Abstract
Long-term potentiation (LTP) is a cellular model for persistent synaptic plasticity in the mammalian brain. Like several forms of memory, long-lasting LTP requires cAMP-mediated activation of protein kinase A (PKA) and is dependent on gene transcription. Consequently, activity-dependent genes such as c-fosthat contain cAMP response elements (CREs) in their 5′ regulatory region have been studied intensely. More recently, arg3.1/arc became of interest, because after synaptic stimulation, arg3.1/arc mRNA is rapidly induced and distributed to dendritic processes and may be locally translated there to facilitate synapse-specific modifications. However, to date nothing is known about the signaling mechanisms involved in the induction of this gene. Here we report that arg3.1/arc is robustly induced with LTP stimulation even at intensities that are not sufficient to activate c-fosexpression. Unlike c-fos, the 5′ regulatory region of arg3.1/arc does not contain a CRE consensus sequence and arg3.1/arc is unresponsive to cAMP in NIH3T3 and Neuro2a cells. However, in PC12 cells and primary cultures of hippocampal neurons, arg3.1/arc can be induced by cAMP and calcium. This induction requires the activity of PKA and mitogen-activated protein kinase, suggesting a neuron-specific pathway for the activation of arg3.1/arc expression.
Keywords: plasticity, long-term memory, hippocampus, LTP, gene induction, Arg3.1, Arc
Like long-term memory, enduring forms of synaptic plasticity including long-term potentiation (LTP) (Bliss and Collingridge, 1993) require alterations in the molecular composition and structure of neurons and are dependent on mRNA and protein synthesis (Goelet et al., 1986; Curran and Morgan, 1987; Sheng and Greenberg, 1990). Plasticity therefore might be achieved by activity-dependent changes in the expression of immediate early genes (IEGs) (Kuhl, 2000). These encode transcription factors (Greenberg et al., 1986; Morgan et al., 1987; Saffen et al., 1988) as well as a set of proteins that have the potential to transduce synaptic activity directly into immediate changes of neural function (Nedivi et al., 1993; Qian et al., 1993; Yamagata et al., 1993; Frey et al., 1996;Kauselmann et al., 1999; Konietzko et al., 1999). A particular role might be played by arg3.1/arc, because this gene is thus far unique among IEGs in that its mRNA is rapidly distributed throughout the dendritic arbor of stimulated neurons (Link et al., 1995; Lyford et al., 1995). Arg3.1/arc, also named BAD-1, was originally identified in differential screens for seizure-stimulated hippocampal mRNAs (Qian et al., 1993; Link et al., 1995; Lyford et al., 1995). However, arg3.1/arc is also robustly induced after LTP induction in the absence of seizures (Link et al., 1995; Lyford et al., 1995), and arg3.1/arc mRNA and protein specifically localize to the field of stimulated synapses (Steward et al., 1998). Moreover, induction of arg3.1/arc in the hippocampus after an exploration paradigm has been demonstrated to occur specifically in neurons engaged in the spatial encoding process (Guzowski et al., 1999), and blocking of arg3.1/arc expression in antisense experiments results in a loss of LTP and impaired hippocampal learning (Guzowski et al., 2000). Although these observations strongly suggest that arg3.1/arc expression is required for synaptic plasticity, nothing is known about the signal transduction pathways that lead to the induction of this gene.
Activity-dependent changes in neuronal function are mediated to a large degree by elevations in intracellular calcium levels. In neurons, calcium ions can stimulate the production of cAMP and the activation of protein kinase A (PKA) (Ginty et al., 1991; Xia et al., 1991; Cali et al., 1994). LTP can be initiated by calcium influx through glutamate-gated NMDA channels (Lynch et al., 1983; Malenka et al., 1992), whereas the late phase of LTP (L-LTP) can be initiated by cAMP, requires the activation of PKA, and is dependent on gene transcription (Frey et al., 1993; Huang et al., 1994; Abel et al., 1997). In addition, accumulating evidence suggests a pivotal role for mitogen-activated protein kinase [(MAPK) also known as extracellular regulated kinase (ERK)] in enduring forms of synaptic plasticity (Impey et al., 1998; Orban et al., 1999). In Aplysia, stimuli that induce long-term facilitation, including cAMP, increase MAPK activity and nuclear localization (Martin et al., 1997). Similarly, MAPK activity is essential for hippocampal LTP (English and Sweatt, 1996, 1997) and learning in the intact animal (Atkins et al., 1998; Berman et al., 1998; Blum et al., 1999), and both PKA and MAPK promote the expression of genes required for the formation of long-term memory (Martin and Kandel, 1996; Impey et al., 1998). An important mediator of such transcriptional changes is the transcription factor cAMP response element-binding (CREB) protein (Dash et al., 1990;Bourtchuladze et al., 1994; Frank and Greenberg, 1994; Yin et al., 1994; Bartsch et al., 1995; Yin et al., 1995; Guzowski and McGaugh, 1997). Therefore the objectives of our studies were to define the roles of the PKA and MAPK pathways in the activation of arg3.1/arc expression.
MATERIALS AND METHODS
Animal preparation and chronic electrophysiology. Adult male Sprague Dawley rats (250–300 gm) were used. Kainic acid (10 mg/kg) was administered by intraperitoneal injection, and animals were killed 1 hr after seizure onset. Control animals were injected with identical volumes of isotonic saline (PBS). Implantation of a monopolar stimulating electrode in the perforant path and a monopolar recording electrode in the hilus of the dentate gyrus was as described (Konietzko et al., 1999). The animals recovered for at least 7 d before being transferred to a chronic recording cage for the actual experiment involving standard in vivo electrophysiological techniques (Staubli and Scafidi, 1997). During the experimental sessions, the current intensity was adjusted (20–100 μA, pulse width 150 μsec, biphasic) to produce a baseline response with a minimal population spike of 0.5–1 mV. Test pulses were delivered at 0.07 Hz for at least 20 min to establish stable baseline potentials, after which either high-frequency stimulation (HFS) or low-frequency stimulation (LFS) was initiated randomly. The 12-train HFS paradigm consisted of 12 trains of 20 msec each at 500 Hz, delivered 10 sec apart (Cole et al., 1989). The 50-train HFS paradigm consisted of five 25-msec-long trains at 400 Hz, delivered 1 sec apart and repeated 10 times with a 1 min interval between repetitions (Abraham et al., 1993; Konietzko et al., 1999). The same number of pulses was delivered for the LFS treatment but at a rate of 0.2 Hz. Potentials were then monitored at 0.07 Hz for 1 hr, after which the rats were decapitated; the brains were removed, frozen on dry ice, and stored at −75°C.
Cell culture, stimulation, and inhibition experiments. NIH3T3 and Neuro2a cells were grown in DMEM (Life Technologies, Gaithersburg, MD) with 10% fetal calf serum (Life Technologies), 100 IU/ml penicillin, and 100 μg/ml streptomycin. PC12 cells were plated on poly-l-lysine-coated (molecular weight > 300,000; Sigma, St. Louis, MO) Petri dishes and were grown in DMEM with 10% horse serum (Boehringer Mannheim, Mannheim, Germany), 5% fetal calf serum (Life Technologies), 100 IU/ml penicillin, and 100 μg/ml streptomycin. All experiments with permanent cell lines were conducted in 60 mm dishes at a cell density of 50–60% confluency. Primary hippocampal neuronal cultures were prepared from 18-d-old Wistar rat embryos as described previously (Papa et al., 1995). Dissociated cells were plated on poly-l-lysine-coated 35 mm dishes in Eagle's MEM with l-alanyl-l-glutamine (Life Technologies), enriched with 0.6% glucose, 5% fetal calf serum, and 5% horse serum, at a density of 1.2 × 106 cells per well. Two days after plating, the medium was changed to one containing 10% horse serum, 20 μg/ml 5′-fluoro-2-deoxyuridine, and 50 μg/ml uridine to stop the proliferation of glial cells. The neuronal cultures were used for experiments at 2 weeks in culture, with no further medium changes. NIH3T3 cells were serum stimulated as described previously for Rat6 cells (Link et al., 1995). Forskolin stimulation of NIH3T3, Neuro2a, PC12 cells, and primary neuronal cultures was performed by adding forskolin (RBI, Natick, MA, or Tocris) dissolved in DMSO to a final concentration of 50 μm. PC12 cells were depolarized by adding KCl to a final concentration of 60 mm. In an alternative protocol, KCl was washed out and replaced by non-KCl-containing medium; however, this did not influence mRNA expression and time course in comparison to continuous exposure to KCl (data not shown). In the case of the alternative protocol, 6 hr before the start of the experiment the medium was changed to exclude stimulation caused by starvation and the addition of serum. The following inhibitors were added to the medium 30 min before stimulation: cycloheximide (Sigma; dissolved in ethanol), final concentration of 10 μg/ml; nifedipine (RBI; dissolved in DMSO), final concentration of 10 μm; H-89 (Biomol; dissolved in DMSO), final concentration of 20 μm; and PD098059 (RBI; dissolved in DMSO), final concentration of 50 μm. Actinomycin D (Sigma; dissolved in DMSO) was added 15 min before stimulation to a final concentration of 3 μg/ml.
