Abstract
Recent work has shown substantial alterations in NMDA receptor subunit expression, assembly, and phosphorylation in the dopamine-depleted striatum of a rodent 6-hydroxydopamine model of Parkinson's disease. These modifications are hypothesized to result from the trafficking of NMDA receptors between subcellular compartments. Here we show that in rat striatal tissues the NR2A and NR2B subunits in the synaptosomal membrane, and not those in the light membrane and synaptic vesicle-enriched compartments, are tyrosine phosphorylated. The dopamine D1 receptor agonist SKF-82958 produces (1) an increase in NR1, NR2A, and NR2B proteins in the synaptosomal membrane fraction; (2) a decrease in NR1, NR2A, and NR2B proteins in the light membrane and synaptic vesicle-enriched fractions; and (3) an increase in the tyrosine phosphorylation of NR2A and NR2B in the synaptosomal membrane compartment. The protein phosphatase inhibitor pervanadate reproduces the alterations in subcellular distribution and phosphorylation, whereas the effects of the dopamine D1 receptor agonist are blocked by genistein, a protein tyrosine kinase inhibitor. Dopamine D1 receptor agonist treatment does not change the subcellular distribution of the AMPA receptor subunits GluR1 or GluR2/3 in the striatum and has no effect on cortical or cerebellar NMDA receptor subunits. These data reveal a rapid dopamine D1 receptor- and tyrosine kinase-dependent trafficking of striatal NMDA receptors between intracellular and postsynaptic sites. The subcellular trafficking of striatal NMDA receptors may play a significant role both in the pathogenesis of Parkinson's disease and in the development of adverse effects of chronic dopaminergic therapy in parkinsonian patients.
Keywords: glutamate receptor, NMDA receptor, dopamine receptor, subcellular compartment, receptor trafficking, tyrosine phosphorylation
NMDA glutamate receptors are heteromeric ligand-gated ion channels assembled from two subunit families: NR1, which consists of eight recognized isoforms that are generated from alternative splicing of a single gene, and NR2, composed of NR2A, NR2B, NR2C, and NR2D, encoded by four distinct genes (Dingledine et al., 1999). In the CNS the properties of NMDA receptors are determined by their subunit composition, phosphorylation, and cellular localization. The composition of NMDA receptors substantially alters the pharmacology of the receptors, in particular their affinities for agonists and antagonists (Laurie and Seeburg, 1994; Lynch et al., 1994; Williams et al., 1994; Lynch and Gallagher, 1996). Both NR1 and NR2 subunits are phosphorylated; the NR1 subunit has several serine phosphorylation sites in the carboxy tail region (Hisatsune et al., 1997; Tingley et al., 1997), whereas the NR2 subunits are phosphorylated at tyrosine residues (Moon et al., 1994;Lau and Huganir, 1995; Dunah et al., 1998). Phosphorylation modulates the activation and deactivation kinetic properties of NMDA receptors as well as other properties (Wang and Salter, 1994; Chen and Leonard, 1996). In the brain, NMDA receptors are found both in the cytoplasm of neurons as well as at excitatory synapses (Petralia et al., 1994). At the postsynaptic membrane, NMDA receptors interact with components of the postsynaptic density, a large macromolecular complex containing both anchoring and signaling elements (Kornau et al., 1995; Kim et al., 1996; Wyszynski et al., 1997; Ziff, 1997; Lin et al., 1998). The mechanisms regulating the subcellular localization and targeting of glutamate receptors to postsynaptic sites are not well defined, but it is clear that the subcellular distribution of the subunits can be regulated by neuronal activity (Rao and Craig, 1997; Lissin et al., 1998; Roche et al., 2000; Zukin et al., 2000; Vissel et al., 2001).
NMDA receptors expressed by neurons in the striatum play an important role in the regulation of movement as they mediate the effects of excitatory inputs from the cerebral cortex and thalamus and exhibit physiologic interactions with dopaminergic input from the nigrostriatal pathway (Di Chiara et al., 1994). In animal models of Parkinson's disease, antagonists of NMDA receptors substantially potentiate the short-term therapeutic effect of dopaminergic agents (Klockgether and Turski, 1990; Morelli et al., 1992; Papa et al., 1993; Kaur and Starr, 1997) and are effective in attenuating the abnormal motor behaviors produced by chronic dopaminergic treatment (Papa et al., 1995; Marin et al., 1996; Blanchet et al., 1997). Recent evidence suggests that the basis for the development of these abnormal motor behaviors may be that long-term treatment with dopaminergic agents modifies the properties of striatal NMDA receptors (Engber et al., 1994; Papa et al., 1995; Marin et al., 1996; Papa and Chase, 1996; Chase et al., 1998).
Recently, we showed that in rats with unilateral 6-hydroxy-dopamine (6-OHDA) lesions of the nigrostriatal pathway, which serves as a model of Parkinson's disease, there is a selective reduction in NMDA heteromers composed of NR1/NR2B subunits in the synaptosomal membranes of the dopamine-depleted striatum (Dunah et al., 2000a). In addition, the serine phosphorylation of NR1 and the tyrosine phosphorylation of NR2B, but not NR2A, are decreased. The alterations were observed only in membrane preparations and not in total striatal homogenate, suggesting a redistribution of NMDA receptor subunits among subcellular compartments. Chronic treatment of lesioned rats with levodopa (l-3,4-dihydroxyphenylalanine) normalized the abundance of the NMDA receptor subunits and increased the phosphorylation of NR1 and NR2 subunits (Dunah et al., 2000a).
On the basis of these observations we hypothesized that the dopamine-dependent phosphorylation of striatal NMDA receptors might be the trigger signal for the trafficking of NMDA receptors from intracellular compartments to the postsynaptic density. In the present study, biochemical protein fractionation, quantitative immunoprecipitation, and immunoblot methods were used to examine the distribution and phosphorylation of NMDA receptors in distinct subcellular compartments of acutely dissected rat striatum and the effect of treatment with pharmacologic agents affecting dopamine receptors or protein phosphorylation.
MATERIALS AND METHODS
Antibodies and pharmacological agents. The subunit-specific monoclonal NR1 (Luo et al., 1997), polyclonal NR2A (Wang et al., 1995), and monoclonal NR2B (Wang et al., 1995) antibodies were generous gifts from Dr. Barry B. Wolfe, Georgetown University (Washington, DC). The polyclonal α-actinin-2 antibody (Wyszynski et al., 1997) was a kind donation from Dr. Morgan Sheng, Harvard Medical School (Boston, MA). The following antibodies were obtained from commercial sources: monoclonal PSD-95 (K28/43; Upstate Biotechnology, Lake Placid, NY); polyclonal NSF (N-ethylmaleimide-sensitive fusion protein) rabbit serum (Synaptic Systems GmbH, Gottingen, Germany); polyclonal GluR1 and GluR2/3 (Chemicon, Temecula, CA); monoclonal syntaxin and synaptophysin (Sigma/RBI, St. Louis, MO/Natick, MA); monoclonal calnexin (Santa Crux Biotechnology, Santa Cruz, CA); monoclonal anti-phosphotyrosine (PY20) and recombinant anti-phosphotyrosine monoclonal (RC20) (Transduction Laboratories, Lexington, KY); horseradish peroxidase-linked goat anti-rabbit and horseradish peroxidase-linked goat anti-mouse (Jackson ImmunoResearch Laboratories, West Grove, PA). The drugs used for treatment of striatal tissues were purchased from the following sources: SKF-82958, SKF-38393, and quinpirole, Sigma/RBI; genistein, Calbiochem; sodium orthovanadate, Acros Organics (Geel, Belgium); hydrogen peroxide, Fisher Scientific (Houston, TX).
Preparation and treatment of dissected rat brain tissues.Experimental protocols involving the use of vertebrate animals were approved by the Massachusetts General Hospital Subcommittee on Research Animal Care and met National Institutes of Health guidelines.
The brains of male Sprague Dawley rats weighing 150–200 gm were removed after decapitation. The striata were dissected and cross-chopped at 200 μm into slices with a McIlwain mechanical tissue chopper in Krebs' buffer [containing (in mm) 118 NaCl, 4.7 KCl, 2 CaCl, 1.2 MgSO4, and 1.2 KH2PO4] that had been equilibrated with 95% O2/5% CO2. The striatal tissues were incubated in the absence (control) or presence (treatment) of SKF-82958 (50 μm), SKF-38393 (100 μm), quinpirole (100 μm), pervanadate (sodium orthovanadate; 200 μm), or genistein (100 μm) in Krebs' buffer at 37°C for 10 min with gentle agitation. Tissues were collected by centrifugation at 700 × g at 4°C and were subjected to subcellular biochemical fractionation. A stock solution of the pervanadate was prepared as described previously (Dunah et al., 1998).
Subcellular fractionation of rat brain tissues. Biochemical fractionation was performed as described previously (Lin et al., 1998;Wyszynski et al., 1998) with minor modifications to allow for the immunoprecipitation of tyrosine-phosphorylated proteins. Dounce homogenates (H) of the pellets in ice-cold TEVP buffer [containing (in mm) 10 Tris-HCl, pH 7.4, 5 NaF, 1 Na3VO4, 1 EDTA, and 1 EGTA] containing 320 mm sucrose was centrifuged at 1000 × g to remove nuclei and large debris (P1). The supernatant (S1) was centrifuged at 10,000 × g to obtain a crude synaptosomal fraction (P2) and subsequently was lysed hypo-osmotically and centrifuged at 25,000 × g to pellet a synaptosomal membrane fraction (LP1). Then the resulting supernatant (LS1) was centrifuged at 165,000 × g to obtain a synaptic vesicle-enriched fraction (LP2). Concurrently, the supernatant (S2) above the crude synaptosomal fraction (P2) was centrifuged at 165,000 × g to obtain a cytosolic fraction (S3) and a light membrane/microsome-enriched fraction (P3; hereafter referred to as light membrane). After each centrifugation the resulting pellet was rinsed briefly with ice-cold TEVP buffer before subsequent fractionations to avoid possible crossover contamination.
Denaturing conditions of protein solubilization for phosphorylation studies. The striatal extracts resulting from subcellular fractionation were solubilized with 1% SDS in TEVP buffer and centrifuged at 15,000 × g for 5 min in a microcentrifuge. Protein concentrations in the supernatants were determined with the Bio-Rad Protein Assay Kit (Hercules, CA) and used for immunoblot and immunoprecipitation studies.
Precoupling antibodies to protein A-Sepharose. The monoclonal anti-phosphotyrosine (PY20) antibody was incubated with protein A-Sepharose beads at a concentration of 20 μg of antibody per 50 μl of hydrated protein A-Sepharose beads for 2 hr at room temperature in 100 mm sodium borate, pH 8.0, with gentle rotation. The beads were washed with 100 mm sodium borate, pH 8.0, and used for immunoprecipitation.
Immunoprecipitation. The solubilized protein samples were diluted 20-fold with immunoprecipitation buffer [containing (in mm) 150 NaCl, 50 Na3SO4, pH 7.2, and 2 EDTA, plus 1% sodium deoxycholate and 1%Triton X-100]. The diluted samples were incubated with 50 μl of the anti-phosphotyrosine antibody-coupled protein A-Sepharose beads for each 100 μg of protein for 3 hr in a cold room with gentle rotation. The immunoprecipitates were washed three times with ice-cold immunoprecipitation buffer after brief centrifugations and were resuspended in a suitable volume of loading buffer (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 50 mm DTT, and 7.5% glycerol). Samples were resolved on SDS-PAGE and immunoblotted for NR1, NR2A, and NR2B proteins.
