Abstract
The initial microglial responses that occur after brain injury and in various neurological diseases are characterized by microglial accumulation in the affected sites of brain that results from the migration and proliferation of these cells. The early-phase signal responsible for this accumulation is likely to be transduced by rapidly diffusible factors. In this study, the possibility of ATP released from injured neurons and nerve terminals affecting cell motility was determined in rat primary cultured microglia. Extracellular ATP and ADP induced membrane ruffling and markedly enhanced chemokinesis in Boyden chamber assay. Further analyses using the Dunn chemotaxis chamber assay, which allows direct observation of cell movement, revealed that both ATP and ADP induced chemotaxis of microglia. The elimination of extracellular calcium or treatment with pyridoxalphosphate-6-azophenyl-2′,4′-disulphonic acid, suramin, or adenosine-3′-phosphate-5′-phosphosulfate did not inhibit ATP- or ADP-induced membrane ruffling, whereas AR-C69931MX or pertussis toxin treatments clearly did so. As an intracellular signaling molecule underlying these phenomena, the small G-protein Rac was activated by ATP and ADP stimulation, and its activation was also inhibited by pretreatment with pertussis toxin. These results strongly suggest that membrane ruffling and chemotaxis of microglia induced by ATP or ADP are mediated by Gi/o-coupled P2Y receptors.
Keywords: microglia, ATP, ADP, membrane ruffling, chemotaxis, Gi/o-coupled P2Y receptors
Accumulated evidence suggests that extracellular ATP functions in various tissues and cells (Dubyak and El-Moatassim, 1993). The roles of extracellular ATP as a neurotransmitter and neuromodulator in the CNS have been well documented. For example, ATP induces excitation and increases in calcium in various neurons in the brain (Edwards et al., 1992; Shen and North, 1993; Chen et al., 1994; Inoue et al., 1995; Nabekura et al., 1995). In addition to the role played by ATP in neurons, effects of ATP on glial cells have also been demonstrated. In astrocytes, for example, DNA synthesis, process formation, and the increase in the expression of glial fibrillary acidic protein (Neary et al., 1994), arachidonic acid release (Chen and Chen, 1998), Erk activation (Neary et al., 1999), and calcium wave propagation (Scemes et al., 2000) were reported to be stimulated by ATP. Ca2+ release from internal stores by ATP stimulation was also reported in oligodendrocytes (Kirischuk et al., 1995). This evidence suggests diverse roles of extracellular ATP in the CNS.
Reports have shown that ATP stimulates microglia, another kind of glial cell in the CNS, to release various biologically active substances, such as interleukin-1β (Ferrari et al., 1996, 1997), plasminogen (Inoue et al., 1998), and tumor necrosis factor-α (Hide et al., 2000). Microglial cell death was also demonstrated after stimulation with high-dose ATP (Ferrari et al., 1999). After neuronal damage, microglia migrate to the affected sites, where they function to secrete a variety of cytokines and neurotrophic factors, to express major histocompatibility complex antigen class II, and in certain cases to perform phagocytic activities (Kreutzberg, 1996; Thomas, 1999). For microglia to function in the proper region of the brain, there must be a mechanism underlying the regulation of microglial motility. In fact, in regions of the facial nucleus affected with neuronal damage after facial nerve axotomy, for instance, the accumulation of microglia resulting from their proliferation and migration was clearly observed (Streit et al., 1988; Graeber et al., 1998; Ito et al., 1998). The search for factors influencing microglial motility is an important issue in understanding the function of microglia in brain pathology. To date, transforming growth factor-β, complement 5a (C5a), epidermal growth factor, and various kinds of chemokines have been reported to enhance microglial motility, and these factors have been postulated as possible chemoattractants for microglia in brain (Yao et al., 1990;Hayashi et al., 1995; Nolte et al., 1996, 1997; Cross and Woodroofe, 1999). In the present study, we demonstrated that extracellular ATP and ADP induced membrane ruffling and cell migration in the manner of chemoattraction, possibly mediated by Gi/o-coupled P2Y receptors.
MATERIALS AND METHODS
Microglial culture. Rat primary cultured microglia were prepared according to the method described previously (Nakajima et al., 1992). In brief, mixed glial culture was prepared from the cerebral cortex of neonatal Wistar rats and maintained for 12–23 d in DMEM (Life Technologies, Grand Island, NY) with 10% fetal bovine serum (Irvine Scientific, Santa Ana, CA). Microglia were obtained as floating cells over the mixed glial culture. The floating cells were collected by a gentle shake and transferred to appropriate dishes or glasses, then the microglia attached to them were used for various assays.
Membrane ruffling. The thus-prepared microglia were attached to glass coverslips (Matsunami, Osaka, Japan) coated with 100 μg/ml of poly-l-lysine (Sigma, St. Louis, MO). After attachment for 2 hr, microglia were washed with serum-free DMEM and starved for 4 hr in the same medium. They were then stimulated with ATP (Yamasashyoyu, Chiba, Japan), ADP (Sigma), UTP (Wako Pure Chemical Industries, Osaka, Japan), and adenosine (Sigma) at 50 μm, or recombinant murine macrophage colony stimulating factor (M-CSF) (R & D Systems, Minneapolis, MN) at 100 ng/ml for 5 min at 37°C. In the control, cells were treated with PBS instead of nucleotides. The reaction was stopped by the addition of PBS containing 3.7% formaldehyde. After fixation for 5 min and washing with PBS, the cells were permeabilized with PBS containing 0.1% Triton X-100 for 5 min and washed three times with PBS. To visualize membrane ruffling, cells were stained with Texas Red-conjugated phalloidin (Texas Red–X Phalloidin) (Molecular Probes, Eugene, OR) and observed under the fluorescence microscope PROVIS AX (Olympus, Tokyo, Japan). In the experiments to determine the effects of inhibitors, cells were preincubated with suramin (Wako) (300 μm), pyridoxal-phosphate-6-azophenyl-2′,4-disulphonic acid tetrasodium salt (PPADS) (Research Biochemicals International, Natick, MA) (300 μm), adenosine-3′-phosphate-5′-phosphosulfate (A3P5PS) (Sigma) (300 μm), or AR-C69931MX (AstraZeneca UK Limited, London, UK) (1 μm) for 10 min after starvation and then stimulated with nucleotides. The cells were also preincubated with pertussis toxin (PTx) (Sigma) (50 ng/ml) for 4 hr.