Generation of stably transfected cell lines. PC12 cells were transfected with the arg3.1/arc promoter constructs [pALΔ1737 + 3′ untranslated region (UTR), pALΔ838 + 3′UTR, or pALΔ177 + 3′UTR] and an expression plasmid carrying a neomycin resistance gene under control of a PGK-1 promoter at a ratio of 10:1 using Lipofectamine reagent (Life Technologies). Selection was performed by addition of 500 μg/ml G418 (Life Technologies) to the culture medium. All G418-resistant clones of each culture dish were pooled.
Cloning of genomic DNA and plasmid constructions. A 21 kb genomic fragment was isolated from a λ phage genomic library (AB-1) prepared from [129/Sv (ev)] embryonic stem cells. Two XhoI fragments were subcloned into pZErO-1 (Invitrogen, San Diego). The first subclone, pλarg-1, contained 10 kb of arg3.1/arc 5′ untranscribed and the whole 3.5 kb transcribed region including the poly(A)+ site. The second subclone, pλarg-2, contained 6.5 kb immediately downstream of the pλarg-1 sequence. From pλarg-1 two smaller fragments were subcloned: a 4.3 kbHindIII–XhoI fragment, containing 838 bp upstream and 3.5 kb downstream of the transcription start site, was inserted into pZErO-1 to obtain pArgGene. A StuI fragment, containing 1737 bp upstream and 477 bp downstream of the transcription start site, was inserted into the EcoRV site of pZErO-1 to yield pArgProm. The luciferase reporter pGL2-Basic Vector (Promega, Madison, WI) was modified in the multiple cloning region by the insertion of a NotI and a SpeI site between theXhoI and BglII sites and the insertion of aNsiI site between the XbaI and MluI sites. To generate pALΔ1737, a bluntedNsiI–SacII fragment from pArgProm, extending from position −1737 to +250 and carrying part of the pZErO-1 polylinker at its 5′ end, was inserted into the bluntedHindIII site of the modified pGL2-Basic Vector. pALΔ838 was obtained by restriction of pALΔ1737 with HindIII, releasing a fragment from position −1737 to −839, and subsequent religation. pALΔ177 was constructed in two steps: in a first step aXhoI–BssHII fragment of pALΔ1737, containing the sequence from position −1737 to −221, was released, and the resulting plasmid was blunted and religated. In a second step, a deletion, comprising position −220 to −178, was introduced using the double-stranded nested deletion kit (Pharmacia) and the NsiI site as the protected site and the NheI site as the nonprotected site. To generate pALΔ1737 + 3′UTR, the XhoI site was removed from the modified pGL2-Basic Vector multiple cloning region of pALΔ1737 by SpeI and NheI restriction and religation of the blunted ends. The arg3.1/arc 3′UTR, contained in the Eco47III–XbaI fragment of pArgGene, was inserted with blunted ends between theClaI–PflMI sites of the modified pALΔ1737, thereby replacing the SV40 3′UTR. In addition a 0.5 kbXhoI–NheI fragment from pλarg-2, containing the genomic sequence immediately downstream of the XhoI site in the Eco47III–XbaI fragment of pArgGene, was inserted into the XhoI–SalI sites after blunting the NheI and SalI ends. pALΔ838 + 3′UTR was obtained by removing position −1737 to −839 from pALΔ1737 + 3′UTR after a MluI–HindIII restriction and blunt-end ligation. To yield pALΔ177 + 3′UTR, theXbaI–EcoRI fragment of pALΔ1737 + 3′UTR was exchanged against the XbaI–EcoRI fragment of pALΔ177.
DNA sequencing and analysis. All plasmid constructs were confirmed by sequencing. The arg3.1/arc genomic sequences were determined from the plasmids pArgProm and pArgGene using multiple synthetic primers and the dideoxynucleotide chain termination method (Sanger et al., 1977) and an ABI Prism sequencer (GenBank accession number AF177701). Analysis of the nucleotide sequence was performed with the GCG software package (University of Wisconsin, Biotechnology Center, Madison, WI). Analysis of potential transcription factor binding sites was assisted by the MatInspector program (Quandt et al., 1995).
S1 analysis. S1 analysis was as described (Kuhl et al., 1987, 1992). The following arg3.1/arc antisense oligonucleotide, S1-arg3.1/arc, was synthesized and gel purified: CGC CGC TGA AGC TAG AGA GGC CCA GAG ACT GCG GCT GCG GGA GAA CTC GCT TGA GCT CTG CAC CGA AAC CGC CAC CAG CGG CTA TTT ATG TGC GCG GGG CCC GT. Forty-one nucleotides were derived from the 5′ untranscribed region to differentiate digested from undigested probe. The S1-arg3.1/arc probe (0.015 pmol) was end labeled with γ-32P-ATP to a specific activity of >2 × 106 cpm/μg using T4 kinase (Life Technologies). Total RNA (50 μg) isolated from 60 min serum-stimulated NIH3T3 cells was used in the S1 nuclease digest. The S1 reaction product was analyzed on a 12% urea-PAGE. M13mp18 DNA was sequenced according to the manufacturer's instructions (USB 70770 sequencing kit; Amersham Pharmacia Biotech, Braunschweig, Germany) and served as a length marker.
RNA preparation, Northern blot analysis, and in situhybridization. Cells were lysed at the indicated times after stimulation. Total RNA was prepared using the RNeasy kit (Qiagen). mRNA was prepared from total RNA using the Oligotex mRNA kit and was additionally desalted with the RNeasy kit (Qiagen). Northern blot analysis, in situ hybridizations, and quantifications of autoradiographs were performed as described (Link et al., 1995). Probes were generated from full-length rat cDNAs: arg3.1/arc (Link et al., 1995), c-fos (Curran et al., 1987), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (Fort et al., 1985).
RESULTS
Arg3.1/arc is robustly induced with plasticity-producing stimulation
Previous studies by Worley and coworkers (Lyford et al., 1995) and by us (Link et al., 1995) established that arg3.1/arc transcript levels are dramatically increased by single seizures induced by pentylenetetrazole or maximal electroconvulsive shock. Moreover, these studies demonstrated that arg3.1/arc can be induced in the absence of seizures by LTP-producing stimulation. A similar responsiveness to synaptic activity has been demonstrated for c-fos, a gene well characterized for the signal transduction pathways contributing to its induction in the nervous system (Curran and Morgan, 1987; Dragunow et al., 1989; Morgan and Curran, 1995). Here we examined the levels of arg3.1/arc transcripts after recurrent kainic acid (KA)-induced seizures and after two in vivo LTP-producing stimulation protocols of different strengths. For comparison we monitored c-fos mRNA levels in the same animals. In situhybridization analysis demonstrated that arg3.1/arc and c-fos differ in their cellular and regional expression patterns (Fig. 1). Constitutive expression of c-fos was low (Fig. 1A), whereas arg3.1/arc was expressed at moderate levels in cortex and hippocampus (Fig. 1B). After KA-induced seizures, both mRNAs were strongly elevated 1 hr after the onset of seizure in cortex, hippocampus, and amygdala. Whereas c-fos transcripts were confined to somata, arg3.1/arc transcripts rapidly translocated to the dendrites of activated neurons of the dentate gyrus. Moreover, we found that c-fos but not arg3.1/arc transcripts were induced in diencephalon (Fig. 1C,D). These results demonstrate that arg3.1/arc and c-fos are expressed by distinct but overlapping populations of neurons. We next examined the responsiveness of arg3.1/arc and c-fos to LTP-producing stimulation. Long-term potentiation can be induced at synapses within the hippocampus by high-frequency orthodromic stimulation (Bliss and Collingridge, 1993). The dentate gyrus granule cells were synaptically stimulated by activating their major afferent projection from the entorhinal cortex using a chronically implanted stimulating electrode (Staubli and Scafidi, 1997). Stimulation of the perforant path at the intensity required to produce a population spike, when administered in 50 or 12 trains at low frequency, did not result in LTP or an increase in arg3.1/arc or c-fos mRNA levels (Fig.1E,F,K,L). By contrast, when LTP was evoked in the granule cells by delivering the same intensity stimuli in 50 or 12 trains at high frequency, arg3.1/arc but not c-fos was consistently induced in the ipsilateral dentate gyrus 1 hr after stimulation (Fig.1H-L,Q,R). The absence of c-fos mRNA induction with weaker LTP-producing stimulation is not a consequence of the short half life of c-fos mRNA, because it was also observed at an earlier time point (Worley et al., 1993). This is contrasted by the robust induction of arg3.1/arc. When stimulus intensities were increased (50-train LTP protocol), one of five animals showed induction of c-fos transcripts, indicating that c-fos can be selectively induced in the stimulated dentate gyrus and that the stronger stimulus parameters used here are presumably close to the threshold of c-fosactivation (Fig. 1H). These results demonstrate that arg3.1/arc and c-fos possess distinct thresholds to synaptic activation and that the induction of arg3.1/arc is most highly associated with LTP. Previous studies have demonstrated different thresholds for the activation of immediate early genes and that c-fos is unresponsive to weak LTP-producing stimulation but can be induced when stimulation intensity is increased and more persistent forms of LTP are generated (Abraham et al., 1993; Worley et al., 1993).
Fig. 1.