Gel electrophoresis, quantitative immunoblotting, and statistical analysis. SDS-PAGE and the transfer of separated proteins to polyvinylidene difluoride (PVDF) membrane were performed as described previously (Wang et al., 1995; Dunah et al., 1996; Luo et al., 1996). For protein separation, 7.5 and 12.5% polyacrylamide gels were used; the concentration of antibodies used for immunoblotting was 1–2 μg/ml. For the quantification of proteins in subcellular fractionation experiments, equal amounts (10 μg) of protein from each fraction were loaded into each lane of the gel. For analysis of tyrosine phosphorylation, 5 μg of each input sample and 40 μg of the corresponding precipitated pellet were loaded in adjacent lanes on the same gel. Bands were visualized on film by enhanced chemiluminescence, and their net intensities were quantified via computer-assisted densitometry (Kodak 1-D System, Rochester, NY). The net intensities of the bands were expressed as a percentage of that in the control striatum. The resulting values were used to calculate group means and reported as means ± SEM. Differences between groups were analyzed by ANOVA with post hoc tests (Scheffe's). For all of the analyses, statistical significance was taken to bep < 0.05.
RESULTS
Characterization of subcellular compartments
A biochemical fractionation approach (Fig.1A) was used to isolate subcellular compartments from brain tissues, and the effectiveness of the subcellular fractionation procedure was evaluated in the striatum by the use of protein markers for subcellular compartments (Fig.1B). Synaptophysin, a synaptic vesicle membrane protein believed to participate in the fusion of synaptic vesicles to the presynaptic membrane (Devoto and Barnstable, 1987), was found to be highly concentrated in the synaptic vesicle-enriched fraction (LP2, lane 9). This protein was also present at lower levels in both the light membrane (P3, lane 5) and synaptosomal membrane (LP1, lane 7) fractions. Syntaxin, a protein that interacts with synaptotagmin and participates in the docking of synaptic vesicles (Bennett et al., 1992; Barinaga, 1993), was enriched in both the light membrane (P3, lane 5) and synaptic vesicle-enriched (LP2, lane 9) fractions, whereas much lower levels were present in the synaptosomal membrane compartment (LP1, lane 7). The calcium-binding protein calnexin, known to interact with newly synthesized glycoproteins in the endoplasmic reticulum (David et al., 1993), was present in the light membrane compartment (P3, lane 5) but was not detected in the synaptosomal membrane (LP1, lane 7) and/or synaptic vesicle-enriched (LP2, lane 9) fractions.
Fig. 1.
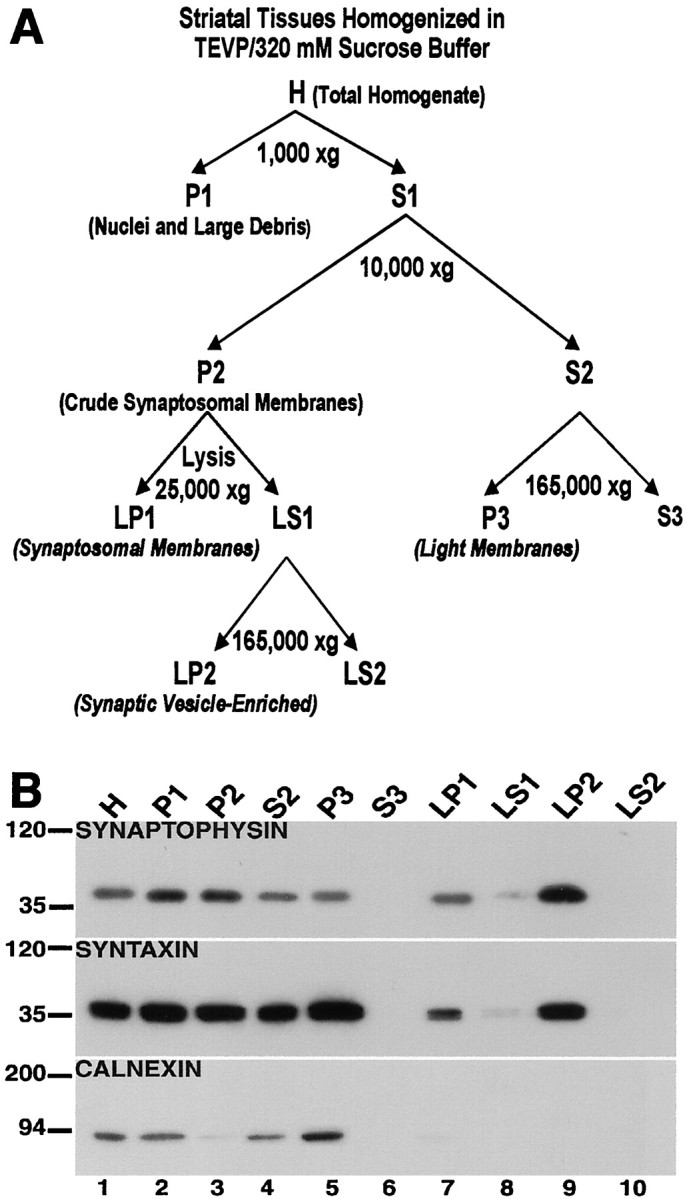
Characterization of fractionated subcellular compartments of the striatum. A, Schematic for the biochemical fractionation. The procedure for the subcellular separation of proteins as depicted in this schematic is described in Materials and Methods. B, Characterization of subcellular compartments. The isolated biochemical fractions from striatal tissues were separated by SDS-PAGE, and the blots were probed with antibodies against synaptophysin (top), syntaxin (middle), and calnexin (bottom). Synaptophysin is highly concentrated in the synaptic vesicle-enriched fraction (LP2, lane 9); syntaxin is enriched in the light membrane (P3, lane 5) and synaptic vesicle-enriched (LP2,lane 9) fractions compared with the synaptosomal membrane (LP1, lane 7); and calnexin is found in the light membrane fraction (P3,lane 5), but not in the synaptosomal membrane (LP1, lane 7) nor synaptic vesicle-enriched (LP2, lane 9) fractions.
Subcellular distribution of NMDA receptor proteins
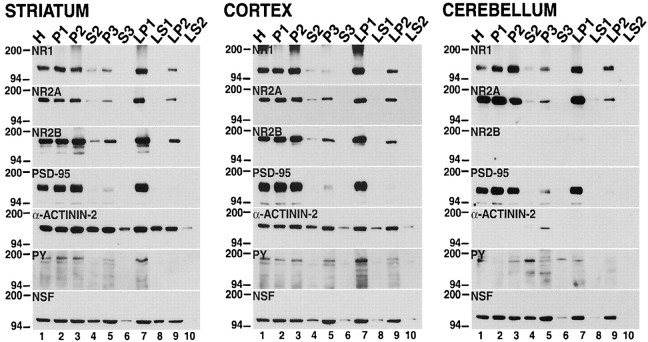
The expression of NMDA receptor subunits in various subcellular compartments was investigated in rat striatal tissues that were homogenized immediately after dissection (Fig. 2). The NR1, NR2A, and NR2B subunits were found in the light membrane (P3,lane 5), synaptosomal membrane (LP1, lane 7), and synaptic vesicle-enriched (LP2,lane 9) fractions. As expected, these proteins were also present in the total homogenate (H, lane 1); cell soma, nuclei, and nuclei-associated membrane (P1, lane 2); crude synaptosomal membrane (P2, lane 3); and the low-speed supernatant (S2, lane 4) fractions; S2 is the parent fraction from which P3 is derived. However, the NMDA subunits were not detectable in the cytosolic or soluble subcellular fractions (S3, LS1,LS2; lanes 6, 8, 10, respectively). In a similar experiment the subcellular distribution of NMDA receptors in the cerebral cortex and cerebellum also was investigated (Fig.2, see cortex and cerebellum). The localization of NMDA receptor subunits NR1, NR2A, and NR2B in these rat brain regions was similar to what was observed in the striatum (Fig. 2, see striatum). As anticipated, the NR2B subunit was not detected in the rat cerebellum, and this concurs with previous studies showing the absence of NR2B expression in this region (Monyer et al., 1994; Wang et al., 1995).
Fig. 2.
NMDA receptor subunits are distributed differentially between subcellular compartments in the rat brain. The tissue samples from the striatum, cortex, and cerebellum were homogenized immediately after dissection, separated into different biochemical fractions as described in Materials and Methods, and resolved on SDS-polyacrylamide gels. Total protein (10 μg) from each fraction was loaded in each lane. The blots were probed with anti-NR1, anti-NR2A, anti-NR2B, anti-PSD-95, anti-α-actinin-2, anti-phosphotyrosine (PY), and anti-NSF antibodies. H, Total homogenate; P1, nuclei and large debris; P2, crude synaptosomal fraction; P3, light membrane fraction;LP1, synaptosomal membrane fraction; LP2, synaptic vesicle-enriched fraction. S2, S3, LS1, andLS2 are supernatants from P2, P3, LP1, and LP2, respectively. The positions and sizes of molecular weight markers are indicated in kilodaltons.
Using the same biochemical fractions, we also examined the subcellular distribution of postsynaptic density-95 protein (PSD-95) and α-actinin-2, proteins that interact with NMDA receptors;N-ethylmaleimide-sensitive fusion protein (NSF), a protein that interacts with AMPA receptors; and total phosphotyrosine (PY) proteins (Fig. 2). The PSD-95 protein and phosphotyrosine proteins were confined to the crude extracts and the membrane compartments, exhibiting a subcellular expression profile similar to the NMDA subunits, except that PSD-95 and phosphotyrosine proteins were not detected in the synaptic vesicle-enriched fraction (LP2,lane 9). α-Actinin-2 and NSF showed a wider subcellular distribution, because they were found in both the membrane and cytosolic subcellular fractions of the striatum (Fig. 2). In the cortex and cerebellum PSD-95, α-actinin-2, and NSF showed a subcellular distribution profile similar to that of the striatum (Fig. 2), except that α-actinin-2 expression was found to be very low in the cerebellum and was detected only in the light membrane fraction (cerebellum; P3, lane 5).
Differential subcellular localization of tyrosine-phosphorylated NMDA receptor subunits in the rat brain
The tyrosine phosphorylation of NMDA receptors in distinct striatal compartments was investigated by immunoprecipitating fractionated protein extracts with anti-phosphotyrosine antibodies and by immunoblotting samples of the input and pellet for NMDA subunits (Fig. 3). In accord with previous studies, tyrosine phosphorylation of NR1 was not detected in any subcellular compartment (Lau and Huganir, 1995; Dunah et al., 2000a). However, tyrosine-phosphorylated striatal NR2A and NR2B subunits were present in the total homogenate (H, lane 2), nuclei and large debris (P1, lane 4), crude synaptosomal membrane (P2, lane 6), and synaptosomal membrane (LP1, lane 14) fractions. Interestingly, the NR2A and NR2B subunits present in the light membrane (P3, lane 10) and synaptic vesicle-enriched (LP2, lane 12) fractions of the striatum did not exhibit detectable tyrosine phosphorylation, even with longer exposure periods. Similar results were obtained in the cerebral cortex and cerebellum, except that the NR2B subunit was not detectable in the cerebellum (Fig. 3, see cortex and cerebellum).