To evaluate extracellular calcium dependency, attached microglia were washed with Ca2+-containing balanced salt solution (BSS) [(in mm): 150 NaCl, 5.0 KCl, 1.2 MgCl2, 25 HEPES, 10 d-glucose, and 1.2 CaCl2] or Ca2+-free BSS [in mm: 150NaCl, 5.0 KCl, 1.2 MgCl2, 25 HEPES, 10d-glucose, and 1 EGTA] (Inoue et al., 1998) and stimulated with nucleotides in the same BSS.
Chemokinesis assay using the Boyden chamber. Chemokinesis of microglia was assessed using the Boyden chamber (Neuroprobe, Bethesda, MD) according to the method described previously (Yokomizo et al., 1997). In brief, polycarbonate filters (5 μm pore) were coated with 10 μg/ml fibronectin (Sigma) in PBS for 60 min. A dry coated filter was installed in the Boyden chamber, whose bottom wells were filled with serum-free DMEM containing nucleotides at the various concentrations indicated. Freshly prepared microglia were suspended in serum-free DMEM containing nucleotides at the same concentration as that in each bottom well, and the cell suspension was placed into the top wells (2–5 × 104 cells/well). The chamber was kept in a CO2 incubator at 37°C for 90 min. The filter was removed and stained with 0.05% crystal violet, 12% formaldehyde, and 10% ethanol in PBS. The cells on the top side of the filter were wiped off, and the number of cells that had migrated to the bottom side was measured at 595 nm with a BioLumin 960 fluorescence/absorbance microassay reader (Pharmacia-LKB, Uppsala, Sweden).
Chemotaxis assay using the Dunn chemotaxis chamber.Chemotaxis was assessed by the Dunn chemotaxis chamber (Weber Scientific International Ltd., Teddington, UK), which allows direct observation of cell movement. The assay was performed according to the method described in the previous report (Webb et al., 1996). In brief, microglia were attached to square coverslips for 2 hr, washed three times with serum-free DMEM, and kept for 4–16 hr until the chemotaxis assay was performed. After starvation, the coverslip was placed over the chamber, whose outer and inner wells were filled with DMEM. The coverslip was sealed with a 1:1 mixture of molten paraffin wax and Vaseline around three edges to leave a slit for exchange of the medium in the outer well. To observe the chemically directed cell migration, the medium in the outer well was exchanged through the slit for DMEM containing 50 μm ATP or ADP. Then the last edge of the coverslip was sealed immediately, and the chamber was set on the stage of a microscope (ECLIPSE TE300; Nikon, Tokyo, Japan), which was maintained at 37°C. Control experiments were performed under the condition in which both outer and inner wells were filled with DMEM.
A region of the bridge was viewed via a CCD video camera (Hamamatsu Photonics, Hamamatsu, Japan), and the phase-contrast images were recorded every 5 min during 1 hr of observation using imaging software (fishPPC; Hamamatsu Photonics). The straight distance between the starting point and the point of a cell reached after 1 hr was measured by plotting the point of the microglial nucleus on a computer display using drawing software. The distance and direction were shown asx,y coordinates on scatter diagrams whose x-axis was positioned parallel to the outer edge of the bridge.
Rac translocation. To determine the translocation of Rac to the region of membrane ruffling, double staining was performed after stimulating the cells with ATP or ADP. After fixation, the cells were incubated with mouse anti-human Rac antibody (Upstate Biotechnology, Lake Placid, NY) and fluorescein isothiocyanate (FITC)-conjugated anti-mouse IgG (BioSource, Sunnyvale, CA). F-actin was stained with Texas Red-conjugated phalloidin. Images were obtained with a confocal laser-scanning microscope CLSM2010 (Pharmacia-LKB).
Pull-down assay of Rac. Activated Rac was measured by the method described previously (Manser et al., 1998; Ohsawa et al., 2000). In brief, the microglia attached to dishes for 2 hr were washed with serum-free DMEM and starved for 4 hr with or without pertussis toxin (50 ng/ml). The starved microglia were stimulated with 50 μm ATP or ADP for 1 min, and lysed in lysis buffer A (25 mm HEPES, pH 7.3, 0.15m NaCl, 5 mmMgCl2, 0.5 mm EGTA, 20 mm β-glycerophosphate, 0.5% Triton X-100, 4% glycerol, 10 mm NaF, 2 mmsodium orthovanadate, 5 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, 10 μm leupeptin, and 10 μmpepstatin). The lysates were centrifuged at 12,000 × g to remove debris. Part of the supernatant was mixed with SDS sample buffer, and the remainder was incubated for 30 min at 4°C with glutathioneS-transferase (GST)-fused p21-activated kinase (PAK) that was coupled to Glutathione Sepharose 4B beads (Pharmacia Biotech). After incubation, the samples were centrifuged at 500 × g and washed twice with lysis buffer A. The precipitated beads were boiled with SDS sample buffer. The total cell lysates and pull-down samples were subjected to SDS-PAGE using 10–20% gradient gel (Daiichi Pure Chemicals, Tokyo, Japan), immunoblotted with anti-Rac antibody and horseradish peroxidase (HRP)-conjugated anti-mouse antibody (Pharmacia), and visualized with ECL Western blotting detection reagents (Pharmacia).
RESULTS
ATP- and ADP-induced membrane ruffling in microglia
We first examined extracellular ATP in terms of morphology and ruffle formation in microglia after 5 min of stimulation with either 50 μm ATP, ADP, UTP or adenosine by staining with Texas Red-conjugated phalloidin. Freshly prepared microglia showed the ramified morphology after 4 hr of incubation in serum-free medium. After the addition of PBS (control), there was no change in morphology (Fig. 1A). However, as shown in Figure 1B, the cells were spread and showed amoeboid-like morphology at 5 min after ATP stimulation. The structure of membrane ruffling was clearly observed by staining with phalloidin. The prominent morphological change that occurred with ruffle formation was also detected with extracellular ADP (Fig. 1C). Although UTP induced obvious morphological change, ruffle formation was not significant (Fig. 1D). The morphological changes of microglia induced by ATP, ADP, and UTP were observed in almost all of the stimulated cells. By contrast, adenosine induced neither morphological change nor ruffle formation (data not shown).
Fig. 1.

Nucleotide-induced membrane ruffling in microglia. The cells were stimulated with PBS (A) or 50 μm ATP (B), ADP (C), or UTP (D) for 5 min. After fixation, the cells were stained with Texas Red-conjugated phalloidin. ATP and ADP clearly induced membrane ruffling (indicated byarrows). Scale bar, 20 μm.