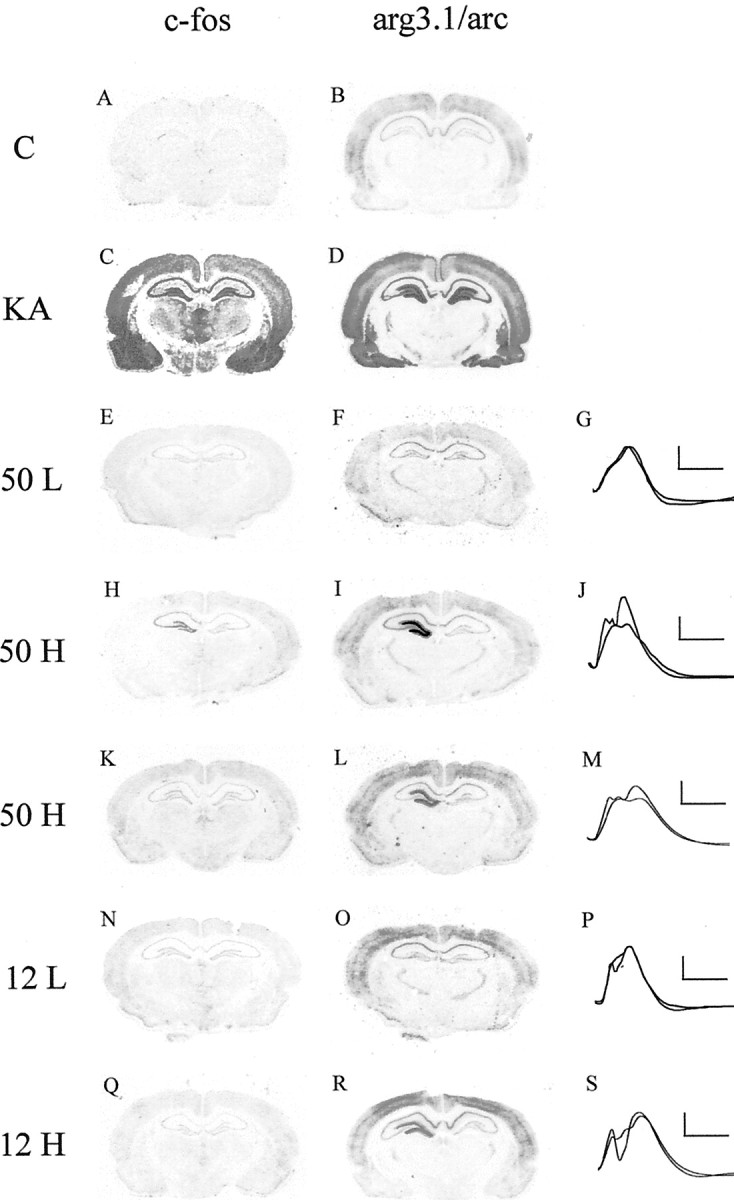
Comparison of arg3.1/arc and c-fosmRNA levels after kainic acid-induced seizures and LTP-producing stimulation. Coronal sections were assayed for arg3.1/arc and c-fos mRNA using in situ hybridization with antisense RNA probes. Representative autoradiographs of three independent experiments are shown. A, B, One hour after saline injection. C, D, One hour after kainic acid (10 mg/kg)-induced seizures.E, F, One hour after 50-train LFS of the perforant path in freely moving rats. H,I, K, L, One hour after 50-train HFS (n = 5). N, O, One hour after 12-train LFS. Q, R, One hour after 12-train HFS. G, J,M, P, S, Superimposed field potentials before and 1 hr after LFS or HFS, respectively. Calibration: 5 mV, 5 msec.
Membrane depolarization increases arg3.1/arc mRNA expression
Membrane depolarization of PC12 cells induces c-fostranscription by calcium influx through voltage-sensitive calcium channels (VSCCs) (Greenberg et al., 1986; Morgan and Curran, 1986). We exposed PC12 cells to potassium chloride and monitored the expression of arg3.1/arc and c-fos mRNA at various times after membrane depolarization (Fig. 2). We will refer to this treatment also as calcium-mediated induction. As was reported earlier (Bartel et al., 1989), the greatest increase in c-fos mRNA levels was observed at 30 min and 1 hr after depolarization. Although present at higher baseline levels, arg3.1/arc mRNA levels were similarly increased, but in contrast to c-fos, they remained elevated for at least 4 hr. Ten hours after stimulation, arg3.1/arc mRNA levels were slightly below control levels. Inhibition of protein synthesis did not abolish the induction of c-fos or arg3.1/arc mRNA, as is typical for IEGs. However, in contrast to c-fos, arg3.1/arc showed no superinduction in the presence of protein synthesis inhibition. This observation resembles arg3.1/arc expression in the hippocampus in vivo, where seizures in the presence of cycloheximide increase arg3.1/arc mRNA without apparent superinduction (Link et al., 1995;Wallace et al., 1998). The lack of superinduction might be the result of a higher stability of arg3.1/arc transcripts, which may also explain the longer time of elevated arg3.1/arc levels compared with c-fos.
Fig. 2.
Depolarization regulates arg3.1/arc and c-fos mRNA levels in PC12 cells. Autoradiograph of Northern blot analysis of arg3.1/arc and c-fostranscripts. Four independent experiments were conducted (n = 4). Six micrograms of RNA isolated from PC12 cells were loaded per lane. The blot was hybridized to a probe specific for arg3.1/arc and a probe specific for c-fos. Hybridization to a probe specific for GAPDH was used as a loading control. Numbers below the lanes indicate the period of 60 mm KCl exposure in hours. Lane C/4, RNA isolated 4 hr after exposure to KCl in the presence of cycloheximide (CHX, 10 μg/ml). Lane C, RNA isolated 4 hr after exposure to CHX only. Note that the increase in arg3.1/arc mRNA levels is maintained for 4 hr and not superinduced by CHX. In contrast, c-fos mRNA is only transiently induced but strongly superinduced in the presence of CHX.
Arg3.1/arc expression is activated by cAMP in PC12 cells but not in NIH3T3 or Neuro2a cells
Pharmacological and genetic experiments have demonstrated that cAMP plays a critical role in L-LTP (Frey et al., 1993; Abel et al., 1997). With an interest in arg3.1/arc involvement in this phenomenon, we determined whether arg3.1/arc expression is responsive to activation by cAMP. PC12 pheochromocytoma cells, NIH3T3 fibroblasts, and Neuro2a neuroblastoma cells were stimulated with forskolin, a strong activator of adenylyl cyclase (Fig.3). We will refer to this treatment also as cAMP-mediated induction. In PC12 cells, arg3.1/arc transcript levels were elevated rapidly by cAMP (Fig. 3A) and increased fourfold (n = 2) and fivefold (n = 6) at 30 min and 1 hour after stimulation, respectively. In contrast to the effects of membrane depolarization, arg3.1/arc transcripts were downregulated below baseline levels by 4 hr after forskolin stimulation. Such a return of arg3.1/arc transcripts to below baseline levels has been observed previously 4 hr after seizure activity in the intact animal and might reflect an active transcriptional shutoff mechanism (Link et al., 1995). As reported elsewhere (Kruijer et al., 1985), c-fos transcript levels were equally increased with a similar time course of induction (Fig. 3A). Forskolin-stimulated increases in arg3.1/arc and c-fos mRNA levels were dependent on transcriptional activation of these genes, because the induction of both genes was completely blocked by actinomycin D, a potent inhibitor of transcription (Fig.3B). In strong contrast, no cAMP-mediated induction of arg3.1/arc was seen in NIH3T3 cells, although c-fostranscript levels were clearly elevated within 30 min after stimulation (Fisch et al., 1989) (Fig. 3C). Similarly, arg3.1/arc was unresponsive to cAMP stimulation in Neuro2a cells, although increased c-fos transcript levels could be observed 1 and 4 hr after stimulation (Fig. 3D). These findings indicate that induction of arg3.1/arc transcription by cAMP is dependent on a signaling pathway that is present in PC12 cells but absent in NIH3T3 or Neuro2a cells. In contrast, c-fos transcripts were readily increased in these cells, indicating that cAMP-mediated induction of c-fos is not solely dependent on the pathway present in PC12 cells.
Fig. 3.