Fig. 3.
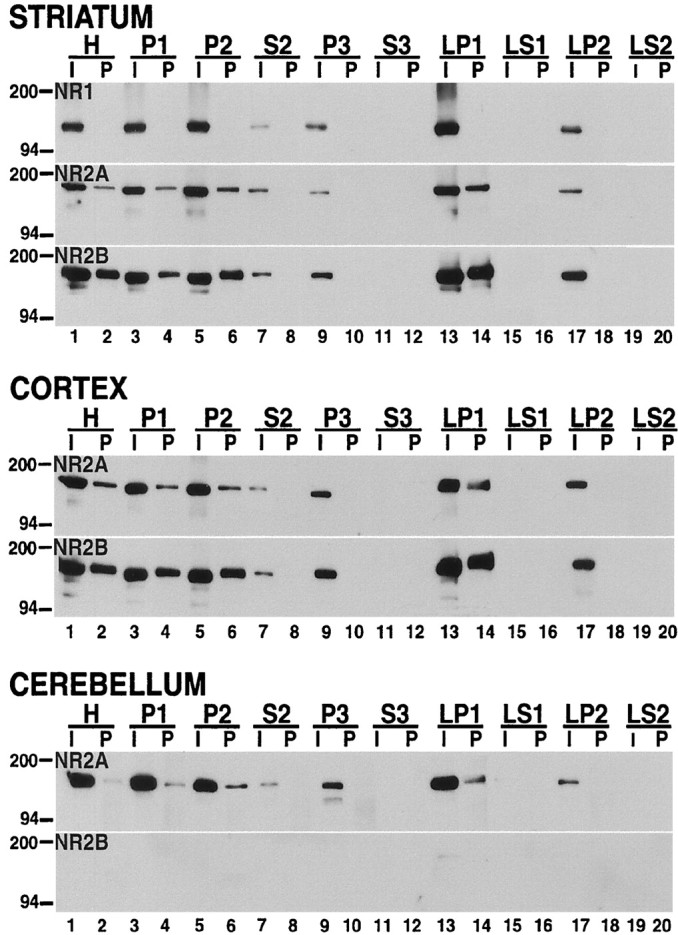
NMDA receptors present in the synaptosomal membrane fraction, but not those in the light membrane and synaptic vesicle-enriched compartments, are tyrosine phosphorylated. Samples from rat striatum (top), cortex (middle), and cerebellum (bottom) were homogenized immediately after dissection, fractionated, solubilized, and immunoprecipitated with anti-phosphotyrosine antibody. The inputs (I;lanes 1, 3, 5, 7, 9, 11, 13, 15, 17, 19; 5 μg) and pellets (P; lanes 2, 4, 6, 8, 10, 12, 14, 16, 18, 20; 40 μg) were separated on SDS-PAGE gels, and the blots were probed with anti-NR1, anti-NR2A, and anti-NR2B antibodies. Tyrosine-phosphorylated NR2A and NR2B subunits were detected in LP1 (lane 13), but not in P3 (lane 10) and LP2 (lane 18) fractions.
A dopamine D1 receptor agonist alters the subcellular distribution and phosphorylation of striatal NMDA receptors
The effect of dopamine receptor activation on the subcellular localization of NMDA receptors was studied by treating rat striatal tissues with specific agonists. These experiments were performed on tissues incubated in vitro with the pharmacologic agents for 10 min. This treatment time was selected on the basis of the earlier studies of Snyder et al. (1998). Similar results were obtained by using a shorter 5 min incubation period (data not shown). The controls for these experiments were tissues incubated under identical in vitro conditions without the addition of pharmacologic agents. A comparison of the data obtained from these control incubations with those described above from tissues processed immediately after dissection indicated that the incubation alone did not alter either the subcellular distribution of the proteins that were examined or the tyrosine phosphorylation of NR2A and NR2B.
Treatment with SKF-82958, a dopamine D1 receptor full agonist (Figs.4, 5), produced significant reductions in the abundance of NR1, NR2A, and NR2B proteins in the light membrane (Figs. 4A, 5,P3) fraction (NR1, 28 ± 5%; NR2A, 25 ± 4%; NR2B, 9 ± 5%; all values expressed as a percentage of control) and the synaptic vesicle-enriched (Figs. 4A, 5,LP2) fraction (NR1, 27 ± 4%; NR2A, 32 ± 4%; NR2B, 15 ± 7%). At the same time there was a marked increase in NR1 (146 ± 5%), NR2A (150 ± 4%), and NR2B (158 ± 8%) subunits in the synaptosomal membrane (Figs. 4A,5, LP1) fraction. There were no significant changes in the relative levels of PSD-95, α-actinin-2, and NSF in the different compartments after SKF-82958 treatment, but there was an apparent elevation of protein tyrosine phosphorylation, especially in the synaptosomal membrane (LP1) fraction (Fig. 4A, Table1).
Fig. 4.

The dopamine D1 receptor agonist SKF-82958 produces subcellular redistribution and an increase in the tyrosine phosphorylation of striatal NMDA receptors. A, Subcellular distribution. Samples from tissues that were incubated for 10 min under control conditions (C) or with 50 μm SKF-82958 (S) were subjected to biochemical fractionation. The subcellular fractions were resolved by SDS-PAGE, and the blots were probed with NR1, NR2A, NR2B, PSD-95, α-actinin-2, NSF, and anti-phosphotyrosine (PY) antibodies. SKF-82958 decreased NR1, NR2A, and NR2B in P3 (lane 10) and LP2 (lane 18) fractions; it increased these subunits in LP1 (lane 14), and it increased phosphotyrosine proteins (PY). B1, B2, Tyrosine phosphorylation. The subcellular-fractionated samples from control (C) and SKF-82958-treated (S) striatal tissues were immunoprecipitated with anti-phosphotyrosine antibody and immunoblotted with anti-NR2A and anti-NR2B. The inputs (I) and pellets (P) for each biochemical fraction are indicated across the top of the figure. SKF-82958 increased tyrosine-phosphorylated NR2A and NR2B in H (lane 4), P1 (lane 8), P2 (lane 12), and LP1 (lane 28) fractions.
Fig. 5.
Densitometric quantification of NMDA receptor subunit NR1, NR2A, and NR2B proteins in striatal tissues that were treated with the dopamine D1 receptor agonist SKF-82958 (left panels) and with a combination of the dopamine D1 receptor agonist SKF-82958 and a protein tyrosine kinase inhibitor genistein (right panels). The exposed films from the experiments depicted in Figures 4A and 10A were scanned and analyzed as described in Materials and Methods. Values on the ordinate represent the relative levels of NR1, NR2A, and NR2B proteins given as a percentage of the control samples. Data are means ± SEM obtained from three rats. Asterisks indicate significant differences between treatment and control samples (p < 0.05, ANOVA). The alteration in the subcellular distribution of NR1, NR2A, and NR2B subunits produced by SKF-82958 was blocked by genistein.
Table 1.
Quantitation of the relative amounts of NR1, NR2A, NR2B, PSD-95, α-actinin-2, and NSF in various subcellular compartments of the striatum after treatment with pharmacologic agents
| Treatment | Proteins | Percentage of control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | P1 | P2 | S2 | P3 | S3 | LP1 | LS1 | LP2 | LS2 | ||
| P3 | S3 | ||||||||||
| SKF-82958 | NR1 | 99 ± 6 | 95 ± 8 | 91 ± 4 | 94 ± 3 | 28 ± 5 | 146 ± 5 | 27 ± 4 | |||
| NR2A | 102 ± 8 | 94 ± 8 | 92 ± 6 | 93 ± 8 | 25 ± 4 | 150 ± 4 | 32 ± 4 | ||||
| NR2B | 98 ± 9 | 103 ± 6 | 91 ± 4 | 92 ± 9 | 9 ± 5 | 158 ± 8 | 15 ± 7 | ||||
| PSD-95 | 106 ± 4 | 95 ± 5 | 98 ± 5 | 99 ± 5 | 103 ± 5 | ||||||
| α-Actinin-2 | 106 ± 5 | 102 ± 4 | 101 ± 5 | 108 ± 6 | 100 ± 4 | 109 ± 6 | 100 ± 7 | 109 ± 8 | 106 ± 8 | 93 ± 8 | |
| NSF | 96 ± 8 | 98 ± 7 | 105 ± 8 | 104 ± 9 | 95 ± 8 | 104 ± 11 | 98 ± 9 | ||||
| SKF-38393 | NR1 | 96 ± 8 | 95 ± 8 | 96 ± 7 | 101 ± 6 | 89 ± 6 | 107 ± 6 | 83 ± 10 | |||
| NR2A | 96 ± 7 | 92 ± 8 | 97 ± 8 | 96 ± 6 | 95 ± 5 | 105 ± 7 | 94 ± 8 | ||||
| NR2B | 96 ± 4 | 95 ± 5 | 108 ± 7 | 107 ± 5 | 90 ± 8 | 116 ± 6 | 91 ± 7 | ||||
| PSD-95 | 103 ± 7 | 101 ± 7 | 96 ± 5 | 105 ± 7 | 103 ± 5 | ||||||
| α-Actinin-2 | 108 ± 4 | 101 ± 4 | 101 ± 7 | 98 ± 4 | 96 ± 5 | 102 ± 3 | 109 ± 4 | 103 ± 4 | 95 ± 7 | 102 ± 6 | |
| NSF | 96 ± 5 | 105 ± 4 | 99 ± 5 | 96 ± 7 | 96 ± 5 | 101 ± 9 | 96 ± 5 | ||||
| Quinpirole | NR1 | 101 ± 4 | 101 ± 6 | 104 ± 6 | 92 ± 7 | 111 ± 7 | 98 ± 9 | 106 ± 8 | |||
| NR2A | 99 ± 10 | 96 ± 9 | 104 ± 9 | 104 ± 6 | 108 ± 8 | 102 ± 5 | 109 ± 9 | ||||
| NR2B | 95 ± 4 | 95 ± 5 | 100 ± 8 | 101 ± 6 | 98 ± 6 | 105 ± 7 | 98 ± 4 | ||||
| PSD-95 | 95 ± 9 | 93 ± 5 | 99 ± 7 | 101 ± 4 | 104 ± 6 | ||||||
| α-Actinin-2 | 97 ± 6 | 108 ± 6 | 100 ± 4 | 103 ± 5 | 107 ± 4 | 97 ± 8 | 94 ± 4 | 102 ± 7 | 106 ± 5 | 108 ± 9 | |
| NSF | 104 ± 6 | 98 ± 9 | 99 ± 8 | 98 ± 6 | 102 ± 5 | 100 ± 4 | 103 ± 5 | ||||
| Pervanadate | NR1 | 95 ± 5 | 99 ± 7 | 92 ± 6 | 92 ± 3 | 11 ± 6 | 165 ± 5 | 21 ± 5 | |||
| NR2A | 104 ± 5 | 108 ± 4 | 93 ± 5 | 93 ± 5 | 6 ± 5 | 142 ± 4 | 17 ± 6 | ||||
| NR2B | 108 ± 6 | 102 ± 7 | 91 ± 6 | 91 ± 4 | 2 ± 4 | 179 ± 5 | 11 ± 5 | ||||
| PSD-95 | 98 ± 4 | 106 ± 10 | 98 ± 8 | 107 ± 5 | 104 ± 7 | ||||||
| α-Actinin-2 | 99 ± 6 | 106 ± 10 | 98 ± 9 | 98 ± 6 | 106 ± 8 | 102 ± 8 | 97 ± 9 | 102 ± 7 | 103 ± 7 | 98 ± 3 | |
| NSF | 99 ± 7 | 96 ± 9 | 98 ± 8 | 102 ± 9 | 101 ± 6 | 99 ± 9 | 105 ± 7 | ||||
| Genistein | NR1 | 96 ± 6 | 103 ± 6 | 108 ± 4 | 104 ± 7 | 94 ± 9 | 92 ± 6 | 106 ± 9 | |||
| NR2A | 100 ± 6 | 95 ± 10 | 100 ± 7 | 102 ± 8 | 102 ± 7 | 101 ± 5 | 98 ± 9 | ||||
| NR2B | 106 ± 7 | 105 ± 7 | 103 ± 6 | 100 ± 7 | 102 ± 5 | 90 ± 4 | 92 ± 8 | ||||
| PSD-95 | 96 ± 5 | 95 ± 5 | 97 ± 5 | 108 ± 6 | 101 ± 8 | ||||||
| α-Actinin-2 | 95 ± 5 | 105 ± 9 | 100 ± 6 | 97 ± 6 | 96 ± 6 | 96 ± 6 | 98 ± 5 | 105 ± 5 | 103 ± 6 | 102 ± 9 | |
| NSF | 102 ± 6 | 100 ± 7 | 94 ± 7 | 98 ± 7 | 97 ± 9 | 98 ± 6 | 103 ± 10 | ||||
| SKF-82958 + Genistein | NR1 | 98 ± 4 | 100 ± 7 | 101 ± 10 | 99 ± 6 | 96 ± 7 | 93 ± 6 | 95 ± 7 | |||
| NR2A | 96 ± 6 | 98 ± 10 | 101 ± 9 | 96 ± 6 | 93 ± 8 | 105 ± 6 | 94 ± 8 | ||||
| NR2B | 97 ± 8 | 98 ± 8 | 103 ± 7 | 96 ± 10 | 92 ± 9 | 94 ± 9 | 92 ± 6 | ||||
| PSD-95 | 94 ± 3 | 99 ± 4 | 101 ± 7 | 104 ± 8 | 102 ± 4 | ||||||
| α-Actinin-2 | 92 ± 5 | 94 ± 5 | 99 ± 5 | 96 ± 4 | 92 ± 6 | 94 ± 9 | 105 ± 3 | 99 ± 9 | 94 ± 5 | 101 ± 7 | |
| NSF | 96 ± 6 | 95 ± 4 | 99 ± 5 | 107 ± 7 | 101 ± 4 | 102 ± 5 | 99 ± 4 | ||||
The results presented in bold indicate significant differences (p < 0.05, ANOVA) between treatment and control samples. The data are given as the means ± SEM obtained from three rats.
