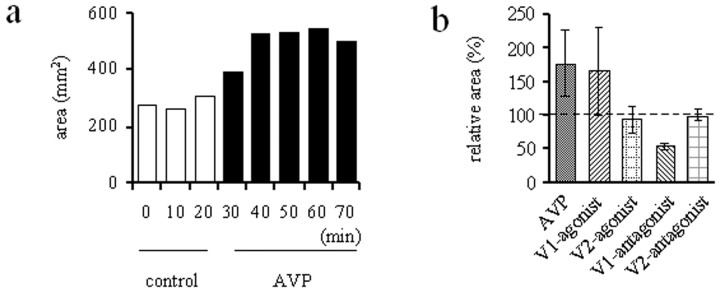
[Arg8]-vasopressin (Fig.3a) (n = 9) and the V1 agonist [Phe2, Orn8]-vasotocin (n = 3) increased the size of the black wave by >50% (Fig. 3b). Signal sizes were determined after 40 min of drug application. The V1 antagonist decreased the size of the black wave (Fig. 3b) (n = 3), i.e., without addition of AVP. This indicates that endogenous V1 agonists exert a tonic facilitatory effect. The V2 agonist [deamino-Cys1,d-Arg8]-vasopressin, and the V2-antagonist [d(CH2)51,d-Ile2, Ile4, Arg8, Ala-NH29]-vasopressin did not affect the size of the black wave (Fig. 3b) (n = 3).
Fig. 3.
Pharmacological influences on the size of the black wave. Slices were electrically stimulated every 10 min with stimulus trains lasting 2 sec. a, Typical experiment with application of AVP. After three control stimulations the slice was superfused with AVP, which caused an increase of the black wave. b, Statistics of effects of AVP, the V1-agonist [(Phe2, Ile3, Orn8)]-vasopressin [(Phe2, Orn8)-vasotocin], the V2 agonist (deamino-Cys1,d-Arg8)-vasopressin, the V1-antagonist Phenylac1, d-Tyr(Me)2, Arg6,8, Lys-NH29)-vasopressin, and the V2-antagonist [d(CH2)51,d-Ile4, Arg8, Ala-NH29]-vasopressin on the maximal extension of the black wave. Signal sizes were determined after 40 min of drug application. V2 agonists or V2 antagonists did not affect the size of the black wave. The difference between values for AVP and V1 agonist is not statistically significant (p > 0.05), but both values (and the value for the V1 antagonist) differ from control level (dashed line) and all other observations (p< 0.05). In this and the following Figures the signal size was expressed in arbitrary areal units as measured on-screen.
Thapsigargin and protein kinase C
To test for the role of intracellular calcium stores, thapsigargin (1 μm), which inhibits the endoplasmic reticulum Ca2+ ATPase and prevents a refill of the Ca2+ stores (Norup et al., 1986), was washed for 60 min. This decreased the size of the black wave evoked by afferent stimulation (Fig.4a,b) (n = 3). In the presence of thapsigargin, AVP no longer had a facilitatory effect (Fig. 4b) (n = 4). Sometimes a rundown of signal size was observed with recording times exceeding 90 min. Figure 4c shows that no such rundown was present within the first 90 min that were used for the experiments in the present study.
Fig. 4.
Changes of IOS sizes after superfusion with thapsigargin. Slices were electrically stimulated every 10 min with stimulus trains lasting 2 sec. a, Thapsigargin decreased the amplitude of the black wave. Three control stimulations were followed by superfusion with thapsigargin for 60 min. b, In the presence of thapsigargin, AVP did not restore or enhance the black wave. Comparison between thapsigargin (60 min after onset of superfusion) and thapsigargin for 30 min followed by thapsigargin plus AVP for 30 min. There was no significant difference (p > 0.05); however, both values differed from control level (dashed line) atp < 0.05. c, Absence of rundown in a typical control experiment with electrical stimulation every 10 min for 90 min. d, Effects of different concentrations of bisindolylmaleimide I (BIS I) together with AVP (500 nm) on size of the black wave. Signal sizes were determined after 60 min of application. There was no significant difference between the two concentrations (p> 0.05); however, both values differed from control level (dashed line) at p < 0.05.