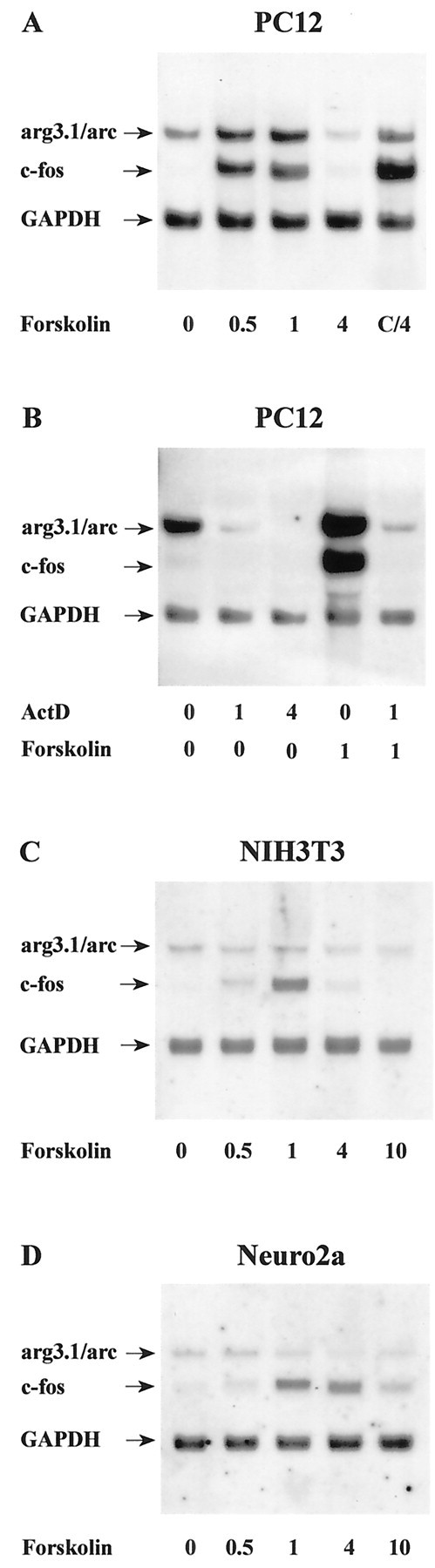
Regulation of arg3.1/arc and c-fostranscription by cAMP. Autoradiographs are of Northern blots. RNA amounts and labels are as in Figure 2. Cells were stimulated with the adenylyl cyclase activator forskolin (50 μm).A, B, RNA isolated from PC12 cells (n = 4). Where indicated, cells in Bwere exposed to 3 mm of the transcription inhibitor actinomycin D (ActD), 1.25 and 4.25 hr before lysis, or 15 min before stimulation with forskolin, respectively.C, RNA isolated from NIH3T3 fibroblasts (n = 2). D, RNA isolated from Neuro2a neuroblastoma cells (n = 2). Note that arg3.1/arc is induced by forskolin in PC12 cells but not in NIH3T3 or Neuro2a cells.
The arg3.1/arc promoter does not contain a CRE consensus sequence
A ubiquitously found cAMP-dependent signaling mechanism is the cAMP/PKA/CREB pathway (Montminy, 1997). For example, in NIH3T3 cells the transcriptional activation of CRE-containing genes such as c-fos is mediated by the PKA-induced phosphorylation of CREB (Fisch et al., 1989; Gonzalez and Montminy, 1989). These observations indicated to us that the arg3.1/arc promoter might not contain a functional CRE site and that other signaling pathways are responsible for its induction by cAMP in PC12 cells. To test this assumption we isolated from a mouse 129/Sv (ev) genomic library a 21 kb fragment containing the arg3.1/arc gene, including 10 kb of the 5′ flanking sequence, the entire transcribed region, and 6.5 kb downstream from the poly(A)+ signal. We determined the transcription start site using nuclease S1 analysis (Kuhl et al., 1987) (Fig.4) and sequenced 1737 bp of the promoter as well as the entire transcribed region (GenBank accession numberAF177701). Figure 5 shows the sequence of the promoter. The first 200 bp upstream from the transcription initiation site are of high GC content (75%), as is typical of a promoter region. At position −28 there is a TATA-box and at position −77 a CAAT-box. Four SP-1 sites were detected, at positions −48, −107, −135, and −184. These elements are presumably responsible for the constitutive activity of the promoter (Dierks et al., 1983). At positions −936 and −1581 we found serum response element (SRE) consensus sequences, which might account for the serum responsiveness of this gene (Link et al., 1995). Similar to c-fos, the core SRE sequence of arg3.1/arc is flanked by sequences that support a stable formation of a ternary complex (Treisman et al., 1992). At positions −205 and −671 we detected AP-1 consensus sequences that are also contained within the c-fos promoter. Despite these similarities to c-fos, 1737 bp of the arg3.1/arc promoter do not contain the CRE consensus sequence TGACGTCA. To test whether this arg3.1/arc promoter segment is inducible by cAMP, we fused it with a luciferase reporter gene and generated stable transfected PC12 cells. In addition, we tested two smaller fragments deleting one or both of the SRE. The SRE can be activated by MAPK in hippocampal cultures by Elk-1 (Xia et al., 1996), and during LTP stimulation Elk-1 is phosphorylated in a MAPK-dependent manner (Davis et al., 2000). The arg3.1/arc promoter contains two SRE consensus sequences. However, our deletion analysis of arg3.1/arc promoter constructs in PC12 cells shows that these SRE sites are without influence on the transcriptional activation of the gene by cAMP. Figure 6 shows that in contrast to the endogenous arg3.1/arc gene all three deletion constructs were only modestly inducible by forskolin. Moreover, transient transfection experiments indicated that the observed weak residual inducibility represents a function of the basic vector itself and not of the inserted arg3.1/arc sequences (data not shown). These experiments indicate that 1737 bp of the arg3.1/arc promoter, containing SRE and AP1 sites, are not sufficient to mediate induction by forskolin. Certain aspects of chromatin structure that are not fully conserved in the transfection assay might be necessary for the controlled expression of arg3.1/arc, or alternatively, additional elements positioned outside of the tested promoter region may be required for induction. Such elements could be CRE sites that are located more distantly.
Fig. 4.
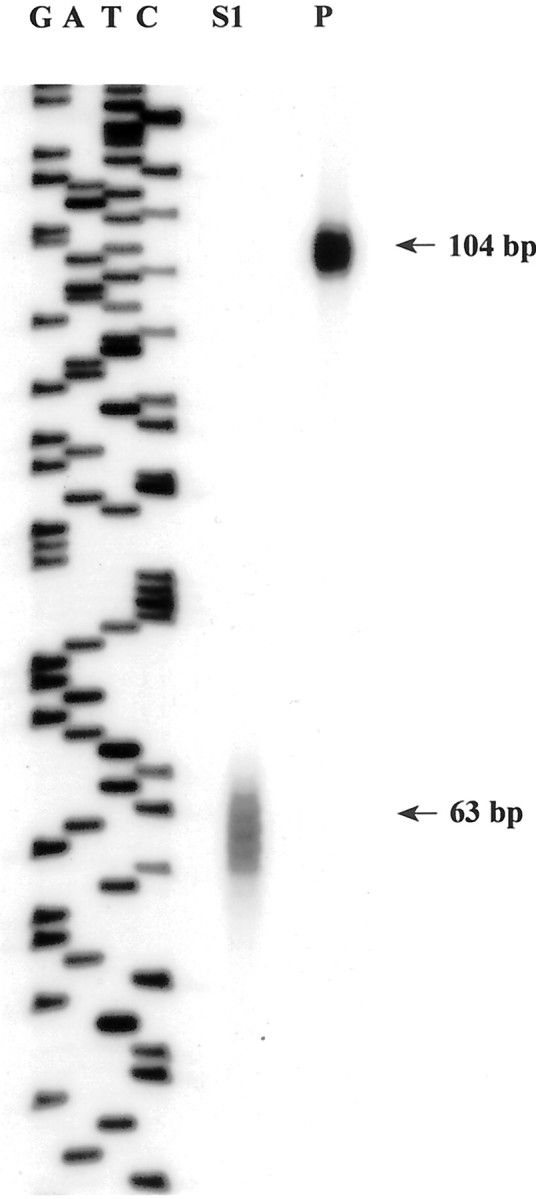
S1 nuclease mapping of the transcription start site. Right lane (P), Undigested S1-arg3.1/arc probe. Middle lane (S1), S1 nuclease-digested S1-arg3.1/arc oligonucleotide after hybridization with total RNA prepared from 60 min serum-stimulated NIH3T3 cells. The sequence of M13 on the left served as a length marker.
Fig. 5.

Sequence of the arg3.1/arc promoter region. The transcription start site is marked with asterisks. Promoter element consensus sequences are boxed. For a detailed description of the regulatory elements, refer to Results. A satellite sequence is underlined. The first codons of the reading frame are translated.
Fig. 6.

Response of the arg3.1/arc promoter to forskolin. Arg3.1/arc promoter deletions extending from position −1737 to +250, −834 to +250, and −177 to +250 were fused to a luciferase reporter. The reporter constructs were used to stably transfect PC12 cells. Cells were stimulated with forskolin for 1 hr. Inducibility of the arg3.1/arc promoter deletion constructs and that of the endogenous arg3.1/arc promoter was determined by Northern blot analyses.A, Autoradiograph of a Northern blot hybridized with a probe specific for luciferase to measure the inducibility of the transfected arg3.1/arc promoter deletions. Hybridization with a probe specific for GAPDH was used as a loading control.Numbers on the left indicate the 5′ deletion endpoints of the arg3.1/arc promoter constructs. Plus signs in the corresponding line indicate the presence of the respective constructs. The first three lanes from theleft contained RNA from forskolin-stimulated cells; thethree lanes on the right contained RNA from mock-stimulated cells. B, Autoradiograph of a Northern blot hybridized with a probe specific for arg3.1/arc to measure the inducibility of the endogenous arg3.1/arc promoter and a probe specific for GAPDH. Labels are as in A.C, Quantification of Northern blots (n = 3). Hybridization signals were normalized against mock-stimulated control and GAPDH signals. Error bars represent the SEM; p < 0.05 compared with control (two-tailed Student's t test). Note that transient transfection experiments indicated that the observed weak residual inducibility represents a function of the basic vector itself and not of the inserted arg3.1/arc sequences (data not shown).