The effect of SKF-82958 treatment on tyrosine phosphorylation of the NMDA receptors present in the striatal compartments was determined by precipitating phosphotyrosine proteins from isolated fractions and probing them with NMDA receptor subunit-specific antibodies (Fig.4B1,B2, Table2). SKF-82958 produced an increase in tyrosine-phosphorylated NR2A and NR2B subunits in the synaptosomal membrane (LP1, lanes 26, 28) compartment to 154 ± 5 and 161 ± 6%, respectively (values expressed as a percentage of control). A similar increase in tyrosine-phosphorylated NR2A and NR2B subunits was observed in the total homogenate (H, lanes 2, 4), nuclei and large debris (P1, lanes 6, 8), and crude synaptosomal membrane (P2, lanes 10, 12) fractions (Fig.4B1, Table 2). Tyrosine-phosphorylated NR2A and NR2B subunits were not detected in the light membrane (P3, lanes 18, 20) and synaptic vesicle-enriched (LP2, lanes 34, 36) fractions (Fig.4B1,B2).
Table 2.
Quantitation of tyrosine-phosphorylated NMDA receptor subunits NR2A and NR2B in striatal subcellular compartments after treatment with pharmacologic agents
| Treatment | Subunits | Percentage of control | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| H | P1 | P2 | S2 | P3 | S3 | LP1 | LS1 | LP2 | LS2 | ||
| SKF-82958 | NR2A | 142 ± 7 | 146 ± 9 | 141 ± 6 | 154 ± 5 | ||||||
| NR2B | 147 ± 10 | 142 ± 6 | 153 ± 6 | 161 ± 6 | |||||||
| SKF-38393 | NR2A | 99 ± 11 | 106 ± 10 | 98 ± 11 | 104 ± 5 | ||||||
| NR2B | 104 ± 8 | 102 ± 8 | 109 ± 7 | 107 ± 7 | |||||||
| Quinpirole | NR2A | 96 ± 6 | 101 ± 9 | 104 ± 5 | 107 ± 6 | ||||||
| NR2B | 98 ± 7 | 105 ± 4 | 104 ± 9 | 97 ± 5 | |||||||
| Pervanadate | NR2A | 174 ± 6 | 176 ± 8 | 171 ± 8 | 183 ± 7 | ||||||
| NR2B | 184 ± 8 | 187 ± 8 | 171 ± 10 | 181 ± 5 | |||||||
| Genistein | NR2A | 52 ± 8 | 51 ± 6 | 49 ± 5 | 44 ± 4 | ||||||
| NR2B | 53 ± 6 | 51 ± 8 | 52 ± 6 | 50 ± 10 | |||||||
| SKF-82958 + Genistein | NR2A | 66 ± 10 | 64 ± 7 | 62 ± 6 | 67 ± 6 | ||||||
| NR2B | 75 ± 8 | 73 ± 7 | 68 ± 6 | 72 ± 8 | |||||||
The data shown in bold indicate significant differences (p < 0.05, ANOVA) between treatment and control groups. The data are given as the means ± SEM obtained from three rats.
Similar experiments were performed with rat cortical and cerebellar tissues. Treatment of these tissues with SKF-82958 produced no apparent alterations in the subcellular distribution of NR1, NR2A, or NR2B subunits. The tyrosine phosphorylation of these NMDA subunits in the various subcellular compartments of the cortex and cerebellum also was unchanged after treatment with the dopamine D1 receptor agonist (data not shown).
In another experiment SKF-38393, a partial agonist of the dopamine D1 receptor, was used to treat striatal tissues. With this pharmacologic agent there was no alteration in the subcellular distribution (Table 1) or tyrosine phosphorylation (Table 2) of striatal NMDA receptors. Similarly, the effects of quinpirole, a dopamine D2 receptor agonist also were examined, and quinpirole produced no significant changes in the subcellular localization of NR1, NR2A, and NR2B receptors (Fig.6A, Table 1) or the phosphorylation of NR2A and NR2B subunits at tyrosine residues (Fig.6B1,B2, Table 2).
Fig. 6.

The dopamine D2 receptor agonist quinpirole does not alter the subcellular distribution and tyrosine phosphorylation of striatal NMDA receptors. A, Subcellular distribution. Samples from tissues that were incubated for 10 min under control conditions (C) or with 100 μmquinpirole (a dopamine D2 receptor agonist; Q) were separated into subcellular fractions and subjected to electrophoresis on SDS-polyacrylamide gels. The blots were probed with NR1, NR2A, NR2B, PSD-95, α-actinin-2, NSF, and anti-phosphotyrosine (PY) antibodies. There were no significant differences in the subcellular distribution of the analyzed proteins between control and quinpirole-treated samples. B1, B2, Tyrosine phosphorylation. Striatal tissues from control (C) and quinpirole-treated (Q) samples were solubilized and precipitated with anti-phosphotyrosine antibody. The inputs (I) and pellets (P) are indicated across the top of the figure. The resulting blots were immunoblotted for the NR2A and NR2B subunits. Quinpirole had no apparent effect on the tyrosine phosphorylation of NR2A and NR2B subunits.
Dopamine D1 receptor agonist does not alter the subcellular distribution or phosphorylation of striatal AMPA receptors
The effect of the dopamine D1 receptor agonist SKF-82958 on the subcellular localization and phosphorylation of striatal AMPA receptor subunits GluR1 and GluR2/3 was investigated (Fig.7). In control tissues (Fig.7A, lanes designated C) the AMPA receptor subunits exhibited a distribution profile similar to that of the NMDA receptor subunits in that they were abundant in the synaptosomal membrane (LP1) but also were found in the light membrane (P3) and synaptic vesicle-enriched (LP2) fractions (Fig. 7A). Like the NMDA receptors, the GluR1 and GluR2/3 subunits found in the synaptosomal membrane (LP1) fraction were tyrosine phosphorylated, whereas those present in the light membrane (P3) and synaptic vesicle-enriched (LP2) fractions were not phosphorylated (Fig.7B1,B2). Interestingly, treatment with SKF-82958 produced no alterations in either the subcellular distribution (Fig. 7A) or the tyrosine phosphorylation of the GluR1 and GluR2/3 subunits (Fig.7B1,B2).
Fig. 7.

The dopamine D1 receptor agonist SKF-82958 had no effect on the subcellular distribution and tyrosine phosphorylation of striatal AMPA receptors. A, Subcellular distribution. Fractionated striatal tissues that were incubated for 10 min under control conditions (C) or with 50 μm SKF-82958 (S) were resolved by SDS-PAGE, and the blots were probed with antibodies specific for GluR1 and GluR2/3. SKF-82958 had no apparent effect on the subcellular distribution of GluR1 and GluR2/3 subunits. B1, B2, Tyrosine phosphorylation. The fractionated striatal samples were precipitated with anti-phosphotyrosine antibody and immunoblotted for GluR1 and GluR2/3. The inputs (I) and pellets (P) are indicated across thetop of the figure. SKF-82958 produced no changes in the tyrosine phosphorylation of GluR1 or GluR2/3.
Inhibition of protein phosphatases alters the subcellular localization and phosphorylation of striatal NMDA receptors
To determine whether enhanced protein phosphorylation was sufficient to produce a redistribution of NMDA receptor subunits among subcellular compartments, we treated striatal tissues with pervanadate, a protein phosphatase inhibitor. Both control and treated tissues were fractionated and immunoblotted for NMDA receptors as well as for PSD-95, α-actinin-2, NSF, and phosphotyrosine proteins (Fig.8A). Pervanadate produced marked reductions in the levels of NR1, NR2A, and NR2B found in the light membrane (P3) fraction (NR1, 11 ± 6%; NR2A, 6 ± 5%; NR2B, 2 ± 4%) and synaptic vesicle-enriched (LP2) fraction (NR1, 21 ± 5%; NR2A, 17 ± 6%; NR2B, 11 ± 5%) and a concomitant increase in NR1 (165 ± 5%), NR2A (142 ± 4%), and NR2B (179 ± 5%) in the synaptosomal membrane (LP1) fraction. There were no significant changes in the subcellular distribution of PSD-95, α-actinin-2, and NSF (Fig.8A, Table 1).
Fig. 8.

