Abstract
This study investigates the physiological properties of parabrachial internal lateral (PBil) neurons that project to the paracentral thalamic (PC) nucleus using antidromic activation and single-unit recording techniques in anesthetized rat. We reported here that most of these neurons responded exclusively to the nociceptive stimulation of large receptive fields with a sustained firing that often outlasted the stimulus up to several minutes. These responses were depressed by intravenous morphine.
Our results demonstrated a novel spino–PBil–PC pathway, which transmits nociceptive messages to the PC nucleus, which in turn projects to the prefrontal cortex. Recent clinical imaging studies showed the important participation of prefrontal cortex in emotional response to pain. This spino–PBil–PC pathway may explain how nociceptive messages reach the prefrontal cortex and thus trigger unbearable aversive aspects of pain.
Keywords: parabrachial area, thalamus, intralaminar nuclei, paracentral nucleus, dorsal horn, nociception
Old clinical reports (Freeman and Watts, 1948), as well as more recent brain imaging studies (Rainville et al., 1997), demonstrated that the prefrontal cortex plays an important role in the processing of aversive component of pain. However, the pathways that carry the nociceptive information to this brain region remain poorly understood. The spinothalamic tract projects primarily to the sensory relay nuclei in the “lateral” thalamus and only moderately to the “medial” thalamic nuclei, which in turn project to the prefrontal regions (Willis et al., 1995). It is generally believed that laminas V/VI of the dorsal horn (a major link of the nociceptive system; Besson and Chaouch, 1987) reach the medial thalamus rather indirectly via spino–reticulo–thalamic pathways. The two main candidates to convey nociceptive messages from the deep spinal laminas to the medial thalamus are the gigantocellular reticular (Gi) nucleus (Casey, 1971; Bowsher, 1976) and the subnucleus reticularis dorsalis (SRD), in the medulla (Villanueva et al., 1996, 1998). Nonetheless, spinal inputs to Gi fit poorly with reticular areas that project to the thalamus (Craig and Dostrovsky, 1999), and SRD neurons project primarily to the ventromedial nucleus (Villanueva et al., 1998; Monconduit et al., 1999) but spare most of the intralaminar thalamus and noticeably the paracentral (PC) nucleus.
Recently, the parabrachial internal lateral nucleus (PBil) has been suggested as a possible nociceptive relay between the deep spinal laminas and a part of the intralaminar thalamus that mainly project to prefrontal compartments (Fig. 1). Indeed, this nucleus receives an extensive input from nociceptive neurons in laminas V/VI of the spinal cord (Kitamura et al., 1993; Bernard et al., 1995; Feil and Herbert, 1995); it projects to the PC and to a lesser extent in other intralaminar thalamic nuclei (see also Fulwiler and Saper, 1984; Hermanson and Blomqvist, 1997b; Bester et al., 1999), and noxious stimulation evoked a marked expression of phospho-cAMP response element-binding protein in and around the PBil (Hermanson and Blomqvist, 1997a). In this study, we investigated the physiological properties of PBil neurons that project to the PC nucleus using antidromic stimulation and single-unit recording techniques.
Fig. 1.

Schematic representation of the experimental design in relation to the spino–PBil–PC nociceptive pathway.A, Antidromic stimulation delivered in the PC nucleus (gray). A′, Terminal labeling in the PC nucleus from Phaseolus vulgarisleucoagglutinin (PHA-L) injection in the PBil (modified fromBester et al., 1999). B, Unitary recording in the PBil nucleus (gray). B′, Terminal labeling in the PBil nucleus from PHA-L injection in spinal reticular lamina V (modified from Bernard et al., 1995). C, Spinal region (gray) that projects densely to the PBil. Scale bars: A′, 1 mm; B′, 500 μm.CL, Central lateral thalamic nucleus; CM, central medial thalamic nucleus; OPC, oval paracentral thalamic nucleus; Rh, rhomboid thalamic nucleus;V/VI, spinal laminas V/VI.
MATERIALS AND METHODS
Animal preparation. Experiments were performed on 59 Sprague Dawley male rats weighing 250–300 gm. The animals were deeply anesthetized with 2% halothane in a nitrous oxide/oxygen mixture (2:3–1:3), paralyzed by an intravenous injection of gallamine triethiodide (Flaxedil), and artificially ventilated using a Palmer pump. The expiratory end tidal CO2 and the core temperature were maintained at ∼4% and 37 ± 0.5°C, respectively. The heart rate and blood pressure were continuously monitored. The animals were mounted in a stereotaxic frame, the head being fixed in a dorsiflexed position (incisor bar elevated 10 mm above the standard position) (Paxinos and Watson, 1998). After surgery, the halothane level was reduced to 0.5–0.7%, and the mixture of nitrous oxide/oxygen was maintained at 2:3–1:3 to achieve the level of anesthesia that was adequate for ethical purposes but did not excessively depress neuronal responses to noxious stimuli (Benoist et al., 1984).