ATP- and ADP-enhanced chemokinesis of microglia in the Boyden chamber
Membrane ruffles are structures that are found primarily at the front edges of migrating cells (Lauffenburger and Horwitz, 1996). To investigate whether the nucleotides that induce membrane ruffling act as a chemoattractant for microglia, a chemotaxis assay using the Boyden chemotaxis chamber was initially performed. In assessing chemotaxis in the Boyden chamber, we usually observe cell migration under two assay conditions, one in which the ligand is in only the bottom compartment and the other in which it is in both the bottom and top compartments. We preliminarily compared the two kinds of microglial migration stimulated by ATP and found that cell migration was enhanced in both cases (data not shown). This preliminary result indicated that ATP-induced cell migration detected in the Boyden chamber is chemokinesis (enhanced migration without chemical gradient). Although we could not evaluate ATP-induced chemotaxis in this assay system, we collected data on the chemokinesis induced by nucleotides. As shown in Figure 2, ATP clearly promoted the chemokinesis of microglia in a dose-dependent manner. ADP exerted a more marked effect than ATP on the chemokinesis of microglia, whereas UTP had no effect (Fig. 2). Unlike nucleotide-induced cell migration, we were able to assess the chemotaxis of microglia in the Boyden chamber by stimulation with C5a. C5a-induced cell migration could be detected only when the ligand was in the bottom compartment. Under this condition, almost the same extent of cell migration was observed between ATP (50 μm)- and C5a (1 μm)-induced chemotaxis at 90 min of incubation (data not shown).
Fig. 2.
Nucleotide-induced chemokinesis of microglia in the Boyden chamber. The cells were exposed to ATP (circle), ADP (square), or UTP (triangle) for 90 min. Nucleotides were added to both top and bottom wells at the concentrations indicated. The absorbance of the stained cells on the bottom side of the filter was measured with a plate reader. Each point and vertical linerepresent the mean and SD for three wells. We confirmed that the three independent experiments showed the same tendency.
ATP- and ADP-induced chemotaxis of microglia in the Dunn chemotaxis chamber
To evaluate whether the nucleotides induced chemotaxis of microglia, we performed another cell migration assay using the Dunn chemotaxis chamber, which allows direct observation of cell movement. The displacements of cells after 1 hr of incubation were plotted asx,y coordinates on scatter diagrams (Fig.3A). As shown in Figure3Aa, microglia have weak motility in the absence of ligands. Compared with that, the great majority of cells that were incubated in the gradient of ATP or ADP migrated toward the source of ligands (Fig.3Ab,Ac), whereas UTP did not induce migration (Fig.3Ad). Two-tailed Student's t tests showed that none of the mean values of x components that were measured for the cells stimulated with ATP (4.07 μm), ADP (7.68 μm), or UTP (−1.76 μm) was significantly different from that of control cells without stimulation (1.58 μm) (p > 0.05). By contrast, the mean value of the y component that was measured for cells stimulated with UTP (1.29 μm) was not significantly different from that without nucleotides (−0.91 μm), whereas each of the mean y components that was measured for the cells stimulated with ATP (53.04 μm) or ADP (51.30 μm) was significantly different from the control (p < 0.0001). Representative images obtained 5 min and 30 min after setting the coverslip in the chamber are presented in Figure 3B. The cells produced membrane ruffles at 5 min, as indicated by arrowheads, and migrated to the ATP gradient (vertically upward) at 30 min. These results indicated that extracellular ATP and ADP promote microglial motility in the manner of chemoattraction, which may have been mediated by P2 receptors.
Fig. 3.
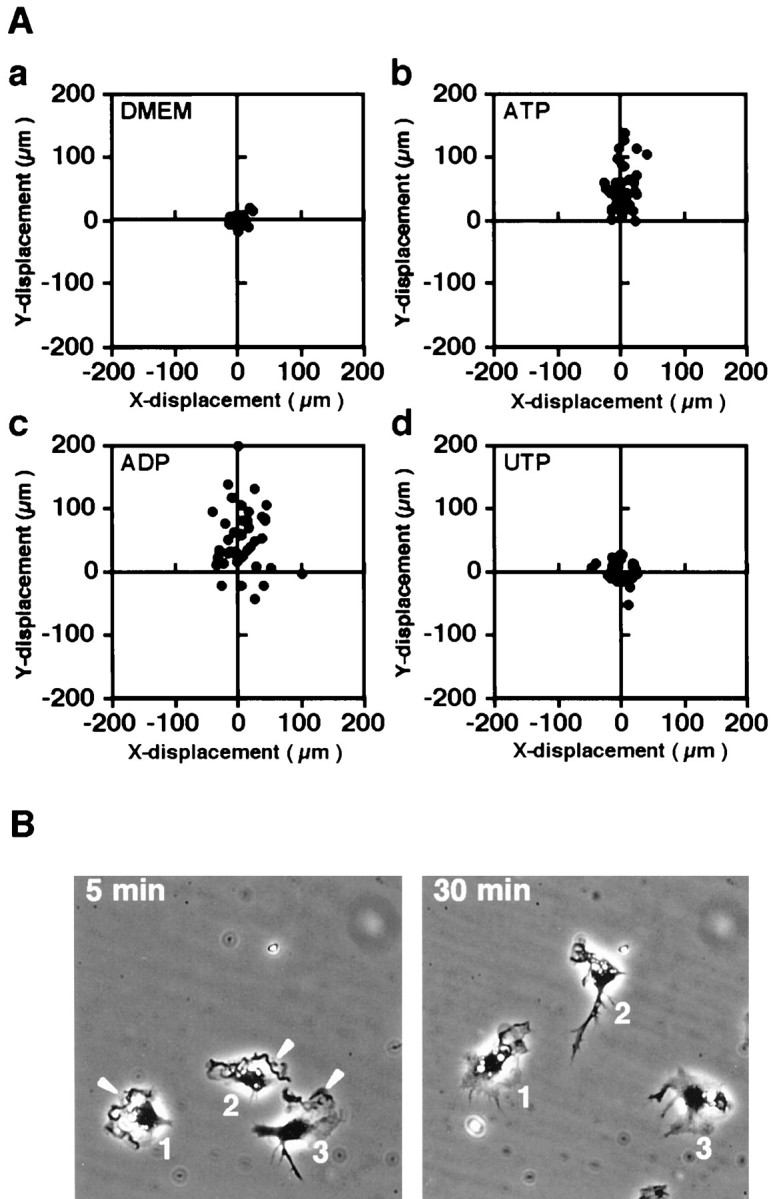
Nucleotide-induced chemotaxis of microglia in the Dunn chemotaxis chamber. A, Vector diagrams of cell displacement at 60 min after setting up the chamber. The cells were incubated in the absence of nucleotides (a) or in the presence of 50 μm ATP (b), ADP (c), or UTP (d) in the outer well. The position of the outer well of the chamber is vertically upward. All diagrams were obtained from a representative experiment using the same lot of microglial culture. We confirmed that the three independent experiments showed the same tendency. B,Displacement and morphological change at 5 and 30 min after setting up the chamber. The cells were incubated in the presence of 50 μm ATP in the outer well. The images of the same area are presented such that the position of the outer well of the chamber is vertically upward. Arrowheads indicate membrane ruffles.