The protein phosphatase inhibitor pervanadate induces subcellular redistribution and an increase in tyrosine phosphorylation of striatal NMDA receptors. A, Subcellular distribution. Striatal samples from tissues that were incubated for 10 min under control conditions (C) or with 200 μm pervanadate (P) were fractionated and resolved on SDS-PAGE gels. The blots were probed with NR1, NR2A, NR2B, PSD-95, α-actinin, NSF, and anti-phosphotyrosine (PY) antibodies. Similar to the effects produced by SKF-82958, pervanadate reduced the levels of NR1, NR2A, and NR2B in P3 (lane 10) and LP2 (lane 18); it increased the levels of these NMDA subunits in LP1 (lane 14) and markedly increased phosphotyrosine proteins (PY). B1, B2, Tyrosine phosphorylation. Samples from the subcellular-fractionated control (C) and pervanadate-treated (P) striatal tissues were immunoprecipitated with anti-phosphotyrosine antibody. The inputs (I) and pellets (P) were electrophoresed and probed for NR2A and NR2B subunits. Tyrosine phosphorylation of NR2A and NR2B was increased in H (lane 4), P1 (lane 8), P2 (lane 12), and LP1 (lane 28).
As shown in Figure 8B2 and Table 2, the treatment of striatal tissues with pervanadate also produced an increase in the tyrosine phosphorylation of the NR2A (183 ± 7%) and NR2B (181 ± 5%) subunits present in the synaptosomal membrane (LP1) fraction. However, even under this condition no tyrosine phosphorylation of the NR2A and NR2B subunits remaining in the light membrane (P3) and synaptic vesicle-enriched (LP2) fractions was detected (Fig. 8B1,B2). This finding was particularly striking when compared with the marked enhancement of tyrosine phosphorylation of proteins other than the NR2A and NR2B subunits in the P3 fraction (Fig. 8A, lane 10).
Blockade of the effects of dopamine D1 receptor activation on NMDA receptors by a protein tyrosine kinase inhibitor
To examine the role of tyrosine phosphorylation in the subcellular redistribution of NMDA receptors after treatment with the dopamine D1 receptor agonist SKF-82958, we pretreated striatal tissues with the protein tyrosine kinase inhibitor genistein. Treatment of striatal tissues with genistein alone produced an apparent decrease in protein tyrosine phosphorylation, especially in the synaptosomal membrane fraction (Fig. 9A, PY, LP1). There was no alteration in the relative levels of NR1, NR2A, or NR2B in the subcellular compartments that were examined (Fig.9A, Table 1). There was, however, an apparent decrease in the tyrosine phosphorylation of NR2A and NR2B in each of the subcellular fractions that contained these subunits (Fig.9B1,B2).
Fig. 9.
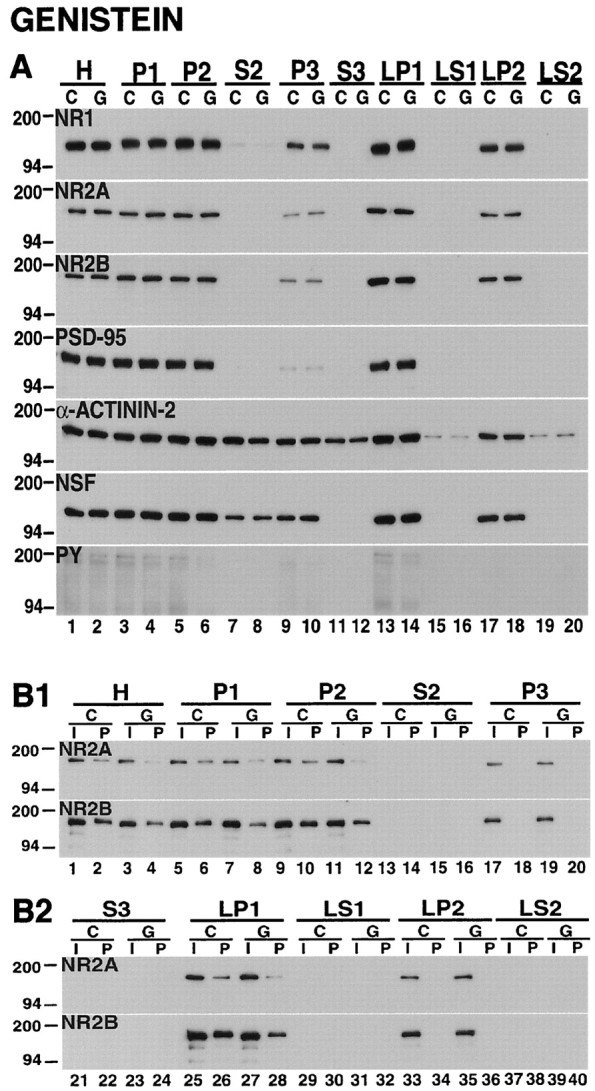
The protein tyrosine kinase inhibitor genistein does not change the subcellular distribution of striatal NMDA receptors but decreases the tyrosine phosphorylation of NR2A and NR2B.A, Subcellular distribution. Fractions from striatal tissues that were incubated for 10 min under control conditions (C) or with 100 μm genistein (G) were electrophoresed on SDS-polyacrylamide gels, and the blots were probed with NR1, NR2A, NR2B, PSD-95, α-actinin-2, NSF, and anti-phosphotyrosine (PY) antibodies. The subcellular localizations of NMDA receptor subunits and the other proteins that were studied were not changed by genistein, but there were reductions in total phosphotyrosine proteins (PY). B1, B2, Tyrosine phosphorylation. The fractionated samples from control (C) and genistein-treated (G) striatal tissues were immunoprecipitated by using anti-phosphotyrosine antibody and were immunoblotted with anti-NR2A and anti-NR2B antibodies. The inputs (I) and pellets (P) are indicated across the top of the figure. Genistein decreased the tyrosine phosphorylation of NR2A and NR2B in H (lane 4), P1 (lane 8), P2 (lane 12), and LP1 (lane 28).
In the striatal tissues that were treated with the dopamine D1 receptor agonist SKF-82958 in the presence of genistein, the changes that were observed in the subcellular distribution of NMDA subunits after treatment with the dopamine D1 receptor agonist alone were no longer apparent (Figs. 5,10A). The abundance of each of the NMDA subunits in the light membrane (P3), synaptosomal membrane (LP1), and synaptic vesicle-enriched (LP2) compartments was identical in control samples and in the samples that were treated with a combination of SKF-82958 and genistein (Fig. 10A, Table 1). In addition, genistein blocked the increase in tyrosine phosphorylation of NR2A and NB2B in each subcellular compartment that was investigated (Fig. 10B1,B2, Table 2).
Fig. 10.
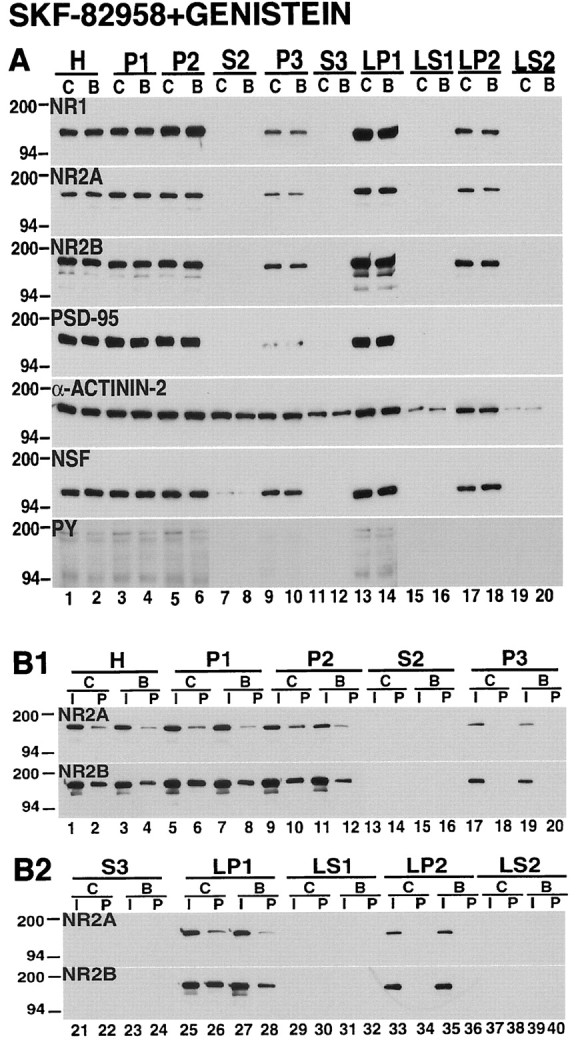
The dopamine D1 receptor agonist-induced alterations in subcellular distribution and tyrosine phosphorylation of striatal NMDA receptors are inhibited by the protein tyrosine kinase inhibitor genistein. A, Subcellular distribution. Samples from tissues that were incubated for 10 min under control conditions (C) or with 50 μmSKF-82958 and 100 μm genistein (B) were subjected to subcellular fractionation. The proteins were separated on SDS-PAGE, and the blots were probed with antibodies against NR1, NR2A, NR2B, PSD-95, α-actinin-2, NSF, and phosphotyrosine proteins (PY). In the presence of genistein, SKF-82958 produced no alteration in the distribution of NMDA receptor subunits. B1, B2, Tyrosine phosphorylation. Solubilized control (C) and treated samples (B) were immunoprecipitated with anti-phosphotyrosine antibody, and the blots were probed with NR2A and NR2B antibodies. The inputs (I) and pellets (P) are indicated across thetop of the figure. Genistein inhibited the increase in tyrosine phosphorylation of NR2A and NR2B produced by the dopamine D1 receptor agonist SKF-82958.
DISCUSSION
The NR1, NR2A, and NR2B subunits in the striatum are confined to biochemical fractions containing cellular membranes, particularly the synaptosomal membrane (LP1), synaptic vesicle-enriched (LP2), and light membrane (P3) compartments. Tyrosine-phosphorylated NR2A and NR2B subunits are found only in the synaptosomal membrane fraction and not in the light membrane and synaptic vesicle-enriched fractions. The dopamine D1 receptor agonist produces a rapid alteration in the distribution of NR1, NR2A, and NR2B proteins, with a reduction in these subunits in the light membrane and synaptic vesicle-enriched compartments and an increase in the synaptosomal membranes. These changes in subcellular distribution are accompanied by an increase in tyrosine phosphorylation of the NR2A and NR2B present in synaptosomal membranes. The alterations in the distribution and phosphorylation of NMDA receptors produced by SKF-82958 are abolished by a protein tyrosine kinase inhibitor and mimicked by a protein phosphatase inhibitor. These observations reveal the existence of a rapid dopamine-dependent mechanism for intracellular trafficking of striatal NMDA receptors and reveal that these effects are mediated via a tyrosine kinase-dependent mechanism.