Recordings. Extracellular unit recordings were made with glass micropipettes (10–15 MΩ) filled with a mixture of NaCl (5%) and Pontamine sky blue dye (2%). Single-unit activity and blood pressure were digitized and monitored on-line using a data acquisition system (CED 1401 with Spike 2 software; Cambridge Electronic Design, Cambridge, UK). To record neurons in the PBil, the micropipettes were inserted in the brain by using the following coordinates: 1.0–3.0 mm rostral to lambda and 1.2–1.7 mm lateral to the midline. The depth was between 5.5 and 7.5 mm from the surface.
Stimulation in the PC thalamic region was applied with a linear array of three concentric monopolar electrodes, and the distance between two adjacent center contacts of the array was 600 μm. The three center contacts (100 μm in diameter; 150 μm in length) could be independently stimulated. The array of antidromic stimulating electrode was inserted into the PC thalamic region on the right side of the brain with the following coordinates: 1–1.4 mm rostral to bregma and 1.5 mm lateral to the midline, with a depth between 5.5 and 7.5 mm. When a unit was backfired from one depth, the site of minimum threshold was determined by moving the stimulating electrode at different depths. Finally, the electrode was placed to the site of minimum threshold for testing the three main criteria of antidromic activation: (1) the stability of the latency, (2) the ability for the evoked response to follow high-frequency stimulation (>500 Hz), and (3) the observation of a systematic collision between the evoked antidromic response and one orthodromic spike.
Natural and electrical cutaneous stimulation. Innocuous mechanical (touch, brushing, rubbing, pressure, and stroking) and proprioceptive (movements of joints) stimuli were applied to the limbs, the tail, and the face. Mechanical and thermal noxious stimuli were applied to the paws, the tail, and the face using calibrated forceps, water bath, or water jet. Graded pressures (2–64 N/cm2; exponential (×2) steps; pressure surface of ∼0.5 cm2) or temperatures (40–52°C; +2°C steps) applied in a 24 sec period were used to determine the encoding properties of the neurons. A delay of 3 min, at least, was used between successive thermal stimuli of the same part of the receptive field.
Electrical square-wave stimuli (2 msec duration) were delivered through pairs of stainless steel stimulating electrodes inserted subcutaneously into the cheeks, the paws, and the tail. The effects of the repeated application of the electrical stimulus (30 per trial, 0.66 Hz) were analyzed with the use of peristimulus histograms (PSTHs).
Histological controls. In most experiments, only one unit per animal was tested. The recording sites of the 113 units antidromically identified or not were each marked by electrophoretic (5 μA direct current; 30 min duration; cathode in the micropipette) deposit of Pontamine sky blue at the tip of the micropipette. The location of stimulating electrodes in the thalamus nucleus were marked by iron deposit at the tip of the electrode (10-μA direct current; 30 sec duration; anode connected to the electrode). At the end of the experiment, the brain was removed and fixed in a mixture of 8 vol of 1% potassium ferricyanide in 10% formalin solution added to 2 vol of 2% acetic acid in 95% alcohol solution for 3–5 d. This procedure induced the formation of Prussian blue staining at the tip of stimulating electrode. The tissue was cut in 100-μm-thick sections and Nissl-stained. Recording and stimulating sites were determined by microscopic examination and then plotted onto a series of camera lucida drawings.
Data analysis. The magnitude of response was defined as the mean firing frequency during the stimulation minus the ongoing activity before the stimulation. The t test and ANOVA test were used for statistical analysis. Data are generally presented as means ± SE.
RESULTS
The results presented below were obtained from 29 PBil neurons that were antidromically activated from the PC nucleus.
The spontaneous activity of the PBil neurons was generally low; most of them (22 of 29) had a low rate of spontaneous activity (<0.5 Hz). Twenty-one neurons were located within the PBil nucleus, and eight were located within 100 μm from its borders (Fig.2). Sixty-five percent of the PBil neurons were nociceptive, i.e., driven by mechanical and thermal stimuli almost only within noxious ranges, and 35% of the PBil neurons were unresponsive.
Fig. 2.
Recording sites of parabrachiothalamic neurons in drawings of coronal sections through the parabrachial area, from caudal to rostral (A). Distance of each level caudal (−) or rostral (+) to the coronal plane in which the inferior colliculus merges with the pons is indicated in micrometers.Open circle, Unresponsive neuron; black circle, nociceptive neuron. B, Microphotograph of Pontamine sky blue deposit labeling the recording site in the PBil. Scale bars: A, B, 1 mm.bc, brachium conjunctivum; cl, parabrachial central lateral nucleus; dl, parabrachial dorsal lateral nucleus; el, parabrachial external lateral nucleus; em, external medial;lcr, parabrachial lateral crescent area;m, medial; Me5, mesencephalic trigeminal nucleus; sl, parabrachial superior lateral nucleus;vl, parabrachial ventral lateral nucleus.
All of the PBil–thalamic neurons fulfilled the criteria for antidromic activation (Fig. 3A–C) (see Materials and Methods). The mean latency was 8.4 ± 0.8 msec (n = 29; range of 2.6–20 msec) (Fig.3F). Such latencies, with an estimated distance of 7 mm between the parabrachial area and the PC nucleus, indicate a slow conduction velocity in the 0.37–2.8 m/sec range. Most low-threshold points for antidromic activation were located in the PC nucleus or in its close vicinity and in the parafascicular nucleus (Fig.3D,E).