To test whether the effect of AVP is mediated through protein kinase C (PKC), the PKC-inhibitor BIS I was given simultaneously with AVP (500 nm) in two different concentrations. BIS I blocked the enhancing effect of AVP and decreased the black wave to below control levels when added at a concentration of 2 μm(n = 5) or 300 nm(n = 4) (Fig. 4d). Signal sizes were determined after 60 min of drug application.
Immunocytochemistry
Immunofluorescence of cortical slices treated with AVP revealed intense AQP4 immunolabeling associated with the pial surface and blood vessels (Fig.5a,b). This pattern of immunolabeling was indistinguishable from that of control slices (data not shown). Extracerebral vessels were consistently unlabeled (Fig. 5b). This is in agreement with the immunogold data (Fig. 5c), which show that AQP4 is expressed by perivascular glial processes but not by endothelial cells. High particle densities were also observed along the glial plasma membranes apposed to pia (data not shown). Other domains of astrocyte plasma membranes were labeled at lower intensities. The pattern of immunolabeling in immersion-fixed cortical slices did not differ from that in perfusion-fixed brain (Fig. 5d). No immunofluorescence or immunogold signals were observed in the absorption controls (data not shown).
Fig. 5.

a, b, Immunofluorescence labeling of AQP4 in a cortical slice treated with vasopressin. a, Strong AQP4 immunolabeling of the glia limitans (double arrows) facing the unlabeled pia, and of glial end feet surrounding the blood vessels (short arrows). p, Pia. b, Higher magnification showing an unlabeled pial vessel (arrow). Scale bar, 20 μm. c, d, Immunogold labeling of AQP4. c, AQP4 labeling of glial end feet surrounding a collapsed vessel in a cortical slice treated with vasopressin. d, Electron micrograph showing AQP4 labeling of glia limitans facing a pial vessel in rat parietal cortex.V, Vessel lumen; B, basal lamina;G, glial process. Scale bar, 0.3 μm.
DISCUSSION
High neuronal activity generates excess K+ that has to be efficiently cleared from the synaptic region. Dissipation through the narrow extracellular space is clearly insufficient (Dietzel et al., 1980), calling for a role of the astroglial syncytium that occupies most of the space between cerebrocortical neurons. Astrocytes are equipped with several mechanisms for K+ handling, the most important of which is K+ spatial buffering. This mechanism can be studied in relative isolation depending on the experimental conditions. Notably, in the present series of studies the low [Cl−] in the incubation medium should prevent any activation of the NaKCl2 transporter (Holthoff and Witte, 1998).
It has long been known that K+ spatial buffering generates osmotic gradients and that these have to be compensated by water redistribution (Dietzel et al., 1980). One piece of evidence for this is the shrinkage of the extracellular space that occurs around active neurons. It follows that any K+ flux through glial syncytia must be associated with a directionally specific water flux (Holthoff and Witte, 2000). This was borne out in the present study. Thus, evoked activity in the deep cortical layers elicited a flux of K+ to the superficial layers of the cortex (as measured by K+-sensitive microelectrodes), and this flux was accompanied by a water flux (recorded as an enlargement of the extracellular space). This water flux originated around the active neurons in layer IV, as indicated by the shrinkage of the extracellular space at this site. The volume changes in the deep and superficial cortical layers occur with a delay of <2 sec after electrical stimulation. The faster dissipation of potassium in upper cortical layers may reflect loss through the cortical surface. Rapid transmembrane water transport seems to be mediated by a specialized class of channel molecules, the aquaporins (Agre et al., 1995). AQP4 occurs in high concentrations in brain (Jung et al., 1994). Recent studies showed that this aquaporin is largely restricted to astrocytes, implying that it should be uniquely suited to facilitate water transport coupled to K+spatial buffering (Nielsen et al., 1997; Nagelhus et al., 1998). Here we demonstrate that AQP4 is expressed by cortical astrocytes and that their expression pattern is maintained in the cortical slice preparation.