Induced expression of arg3.1/arc is dependent on the activation of PKA
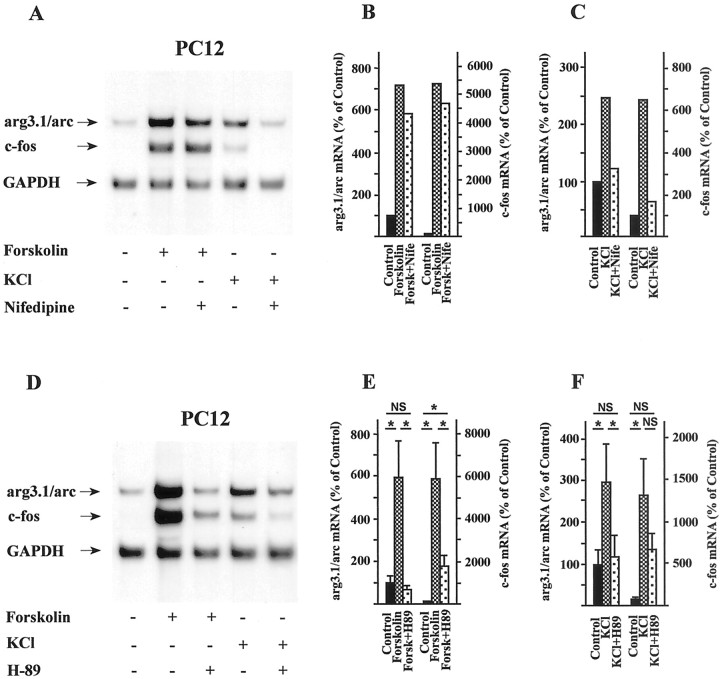
To delineate the signaling pathways that are required for the activation of arg3.1/arc transcription, we made use of various pharmacological inhibitors. Nifedipine, a blocker of VSCCs (Toll, 1982), effectively blocked the induction elicited by membrane depolarization of c-fos (Morgan and Curran, 1986) and arg3.1/arc but had no significant effect on the cAMP-induced expression of either gene (Fig.7A–C). These results demonstrate that calcium influx through VSCCs is necessary for membrane depolarization-induced expression of arg3.1/arc mRNA. We next asked whether the calcium- and cAMP-induced expression of arg3.1/arc mRNA in PC12 cells is dependent on PKA. H89 is a potent and selective inhibitor of PKA but does not inhibit CaM kinase, protein kinase C, casein kinase II, or cGMP-dependent protein kinase at the concentrations used in the present experiments (Chijiwa et al., 1990). In PC12 cells both KCl- and forskolin-induced expression of arg3.1/arc mRNA was completely abolished in the presence of the PKA inhibitor H-89. Similarly, induced expression of c-fos was also greatly diminished (Ginty et al., 1991) (Fig.7D–F). The small remaining increase in c-fos mRNA levels in the presence of the PKA blocker might be accounted for by cAMP-mediated activation of VSCCs (Sculptoreanu et al., 1993), leading to calcium influx and stimulation of calcium–calmodulin-dependent kinases, which phosphorylate CREB (Sheng et al., 1991; Deisseroth et al., 1996). Alternatively, it is conceivable that the inhibitor might have an effect on protein synthesis and thereby stabilize c-fos mRNA. In any case, these data indicate that calcium- and cAMP-induced expression of arg3.1/arc and c-fos mRNA is dependent on the activation of PKA.
Fig. 7.
Induced levels of arg3.1/arc and c-fos mRNA can be blocked by calcium channel and protein kinase A inhibitors in PC12 cells. PC12 cells were pretreated for 30 min with the indicated inhibitors and subsequently stimulated by the addition of either KCl or forskolin. A,D, Autoradiographs of Northern blots hybridized to probes specific for arg3.1/arc, c-fos, and GAPDH.B, E, Quantification of hybridization signals after forskolin stimulation. C,F, Quantification of hybridization signals after KCl depolarization. A–C, Effect of the calcium channel blocker nifedipine (10 μm) on induced arg3.1/arc and c-fos mRNA levels (n= 2). D–F, Effect of the protein kinase A blocker H-89 (20 μm) on induced arg3.1/arc and c-fos mRNA levels (n = 3). Error bars represent SEM; *p < 0.05 (two-tailed Student's t test); NS, not significant,p > 0.05. The induction of arg3.1/arc mRNA by forskolin and KCl was completely blocked by the PKA inhibitor.
cAMP-induced expression of arg3.1/arc is dependent on the activation of MAPK
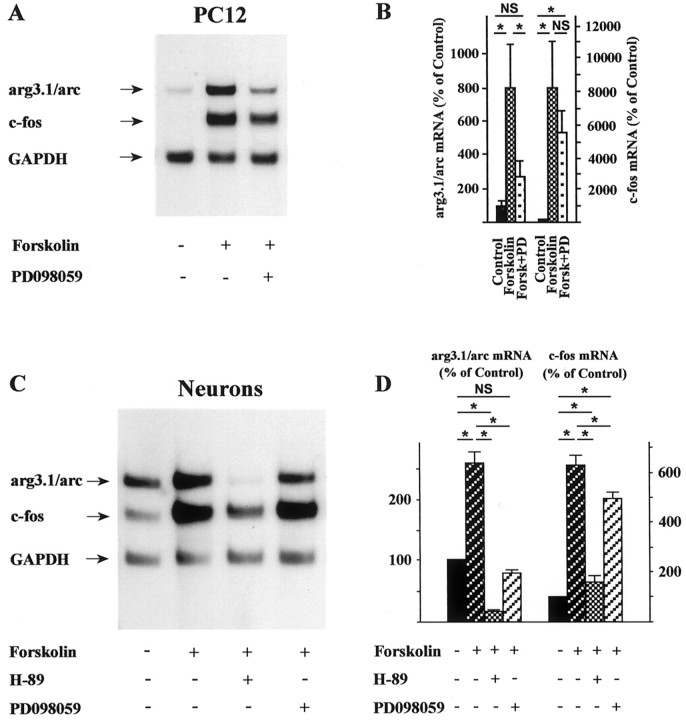
After cAMP stimulation PKA can activate MAPK in PC12 cells (Vossler et al., 1997). In this instance, PKA phosphorylates the small G-protein Rap1 and increases GTP loading (Altschuler and Lapetina, 1993). This in turn leads to the activation of B-Raf, which can phosphorylate the MAPK/ERK kinase (MEK) and finally results in the activation of MAPK. Interestingly, this pathway is absent in NIH3T3 cells, which do not express B-Raf at significant levels (Vossler et al., 1997). With an interest in the possibility that cAMP/PKA-mediated induction of arg3.1/arc is dependent on this signaling mechanism, the effect of the selective MEK inhibitor PD098059 (Alessi et al., 1995) was examined. In the presence of the MEK inhibitor, cAMP-mediated induction of arg3.1/arc in PC12 cells was greatly reduced, whereas the induction of c-fos was largely retained (Fig.8A,B). Similar to PC12 cells (Figs. 7D,E,8A,B), in primary cultures of hippocampal neurons, expression of both arg3.1/arc and c-fosmRNA was strongly induced by forskolin (Fig.8C,D). The PKA blocker H-89 dramatically reduced cAMP-induced arg3.1/arc mRNA levels to below constitutive levels. Induced transcription of c-fos was also greatly reduced. In neurons pretreated with the MEK inhibitor PD098059, activation of arg3.1/arc transcription by forskolin was blocked. In contrast, the activation of c-fos by cAMP was only slightly attenuated by the MEK inhibitor (Fig. 8C,D).
Fig. 8.
Forskolin-induced arg3.1/arc mRNA levels are blocked by MAPK/ERK kinase inhibitor in PC12 cells and primary cultures of hippocampal neurons. PC12 cells and primary cultures of hippocampal neurons were pretreated with the MAPK/ERK kinase blocker PD098059 (50 μm) and subsequently stimulated by forskolin (n = 3). Hippocampal neurons were also pretreated with the PKA blocker H-89 (compare with Fig.7D,E). A,C, Autoradiographs of Northern blots loaded with RNA from PC12 cells (A) and RNA from hippocampal neurons (C). Hybridization was with probes specific for arg3.1/arc, c-fos, and GAPDH.B,D, Quantification of arg3.1/arc and c-fos hybridization signals from analyses of PC12 cells and hippocampal neurons, respectively. Error bars represent SEM; *p < 0.05 (two-tailed Student'st test); NS, not significant,p > 0.05. Although the induction of arg3.1/arc mRNA by forskolin was blocked in PC12 cells and hippocampal neurons by the MAPK/ERK kinase inhibitor, induction of c-fos mRNA was affected to a much lesser extent.
DISCUSSION
Activity-dependent alterations in synaptic efficacy are thought to underlie learning and memory, epileptogenesis, drug addiction, and several neurological diseases (Nestler and Aghajanian, 1997; Milner et al., 1998). To stabilize changes in synaptic strength, neurons activate a program of gene expression that results in alterations of their molecular composition and structure. Among activity-dependent genes, arg3.1/arc is thus far unique, because its mRNA has the potential to be locally translated at stimulated synapses and consequently might play a key role in synapse-specific modifications (Kuhl and Skehel, 1998). Importantly, arg3.1/arc is the first and only gene with expression that has been directly linked to information processing (Guzowski et al., 1999). Moreover, arg3.1/arc is reliably induced with LTP-producing stimulation. The strict association of induced arg3.1/arc expression in cells that have established LTP further indicates that arg3.1/arc plays a role in the maintenance or consolidation phase of this process. In this study we analyzed the signaling pathways that contribute to the induction of arg3.1/arc. Of particular interest to us was the examination of signaling pathways that had been shown to be important for the establishment of long-lasting synaptic plasticity.