Fig. 3.
Antidromic activation of one parabrachiothalamic neuron recorded in the PBil. A, Superimposition of five antidromic spikes; note the perfect stability of the latency.B, High-frequency stimulation (5 pulses, 600 Hz); note the capacity of the antidromic response to follow high-frequency stimulation. C, Collision test. Filled circle shows the expected location of the antidromic spike, andblack triangle indicates the antidromic shock.C1, The orthodromic spike fired before the 2t + r collision period, and the antidromic spike occurred with 11 msec latency. C2–C4, The orthodromic spike fired within the 2t +r collision period, and the antidromic spike did not occur. D, Microphotograph of the corresponding Prussian blue point in the paracentral nucleus. E1,E2, Antidromic activation sites with thresholds <200 μA (asterisks) and >200 μA (black dots). F, Distribution histogram of the antidromic latencies. Scale bar (in D), 1 mm.CM, Central medial thalamic nucleus; fr, fasciculus retroflexus; ml, medial lemniscus;OPC, oval paracentral thalamic nucleus;Pf, parafascicula thalamic nucleus; Po, posterior thalamic nuclear group; VPM, ventral posteromedial thalamic nucleus; VPPC, ventral posterior parvicellular thalamic nucleus.
Response to natural stimulation of PBil nociceptive neurons
Innocuous thermal (temperature of ≤44°C) or mechanical (touch or light brush; pressure of ≤4 N/cm2) stimuli were generally ineffective, and the discharges were observed only near the nociceptive threshold (Fig.4). The application of noxious stimuli (temperature of >44°C; pressure of >4 N/cm2) gave rise to a rapid tonic discharge throughout the stimulation period (Fig. 4) that often (56%) lasted many seconds after the termination of the stimulus (Fig.4A,B2). These marked afterdischarges lasted 74 ± 13 sec after thermal stimuli and 153 ± 29 sec after mechanical stimuli.
Fig. 4.
Responses of two PBil–PC neurons (one inA, the other in B) to innocuous (near the pain threshold) and noxious stimuli. Thermal (A1,B1) and mechanical (A2,B2) stimuli were applied for 24 secbetween the two arrows. Note that, in each case, the weak response to innocuous stimuli and the heavy and tonic response to noxious stimuli was followed by marked (A) and moderate (B) afterdischarges.
Among the 19 nociceptive neurons, 10 had a large receptive field (the entire body), seven had a medium receptive field (two paws or several regions but not the entire body), and only two had a small receptive field (one part of the body). All receptive fields included a restricted area of the body from which we could obtain a more intense activation, the preferential receptive field (PRF).
Encoding properties of the PBil nociceptive neurons
The stimulus–response curves of individual neurons are in Figure5A1–B1. They demonstrated a similar feature: they were monotonic and positive from the threshold to the maximum response, 48°C for the thermal stimuli and 16 N/cm2 for the mechanical. After these points, the curves were clearly decreasing.
Fig. 5.
Stimulus–response curves of PBil–PC neurons.A1, Responses of individual neurons to graded thermal stimuli. A2, Mean stimulus–response curve to graded thermal stimuli (solid line). B1, Response of individual neurons to graded mechanical stimuli.B2, Mean stimulus–response curve to graded mechanical stimuli (solid line). In both A2 andB2, broken line illustrates, for comparison, the curves obtained in a previous study (Matsumoto et al., 1996) from external parabrachial area. Ordinate, Mean frequency of response; abscissa, stimulus temperature or pressure. Note that the pain thresholds can be estimated at ∼45°C and between 4 and 8 N/cm2.
The mean thermal thresholds of the PBil neurons was 44.8 ± 0.6°C (n = 15). The mean thermal curve could be divided in three phases (Fig. 5A2). In the first phase, between 40 and 44°C, around the threshold, the slope of the curve was positive and increased slowly. In the second phase, from 44 up to 48°C, the curve increased strongly and the slope was very steep. Then, in the last phase, between 48 and 52°C, the curve distinctly decreased and the slope became negative. From 42 up to 48°C, this curve is quite identical to the curve obtained in a previous work, in the external parabrachial area (PBe) (see Discussion). However, after this point, a marked difference appeared; the PBe curve still increased, whereas the PBil curve decreased.
The mean mechanical thresholds of the PBil neurons was 8 ± 2.8 N/cm2 (n = 8). The mean mechanical curve could be divided in two phases (Fig. 5B2). The slope of the curve regularly increased in the first phase from 2 N/cm2 up to the maximum (16 N/cm2). Beyond this point, the slope of the curve decreased and became negative. This curve is noticeably different from the PBe mechanical curve obtained in a previous work. In the low-pressure range, the PBil curve increases more than the PBe curve. After 16 N/cm2, the PBil curve decreases, whereas the PBe curve is still increasing (see Discussion).