The present experimental approach is based on the idea that evoked K+ fluxes can be used to drive AQP4-mediated water transport. Even if high frequency stimulation is used, our approach is likely to mimic the physiological situation much more closely than alternative procedures based on cultures or cell lines challenged with hypo-osmotic or hyperosmotic media. What is lost in the present model compared with the in vivo situation is the access to the blood stream and subarachnoidal space, which are believed to be the ultimate sinks of excess K+. However, when using IOSs as a measure of water transport, leaving out the ultimate sinks is turned to an advantage because this is likely to accentuate the volume changes of the extracellular space.
Here we tested our hypothesis that vasopressin may exert an important regulatory role on brain water transport. Vasopressin was chosen for the following reasons. First, vasopressin is endogenous to the brain (Landgraf, 1992), and the brain has an intrinsic vasopressin-containing fiber system (Buijs, 1978; de Vries and Miller, 1998). Second, V1 vasopressin receptors are widely distributed throughout the cerebral cortex (Chen et al., 1993; Brinton, 1999). Third, vasopressin increases the rate of osmotically induced volume changes of astrocytes in culture (Sarfaraz and Fraser, 1999). Our results are compatible with the following scheme. K+ released consequent to the evoked neuronal activity accumulates in the extracellular space of layer IV. Some of this K+ is cleared by spatial buffering. In the presence of AVP, water transport through astrocytes will be facilitated, allowing the cells to better compensate for the osmotic gradients set up by K+ transport. In other words, vasopressin will enable a radial water flux even lateral to the stimulating electrode, where the evoked neuronal activity is lower and the potassium driving force smaller than in the cortex perpendicular to the site of stimulation. This will be recorded as an enhanced tangential spread of the IOS in the superficial as well as in the deep layers of the cortex (representing an increase and decrease, respectively, of the extracellular space volume). In agreement with the involvement of the glial syncytium, pharmacological decoupling of the glial cells abolishes the widening of the extracellular space in superficial cortical layers (Holthoff and Witte, 2000).
V1a receptors and water redistribution
The present data indicate that the effect of vasopressin is mediated through V1a receptors rather than V2 receptors. Preincubation with a V1a antagonist caused a decrease in the black wave even under basal stimulation conditions (i.e., in the absence of any other drugs in the medium). These data suggest that there is a tonic, V1a receptor-mediated facilitation of water permeability. This tonic stimulation could be provided by AVP released from nerve fibers intrinsic to the cerebral cortex (Sofroniew, 1983) or by AVP diffusing into the cortex via the extracellular space. In vivo the extracellular AVP level may reach nanomolar levels (Robinson, 1983;Landgraf, 1992), attesting to the physiological relevance of the present AVP concentration. Local vasopressin release in brain tissue is increased by high K+ (Landgraf, 1992).
Intracellular signaling mechanisms
The V1a receptors are coupled via G-proteins to phospholipase C (Thibonnier et al., 1994). Thus one possibility is that the effects of V1a stimulation on extracellular volume are mediated through an IP3-dependent release of Ca2+ from internal stores. In support of this we could demonstrate that a depletion of these stores by use of thapsigargin abolished the facilitatory effect of vasopressin on water redistribution.
V1a receptor stimulation also causes activation of PKC. By use of a PKC inhibitor (BIS I together with AVP), evidence was provided that vasopressin exerts part of its facilitatory effect through this signaling pathway. With a BIS I concentration of 300 nm and 2 μm (which would inhibit all major PKC subtypes), a reduction of the black wave was observed. The signals became even smaller than under control conditions, possibly relating to the fact that the effects of the basal level of vasopressin were also antagonized by the inhibitor. This is in contrast to the observation ofYang and Verkman (1997), who found no change in AQP4 activity after PKC activation. However, the latter study was performed in oocytes that may have different PKC isoforms or PKC substrates. It remains to be shown whether PKC acts through phosphorylation of AQP4 or, more indirectly, through phosphorylation of one or several molecules upstream in the signal transduction pathway. AQP4 is known to contain three putative phosphorylation sites, and studies are under way to resolve whether these are targeted by V1 receptor stimulation.
That vasopressin has some effects on other membrane proteins cannot be excluded. In fact, in the absence of specific blockers there is as yet no direct evidence that the observed effects are mediated by aquaporin-4. An involvement of gap junctions is unlikely. PKC reduces gap junction conductance (Greenfield et al., 1990), and intracellular calcium, at least at high concentrations, decreases junctional permeability (Blomstrand et al., 1999). This contrasts with our findings that both PKC and intracellular calcium are involved in the facilitation of water redistribution.