Determination of subtype of P2 receptors
P2 receptors have been divided into two types, ionotropic P2X and G-protein-coupled P2Y receptors (Barnard et al., 1997). P2X receptors act as ligand-gated, nonselective cation channels. We have previously demonstrated that ATP-induced plasminogen release from microglia depends on extracellular-Ca2+ influx via P2X7 receptors (Inoue et al., 1998). To identify the types of P2 receptors involved in ATP- and ADP-induced membrane ruffling, we examined the effect of extracellular calcium deprivation. Unlike the plasminogen release (Inoue et al., 1998), ATP and ADP induced membrane ruffling in Ca2+-free BSS as well as in Ca2+-containing BSS (Fig.4). These results suggest that the membrane ruffling induced by ATP and ADP is not dependent on extracellular Ca2+ influx via ionotropic P2X receptors, but is mediated by P2Y receptors.
Fig. 4.
Effect of extracellular calcium deprivation on membrane ruffling induced by ATP or ADP. Cells were stimulated with PBS (A, D), 50 μm ATP (B, E), or 50 μm ADP (C, F) in BSS with (A–C) or without (D–F) calcium. After stimulation, the cells were fixed and stained with Texas Red-conjugated phalloidin. Arrowheads indicate membrane ruffles. Scale bar, 20 μm.
In our experiments, ATP and ADP acted as potent agonists, whereas UTP exerted only a slight effect on membrane ruffling. Some reports have shown that human P2Y1, among various P2Y receptors, is activated by ADP and that mouse P2Y2 is equally activated by ATP and UTP (Lustig et al., 1993; Léon et al., 1997). Although some discrepancy exists regarding the potency of UTP, we speculated that P2Y1 or P2Y2 might be a possible receptor for ADP- or ATP-induced membrane ruffling and cell migration. We examined this possibility using three kinds of selective antagonists, suramin, PPADS, and A3P5PS. Charlton et al. (1996)reported that suramin caused antagonistic effects on turkey P2Y1 and human P2Y2expressed in human astrocytoma cell line 1321N1, whereas PPADS antagonized the effects via P2Y1 and not P2Y2. A3P5PS was reported as a selective antagonist of the P2Y1 receptor (Boyer et al., 1996). By contrast, pretreatment with a rather high dose (300 μm) of PPADS, suramin, or A3P5PS did not inhibit the ATP- and ADP-induced membrane ruffling in microglia, as shown in Figure5. These data suggest that neither P2Y1 nor P2Y2 plays a main role in the ADP- and ATP-evoked responses in microglia.
Fig. 5.

Effects of PPADS, suramin, and A3P5PS on nucleotide-induced membrane ruffling. Microglia were stimulated with 50 μm ATP (B, E, H, K) or ADP (C, F, I, L) for 5 min after 10 min of pretreatment with PBS (A–C), 300 μm suramin (D–F), 300 μm PPADS (G–I), or 300 μm A3P5PS (J–L). After fixation, the cells were stained with Texas Red-conjugated phalloidin. Arrowheadsindicate membrane ruffles. Scale bar, 20 μm.
Inhibition of membrane ruffling by AR-C69931MX and pertussis toxin
P2TAC receptors, which are highly sensitive to ADP but distinct from P2Y1, have recently been predicted to be present in platelets (Daniel et al., 1998), although they have not yet been cloned. Therefore, we examined the effect of AR-C69931MX, a potent and selective antagonist against P2TAC receptors (Ingall et al., 1999; Ishii-Watabe et al., 2000), on ATP- and ADP-induced membrane ruffling. Pretreatment with 1 μmAR-C69931MX completely inhibited ATP- and ADP-induced membrane ruffling in microglia, whereas the same treatment did not inhibit M-CSF-induced membrane ruffling (Fig. 6). The antagonistic effect of AR-C69931MX was further confirmed against the poorly hydrolyzable analog ATPγS (50 μm) (data not shown). These receptors were also reported to be PTx-sensitive (Daniel et al., 1998). To verify the possibility of this type of P2 receptor being involved, we further examined the effects of PTx on ATP- and ADP-induced membrane ruffling and chemokinesis. The 4 hr pretreatment with PTx at 50 ng/ml completely inhibited membrane ruffling induced by ATP and ADP (Fig. 7), whereas M-CSF-induced membrane ruffling, which is well known to be mediated by a tyrosine kinase receptor, Fms, was not affected by PTx. Furthermore, the pretreatment with PTx also inhibited ATP-induced chemokinesis in the Boyden chamber assay (Fig. 8). These results strongly suggest that Gi/o-coupled P2Y receptors, most likely P2TAC receptors, are involved in the membrane ruffling and chemotaxis of microglia induced by ATP or ADP, although precise identification of the specific receptor remains unclear.
Fig. 6.
Effect of AR-C69931MX on nucleotide-induced membrane ruffling. Microglia were stimulated with 50 μmATP (B, F) or ADP (C, G) or 100 ng/ml M-CSF (D, H) for 5 min after 10 min of pretreatment with PBS (A–D) or 1 μm AR-C69931MX (E–H). After fixation, the cells were stained with Texas Red-conjugated phalloidin.Arrowheads indicate membrane ruffling. Scale bar, 20 μm.
Fig. 7.
Effects of PTx on nucleotide-induced membrane ruffling. Microglia were stimulated with PBS (A, E), 50 μm ATP (B, F), 50 μmADP (C, G), or 100 ng/ml M-CSF (D, H) for 5 min after 4 hr of pretreatment with PBS (A–D) or 50 ng/ml pertussis toxin (E–H). After fixation, the cells were stained with Texas Red-conjugated phalloidin. Arrowheadsindicate membrane ruffles. Scale bar, 20 μm.