Responses to electrical stimulation of PBil nociceptive neurons
All nociceptive neurons tested responded to suprathreshold transcutaneous electrical stimulation applied to the receptive field. As in the illustrated case, the repeated electrical stimulation of very high intensity (30 mA) induced generally a progressive increase of firing in neurons that did not discharged initially (Fig.6A,B). A short silent period after the electrical stimuli and during 100–300 msec was nonetheless often observed (Fig. 6A2). Moreover, most of the neurons excited by electrical stimuli exhibited after the end of a natural noxious stimulus a strong and lasting afterdischarge that stopped, by its own, after 1 or several minutes (Fig. 6C).
Fig. 6.
Response of one nociceptive PBil–PC neuron to transcutaneous electrical stimulation (30 mA, 2 msec duration).A1, PSTH made without stimulation. A2, PSTH made from responses to repetitive electrical stimulation (0.66 Hz, 30 trials) applied in the preferential receptive field.B1, Electrical stimulation (0.66 Hz, 30 trials; eachline is one shock). B2, Continuous response of the neuron to transcutaneous electrical stimulation (synchronous to B1). C, Response of the same neuron to noxious thermal stimulus (46°C) applied for 24 secbetween the two arrows.
Effect of morphine on PBil nociceptive neurons
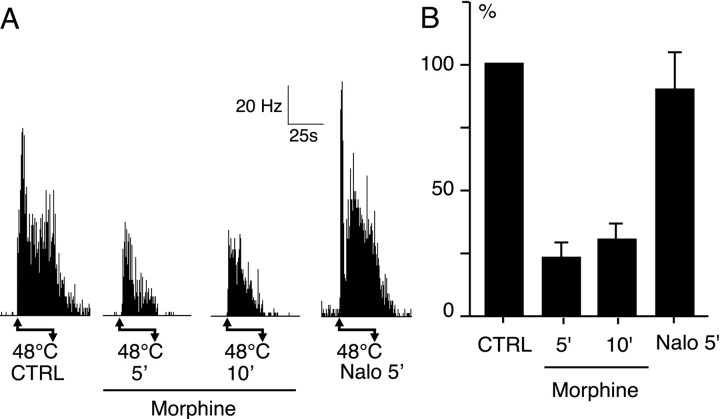
The effects of intravenous injection of morphine (3 mg/kg) were tested on the response to noxious heat (48°C) applied in the PRF. Morphine had clear depressive effects shown in an individual example in Figure 7A. The effect of morphine on ongoing activity was not noticeable because it was often very low or absent. On the other hand, in all cases, the injection of naloxone induced a marked increase of the ongoing activity, suggesting indirectly that the spontaneous activity could be decreased by morphine. The mean histogram (Fig. 7B) summarizes the individual data. Before the morphine injection, the mean frequency of the control response to 48°C was 22 ± 8 Hz (n = 5). Five and then 10 min after 3 mg/kg of intravenous morphine, the responses were markedly and significantly (p< 0.001) reduced to 23 ± 6% and then to 31 ± 7% of their initial value, respectively. After naloxone, the responses recovered to 90 ± 15% of the control value.
Fig. 7.
Depressive effect of intravenous morphine (3 mg/kg) on the effect of PBil neurons. A, Effect in one individual case. Noxious stimuli (48°C) was applied for 24 secbetween the arrows. 5′,10′, Nalo 5′, Responses 5 and 10 min after morphine and 5 min after naloxone administration.B, Mean effect of morphine in percent of the control response. CTRL, Mean control response of PBil neurons (n = 5). 5′, 10′,Nalo 5′, Mean response of the same neurons 5 and 10 min after morphine and 5 min after naloxone administration.
DISCUSSION
Here we showed that most of PBil–intralaminar neurons convey and encode cutaneous nociceptive information to the intralaminar thalamus. The results of the antidromic stimulation further support anatomical data demonstrating that the PBil projects precisely to the intralaminar thalamus (primarily to the PC nucleus as shown in Fig.1A′ and to a lesser extent to the parafascicular and centromedial nuclei) (Fulwiler and Saper, 1984; Hermanson and Blomqvist, 1997b; Bester et al., 1999).
Nociceptive processing in the spino–PBil–PC pathway
PBil–intralaminar neurons convey cutaneous nociceptive information that arises in large cutaneous receptive fields. They display long afterdischarges after intense and moderate nociceptive stimuli and never respond to clearly innocuous stimuli. The mean thermal 44.8 ± 0.6°C (n = 15) and mechanical 8 ± 2.8 N/cm2 (n = 8) thresholds are close to human pain thresholds (∼45°C and ∼7 N/cm2) (Hardy et al., 1967), but the encoding properties were limited to a narrow range of ∼44–48°C for thermal and 4–16 N/cm2 for mechanical stimuli. Furthermore, the response of PBil–PC neurons to noxious stimuli is clearly depressed by intravenous morphine (3 mg/kg). Based on these findings, we suggest that PBil–PC neurons are involved in the processing of nociceptive information.