Subcellular distribution of NMDA receptors and associated proteins in the striatum
In the rat striatum, cortex, and cerebellum the NMDA subunits NR1, NR2A, and NR2B are present in three membrane-associated compartments (LP1, LP2, and P3) and were undetected in cytosolic fractions. This is consistent with anatomical studies showing that NMDA receptor proteins are present both at postsynaptic sites and in intracellular locations, where they often are associated with membranous structures (Petralia et al., 1994). The LP1 fraction contains postsynaptic density, an organized cytoskeletal structure located within the postsynaptic neuron at sites of excitatory synapses that contain both NMDA and non-NMDA glutamate receptors, and a variety of other proteins involved in receptor anchoring, signaling, and modulation (Kennedy, 1993; Ziff, 1997). The LP2 contains synaptic vesicles and other vesicles that function as trafficking organelles for several membrane proteins, including neurotransmitter receptors (Sudhof, 1995; Takamori et al., 2000), whereas P3 contains cellular organelles that include endoplasmic reticulum, Golgi apparatus, and synaptic mitochondria (Schapira, 1998). The PSD-95, an integral component of the postsynaptic density, was highly concentrated in the synaptosomal membrane (LP1) fraction but was absent in the cytosolic and synaptic vesicle-enriched (LP2) compartments. In contrast, α-actinin-2 was found in both membrane-associated and cytosolic compartments. Both proteins are known to link NMDA receptors physically and functionally to the postsynaptic density (Kornau et al., 1995; Wyszynski et al., 1998), although our recent report revealed that only a minor but perhaps functionally significant fraction of the α-actinin-2 present in rat striatum is bound directly to NMDA receptors (Dunah et al., 2000b).
Trafficking of NMDA receptors from intracellular compartments to the synaptic membrane
Mounting evidence suggests that the activities of the striatum are regulated by complex interactions between dopamine and NMDA receptors (Di Chiara et al., 1994; Cepeda and Levine, 1998;Schoffelmeer et al., 2000). We found that the dopamine D1 receptor agonist SKF-82958 produced a rapid redistribution of NMDA subunits among subcellular compartments of the striatum. This process of receptor redistribution appears to be specific for the NMDA subunits, because the AMPA receptor subunits GluR1 and GluR2/3, as well as PSD-95 and α-actinin-2, proteins that interact with NMDA receptors (Kornau et al., 1995; Wyszynski et al., 1998), and NSF, a protein that binds to AMPA receptors (Nishimune et al., 1998; Osten and Ziff, 1999), were not altered. In addition, the effect of the dopamine D1 agonist on NMDA receptor distribution and phosphorylation was not observed in either the cerebral cortex or the cerebellum. This may result from the existence of a lower density of D1 dopamine receptors in the cortex and the virtual lack of expression of these receptors in the cerebellum (De Keyser, 1993; Sealfon and Olanow, 2000). However, it is also possible that there are unique anatomical and structural properties of striatal neurons that are required for this interaction of glutamatergic and dopaminergic transmission (Cepeda and Levine, 1998; Schoffelmeer et al., 2000).
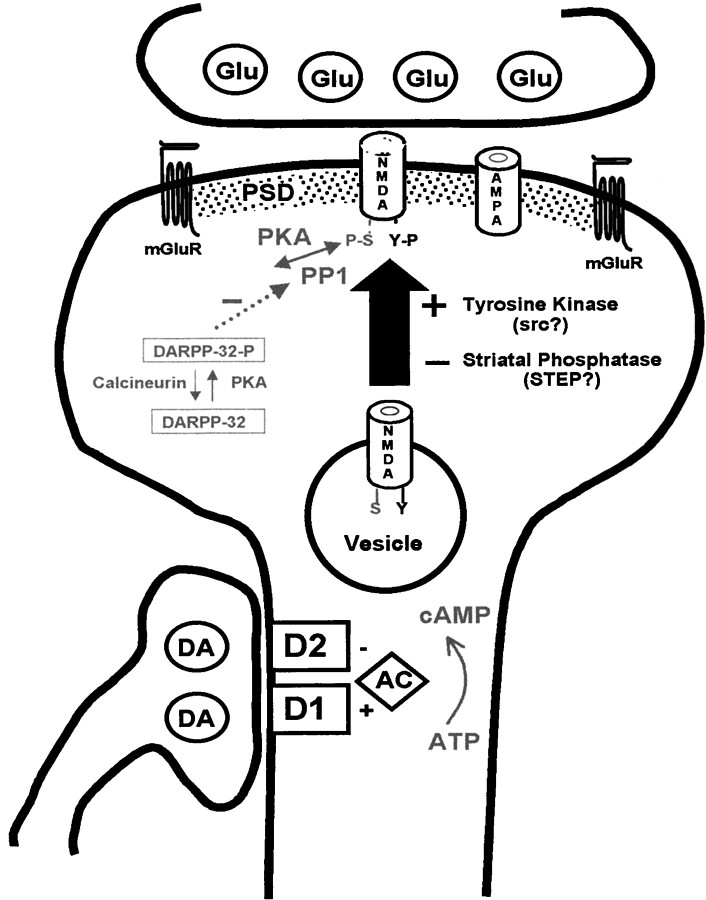
We propose that the alterations in subcellular distribution reflect the dopamine D1 receptor-induced trafficking of NR1, NR2A, and NR2B proteins from the light membrane and synaptic vesicle-enriched compartments to the synaptic membranes (Fig.11). Because we analyzed only the total receptor proteins in each compartment and did not follow individual subunits directly over time, the involvement of a more complex mechanism of subcellular redistribution of receptors cannot be excluded. For instance, the observed changes could arise from enhanced degradation of the NMDA receptors in the light membrane and synaptic vesicle-enriched compartments, together with the insertion of receptors into the synaptic membranes from other sources that are not identifiable by the present experimental approach.
Fig. 11.
Model for the trafficking of NMDA receptors at the corticostriatal synapse. Illustrated is a dendritic spine of a striatal projection neuron receiving input from a cortical axon, using glutamate as a transmitter (Glu) at the head of the spine, and from a nigrostriatal axon, using dopamine (DA) as a transmitter and forming a synapse on the shaft of the spine. The effects of glutamate are mediated by NMDA, AMPA, and metabotropic glutamate receptors (mGluR). These are linked to each other and to additional signaling molecules via the proteins of the postsynaptic density (PSD). Dopaminergic inputs are mediated by the dopamine D1 and D2 receptors. These receptors have reciprocal effects on the formation of cAMP by adenylyl cyclase (AC), but most striatal neurons express a preponderance of a single dopamine receptor type, depending on whether they contribute to the direct or indirect pathways. In addition to the NMDA receptors that are present at the excitatory synapse, our data suggest that there is a pool of assembled receptors within intracellular vesicular compartments. The vesicle-associated receptors are characterized by the absence of tyrosine (Y) phosphorylation of the NR2A and NR2B subunits. Activation of dopamine D1 receptors leads (via mechanisms that are not well defined) to tyrosine phosphorylation of the vesicular NMDA receptor subunits by a tyrosine kinase [perhaps a member of the src family reported to phosphorylate NMDA subunits at tyrosine residues (Suzuki and Okumura-Noji, 1995)] and insertion of the receptors into the synaptic membrane. There is also a tonically active tyrosine phosphatase, which may be a member of the STEP family. The diagram also illustrates the serine (S) phosphorylation of the NR1 subunit, which is regulated both by the direct action of cAMP on protein kinase A (PKA) as well as by the DARPP-32 pathway; phosphorylation of DARPP-32 by PKA inhibits the dephosphorylation of NR1 by protein phosphatase-1 (PP-1; Snyder et al., 1998). Although protein kinase C has been reported to modulate the trafficking of NMDA receptors (Lan et al., 2001), the precise role of cAMP in the phosphorylation of NMDA subunits at tyrosine residues is not known. At present it is also uncertain whether serine phosphorylation plays a role in the trafficking of NMDA receptors at the corticostriatal synapse.
Recent studies have provided ample evidence that redistribution is an important mechanism for regulating AMPA receptors. Both synaptic activity and the increased activity of calcium calmodulin-dependent protein kinase II can rapidly induce the trafficking of GFP-tagged GluR1 receptors to the postsynaptic membrane (Shi et al., 1999; Hayashi et al., 2000). The recruitment of GluR1 receptors to the postsynaptic density has been found to increase AMPA receptor-mediated synaptic transmission in long-term potentiation (Hayashi et al., 2000). Similarly, insulin causes GABAA receptors to translocate rapidly from intracellular compartments to the plasma membrane, an effect probably mediated by phosphorylation of the receptors at tyrosine residues (Wan et al., 1997). Use-dependent downregulation of NMDA receptors appears to involve a clathrin-mediated process of endocytosis (Vissel et al., 2001), and activators of protein kinase C, including insulin, recruit new NMDA receptors to the cell surface via a process involving vesicular trafficking (Lan et al., 2001; Scott et al., 2001; Skeberdis et al., 2001).
Striatal NMDA receptor trafficking requires tyrosine phosphorylation
Our findings directly implicate tyrosine phosphorylation in the subcellular redistribution of striatal NMDA receptors. Consistent with previous reports (Moon et al., 1994; Lau et al., 1995; Dunah et al., 1998), the NR2A and NR2B subunits, but not NR1, in striatum were found to be tyrosine-phosphorylated. After analysis of specific subcellular compartments, tyrosine-phosphorylated NR2A and NR2B subunits in the synaptosomal membrane, but not in the light membrane and synaptic vesicle-enriched fractions, were detected. Treatment with the dopamine D1 agonist SKF-82958 produced an increase in tyrosine-phosphorylated NR2A and NR2B in the synaptosomal membrane fraction. This effect was mimicked by pervanadate, a nonspecific inhibitor of protein phosphatases, which also increased the tyrosine phosphorylation of many membrane-associated proteins. Interestingly, even after a pharmacologic blockade of protein phosphatases, tyrosine phosphorylation of NR2A or NR2B in the light membrane or synaptic vesicle-enriched fractions was not observed. The effects of SKF-82958 on both subcellular distribution and tyrosine phosphorylation of NR2A and NR2B were blocked by genistein, a protein tyrosine kinase inhibitor.
We suggest, therefore, that tyrosine phosphorylation of the pool of NR2A and NR2B receptors in the light membrane and synaptic vesicle-enriched fractions functions as a trigger for the redistribution of these subunits from intracellular compartments to the synaptosomal membrane. This phenomenon would account for the rapidity of subcellular redistribution of the receptors and the inability to detect tyrosine-phosphorylated NR2A and NR2B in the light membrane and synaptic vesicle-enriched fractions. The inhibition of protein phosphatases with orthovanadate triggers redistribution, suggesting the existence of a tonically active phosphatase activity. Interestingly, genistein can block the effect of dopamine D1 receptor activation but does not alter the basal distribution of the receptor subunits in the 10 min time period that was studied, suggesting that constitutive trafficking of subunits to the synaptosomal membrane is either a much slower process or uses a different tyrosine kinase-independent mechanism.
The regulatory steps between the activation of dopamine D1 receptors and the tyrosine phosphorylation of NR2A and NR2B subunits are understood incompletely. In other systems, however, members of the src family of protein tyrosine kinases, in particular the fyn kinases, have been shown to phosphorylate NMDA subunits (Gurd, 1985; Moon et al., 1994; Suzuki and Okumura-Noji, 1995), and this resulted in increased channel activity of the receptors (Köhr and Seeburg, 1996); fyn kinase knock-out mice have been reported to be deficient in certain forms of long-term potentiation (Grant et al., 1992). The striking increase in NR2A and NR2B tyrosine phosphorylation with pervanadate suggests tonic activity of a protein phosphatase in the striatum. Potential candidates for this role are the striatal-enriched phosphatases (STEP), which are abundant in striatal neurons and are found in association with NMDA receptor subunits (Boulanger et al., 1995).