Fig. 8.
Effect of PTx on ATP-induced chemokinesis in the Boyden chamber. The chemokinesis assay was performed in the presence of 50 μm ATP with (white column) or without (black column) pertussis toxin treatment. Cells were pretreated with 50 ng/ml PTx for 4 hr.
ATP- and ADP-induced Rac activation in microglia
Finally, to determine whether ATP- and ADP-induced membrane ruffling and chemotaxis of microglia are mediated by Gi/o-coupled P2Y receptors, intracellular signaling was investigated. It has been well established that the Rho family of small G-proteins are key molecules in the reorganization of actin cytoskeleton (Hall, 1998). Among the Rho family, Rac is known to be activated when cells form membrane ruffles and lamellipodia (Ridley et al., 1992; Ohsawa et al., 2000). Activated Rac was found to be translocated to the membrane after stimulation (Ridley et al., 1992;Bokoch et al., 1994). Thus, we performed double staining to examine the translocation of Rac after ATP or ADP stimulation by using Texas Red-conjugated phalloidin (red) and anti-Rac antibody visualized with FITC-conjugated secondary antibody (green) (Fig.9A). We could detect the translocation of Rac to the ruffling region colocalized with phalloidin staining 5 min after stimulation.
Fig. 9.

Rac activation induced by ATP and ADP.A, Translocation of Rac. The cells were stimulated with PBS (a–c), 50 μm ATP (d–f), or 50 μm ADP (g–i) for 5 min and stained with Rac antibody and FITC-conjugated anti-mouse IgG (a, d, g) and Texas Red-conjugated phalloidin (b, e, h). Merged photographs show the colocalization of Rac and F-actin in the ruffling region (f, i), as indicated by arrows. Scale bar, 10 μm. B, Pull-down assay of activated Rac. Cells were stimulated with PBS (control), 50 μm ATP, or 50 μm ADP for 1 min, and pull-down assay was performed as described in Materials and Methods. Although the total amount of Rac in the cell lysate was the same for each stimulation, activated Rac was increased in the pull-down samples from ATP- and ADP-stimulated cells.
To confirm Rac activation, activated Rac was biochemically measured. Because activated Rac is known to bind to PAK kinase (Manser et al., 1998), a pull-down assay was performed using GST-fused PAK to detect the GTP-bound form of Rac. In samples from cells that were stimulated with ATP or ADP for 1 min, more of the active form of Rac was pulled down as compared with that in control cells (Fig. 9B). At the same time, we confirmed that the total amount of Rac in the cell lysates in controls was the same as that in the nucleotide-treated cells (Fig. 9B). Moreover, pretreatment of the cells with PTx completely blocked the activation of Rac induced by ATP or ADP (Fig. 10). These results further support the idea that ATP- and ADP-induced membrane ruffling and chemotaxis are mediated by Gi/o-coupled P2Y receptors.
Fig. 10.
Effect of PTx on the nucleotide-induced activation of Rac. Pull-down assay was performed, as described in Materials and Methods. Microglia were stimulated with PBS (control), 50 μm ADP, 50 μm ATP, or 100 ng/ml M-CSF for 1 min after 4 hr of treatment with (+) or without (−) PTx at 50 ng/ml.
DISCUSSION
In the present study, we demonstrated that extracellular ATP and ADP strongly enhanced the formation of membrane ruffles and chemotaxis of microglia. Nucleotide-induced chemotaxis has been reported in rat mast cells (McCloskey et al., 1999) and human neutrophils (Verghese et al., 1996) by using the Boyden chamber assay. We found that ATP and ADP enhanced chemokinesis in the Boyden chamber assay. Although we could not determine whether nucleotides played a role as chemoattractants on microglia by the Boyden chamber assay, the Dunn chemotaxis chamber, which allows the direct observation of cell migration, revealed that the nucleotides had the potency to induce chemotaxis of microglia. The reason we were unable to detect the chemotactic activity of nucleotides in the Boyden chamber assay seems to be partly that the low molecular weight nucleotides easily diffuse from the bottom to the top compartment of the Boyden chamber. Considering the strong effect of ADP, it is highly probable that the effects of ATP actually depend on the metabolite ADP, which may be easily produced during incubation. However, the possibility is very slight because ATPγS (10 μm) had the same effect as ATP (data not shown).
Considering the physiological effect of nucleotides on microglia, it is important to identify P2 receptor subtypes. P2 receptors have been divided into two types, ionotropic P2X, and G-protein-coupled P2Y receptors (Barnard et al., 1997). Although no study has clarified the profile of P2-receptor subtype expression in microglia, the existence of several kinds of P2Y and P2X receptors has been suggested from electrophysiological studies (Nörenberg et al., 1997; Visentin et al., 1999). The biological effects of ATP on microglia have been suggested to be mediated mainly by P2X7 receptors (Ferrari et al., 1996, 1997, 1999; Inoue et al., 1998; Hide et al., 2000). The present study, however, suggests the involvement of P2Y receptors in nucleotide-induced ruffle formation and cell migration, based on the experiments of extracellular-calcium elimination and AR-C69931MX or PTx treatment.
To date, seven G-protein-coupled P2Y receptors have been cloned in mammalian species, some of which have not been fully characterized in nature (Alexander et al., 1999). Suramin, PPADS, and A3P5PS are commonly used for the pharmacological classification of the subtypes of P2Y receptors. Suramin is an antagonist for both P2Y1 and P2Y2, whereas PPADS antagonizes P2Y1 but not P2Y2 (Charlton et al., 1996). A3P5PS is a potent and selective antagonist of P2Y1 (Boyer et al., 1996). Although we examined the effects of these antagonists on ATP- and ADP-induced membrane ruffling in microglia, we did not observe any inhibitory effect. Based on the previous reports described above, neither P2Y1 nor P2Y2 would be involved in nucleotide-induced membrane ruffling of microglia.