Here we demonstrate that transcription of arg3.1/arc mRNA in neurons can be induced by calcium and cAMP. This inducibility is strictly dependent on the activation of PKA and the MAPK/ERK kinase signaling pathways, which have been demonstrated to play specific roles in learning and memory and synaptic plasticity (Frey et al., 1993; Abel et al., 1997; English and Sweatt, 1997; Martin et al., 1997; Berman et al., 1998; Blum et al., 1999; Coogan et al., 1999; Rosenblum et al., 2000). Moreover, our experiments suggest indirectly that induction of arg3.1/arc may not require the transcription factor CREB. This assumption is based on three independent observations, but alternative explanations may exist, as indicated below.
First, CRE sites are typically located within the first few hundred nucleotides of cAMP-responsive genes (Sassone-Corsi, 1995; Montminy, 1997). We did not detect a CRE site within 1737 bp of the arg3.1/arc promoter or the entire transcribed region of 3.5 kb. At position +111 the arg3.1/arc promoter contains a single CGTCA sequence; however, such an incomplete CRE site is not active (Fink et al., 1988) and does not bind to CREB by itself (Nichols et al., 1992). However, we note that 1737 bp of the arg3.1/arc promoter proved unresponsive to forskolin stimulation in transfection experiments; therefore, these data do not exclude the possibility that unidentified CRE sites lie outside of this region. Second, in PC12 cells and hippocampal neurons, both arg3.1/arc and c-fos transcripts are strongly induced by cAMP. In strong contrast, although CREB is activated by cAMP stimulation in NIH3T3 and Neuro2a cells and the transcription of the CRE-containing c-fos gene is consequently induced (Fisch et al., 1989;Gonzalez and Montminy, 1989), we observed no induction of arg3.1/arc in these two cell lines. Third, in cAMP-stimulated PC12 cells and hippocampal neurons that had been pretreated with a MAPK/ERK kinase inhibitor, the induction of arg3.1/arc was effectively blocked. Similar to NIH3T3 cells in which B-Raf is not expressed at levels sufficient to activate MAPK/ERK kinase (Vossler et al., 1997), cAMP can mediate induction of c-fos transcription in the MAPK/ERK kinase inhibitor-treated neurons, presumably through the PKA/CREB or calcium–calmodulin kinase/CREB pathway (Deisseroth et al., 1996). These data argue, at face value, against an involvement of CREB in the induction of arg3.1/arc. However, an alternative explanation may exist if we assume that the expression of arg3.1/arc is under both positive and negative control. In such a scenario cAMP-induced expression of arg3.1/arc would be under the control of a repressor and a positive activator, which may be CREB. Release of the repressor would depend strictly on the MAPK pathway, and in cell lines that carry a defect in this pathway, arg3.1/arc would be unresponsive to stimulation, although CREB was activated.
Phosphorylation of CREB directed by cAMP- and calcium-influenced signaling pathways has been shown to be important in the neuronal signaling processes leading to the formation and retention of long-term memory in invertebrates and vertebrates (Dash et al., 1990;Bourtchuladze et al., 1994; Bartsch et al., 1995; Yin and Tully, 1996;Guzowski and McGaugh, 1997). Although CREB plays a crucial role in memory in Aplysia and Drosophila, recent studies on mice with hypomorphic CREB alleles suggest that more complex pathways may exist in mammals (Gass et al., 1998). Moreover, the transcription factor CREB is activated by diverse extracellular stimuli through multiple signaling cascades and in addition to synaptic plasticity seems to play a role in developmental and adaptive responses (Datta et al., 1999; Finkbeiner, 2000; Walton and Dragunow, 2000). Although the programs of gene expression in these distinct biological functions of CREB might overlap and specificity might be brought about by cooperation with other transcriptional factors, our studies suggest that in addition parallel pathways may exist. To our knowledge arg3.1/arc is the first activity-dependent gene with stimulus-dependent transcriptional activation that relies solely on the MAPK/ERK pathway. It will be interesting to determine whether other plasticity-associated genes are regulated in the same manner.
Footnotes
R.W. and P.W. were supported by the Graduiertenkolleg Grant GRK255 to D.K. This research was supported by Deutsche Forschungsgemeinschaft Grants SFB444 and FOR296 to D.K. We thank Drs. U. Müller and C. Weissmann for library AB-1, and Drs. N. Irwin and L. Benowitz for PC12 cells.
Correspondence should be addressed to Dietmar Kuhl, Zentrum für Molekulare Neurobiologie Hamburg, Martinistrasse 52, 20246 Hamburg, Germany. E-mail: dietmar.kuhl@zmnh.uni-hamburg.de.
R. Waltereit's present address: Department of Neurology, University of Tuebingen, Hoppe-Seyler-Strasse 3, 72076 Tuebingen, Germany.
G. Kauselmann's present address: Artemis Pharmaceuticals GmbH, Neurather Ring 1, 51063 Cologne, Germany.
M. Bundman's present address: Institute for Experimental Pathology, University of Muenster, Von-Esmarch-Strasse 56, 48149 Muenster, Germany.
REFERENCES
- 1.Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R. Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell. 1997;88:615–626. doi: 10.1016/s0092-8674(00)81904-2. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WC, Mason SE, Demmer J, Williams JM, Richardson CL, Tate WP, Lawlor PA, Dragunow M. Correlations between immediate early gene induction and the persistence of long-term potentiation. Neuroscience. 1993;56:717–727. doi: 10.1016/0306-4522(93)90369-q. [DOI] [PubMed] [Google Scholar]
- 3.Alessi DR, Cuenda A, Cohen P, Dudley DT, Saltiel AR. PD 098059 is a specific inhibitor of the activation of mitogen-activated protein kinase kinase in vitro and in vivo. J Biol Chem. 1995;270:27489–27494. doi: 10.1074/jbc.270.46.27489. [DOI] [PubMed] [Google Scholar]
- 4.Altschuler D, Lapetina EG. Mutational analysis of the cAMP-dependent protein kinase-mediated phosphorylation site of Rap1b. J Biol Chem. 1993;268:7527–7531. [PubMed] [Google Scholar]
- 5.Atkins CM, Selcher JC, Petraitis JJ, Trzaskos JM, Sweatt JD. The MAPK cascade is required for mammalian associative learning. Nat Neurosci. 1998;1:602–609. doi: 10.1038/2836. [DOI] [PubMed] [Google Scholar]
- 6.Bartel DP, Sheng M, Lau LF, Greenberg ME. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989;3:304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- 7.Bartsch D, Ghirardi M, Skehel PA, Karl KA, Herder SP, Chen M, Bailey CH, Kandel ER. Aplysia CREB2 represses long-term facilitation: relief of repression converts transient facilitation into long-term functional and structural change. Cell. 1995;83:979–992. doi: 10.1016/0092-8674(95)90213-9. [DOI] [PubMed] [Google Scholar]
- 8.Berman DE, Hazvi S, Rosenblum K, Seger R, Dudai Y. Specific and differential activation of mitogen-activated protein kinase cascades by unfamiliar taste in the insular cortex of the behaving rat. J Neurosci. 1998;18:10037–10044. doi: 10.1523/JNEUROSCI.18-23-10037.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 10.Blum S, Moore AN, Adams F, Dash PK. A mitogen-activated protein kinase cascade in the CA1/CA2 subfield of the dorsal hippocampus is essential for long-term spatial memory. J Neurosci. 1999;19:3535–3544. doi: 10.1523/JNEUROSCI.19-09-03535.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bourtchuladze R, Frenguelli B, Blendy J, Cioffi D, Schutz G, Silva AJ. Deficient long-term memory in mice with a targeted mutation of the cAMP- responsive element-binding protein. Cell. 1994;79:59–68. doi: 10.1016/0092-8674(94)90400-6. [DOI] [PubMed] [Google Scholar]
- 12.Cali JJ, Zwaagstra JC, Mons N, Cooper DM, Krupinski J. Type VIII adenylyl cyclase. A Ca2+/calmodulin-stimulated enzyme expressed in discrete regions of rat brain. J Biol Chem. 1994;269:12190–12195. [PubMed] [Google Scholar]
- 13.Chijiwa T, Mishima A, Hagiwara M, Sano M, Hayashi K, Inoue T, Naito K, Toshioka T, Hidaka H. Inhibition of forskolin-induced neurite outgrowth and protein phosphorylation by a newly synthesized selective inhibitor of cyclic AMP-dependent protein kinase, N-[2-(p-bromocinnamylamino)ethyl]-5- isoquinolinesulfonamide (H-89), of PC12D pheochromocytoma cells. J Biol Chem. 1990;265:5267–5272. [PubMed] [Google Scholar]