Does vasopressin alter the expression pattern of AQP4?
So far we have tacitly assumed that any regulation of AQP4-mediated water flux must be caused by an allosteric modulation of the AQP4 channel. However, there is an alternative possibility: namely, that the changes in water permeability are caused by alterations in AQP4 expression. The latter idea is not supported by the present immunocytochemical analysis, which failed to provide any evidence of a vasopressin-induced translocation of AQP4 to the plasma membrane. In fact, similar to the situation in other cell populations (Nielsen et al., 1997), most of the AQP4 molecules in cortical astrocytes are located at the cell surface even in basal conditions. In contrast, our immunogold data cannot rule out the possibility that vasopressin causes a subtle reorganization of the plasma membrane AQP4 molecules within the individual membrane compartments. A combined freeze-fracture/immunogold approach (Rash et al., 1998) would be required to settle this issue.
Conclusion
Our data are consistent with the idea that vasopressin through V1a receptors facilitates an activity-dependent flux of water through the cortical astrocyte syncytium. The rapid time course of the volume changes suggests that the effect of vasopressin is mediated by modulation of a specialized water channel, most probably aquaporin-4.In vivo, the efflux pathway described here will be interfaced to extracerebral fluid spaces. Because water flux through AQP4 is osmotically driven, this pathway will also admit water movement in the opposite direction. In fact, it is known that vasopressin causes a net increase in brain water content, accompanying a net electrolyte accumulation (DePasquale et al., 1989). An important task for further studies will be to resolve whether the present regulatory mechanisms may be useful therapeutic targets in brain edema.
Footnotes
This work was supported by the Deutsche Forschungsgemeinschaft (SFB 194 and 1849), the Düsseldorf Entrepreneurs Foundation, the Norwegian Research Council, and the Letten F. Saugstad's Foundation. We thank C. Bruehl and K. Schiene for helpful discussions and S. Hamm, D. Steinhoff, and Milan Srejic for technical assistance.
H.N. and M.A.-M. contributed equally to this paper.
Correspondence should be addressed to Prof. Dr. Otto W. Witte, Neurologische Klinik, Heinrich Heine Universität, Moorenstrasse 5, D40225 Düsseldorf, Germany. E-mail:witteo@uni-duesseldorf.de.
REFERENCES
- 1.Agre P, Brown D, Nielsen S. Aquaporin water channels: unanswered questions and unresolved controversies. Curr Opin Cell Biol. 1995;7:472–483. doi: 10.1016/0955-0674(95)80003-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken PG, Fayuk D, Somjen GG, Turner DA. Use of intrinsic optical signals to monitor physiological changes in brain tissue slices. Methods. 1999;18:91–103. doi: 10.1006/meth.1999.0762. [DOI] [PubMed] [Google Scholar]
- 3.Blomstrand F, Aberg ND, Eriksson PS, Hansson E, Ronnback L. Extent of intercellular calcium wave propagation is related to gap junction permeability and level of connexin-43 expression in astrocytes in primary cultures from four brain regions. Neuroscience. 1999;92:255–265. doi: 10.1016/s0306-4522(98)00738-6. [DOI] [PubMed] [Google Scholar]
- 4.Buijs RM. Intra- and extrahypothalamic vasopressin and oxytocin pathways in the rat. Pathways to the limbic system, medulla oblongata and spinal cord. Cell Tissue Res. 1978;192:423–435. doi: 10.1007/BF00212323. [DOI] [PubMed] [Google Scholar]
- 5.Chen C, Diaz Brinton RD, Shors TJ, Thompson RF. Vasopressin induction of long-lasting potentiation of synaptic transmission in the dentate gyrus. Hippocampus. 1993;3:193–203. doi: 10.1002/hipo.450030211. [DOI] [PubMed] [Google Scholar]