There is evidence for a dopamine-regulated phosphorylation of the NR1 subunit at serine residues regulated by the phosphoprotein DARPP-32 (dopamine and cAMP-regulated phosphoprotein; Snyder et al., 1998). Phosphorylation at serine890, but not at serine896 or serine897, produced a redistribution of NR1 receptors from intracellular sites to the cell surface (Tingley et al., 1997). Thus, serine phosphorylation of the NR1 subunit in these receptor complexes may have a role in the trafficking of striatal NMDA receptors, but the ability of genistein to block receptor redistribution suggests that tyrosine phosphorylation is essential to this phenomenon.
NMDA receptor trafficking and parkinsonism
The present findings complement earlier studies of dopamine-dependent modifications of NMDA receptors from our laboratory and others (Oh et al., 1998; Dunah et al., 2000a). In a previous study we analyzed the biochemical properties of striatal NMDA receptors in a rat model of unilateral 6-OHDA depletion. At 14 d postlesion we found significant reductions in NR1 and NR2B in the synaptosomal membranes, and treatment with levodopa for 21 d normalized both subunits (Dunah et al., 2000a). Although this in vivo study was performed over a longer time period, both the magnitude and direction of subcellular distribution of NMDA receptors are comparable with the results that have been described in the present study. Dopamine-dependent alterations in NMDA subunits, particularly the increased association of these receptors with corticostriatal synapses, may underlie the adverse motor effects that have been observed in patients with Parkinson's disease treated with dopaminergic drugs, which are the primary sources of ongoing disability in this neurodegenerative disease. The development of approaches targeted at modifying the trafficking of NMDA receptors in the striatum may prove to be useful in the treatment of Parkinson's disease.
Footnotes
This work was supported by National Institutes of Health Grant NS34361 (D.G.S.) and a grant from The National Parkinson Foundation (A.W.D.). We thank Dr. Barry B. Wolfe for the generous gifts of NR1, NR2A, and NR2B antibodies; Dr. Morgan Sheng for the kind donation of α-actinin-2 antibody; and Dr. Michael Wyszynski for very helpful comments on this manuscript.
Correspondence should be addressed to Dr. David G. Standaert, Massachusetts General Hospital, CNY B114-2000, Charlestown, MA 02129-4404. E-mail: standaert@helix.mgh.harvard.edu.
REFERENCES
- 1.Barinaga M. Secrets of secretion revealed. Science. 1993;260:487–489. doi: 10.1126/science.8475382. [DOI] [PubMed] [Google Scholar]
- 2.Bennett MK, Calakos N, Scheller RH. Syntaxin: a synaptic protein implicated in docking of synaptic vesicles at presynaptic active zones. Science. 1992;257:255–259. doi: 10.1126/science.1321498. [DOI] [PubMed] [Google Scholar]
- 3.Blanchet PJ, Papa SM, Metman LV, Mouradian MM, Chase TN. Modulation of levodopa-induced motor response complications by NMDA antagonists in Parkinson's disease. Neurosci Biobehav Rev. 1997;21:447–453. doi: 10.1016/s0149-7634(96)00038-3. [DOI] [PubMed] [Google Scholar]
- 4.Boulanger LM, Lombroso PJ, Raghunathan A, During MJ, Wahle P, Naegele JR. Cellular and molecular characterization of a brain-enriched protein tyrosine phosphatase. J Neurosci. 1995;15:1532–1544. doi: 10.1523/JNEUROSCI.15-02-01532.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cepeda C, Levine MS. Dopamine and N-methyl-d-aspartate receptor interactions in the neostriatum. Dev Neurosci. 1998;20:1–18. doi: 10.1159/000017294. [DOI] [PubMed] [Google Scholar]
- 6.Chase TN, Oh JD, Blanchet PJ. Neostriatal mechanisms in Parkinson's disease. Neurology. 1998;51:S30–S35. doi: 10.1212/wnl.51.2_suppl_2.s30. [DOI] [PubMed] [Google Scholar]
- 7.Chen C, Leonard JP. Protein tyrosine kinase-mediated potentiation of currents from cloned NMDA receptors. J Neurochem. 1996;67:194–200. doi: 10.1046/j.1471-4159.1996.67010194.x. [DOI] [PubMed] [Google Scholar]
- 8.David V, Hochstenbach F, Rajagopalan S, Brenner MB. Interaction with newly synthesized and retained proteins in the endoplasmic reticulum suggests a chaperone function for human integral membrane protein IP90 (calnexin). J Biol Chem. 1993;268:9585–9592. [PubMed] [Google Scholar]
- 9.De Keyser J. Subtypes and localization of dopamine receptors in human brain. Neurochem Int. 1993;22:83–93. doi: 10.1016/0197-0186(93)90001-l. [DOI] [PubMed] [Google Scholar]
- 10.Devoto SH, Barnstable CJ. Cellular and molecular biology of hormone- and neurotransmitter-containing secretory vesicles. Ann NY Acad Sci. 1987;493:1–590. [PubMed] [Google Scholar]
- 11.Di Chiara G, Morelli M, Consolo S. Modulatory functions of neurotransmitters in the striatum: ACh/dopamine/NMDA interactions. Trends Neurosci. 1994;17:228–233. doi: 10.1016/0166-2236(94)90005-1. [DOI] [PubMed] [Google Scholar]
- 12.Dingledine R, Borges K, Bowie D, Traynelis SF. The glutamate receptor ion channels. Pharmacol Rev. 1999;51:7–61. [PubMed] [Google Scholar]
- 13.Dunah AW, Yasuda RP, Luo J, Wang Y-H, Davila-Garcia MI, Gbadegesin M, Vicini S, Wolfe BB. Regional and ontogenetic expression of the NMDA receptor subunit NR2D protein in rat brain using a subunit-specific antibody. J Neurochem. 1996;67:2335–2345. doi: 10.1046/j.1471-4159.1996.67062335.x. [DOI] [PubMed] [Google Scholar]
- 14.Dunah AW, Yasuda RP, Wolfe BB. Developmental regulation of tyrosine phosphorylation of the NR2D NMDA glutamate receptor subunit in the rat central nervous system. J Neurochem. 1998;71:1926–1934. doi: 10.1046/j.1471-4159.1998.71051926.x. [DOI] [PubMed] [Google Scholar]
- 15.Dunah AW, Wang Y-H, Yasuda RP, Kameyama K, Huganir RL, Wolfe BB, Standaert DG. Alterations in subunit expression, composition, and phosphorylation of striatal N-methyl-d-aspartate glutamate receptors in a rat 6-OHDA model of Parkinson's disease. Mol Pharmacol. 2000a;57:342–352. [PubMed] [Google Scholar]
- 16.Dunah AW, Wyszynski M, Martin DM, Sheng M, Standaert DG. α-Actinin-2 in rat striatum: localization and interaction with NMDA glutamate receptor subunits. Brain Res Mol Brain Res. 2000b;79:77–87. doi: 10.1016/s0169-328x(00)00102-9. [DOI] [PubMed] [Google Scholar]
- 17.Engber TM, Papa SM, Boldry RC, Chase TN. NMDA receptor blockade reverses motor response alterations induced by levodopa. NeuroReport. 1994;5:2586–2588. doi: 10.1097/00001756-199412000-00045. [DOI] [PubMed] [Google Scholar]
- 18.Grant SG, O'Dell TJ, Karl KA, Stein PL, Soriano P, Kandel ER. Impaired long-term potentiation, spatial learning, and hippocampal development in fyn mutant mice. Science. 1992;258:1903–1910. doi: 10.1126/science.1361685. [DOI] [PubMed] [Google Scholar]
- 19.Gurd JW. Phosphorylation of the postsynaptic density glycoprotein gp180 by endogenous tyrosine kinase. Brain Res. 1985;333:385–388. doi: 10.1016/0006-8993(85)91599-9. [DOI] [PubMed] [Google Scholar]
- 20.Hayashi Y, Shi SH, Esteban JA, Piccini A, Poncer JC, Malinow R. Driving AMPA receptors into synapses by LTP and CaMKII: requirement for GluR1 and PDZ domain interaction. Science. 2000;287:2262–2267. doi: 10.1126/science.287.5461.2262. [DOI] [PubMed] [Google Scholar]
- 21.Hisatsune C, Umemori H, Inoue T, Michikawa T, Kohda K, Mikoshiba K, Yamamoto T. Phosphorylation-dependent regulation of N-methyl-d-aspartate receptors by calmodulin. J Biol Chem. 1997;272:20805–20810. doi: 10.1074/jbc.272.33.20805. [DOI] [PubMed] [Google Scholar]
- 22.Kaur S, Starr MS. Differential effects of intrastriatal and intranigral injections of glutamate antagonists on motor behaviour in the reserpine-treated rat. Neuroscience. 1997;76:345–354. doi: 10.1016/s0306-4522(96)00407-1. [DOI] [PubMed] [Google Scholar]
- 23.Kennedy MB. The postsynaptic density. Curr Opin Neurobiol. 1993;3:732–737. doi: 10.1016/0959-4388(93)90145-o. [DOI] [PubMed] [Google Scholar]
- 24.Kim E, Cho K-O, Rothschild A, Sheng M. Heteromultimerization and NMDA receptor-clustering activity of chapsyn-110, a member of the PSD-95 family of proteins. Neuron. 1996;17:103–113. doi: 10.1016/s0896-6273(00)80284-6. [DOI] [PubMed] [Google Scholar]
- 25.Klockgether T, Turski L. NMDA antagonists potentiate anti-parkinsonian actions of l-DOPA in monoamine-depleted rats. Ann Neurol. 1990;28:539–546. doi: 10.1002/ana.410280411. [DOI] [PubMed] [Google Scholar]
- 26.Köhr G, Seeburg PH. Subtype-specific regulation of recombinant NMDA receptor channels by protein tyrosine kinases of the src family. J Physiol (Lond) 1996;492:445–452. doi: 10.1113/jphysiol.1996.sp021320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kornau HC, Schenker LT, Kennedy MB, Seeburg PH. Domain interaction between NMDA receptor subunits and the postsynaptic density protein PSD-95. Science. 1995;269:1737–1740. doi: 10.1126/science.7569905. [DOI] [PubMed] [Google Scholar]
- 28.Lan J, Skeberdis VA, Jover T, Grooms SY, Lin Y, Araneda RC, Zheng X, Bennett MV, Zukin RS. Protein kinase C modulates NMDA receptor trafficking and gating. Nat Neurosci. 2001;4:382–390. doi: 10.1038/86028. [DOI] [PubMed] [Google Scholar]
- 29.Lau LF, Huganir RL. Differential tyrosine phosphorylation of N-methyl-d-aspartate receptor subunits. J Biol Chem. 1995;270:20036–20041. doi: 10.1074/jbc.270.34.20036. [DOI] [PubMed] [Google Scholar]
- 30.Laurie DJ, Seeburg PH. Ligand affinities at recombinant N-methyl-d-aspartate receptors depend on subunit composition. Eur J Pharmacol. 1994;268:335–345. doi: 10.1016/0922-4106(94)90058-2. [DOI] [PubMed] [Google Scholar]