- 14.Cole AJ, Saffen DW, Baraban JM, Worley PF. Rapid increase of an immediate early gene messenger RNA in hippocampal neurons by synaptic NMDA receptor activation. Nature. 1989;340:474–476. doi: 10.1038/340474a0. [DOI] [PubMed] [Google Scholar]
- 15.Coogan AN, O'Neill LA, O'Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93:57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- 16.Curran T, Morgan JI. Memories of fos. BioEssays. 1987;7:255–258. doi: 10.1002/bies.950070606. [DOI] [PubMed] [Google Scholar]
- 17.Curran T, Gordon MB, Rubino KL, Sambucetti LC. Isolation and characterization of the c-fos(rat) cDNA and analysis of post-translational modification in vitro. Oncogene. 1987;2:79–84. [PubMed] [Google Scholar]
- 18.Dash PK, Hochner B, Kandel ER. Injection of the cAMP-responsive element into the nucleus of Aplysia sensory neurons blocks long-term facilitation. Nature. 1990;345:718–721. doi: 10.1038/345718a0. [DOI] [PubMed] [Google Scholar]
- 19.Datta SR, Brunet A, Greenberg ME. Cellular survival: a play in three Akts. Dev. 1999;13:2905–2927. doi: 10.1101/gad.13.22.2905. [DOI] [PubMed] [Google Scholar]
- 20.Davis S, Vanhoutte P, Pages C, Caboche J, Laroche S. The MAPK/ERK cascade targets both Elk-1 and cAMP response element-binding protein to control long-term potentiation-dependent gene expression in the dentate gyrus in vivo. J Neurosci. 2000;20:4563–4572. doi: 10.1523/JNEUROSCI.20-12-04563.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 22.Dierks P, van Ooyen A, Cochran MD, Dobkin C, Reiser J, Weissmann C. Three regions upstream from the cap site are required for efficient and accurate transcription of the rabbit beta-globin gene in mouse 3T6 cells. Cell. 1983;32:695–706. doi: 10.1016/0092-8674(83)90055-7. [DOI] [PubMed] [Google Scholar]
- 23.Dragunow M, Abraham WC, Goulding M, Mason SE, Robertson HA, Faull RL. Long-term potentiation and the induction of c-fos mRNA and proteins in the dentate gyrus of unanesthetized rats. Neurosci Lett. 1989;101:274–280. doi: 10.1016/0304-3940(89)90545-4. [DOI] [PubMed] [Google Scholar]
- 24.English JD, Sweatt JD. Activation of p42 mitogen-activated protein kinase in hippocampal long term potentiation. J Biol Chem. 1996;271:24329–24332. doi: 10.1074/jbc.271.40.24329. [DOI] [PubMed] [Google Scholar]
- 25.English JD, Sweatt JD. A requirement for the mitogen-activated protein kinase cascade in hippocampal long term potentiation. J Biol Chem. 1997;272:19103–19106. doi: 10.1074/jbc.272.31.19103. [DOI] [PubMed] [Google Scholar]
- 26.Fink JS, Verhave M, Kasper S, Tsukada T, Mandel G, Goodman RH. The CGTCA sequence motif is essential for biological activity of the vasoactive intestinal peptide gene cAMP-regulated enhancer. Proc Natl Acad Sci USA. 1988;85:6662–6666. doi: 10.1073/pnas.85.18.6662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Finkbeiner S. CREB couples neurotrophin signals to survival messages. Neuron. 2000;25:11–14. doi: 10.1016/s0896-6273(00)80866-1. [DOI] [PubMed] [Google Scholar]
- 28.Fisch TM, Prywes R, Simon MC, Roeder RG. Multiple sequence elements in the c-fos promoter mediate induction by cAMP. Genes Dev. 1989;3:198–211. doi: 10.1101/gad.3.2.198. [DOI] [PubMed] [Google Scholar]
- 29.Fort P, Marty L, Piechaczyk M, el Sabrouty S, Dani C, Jeanteur P, Blanchard JM. Various rat adult tissues express only one major mRNA species from the glyceraldehyde-3-phosphate-dehydrogenase multigenic family. Nucleic Acids Res. 1985;13:1431–1442. doi: 10.1093/nar/13.5.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Frank DA, Greenberg ME. CREB: a mediator of long-term memory from mollusks to mammals. Cell. 1994;79:5–8. doi: 10.1016/0092-8674(94)90394-8. [DOI] [PubMed] [Google Scholar]
- 31.Frey U, Huang YY, Kandel ER. Effects of cAMP simulate a late stage of LTP in hippocampal CA1 neurons. Science. 1993;260:1661–1664. doi: 10.1126/science.8389057. [DOI] [PubMed] [Google Scholar]
- 32.Frey U, Muller M, Kuhl D. A different form of long-lasting potentiation revealed in tissue plasminogen activator mutant mice. J Neurosci. 1996;16:2057–2063. doi: 10.1523/JNEUROSCI.16-06-02057.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gass P, Wolfer DP, Balschun D, Rudolph D, Frey U, Lipp HP, Schutz G. Deficits in memory tasks of mice with CREB mutations depend on gene dosage. Learn Mem. 1998;5:274–288. [PMC free article] [PubMed] [Google Scholar]
- 34.Ginty DD, Glowacka D, Bader DS, Hidaka H, Wagner JA. Induction of immediate early genes by Ca2+ influx requires cAMP-dependent protein kinase in PC12 cells. J Biol Chem. 1991;266:17454–17458. [PubMed] [Google Scholar]
- 35.Goelet P, Castellucci VF, Schacher S, Kandel ER. The long and the short of long-term memory—a molecular framework. Nature. 1986;322:419–422. doi: 10.1038/322419a0. [DOI] [PubMed] [Google Scholar]
- 36.Gonzalez GA, Montminy MR. Cyclic AMP stimulates somatostatin gene transcription by phosphorylation of CREB at serine 133. Cell. 1989;59:675–680. doi: 10.1016/0092-8674(89)90013-5. [DOI] [PubMed] [Google Scholar]
- 37.Greenberg ME, Ziff EB, Greene LA. Stimulation of neuronal acetylcholine receptors induces rapid gene transcription. Science. 1986;234:80–83. doi: 10.1126/science.3749894. [DOI] [PubMed] [Google Scholar]
- 38.Guzowski JF, McGaugh JL. Antisense oligodeoxynucleotide-mediated disruption of hippocampal cAMP response element binding protein levels impairs consolidation of memory for water maze training. Proc Natl Acad Sci USA. 1997;94:2693–2698. doi: 10.1073/pnas.94.6.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- 40.Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Huang YY, Li XC, Kandel ER. cAMP contributes to mossy fiber LTP by initiating both a covalently mediated early phase and macromolecular synthesis-dependent late phase. Cell. 1994;79:69–79. doi: 10.1016/0092-8674(94)90401-4. [DOI] [PubMed] [Google Scholar]
- 42.Impey S, Obrietan K, Wong ST, Poser S, Yano S, Wayman G, Deloulme JC, Chan G, Storm DR. Cross talk between ERK and PKA is required for Ca2+ stimulation of CREB-dependent transcription and ERK nuclear translocation. Neuron. 1998;21:869–883. doi: 10.1016/s0896-6273(00)80602-9. [DOI] [PubMed] [Google Scholar]
- 43.Kauselmann G, Weiler M, Wulff P, Jessberger S, Konietzko U, Scafidi J, Staubli U, Bereiter-Hahn J, Strebhardt K, Kuhl D. The polo-like protein kinases Fnk and Snk associate with a Ca(2+)- and integrin-binding protein and are regulated dynamically with synaptic plasticity. EMBO J. 1999;18:5528–5539. doi: 10.1093/emboj/18.20.5528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Konietzko U, Kauselmann G, Scafidi J, Staubli U, Mikkers H, Berns A, Schweizer M, Waltereit R, Kuhl D. Pim kinase expression is induced by LTP-stimulation and required for the consolidation of enduring LTP. EMBO J. 1999;18:3359–3369. doi: 10.1093/emboj/18.12.3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kruijer W, Schubert D, Verma IM. Induction of the proto-oncogene fos by nerve growth factor. Proc Natl Acad Sci USA. 1985;82:7330–7334. doi: 10.1073/pnas.82.21.7330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Kuhl D. Learning about activity-dependent genes. In: Baudry M, Davis JL, Thompson RF, editors. Advances in synaptic plasticity. MIT; Boston: 2000. pp. 1–31. [Google Scholar]
- 47.Kuhl D, Skehel P. Dendritic localization of mRNAs. Curr Opin Neurobiol. 1998;8:600–606. doi: 10.1016/s0959-4388(98)80087-1. [DOI] [PubMed] [Google Scholar]
- 48.Kuhl D, de la Fuente J, Chaturvedi M, Parimoo S, Ryals J, Meyer F, Weissmann C. Reversible silencing of enhancers by sequences derived from the human IFN-alpha promoter. Cell. 1987;50:1057–1069. doi: 10.1016/0092-8674(87)90172-3. [DOI] [PubMed] [Google Scholar]