It must be borne in mind that the PBil nucleus is anatomically distinct from another parabrachial area, the PBe, which was involved previously in nociceptive processing (Bernard and Besson, 1990; Bester et al., 1995) and which includes the lateral crescent, the external lateral, the dorsal lateral, the superior lateral, and the external medial parabrachial nuclei [Fulwiler and Saper, 1984, their Figs. 1, 2;Bester et al., 1997, their Fig. 1]. The PBil receives its nociceptive inputs from the spinal laminas V/VI and projects only to the intralaminar thalamic nuclei (see references in introductory remarks), whereas the PBe receives its inputs from the spinal lamina I (Cechetto et al., 1985; Bernard et al., 1995; Feil and Herbert, 1995; Craig and Dostrovsky, 1999 and references therein) and projects primarily to the amygdala and the hypothalamus (Saper and Loewy, 1980; Fulwiler and Saper, 1984; Bernard et al., 1993; Bester et al., 1997). Importantly, the nociceptive properties of the PBil nociceptive neurons are noticeably different from those of PBe nociceptive-specific neurons: the PBil neurons encode noxious stimuli in a narrower range (close to the nociceptive threshold) (Fig. 5) and have a tendency to be more sensitive to mechanical stimuli around the nociceptive threshold than the PBe neurons. The afterdischarges of PBil neurons were much more marked than those of PBe neurons.
The afterdischarge fitted well with the marked windup observed in PBil neurons when using electrical stimulation: the absence of response to the firsts electrical shocks followed by a progressive increase of firing that persists a long time after the end of the stimulation period. This indicates that PBil neurons could either respond adequately to prolonged noxious stimulus and/or signal pain after the interruption of the nociceptive stimulation. Another important feature of PBil neurons is that their highest response arises to a specific nociceptive strength (48°C and 16 N/cm2), which corresponds, at least for thermal modality (Neisser, 1959), to the threshold of unbearable pain. Above and below this point, the firing was clearly less intense. Thus, it is tempting to speculate that PBil neurons would be tuned to indicate the threshold of unbearable pain. Beyond the tuned nociceptive intensity, PBil firing within narrow ranges would avoid saturating the corresponding thalamo–cortical network.
The particular responsiveness of PBil neurons cannot be totally explained by the nociceptive input they receive from the medial and the lateral reticular portion of laminas V/VI (Kitamura et al., 1993;Bernard et al., 1995; Feil and Herbert, 1995) because most neurons recorded in laminas V/VI are nociceptive of wide dynamic range (Menétrey et al., 1977, 1979, 1984; Dado et al., 1994), whereas most PBil neurons are nociceptive with a narrower dynamic range. There are two possible explanations.
(1) The spino–PBil neurons, which have not been studied physiologically, may be either mostly nociceptive-specific (such neurons were also observed in laminas V/VI) or have properties closer to those of PBil neurons.
(2) The dynamic of response observed in the present study could result from specific filtering gains by local modulation at the PBil level. The properties of the local network could be linked, at least in part, to a particular synaptic transmission in the PBil: it is the only parabrachial subnucleus containing AMPA glutamate receptors of the GluRD/4 type (Chamberlin and Saper, 1995; Guthmann and Herbert, 1999), the highest density fitting especially well with the dorsal location of spinal laminas V/VI input in the dorsal aspect of the PBil (Fig.1B′). This second explanation fits rather well with the hypothesis of a specific PBil tuning. In the framework of this hypothesis, the windup observed at the PBil level could be related to the windup of the spinal wide dynamic range neurons plus a PBil synaptic filter.
No previous electrophysiological study has focused on the caudal PC nucleus, the main target of the PBil. However, recordings in and around the intralaminar nuclei, including the PC, showed that a number of neurons responded to noxious stimuli from a very large receptive field (Dong et al., 1978; Dostrovsky and Guilbaud, 1990). Furthermore,Rinaldi et al. (1991) observed spontaneous hyperactivity in intralaminar thalamic nuclei in human suffering of chronic pain associated with deafferentation. All of these data support the involvement of the spino–PBil–PC pathway in nociceptive processing.
Functional considerations
The projection targets of PC thalamic nuclei are the striatum and the cortex (Berendse and Groenewegen, 1991). The stimulation of intralaminar nuclei induces cortical recruiting responses (Morison and Dempsey, 1942; Jasper, 1960) that are usually accompanied by an increase of the cortical responsiveness to peripheral stimuli (Li et al., 1955). Because the PBil–PC neurons are excited just above the nociceptive threshold with a long-lasting afterdischarge, they could increase durably, via a lasting excitation of PC neurons, the responsiveness of the corresponding striatal–cortical compartment as soon as the pain threshold is reached.
The PC nucleus has been involved in alertness and high vigilance states (Glenn and Steriade, 1982; Kinomura et al., 1996). The cortical target of the PC, namely the lateral orbital, the lateral agranular, and the dorsomedial prefrontal areas (Berendse and Groenewegen, 1991), seems to play an important role in high cognitive functions (Aggleton et al., 1995), as well as in modulation of aggressive behavior, emotional states, and associated autonomic regulations (Frysztak and Neafsey, 1994; Giancola, 1995; Morgan and LeDoux, 1995). Consequently, the spino–PBil–PC–prefrontal nociceptive pathway could be involved, through an arousal of prefrontal (striatal) compartments, in cognitive, attentional, and emotional strategies to cope with noxious stimulation.