- 6.DePasquale M, Patlak CS, Cserr HF. Brain ion and volume regulation during acute hypernatremia in Brattleboro rats. Am J Physiol (Lond) 1989;256:F1059–F1066. doi: 10.1152/ajprenal.1989.256.6.F1059. [DOI] [PubMed] [Google Scholar]
- 7.de Vries GJ, Miller MA. Anatomy and function of extrahypothalamic vasopressin systems in the brain. Prog Brain Res. 1998;119:3–20. doi: 10.1016/s0079-6123(08)61558-7. [DOI] [PubMed] [Google Scholar]
- 8.Diaz Brinton R. Vasopressin in the mammalian brain: the neurobiology of a mnemonic peptide. Prog Brain Res. 1998;119:177–199. doi: 10.1016/s0079-6123(08)61570-8. [DOI] [PubMed] [Google Scholar]
- 9.Dietzel I, Heinemann U, Hofmeier G, Lux HD. Transient changes in the size of the extracellular space in the sensorimotor cortex of cats in relation to stimulus-induced changes in potassium concentration. Exp Brain Res. 1980;40:432–439. doi: 10.1007/BF00236151. [DOI] [PubMed] [Google Scholar]
- 10.Greenfield LJJ, Hackett JT, Linden J. Xenopus oocyte K+ current. III. Phorbol esters and pH regulate current at gap junctions. Am J Physiol. 1990;259:C792–C800. doi: 10.1152/ajpcell.1990.259.5.C792. [DOI] [PubMed] [Google Scholar]
- 11.Hansen AJ, Olsen CE. Brain extracellular space during spreading depression and ischemia. Acta Physiol Scand. 1980;108:355–365. doi: 10.1111/j.1748-1716.1980.tb06544.x. [DOI] [PubMed] [Google Scholar]
- 12.Holthoff K, Witte OW. Intrinsic optical signals in rat neocortical slices measured with near-infrared dark-field microscopy reveal changes in extracellular space. J Neurosci. 1996;16:2740–2749. doi: 10.1523/JNEUROSCI.16-08-02740.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holthoff K, Witte OW. Intrinsic optical signals in vitro: a tool to measure alterations in extracellular space with two-dimensional resolution. Brain Res Bull. 1998;47:649–655. doi: 10.1016/s0361-9230(98)00135-x. [DOI] [PubMed] [Google Scholar]
- 14.Holthoff K, Witte OW. Directed spatial potassium redistribution in rat neocortex. Glia. 2000;29:288–292. doi: 10.1002/(sici)1098-1136(20000201)29:3<288::aid-glia10>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 15.Holthoff K, Dodt HU, Witte OW. Changes in intrinsic optical signal of rat neocortical slices following afferent stimulation. Neurosci Lett. 1994;180:227–230. doi: 10.1016/0304-3940(94)90526-6. [DOI] [PubMed] [Google Scholar]
- 16.Huang B, Karwoski CJ. Light-evoked expansion of subretinal space volume in the retina of the frog. J Neurosci. 1992;12:4243–4252. doi: 10.1523/JNEUROSCI.12-11-04243.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung JS, Bhat RV, Preston GM, Guggino WB, Baraban JM, Agre P. Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance. Proc Natl Acad Sci USA. 1994;91:13052–13056. doi: 10.1073/pnas.91.26.13052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landgraf R. Central release of vasopressin: stimuli, dynamics, consequences. Prog Brain Res. 1992;91:29–39. doi: 10.1016/s0079-6123(08)62313-4. [DOI] [PubMed] [Google Scholar]
- 19.Manley GT, Fujimura M, Ma T, Noshita N, Filiz F, Bollen AW, Chan P, Verkman AS. Aquaporin-4 deletion in mice reduces brain edema after acute water intoxication and ischemic stroke. Nat Med. 2000;6:159–163. doi: 10.1038/72256. [DOI] [PubMed] [Google Scholar]