- 31.Lin JW, Wyszynski M, Madhavan R, Sealock R, Kim JU, Sheng M. Yotiao, a novel protein of neuromuscular junction and brain that interacts with specific splice variants of NMDA receptor subunit NR1. J Neurosci. 1998;18:2017–2027. doi: 10.1523/JNEUROSCI.18-06-02017.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lissin DV, Gomperts SN, Carroll RC, Christine CW, Kalman D, Kitamura M, Hardy S, Nicoll RA, Malenka RC, von Zastrow M. Activity differentially regulates the surface expression of synaptic AMPA and NMDA glutamate receptors. Proc Natl Acad Sci USA. 1998;95:7097–7102. doi: 10.1073/pnas.95.12.7097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Luo JH, Bosy TZ, Wang YH, Yasuda RP, Wolfe BB. Ontogeny of NMDA R1 subunit protein expression in five regions of rat brain. Brain Res Dev Brain Res. 1996;92:10–17. doi: 10.1016/0165-3806(95)00191-3. [DOI] [PubMed] [Google Scholar]
- 34.Luo JH, Wang Y, Yasuda RP, Dunah AW, Wolfe BB. The majority of N-methyl-d-aspartate receptor complexes in adult rat cerebral cortex contain at least three different subunits (NR1/NR2A/NR2B). Mol Pharmacol. 1997;51:79–86. doi: 10.1124/mol.51.1.79. [DOI] [PubMed] [Google Scholar]
- 35.Lynch DR, Gallagher MJ. Inhibition of N-methyl-d-aspartate receptors by haloperidol: developmental and pharmacological characterization in native and recombinant receptors. J Pharmacol Exp Ther. 1996;279:154–161. [PubMed] [Google Scholar]
- 36.Lynch DR, Anegawa NJ, Verdoorn T, Pritchett DB. N-methyl-d-aspartate receptors: different subunit requirements for binding of glutamate antagonists, glycine antagonists, and channel-blocking agents. Mol Pharmacol. 1994;45:540–545. [PubMed] [Google Scholar]
- 37.Marin C, Papa S, Engber TM, Bonastre M, Tolosa E, Chase TN. MK-801 prevents levodopa-induced motor response alterations in parkinsonian rats. Brain Res. 1996;736:202–205. doi: 10.1016/0006-8993(96)00693-2. [DOI] [PubMed] [Google Scholar]
- 38.Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–540. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- 39.Moon IS, Apperson ML, Kennedy MB. The major tyrosine-phosphorylated protein in the postsynaptic density fraction is N-methyl-d-aspartate receptor subunit 2B. Proc Natl Acad Sci USA. 1994;91:3954–3958. doi: 10.1073/pnas.91.9.3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Morelli M, Fenu S, Pinna A, Di Chiara G. Opposite effects of NMDA receptor blockade on dopaminergic D1- and D2-mediated behavior in the 6-hydroxydopamine model of turning: relationship with c-fos expression. J Pharmacol Exp Ther. 1992;260:402–408. [PubMed] [Google Scholar]
- 41.Nishimune A, Isaac JTR, Molnar E, Noel J, Nash SR, Tagaya M, Collingridge GL, Nakanishi S, Henley JM. NSF binding to GluR2 regulates synaptic transmission. Neuron. 1998;21:87–97. doi: 10.1016/s0896-6273(00)80517-6. [DOI] [PubMed] [Google Scholar]
- 42.Oh JD, Russell DS, Vaughan CL, Chase TN (1998) Enhanced tyrosine phosphorylation of striatal NMDA receptor subunits: effect of dopaminergic denervation and l-DOPA administration. Brain Res 813. [DOI] [PubMed]
- 43.Osten P, Ziff EB. AMPA receptor forms a biochemically functional complex with NSF and α- and β-SNAPs. Ann NY Acad Sci. 1999;868:558–560. doi: 10.1111/j.1749-6632.1999.tb11328.x. [DOI] [PubMed] [Google Scholar]
- 44.Papa SM, Chase TN. Levodopa-induced dyskinesias improved by a glutamate antagonist in parkinsonian monkeys. Ann Neurol. 1996;39:574–578. doi: 10.1002/ana.410390505. [DOI] [PubMed] [Google Scholar]
- 45.Papa SM, Engber TM, Boldry RC, Chase TN. Opposite effects of NMDA and AMPA receptor blockade on catalepsy induced by dopamine receptor antagonists. Eur J Pharmacol. 1993;232:247–253. doi: 10.1016/0014-2999(93)90781-c. [DOI] [PubMed] [Google Scholar]
- 46.Papa SM, Boldry RC, Engber TM, Kask AM, Chase TN. Reversal of levodopa-induced motor fluctuations in experimental parkinsonism by NMDA receptor blockade. Brain Res. 1995;701:13–18. doi: 10.1016/0006-8993(95)00924-3. [DOI] [PubMed] [Google Scholar]
- 47.Petralia RS, Yokotani N, Wenthold RJ. Light and electron microscope distributions of the NMDA receptor subunit NMDAR1 in the rat nervous system using a selective anti-peptide antibody. J Neurosci. 1994;14:667–696. doi: 10.1523/JNEUROSCI.14-02-00667.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rao A, Craig AM. Activity regulates the synaptic localization of the NMDA receptor in hippocampal neurons. Neuron. 1997;19:801–812. doi: 10.1016/s0896-6273(00)80962-9. [DOI] [PubMed] [Google Scholar]
- 49.Roche KW, Standley S, McCallum J, Ly CD, Wenthold RJ. NMDA receptor internalization is regulated by PSD-95. Soc Neurosci Abstr. 2000;26:1918. [Google Scholar]
- 50.Schapira AH. Mitochondrial dysfunction in neurodegenerative disorders. Biochim Biophys Acta. 1998;1366:225–233. doi: 10.1016/s0005-2728(98)00115-7. [DOI] [PubMed] [Google Scholar]
- 51.Schoffelmeer AN, Vanderschuren LJ, De Vries TJ, Hogenboom F, Wardeh G, Mulder AH. Synergistically interacting dopamine D1 and NMDA receptors mediate nonvesicular transporter-dependent GABA release from rat striatal medium spiny neurons. J Neurosci. 2000;20:3496–3503. doi: 10.1523/JNEUROSCI.20-09-03496.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Scott DB, Blanpied TA, Swanson GT, Zhang C, Ehlers MD. An NMDA receptor ER retention signal regulated by phosphorylation and alternative splicing. J Neurosci. 2001;21:3063–3072. doi: 10.1523/JNEUROSCI.21-09-03063.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sealfon SC, Olanow CW. Dopamine receptors: from structure to behavior. Trends Neurosci. 2000;23:S34–S40. doi: 10.1016/s1471-1931(00)00025-2. [DOI] [PubMed] [Google Scholar]
- 54.Shi SH, Hayashi Y, Petralia RS, Zaman SH, Wenthold RJ, Svoboda K, Malinow R. Rapid spine delivery and redistribution of AMPA receptors after synaptic NMDA receptor activation. Science. 1999;284:1811–1816. doi: 10.1126/science.284.5421.1811. [DOI] [PubMed] [Google Scholar]
- 55.Skeberdis VA, Lan J, Zheng X, Zukin RS, Bennett MV. Insulin promotes rapid delivery of N-methyl-d-aspartate receptors to the cell surface by exocytosis. Proc Natl Acad Sci USA. 2001;98:3561–3566. doi: 10.1073/pnas.051634698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Snyder GL, Fienberg AA, Huganir RL, Greengard P. A dopamine/D1 receptor/protein kinase A/dopamine- and cAMP-regulated phosphoprotein (Mr 32 kDa)/protein phosphatase-1 pathway regulates dephosphorylation of the NMDA receptor. J Neurosci. 1998;18:10297–10303. doi: 10.1523/JNEUROSCI.18-24-10297.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sudhof TC. The synaptic vesicle cycle: a cascade of protein–protein interactions. Nature. 1995;375:645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- 58.Suzuki T, Okumura-Noji K. NMDA receptor subunits ε1 (NR2A) and ε2 (NR2B) are substrates for fyn in the postsynaptic density fraction isolated from the rat brain. Biochem Biophys Res Commun. 1995;216:582–588. doi: 10.1006/bbrc.1995.2662. [DOI] [PubMed] [Google Scholar]
- 59.Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20:4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tingley WG, Ehlers MD, Kameyama K, Doherty C, Ptak JB, Riley CT, Huganir RL. Characterization of protein kinase A and protein kinase C phosphorylation of the N-methyl-d-aspartate receptor NR1 subunit using phosphorylation site-specific antibodies. J Biol Chem. 1997;272:5157–5166. doi: 10.1074/jbc.272.8.5157. [DOI] [PubMed] [Google Scholar]
- 61.Vissel B, Krupp JJ, Heinemann SF, Westbrook GL. A use-dependent tyrosine dephosphorylation of NMDA receptors is independent of ion flux. Nat Neurosci. 2001;4:587–596. doi: 10.1038/88404. [DOI] [PubMed] [Google Scholar]
- 62.Wan Q, Xiong ZG, Man HY, Ackerley CA, Braunton J, Lu WY, Becker LE, MacDonald JF, Wang YT. Recruitment of functional GABAA receptors to postsynaptic domains by insulin. Nature. 1997;388:686–690. doi: 10.1038/41792. [DOI] [PubMed] [Google Scholar]
- 63.Wang Y-H, Bosy TZ, Yasuda RP, Grayson DR, Vicini S, Pizzorusso R, Wolfe BB. Characterization of NMDA receptor subunit-specific antibodies: distribution of NR2A and NR2B receptor subunits in the rat brain and ontogenic profile in cerebellum. J Neurochem. 1995;65:176–183. doi: 10.1046/j.1471-4159.1995.65010176.x. [DOI] [PubMed] [Google Scholar]
- 64.Wang YT, Salter MW. Regulation of NMDA receptors by tyrosine kinases and phosphatases. Nature. 1994;369:233–235. doi: 10.1038/369233a0. [DOI] [PubMed] [Google Scholar]
- 65.Williams K, Zappia A, M, Pritchett DB, Shen YM, Molinoff PB. Sensitivity of the N-methyl-d-aspartate receptor to polyamines is controlled by NR2 subunits. Mol Pharmacol. 1994;45:803–809. [PubMed] [Google Scholar]
- 66.Wyszynski M, Lin J, Rao A, Nigh E, Beggs AH, Craig AM, Sheng M. Competitive binding of α-actinin and calmodulin to the NMDA receptor. Nature. 1997;385:439–442. doi: 10.1038/385439a0. [DOI] [PubMed] [Google Scholar]
- 67.Wyszynski M, Kharazia V, Shanghvi R, Rao A, Beggs AH, Craig AM, Weinberg R, Sheng M. Differential regional expression and ultrastructural localization of α-actinin-2, a putative NMDA receptor anchoring protein, in rat brain. J Neurosci. 1998;18:1383–1392. doi: 10.1523/JNEUROSCI.18-04-01383.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ziff EB. Enlightening the postsynaptic density. Neuron. 1997;19:1163–1174. doi: 10.1016/s0896-6273(00)80409-2. [DOI] [PubMed] [Google Scholar]
- 69.Zukin RS, Lan JY, Skeberdis VA, Opitz T, Bennett MVL. Activation of mGluR1 regulates NMDA channel gating and recruits new NMDA receptors to the cell surface. Soc Neurosci Abstr. 2000;26:A806. [Google Scholar]