Although the switch from the rat to the human remains hazardous, it seems reasonable to hypothesize that the spino–PBil–PC–prefrontal nociceptive pathway exists and could contribute to emotional aspects of pain in human. Surgery to relieve intractable pain may enlighten us about the role of this system. Freeman and Watts (1948) observed that a prefrontal lobotomy makes patients tolerant to their chronic unbearable pain. These authors report that “Following operation he continued to have pain and to react to them as before, but the haunting fear of them disappeared and thus changed his entire outlook on life” and more generally “it does not relieve the pain but rather the disabling reaction to pain, the fear of pain.” Thus, it is tempting to hypothesize that the spino–PBil–PC–prefrontal nociceptive pathway could contribute to trigger among the most aversive emotional aspects of pain (a dreadful feeling and the haunting fear of pain) that makes the life of chronic painful patient so unbearable.
The existence of a pain pathway directed to the prefrontal cortex fits well with the cognitive–emotional framework of Damasio's group (Bechara et al., 2000). Indeed the finding of the spino–PBil–PC–prefrontal nociceptive pathway supports the involvement of the prefrontal cortex in pain processing. Thus, according to Damasio's theory, it becomes easier to believe that the prefrontal cortex might also generate “pseudopainful sensation,” associating them to potentially dangerous ways and/or bad decisions in the life of an individual.
Footnotes
This work was supported by a grant from the Institut National de la Santé et de la Recherche Médicale and the Institut UPSA de la douleur (Paris, France). We thank Dr. R. Burstein for advice in the preparation of this manuscript, J. Martin for histology, and R. Rambur for photography.
Correspondence should be addressed to Dr. Jean-François Bernard, Institut National de la Santé et de la Recherche Médicale U-161, 2 rue d'Alésia, F-75014 Paris, France. E-mail:jfbernard@broca.inserm.fr.
REFERENCES
- 1.Aggleton JP, Neave N, Nagle S, Sahgal A. A comparison of the effects of medial prefrontal, cingulate cortex, and cingulum bundle lesions on tests of spatial memory: evidence of a double dissociation between frontal and cingulum bundle contributions. J Neurosci. 1995;15:7270–7281. doi: 10.1523/JNEUROSCI.15-11-07270.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10:295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 3.Benoist JM, Kayser V, Gautron M, Guilbaud G. Letter to the editor. Pain. 1984;18:410–411. [Google Scholar]
- 4.Berendse HW, Groenewegen HJ. Restricted cortical termination fields of the midline and intralaminar thalamic nuclei in the rat. Neuroscience. 1991;42:73–102. doi: 10.1016/0306-4522(91)90151-d. [DOI] [PubMed] [Google Scholar]
- 5.Bernard JF, Besson JM. The spino(trigemino)pontoamygdaloid pathway: electrophysiologica l evidence for an involvement in pain processes. J Neurophysiol. 1990;63:473–490. doi: 10.1152/jn.1990.63.3.473. [DOI] [PubMed] [Google Scholar]
- 6.Bernard JF, Alden M, Besson JM. The organization of the efferent projections from the pontine parabrachial area to the amygdaloid complex: a Phaseolus vulgaris leucoagglutinin (PHA-L) study in the rat. J Comp Neurol. 1993;329:201–229. doi: 10.1002/cne.903290205. [DOI] [PubMed] [Google Scholar]
- 7.Bernard JF, Dallel R, Raboisson P, Villanueva L, Le Bars D. Organization of the efferent projections from the spinal cervical enlargement to the parabrachial area and periaqueductal gray: a PHA-L study in the rat. J Comp Neurol. 1995;353:480–505. doi: 10.1002/cne.903530403. [DOI] [PubMed] [Google Scholar]
- 8.Besson JM, Chaouch A. Peripheral and spinal mechanisms of nociception. Physiol Rev. 1987;67:67–185. doi: 10.1152/physrev.1987.67.1.67. [DOI] [PubMed] [Google Scholar]
- 9.Bester H, Menendez L, Besson JM, Bernard JF. The spino(trigemino)parabrachiohypothalamic pathway: electrophysiological evidence for an involvement in pain processes. J Neurophysiol. 1995;73:568–585. doi: 10.1152/jn.1995.73.2.568. [DOI] [PubMed] [Google Scholar]