- 49.Kuhl D, Kennedy TE, Barzilai A, Kandel ER. Long-term sensitization training in Aplysia leads to an increase in the expression of BiP, the major protein chaperon of the ER. J Cell Biol. 1992;119:1069–1076. doi: 10.1083/jcb.119.5.1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate early gene is regulated by synaptic activity. Proc Natl Acad Sci USA. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- 52.Lynch G, Larson J, Kelso S, Barrionuevo G, Schottler F. Intracellular injections of EGTA block induction of hippocampal long-term potentiation. Nature. 1983;305:719–721. doi: 10.1038/305719a0. [DOI] [PubMed] [Google Scholar]
- 53.Malenka RC, Lancaster B, Zucker RS. Temporal limits on the rise in postsynaptic calcium required for the induction of long-term potentiation. Neuron. 1992;9:121–128. doi: 10.1016/0896-6273(92)90227-5. [DOI] [PubMed] [Google Scholar]
- 54.Martin KC, Kandel ER. Cell adhesion molecules, CREB, and the formation of new synaptic connections. Neuron. 1996;17:567–570. doi: 10.1016/s0896-6273(00)80188-9. [DOI] [PubMed] [Google Scholar]
- 55.Martin KC, Michael D, Rose JC, Barad M, Casadio A, Zhu H, Kandel ER. MAP kinase translocates into the nucleus of the presynaptic cell and is required for long-term facilitation in Aplysia. Neuron. 1997;18:899–912. doi: 10.1016/s0896-6273(00)80330-x. [DOI] [PubMed] [Google Scholar]
- 56.Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- 57.Montminy M. Transcriptional regulation by cyclic AMP. Annu Rev Biochem. 1997;66:807–822. doi: 10.1146/annurev.biochem.66.1.807. [DOI] [PubMed] [Google Scholar]
- 58.Morgan JI, Curran T. Role of ion flux in the control of c-fos expression. Nature. 1986;322:552–555. doi: 10.1038/322552a0. [DOI] [PubMed] [Google Scholar]
- 59.Morgan JI, Curran T. Immediate-early genes: ten years on. Trends Neurosci. 1995;18:66–67. [PubMed] [Google Scholar]
- 60.Morgan JI, Cohen DR, Hempstead JL, Curran T. Mapping patterns of c-fos expression in the central nervous system after seizure. Science. 1987;237:192–197. doi: 10.1126/science.3037702. [DOI] [PubMed] [Google Scholar]
- 61.Nedivi E, Hevroni D, Naot D, Israeli D, Citri Y. Numerous candidate plasticity-related genes revealed by differential cDNA cloning. Nature. 1993;363:718–722. doi: 10.1038/363718a0. [DOI] [PubMed] [Google Scholar]
- 62.Nestler EJ, Aghajanian GK. Molecular and cellular basis of addiction. Science. 1997;278:58–63. doi: 10.1126/science.278.5335.58. [DOI] [PubMed] [Google Scholar]
- 63.Nichols M, Weih F, Schmid W, DeVack C, Kowenz-Leutz E, Luckow B, Boshart M, Schutz G. Phosphorylation of CREB affects its binding to high and low affinity sites: implications for cAMP induced gene transcription. EMBO J. 1992;11:3337–3346. doi: 10.1002/j.1460-2075.1992.tb05412.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Orban PC, Chapman PF, Brambilla R. Is the Ras-MAPK signalling pathway necessary for long-term memory formation? Trends Neurosci. 1999;22:38–44. doi: 10.1016/s0166-2236(98)01306-x. [DOI] [PubMed] [Google Scholar]
- 65.Papa M, Bundman MC, Greenberger V, Segal M. Morphological analysis of dendritic spine development in primary cultures of hippocampal neurons. J Neurosci. 1995;15:1–11. doi: 10.1523/JNEUROSCI.15-01-00001.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Qian Z, Gilbert ME, Colicos MA, Kandel ER, Kuhl D. Tissue-plasminogen activator is induced as an immediate-early gene during seizure, kindling and long-term potentiation. Nature. 1993;361:453–457. doi: 10.1038/361453a0. [DOI] [PubMed] [Google Scholar]
- 67.Quandt K, Frech K, Karas H, Wingender E, Werner T. MatInd and MatInspector: new fast and versatile tools for detection of consensus matches in nucleotide sequence data. Nucleic Acids Res. 1995;23:4878–4884. doi: 10.1093/nar/23.23.4878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rosenblum K, Futter M, Jones M, Hulme EC, Bliss TV. ERKI/II regulation by the muscarinic acetylcholine receptors in neurons. J Neurosci. 2000;20:977–985. doi: 10.1523/JNEUROSCI.20-03-00977.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saffen DW, Cole AJ, Worley PF, Christy BA, Ryder K, Baraban JM. Convulsant-induced increase in transcription factor messenger RNAs in rat brain. Proc Natl Acad Sci USA. 1988;85:7795–7799. doi: 10.1073/pnas.85.20.7795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Sanger F, Nicklen S, Coulson AR. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Sassone-Corsi P. Transcription factors responsive to cAMP. Annu Rev Cell Dev Biol. 1995;11:355–377. doi: 10.1146/annurev.cb.11.110195.002035. [DOI] [PubMed] [Google Scholar]
- 72.Sculptoreanu A, Scheuer T, Catterall WA. Voltage-dependent potentiation of L-type Ca2+ channels due to phosphorylation by cAMP-dependent protein kinase. Nature. 1993;364:240–243. doi: 10.1038/364240a0. [DOI] [PubMed] [Google Scholar]
- 73.Sheng M, Greenberg ME. The regulation and function of c-fos and other immediate early genes in the nervous system. Neuron. 1990;4:477–485. doi: 10.1016/0896-6273(90)90106-p. [DOI] [PubMed] [Google Scholar]
- 74.Sheng M, Thompson MA, Greenberg ME. CREB: a Ca(2+)-regulated transcription factor phosphorylated by calmodulin-dependent kinases. Science. 1991;252:1427–1430. doi: 10.1126/science.1646483. [DOI] [PubMed] [Google Scholar]
- 75.Staubli U, Scafidi J. Studies on long-term depression in area CA1 of the anesthetized and freely moving rat. J Neurosci. 1997;17:4820–4828. doi: 10.1523/JNEUROSCI.17-12-04820.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- 77.Toll L. Calcium antagonists high-affinity binding and inhibition of calcium transport in a clonal cell line. J Biol Chem. 1982;257:13189–13192. [PubMed] [Google Scholar]
- 78.Treisman R, Marais R, Wynne J. Spatial flexibility in ternary complexes between SRF and its accessory proteins. EMBO J. 1992;11:4631–4640. doi: 10.1002/j.1460-2075.1992.tb05565.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vossler MR, Yao H, York RD, Pan MG, Rim CS, Stork PJ. cAMP activates MAP kinase and Elk-1 through a B-Raf- and Rap1-dependent pathway. Cell. 1997;89:73–82. doi: 10.1016/s0092-8674(00)80184-1. [DOI] [PubMed] [Google Scholar]
- 80.Wallace CS, Lyford GL, Worley PF, Steward O. Differential intracellular sorting of immediate early gene mRNAs depends on signals in the mRNA sequence. J Neurosci. 1998;18:26–35. doi: 10.1523/JNEUROSCI.18-01-00026.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Walton MR, Dragunow I. Is CREB a key to neuronal survival? Trends Neurosci. 2000;23:48–53. doi: 10.1016/s0166-2236(99)01500-3. [DOI] [PubMed] [Google Scholar]
- 82.Worley PF, Bhat RV, Baraban JM, Erickson CA, McNaughton BL, Barnes CA. Thresholds for synaptic activation of transcription factors in hippocampus: correlation with long-term enhancement. J Neurosci. 1993;13:4776–4786. doi: 10.1523/JNEUROSCI.13-11-04776.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Xia Z, Dudek H, Miranti CK, Greenberg ME. Calcium influx via the NMDA receptor induces immediate early gene transcription by a MAP kinase/ERK-dependent mechanism. J Neurosci. 1996;16:5425–5436. doi: 10.1523/JNEUROSCI.16-17-05425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Xia ZG, Refsdal CD, Merchant KM, Dorsa DM, Storm DR. Distribution of mRNA for the calmodulin-sensitive adenylate cyclase in rat brain: expression in areas associated with learning and memory. Neuron. 1991;6:431–443. doi: 10.1016/0896-6273(91)90251-t. [DOI] [PubMed] [Google Scholar]
- 85.Yamagata K, Andreasson KI, Kaufmann WE, Barnes CA, Worley PF. Expression of a mitogen-inducible cyclooxygenase in brain neurons: regulation by synaptic activity and glucocorticoids. Neuron. 1993;11:371–386. doi: 10.1016/0896-6273(93)90192-t. [DOI] [PubMed] [Google Scholar]
- 86.Yin JC, Tully T. CREB and the formation of long-term memory. Curr Opin Neurobiol. 1996;6:264–268. doi: 10.1016/s0959-4388(96)80082-1. [DOI] [PubMed] [Google Scholar]
- 87.Yin JC, Wallach JS, Del VM, Wilder EL, Zhou H, Quinn WG, Tully T. Induction of a dominant negative CREB transgene specifically blocks long-term memory in Drosophila. Cell. 1994;79:49–58. doi: 10.1016/0092-8674(94)90399-9. [DOI] [PubMed] [Google Scholar]
- 88.Yin JC, Del VM, Zhou H, Tully T. CREB as a memory modulator: induced expression of a dCREB2 activator isoform enhances long-term memory in Drosophila. Cell. 1995;81:107–115. doi: 10.1016/0092-8674(95)90375-5. [DOI] [PubMed] [Google Scholar]