Recently, Gi/o-coupled P2Y receptors, designated P2TAC receptors, which are highly sensitive to ADP, were predicted in platelets (Daniel et al., 1998), although they have not yet been cloned. Based on the results showing that the effects of nucleotides on microglial motility were completely blocked by either AR-C69931MX or PTx pretreatment, this type of Gi/o-coupled receptor is postulated as a candidate for the P2Y receptors involved in membrane ruffling and chemotaxis. However, there are a few reports suggesting that P2Y2 is partially sensitive to PTx in the stable expression system of 1321N1 astrocytoma cells (Parr et al., 1994) and in human erythroleukemia cells (Baltensperger and Porzig, 1997). P2Y1 was also suggested to be PTx-sensitive in astrocytes (Chen et al., 1998), whereas Schachter et al. (1997) clarified the uncoupling of P2Y1 to Gi/o by using the system of expression in 1321N1 cells. Under these circumstances, in which contradictory reports have been issued, the specific Gi/o-coupled subtype of P2Y receptors involved in the present effects cannot be defined. Further characterization of P2Y receptors, including cloning of the P2TAC receptor is needed.
With regard to the intracellular signaling downstream of the Gi/o-coupled P2Y receptors, we were able to detect Rac activation in microglia when the cells formed membrane ruffles after ATP or ADP stimulation. Furthermore, PTx-induced inhibition of Rac activation indicates a signal cascade from Gi/o-coupled-P2Y receptors to Rac activation. Rac is a member of the Rho family of small G-proteins, and Rac activation is known to induce lamellipodia and membrane ruffles in various kinds of cells (Ridley et al., 1992; Hall, 1998). There has been cumulative evidence suggesting of the activation of Rac via heterotrimeric G-proteins. Ma et al. (1998) suggested that cytoskeletal reorganization by fMLP (N-formyl-Met-Leu-Phe) is dependent on Rac, Vav, a guanosine exchange factor of Rac, and phosphoinositide 3-kinase γ (PI3Kγ), in Cos-7SH cells. Although Rac activation still occurred in neutrophils obtained from PI3Kγ and PLCβ2/3 knock-out mice, chemokine-mediated chemotaxis was impaired in PI3Kγ knock-out mice (Li et al., 2000). Such signaling molecules may be involved in microglia. The possibility of the present findings having been physiologically involved with the function of microglia in the pathological states of the brain may be considered.
In brain with damaged neurons and astrocytes, large amounts of nucleotides are released from these cells (Dubyak and El-Moatassim, 1993; Neary et al., 1996). The extracellular nucleotides, which are easily diffused and rapidly catalyzed by ATPases, may play a role in modulating the microglial function of the brain in the early phase of pathology. We presented a novel effect of nucleotides on microglia, that is, the induction of chemotaxis via Gi/o-coupled P2Y receptors. Considering former studies that have revealed the biological effects of nucleotides on microglia such as the release of IL-1β, plasminogen, and TNF-α via P2X7 (Ferrari et al., 1996, 1997, 1999; Inoue et al., 1998; Hide et al., 2000), our results suggest that two distinct P2X and P2Y receptor subtypes are involved in the diverse functions of microglia, such as scavenging and neuroprotective actions, in pathological states.
Footnotes
This work was supported by a grant from the Organization for Pharmaceutical Safety and Research and by a Grant-in-Aid for the Scientific Research on Priority Areas from the Ministry of Education, Science, Sports, and Culture of Japan. AR-C69931MX was kindly supplied from AstraZeneca UK Limited (London, UK).
Correspondence should be addressed to Shinichi Kohsaka, Department of Neurochemistry, National Institute of Neuroscience, 4-1-1 Ogawa-higashi, Kodaira, Tokyo 187-8502, Japan. E-mail:kohsaka@ncnp.go.jp.
REFERENCES
- 1.Alexander S, Peters J, Mead A, Lewis S. Receptor & ion channel nomenclature supplement, Ed 10, Trends Pharmacol Sci, pp 64–69. Elsevier Science; London: 1999. [Google Scholar]
- 2.Baltensperger K, Porzig H. The P2U purinoceptor obligatorily engages the heterotrimeric G protein G16 to mobilize intracellular Ca2+ in human erythroleukemia cells. J Biol Chem. 1997;272:10151–10159. doi: 10.1074/jbc.272.15.10151. [DOI] [PubMed] [Google Scholar]
- 3.Barnard EA, Simon J, Webb TE. Nucleotide receptors in the nervous system. An abundant component using diverse transduction mechanisms. Mol Neurobiol. 1997;15:103–129. doi: 10.1007/BF02740631. [DOI] [PubMed] [Google Scholar]
- 4.Bokoch GM, Bohl BP, Chuang TH. Guanine nucleotide exchange regulates membrane translocation of Rac/Rho GTP-binding proteins. J Biol Chem. 1994;269:31674–31679. [PubMed] [Google Scholar]
- 5.Boyer JL, Romero-Avila T, Schachter JB, Harden TK. Identification of competitive antagonists of P2Y1 receptor. Mol Pharmacol. 1996;50:1323–1329. [PubMed] [Google Scholar]
- 6.Charlton SJ, Brown CA, Weisman GA, Turner JT, Erb L, Boarder MR. PPADS and suramin as antagonists at cloned P2Y- and P2U-purinoceptors. Br J Pharmacol. 1996;118:704–710. doi: 10.1111/j.1476-5381.1996.tb15457.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen WC, Chen C-C. ATP-induced arachidonic acid release in cultured astrocytes is mediated by Gi protein coupled P2Y1 and P2Y2 receptors. Glia. 1998;22:360–370. doi: 10.1002/(sici)1098-1136(199804)22:4<360::aid-glia5>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 8.Chen ZP, Levy A, Lightman SL. Activation of specific ATP receptors induces a rapid increase in intracellular calcium ions in rat hypothalamic neurons. Brain Res. 1994;641:249–256. doi: 10.1016/0006-8993(94)90151-1. [DOI] [PubMed] [Google Scholar]