- 10.Bester H, Besson JM, Bernard JF. Organisation of the efferent projections from the parabrachial area to the hypothalamus: a PHA-L study in the rat. J Comp Neurol. 1997;383:245–281. doi: 10.1002/(sici)1096-9861(19970707)383:3<245::aid-cne1>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- 11.Bester H, Bourgeais L, Villanueva L, Besson JM, Bernard JF. Differential projections to the intralaminar, gustatory thalamus from the parabrachial area: a PHA-L study in the rat. J Comp Neurol. 1999;405:421–449. [PubMed] [Google Scholar]
- 12.Bowsher D. Role of the reticular formation in response to noxious stimulation. Pain. 1976;2:361–378. doi: 10.1016/0304-3959(76)90079-8. [DOI] [PubMed] [Google Scholar]
- 13.Casey KL. Response of bulboreticular units to somatic stimuli eliciting escape behaviour in the cat. Int J Neurosci. 1971;2:15–28. doi: 10.3109/00207457109146989. [DOI] [PubMed] [Google Scholar]
- 14.Cechetto DF, Standaert DG, Saper CB. Spinal and trigeminal dorsal horn projections to the parabrachial nucleus in the rat. J Comp Neurol. 1985;240:153–160. doi: 10.1002/cne.902400205. [DOI] [PubMed] [Google Scholar]
- 15.Chamberlin NL, Saper CB. Differential distribution of AMPA-selective glutamate receptor subunits in the parabrachial nucleus of the rat. Neuroscience. 1995;68:435–443. doi: 10.1016/0306-4522(95)00129-7. [DOI] [PubMed] [Google Scholar]
- 16.Craig AD, Dostrovsky JO. Medulla to thalamus. In: Wall PD, Melzack R, editors. Textbook of pain, Ed 4. Churchill Livingstone; London: 1999. pp. 183–214. [Google Scholar]
- 17.Dado RJ, Katter JT, Giesler GJ. Spinothalamic and spinohypothalamic tract neurons in the cervical enlargement of rats. II. responses to innocuous and noxious mechanical and thermal stimuli. J Neurophysiol. 1994;71:981–1002. doi: 10.1152/jn.1994.71.3.981. [DOI] [PubMed] [Google Scholar]
- 18.Dong WK, Ryu H, Wagman IH. Nociceptive responses of neurons in medial thalamus and their relationship to spinothalamic pathways. J Neurophysiol. 1978;41:1592–1613. doi: 10.1152/jn.1978.41.6.1592. [DOI] [PubMed] [Google Scholar]
- 19.Dostrovsky JO, Guilbaud G. Nociceptive responses in medial thalamus of the normal and arthritic rat. Pain. 1990;40:93–104. doi: 10.1016/0304-3959(90)91056-O. [DOI] [PubMed] [Google Scholar]
- 20.Feil K, Herbert H. Topographic organization of spinal and trigeminal somatosensory pathways to the rat parabrachial and Kolliker-Fuse nuclei. J Comp Neurol. 1995;353:506–528. doi: 10.1002/cne.903530404. [DOI] [PubMed] [Google Scholar]
- 21.Freeman W, Watts JW. Pain mechanisms and the frontal lobes: a study of prefrontal lobotomy for intractable pain. Ann Int Med. 1948;28:747–754. doi: 10.7326/0003-4819-28-4-747. [DOI] [PubMed] [Google Scholar]
- 22.Frysztak RJ, Neafsey EJ. The effect of medial frontal cortex lesions on cardiovascular conditioned emotional responses in the rat. Brain Res. 1994;643:181–193. doi: 10.1016/0006-8993(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 23.Fulwiler CE, Saper CB. Subnuclear organization of the efferent connections of the parabrachial nucleus in the rat. Brain Res. 1984;319:229–259. doi: 10.1016/0165-0173(84)90012-2. [DOI] [PubMed] [Google Scholar]
- 24.Giancola PR. Evidence for dorsolateral and orbital prefrontal cortical involvement in the expression of aggressive behavior. Aggress Behav. 1995;21:431–450. [Google Scholar]
- 25.Glenn LL, Steriade M. Discharge rate and excitability of cortically projecting intralaminar thalamic neurons during waking and sleep states. J Neurosci. 1982;2:1387–1404. doi: 10.1523/JNEUROSCI.02-10-01387.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Guthmann A, Herbert H. In situ hybridization analysis of flip/flop splice variants of AMPA-type glutamate receptor subunits in the rat parabrachial and Kolliker-Fuse nuclei. Mol Brain Res. 1999;74:145–157. doi: 10.1016/s0169-328x(99)00281-8. [DOI] [PubMed] [Google Scholar]
- 27.Hardy JD, Wolff HG, Goodell H. Pain sensations and reactions. Hafner; New York: 1967. [Google Scholar]
- 28.Hermanson O, Blomqvist A. Differential expression of the AP-1/CRE-binding proteins Fos and CREB in preproenkephalin mRNA-expressing neurons of the rat parabrachial nucleus after nociceptive stimulation. Mol Brain Res. 1997a;51:188–196. doi: 10.1016/s0169-328x(97)00236-2. [DOI] [PubMed] [Google Scholar]