- 20.Nagelhus EA, Veruki ML, Torp R, Haug FM, Laake JH, Nielsen S, Agre P, Ottersen OP. Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Muller cells and fibrous astrocytes. J Neurosci. 1998;18:2506–2519. doi: 10.1523/JNEUROSCI.18-07-02506.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nagelhus EA, Horio Y, Inanobe A, Fujita A, Haug FM, Nielsen S, Kurachi Y, Ottersen OP. Immunogold evidence suggests that coupling of K+ siphoning and water transport in rat retinal Muller cells is mediated by a coenrichment of Kir4.1 and AQP4 in specific membrane domains. Glia. 1999;26:47–54. doi: 10.1002/(sici)1098-1136(199903)26:1<47::aid-glia5>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 22.Nicholson C, Phillips JM. Ion diffusion modified by tortuosity and volume fraction in the extracellular microenvironment of the rat cerebellum. J Physiol (Lond) 1981;321:225–257. doi: 10.1113/jphysiol.1981.sp013981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nielsen S, Nagelhus EA, Amiry-Moghaddam M, Bourque C, Agre P, Ottersen OP. Specialized membrane domains for water transport in glial cells: high-resolution immunogold cytochemistry of aquaporin-4 in rat brain. J Neurosci. 1997;17:171–180. doi: 10.1523/JNEUROSCI.17-01-00171.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Norup E, Smitt UW, Christensen SB (1986) The potencies of thapsigargin and analogues as activators of rat peritoneal mast cells. Planta Med 251–255. [DOI] [PubMed]
- 25.Ransom BR, Yamate CL, Connors BW. Activity-dependent shrinkage of extracellular space in rat optic nerve: a developmental study. J Neurosci. 1985;5:532–535. doi: 10.1523/JNEUROSCI.05-02-00532.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rash JE, Yasumura T, Hudson CS, Agre P, Nielsen S. Direct immunogold labeling of aquaporin-4 in square arrays of astrocyte and ependymocyte plasma membranes in rat brain and spinal cord. Proc Natl Acad Sci USA. 1998;95:11981–11986. doi: 10.1073/pnas.95.20.11981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Robinson IC. Neurohypophysial peptides in cerebrospinal fluid. Prog Brain Res. 1983;60:129–145. doi: 10.1016/S0079-6123(08)64381-2. [DOI] [PubMed] [Google Scholar]
- 28.Sarfaraz D, Fraser CL. Effects of arginine vasopressin on cell volume regulation in brain astrocyte in culture. Am J Physiol. 1999;276:E596–E601. doi: 10.1152/ajpendo.1999.276.3.E596. [DOI] [PubMed] [Google Scholar]
- 29.Sofroniew MV. Morphology of vasopressin and oxytocin neurones and their central and vascular projections. Prog Brain Res. 1983;60:101–114. doi: 10.1016/S0079-6123(08)64378-2. [DOI] [PubMed] [Google Scholar]
- 30.Takumi Y, Nagelhus EA, Eidet J, Matsubara A, Usami S, Shinkawa H, Nielsen S, Ottersen OP. Select types of supporting cell in the inner ear express aquaporin-4 water channel protein. Eur J Neurosci. 1998;10:3584–3595. doi: 10.1046/j.1460-9568.1998.00360.x. [DOI] [PubMed] [Google Scholar]
- 31.Thibonnier M, Auzan C, Madhun Z, Wilkins P, Berti-Mattera L, Clauser E. Molecular cloning, sequencing, and functional expression of a cDNA encoding the human V1a vasopressin receptor. J Biol Chem. 1994;269:3304–3310. [PubMed] [Google Scholar]
- 32.Van Lookeren CM, Oestreicher AB, Buma P, Verkleij AJ, Gispen WH. Ultrastructural localization of adrenocorticotrophic hormone and the phosphoprotein B-50/growth-associated protein 43 in freeze-substituted, Lowicryl HM20-embedded mesencephalic central gray substance of the rat. Neuroscience. 1991;42:517–529. doi: 10.1016/0306-4522(91)90394-4. [DOI] [PubMed] [Google Scholar]
- 33.Wen H, Nagelhus EA, Amiry-Moghaddam M, Agre P, Ottersen OP, Nielsen S. Ontogeny of water transport in rat brain: postnatal expression of the aquaporin-4 water channel. Eur J Neurosci. 1999;11:935–945. doi: 10.1046/j.1460-9568.1999.00502.x. [DOI] [PubMed] [Google Scholar]
- 34.Yang B, Verkman AS. Water and glycerol permeabilities of aquaporins 1–5 and MIP determined quantitatively by expression of epitope-tagged constructs in Xenopus oocytes. J Biol Chem. 1997;272:16140–16146. doi: 10.1074/jbc.272.26.16140. [DOI] [PubMed] [Google Scholar]