- 9.Cross AK, Woodroofe MN. Chemokines induce migration and changes in actin polymerization in adult rat brain microglia and a human fetal microglial cell line in vitro. J Neurosci Res. 1999;55:17–23. doi: 10.1002/(SICI)1097-4547(19990101)55:1<17::AID-JNR3>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 10.Daniel JL, Dangelmaier C, Jin J, Ashby B, Smith JB, Kunapuli SP. Molecular basis for ADP-induced platelet activation. J Biol Chem. 1998;273:2024–2029. doi: 10.1074/jbc.273.4.2024. [DOI] [PubMed] [Google Scholar]
- 11.Dubyak GR, El-Moatassim C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 12.Edwards FA, Gibb AJ, Colquhoun D. ATP receptor-mediated synaptic currents in the central nervous system. Nature. 1992;359:144–147. doi: 10.1038/359144a0. [DOI] [PubMed] [Google Scholar]
- 13.Ferrari D, Villalba M, Chiozzi P, Falzoni S, Ricciardi-Castagnoli P, Di Virgillio F. Mouse microglial cells express a plasma membrane pore gated by extracellular ATP. J Immumol. 1996;156:1531–1539. [PubMed] [Google Scholar]
- 14.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of Interleukin-1β release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ferrari D, Los M, Bauer MKA, Vandenabeele P, Wesselborg S, Schulze-Osthoff K. P2Z purinoceptor ligation induces activation of caspases with distinct roles in apoptotic and necrotic alterations of cell death. FEBS Lett. 1999;447:71–75. doi: 10.1016/s0014-5793(99)00270-7. [DOI] [PubMed] [Google Scholar]
- 16.Graeber MB, López-Redondo F, Ikoma E, Ishikawa M, Imai Y, Nakajima K, Kreutzberg GW, Kohsaka S. The microglia/macrophage response in the neonatal rat facial nucleus following axotomy. Brain Res. 1998;813:241–253. doi: 10.1016/s0006-8993(98)00859-2. [DOI] [PubMed] [Google Scholar]
- 17.Hall A. Rho GTPases and the actin cytoskeleton. Science. 1998;279:509–514. doi: 10.1126/science.279.5350.509. [DOI] [PubMed] [Google Scholar]
- 18.Hayashi M, Luo Y, Laning J, Strieter RM, Dorf ME. Production and function of monocyte chemoattractant protein-1 and other β-chemokines in murine glial cells. J Neuroimmunol. 1995;60:143–150. doi: 10.1016/0165-5728(95)00064-9. [DOI] [PubMed] [Google Scholar]
- 19.Hide I, Tanaka M, Inoue A, Inoue K, Kohsaka S, Nakata Y. Extracellular ATP triggers TNF-α release from rat microglia. J Neurochem. 2000;75:965–972. doi: 10.1046/j.1471-4159.2000.0750965.x. [DOI] [PubMed] [Google Scholar]
- 20.Ingall AH, Dixon J, Bailey A, Coombs ME, Cox D, Mclnally JI, Hunt SF, Kindon ND, Teobald BJ, Willis PA, Humphries RG, Leff P, Clegg JA, Smith JA, Tomlinson W. Antagonists of the platelet P2T receptor: a novel approach to antithrombotic therapy. J Med Chem. 1999;42:213–220. doi: 10.1021/jm981072s. [DOI] [PubMed] [Google Scholar]
- 21.Inoue K, Koizumi S, Nakazawa K. Glutamate-evoked release of adenosine 5′-triphosphate causing an increase in intracellular calcium in hippocampal neurones. NeuroReport. 1995;6:437–440. doi: 10.1097/00001756-199502000-00008. [DOI] [PubMed] [Google Scholar]
- 22.Inoue K, Nakajima K, Morimoto T, Kikuchi Y, Koizumi S, Illes P, Kohsaka S. ATP stimulation of Ca2+-dependent plasminogen release from cultured microglia. Br J Pharmacol. 1998;123:1304–1310. doi: 10.1038/sj.bjp.0701732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii-Watabe A, Uchida E, Mizuguchi H, Hayakawa T. On the mechanism of plasmin-induced platelet aggregation. Biochem Pharmacol. 2000;59:1345–1355. doi: 10.1016/s0006-2952(00)00279-3. [DOI] [PubMed] [Google Scholar]
- 24.Ito D, Imai Y, Ohsawa K, Nakajima K, Fukuuchi Y, Kohsaka S. Microglia-specific localisation of a novel calcium binding protein, Iba1. Mol Brain Res. 1998;57:1–9. doi: 10.1016/s0169-328x(98)00040-0. [DOI] [PubMed] [Google Scholar]
- 25.Kirischuk S, Scherer J, Kettenmann H, Verkhratsky A. Activation of P2-purinoreceptors triggered Ca2+ release from InsP3-sensitive internal stores in mammalian oligodendrocytes. J Physiol (Lond) 1995;483:41–57. doi: 10.1113/jphysiol.1995.sp020566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kreutzberg GW. Microglia: a sensor for pathological events in the CNS. Trends Neurosci. 1996;19:312–318. doi: 10.1016/0166-2236(96)10049-7. [DOI] [PubMed] [Google Scholar]
- 27.Lauffenburger DA, Horwitz AF. Cell migration: a physically integrated molecular process. Cell. 1996;84:359–369. doi: 10.1016/s0092-8674(00)81280-5. [DOI] [PubMed] [Google Scholar]
- 28.Léon C, Hechler B, Vial C, Leray C, Cazenave JP, Gachet C. The P2Y1 receptor is an ADP receptor antagonized by ATP and expressed in platelets and megakaryoblastic cells. FEBS Lett. 1997;403:26–30. doi: 10.1016/s0014-5793(97)00022-7. [DOI] [PubMed] [Google Scholar]
- 29.Li Z, Jiang H, Xie W, Zhang Z, Smrcka AV, Wu D. Roles of PLC-β2 and -β3 and PI3Kγ in chemoattractant-mediated signal transduction. Science. 2000;287:1046–1049. doi: 10.1126/science.287.5455.1046. [DOI] [PubMed] [Google Scholar]
- 30.Lustig KD, Shiau AK, Brake AJ, Julius D. Expression cloning of an ATP receptor from mouse neuroblastoma cells. Proc Natl Acad Sci USA. 1993;90:5113–5117. doi: 10.1073/pnas.90.11.5113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ma AD, Metjian A, Bagrodia S, Taylor S, Abrams CS. Cytoskeletal reorganization by G protein-coupled receptors is dependent on phosphoinositide 3-kinase γ, a Rac guanosine exchange factor, and Rac. Mol Cell Biol. 1998;18:4744–4751. doi: 10.1128/mcb.18.8.4744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manser E, Loo T-H, Koh C-G, Zhao Z-S, Chen X-Q, Tan L, Tan I, Leung T, Lim L. PAK kinases are directly coupled to the PIX family of nucleotide exchange factors. Mol Cell. 1998;1:183–192. doi: 10.1016/s1097-2765(00)80019-2. [DOI] [PubMed] [Google Scholar]