- 29.Hermanson O, Blomqvist A. Preproenkephalin messenger RNA-expressing neurons in the rat parabrachial nucleus: subnuclear organization and projections to the intralaminar thalamus. Neuroscience. 1997b;81:803–812. doi: 10.1016/s0306-4522(97)00241-8. [DOI] [PubMed] [Google Scholar]
- 30.Jasper HH. Unspecific thalamocortical relations. In: Field J, Magoun HW, Hall VE, editors. Handbook of physiology, Sec 1, Vol 2. American Physiological Society; Washington, DC: 1960. pp. 1307–1321. [Google Scholar]
- 31.Kinomura S, Larsson J, Gulyas B, Roland PE. Activation by attention of the human reticular formation and thalamic intralaminar nuclei. Science. 1996;271:512–515. doi: 10.1126/science.271.5248.512. [DOI] [PubMed] [Google Scholar]
- 32.Kitamura T, Yamada J, Sato H, Yamashita K. Cells of origin of the spinoparabrachial fibers in the rat: a study with fast blue and WGA-HRP. J Comp Neurol. 1993;328:449–461. doi: 10.1002/cne.903280310. [DOI] [PubMed] [Google Scholar]
- 33.Li CL, Cullen C, Jasper HH. Laminar microelectrode analysis of cortical unspecific recruiting responses and spontaneous rhythms. J Neurophysiol. 1955;19:131–143. doi: 10.1152/jn.1956.19.2.131. [DOI] [PubMed] [Google Scholar]
- 34.Matsumoto N, Bester H, Menendez L, Besson JM, Bernard JF. Changes in the responsiveness of parabrachial neurons in the arthritic rat: an electrophysiological study. J Neurophysiol. 1996;76:4113–4126. doi: 10.1152/jn.1996.76.6.4113. [DOI] [PubMed] [Google Scholar]
- 35.Menétrey D, Giesler GJ, Besson JM. An analysis of response properties of spinal cord dorsal horn neurones to nonnoxious and noxious stimuli in the spinal rat. Exp Brain Res. 1977;27:15–33. doi: 10.1007/BF00234822. [DOI] [PubMed] [Google Scholar]
- 36.Menétrey D, Chaouch A, Besson JM. Responses of spinal cord dorsal horn neurones to non-noxious and noxious cutaneous temperature changes in the spinal rat. Pain. 1979;6:265–282. doi: 10.1016/0304-3959(79)90048-4. [DOI] [PubMed] [Google Scholar]
- 37.Menétrey D, De Pommery J, Besson JM. Electrophysiological characteristics of lumbar spinal cord neurons backfired from lateral reticular nucleus in the rat. J Neurophysiol. 1984;52:595–611. doi: 10.1152/jn.1984.52.4.595. [DOI] [PubMed] [Google Scholar]
- 38.Monconduit L, Bourgeais L, Bernard JF, Le Bars D, Villanueva L. Ventromedial thalamic neurons convey nociceptive signals from the whole body surface to the dorsolateral neocortex. J Neurosci. 1999;19:9063–9072. doi: 10.1523/JNEUROSCI.19-20-09063.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- 40.Morison RS, Dempsey EW. A study of thalamo-cortical relations. Am J Physiol. 1942;135:281–292. [Google Scholar]
- 41.Neisser U. Temperature thresholds for cutaneous pain. J Appl Physiol. 1959;14:368–372. doi: 10.1152/jappl.1959.14.3.368. [DOI] [PubMed] [Google Scholar]
- 42.Paxinos G, Watson C. The rat brain in stereotaxic coordinates. Ed 4. Academic; Sydney: 1998. [Google Scholar]
- 43.Rainville P, Duncan GH, Price DD, Carrier B, Bushnell MC. Pain affect encoded in human anterior cingulate but not somatosensory cortex. Science. 1997;277:968–971. doi: 10.1126/science.277.5328.968. [DOI] [PubMed] [Google Scholar]
- 44.Rinaldi PC, Young RF, Albe-Fessard D, Chodakiewitz J. Spontaneous neuronal hyperactivity in the medial and intralaminar thalamic nuclei of patients with deafferentation pain. J Neurosurg. 1991;74:415–421. doi: 10.3171/jns.1991.74.3.0415. [DOI] [PubMed] [Google Scholar]
- 45.Saper CB, Loewy AD. Efferent connections of the parabrachial nucleus in the rat. Brain Res. 1980;197:291–317. doi: 10.1016/0006-8993(80)91117-8. [DOI] [PubMed] [Google Scholar]
- 46.Villanueva L, Bouhassira D, Le Bars D. The medullary subnucleus reticularis dorsalis (SRD) as a key link in both the transmission and modulation of pain signals. Pain. 1996;67:231–240. doi: 10.1016/0304-3959(96)03121-1. [DOI] [PubMed] [Google Scholar]
- 47.Villanueva L, Desbois C, Le Bars D, Bernard JF. Organization of diencephalic projections from the medullary subnucleus reticularis dorsalis and the adjacent cuneate nucleus: a retrograde and anterograde tracer study in the rat. J Comp Neurol. 1998;390:133–160. [PubMed] [Google Scholar]
- 48.Willis WD, Westlund KN, Carlton SM. Pain. In: Paxinos G, editor. The rat nervous system, Ed 2. Academic; Sydney: 1995. pp. 725–750. [Google Scholar]