- 33.McCloskey MA, Fan Y, Luther S. Chemotaxis of rat mast cells toward adenine nucleotides. J Immunol. 1999;163:970–977. [PubMed] [Google Scholar]
- 34.Nabekura J, Ueno S, Ogawa T, Akaike N. Colocalization of ATP and nicotinic ACh receptors in the identified vagal preganglionic neurone of rat. J Physiol (Lond) 1995;489 2:519–527. doi: 10.1113/jphysiol.1995.sp021069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nakajima K, Shimojo M, Hamanoue M, Ishiura S, Sugita H, Kohsaka S. Identification of elastase as a secretory protease from cultured rat microglia. J Neurochem. 1992;58:1401–1408. doi: 10.1111/j.1471-4159.1992.tb11356.x. [DOI] [PubMed] [Google Scholar]
- 36.Neary JT, Baker L, Jorgensen SL, Norenberg MD. Extracellular ATP induces stellation and increases glial fibrillary acidic protein content and DNA synthesis in primary astrocyte cultures. Acta Neuropathol. 1994;87:8–13. doi: 10.1007/BF00386249. [DOI] [PubMed] [Google Scholar]
- 37.Neary JT, Rathbone MP, Cattabeni F, Abbracchio MP, Burnstock G. Trophic actions of extracellular nucleotides and nucleosides on glial and neuronal cells. Trends Neurosci. 1996;19:13–18. doi: 10.1016/0166-2236(96)81861-3. [DOI] [PubMed] [Google Scholar]
- 38.Neary JT, Kang Y, Bu Y, Yu E, Akong K, Peters CM. Mitogenic signaling by ATP/P2Y purinergic receptors in astrocytes: involvement of a calcium-independent protein kinase C, extracellular signal-regulated protein kinase pathway distinct from the phosphatidylinositol-specific phospholipase C/calcium pathway. J Neurosci. 1999;19:4211–4220. doi: 10.1523/JNEUROSCI.19-11-04211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nolte C, Möller T, Walter T, Kettenmann H. Complement 5a controls motility of murine microglial cells in vitro via activation of an inhibitory G-protein and the rearrangement of the actin cytoskeleton. Neuroscience. 1996;73:1091–1107. doi: 10.1016/0306-4522(96)00106-6. [DOI] [PubMed] [Google Scholar]
- 40.Nolte C, Kirchhoff F, Kettenmann H. Epidermal growth factor is a motility factor for microglial cells in vitro: evidence for EGF receptor expression. Eur J Neurosci. 1997;9:1690–1698. doi: 10.1111/j.1460-9568.1997.tb01526.x. [DOI] [PubMed] [Google Scholar]
- 41.Nörenberg W, Cordes A, Blöhbaum G, Fröhlich R, Illes P. Coexistence of purino- and pyrimidinoceptors on activated rat microglial cells. Br J Pharmacol. 1997;121:1087–1098. doi: 10.1038/sj.bjp.0701241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohsawa K, Imai Y, Kanazawa H, Sasaki Y, Kohsaka S. Involvement of Iba1 in membrane ruffling and phagocytosis of macrophage/microglia. J Cell Sci. 2000;113:3073–3084. doi: 10.1242/jcs.113.17.3073. [DOI] [PubMed] [Google Scholar]
- 43.Parr CE, Sullivan DM, Paradiso AM, Lazarowski ER, Burch LH, Olsen JC, Erb L, Weisman GA, Boucher RC, Turner JT. Cloning and expression of a human P2U nucleotide receptor, a target for cystic fibrosis pharmacotherapy. Proc Natl Acad Sci USA. 1994;91:3275–3279. doi: 10.1073/pnas.91.8.3275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridley AJ, Paterson HF, Johnston CL, Diekmann D, Hall A. The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell. 1992;70:401–410. doi: 10.1016/0092-8674(92)90164-8. [DOI] [PubMed] [Google Scholar]
- 45.Scemes E, Suadicani SO, Spray DC. Intercellular communication in spinal cord astrocytes: fine tuning between gap junctions and P2 nucleotide receptors in calcium wave propagation. J Neurosci. 2000;20:1435–1445. doi: 10.1523/JNEUROSCI.20-04-01435.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schachter JB, Boyer JL, Li Q, Nicholas RA, Harden TK. Fidelity in functional coupling of the rat P2Y1 receptor to phospholipase C. Br J Pharmacol. 1997;122:1021–1024. doi: 10.1038/sj.bjp.0701479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Shen K-Z, North RA. Excitation of rat locus coeruleus neurons by adenosine 5′-triphosphate: ionic mechanism and receptor characterization. J Neurosci. 1993;13:894–899. doi: 10.1523/JNEUROSCI.13-03-00894.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Streit WJ, Graeber MB, Kreutzberg GW. Functional plasticity of microglia: a review. Glia. 1988;1:301–307. doi: 10.1002/glia.440010502. [DOI] [PubMed] [Google Scholar]
- 49.Thomas WE. Brain macrophages: on the role of pericytes and perivascular cells. Brain Res Rev. 1999;31:42–57. doi: 10.1016/s0165-0173(99)00024-7. [DOI] [PubMed] [Google Scholar]
- 50.Verghese MW, Kneisler TB, Boucheron JA. P2U agonists induce chemotaxis and actin polymerization in human neutrophils and differentiated HL60 cells. J Biol Chem. 1996;271:15597–15601. doi: 10.1074/jbc.271.26.15597. [DOI] [PubMed] [Google Scholar]
- 51.Visentin S, Renzi M, Frank C, Greco A, Levi G. Two different ionotropic receptors are activated by ATP in rat microglia. J Physiol (Lond) 1999;519:723–736. doi: 10.1111/j.1469-7793.1999.0723n.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Webb SE, Pollard JW, Jones GE. Direct observation and quantification of macrophage chemoattraction to the growth factor CSF-1. J Cell Sci. 1996;109:793–803. doi: 10.1242/jcs.109.4.793. [DOI] [PubMed] [Google Scholar]
- 53.Yao J, Harvath L, Gilbert DL, Colton CA. Chemotaxis by a CNS macrophage, the microglia. J Neurosci Res. 1990;27:36–42. doi: 10.1002/jnr.490270106. [DOI] [PubMed] [Google Scholar]
- 54.Yokomizo T, Izumi T, Chang K, Takuwa Y, Shimizu T. A G-protein-coupled receptor for leukotriene B4 that mediates chemotaxis. Nature. 1997;387:620–624. doi: 10.1038/42506. [DOI] [PubMed] [Google Scholar]








